Abstract
All schoolchildren know how often they breathe, but even experts don’t know exactly why. The aim of this publication is to develop a model of the resting spontaneous breathing rate using physiological, physical and mathematical methods with the aid of the principle that evolution pushes physiology in a direction that is as economical as possible. The respiratory rate then follows from an equation with the parameters -production rate of the organism, resistance, static compliance and dead space of the lungs, the inspiration duration: expiration duration - ratio and the end-expiratory fraction. The derivation requires exclusively secondary school mathematics. Using the example of an adult human or a newborn child, data from the literature then result in normal values for their breathing rate at rest. The reason for the higher respiratory rate of a newborn human compared to an adult is the relatively high -production rate together with the comparatively low compliance of the lungs. A side result is the fact that the common alveolar pressure throughout the lungs and the common time constant is a consequence of the economical principle as well. Since the above parameters are not human-specific, there is no reason to assume that the above equation could not also be applicable to many animals breathing through lungs within a thorax, especially mammals. Not only physiology and biology, but also medicine, could benefit: Applicability is being discussed in pulmonary function diagnostics, including pathophysiology. However, the present publication only claims to be a theoretical concept of the spontaneous quiet breathing rate. In the absence of comparable animal data, this publication is intended to encourage further scientific tests.
Keywords: Respiratory rate, Evolutionary economy, Respiratory physiology, Volume flow pattern, -production rate, Static compliance, Airway resistance, End-expiratory fraction, Time constant, Alveolar pressure
Introduction
The aim of this publication is to develop a model of the resting spontaneous breathing rate that is as simple as possible using physiological, physical and mathematical methods with the aid of the principle that evolution pushes physiology in a direction that is as economical as possible. The respiratory rate then follows thereof from an equation with the parameters production rate of the organism, resistance R, static compliance C and dead space of the lungs, the inspiration duration: expiration duration ratio I : E and the end-expiratory -fraction CO In order to make this publication readable for many, only elementary analysis without numerical methods shall be used, for the main result, which is also helpful for intuition.
In the following, well-known terms and facts of respiratory physiology are sometimes repeated with full intent in order to transcribe them into a physical language with mathematical aids. This is necessary because in respiratory physiology, for example, an inflection point has a different meaning than in mathematics, and if compliance is defined as a difference quotient as usual, this is introduced below as a differential quotient. Even physical units can sometimes not be translated in medical publications, or only with difficulty (Schramm 2010), for example the unit gm for a work in studies (Otis et al. 1950; Crosfill and Widdicombe 1961) which use clinical data to determine the breathing rate at rest. A certain analogy between lung physiology and electrical engineering (Campbell and Brown 1963) can be helpful here (Table 1).
Table 1.
Analogy (“in a sense even an isomorphism”) between pulmonary physiology and electrical engineering
| Pulmonary physiology | Electrical engineering |
|---|---|
| Volume V | Charge Q |
| Pressure difference | Voltage difference |
| Volume flow dV / dt | Current I = dQ / dt |
| Airway resistance | Resistance / I(=Ohm’s law) |
| Resistance pressure | Voltage across a resistor |
| Static compliance C(p) = dV / dp | Capacity / |
| Compliance pressure | Voltage across a capacitor |
| Time constant | Time constant |
| Compliance work | Energy stored in a capacitor |
| Passive expiration function at rest | RC-discharging function |
| Passive expiration pressure difference at rest | RC-discharging voltage difference: |
| Serial resistances | Serial resistances |
| Parallel resistances | Parallel resistances |
| Resistance power | Resistance power |
| Parallel compliance | Parallel capacitance |
Any line on the left that contains the airway resistance R, requires laminar flow. In the case of a capacitor, the change in charge leads to a proportional change in voltage , since the capacitance C usually does not change during loading. However, the compliance of the lungs and thorax is not constant but a function C(p) of the pressure p, which is why, in contrast to electrical engineering, the transition from the differential quotient to the derivative dV/dp is relevant in lung physiology. However, under spontaneous breathing at rest C(p) may by considered as constant
Total work of breathing
A survival advantage, especially in the energy balance, is certainly not a disadvantage in times of low food resources and consequently an advantage over living beings that are less economical in this regard. Therefore, the following "postulate of evolutionary economy" is assumed:
Postulate: Evolution pushes physiology in a direction that is as economical as possible. (P)
Consequently, it can be assumed that evolution has minimized the total respiratory power (work/time) as well. Hence, many authors (Otis et al. 1950; Kerem 1996; Noël and Mauroy 2019) have assumed that the breathing power must be as low as possible during spontaneous breathing.
In purely physical terms, the work of breathing is composed of
Compliance work (= work to expand the lungs against the lung and chest wall elastic forces)
Resistance work (= work to overcome the airway resistance R)
Tissue resistance work ( = work to overcome the viscosity of the lung and chest wall structures)
Acceleration work of the lungs, chest wall and breathing gas
additively.
The power required for acceleration is, however, only relevant in diving medicine during deep dives (Dwyer et al. 1977) together with large respiratory minute volumes , but negligible at rest under one atmosphere of air pressure (Rohrer 1925), as a simple calculation at the end will show as well. The tissue resistance work will be discussed below together with the airway resistance R.
The ambient pressure is usually chosen as the reference pressure in (respiratory) physiology and biology. Consequently, you can replace with zero nearly everywhere in this paper (except e.g. in the definition of the fraction and the ideal gas equation).
Definition
The pleural pressure is the pressure within the narrow space (=pleural space) between lungs- and chest wall pleura.
Definition
The pressure within all the alveoli (= alveolar space) is denoted alveolar pressure .
The next but one subsection shows that is a common pressure for all the alveoli, which lastly follows from (P) as well.
There is no doubt among physiologists that spontaneous expiration at rest is passive (Levitzky et al. 2018; Lumb 2016a). Since this is also the case under controlled positive pressure ventilation and, moreover, volumes, pressures and volume flows can be measured very easily under mechanical ventilation, not only analogies between pulmonary physiology and electrical engineering, but also analogies between spontaneous breathing and mechanical ventilation will often be helpful in the following.
During positive pressure mechanical ventilation the ambient pressure in front of the lungs (mouth, nose or trachea) seemingly changes, which is responsible for the volume flow . During spontaneous breathing however, it is the changing pleural pressure which is responsible for the volume flow and the alveolar pressure changes the sign with respect to the ambient pressure between inspiration and expiration, which is not the case under positive pressure ventilation. This is ultimately why this type of mechanical ventilation is known as positive pressure ventilation. Side-note: During ventilation with the historical iron lung the ambient pressure of the whole body except the head is responsible for the volume flow, which actually is more physiologic.
Definition
During positive pressure ventilation the pressure difference between the (seemingly changing ambient) pressure in front of the lungs (e.g. within the ventilation device: endotracheal tube, laryngeal mask, ...) respectively face (e.g. face mask, ...) and the alveolar pressure is called (positive) ventilation pressure .
The -partial pressure within the organism is responsible for the respiratory drive at least under one atmosphere of air pressure. Therefore the balance is being discussed first, which will yield a side condition for the calculation of the respiratory rate needed later.
The balance of the organism and the alveolar ventilation
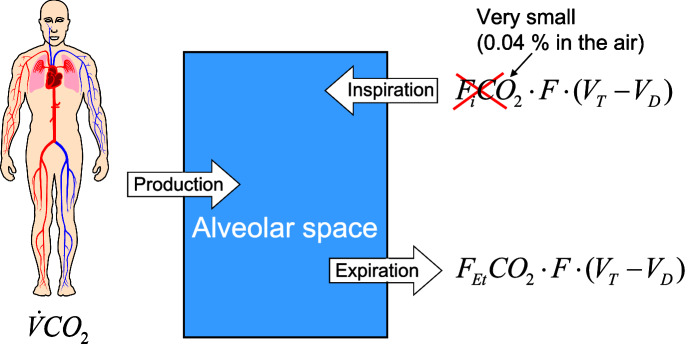
A slight change in the -partial pressure of the breathing air at sea level, e.g. due to weather conditions, undoubtedly has a negligible influence on the tidal volume and the breathing rate F. However, this changes with increasing altitude (Duffin 2007; Weil et al. 1970). On Mt. Everest the respiratory minute volume even takes on considerable size (Pugh 1957; Weil et al. 1970), which is why an air pressure of 760 mmHg is assumed in the following. If the organism is viewed as a black box (Fig. 1), the balance can be summarized in the equation:
if the excretion via the kidneys (bicarbonate or in physical solution) is neglected, especially since the latter is at least a factor of 10 less than the output via expiration.
Fig. 1.
balance if the (human) organism is viewed as a black box. With respectively CO the inspiratory or end-tidal fraction is referred to and is the alveolar ventilation. The -production rate is denoted
Definition
The anatomical dead space, such as the nose, mouth, trachea and the bronchial tree, is the part of the tidal volume that does not participate in gas exchange, which is predetermined by the anatomy. If, in pathophysiology, there are additional components that do not participate in gas exchange, the entire dead space is then referred to as total dead space.
The part of the minute volume that takes part in gas exchange in the lungs is accordingly and is referred to as alveolar ventilation.
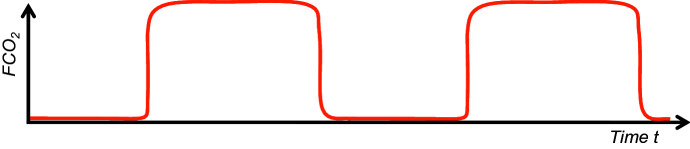
The fraction of a gas is defined as the quotient of the partial pressure of any gas and the total pressure , which is always 760mmHg here. Let be the inspiratory, the end-tidal -fraction (Fig. 2) and the production rate (in a volume / time unit), then the above balance equation can be rewritten as follows:
The inspiratory -fraction , compared to the physiological expiratory -fraction CO (in humans approx. 5.2%, see Table 2) is at least a factor of 10 (in humans approx. 130) lower. Therefore, the inspiratory proportion in the above equation can be ignored. Contrary to the mathematical and physical convention, approximations will continue to be noted with an equal sign in the following. Then the following “alveolar ventilation equation” applies (West 2007):
| 1 |
Physiologically, the is assumed to be representative for the alveolar gas. Therefore, the “inverse proportionality” between the alveolar partial pressure of and the alveolar ventilation is often graphically depicted in physiology textbooks, usually without citing (1), for example on p. 514 Fig. 40-5 in the book of Hall (2020). Because of the extremely rapid diffusion of through the alveolar membrane as a result of the very well solubility of in aqueous body fluids (Fenn et al. 1946; Weingarten 1990), the arterial correlates very well with the alveolar partial pressure of , which is apparently why Chambers et al. (2019) on p.46 replaced the index Et (=end-tidal) in equation (1) with the arterial one.
Fig. 2.
Idealized course of an end-tidal curve (Weingarten 1990) under ventilation or spontaneous breathing. This function is similar to a periodic step function. The upper tangent parallel to the t-axis corresponds to the value CO and the lower one to the value
Table 2.
The data dead space , end-tidal -fraction , production rate of the organism , duration of inspiration : duration of expiration I : E-ratio, airway resistance R, static compliance C of the lungs and thorax close to the FRC within this table are derived from the literature (Galetke07; Long and Santhanam 2017; Butler et al. 1957; Guo et al. 2005; Huang et al. 2016; Levitzky et al. 2018; Pasquis et al. 1976; Boggs and Tenney 1984; Weingarten 1990; Lumb 2016b; Joehr 1993). The respiratory rate of an adult human and a newborn baby weighing about 3.5 kg in the penultimate column is the solution of equation (29) using these data. For example the adult gives The Factor is the conversion coefficient from ml. to liter respectively sec. to min. The associated tidal volume in the last column follows from (22) and the product is then the related minute volume. The above data allow the calculation of the entire physical respiratory power (27) of the adult human (30.64 mW) and the human newborn (3.84 mW) as well
| ml | CO % | ml/min | I : E ratio | R mbar/(l/s) | C ml/mbar | 1/min | ml | |
|---|---|---|---|---|---|---|---|---|
| Adult human | 160 | 5.2 | 196.8 | 0.5 | 3 | 100 | 13.2 | 446.7 |
| Newborn human | 7.2 | 5.2 | 19.1 | 0.67 | 30 | 3 | 33.9 | 18.05 |
The production rate depends on the nutritional-dependent respiratory quotient RQ and on the -consumption rate and the latter in the resting state (due to the aerobic metabolism in this state) on the caloric equivalent and the constant basal metabolic rate. Admittedly the basal metabolic rate includes the resting respiratory power as well and surely depends on F. However, in the resting state makes up only a small fraction of the basal metabolic rate. (Using the example of humans, this will be shown later.) Moreover from the postulate (P) at the resting respiratory rate is to be expected. Both together justify the assumption that is (almost) constant within a neighbourhood of , which therefore will be assumed in this paper.
The dead space given by the anatomy (or pathophysiology) and age can be regarded as a constant in the following context and the physiological homeostasis within the organism is responsible for a largely constant end-tidal -fraction CO.
The respiratory rate F in (1) can vary by adjusting the tidal volume in order to maintain a physiological homeostasis in the organism. It is known, however, that the breathing rate of the organism at rest on average within a range, depending on age, is largely predetermined by biology, physiology or pathophysiology. The question therefore arises which parameters determine the breathing rate at rest and thus also determine the tidal volume as well. Interestingly, this question can be answered with relatively simple aids, at least under physiological conditions.
Minimum of the respiratory compliance work and power
The elastic work to expand lungs and chest wall is being discussed first. Let TLC be the total lung capacity, RV the residual volume and FRC the functional residual capacity (= RV + expiratory reserve volume), which is the physiologic resting (or balance) position volume in the lungs at the end of expiration (Fig. 3).
Fig. 3.
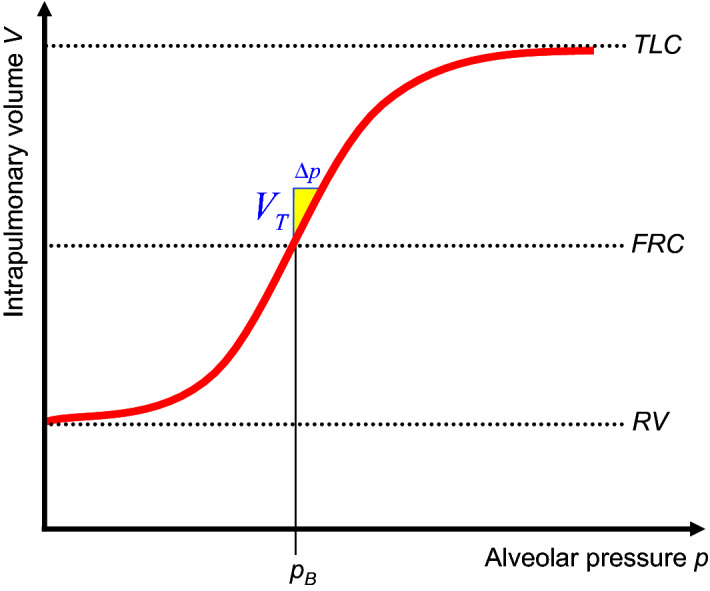
TLC is the total lung capacity, RV the residual volume, the physiologic functional residual capacity and is the ambient pressure. If under very slow ventilation, i.e. quasistatically, the tidal volume is pushed into the lungs starting from the FRC, then the alveolar (=intrapulmonary) pressure increases by and the intrapulmonary volume follows this strictly increasing function V(p), which is called static. Both elastic structures of the lungs and the thorax surrounding the lungs are responsible for the course of this function
Quasi-static means that the parameters are independent of the time t: . Hence, pressure changes, which are caused by the volume flow should be as small as possible. From (9) , (16) or (17) then follows . This is why the index A respectively V will be suppressed within this subsection. Since the pressure p and the volume V pushed into or released from the lungs can easily be measured during positive pressure mechanical ventilation, the function V(p) of the intrapulmonary volume V as a function of the ventilation pressurep can be easily ascertained quasi-statically for and V(p) is then referred to as static (Fig. 3).
Definition
A PEEP is that pressure which is positive compared to the ambient pressure in the alveolar space at the end of expiration: .
A PEEP is usually specified on a ventilator using an adjusting screw or can be caused pathophysiologically by an increased airway resistance in the dead space, for example in chronic obstructive pulmonary disease (COPD) patients or during an asthma attack, but also by resistances in the ventilation device (endotracheal tube, laryngeal mask, ...). As shown below, using the example of the human data from Table 2, there is (almost) no PEEP under physiological quiet breathing. Consequently, (based on the above convention) physiologically applies.
The function V(p) in Figure 3 was therefore created with particularly slow inspiration or expiration. You cannot breathe in beyond the TLC and you cannot breathe out below the RV. Consequently, an S-shaped course (Rahn et al. 1946; Tepper and Costa 2015; Bryan and Wohl 2011) (s. Figure 3) of the function V(p) is to be expected, which therefore has exactly one inflection point. Many publications e.g. (Grinnan and Truwit 2005) as well as textbooks (Kacmarek and Dean 2019; Campbell and Brown 1963; Exline et al. 2020), on the other hand, define on V(p) an upper and a lower inflection point, which, however, are not inflection points in the mathematical sense and are therefore not referred to as inflection points in the following. The mathematical inflection point of the function V(p) is in any case depicted in textbooks (Chatburn 2003; Bryan and Wohl 2011) and publications (Rahn et al. 1946) close to the FRC.
Both elastic structures of the lungs and the thorax surrounding the lungs are responsible for the course of V(p). Starting from the resting position FRC, it is physically evident that the volume of the lungs (strictly) increases while the pressure within the lungs (strictly) increases and the other way around: Starting from the FRC, the volume of the lungs (strictly) decreases while the pressure within the lungs (strictly) decreases. Therefore, as can be seen from Figure 3, V(p) increases strictly and consequently, the inverse function p(V) exists.
From a physical point of view, changing any pressure p on a null set would have no effect on the volume V and all other parameters (s. Table 1) in this paper are in some sense derived from these two and the time t. Hence, let all occurring variables be sufficient smooth which is biologically meaningful as well. For example, let us in the following assume that V(p) is at least doubly continuously differentiable (i.e . In physics, especially thermodynamics this assumption is usually accepted as well.
The location of the inflection point of V(p) is being discussed now. The slope, i.e. the first derivative of V(p), is referred to here as static compliance. (Note: Often, however, in publications or textbooks the difference quotient is defined as the static compliance.)
If, as an exception, mechanical ventilation is carried out without PEEP, then the pressure increases during the inspiration phase, starting from with a correspondingly slow ventilation, depending on p(V), in order to passively fall back to in the expiration phase. With positive pressure ventilation, the tidal volume is pushed into the lungs during each inspiration. Since , the inspiratory quasi-static work of breathing per breath, which is referred to here as compliance work, is:
| 2 |
because the product of a volume and a pressure difference has the physical unit of a work.
The following few lines will justify the assumption that C(p) is approximately constant and highest within a neighbourhood of the FRC. The tangent of V(p) at is the straight line equation:
| 3 |
The slope C of this tangent is of course . With this linearization the inspiratory compliance work (2) can be rewritten:
| 4 |
which (approximately) corresponds to the yellow colored triangle in Figure 3 with the area as usually given in textbooks (Hall 2020). (This work is equivalent to the energy content of a capacitor in electrical engineering.) The pressure difference
| 5 |
is called compliance pressure in respiratory text books (which is equivalent to the voltage across a capacitor in electrical engineering) and the associated compliance power is:
| 6 |
because the work (4) has to be done for each inspiration. With quasi-static spontaneous breathing (due to the conservation of energy theorem) the same physical work respectively power has to be exerted by the organism, consequently the equations (4) and (6) analogously apply to spontaneous breathing as well.
With (P) it makes sense for (4) to assume a minimum under quiet spontaneous breathing, which at the same time minimizes (6) when the breathing rate F (and due to (1) as well) is initially given. However, this is only possible if C is as large as biologically possible, or in a mathematical language: The parameter C in (3) has to be a maximum of the function at the point , which means that applies and consequently the 2nd derivative disappears. Exactly this defines mathematically an inflection point of the function V(p) at the point , which justifies the above assumption in retrospect and the course of the function V(p) in Figure 3 as well as in textbooks (Chatburn 2003; Bryan and Wohl 2011) and publications (Rahn et al. 1946). This is surely why Fernandez et al. (1993) writes that C is constant at the FRC and Chambers et al. (2019) on p. 51 write in their book that lung compliance is at its highest at FRC, however, both without citing a reference. On the other hand, the above result strenghtens the trust in (P).
The above simple derivation has not been published until now, possibly because the result (4) is obvious anyway.
By the way, the resting volume of the isolated thorax is larger than the , because the physiologic resting volume of the isolated lungs disappears. Nevertheless, quasistatic linear approximations and
| 7 |
(the last equation within the range of quiet breathing) similar to (3) (West 2012; Lumb 2016b) are well known, wherein and are the compliances of the isolated thorax respectively isolated lungs. Physiologically the volume of the pleural space is negligible and the volume of the empty lung is much smaller than the FRC. Hence, , and V are almost equal, which is why these volume indices will be suppressed in the following. Often the serial addition equation is quoted in respiratory physiology text books (Levitzky et al. 2018; West 2012) however, this presupposes , which is not the case. In an electrical engineering language: The lungs can be compared with an electrolytic capacitor, since only a positive volume [ charge] can be applied, however the thorax (like any other capacitor) can be “charged” with a positive or negative volume. Since the following is true: The lung within the thorax, both together in the resting (= balance) position, unloads the thorax by the volume while the thorax keeps the lungs in a loaded position with the volume FRC. This is why the pleural pressure has to be negative compared to the ambient pressure in this resting position. As already mentioned, the FRC is the physiologic resting (= balance) position of the lungs (within the thorax) at the end of expiration. If we apply the inverse function p(V) to this results to . Hence, holds at the end of expiration. Even more, it is known that holds during the entire respiratory period, especially during quiet expiration as well (Hall 2020; West 2012), which is often an argument that quiet expiration is passive.
If ventilation was previously considered to be quasi-static, then this should no longer apply in the following, especially when discussing airway resistance. Consequently, does not apply any more.
Theoretical considerations on the type of volume flow within the dead space, the alveolar pressure and the respiratory time constant
This and the following subsection are not (or only indirectly) included in the calculation of the main result (29). The volume in the previous subsection was mainly a function of the pressure , from now on, this volume is mainly a function of the time t. Therefore, strictly speaking it would be more appropriate to use the notion of partial derivatives, however, due to convention in physiology, let us exceptionally dispense with the mathematically exact notion. A turbulent flow leads to a considerably higher flow resistance, which obviously contradicts (P) at least in the resting state. Nevertheless a turbulent resistor summand of the form (17) was the basis for the results in previous publication (Otis et al. 1950) dealing with the human respiratory rate. Due to (P), however, it is more likely that the flow within the dead space is laminar, at least for the most part, at rest. This assumption shall now be evaluated and will be shown indirectly in the next subsection using the example of expiration under mechanical ventilation. Then the law of Hagen-Poiseuille applies in each individual dead space section with a cross-section assumed to be nearly circular, because breathing gases (such as air) are Newtonian fluids. It would now be easy to derive the Hagen-Poiseuille law from even simpler physical laws in a few lines, but this should be dispensed with here, since this can be found in physics textbooks. The resistance R of the considered dead space section, bronchus, bronchiolus, trachea, pharynx or nose is therefore dependent on the pipe geometry (length, diameter) as well as the dynamic viscosity of the breathing gas. The pressure difference which arises from the volume flow at R is then directly proportional to :
| 8 |
In respiratory physiology this is referred to as resistance pressure. The analogue to (8) in electrical engineering is Ohm’s law . Starting from the alveolar space, the bronchial tree, including pharynx and nose, can be viewed as a network (which is rather a tree) of resistors. Analogous to electrical engineering, from the linearity between and in each individual dead space section with a resistance R then follows that two serial resistances and must be added and two parallel resistances and follow the law . If these serial and parallel addition rules are applied iteratively throughout the entire resistance network, then the resistance R in (8) may also be viewed as the resistance of the entire airway system, which is then usually referred to as airway resistance R. This is to be defined in the following, with in (8) then being the difference between the ambient pressure and the alveolar pressure (Fig 4) provided that is a common pressure for all alveoli:
| 9 |
(Under spontaneous breathing is the constant barometric pressure and under positive pressure ventilation has to be replaced with the seemingly changing ambient pressure in front of the face respectively lungs.) Hence, both the volume flow (as usually defined in respiratory physiology) and the pressure are positive during inspiration and negative during passive expiration.
Fig. 4.
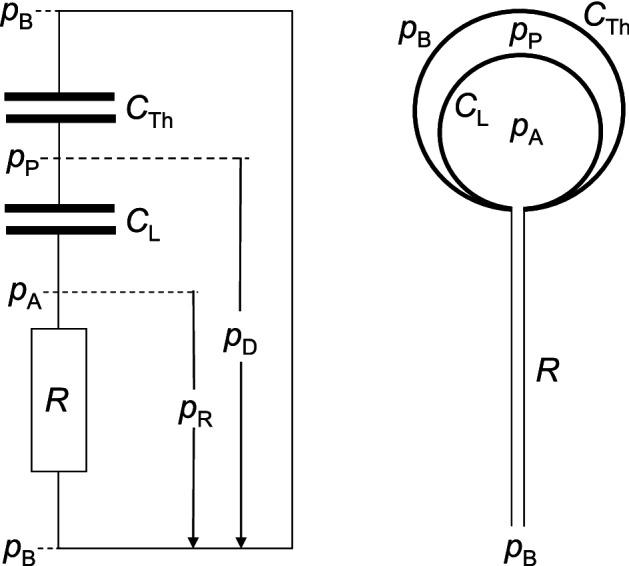
Simplified respiratory “circuit diagram”: R is the airway resistance, C is the compliance of the lungs respectively thorax and p is the pressure variable. The index P is short for pleural, A for alveolar, B for barometric (or base), R for airway resistance, L for lung, Th for thorax and D for driving pressure. The pleural pressure (and hence as well) is a common pressure for all alveoli. In reality there is almost no space between the lungs and the thorax
Admittedly, the geometry of the nose differs largely from a pipe and the flow within the nose might not be laminar. This is possibly why, we usually run with an open mouth. However it is assumed here that the intranasal flow is at least mostly laminar, in any case at rest, since in this state our mouth usually remains closed.
Definition
The difference between the ambient pressure and pleural pressure is called driving-pressure (s. Fig. 4).
The driving-pressure is responsible for ventilation under spontaneous breathing and is in some sense the spontaneous ventilation “analogue” to the ventilation pressure during positive pressure mechanical ventilation.
Since the pleural space, which is filled with a small amount of serous fluid, surrounds the lungs, the driving pressure is an (at least largely) common pressure for all alveoli. In the following it is assumed that is a common pressure for all alveoli.
At the internal end of the resistance network the alveoli are located. If we compare the lungs to a tree, then the branches correspond to the bronchial tree and the leaves to the alveoli. From a distance, trees of the same species look similar, but the leaves (like the alveoli) are still differently shaped and sized. It is unlikely that physiology would (modulo gravitational effects) preferentially ventilate individual alveoli, thus disadvantage others. Hence, from a macroscopic point of view, the lung is almost homogeneous. Concerning ventilation, the units (alveoli, resistors) of the lungs are large enough, so that quantum effects should only play a minor role. Hence, let us assume the following postulate:
Postulate: Each lung segment (e.g. alveolus with associated resistor) follows the same macroscopic physical equations (7) and (8) in any case lastly within the entire lung.
There of doesn’t already follow that the product of the parameters in (7) and (8) is homogeneous throughout the lungs, since the alveoli are differently shaped and sized and the resistance tree is a very complex one.
At first, let us assume that the resistors R and the compliances C in (7) and (8) are constant. This assumption surely is justified during quiet breathing, but not under physical exercise. Hence, this assumption shall be corrected at the end of this subsection.
Although the equations (7) and (8) might be valid only within the association of the lung, nevertheless, let us build up the lungs step by step from its alveoli and its resistors by using these two equations.
First of all, let us assume that the lungs consisted of only 2 alveoli with compliances and which are each parallel connected to the internal end of the trachea via the resistors and . Let us further assume that the alveolar pressure is not a common pressure within these 2 alveoli, then at the beginning and at the end of each inspiration or expiration (i.e. at the time when the volume-flow at the outer end of the nose equals zero) pressure equalization between these 2 alveoli would take place. This of course additionally requires breathing work, which contradicts (P) and simultaneously raises the following question: What dimensioning of the bronchial tree is necessary, to prevent this additional breathing work of course during physical exertion as well and consequently for every respiratory rate and breathing pattern? Pressure equalization between alveoli does not take place, if the pressure within the alveoli is always the same. Since the lung itself does not have any information about the very next following respiratory rate, this specifies the above question: Which structure of the lungs is necessary, to keep a common pressure (for all driving pressure patterns) within the alveoli?
A sufficient condition surely is that the compliances and resistances are all equal. Consequently, let us assume that the lungs consisted again of only 2 alveoli, however with the compliances which are each parallel connected to the outer end of the nose via the 2 resistors . Since the driving-pressure for these two alveoli is always the same, these 2 alveoli are ventilated synchronously and due to symmetry there cannot be any alveolar pressure difference between them. Since the alveolar pressure is the pressure “between” these and the total compliance of this 2-alveoli-sample lung and the total inverse resistance follow from the symmetry as well. The analogy between respiratory physiology and electrical engineering supports the last conclusion. (s. Table 1).
Definition
The product of any laminar-flow resistance R (the one in (8)) connected in series to an alveolus with the compliance C has the physical unit of a time, which is why is called time constant.
The parameter is equivalent to the time constant in electrical engineering.
Claim
Parallel connection of any alveoli with the same time constant does not change the time constant .
Proof
For the above symmetric 2-alveoli (connected to the outer end of the nose) sample lung with the time constant of this small lung is . The same proof applies for any (-alveoli sample lung, all parallel connected to the outer end of the nose with their resistor . Consequently, (apart from units) again for a 2-alveoli sample lung with compliances and thus with rational numbers (which is sufficient for physiology), since: . This again iteratively applies to any number of any alveoli each with the same time constant connected in parallel with the resistor to the outer end of the nose.
Definition
In the following a RC-element (R, C) is defined to be the connection of one alveolus with the compliance C to its resistor R or equivalently in electrical engineering the serial connection of one capacitor C to one resistor R.
The restriction to rational numbers is not necessary: Because of the analogy between electrical engineering and respiratory physiology (s. Table 1) the complex impedance calculus in electrical engineering (Quade 1937) can be applied here as well. But even more:
Claim
Two parallel connected RC-elements (, respectively , are equivalent to the one RC-element (, if and only if the time constants of the 2 parallel connected RC-elements match.
(Equivalent in the following sense: From a black box view these behave equivalently if sine-waves of any frequency are applied to them.)
Proof
Let us begin with the easier direction: Hence, let and let be arbitrary, then a simple calculation shows that the following complex calculus equation holds: . Thereof follows as well.
Now the proof into the other direction: Let , then holds only if , since: The imaginary part and the real part have to hold separately. After bringing each equation to a common divisor the polynomials in the variable in the nominator have to match on both sides of the equal sign for each -part with . Thereof , , and of course as well follow.
This calculus is called complex, since occurs in the calculation and the parameter is called sinusoidal angular frequency. This proof is admittedly a little more general and but hides the above shown symmetry.
Claim
Let , then k parallel connected RC-elements , are equivalent to the one RC-element (, if and only if the time constants of all the k parallel connected RC-elements match (Fig. 5).
Fig. 5.

The parallel connected RC-elements behave equivalently to the one RC-element (with and if and only if the time constants of all the parallel connected RC-elements match. Hence, a lung with many alveoli, all parallel connected with their resistor to the outer end of the nose behaves equivalently to a lung with one alveolus with connected with its resistor to the outer end of the nose if and only if all have the same time constant
Proof
This follows from the above claim by induction from to n.
Definition
Let us from now on call this RC-element the equivalent one to the parallel connection of these RC-elements .
If a sine wave of any frequency is applied to parallel connected RC-elements, this simple “lung” behaves equivalently to the one equivalent RC-element (= even a simpler lung with only one alveolus connected to its resistor) if and only if all the parallel connected RC-elements have the same time constant .
Let be any twice continuous differentiable breathing pattern within . It is well known that Fourier series converge uniformly to . From electrical engineering it is well known that differential equations of RC-elements are linear, consequently the superposition principle holds.
Corollary
Parallel connected RC-elements behave for all in the same manner as the one equivalent RC-element if and only if all the parallel connected RC-elements have the same time constant .
Proof
From a theoretical (or better electro-technical) point of view, all sine (and cosine) functions are a subset of . The other direction follows from the superposition principle, since the Fourier series of any (within any compact interval of the finite life) is composed of sine (and cosine) functions.
By the way, from a biological point of view, the condition is practically always fulfilled, since (due to the Stone-Weierstrass theorem) every continuous function defined on a closed interval can be uniformly approximated as closely as desired by a polynomial function and polynomials are even analytic.
Corollary
Let , then k parallel RC-elements behave even for all equivalently to the one equivalent RC-element if and only if all RC-elements have the same time constant. (The one equivalent RC-element of course again has this time constant.)
Corollary
The alveolar pressure within a 2-alveoli (each alveolus connected by its resistor to the outer end of the nose) sample lung is a common pressure for all if the two alveoli have the same time constant.
Proof
(Of course these 2-alveoli behave like 2 parallel connected RC-elements.) This follows from the above symmetry proof or the fact that this 2-alveoli sample lung is equivalent to the equivalent 1-alveolus sample lung and the pressure within this last one is by definition a common pressure.
If the time constants differ: , then there exists a breathing pattern , so that the alveolar pressure still remains a common pressure, namely , which is of course biologically irrelevant (and is an exceptional case anyway). The elaborate proof that this is an exceptional case shall be dispensed here, since this result is not necessary in the following. Nevertheless let us study the following example:
Claim
If the time constants differ, then there exist breathing patterns, so that the alveolar pressure within the above 2-alveoli sample lung is not always a common pressure.
Proof
Let and the lungs shall consist of the 2 alveoli with the compliances and which are each parallel connected to the outer end of the nose via their resistors and . The analogy between electrical engineering and respiratory physiology (s. Table 1) shows that during passive expiration the following alveolar pressure difference for all applies, whereby and are the alveolar pressures within these alveoli at time . This pressure difference disappears of course only if , since the right hand side of is a function of the time t, whereas the left hand side is constant.
Hence, a common time constant is a necessary condition to keep a common alveolar pressure within this small sample lung.
Corollary
The alveolar pressure is not a common pressure for all of the above n-alveoli (connected to the outer end of the nose) sample lung if (only) one time constant differs from all the other ones.
Proof
Collect all the alveoli with the same time constant to 1 equivalent RC-lung (better sub-lung). This corollary then follows from the last one.
Corollary
The alveolar pressure of a n-alveoli (each connected to the outer end of the nose) sample lung is a common pressure for all alveoli and all , only if all alveoli have the same time constant.
Claim
Provided that the lungs consist of alveoli, each parallel connected to the outer end of the nose via their resistors, all having the same time constant, then this lung is ventilated synchronously.
Proof
Formally this n alveoli sample lung is equivalent to a 1-alveoli sample lung, hence this again follows from symmetry.
This is provisionally the above mentioned dimensioning condition for this very small and simple “bronchial tree”.
Now back to biology: In reality, the alveoli are jointly ventilated via the resistance tree (bronchioles, bronchi, trachea and nose), but this must not change the time constants and the common alveolar pressure, because:
Let us again assume that the alveolar pressure is not common within all the alveoli, then again at the beginning and at the end of each inspiration or expiration pressure equalization between the alveoli would take place. This additionally requires breathing work which again contradicts (P). Consequently, the direction of the volume flow has to be the same within the entire resistance tree. This direction of course changes between the ventilation phases inspiration respectively expiration. The joint ventilation throughout this resistance tree is then of course as well responsible for the synchronous ventilation and is therefore a dimensioning-condition for the resistance tree as well.
This dimensioning-condition shall now be discussed.
Claim
Let us assume that the lungs again consisted of only 2 alveoli with the compliances and which are each parallel connected via their resistors and , however this time not to the (outer end of the) nose but to the (internal end of the) trachea with a resistor Then the time constants are still equal and the alveolar pressure is still a common pressure within these 2 alveoli.
Proof
The proof is exactly the same, as before. The only difference is: The (common) ambient pressure (at the nose) has to be replaced with the (common) pressure at the internal end of , which is the connection point to and . More precisely: The former driving pressure is now (better: sub-driving pressure). Consequently, and formally the and can be replaced with and whereby holds. The serial connection of to this 2 alveoli sample lung (which is formally equivalent to the 1-alveolus sample lung with the equivalent -element) does not affect the fact that remains a common pressure. From the viewpoint at the outer end of , the time constants increase a little to , but it is physically evident that (due to symmetry) these 2 time constants and consequently still remain equal.
This proof does not change, if more than 2 alveoli (e.g. alveoli) each parallel connected via their resistors are (at the same branching point) connected to . In reality this is often the case. Consequently, each of them again has to have the same time constant and again this small lung can formally be replaced by the equivalent 1-alveolus lung with the same time constant . From the viewpoint at the outer end of , the time constants again increase a little to .
The entire lung can now be composed from this small bronchial tree by iterating the above proof (at each branching point) throughout the entire real resistance tree. The above resistor is the parameter, which needs then to be adapted at each step. Consequently, the dimensioning-condition for the whole bronchial tree is ultimately a consequence of (P) as well.
Of course, the time constant of the whole lung is larger, than the time constant of each isolated lung segment (in the broadest and not anatomical sense). The resistance of the trachea, pharynx and nose is then the last time constant increasing step.
Corollary
The alveolar pressure is a common pressure within the lungs only if all alveoli have the same time constant.
The following few lines are a little bit hypothetical and are not at all necessary for the results of this paper. In all the proofs of this subsection all occurring C and R were assumed to be independent of the volume within the lungs, which approximately is true for small tidal volumes like those at rest. However, like C(p) (s. Fig. 3), is not constant, but a function of the pressure within the isolated lungs (West 2012; Lumb 2016b). By using the same arguments as in the previous subsection, the inverse function exists as well. Hence, is also a function of the volume within the lungs. It goes without saying, that and are different functions, nevertheless let us denote them with the same letter as long as there is no risk of confusion. Moreover, it is known that at least close to the residual volume RV or the total lung capacity TLC the airway resistance depends also on the intrapulmonary volume (West 2012). Hence, the equations (7) and (8) for tidal volumes large enough (e.g during physical exertion) have to be adapted in the following way:
The postulated homogeneity must not change with the volume within the lungs. Hence, the parameter has to be the same for each lung segment (in the broadest and not anatomical sense). Otherwise, homogeneity would be violated. Of course, the functions respectively (apart from the physical unit) need not to be the same. Again, thereof doesn’t already follow that is a homogeneous function for all alveoli, since as mentioned above, these are differently shaped and sized and the resistance tree is a very complex one. Hence, all the C, , , , R within this subsection have to be replaced with , , respective , . This presupposes that the serial and parallel addition laws of compliances respectively resistors are still valid. But this trivially is the case and the proof of them changes only slightly. Since the charge Q is the electrical engineering analogue to the volume V, all resistors and capacitors now homogeneously depend on . For example the parallel addition law of these -dependent resistors and : Due to Ohm’s law and due to Kirchhoff‘s current law holds, whereof := = = follows. Hence, is true. The proof doesn’t even change if U is replaced with . The serial addition law of homogeneously -dependent capacitors and gives the analogous result as well: Denote q the charge (of course) on both capacitors, then and , hence := . Consequently, follows. Again, the proof doesn’t change if q is replaced with or . The proofs of the other serial and parallel addition laws are even simpler. The proofs of , and the other serial and parallel addition laws for respiratory physiology are of course equivalent to the ones in this obscure electrical engineering. The proof of the impedance calculus is a little more elaborate, but can be done in the same way. Only the proof using the expiratory breathing pattern can be applied within a small neighbourhood of any point of , but this is sufficient.
Corollary
The “time constant” of the isolated lungs is a homogeneous function of the intrapulmonary volume V i.e. all the alveoli (and the lung itself) obey the same “time constant” function.
Otherwise during physical exertion the alveolar pressure would not be a common pressure within all the alveoli and moreover, would contradict (P) in this activity state. However, the present paper deals only with resting spontaneous breathing, hence this corollary is only a side result. Nevertheless, this corollary could partially explain the course of spirometry functions in medicine.
This corollary has of course to be biologically and clinically evaluated first, which ends the hypothetical lines.
From a mathematical point of view, the time constant of all the alveoli within the lungs might possibly not be exactly the same. From a biological point of view, despite the fact that the pleural pressure is a common pressure for the lungs, small differences in the driving pressure might lead to small differences in the alveolar pressure . However, the elastic septa between the bronchioles and in particular between the alveoli are so thin (Peake and Pinkerton 2015) that pressure equalization of small alveolar pressure differences takes place almost immediately.
Consequently, ultimately following from (P), it can be concluded that the alveolar pressure is an (at least largely) common pressure within real lungs and all alveoli have (almost) the same time constant (or “hypothetical time constant” function). This is now the answer to the above given questions.
The next question arises now: How can the laminar flow assumption (8) be proven?
Claim
The passive (laminar-flow) expiration function of an 1-alveolus sample lung with the constant compliance connected to a resistor R is the following exponential function:
| 10 |
Proof
The analogy between pulmonary physiology and electrical engineering shown in Table 1 gives this result. V(0) is of course the volume within the alveolus at the beginning of expiration. The proof will be dispensed here, since the analog proof can be found in every electric engineering textbook and the next subsection derives this proof for the lung within the thorax anyway.
During quiet spontaneous breathing the compliance is almost constant within a neighbourhood of the FRC and the constancy of R within the quiet breathing range will be discussed in the next subsection. Hence, due to the derived homogeneity of together with the common alveolar pressure the following holds:
Corollary
The passive quiet expiration function of any real (isolated) lung in biology is an exponential function (10).
However, no lung is isolated during spontaneous breathing. Due to (3) within a neighbourhood of the FRC the compliance C is almost constant as well and during quiet spontaneous breathing the tidal volume remains within the constant range of C. The serial connection of the thorax compliance to the lung compliance surely changes the time constant of all the alveoli, however the time constants of all the alveoli still remain equal. Hence the constant in (10) can be replaced with the constant C if remains small enough. This surely is the case during passive quiet spontaneous expiration. Note: This argumentation does not need the above mentioned compliance serial addition equation . The electrical engineering argument would be: We just replace one capacitor by another one.
Consequently, the time constant of the lungs within the thorax during spontaneous breathing is . This result can now be used as a test for the laminar flow assumption (8), which will be the subject of the next subsection.
Hence, during spontaneous breathing at rest, real lungs behave like an 1-alveoli sample lung, consequently all alveoli are even ventilated synchonously.
Nevertheless, there are gravitational physiologic and (patho)physiologic factors (e.g. mucus) influencing the airway resistance or compliance, which is probably why nature has invented the bronchial muscles for fine adaptation of the individual alveoli time constants. Moreover, since is slightly different for inspiration and expiration (West 2012), this fine adaptation should depend on the direction of the volume flow. The last seems to be clinically unproven until now and should be evaluated in the future. Disturbances, like asthma bronchiale seem to support the existence of a flow direction dependent fine adaptation.
Interestingly, these theoretical considerations concerning the time constant, synchronous ventilation and common alveolar pressure (due to (P)) were not published until now. Possibly this is why, the results of equation (17) are still regarded to be valid and consequently the calculation of the respiratory rate at rest (Otis et al. 1950) were not reevaluated until now.
Passive expiratory volume-function and flow-pattern of the lungs
In the following, the time course of the volume V(t) and the flow pattern during quiet passive expiration is to be clarified. The theoretical considerations in the previous subsection led (due to (P)) to the fact that the entire lung within the thorax has a (largely) unique time constant and the alveolar pressure is a common pressure for all alveoli. This theoretical result shall now be evaluated. As mentioned above, expiration is passive both during spontaneous breathing and controlled ventilation, the energy required for this being obtained from (4). Mechanical controlled ventilation is sometimes performed under muscle relaxation. Hence, as a test for equation (10), controlled ventilation (with regard to spontaneous breathing exceptionally without PEEP) is better suited. The electrical engineering analogy to the passive expiration is the unloading of a capacitor, which leads due to (3): and (9) to the first order homogeneous linear differential equation . Another argument, leading to this equation is the application of Kirchhoff’s voltage law, which corresponds to the Kirchhoff’s pressure law in respiratory physiology. After separation of variables the equation gives:
| 11 |
, wherein is the time constant of the lungs within the thorax. Based on the initial condition , which corresponds to the intrapulmonary volume at the beginning of expiration follows and for sufficiently large t (since ventilation was performed without PEEP) the FRC is the “asymptote” of V(t) until the next inspiration. As expected, the time course of the intrapulmonary volume during expiration is an exponential one:
| 12 |
Since the FRC ultimately remains within the lungs under mechanical ventilation, the function is specified in textbooks (Davies et al. 2005; Chatburn 2003), publications (Bergman 1969; Botsis and Mantas 2003; Ashutosh and Keighley 1978) and also shown on modern ventilators as the passive expiration curve (Fig. 6). This result was even already published by Brody in 1954 (Brody 1954).
Fig. 6.
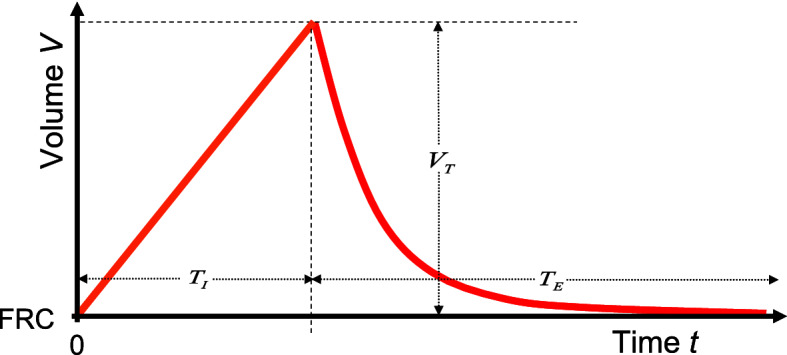
After inspiration with a constant volume flow starting from the intrapulmonary volume FRC to the volume , expiration takes place passively with the time constant under ventilation. The FRC is the “asymptote” of the expiratory function and is almost reached again after about 5 because . This volume-controlled, flow-constant ventilation serves as an idealized model for spontaneous quiet breathing. The quotient is called I : E-ratio
The time constant was recorded on adult animals (Robinson and Sorenson 1978) and on several newborn mammals (Mortola 1983; Mortola et al. 1985) as well, which is why the result (12) of course is not human-specific. Completely analog to the unloading of a capacitor, about of the tidal volume escaped from the lungs after one and after the FRC is almost reached again because . These two percentages are published in respiratory medicine textbooks often without citing (11) (Cairo 2012).
From (12) the volume flow pattern:
| 13 |
which usually is also monitored on modern ventilators and with (5) the pressure gradient (9):
| 14 |
follow an exponential function as well. The signs indicate the direction of flow and pressure gradient.
The product of the volume flow and the pressure difference which this causes across a resistor R has the physical unit of a power. The time integral of the power is therefore the work which is passively done on the airway resistance R during expiration and ultimately converted into heat. As you can see for yourself by inserting (13) and (14), this work corresponds exactly to (4).
Let denote the expiratory duration and the inspiratory duration, then e.g. the data of adult humans from Table 2 show that
| 15 |
Because of , the upper limit in the above integral can be replaced by with a negligible error. By the way, the data from Table 2, show, that the quotients (15) for the human newborn are even larger. This last fact shall be discussed later in connection with the ratio (s. Fig. 6).
From (12) it follows with (15) that at the end of expiration and the quasi-static condition also applies with a comparatively small error and therefore physiologically (almost) the entire tidal volume has left the lungs at the end of expiration. Hence, there is no physiological PEEP, a well known fact. Concerning spontaneous breathing, because of the change in sign of the continuous intrapulmonary pressure curve (with the intermediate value theorem), there must even be exactly one point on V(p) for which applies.
If the assumption of a laminar flow in the dead space, which led to (8), were wrong, then the expiration equation would not follow an exponential function, because a turbulent flow typically shows a resistance behavior that does not depend linearly but approximately on the square (Lumb 2016c) of the volume flow :
| 16 |
An exponential expiratory behavior as illustrated in Figure 6 can never be achieved with this - the expiration would be considerably more slowly. However, since the exponential volume curve (12) is described under ventilation during passive expiration (Davies et al. 2005; Chatburn 2003; Bergman 1969; Botsis and Mantas 2003; Ashutosh and Keighley 1978; Mireles-Cabodevila 2020) and is also displayed in this way on ventilators, it can, conversely be assumed that turbulent flow behavior at rest, if at all, plays a subordinate role at best, which justifies the laminar flow assumption and consequently (8) in retrospect. Of course, this is not a physical but biological proof, nevertheless the well known fact (12) sufficiently supports the previous theoretical considerations, which ultimately base on (8).
Incidentally, with a turbulent flow (16) would have the consequence that the “time constant” has the physical unit , so the term time constant would no longer be appropriate and if
| 17 |
were to apply, then could no longer be assigned a clear physical unit. Nevertheless, this equation (17) was used for the calculation of the respiratory rate at rest in previous publications (Otis et al. 1950) and the results thereof are still expected to be valid.
Concerning (11) this step is only allowed, if the time constant RC is independent of the volume V during quiet breathing. For the compliance C with the linearization (3) at the inflection point of V(p) this surely is the case. The nose, pharynx, throat, trachea and bronchi, which are surrounded by bones or cartilage (Peake and Pinkerton 2015), are mainly responsible for R (West 2012), while the bronchioles only make a very small contribution to R. Consequently, this mainly responsible part for R should not (or only insignificantly) depend on the volume V in the lung, at least during quiet breathing. Nevertheless, if R depends on the volume already for then an exponential expiratory behavior like (12) or (13) could not be achieved. By the way, none of the publications (Otis et al. 1950; Crosfill and Widdicombe 1961; Mead 1960; Kerem 1996; Noël and Mauroy 2019) that deal with the resting respiratory rate did even mention this topic.
The dynamic viscosity of the breathing gas is caused by the internal friction of the gas. The internal friction (responsible for the tissue resistance work) within the lung tissue and chest wall structures or between the lungs and thorax usually plays a subordinate role, which is why it should either be neglected for the sake of simplicity or could be regarded as being subsumed in R (Bates 2015; Bates et al. 1988), as will be suggested here. Assuming this resistance work to overcome the viscosity of the lung and chest wall structures during passive expiration would not show an approximately linear resistance behavior analogous to (8), this behavior would also disturb the exponential course of (12). However, since (12) is described in the literature and shown in this way on ventilators, it can be assumed, conversely that R in (8) actually includes all of the above-mentioned friction and that this resistance R also (predominantly) depends linearly (Bates 2015) on , especially since only this linearity in (8) leads to the exponential functions (12), (13) and (14). Moreover, the simple derivation (13) would not be valid any more and a deviation from the exponential function would be more obvious in the course of (13).
The quotients (15) are based on human data, which is why the objection could be raised that in evolution, humans could be an exception in this regard. But this is very unlikely. Note: Even halving the quotients in (15) would only change the further discussion slightly.
In any case, it would be a waste of energy in the sense of (P) if the compliance work (4) for passive expiration were not recovered analogously to ventilation while breathing spontaneously at rest. It is therefore to be assumed that passive expiration with quiet spontaneous breathing also follows at least approximately the exponential function (12). For the sake of simplicity, this should in any case be assumed here. A discussion about this assumption could develop in subsequent publications, but this does not (or at best only insignificantly) change the respiratory power, since quiet expiration is passive (Levitzky et al. 2018; Lumb 2016a).
Inspiratory flow pattern and resistance power
In the following, the course of the volume flow pattern during inspiration within is now to be explored. For biological reasons shall be a continuous function. The (positive) pressure gradient between the environment and the alveolar space caused by the respiratory muscles during inspiration under spontaneous breathing leads to a (positive) volume flow of the breathing gas. The inspiration consequently requires to overcome the airway resistance R in (9) the power , which is referred to here as the resistance power . (This power is equivalent to in electrical engineering.) With (9) it follows (s. Table 1):
| 18 |
If the entire time available for inspiration is used for a constant inspiratory volume flow , which is required to inhale the tidal volume into the lungs, then:
| 19 |
follows. With mechanical ventilation one would describe this as constant flow ventilation. In physiology, as already mentioned above, the I : E breathing time ratio is defined as the quotient (s. Fig. 6) and because of the following applies:
| 20 |
as
| 21 |
First of all, let the respiratory rate F (within a neighbourhood of the resting respiratory rate but also the I : E-ratio and thus also due to (20) be given. With (1) it follows that with normal quiet spontaneous breathing the tidal volume
| 22 |
is then fixed as well, because (as discussed above) is largely constant in the resting state. Even if the constant volume flow (19) will ultimately turn out to be the power-minimized one, let us now assume that is not constant, but an arbitrary function of time t within the period . Overall, of course, the inspiratory volume flow has to fulfill:
| 23 |
The inspiratory resistance power (18), which now has to be averaged (i.e. integrated and divided by the integration interval) over , is thus:
| 24 |
wherein R is given by the anatomy and was therefore (as a constant) pulled in front of the integral. Analogous to the procedure for the compliance power, (24) should now be minimized again because of (P). One could object here that these minimizations might not be independent. However, except and consequently because of (22) (within a neighbourhood of there are no common parameters in (6) and (24). This is why, the “connecting”-parameter F (or requires another use of (P).
The constant volume flow (19) results in the power
| 25 |
Doubling the volume flow only needs the time to fulfill (23), but double the power , which intuitively suggests that must fill the entire available time period . What is sought, is a function of the time t within the period which, under the side condition (23), minimizes the inspiratory resistance power (24). Claim: The solution of this variation problem is already (19) with the power (25). Proof: It is now assumed, however that is even better regarding power minimalism, where is any function within which, because of (23), has to fulfill the condition . The function g(t) of course has to be real and continuous for physiological or biological reasons. With this assumption, the inspiratory resistance power = applies. The first summand equals (25), the second one disappears because of the above condition, since may be pulled in front of the integral. However, because of , the third term doesn’t make the inspiratory resistance power smaller than (25). The above assumption is therefore wrong. Consequently, the inspiratory flow constant function (19) together with the associated resistance power (25) proves as the minimal solution. Interestingly, none of the authors, who have chosen the respiratory rate as their research subject, used this result.
From (15) it also follows that (19) (at least in humans) is about 5 times smaller than at the beginning of expiration, i.e. at time in (13), which is why (19) cannot have a turbulent character and therefore (9) and (18) could also be used for inspiration.
The linear inspiratory volume function as shown in Fig. 4 then follows from (19) by integration with the initial condition which corresponds to the intrapulmonary volume at the end of the previous expiration.
The postulate (P) therefore leads, with spontaneous breathing at rest, to the constant inspiratory volume flow (19), which is accordingly to be assumed here in the following. However, a discussion about this now power-minimized inspiratory volume flow pattern could still develop in subsequent publications and (19) will actually be modified slightly below.
Minimum of the entire physical respiratory power
Because of the passivity of the spontaneous expiration, the organism no longer has to provide (18) in this breathing phase. It follows from this that for the expansion from (25) to the entire breathing period , only the addition of zero expended resistance power during the passive expiratory duration is necessary. This is taken into account in the following by multiplying (25) with , which corresponds to an averaging of the power (25) over the entire breathing period.
The entire physical respiratory power P to be expended by the organism is thus composed of the two components (6) and the averaging of (25): and with (20) it follows:
| 26 |
The variable can be substituted for the respiratory rate F with (22) (or vice versa). The first choice seems to be simpler. It then follows for the entire respiratory physical power to be provided by the organism:
| 27 |
This equation was not published until now. From a physical point of view the side condition is obvious. At this point one could ask whether there is an optimal compliance C (modulo the discussion in the second subsection) and an optimal resistance R? The answer is quite simple: and , since both together lead to . This result of course is biologically impossible and would imply = 0 and = 0. Animals with a body covered by fur have limited ability to sweat, relying heavily on panting. A close to and high F is characteristic of panting causing more air to ventilate the dead-space in order to increase heat loss by water evaporation without increasing -loss. Due to this physiological dead space ventilation and of course due to the huge necessary gas exchange surface as well, which requires a bronchial tree, the same impossibility applies to the optimal dead space . Questions like these seemingly are irrelevant in this context. Therefore, the parameters R, C and are given by the anatomy (or pathophysiology) and consequently constant within this context. The -homeostasis of the organism requires an (at least largely) constant and is also constant (with the already mentioned restrictions) under spontaneous breathing at rest. So far, the respiratory rate F and the I : E-ratio have been considered constant as well. This should initially continue to apply to the I : E-ratio. After this little sense of achievement, the question arises as to which the breathing rate F in the sense of (P) could be optimal. In order to obtain the minimum of the respiratory power, (27) must be derived with respect to the respiratory rate F in order to then calculate the optimal respiratory rate from . It is then to be checked whether this leads to a minimum of (27) and, hopefully, not to a maximum. Let be the constant energy conversion efficiency of respiration (Campbell et al. 1957) at rest, then from of course follows as well, since . Consequently, (as discussed above) is largely independent of F within a neighbourhood of as well. Assuming that , for example the human data in Table 2 and the following result then show that accounts for less then 0.25% of the basal metabolic rate in this simple model of spontaneous breathing at rest.
To keep it general, all the 6 parameters , I : E-ratio, , , C and R in (27) have not yet been specified, since these parameters are not human-specific. (The I : E-ratio will be discussed below.) The results obtained should therefore apply more widely in biology, possibly even for most lung-breathing animals with a thorax, especially mammals. A whale or other mammals who predominantly live in water also use the buoyancy of the air in the lungs, which admittedly still would have to be included in the above considerations.
After deriving, multiplying by and then setting equal to zero, (27) results in a 3rd degree polynomial in the breathing rate F, whose positive real zero has to be calculated:
| 28 |
Every polynomial of n-th degree is known to have n solutions, so the above equation has 3 solutions, which could also be complex. Undoubtedly, only a positive real solution is physiologically meaningful. The polynomial (in the variable F) on the right-hand side of (28) has only real coefficients and as a real polynomial of degree 3 it has at least one real solution.
The question that immediately arises: Is there a positive solution at all? This question can be answered in the affirmative, because: The multiplication with could have yielded the trivial solution . This multiplication was allowed, however, since is biologically only possible for and due to ultimately only for . The latter is indeed a solution for living beings for a short period of time that hibernate (e.g. hamster) and take long pauses in breathing in this state. (Side note: Since not only the metabolism but also the urine excretion is reduced in hibernation, the above approximation of the balance also applies in hibernation.) However, a human with is subject to resuscitation, but lowering the body temperature also reduces oxygen consumption in humans substantially, so that is possible for a short time during hypothermic cardiac arrest in the cardiac operating room - but not under spontaneous breathing as in hibernators.
If F in (28) increases starting from 0, then the first two summands always remain positive increasing and together reach the value exactly once, which is why (28) has exactly one positive real solution provided that . This solution is therefore unique, which is physiologically reasonable, because: Assuming (28) actually had 3 positive real solutions, then the organism would be spoiled for choice between 3 different optimized breathing rates and even worse, evolution would not have focused on one stable respiratory rate, but could have established a metastable level.
Every polynomial of degree less than or equal to 4 can be solved with radicals. A simple solution of (28) is , which cannot be the biological one. However, dividing of (28) by results in a quadratic equation and the unique real positive root of this quadratic equation is:
| 29 |
This equation has never been published before as well. The second derivative of (27): hasn’t negative coefficients and since the biological relevant solution (29) is always positive (or in the extreme case almost 0), this happily is a minimum of (27). The associated tidal volume is then to be calculated from (22). Since remains constant under spontaneous breathing at rest with the respiratory rate , the minute volume is also constant under the requirement of a physiological homeostasis and thus a largely constant . With (19) such ventilation is called constant flow volume-controlled, which suggests the function V(t) in Figure 6 as an idealized simple model of spontaneous breathing at rest. Surely there exists no simpler one with comparable results.
Acceleration power and other neglects
A secondary result during the derivation of the Hagen-Poiseuille law is the fact that the maximum velocity in the middle of a laminar flow in a pipe is exactly twice as large as the mean flow velocity . It is known that the velocity of the breathing gas is highest in the main bronchi and the trachea. Let r be the radius of the trachea (Pinkerton et al. 2015) (in human adults 13 mm), then because of (19) is the mean velocity in the trachea during spontaneous inspiration at rest. In order to accelerate the breathing gas with the mass m to the maximum speed the energy is required. The inspiratory acceleration power necessary to reach the kinetic energy of the breathing gas with the density for every breathing period, even at the maximum velocity for air (, is (as a simple calculation shows using the human data in Table 2) at least a factor of 10 less than (27) and could therefore be neglected.
The acceleration of the lungs and chest wall between the breathing phases is comparably low, because the accelerated mass is (compared to the breathing gas) indeed a factor of 10 larger, but the square of the maximum velocity is about the same factor 10 less.
As mentioned before, nose, pharynx, throat, trachea and bronchi are surrounded by bones or cartilage (Peake and Pinkerton 2015), which are mainly responsible for R, while the bronchioles only make a very small contribution to this (West 2012). Therefore, analogous to the lungs themselves, this dead space at the transition between the breathing phases holds back a very small volume and accordingly influences the volume flow. The expiratory pressure gradient (14) is almost zero at the end of expiration, however due to (9) the volume flow (19) causes an inspiratory resistance pressure gradient which, due to (5) and (15), is about 5 times smaller than the compliance pressure (5). Nevertheless, this pressure gradient is still found at the end of inspiration mainly in this inelastic part of the dead space with an admittedly very low (here negligible) compliance , at least . With a constant body temperature, an isothermic process can be assumed within the lungs, hence the Boyle-Mariotte law is applicable within the dead space, consequently approximately holds, whereof follows. For example the adult human data in Table 2 give . Hence, in retrospect Kirchhoff’s current (which corresponds in respiratory physiology to Kirchhoff’s volume flow) law was at least approximately applicable. Even if is not negligible and would be 5 to 10 times larger, the inspiratory resistance pressure gradient can not significantly affect (22) and (29). Nevertheless, the inspiratory volume flow is no longer constant at the very end and the very beginning of inspiration, rather the corners and edges in Figure 6 like a “mollifier” are rounded off, so that the volume curve comes closer to that shown in physiology textbooks (Hall 2020).
With all the neglects and approximations made so far, no exact result can be expected by (29). The art of applied mathematics in the natural sciences is to choose such neglects and approximations in such a way that the resulting as simple as possible formalism still describes nature as well as possible. In subsequent publications, however, these approximations could be analyzed more precisely using more complex models.
Example: The respiratory rate of an adult human and a human newborn baby
In the literature, is rarely given, which is why in Table 2 this parameter was calculated from the -consumption rate with the respiratory quotient RQ. Using the example of humans, the results in the penultimate and last column in Table 2 should reinforce confidence in the concept that led to (29). Moreover, for the newborn human there are (apart from descriptive studies) no clinical studies at all, which predict or estimate the respiratory rate at rest. However, the present publication only claims to be a theoretical concept for the spontaneous breathing rate in the resting state. The compliance C increases with body weight and age (Bolle et al. 2008; Gaultier et al. 1984; Rendas et al. 1978) as can be seen in Table 2 as well. However, in the absence of (most of the other comparable) animal data analogous to Table 2, this publication is intended to encourage (28) further scientific tests.
Quantitative influencing factors on the respiratory rate
At least as interesting, is the following question: How big is the influence of the 6 parameters in (29) on the optimal , or to put it somewhat more mathematically while keeping it dimensionless: How does change, if only one of the 6 parameters changes slightly, which leads to the linear ansatz: , in which and the parameters , which led to the solution , but not and , are regarded as constant. It is now necessary to calculate these 6 dimensionless factors . A little rewritten and for follows where is the partial derivative of according to the parameter at the point which led to .
For example:
Note: Since is largely constant within a small neighbourhood of the above partial derivative is surely valid for the parameter .
Claim
Since the parameters C and R occur in (29) exclusively together in form of the product , the following holds: . The same symmetry is true for , and as well and consequently holds too.
Proof
Let , then consequently and analogously . Therefore and dividing by gives the first result. Let now , then only a slightly changed proof gives and again dividing by gives the negative sign result.
Corollary
and (s. Table 3).
Table 3.
Dimensionless factors at the point of these parameters given in Table 2. Because of are always positive, the sign of is a parameter for the “correlation” direction between and . The parameters and I : E–ratio therefore “correlate” positively with , with having a significantly stronger effect on . All other parameters “correlate” negatively with . Due to symmetries of (29) and applies
| I : E | R | C | ||||
|---|---|---|---|---|---|---|
| Adult human | 0.69 | 0.69 | 0.69 | 0.21 | 0.31 | 0.31 |
| Newborn human | 0.75 | 0.75 | 0.75 | 0.15 | 0.25 | 0.25 |
Claim
Let , , and , then .
Proof
Let , then f(x) is a strictly increasing (at least) function for and moreover . Let now , then g(x) has the same properties. Due to the chain rule, the composition of functions is again a function. Moreover, the composition of strictly increasing functions is again a strictly increasing function, consequently g(f(x)) is a strictly increasing (at least) function for . Hence, because (29) as a function of the parameter I : E has the same property.
Corollary
Within the biologically relevant range the function (29) is strictly positive if . Consequently the I : E-ratio “correlates” positively with (s. Table 3).
Similar proofs can be used for all the other parameters at least within a small neighbourhood close to the biologically relevant (for humans given in Table 2).
Concerning the human data in Table 2, the quantitative effect in terms of the of these 6 parameters on is summarized in Table 3, which is the basis for the first sentence in the summary. If one only looks at the sign of the , then (because all are positive) an increase in or I : E-ratio increases the respiratory rate with having a significantly stronger effect on , whereas the parameter , , R and C “correlate” negatively with the respiratory rate . This explains the relatively high quiet breathing rate of a human newborn compared to an adult: A newborn produces about twice as much per kg body weight and time unit due to the approximately twice as high (Long and Santhanam 2017), whereby the compliance C is considerably lower (Huang et al. 2016; Kerem 1996). The relatively large head is responsible for a somewhat larger -ratio (Numa and Newth 1996), hence the relatively larger anatomical dead space as well as the relatively higher resistance R reduce the breathing rate .
Again using the example of adult humans then (15) together with (21) gives a dimensionless factor and the data from Table 2 show that:
| 30 |
In adults it has been noted that the I : E-ratios tend to be approximately the same 1:2 inter-specifically (Boggs and Tenney 1984). The time constants are of course given by the anatomy, physiology and the age. The parameters and / or the -ratio in (30) could therefore accordingly be increased without adversely affecting expiration (12) since . As shown above, is a continuous strictly increasing function of the parameter I : E within the biologically relevant range provided that . Consequently, there must be exactly one I : E-ratio for the (non hibernating) newborn, so that the equal sign applies in (30). However, this solution can only be calculated numerically. With the daring hypothesis that this equal sign applies in (30) and the right side of (30) defines a dimensionless number for each species, the I : E-ratio and for the newborn or the child could be calculated from (30). For the human newborn the would then slightly increase to 35.05/min, which is still within the normal range. At the same time the respiratory power (27) of the newborn would then even decrease by about 4.6%. Side-note: Concerning the I:E-ratio none of the authors, who have chosen the respiratory rate as their subject, used this parameter.
Discussion in the light of previous publications
In 1950, ventilation was only possible with the historical iron lung, e.g. the Drinker respirator, in which test subjects were ventilated as part of a study (Otis et al. 1950). The authors used clinical data, in some cases even with added , to minimize the respiratory power with mathematical aids in order to calculate an optimal respiratory rate as well, assuming that the breathing pattern is a sine wave. However, their method cannot be generalized in a comparable way, which is why the respiratory rate of a newborn human could not be calculated, especially since no newborns were included into the study. In addition, the power unit GM.CM/MIN selected in this study is hardly comprehensible today and, according to today’s standards, would no longer have the physical unit of a power (Schramm 2010). In this publication (and also subsequent publications) there is also a term like the one in (17), which, as shown above, no longer allows the concept of a time constant . Moreover, this publication does not reveal to what extent the discussed parameters ultimately influence the resting respiratory rate. The term lung respective thorax compliance was not yet developed in its present form in 1950, however, the elastic work was already known by nearly the same authors (Rahn et al. 1946). The mathematical methods, such as the Euler-Lagrange formalism, were fully developed at this time, but the calculus of variations was not used.
In a similar clinical study based on Otis’ work (Otis et al. 1950), Mead (Mead 1960) examined both guinea pigs and human subjects, but ultimately came to the conclusion that the optimal respiratory rate is not achieved by minimizing respiratory power, but rather by the force to be applied to the respiratory muscles. Crosfill and Widdicombe (1961) have each derived an equation for minimizing respiratory performance and an equation for minimizing the mean force to be exerted by the respiratory muscles by assuming the alveolar ventilation to be constant. However should have been included in the derivation of the optimal breathing rate as a side condition. Bates and Milic-Emili (Bates and Milic-Emili 1993) set out to determine if the viscoelastic properties of the lung could better refine the determinants of optimal breathing frequency, however, during quiet breathing as well as exercise. They simulated the lung using computational methods. To their surprise, they concluded that the viscoelastic properties of the respiratory system tissues do not significantly alter the prediction of optimal breathing frequency in humans or dogs and inspiratory work was a better predictor of optimal breathing frequency than an inspiratory pressure-time integral.
The quantitative measurement of was already described by Tyndall in 1862 (Tyndall 1862) and the infrared gas analysis of air (Luft 1943) in 1943, but at that time it was still not used in biology or medicine for the continuous measurement of the (Jaffe 2008) or, if at all, only with great technical effort (Miller et al. 1950), which is why the above publications have not included (1) in their considerations. Noël and Mauroy (2019) has integrated the gas transport into his model, however, thus buying in other difficulties and was therefore unable to answer all the raised questions. Kerem (1996) completely dispensed with mathematics in order to describe qualitative influences of the parameters , , R, C, but also other possible influencing factors on the respiratory rate in small children and newborns, so quantitative data are completely lacking. With regard to the volume flow Li and Wassim (2012) have chosen a minimizing approach for both the inspiratory and the expiratory airflow in a non-linear multi-compartment numerical model. Under additional assumptions, they achieved results for the respiratory flow that differ completely from (13) and (19).
Limits under physical exercise
As already mentioned, expiration is passive under quiet spontaneous breathing. This assumption generally no longer applies under physical exercise, because in the latter case the respiratory gas (usually air) is actively exhaled via the respiratory muscles. In order to maintain a physiological homeostasis in the organism due to (1), an increased alveolar ventilation is necessary with increased physical performance because of the excess production rate coupled to the aerobic metabolism. As everyone knows, this requires an increase in the tidal volume (via the inspiratory and expiratory reserve volume) as well as the respiratory rate F. Because of (21) the higher respiratory rate F shortens the expiration time and due to (12) both, the increased tidal volume and the shorter are responsible for the active accelerated expiration by increasing the intrathoracic pressure by the respiratory muscles. Therefore expiration is no longer passive under an appropriate higher level of physical performance. Incidentally, the expiratory reserve volume can never be exhaled without the aid of the respiratory muscles. For this reason (28) can only apply in an (at least approximately) resting state.
Another limit of (28) is the following: It is known that the lactate concentration within the organism increases under heavy physical exertion, which leads to a metabolic acidosis shifting the balance  to the left and thus causing an increased release from the bicarbonate buffer, which then has to be included in the balance addressed in the beginning. This is why even the RQ can sometimes rise above the value 1 independent of nourishment, however only when almost physical exhaustion occurs.
to the left and thus causing an increased release from the bicarbonate buffer, which then has to be included in the balance addressed in the beginning. This is why even the RQ can sometimes rise above the value 1 independent of nourishment, however only when almost physical exhaustion occurs.
Possible applications in medicine
Since all schoolchildren already know their breathing rate at rest, the practical benefit of the resting breathing rate is low and the present publication only appears to be of academic interest. The parameters , , I : E-ratio, which occur in (29) can be measured with simple aids and also , C and R (the latter with a body plethysmograph) can still be recorded without much effort. Moreover, using (1) one of the parameters , or can be substituted in (29) by the easily measurable tidal volume and the dead space can be determined from the Enghoff-modified Bohr equation (Enghoff 1938; Bohr 1891). If 6 of the 7 parameters (including in (29) are known, then the 7th can be calculated therefrom, which is why equation (29) can be of interest not only in physiology and biology, but under certain circumstances also in medicine. Since C and R are routinely measured in a body plethysmograph, (29) could, for example, extend this pulmonary instrument.
It cannot be ruled out that this equation could also become valid in parts of the pathophysiology, which, however, would have to be proven in clinical studies: It is known that the airway resistance R is increased during an asthma attack, which results in an extended time constant and leads due to (12) to an extended expiratory duration. The time is then no longer sufficient to passively exhale the tidal volume , consequently . In this case, under ventilation, the increased intrapulmonary volume is called trapped volume. Because of (3) and (8) this leads to a PEEP, which is then referred to as intrinsic PEEP (sometimes called auto-PEEP). In order to counteract this, the patient has no choice but to actively expire during an asthma attack, i.e. supported by the respiratory muscles (and the PEEP), which is why (28) can no longer be valid in an asthma attack. However, an asthma attack in the body plethysmograph is the exception anyway. A comparable pathophysiology can be observed in COPD patients. In COPD patients, however, the permanently high airway resistance R and moreover, a permanently increased compliance C (due to a loss of elasticity of the lungs) leads to a permanently extended time constant . The resulting (due to (13)) permanently lower expiration flow leads to a permanently increased FRC with all its consequences such as high intrathoracic pressure, barrel chest, ... therefore equation (28) would have to be modified accordingly for COPD patients. Moreover, in a COPD diseased lung, certain portions may be more affected than others, therefore is not homogeneous within the lungs any more. Consequently, the alveolar pressure cannot be a common pressure for all the alveoli any more and pressure equalization during and between the breathing phases is the consequence, requiring additional breathing work.
It can be assumed that hypoxia from a pulmonary origin affects the minute volume in a similar way as described by Duffin in altitude medicine (Duffin 2007). For patients with lung diseases without intrinsic PEEP and still sufficiently high arterial -partial pressure, however, nothing should diminish the applicability of (29). Pneumonia, for example, reduces the compliance C (Somerson et al. 1971) and the accompanying rise in body temperature increases and thus . Both parameters lead to an increase of , a clinically age-old finding.
Since the organism prefers a normal pH value rather than a -homeostasis, which in pathophysiology is referred to as the respiratory compensation of a metabolic disorder, there are also deviations from the norm value, which due to (1) and (29) accordingly affect without primary lung diseases (Kußmaul 1874). Hence, metabolic disorders might be applications of (29) as well.
The applicability of (29) for these pathophysiologies of course should be first evaluated in clinical studies.
As already mentioned in the theoretical subsection, the “hypothetical time constant function” could partially explain the course of spirometry functions in medicine.
Final remarks
Although the translation of respiratory physiology into a primarily physical and mathematical language together with a repeatedly applied postulate from the theory of evolution using the example of humans has led to expected results, it must not be overlooked that evolution has spent millions of years on a human being and the surrounding wildlife. Even if the breathing mechanics is only a very tiny part of it, the attempt to describe this part in a few lines of evolution, biology, physiology, physics and mathematics can just remain an attempt, everything else would be presumptuous. But if this publication helps to better understand the mechanics of breathing, especially spontaneous breathing at rest, or perhaps even to stimulate further reflection and publication, it will perhaps help that not only the physiology of humans, but also the biology of many living beings to become a tiny bit richer.
List of symbols
- C(p)
Static compliance = dV/dp
- C
Static compliance at the inflection point of V(p)
- F
Respiratory rate =
Power-minimized respiratory rate at rest
-fraction =
Inspiratory -fraction
Endtidal -fraction
- FRC
Functional residual capacity
- I : E
Inspiratory duration / expiratory duration =
- m
Mass
- p
Pressure
Compliance pressure
Resistance pressure
Ambient pressure
- P
Physical power
- PEEP
Postitive end-expiratory pressure
Compliance power
Resistance power
- R
Airway-resistance
- RQ
Respiratory quotient =
- RV
Residual volume
- t
Time
Time constant =
Inspiratory duration
Expiratory duration
- TC
Total lung capacity
- v
Velocity
Mean velocity
- V
Volume
- V(p)
Static “pressure-volume” function
-consumption rate
-production rate =
Tidal volume
Power-minimized tidal volume at rest
Dead space
- dV/dt
Volume flow
Compliance work
- W
Physical work
Funding
This project was not funded.
Declarations
Conflict of interest
The author declares that he has no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ashutosh K, Keighley JF. Passive expiration as a test of lung function. Thorax. 1978;33(6):740–6. doi: 10.1136/thx.33.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JH, Ludwig MS, Sly PD, Brown K, Martin JG, Fredberg JJ. Interrupter resistance elucidated by alveolar pressure measurement in open-chest normal dogs. J Appl Physiol. 1988;65(1):408–14. doi: 10.1152/jappl.1988.65.1.408. [DOI] [PubMed] [Google Scholar]
- Bates JH, Milic-Emili J. Influence of the viscoelastic properties of the respiratory system on the energetically optimum breathing frequency. Ann Biomed Eng. 1993;21(5):489–99. doi: 10.1007/BF02584331. [DOI] [PubMed] [Google Scholar]
- Bates Jason HT (2015) Mechanical Properties of the Lung. In: Parent RA (ed) Comparative Biology of the Normal Lung. Academic Press Inc
- Bergman NA. Properties of passive exhalations in anesthetized subjects. Anesthesiology. 1969;30(4):378–87. doi: 10.1097/00000542-196904000-00005. [DOI] [PubMed] [Google Scholar]
- Boggs DF, Tenney SM. Scaling respiratory pattern and respiratory drive. Respiration Physiology. 1984;58(3):245–251. doi: 10.1016/0034-5687(84)90001-x. [DOI] [PubMed] [Google Scholar]
- Bohr C. Ueber die Lungenathmung. Skand Arch Physiol. 1891;2:236–268. doi: 10.1111/j.1748-1716.1891.tb00581.x. [DOI] [Google Scholar]
- Bolle I, Eder G, Takenaka S, Ganguly K, Karrasch S, Zeller C, Neuner M, Kreyling WG, Tsuda A, Schulz H. Postnatal lung function in the developing rat. J Appl Physiol. 2008;104(4):1167–76. doi: 10.1152/japplphysiol.00587.2007. [DOI] [PubMed] [Google Scholar]
- Botsis T, Mantas J. Mathematical modelling for the study of respiratory mechanics. Stud Health Technol Inform. 2003;95:9–14. [PubMed] [Google Scholar]
- Brody AW. Mechanical Compliance and Resistance of the Lung-Thorax Calculated From the Flow Recorded During Passive Expiration. Am J Physiol. 1954;178(2):189–96. doi: 10.1152/ajplegacy.1954.178.2.189. [DOI] [PubMed] [Google Scholar]
- Bryan AC, Wohl Mary EB (2011) Wiley Online Library Handbook of Physiology, The Respiratory System, Mechanics of Breathing, Supplement 12. Respiratory Mechanics in Children
- Butler J, White HC, Arnott WM. The pulmonary compliance in normal subjects. Clin Sci. 1957;16(4):709–29. [PubMed] [Google Scholar]
- Cairo JM (2012) How a Breath Is Delivered. In: Cairo JM (ed) Pilbeams Mechanical Ventilation Physiological and Clinical Applications. Elsevier
- Campbell D, Brown J. The electrical analogue of the lung. Brit. J. Anaesth. 1963;35:684. doi: 10.1093/bja/35.11.684. [DOI] [Google Scholar]
- Campbell EJ, Westlake EK, Cherniack RM. Simple Methods of Estimating Oxygen Consumption and Efficiency of the Muscles of Breathing. J Appl Physiol. 1957;11(2):303–8. doi: 10.1152/jappl.1957.11.2.303. [DOI] [PubMed] [Google Scholar]
- Chambers D, Huang Ch, Matthews G (2019) Basic Physiology for Anaesthetists, 2nd edn. Cambridge University Press
- Chatburn R (2003) Mandu Press Ltd. Fundamentals of Mechanical Ventilation. A short course on the theory of mechanical ventilators
- Crosfill ML, Widdicombe JG. Physical characteristics of the chest and lungs and the work of breathing in different mammalian species. J Physiol. 1961;158(1):1–14. doi: 10.1113/jphysiol.1961.sp006750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DS, Gappa M, Rosenfeld M (2005) Respiratory Mechanics. In: Hammer J, Eber E (ed) Paediatric Pulmonary Function Testing, (Progress in Respiratory Research, Vol. 33). Karger Publishers
- Duffin J. Measuring the ventilatory response to hypoxia. J Physiol. 2007;584(1):285–293. doi: 10.1113/jphysiol.2007.138883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J, Saltzman HA, O’Bryan R. Maximal physical-work capacity of man at 43.4 ATA. Undersea Biomed Res. 1977;4(4):359–72. [PubMed] [Google Scholar]
- Enghoff H. Volumen inefficax. Bemerkungen zur Frage des schädlichen Raumes. Upsala Läk.-Fören Förh. 1938;44:191–218. [Google Scholar]
- Exline MC, Mireles-Cabodevila E, Hite RD (2020) Acute Respiratory Distress Syndrome. In: Kacmarek RM, Stoller JK, Heuer Al (ed) Egan’s Fundamentals of Respiratory Care, 12th edn. Mosby
- Fenn WO, Rahn H, Otis AB. A theoretical study of the composition of the alveolar air at altitude. Am J Physiol. 1946;146:637–53. doi: 10.1152/ajplegacy.1946.146.5.637. [DOI] [PubMed] [Google Scholar]
- Fernandez R, Blanch L, Artigas A. Inflation static pressure-volume curves of the total respiratory system determined without any instrumentation other than the mechanical ventilator. Intensive Care Med. 1993;19(1):33–8. doi: 10.1007/BF01709275. [DOI] [PubMed] [Google Scholar]
- Galetke W, Feier C, Muth T, Ruehle K-H, Borsch-Galetke E, Randerath W. Reference values for dynamic and static pulmonary compliance in men. Respir Med. 2007;101(8):1783–9. doi: 10.1016/j.rmed.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Gaultier C, Harf A, Lorino AM, Atlan G. Lung mechanics in growing guinea pigs. Respir Physiol. 1984;56(2):217–28. doi: 10.1016/0034-5687(84)90105-1. [DOI] [PubMed] [Google Scholar]
- Grinnan DC, Truwit JD. Clinical review Respiratory mechanics in spontaneous and assisted ventilation. Critical Care. 2005;9(5):472–84. doi: 10.1186/cc3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YF, Herrmann F, Michel JP, Janssens JP. Normal values for respiratory resistance using forced oscillation in subjects65 years old. Eur Respir J. 2005;26(4):602–8. doi: 10.1183/09031936.05.00010405. [DOI] [PubMed] [Google Scholar]
- Hall JE. Pulmonary Ventilation. In: Hall JE, Guyton AC, editors. Guyton and Hall Textbook of Medical Physiology. 14. Saunders Company: W.B; 2020. [Google Scholar]
- Li Hancao, Wassim MH (2012) Optimal determination of respiratory airflow patterns using a nonlinear multicompartment model for a lung mechanics system. Comput Math Methods Med :ID 165946. 10.1155/2012/165946 [DOI] [PMC free article] [PubMed]
- Huang J, Zhang H, Zhang M, Zhang X, Wang L. Reference values for resistance and compliance based on the single occlusion technique in healthy infants from Southeast China. J Thorac Dis. 2016;8(3):513–9. doi: 10.21037/jtd.2016.02.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe MB. Infrared measurement of carbon dioxide in the human breath: ‘breathe-through’ devices from Tyndall to the present day. Anesth Analg. 2008;107(3):890–904. doi: 10.1213/ane.0b013e31817ee3b3. [DOI] [PubMed] [Google Scholar]
- Joehr M (1993) Techniken und Material. In: Joehr M (ed) Kinderanaesthesie, 2nd edn. Gustav Fischer
- Kacmarek RM, Dean RH (2019) Mc Graw Hill Education, 4th edn. Essentials of mechanical ventilation
- Kerem E (1996) Why do infants and small children breathe faster? Pediatr Pulmonol 21(1):65–8. 10.1002/1099-0496(199601)21:1$<$65::aid-ppul1950210104$>$3.0.co [DOI] [PubMed]
- Kußmaul A. Zur Lehre vom Diabetes mellitus. Über eine eigenthümliche Todesart bei Diabetischen, über Acetonämie, Glycerin-Behandlung des Diabetes und Einspritzungen von Diastase in’s Blut bei dieser Krankheit. Deutsches Archiv für klinische Medicin. Leipzig. 1874;14:1–46. [Google Scholar]
- Levitzky MG (2018) New York: McGraw-Hill Education. Pulmonary Physiology 9th ed
- Long Justin B, Santhanam Suresh. Neonatal Anesthesia. In: Barash PH, Cullen N, Stoelting RK, editors. Clinical Anesthesia. 8. Philadelphia: Wolters Kluver/Lippincott Williams & Wilkins; 2017. [Google Scholar]
- Luft K. Über eine neue Methode zur Registrierung der Gasanalyse mit Hilfe der Absorption ultraroter Strahlen ohne spektrale Zerlegung. Zeitschr f Techn Phys. 1943;24:97–104. [Google Scholar]
- Lumb AB. Pulmonary Ventilation. In: Lumb AB, editor. Nunn’s Applied Respiratory Physiology. 8. Elsevier; 2016. [Google Scholar]
- Lumb AB (2016b) Elastic forces and Lung Volumes. In: Lumb AB (ed) Nunn’s Applied Respiratory Physiology, 8th edn
- Lumb AB (2016c) Respiratory System Resistance. In: Lumb AB (ed) Nunn’s Applied Respiratory Physiology, 8th edn
- Mead J. Control of respiratory frequency. J Appl Physiol. 1960;15(3):325. doi: 10.1152/jappl.1960.15.3.325. [DOI] [Google Scholar]
- Miller FA, Hemingway A, Nier AO, Knight RT, Brown EB, Varco RL. The development of, and certain clinical applications for, a portable mass spectrometer. J. thorac. Surg. 1950;20:714–728. doi: 10.1016/S0096-5588(20)31540-3. [DOI] [PubMed] [Google Scholar]
- Mireles-Cabodevila E (2020) Ventilation. In: Kacmarek RM, Stoller JK, Heuer Al (ed) Egan’s Fundamentals of Respiratory Care, 12th edn. Mosby
- Mortola JP. Comparative aspects of the dynamics of breathing in newborn mammals. J Appl Physiol Respir Environ Exerc Physiol. 1983;54(5):1229–35. doi: 10.1152/jappl.1983.54.5.1229. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Magnante D, Saetta M. Expiratory pattern of newborn mammals. J Appl Physiol. 1985;58(2):528–33. doi: 10.1152/jappl.1985.58.2.528. [DOI] [PubMed] [Google Scholar]
- Noël F, Mauroy B. Interplay Between Optimal Ventilation and Gas Transport in a Model of the Human Lung. Front Physiol. 2019;26(10):488. doi: 10.3389/fphys.2019.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numa AH, Newth CJ. Anatomic dead space in infants and children. J Appl Physiol. 1996;80(5):1485–9. doi: 10.1152/jappl.1996.80.5.1485. [DOI] [PubMed] [Google Scholar]
- Otis AB, Fenn WO, Rahn H. Mechanics of breathing in man. J Appl Physiol. 1950;2(11):592–607. doi: 10.1152/jappl.1950.2.11.592. [DOI] [PubMed] [Google Scholar]
- Pasquis P, Denis P, Hellot MF, Lefrancois R. Reference values of pulmonary compliance. Bull Eur Physiopathol Respir. 1976;12(5):659–68. [PubMed] [Google Scholar]
- Peake Janice L, Pinkerton Kent E. Gross and Subgross Anatomy of Lungs, Pleura, Connective Tissue Septa, Distal Airways, and Structural Units. In: Parent RA, editor. Comparative Biology of the Normal Lung. Academic Press Inc; 2015. [Google Scholar]
- Pinkerton KE, Van Winkle LS, Plopper CG, Smiley-Jewell S, Covarrubias EC, McBride JT (2015) Architecture of the Tracheobronchial Tree. In: Parent RA (ed) Comparative Biology of the Normal Lung
- Pugh LG. Resting ventilation and alveolar air on Mount Everest: with remarks on the relation of barometric pressure to altitude in mountains. J Physiol. 1957;135(3):590–610. doi: 10.1113/jphysiol.1957.sp005733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn H, Otis AB, Chadwick Leigh E, Fenn Wallace O. The pressure-volume diagram of the thorax and lung. Am J Physiol. 1946;146(2):161–78. doi: 10.1152/ajplegacy.1946.146.2.161. [DOI] [PubMed] [Google Scholar]
- Rendas A, Branthwaite M, Reid L. Growth of pulmonary circulation in normal pigdstructural analysis and cardiopulmonary function. J Appl Physiol. 1978;45(5):806–17. doi: 10.1152/jappl.1978.45.5.806. [DOI] [PubMed] [Google Scholar]
- Robinson NE, Sorenson PR. Collateral flow resistance and time constants in dog and horse lungs. J Appl Physiol Respir Environ Exerc Physiol. 1978;44(1):63–8. doi: 10.1152/jappl.1978.44.1.63. [DOI] [PubMed] [Google Scholar]
- Rohrer F (1925) Berlin: Julius-Springer. Handbuch der normalen und pathologischen Physiologie
- Schramm W. The units of measurement of the ventricular stroke work: a review study. J Clin Monit Comput. 2010;24(3):213–7. doi: 10.1007/s10877-010-9234-4. [DOI] [PubMed] [Google Scholar]
- Somerson NL, Kontras SB, Pollack JD, Weiss HS. Pulmonary compliance: alteration during infection. Science. 1971;171(3966):66–8. doi: 10.1126/science.171.3966.66. [DOI] [PubMed] [Google Scholar]
- Tepper JS, Costa DL. Methods, Measurements, and Interpretation of Animal Lung Function in Health and Disease. In: Parent RA, editor. Comparative Biology of the Normal Lung. Academic Press Inc; 2015. [Google Scholar]
- Tyndall J. On the Absorption and Radiation of Heat by Gaseous Matter. Second Memoir. Philosophical Transactions of the Royal Society of London. 1862;152:59–98. doi: 10.1098/rstl.1862.0008. [DOI] [Google Scholar]
- Weil JV, Byrne-Quinn E, Sodal IE, Friesen WO, Underhill B, Filley GF, Grover RF. Hypoxic ventilatory drive in normal man. J Clin Invest. 1970;49(6):1061–72. doi: 10.1172/JCI106322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. Respiratory monitoring of carbon dioxide and oxygen: a ten-year perspective. J Clin Monit. 1990;6(3):217–25. doi: 10.1007/BF02832150. [DOI] [PubMed] [Google Scholar]
- West JB (2007) Normal Physiology: Hypoxia. In: West JB (ed) Pulmonary Physiology and Pathophysiology An Integrated, Case-Based Approach, 2nd edn. Williams & Wilkins, Woltes Kliver, Lippincott
- West JB (2012) Mechanics of Breathing. In: West JB (ed) Respiratory Physiology The Essentials, 9th edn. Williams & Wilkins, Woltes Kliver, Lippincott