Abstract
Introduction
Duchenne muscular dystrophy (DMD) is a progressive disease that leads to damage of muscle and myocardium due to genetic abnormalities in the dystrophin gene. In utero cell transplantation that might facilitate allogenic transplantation is worth considering to treat this disease.
Methods
We performed allogeneic in utero transplantation of GFP-positive myoblasts and adipose-derived mesenchymal stem cells into murine DMD model animals. The transplantation route in this study was fetal intraperitoneal transplantation and transplacental transplantation. Transplanted animals were examined at 4-weeks old by immunofluorescence staining and RT-qPCR.
Results
No GFP-positive cells were found by immunofluorescence staining of skeletal muscle and no GFP mRNA was detected by RT-qPCR in any animal, transplantation method and cell type. Compared with previous reports, myoblast transplantation exhibited an equivalent mortality rate, but adipose-derived stem cell (ASC) transplantation produced a higher mortality rate.
Conclusions
In utero transplantation of myoblasts or ASCs to murine models of DMD does not lead to engraftment and, in ASC transplantation primarily, frequently results in fetal death.
Keywords: Fetal therapy, In utero transplantation, Myoblasts, Adipose-derived mesenchymal stem cells, Duchenne muscular dystrophy
1. Introduction
Duchenne muscular dystrophy (DMD) is a progressive disease that leads to damage of muscle and myocardium due to genetic abnormalities of the dystrophin gene [1], and is currently treated with corticosteroids [2]. Very recently, other novel drugs are approved. Furthermore, experimental gene therapies are seeking to address each specific mutation [3]. While these therapies have had some efficacy, treatment of patients with mutations not eligible for the approved gene therapy is limited to steroids and symptomatic treatment, which is focused on alleviation of symptoms.
Cell therapy for DMD has been reported with various cell types, both in preclinical animal studies and in humans, and is promising as it is applicable to any genetic mutations [4]. It has been reported that, at the animal experimental level, animal models of DMD receiving mesenchymal stem cells (MSCs) intravenously or intra-arterially show suppression of tissue necrosis and appearance of dystrophin-positive muscle cells [[5], [6], [7], [8]]. Reports from human clinical trials using intravenous systemic administration of cardiosphere-derived cells show delays in the decline of motor and cardiac function [9]. There are also reports of improvement in motor and respiratory functions in combination with intra-arterial and intramuscular injections of MSCs [10].
One human study reports intravenously transplanted cells being engrafted into recipient’s myofibers [11]. On the other hand, several reports describe intravascularly administered stem cells observed in recipient’s muscle fiber and differentiating or fusing into muscle in animal models [7,8,[12], [13], [14]]. However, even if intravascularly administered stem cells do not migrate, differentiate, and fuse into skeletal muscle to extents that slow disease progression, both paracrine and endocrine effects of MSC are expected to reduce inflammation, necrosis and fibrosis [15].
Allogenic stem cell transplantation in adults has the inherent disadvantage that transplanted cells may be rejected by recipient immune cells. To compensate for this, immunosuppressive drugs have been used in some human clinical studies [16]. In utero cell transplantation performed in the present study is a treatment transplanting cells into the fetus via the umbilical vein or other means. The advantage of this treatment is that the immunologically naïve state of fetal immune cells may not reject the transplanted cells [[17], [18], [19], [20], [21], [22], [23]]. This means that the use of immunosuppressive drugs may not be necessary, and the administration of normal stem cells during organ development may result in the restoration of a normal cell nucleus in organ development to mitigate the mutation.
Human clinical trials have already been conducted in osteogenesis imparfecta (OI) and severe combined immune deficiency (SCID). Osteogenesis imperfecta is a group of phenotypically and molecularly heterogeneous inherited connective tissue diseases with similar skeletal abnormalities that cause bone fragility and deformity [24], and SCID is a prenatal disorder of T cell development caused by various gene mutations [25]. In OI, booster administration in both the fetal and neonatal period allowed donor cells to establish in the bone, reducing fractures and improving mobility [22,26,27]. In addition, in SCID, donor cells were observed in the blood after fetal administration, and T cells originally lacking in this disease were observed in the blood, making treatment unnecessary after birth [[28], [29], [30], [31]].
In humans, drugs are often administered through the umbilical vein, while red blood cells are sometimes administered into the abdominal cavity of the fetus, both of which proved to be safe to a certain degree [32]. In mice, however, the umbilical vein is narrow and difficult to administer, so intraperitoneal and transplacental routes of administration have been reported. There have been several reports on intraperitoneal administration in rodents [19,20,[33], [34], [35], [36], [37], [38], [39], [40], [41], [42]]. As for transplacental administration, transplacental fetal hematopoietic stem cell transplantation into model animals lacking hematopoietic stem cells has been reported [18].
In utero cell transplantation for muscular dystrophy has only been reported in animal studies. One is the transplantation of mononuclear cell layers from the bone marrow blood of adult mice or fetal hepatocytes into mdx mouse fetuses. Among the nuclei of the whole muscle fibers, 0.4–3% of nuclei was donor origin at 4 weeks old [33]. In another study, human fetal MSCs were transplanted into mdx mouse fetuses, and among the nuclei of the whole muscle fibers, 0.5–1% of nuclei was donor origin at 6 weeks old [19].
In order to acquire proof of concept of complex disease and therapy, validated animal models are essential. For animal models of congenital diseases, transgenic mice have been often utilized. Therefore, the safety and efficacy of in utero cell transplantation should be confirmed in an appropriate murine model. However, compared with human embryo and infants, murine counterparts are too small. In the present study, we examined cell transplantation to newborn mouse models in addition to both in utero cell and in utero intraplacental transplantation.
2. Materials and methods
2.1. Ethics
All experiments were approved by the Ethics Committee of Tokyo Women's Medical University, Tokyo, Japan, and The University of Tokyo, Tokyo, Japan, and animal care was based on guidelines from the Science Council of Japan. This study was carried out in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
2.2. Animals
C57BL/10-mdx mice and Wistar rats were purchased from CLEA Japan Inc., Japan. Enhanced GFP transgenic C57BL/6 mouse and SD rats were purchased from Japan SLC Inc., Japan. W-Dmdem1Kykn (DMD rats) were established and provided by The University of Tokyo [43,44]. Mice were housed in the Institute of Laboratory Animals, Tokyo Women's Medical University. Rats were housed in the Institute of Advanced Biomedical Engineering and Science, Tokyo, Japan or at the University of Tokyo. Both murine species were housed in separate cages, with no more than 5 mice/cage and no more than 2 rats/cage, with 12-h light/dark cycles. Laboratory chow and water were given ad libitum.
2.3. Genotyping of DMD rat
At 3 weeks of age 2 mm of tail tip was obtained from transplanted rat. Then genotyping by PCR was performed using the primers listed below [43], and only males with the mutation were subjected to further analyses: forward AGTTTCCATCAATAGCCATACCAAA and reverse TCTCAGTGTACAAGTGTGACGAACA.
2.4. Cell culture
Cell culture was performed using the following culture media: 1) Ham’s F-10 Nutrient Mix (Life Technologies, Carlsbad, Canada) supplemented with 2 ng/mL basic fibroblast growth factor (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), 10% fetal bovine serum (FBS, Life Technologies), and 1% penicillin-streptomycin (PS, FUJIFILM Wako Pure Chemical Corporation) for myoblast culture; 2) DMEM/F-12, GlutaMAX™ supplement with 10% FBS and 1% PS for rat ASC culture (Life Technologies); 3) KBM ADSC-1 (Kohjin Bio Co., Saitama, Japan) for mouse ASC culture.
2.5. Myoblast primary culture
Six-week old enhanced GFP transgenic C57BL/6 mice were anesthetized with isoflurane and sacrificed by exsanguination from the heart. Connective tissue, blood vessels, and fat were removed from skeletal muscle collected from the lower limbs. The collected muscle tissue was placed in Hanks' balanced salt solution (HBSS-, FUJIFILM Wako Pure Chemical Corporation) containing 1% PS and gently shaken to avoid contamination. Myoblast-containing cell suspensions were prepared using the MACS skeletal muscle dissociation kit for mouse and rat (Miltenyi Biotec, North Rhine, Germany). Then, mouse myoblasts were isolated by MACS with a purity of 98.5%, using the satellite cell isolation kit (Miltenyi Biotec), according to the manufacturers’ protocols [45]. Obtained cells were expanded on cell culture dishes coated with type I collagen solution (FUJIFILM Wako Pure Chemical Corporation) with myoblast culture medium. Since Motohashi et al. reported that these myoblasts engrafted to muscle when intramuscularly injected, it is assumed that the cells in this study, obtained by the same method, would also be able to engraft if they migrated into the muscle [45].
2.6. ASC primary culture
Six-week old enhanced GFP transgenic C57BL/6 mice or SD rats were sacrificed by CO2 inhalation. Abdominal fat was harvested and washed in Hanks' balanced salt solution (HBSS-, FUJIFILM Wako Pure Chemical Corporation) containing 1% penicillin-streptomycin and gently shaken to avoid contamination. Purification method for ASCs was performed according to a previous report [46]. Briefly, collected fat tissue was digested by type II collagenase (Sigma-Aldrich, St. Louis, USA) at 37 °C with gentleMACS™ Octo Dissociator with Heaters. Cells obtained by centrifugation were seeded onto type I collagen-coated dishes described below (FUJIFILM Wako Pure Chemical Corporation) and cultured in ASC medium. Cells used for transplantation in present study were all at passage 4–6.
2.7. Intraperitoneal in utero transplantation to mdx mice
Pregnant mdx mice (E14.5) were anesthetized on a heater (37 °C) with inhalation of isoflurane and laparotomized. Myoblasts (5.0 × 105 cells) or ASCs (1.0 × 106 cells) were resuspended in 20 μl saline solution and loaded in a micro-syringe with a 36-gauge needle (both provided by TERUMO, Tokyo, Japan). The needle was inserted through uterus into the fetal abdominal cavity (Fig. 1a). Then, a given cell suspension was injected slowly into fetal abdominal cavity. During the procedure, the uterus was moistened with warm saline to prevent drying and loss of body temperature. The uteri were returned to the abdominal cavity and the abdomen was closed with a 4-0 braided absorbable suture after all fetuses had been injected. The transplanted mice were delivered by cesarean section at 19.5 days gestation and raised until 4 weeks of age for analysis.
Fig. 1.
Photographs of various in utero cell transplantation techniques: (a) Intraperitoneal transplantation to the mdx mouse fetus. Fetuses were seen through the outside of the uterus, and the cell suspension was injected into the abdominal cavity of the fetus. (b) Placental transplantation of the mdx mouse. Injections were made into the placenta from outside the uterus using a glass tube for embryo transfer connected to a femtojet. (c) Placental transplantation of Wistar rats: a 36G needle with microsyringe was used to inject cells into the placenta by puncturing about 6 mm from the outside the uterus.
2.8. Transplacental in utero transplantation to mdx mice
Injection pipettes were prepared according to a previous report [37]. Briefly, the glass tube (NARISHIGE, Tokyo, Japan) was formed thin using a pipette puller (PC-100, NARISHIGE), and the tip was formed into a needle shape using a diamond sharping wheel. 5.0 × 104 myoblasts or 2.0 × 104 ASCs were resuspended in 2 μl saline solution and loaded in injection pipette. Then, an injection pipette was connected to electronic microinjector (FemtoJet 4, Eppendorf, Hmburg, Germany). Pregnant mdx mice (E11.5) were anesthetized on a heater with inhalation of isoflurane and laparotomized. Placentas seen from outside the uterus were punctured with a pipette to a depth of 2 mm vertically and cells were injected with a microinjector (Fig. 1b). During the procedure, the uterus was moistened with warm saline to prevent dryness and loss of body temperature. After all fetuses were injected, the uterus was returned to the abdominal cavity and the abdomen was closed with a 4-0 braided absorbable suture. The transplanted mice were delivered by cesarean section at 19.5 days gestation and raised until 4 weeks of age for analysis.
2.9. Intraperitoneal in utero transplantation to DMD rats
Pregnant DMD rats (E16.5) were anesthetized on a heater with inhalation of isoflurane and laparotomized. ASCs (1.0 × 106 cells) were injected into the fetus abdominal cavity by the same procedure as mdx mouse intraperitoneal in utero transplantation. Rats born by vaginal delivery were subject to genotyping, and male rats carrying the mutation were raised until 4 weeks of age for analysis.
2.10. Transplacental in utero transplantation to Wistar rats
Pregnant Wistar rat (E14.5) was anesthetized on a body heater with inhalation of isoflurane and laparotomized. Since we did not have a Femto jet available for rats at our facility, 5.0 × 105 ASCs were resuspended in 5 μl saline solution and loaded into a micro syringe with 36-gauge needle. Illuminating the uterus from the back with LED white light, the placenta was visible through the uterine wall (Fig. 1c). They were punctured to a depth of 6 mm vertically and injected slowly. Two mothers were used for this preliminary experiment: one is for the sampling the day after transplantation and the other is for 4 days later from transplantation.
2.11. Intraperitoneal transplantation to wild type neonatal rats
GFP-positive ASCs (5.0 × 106 cells) were resuspended in 100 μl of warm normal saline and transplanted intraperitoneally to 1-day old Wistar rats with 30-gauge needle (Nipro, Osaka, Japan). Total of 6 neonates were sacrificed on 3, 4, 5, 6, 7, 14-days old by decapitation or CO2 inhalation. Total leg muscle and lung were collected and subjected to RT-qPCR.
2.12. Immunofluorescence staining
The sampled fetus or placenta tissues were frozen in OTC compound in cryomolds. On the other hand, sampled skeletal muscles were fixed on a corkboard with tragacanth gum and then frozen in isopentane at the temperature of liquid nitrogen. 8-μm-thick cryo-tissue sections were prepared using a cryostat, fixed with acetone for 5 min, and subjected to immunofluorescence staining as follows. After washing with PBS, tissue sections were incubated with 0.5% Triton-X (Sigma-Aldrich) in PBS for 10 min for permeabilization, washed with PBS, blocked using Blocking One Histo (NACALAI TESQUE, Kyoto, Japan) for 15 min, incubated with primary antibodies (rabbit anti-GFP and mouse anti-dystrophin, 1:100 diluted, Abcam, Cambridge, UK), washed, and incubated with Alexa-Fluor 488-conjugated anti-rabbit IgG antibody (1:200 diluted, Abcam) and Alexa-Fluor 588-conjugated anti-mouse IgG antibody. The immunostained tissue sections were observed under a confocal laser scanning fluorescence microscope (FV1200, Olympus, Tokyo, Japan) and Cell Sens Standard software (FV1-ASW, Olympus).
2.13. RT-qPCR
Transplanted mice were raised until 4-weeks old and their skeletal muscles (diaphragm, quadriceps, tibialis anterior) were harvested. These muscles were subjected to total RNA isolation and gene expression analyses by TaqMan PCR. Total RNA was purified with RNeasy plus mini kit (QIAGEN, Venlo, Netherlands), according to the manufacturer’s protocol. Further, cDNA was synthesized with ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan). RT-qPCR was performed using ViiA 7 real time PCR system (Life Technologies), TaqMan Fast Advanced Master Mix and TaqMan probes for enhanced GFP and GAPDH (Life Technologies). In all analyses, the mRNA extracted from GFP mice or rats was utilized as a positive control.
3. Results
3.1. Intraperitoneal in utero transplantation to mdx mouse
As a control experiment, we conducted intraperitoneal in utero administration of only normal saline to mdx murine fetuses, and confirmed that this process was not fatal and that the fetuses were fully developed and normally born at term. Then, a total of 22 fetuses were subjected to myoblast intraperitoneal transplantation and 21 fetuses were subject to ASC intraperitoneal transplantation. Nine neonates for myoblast transplanted group and 1 neonate for ASC transplanted group were born by surgical cesarean section at 19.5 days gestation; that is, the neonatal survival rate was 41% for myoblasts and 4.8% for ASC (Table 1). At 4-weeks of age, neither RT-qPCR nor immunofluorescence staining of skeletal muscle showed positive evidence of enhanced GFP (Fig. 2). Treated fetuses that died during pregnancy showed necrosis and absorbed at 19.5 days gestation (Fig. 3a).
Table 1.
Number of trials and survival rates of in utero cell transplantation. For mdx mice and DMD rats, analysis was performed at 4 weeks of age. No GFP-positive cells or GFP mRNA were detected by immunofluorescence staining or RT-qPCR of muscle. Preliminary placental transplantation experiments on Wistar rats resulted in death of all fetuses.
| Species | Method | Cell type | Number of fetuses (mothers) | Number of births | Survival rate(%) |
|---|---|---|---|---|---|
| mdx mouse | ip | myoblast | 22(3) | 9 | 41 |
| ASC | 21(3) | 1 | 4.8 | ||
| pl | myoblast | 7(1) | 6 | 86 | |
| ASC | 8(1) | 0 | 0 | ||
| DMD Rat | ip | ASC | 41(3) | 17 | 41 |
| Wistar Rat | pl | ASC | 40(3) | ||
ip: intraperitoneal administration, pl: placental administration, ASC: adipose-derived stem cell.
Fig. 2.
Immunofluorescence staining of transplanted murine quadriceps. Mice transplanted by the various cell methods were sacrificed at 4-weeks old and subjected to immunofluorescent staining. Quadriceps, tibialis anterior, and diaphragm muscles were stained, and the quadriceps muscle is presented in this figure. No rats were GFP-positive other than GFP rats stained as controls. Bars = 100 μm.
Fig. 3.
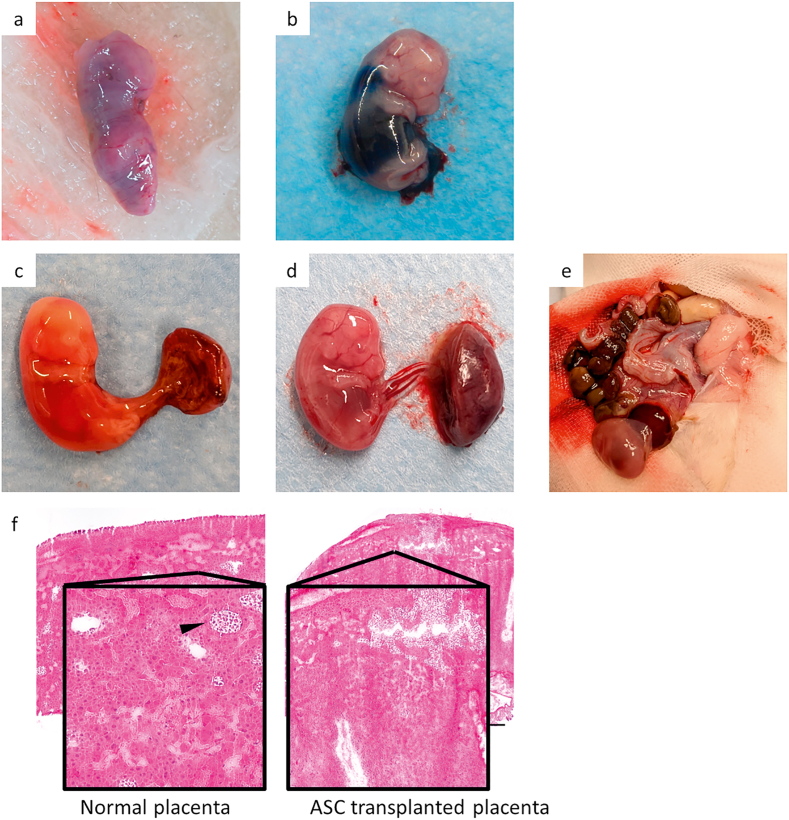
Photographs of in utero-transplanted fetuses. (a) Dead fetus from myoblast intraperitoneally transplanted mdx mouse (E19.5). As seen from outside the uterus, the dead fetuses were atrophying and being absorbed. (b) Fetus of Wistar rats intraperitoneally injected with Evans blue dye (E16.5). (c) Dead fetus of Wistar rat one day after placental transplantation of ASCs (E17.5). Compared to Fig. 2e, blood flow was disrupted and the fetus was whitened, suggesting that the placental transplantation of ASCs may have resulted in destruction and loss function of placenta. (d) E17.5 fetus saline-injected control. (e) Atrophying fetus and uterus of Wistar rats three days after placental transplantation of ASCs (E19.5). (f) HE staining of normal placenta (E16.5) and placenta after placental transplantation of ASCs (E16.5). Cell injection into the placenta results in disruption of structures such as chorionic villi and lacuna (arrow). Bars = 1000 μm.
3.2. Transplacental in utero transplantation to mdx mouse
Total of 7 murine fetuses were subject to myoblast transplacental transplantation and 8 fetuses were subject to ASC intraperitoneal transplantation. Six full-term neonates from the myoblast-transplanted group were delivered by cesarean section at 19.5 days gestation. On the other hand, all fetuses in ASC transplacental transplantation group died; that is, survival rates were 86% for myoblast and 0% for ASC, respectively (Table 1). At 4-weeks of age neither RT-qPCR or immunofluorescence staining of skeletal muscle showed positive evidence of enhanced GFP (Fig. 2).
3.3. Intraperitoneal in utero injection to Wistar rats
Intraperitoneal in utero injection of 20 μL Evans Blue dye (FUJIFILM Wako Pure Chemical Corporation) was performed on E14.5 Wistar rat fetuses. Fig. 3b shows a sample fetus 30 min post-injection. The clearly blue-colored abdomen proves the success of the intraperitoneal injection.
3.4. Intraperitoneal in utero transplantation to DMD rats
Total of 41 fetuses were subject to ASC intraperitoneal transplantation: 17 neonates was born by normal vaginal delivery; a survival rate of 41% (Table 1). Genotyping revealed that 4 neonates retained the dystrophin gene mutation. At 4-weeks of age, neither RT-qPCR nor immunofluorescence staining of skeletal muscle showed positive evidence of enhanced GFP (Fig. 2).
3.5. Transplacental in utero transplantation of ASC to Wistar rats
Because of the high mortality rate of ASC transplantation, we discontinued transplantation in DMD rats that do not have a high probability of having mutations and to conduct a preliminary experiment in Wistar rats. ASC-transplanted E17.5 fetuses subjected to placental transplantation on E16.5 died (Fig. 3c). Compared to normal control fetuses (Fig. 3d), blood flow was disrupted, and the fetus was whitened. ASC-transplanted E19.5 fetuses subject to placental transplantation on E16.5 were also dead and absorbed. Atrophying and absorbed fetuses were observed (Fig. 3e). Also, compared to normal placenta (Fig. 3f), HE staining of transplanted placenta revealed disruption of structures such as chorionic villi and lacuna (Fig. 3f).
3.6. Neonatal intraperitoneal transplantation to Wistar rats
GFP-positive ASCs (5.0 × 106 cells) were transplanted intraperitoneally to 1-day old Wistar rats. Total leg muscle and lung tissues were collected and subjected to RT-qPCR over time post-injection. 3-day old leg muscle and lung samples showed GFP-positive signal and lungs of 14-day old also showed GFP-positive signals enhanced with GFP gene expression by RT-qPCR (Table 2).
Table 2.
RT-qPCR results from ASC-transplanted neonates of Wistar rats. GFP-positive ASCs (5.0 × 106 cells per fetus) were transplanted intraperitoneally to 1-day old Wistar rats. Presence of GFP mRNA in lung and leg muscle tissues over time indirectly indicates that intraperitoneal cell administration to the neonates allows the administered GFP-cells to migrate into the bloodstream. Each value represents Ct value, and number in () represents relative expression compared to GAPDH (%).
| 3 | 4 | 5 | 6 | 7 | 14 (day old) | |
|---|---|---|---|---|---|---|
| Leg | 35.8 (0.0760) | (−) | (−) | (−) | (−) | (−) |
| Lung | 33.1 (1.62) | (−) | (−) | (−) | (−) | 37.6 (7.85) |
4. Discussion
In the early fetal development period, the immune system remains immature, and therefore immune rejection of transplanted cells does not occur [47]. We hypothesized that transplantation of allogeneic cells at this point would allow long-term engraftment without the need for immunosuppressive drugs, and transplantation was performed in fetuses of DMD model rodents, but the transplanted cells were not viable and showed a high mortality rate (Table 1).
In this study, fetal cell transplantation was performed with two different cell types and two different administration methods in two different murine models, but no GFP-positive cells were found by immunofluorescence staining, and no GFP mRNA was detected by RT-qPCR. Previous studies on fetal cell transplantation have reported intraperitoneal transplantation of mouse bone marrow and fetal liver cells [33] and human fetal MSCs [19] into mdx mouse fetuses, with fetal mortality results similar to those of this study. This high mortality rate is attributed to fetal removal from the abdominal cavity and ex situ canula puncture as a fundamentally unsafe procedure with significant risk of hypothermia and hemorrhage. Higher mortality of ASC fetal treatment over that of myoblasts may be due to the higher thrombogenic risk of ASCs resulting in impaired blood flow [48,49] but this is not confirmed. MSC administration in blood induces platelet aggregation with MSCs included in the aggregates, which may induce thrombosis [50]. The highest mortality rate for ASC transplacental administration may also be due to embolization of ASCs and thrombosis triggered by ASCs, resulting in the destruction of the placenta. The pathological observation of placental destruction after ASC transplantation also supports this hypothesis. Contrary to our present study, fetal intraperitoneal transplantation in utero to mdx mice was reported to be successful with murine bone marrow mononuclear cells, mouse fetal liver cells [33], and human fetal MSCs [19]. On the other hand, transplanted ASCs and myoblasts failed to engraft even in the surviving cases in this current study. ASCs, as mentioned above, can trigger thrombosis and may have been disadvantaged in intraperitoneal migration. As for myoblasts, their lack of migratory ability through endothelial layers of vessels may have prevented them from reaching the muscles [51], even if they were able to migrate from the abdominal cavity into the fetal vasculature. Intravenously transplanted ASCs and myoblasts are reported to migrate to inflammatory sites [51], but in mdx mice, pathological changes in muscle do not occur until approximately 3 weeks after birth [52], and mechanical muscle destruction does not occur during the fetal period without muscle movement. Even if the administered stem cells successfully migrated into the bloodstream, they would not have extravasated from the blood vessels to further contribute to muscle formation without inflammation. Cell migration from the abdominal cavity to the blood is reported to be via the lymphatic vessels and into the venous system [53]. It is also reported that cell migration to the lymphatic vessels is accomplished by intermittent increases in abdominal pressure due to moderate muscle tone of the abdominal wall and movement of the diaphragm [54]. In contrast, the fetus does not make physiologically mature oscillatory respiratory motions similar to that of adult, as it obtains oxygen from the mother via the placenta and is also thought to have little muscle tone in the abdominal wall [55,56]. Therefore, it is possible that increases in abdominal pressure did not occur, and requisite administered cell migration into the vessels did not occur in this study. These factors, i.e., lack of abdominal pressure in the fetus, lack of stem cell migratory capacity, and known tendency of ASCs to form emboli, may have resulted in unsuccessful fetal cell transplantation in the present study.
Since the observed high mortality rate in fetal cell transplantation could be attributed to the limited size of the murine fetal vasculature and cell transport issues, we also used DMD rats for intraperitoneal fetal cell transplantation. As expected, mortality decreased as these animals became larger, but no viable grafted cells were observed. Considering that highly intense tissue destruction does not occur even in 13 weeks in DMD rats [43], it is possible that inflammation in the muscle did not occur during the early fetal period, thus preventing cell migration and engraftment, even if they migrated into the blood. As for the neonatal transplantation, 5.0 × 106 cells were administered intraperitoneally and GFP mRNA was detected in the lungs and muscles. Although these GFP-positive result in muscle may have actually reflected cells retained in blood vessels and in blood contained therein, observations combined with GFP-positive results also in the lungs confirm that exogenous cells administered intraperitoneally migrated to blood vessels. Since cells administered are the same, we can conclude that our hypothesis that the cells did not migrate into the blood due to lack of mature respiratory movement and muscle tone is at least a contributing factor to the observed failure of fetal cell transplantation.
Although ASCs and myoblast fetal cell transplantation failed in this study, this does not mean that fetal cell transplantation completely lacks developmental potential. In human clinical practice, successful fetal cell transplantation has already been reported in OI [26] and SCID [23]. These treatments were performed via the umbilical vein, which is different from intraperitoneal administration. In mice and rats, the umbilical cord is thin and technically difficult to access and reproduce, so we were unable to perform this technique in this study to assert full equivalence with human clinical practice.
5. Conclusions
We performed in utero transplantation of myoblasts or ASCs to murine models of DMD by fetal intraperitoneal transplantation and transplacental transplantation but did not lead to engraftment. Furthermore, in ASC transplantation primarily, frequently resulted in fetal death. However, this does not mean that in utero cell transplantation of myoblasts or ASCs is not promising for DMD, since different route of administration is used in human in utero cell transplantation.
Author contributions
Y.K. designed the study, performed the experiments, analyzed the data, and drafted the manuscript. Y.T. and M.I. helped experimental procedure and advised experimental methods. J.H., R.T., K.Y., H.H., N.S. and K.I. advised experimental methods. M.Y. conceived of the study and supervised all experiments. All authors critically revised the report, commented on the manuscript, and approved the final submission content.
Declaration of competing interest
The authors declare no conflicts of interest associated with this manuscript.
Acknowledgements
This study was in part supported by the Institute of Laboratory Animals (ILA), Tokyo Women’s Medical University, Japan for animal experiments. We thank Dr. M. Hamada, Department of Anatomy and Embryology, Faculty of Medicine, University of Tsukuba, Japan for instructing the transplacental in utero transplantation. We are indebted to Professor D.W. Grainger, University of Utah, USA for technical review. This study was partially supported by the Morinaga Foundation for Health and Nutrition to Y. Kihara and Research on dissemination of best practicable care for muscle dystrophy (21FC1006).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Mercuri E., Bönnemann C.G., Muntoni F. Muscular dystrophies. Lancet. 2019;394:2025–2038. doi: 10.1016/S0140-6736(19)32910-1. [DOI] [PubMed] [Google Scholar]
- 2.Manzur A.Y., Kuntzer T., Pike M., Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD003725.pub3.Cd003725. [DOI] [PubMed] [Google Scholar]
- 3.Roshmi R.R., Yokota T. Pharmacological profile of viltolarsen for the treatment of duchenne muscular dystrophy: a Japanese experience. Clin Pharmacol. 2021;13:235–242. doi: 10.2147/CPAA.S288842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nitahara-Kasahara Y., Takeda Si, Okada T. Cell therapeutic approaches using multipotent mesenchymal stromal cells for muscular dystrophy. Inflamm Regen. 2014;34:198–205. [Google Scholar]
- 5.Sitzia C., Farini A., Jardim L., Razini P., Belicchi M., Cassinelli L., et al. Adaptive immune response impairs the efficacy of autologous transplantation of engineered stem cells in dystrophic dogs. Mol Ther. 2016;24:1949–1964. doi: 10.1038/mt.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y., Zhu Y., Li Y., Cao J., Zhang H., Chen M., et al. Long-term engraftment of myogenic progenitors from adipose-derived stem cells and muscle regeneration in dystrophic mice. Hum Mol Genet. 2015;24:6029–6040. doi: 10.1093/hmg/ddv316. [DOI] [PubMed] [Google Scholar]
- 7.Li Z., Liu H.Y., Lei Q.F., Zhang C., Li S.N. Improved motor function in dko mice by intravenous transplantation of bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2011;13:69–77. doi: 10.3109/14653249.2010.510502. [DOI] [PubMed] [Google Scholar]
- 8.Feng S.W., Lu X.L., Liu Z.S., Zhang Y.N., Liu T.Y., Li J.L., et al. Dynamic distribution of bone marrow-derived mesenchymal stromal cells and change of pathology after infusing into mdx mice. Cytotherapy. 2008;10:254–264. doi: 10.1080/14653240802020381. [DOI] [PubMed] [Google Scholar]
- 9.McDonald C.M., Marbán E., Hendrix S., Hogan N., Ruckdeschel Smith R., Eagle M., et al. Repeated intravenous cardiosphere-derived cell therapy in late-stage Duchenne muscular dystrophy (HOPE-2): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2022;399:1049–1058. doi: 10.1016/S0140-6736(22)00012-5. [DOI] [PubMed] [Google Scholar]
- 10.Dai A., Baspinar O., Yeşilyurt A., Sun E., Aydemir Ç., Öztel O.N., et al. Efficacy of stem cell therapy in ambulatory and nonambulatory children with Duchenne muscular dystrophy - phase I-II. Degener Neurol Neuromuscul Dis. 2018;8:63–77. doi: 10.2147/DNND.S170087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gussoni E., Bennett R.R., Muskiewicz K.R., Meyerrose T., Nolta J.A., Gilgoff I., et al. Long-term persistence of donor nuclei in a Duchenne muscular dystrophy patient receiving bone marrow transplantation. J Clin Invest. 2002;110:807–814. doi: 10.1172/JCI16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siemionow M., Malik M., Langa P., Cwykiel J., Brodowska S., Heydemann A. Cardiac protection after systemic transplant of dystrophin expressing chimeric (DEC) cells to the mdx mouse model of duchenne muscular dystrophy. Stem Cell Rev Rep. 2019;15:827–841. doi: 10.1007/s12015-019-09916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rong-Qing Pang, He Jie, Zhang Yong-Yun, Fu Xiong, Ruan Guang-Ping, Zhu Xiang-Qing, et al. Systemic delivery of human bone marrow embryonic-like stem cells improves motor function of severely affected dystrophin/utrophin-deficient mice. Cytotherapy. 2014;16 doi: 10.1016/j.jcyt.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Harada A., Goto M., Kato A., Takenaka-Ninagawa N., Tanaka A., Noguchi S., et al. Systemic supplementation of collagen VI by neonatal transplantation of iPSC-derived MSCs improves histological phenotype and function of col6-deficient model mice. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.790341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S.E., Jeong J.B., Oh S.J., Kim S.J., Kim H., Choi A., et al. Wharton's jelly-derived mesenchymal stem cells reduce fibrosis in a mouse model of duchenne muscular dystrophy by upregulating microRNA 499. Biomedicines. 2021;9:1089. doi: 10.3390/biomedicines9091089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skuk D., Goulet M., Roy B., Chapdelaine P., Bouchard J.P., Roy R., et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- 17.Shields L.E., Lindton B., Andrews R.G., Westgren M. Fetal hematopoietic stem cell transplantation: a challenge for the twenty-first century. J Hematother Stem Cell Res. 2002;11:617–631. doi: 10.1089/15258160260194767. [DOI] [PubMed] [Google Scholar]
- 18.Jeon H., Asano K., Wakimoto A., Kulathunga K., Tran M.T.N., Nakamura M., et al. Generation of reconstituted hemato-lymphoid murine embryos by placental transplantation into embryos lacking HSCs. Sci Rep. 2021;11:4374. doi: 10.1038/s41598-021-83652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan J., Waddington S.N., O'Donoghue K., Kurata H., Guillot P.V., Gotherstrom C., et al. Widespread distribution and muscle differentiation of human fetal mesenchymal stem cells after intrauterine transplantation in dystrophic mdx mouse. Stem Cell. 2007;25:875–884. doi: 10.1634/stemcells.2006-0694. [DOI] [PubMed] [Google Scholar]
- 20.Witt R.G., Kreger E.M., Buckman L.B., Moradi P.W., Ho P.T., Derderian S.C., et al. Systemic multilineage engraftment in mice after in utero transplantation with human hematopoietic stem cells. Blood Adv. 2018;2:69–74. doi: 10.1182/bloodadvances.2017011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loewendorf A.I., Csete M., Flake A. Immunological considerations in in utero hematopoetic stem cell transplantation (IUHCT) Front Pharmacol. 2014;5:282. doi: 10.3389/fphar.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz E.M., Gordon P.L., Koo W.K., Marx J.C., Neel M.D., McNall R.Y., et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flake A.W., Roncarolo M.G., Puck J.M., Almeida-Porada G., Evans M.I., Johnson M.P., et al. Treatment of X-linked severe combined immunodeficiency by in utero transplantation of paternal bone marrow. N Engl J Med. 1996;335:1806–1810. doi: 10.1056/NEJM199612123352404. [DOI] [PubMed] [Google Scholar]
- 24.Forlino A., Marini J.C. Osteogenesis imperfecta. Lancet. 2016;387:1657–1671. doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cossu F. Genetics of SCID. Ital J Pediatr. 2010;36:76. doi: 10.1186/1824-7288-36-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Blanc K., Gotherstrom C., Ringden O., Hassan M., McMahon R., Horwitz E., et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79:1607–1614. doi: 10.1097/01.tp.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- 27.Gotherstrom C., Westgren M., Shaw S.W., Astrom E., Biswas A., Byers P.H., et al. Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: a two-center experience. Stem Cells Transl Med. 2014;3:255–264. doi: 10.5966/sctm.2013-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wengler G.S., Lanfranchi A., Frusca T., Verardi R., Neva A., Brugnoni D., et al. In-utero transplantation of parental CD34 haematopoietic progenitor cells in a patient with X-linked severe combined immunodeficiency (SCIDX1) Lancet. 1996;348:1484–1487. doi: 10.1016/s0140-6736(96)09392-0. [DOI] [PubMed] [Google Scholar]
- 29.Magnani A., Jouannic J.M., Rosain J., Gabrion A., Touzot F., Roudaut C., et al. Successful in utero stem cell transplantation in X-linked severe combined immunodeficiency. Blood Adv. 2019;3:237–241. doi: 10.1182/bloodadvances.2018023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westgren M., Ringden O., Bartmann P., Bui T.H., Lindton B., Mattsson J., et al. Prenatal T-cell reconstitution after in utero transplantation with fetal liver cells in a patient with X-linked severe combined immunodeficiency. Am J Obstet Gynecol. 2002;187:475–482. doi: 10.1067/mob.2002.123602. [DOI] [PubMed] [Google Scholar]
- 31.Westgren M., Ringdén O., Bartmann P., Bui T.-H., Lindton B., Mattsson J., et al. Prenatal T-cell reconstitution after in utero transplantation with fetal liver cells in a patient with X-linked severe combined immunodeficiency. Am J Obstet Gynecol. 2002;187:475–482. doi: 10.1067/mob.2002.123602. [DOI] [PubMed] [Google Scholar]
- 32.Lindenburg I.T., van Kamp I.L., Oepkes D. Intrauterine blood transfusion: current indications and associated risks. Fetal Diagn Ther. 2014;36:263–271. doi: 10.1159/000362812. [DOI] [PubMed] [Google Scholar]
- 33.Mackenzie T.C., Shaaban A.F., Radu A., Flake A.W. Engraftment of bone marrow and fetal liver cells after in utero transplantation in MDX mice. J Pediatr Surg. 2002;37:1058–1064. doi: 10.1053/jpsu.2002.33844. [DOI] [PubMed] [Google Scholar]
- 34.Chen C.P., Liu S.H., Huang J.P., Aplin J.D., Wu Y.H., Chen P.C., et al. Engraftment potential of human placenta-derived mesenchymal stem cells after in utero transplantation in rats. Hum Reprod. 2009;24:154–165. doi: 10.1093/humrep/den356. [DOI] [PubMed] [Google Scholar]
- 35.Chen X., Gong X.L., Katsumata M., Zeng Y.T., Huang S.Z., Zeng F. Hematopoietic stem cell engraftment by early-stage in utero transplantation in a mouse model. Exp Mol Pathol. 2009;87:173–177. doi: 10.1016/j.yexmp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Misra M.V., Gutweiler J.R., Suh M.Y., Twark C.M., Valim C., Perez-Atayde A., et al. A murine model of graft-vs-host disease after in utero hematopoietic cell transplantation. J Pediatr Surg. 2009;44:1102–1107. doi: 10.1016/j.jpedsurg.2009.02.033. ; discussion 7. [DOI] [PubMed] [Google Scholar]
- 37.Nijagal A., Le T., Wegorzewska M., Mackenzie T.C. A mouse model of in utero transplantation. J Vis Exp. 2011 doi: 10.3791/2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nijagal A., Wegorzewska M., Jarvis E., Le T., Tang Q., MacKenzie T.C. Maternal T cells limit engraftment after in utero hematopoietic cell transplantation in mice. J Clin Invest. 2011;121:582–592. doi: 10.1172/JCI44907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nijagal A., Derderian C., Le T., Jarvis E., Nguyen L., Tang Q., et al. Direct and indirect antigen presentation lead to deletion of donor-specific T cells after in utero hematopoietic cell transplantation in mice. Blood. 2013;121:4595–4602. doi: 10.1182/blood-2012-10-463174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang C.M., Gong X.L., Qiu J., Tang H.X., Gong Z.J., Huang S.Z., et al. Engraftment of genetically modified human amniotic fluid-derived progenitor cells to produce coagulation factor IX after in utero transplantation in mice. Cell Biol Int. 2013;37:420–429. doi: 10.1002/cbin.10037. [DOI] [PubMed] [Google Scholar]
- 41.Lin K.Y., Peng S.Y., Chou C.J., Wu C.C., Wu S.C. Engraftment of mouse amniotic fluid-derived progenitor cells after in utero transplantation in mice. J Formos Med Assoc. 2015;114:1105–1115. doi: 10.1016/j.jfma.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Shangaris P., Loukogeorgakis S.P., Blundell M.P., Petra E., Shaw S.W., Ramachandra D.L., et al. Long-term hematopoietic engraftment of congenic amniotic fluid stem cells after in utero intraperitoneal transplantation to immune competent mice. Stem Cells Dev. 2018;27:515–523. doi: 10.1089/scd.2017.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura K., Fujii W., Tsuboi M., Tanihata J., Teramoto N., Takeuchi S., et al. Generation of muscular dystrophy model rats with a CRISPR/Cas system. Sci Rep. 2014;4:5635. doi: 10.1038/srep05635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugihara H., Teramoto N., Nakamura K., Shiga T., Shirakawa T., Matsuo M., et al. Cellular senescence-mediated exacerbation of Duchenne muscular dystrophy. Sci Rep. 2020;10 doi: 10.1038/s41598-020-73315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motohashi N., Asakura Y., Asakura A. Isolation, culture, and transplantation of muscle satellite cells. J Vis Exp. 2014 doi: 10.3791/50846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryu B., Sekine H., Homma J., Kobayashi T., Kobayashi E., Kawamata T., et al. Allogeneic adipose-derived mesenchymal stem cell sheet that produces neurological improvement with angiogenesis and neurogenesis in a rat stroke model. J Neurosurg. 2019;132:442–455. doi: 10.3171/2018.11.JNS182331. [DOI] [PubMed] [Google Scholar]
- 47.Haynes B.F., Scearce R.M., Lobach D.F., Hensley L.L. Phenotypic characterization and ontogeny of mesodermal-derived and endocrine epithelial components of the human thymic microenvironment. J Exp Med. 1984;159:1149–1168. doi: 10.1084/jem.159.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tatsumi K., Ohashi K., Matsubara Y., Kohori A., Ohno T., Kakidachi H., et al. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem Biophys Res Commun. 2013;431:203–209. doi: 10.1016/j.bbrc.2012.12.134. [DOI] [PubMed] [Google Scholar]
- 49.Coppin L., Sokal E., Stéphenne X. Thrombogenic risk induced by intravascular mesenchymal stem cell therapy: current status and future perspectives. Cells. 2019;8:1160. doi: 10.3390/cells8101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheriff L., Alanazi A., Ward L.S.C., Ward C., Munir H., Rayes J., et al. Origin-specific adhesive interactions of mesenchymal stem cells with platelets influence their behavior after infusion. Stem cells (Dayton, Ohio) 2018;36:1062–1074. doi: 10.1002/stem.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cossu G., Sampaolesi M. New therapies for muscular dystrophy: cautious optimism. Trends Mol Med. 2004;10:516–520. doi: 10.1016/j.molmed.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Bulfield G., Siller W.G., Wight P.A., Moore K.J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Courtice F.C., Harding J., Steinbeck A.W. The removal of free red blood cells from the peritoneal cavity of animals. Aust J Exp Biol Med Sci. 1953;31:215–225. doi: 10.1038/icb.1953.26. [DOI] [PubMed] [Google Scholar]
- 54.Courtice F.C., Morris B. The effect of diaphragmatic movement on the absorption of protein and of red cells from the pleural cavity. Aust J Exp Biol Med Sci. 1953;31:229–238. [PubMed] [Google Scholar]
- 55.Koos B.J., Rajaee A. Springer New York; New York, NY: 2014. Fetal Breathing Movements and Changes at Birth; pp. 89–101. [DOI] [PubMed] [Google Scholar]
- 56.Racca A.W., Beck A.E., Rao V.S., Flint G.V., Lundy S.D., Born D.E., et al. Contractility and kinetics of human fetal and human adult skeletal muscle. J Physiol. 2013;591:3049–3061. doi: 10.1113/jphysiol.2013.252650. [DOI] [PMC free article] [PubMed] [Google Scholar]