Abstract
The circulating levels of immune activation markers, including neopterin, tumor necrosis factor receptor type II, and interleukin-2 receptors, are increased in human immunodeficiency virus-infected patients. We show here that highly active antiretroviral therapy significantly decreased neopterin levels. This effect is reversible, since neopterin levels increased after the arrest of treatment. Their determination may be useful in the evaluation of the efficacy of antiretroviral therapy.
A profound dysregulation of immunological parameters is observed during the course of human immunodeficiency virus (HIV) infection. The administration of antiretroviral therapy combining two inhibitors of reverse transcriptase (RT) and one protease inhibitor (highly active antiretroviral therapy [HAART]) causes a dramatic decline in AIDS-related morbidity and mortality (14). HAART reduces plasma HIV load and increases the CD4+-T-cell count (6). We recently reported that HAART reduces CD16high monocytes, which are considered inflammatory monocytes (1). HAART normalizes the function of progenitor cells (13) and restores CD4+-T-cell functions (3, 8). On the other hand, progression of HIV infection is related to increases in the following circulating markers of immune activation: soluble tumor necrosis factor receptor type II (sTNF-RII) (2), soluble interleukin-2 receptors (sIL-2R) (10), and monocyte activation markers, such as neopterin (12). Our goal in this study was to evaluate the impact of antiretroviral therapies on circulating markers of immune activation.
Informed consent was obtained from all HIV-infected patients and from healthy HIV-negative individuals who were investigated as controls. The clinical stages of disease were determined according to the Centers for Disease Control and Prevention definition. Clinical and biological features concerning the HIV-positive patients were described elsewhere (1). The patients were divided into four groups. The first group comprised 20 patients (15 men and 5 women; mean age, 36 years; range, 21 to 53 years) with a plasma viral load of 197 × 106 ± 81 × 106 copies liter−1 and naive of any antiretroviral treatment. Opportunistic infections (cytomegalovirus, Toxoplasma gondii, Cryptococcus neoformans, herpes simplex virus) were observed in 11 patients. The second group, consisting of 45 patients (28 men and 17 women; mean age, 35 years; range, 26 to 51 years), received two RT inhibitors during an average of 10 months (range, 6 to 20 months) at the time of blood sampling (plasma viral load, 80 × 106 ± 28 × 106 copies liter−1). The third group, consisting of 35 patients (25 men and 10 women; mean age, 36 years; range, 22 to 50 years), received a combination of two RT inhibitors and one protease inhibitor (plasma viral load, 35 × 106 ± 23 × 106 copies liter−1). The mean treatment duration was 6 months (range, 1 to 10 months) at the time of sampling. The fourth group consisted of 20 noncompliant patients (13 men and 7 women; mean age, 34 years; range, 28 to 46 years) who had recently (<3 months) stopped their treatment, and their plasma viral load remained low (22 × 106 ± 7 × 106 copies liter−1). Healthy HIV-negative individuals consisted of 26 men and 14 women with a mean age of 34 years (range, 20 to 48 years).
Blood was collected by venipuncture in EDTA anticoagulant tubes, and the resulting plasma was aliquoted and stored at −80°C within 2 h of collection. Samples were thawed and then refrozen once before being discarded. Circulating markers of leukocyte activation were measured by commercial enzyme immunoassays (EIA) as previously described (5). Procedures for quality control, including in-house reference samples, tests of intra- and interassay variability, and assays performed in duplicate, were conducted according to Aziz and others' recommendations (4). The sensitivity of the neopterin detection kit (IBL; BioAdvance, Emerainville, France) was 2.5 ng ml−1. The detection limit of the sTNF-RII kit (R&D Systems, Abingdon, United Kingdom) was 10 pg ml−1. sIL-2R and sCD23 detection kits were from T Cell Diagnostics (BioAdvance). The sensitivities of the assays were 24 and 1.5 U ml−1, respectively. The intra- and interassay coefficients of variation of the EIA kits ranged between 5 and 10%. Results are expressed as means ± standard errors. The results were compared by variance analysis and nonparametric tests. The likelihood of significant difference was >95%.
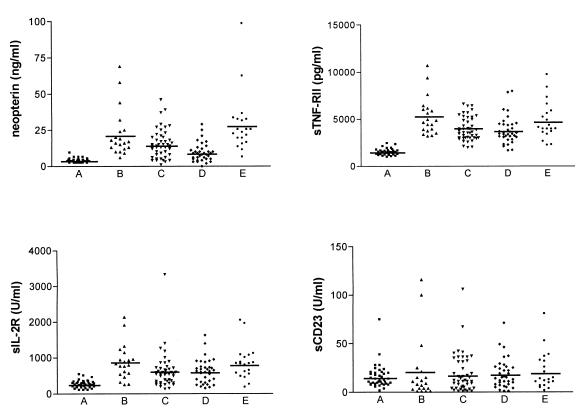
A significant increase in circulating levels of neopterin (P < 0.0001), sTNF-RII (P < 0.001), and sIL2-R (P < 0.001) was found in HIV-positive patients compared to HIV-negative controls (Fig. 1, columns A and B). This is in accordance with other reports which show increased levels of neopterin, sTNF-RII, and sIL-2R during the course of HIV infection (9, 15, 20). This increase was specific, since sCD23, an activation marker of B cells, was not affected in HIV-positive patients (Fig. 1, columns A and B). The levels of activation markers did not depend on the clinical stage of HIV infection, opportunistic infections, or the route of acquisition of HIV infection, sexual or blood route (in drug abusers). In patients with a high viral load (>100 × 106 copies liter−1) the neopterin levels were significantly (P < 0.05) higher than in patients with a low viral load (<20 × 106 copies liter−1) (Table 1), while the levels of sTNF-RII and sIL-2R were not related to HIV load. Our results are in agreement with some studies in which the levels of plasma neopterin correlate with plasma HIV load (18) and increase early in HIV infection (12); this increase precedes CD4+-T-cell decline (17). The high levels of circulating neopterin persist throughout the course of disease (12) and seem to predict more efficiently HIV-related mortality than disease progression (16, 21).
FIG. 1.
Effect of antiretroviral therapies on activation markers. The circulating levels of activation markers were determined by EIA. They were studied for healthy individuals (A), HIV-positive patients naive of treatment (B), patients treated with two RT inhibitors (C), patients treated with HAART (D), and patients who had stopped their treatment (E). Each symbol represents the amount of circulating marker in each individual. Horizontal bar, mean value.
TABLE 1.
Activation markers and viral loada
| Activation marker | Marker level in patients with viral load
|
|
|---|---|---|
| Low (<20 × 106 copies liter−1) | High (>100 × 106 copies liter−1) | |
| Neopterin (ng ml−1) | 9.1 ± 1.5 (2–20) | 27.3 ± 4.1 (8–58)* |
| sTNF-RII (pg ml−1) | 4,337 ± 423 (2,931–7,781) | 4,822 ± 491 (1,398–8,762) |
| sIL-2R (U ml−1) | 759 ± 165 (132–1,466) | 946 ± 162 (315–1,860) |
| sCD23 (U ml−1) | 26.5 ± 5.7 (6–62) | 24.4 ± 7.2 (1–100) |
The circulating levels of immune activation markers were studied for patients with a low viral load (n = 13) and patients with a high viral load (n = 15). The results are expressed as means ± standard errors (ranges in parentheses) and were compared using the Mann-Whitney U test. *, P < 0.05 compared with the value for low-viral-load patients.
The effect of therapy on circulating levels of activation markers associated with HIV infection was assessed in patients treated with RT inhibitors and patients undergoing HAART (Fig. 1, columns C and D). RT inhibitor treatment decreased circulating levels of neopterin (15.6 ± 1.5 ng ml−1 for treated patients versus 22.3 ± 3.7 ng ml−1 for naive patients, P < 0.04), sTNF-RII (3,964 ± 186 versus 5,309 ± 454 pg ml−1, P < 0.006), and sIL-2R (624 ± 75 versus 884 ± 110 U ml−1, P < 0.009) by about 30%. HAART was more potent than RT inhibitor treatment for reducing the circulating levels of neopterin (9.5 ± 1.1 ng ml−1 versus 15.6 ± 1.5 ng ml−1, P < 0.02). Nevertheless, the neopterin levels remained higher than in HIV-negative controls (3.8 ± 0.2 ng ml−1). In addition, a follow-up of four HIV-positive patients was performed before antiretroviral treatment and after 6 months of HAART. The neopterin levels were 20.8 ± 4.2 ng ml−1 before HAART and decreased to 11.9 ± 3.6 ng ml−1 after HAART. The effect of HAART on neopterin was specific, since HAART did not diminish the levels of sTNF-RII (3,810 ± 246 pg ml−1) and sIL-2R (620 ± 57 U ml−1) compared to RT inhibitor treatment. Our results are in accordance with those of Daniel et al., which show that HAART decreases plasma neopterin levels in HIV-infected hemophilia patients (7). The effect of each antiretroviral treatment on activation markers is reversible. The activation markers were studied for patients who had recently (less than 3 months) stopped their treatment and had a plasma viral load lower than 30 × 106 copies liter−1. The levels of neopterin, sTNF-RII, and sIL-2R became similar to those observed in HIV-positive patients naive of treatment (Fig. 1, column E). We also reported that the effect of HAART on cytokine release by monocytes is reversible after the arrest of the treatment (1). Thus, we suggest that only prolonged HAART can normalize neopterin levels. The decrease of circulating levels of neopterin, which reflects the activation state of monocytes (11), may be related to recent findings of our group obtained with the same patients (1). Monocytes from HIV-positive patients naive of treatment exhibit an activated pattern. First, they release large amounts of TNF, IL-1β, IL-6, and IL-10. Second, a monocyte subset corresponding to an activated phenotype, i.e., CD16high monocytes, is expanded in HIV infection (1, 19). RT inhibitor treatment and HAART down-modulate cytokine production, but only HAART decreases the percentage of CD16high monocytes. Hence, it is likely that HAART controls the activation of monocytes associated with HIV infection.
In conclusion, circulating levels of neopterin, which were related to HIV load, were specifically affected by HAART. The maintenance of monocyte activation may contribute to the therapeutic failure of HAART due to the toxicity of antiretroviral drugs, the development of viral resistance, or the lack of adherence to the prescribed regimen. Hence, the measurement of neopterin may be useful in the evaluation of the effect of antiretroviral therapies on host response.
Acknowledgments
This work was supported by grants from the Agence Nationale de Recherches sur le SIDA, Sidaction (Fondation pour la Recherche Médicale), and the Conseil Général des Bouches-du-Rhône, France.
REFERENCES
- 1.Amirayan-Chevillard N, Tissot Dupont H, Capo C, Brunet C, Dignat-George F, Obadia Y, Gallais H, Mege J L. Impact of highly active antiretroviral therapy on cytokine production and monocyte subsets in HIV-infected patients. Clin Exp Immunol. 2000;120:107–112. doi: 10.1046/j.1365-2249.2000.01201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aukrust P, Liabakk N B, Müller F, Lien E, Espevik T, Froland S S. Serum levels of tumor necrosis factor-α (TNF-α) and soluble TNF receptors in human immunodeficiency virus type 1 infection. Correlations to clinical immunologic and virologic parameters. J Infect Dis. 1994;169:420–424. doi: 10.1093/infdis/169.2.420. [DOI] [PubMed] [Google Scholar]
- 3.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debré P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 4.Aziz N, Nishanian P, Fahey J L. Levels of cytokines and immune activation markers in plasma in human immunodeficiency virus infection: quality control procedures. Clin Diagn Lab Immunol. 1998;5:755–761. doi: 10.1128/cdli.5.6.755-761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capo C, Amirayan N, Ghigo E, Raoult D, Mege J L. Circulating cytokine balance and activation markers of leucocytes in Q fever. Clin Exp Immunol. 1999;115:120–123. doi: 10.1046/j.1365-2249.1999.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collier A C, Coombs R W, Schoenfeld D A, Bassett R L, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe V J, Friedman H M, Merigan T C, Reichman R C, Hooper C, Corey L. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. N Engl J Med. 1996;334:1011–1017. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 7.Daniel V, Süsal C, Melk A, Weimer R, Kröpelin M, Zimmermann R, Huth-Kühne A, Uhle C, Opelz G. Reduction of viral load and immune complex load on CD4+ lymphocytes as a consequence of highly active antiretroviral treatment (HAART) in HIV-infected hemophilia patients. Immunol Lett. 1999;69:283–289. doi: 10.1016/s0165-2478(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 8.David D, Bani L, Moreau J L, Treilhou M P, Nakarai T, Joussemet M, Ritz J, Dupont B, Pialoux G, Theze J. Regulatory dysfunction of the interleukin-2 receptor during HIV infection and the impact of triple combination therapy. Proc Natl Acad Sci USA. 1998;95:11348–11353. doi: 10.1073/pnas.95.19.11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahey J L, Taylor J M G, Manna B, Nishanian P, Aziz N, Giorgi J V, Detels R. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS. 1998;12:1581–1590. doi: 10.1097/00002030-199813000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann B, Nishanian P, Fahey J L, Esmail I, Jackson A L, Detels R, Cumberland W. Serum increases and lymphoid cell surface losses of IL-2 receptor CD25 in HIV-1 infection: distinctive parameters of HIV-1-induced changes. Clin Immunol Immunopathol. 1991;61:212–224. doi: 10.1016/s0090-1229(05)80025-x. [DOI] [PubMed] [Google Scholar]
- 11.Huber C, Batchelor J R, Fuchs D, Hausen A, Lang A, Niederwieser D, Reibnegger G, Swetly P, Troppmair J, Wachter H. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984;160:310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melmed R N, Taylor J M, Detels R, Bozorgmehri M, Fahey J L. Serum neopterin changes in HIV-1 infected subjects: indicator of significant pathology, CD4 T cell changes and the development of AIDS. J Acquir Immune Defic Syndr. 1989;2:70–76. [PubMed] [Google Scholar]
- 13.Nielsen S D, Ersboll A K, Mathiesen L, Nielsen J O, Hansen J E S. Highly active antiretroviral therapy normalizes the function of progenitor cells in human immunodeficiency virus-infected patients. J Infect Dis. 1998;178:1299–1305. doi: 10.1086/314464. [DOI] [PubMed] [Google Scholar]
- 14.Palella F J, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman J D, Holmberg S D. Declining morbidity and mortality among patients with advanced immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 15.Plaeger S, Bass H Z, Nishanian P, Thomas J, Aziz N, Detels R, King J, Cumberland W, Kemeny M, Fahey J. The prognostic significance in HIV infection of immune activation represented by cell surface antigen and plasma activation marker changes. Clin Immunol. 1999;90:238–246. doi: 10.1006/clim.1998.4646. [DOI] [PubMed] [Google Scholar]
- 16.Sacktor N, Liu X, Popescu M, Marder K, Stern Y, Mayeux R. Serum neopterin level predicts HIV-related mortality but not progression to AIDS or development of neurological disease in gay men and parenteral drug users. Arch Neurol. 1995;52:676–679. doi: 10.1001/archneur.1995.00540310046015. [DOI] [PubMed] [Google Scholar]
- 17.Salazar-Gonzales J F, Martinez-Maza O, Nishanian P, Aziz N, Shen L P, Grosser S, Taylor J, Detels R, Fahey J L. Increased immune activation precedes the inflection point of CD4 T cells and the increased serum virus load in human immunodeficiency virus infection. J Infect Dis. 1998;178:423–430. doi: 10.1086/515629. [DOI] [PubMed] [Google Scholar]
- 18.Stein D S, Lyles R H, Graham N M H, Tassoni C J, Margolick J B, Phair J P, Rinaldo C, Detels R, Saah A, Bilello J. Predicting clinical progression or death in subjects with early-stage human immunodeficiency virus (HIV) infection: a comparative analysis of quantitation of HIV RNA soluble tumor necrosis factor type II receptors, neopterin and β2-microglobulin. J Infect Dis. 1997;176:1161–1167. doi: 10.1086/514108. [DOI] [PubMed] [Google Scholar]
- 19.Thieblemont N, Weiss L, Sadeghi H M, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 20.Tsoukas C M, Bernard N F. Markers predicting progression of human immunodeficiency virus-related disease. Clin Microbiol Rev. 1994;7:14–28. doi: 10.1128/cmr.7.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zangerle R, Steinhuber S, Sarcletti M, Dierich M P, Wachter H, Fuchs D, Möst J. Serum HIV-1 RNA levels compared to soluble markers of immune activation to predict disease progression in HIV-1-infected individuals. Int Arch Allergy Immunol. 1998;116:228–239. doi: 10.1159/000023949. [DOI] [PubMed] [Google Scholar]