Abstract
Distinct cell types are generated at specific times during brain development and are regulated by epigenetic, transcriptional and newly emerging epitranscriptomic mechanisms. RNA modifications are known to affect many aspects of RNA metabolism and have been implicated in the regulation of various biological processes and in disease. Recent studies imply that dysregulation of the epitranscriptome may be significantly associated with neuropsychiatric, neurodevelopmental, and neurodegenerative disorders. Here we review the current knowledge surrounding the role of the RNA modifications N6-methyladenosine, 5-methylcytidine, pseudouridine, A-to-I RNA editing, 2’O-methylation, and their associated machinery, in brain development and human diseases. We also highlight the need for the development of new technologies in the pursuit of directly mapping RNA modifications in both genome- and single-molecule-level approach.
Introduction
Over 170 chemical modifications of eukaryotic RNA are known, together making up what is referred to as the epitranscriptome 1. RNA modifications were first described in abundant RNA species such as rRNA and tRNA; however, with the advent of next generation sequencing technologies, RNA modifications have begun to be identified in a transcriptome-wide manner. Generally, RNA modifications have been shown to affect RNA metabolism through regulation of splicing 2, 3 mRNA export 4 mRNA stability 5, 6 mRNA translation 7-10, and miRNA processing 11, 12. N6-methyladenosine is the most characterized modification and proteins that install (‘writers’), remove (‘eraser’), and specifically recognize (‘readers’) the modification have been identified. Interestingly, the characterization of ‘writers’ and ‘erasers’ for a few RNA modifications has provided evidence that, like DNA methylation and protein phosphorylation, mRNA is subjected to dynamic, reversible chemical modification. These dynamic mRNA modifications represent an additional mechanism by which function and expression can be regulated at the mRNA level. Identifying ‘writers’, ‘readers’ and ‘erasers’ has expanded our knowledge regarding the potential role of particular RNA modifications in cellular, developmental, and disease processes 13, 14.
Proper function of the nervous system is maintained through tight, accurate regulation. For example, normal brain development requires correct signals to be initiated at specific times to determine the required numbers of neural and non-neural cell types in the appropriate location. Indeed, layers of regulation are needed to ensure that the precise mechanisms are executed in a time-sensitive manner. One of these layers of regulation is epigenetic modifications. For example, DNA methylation and histone modification-mediated gene regulation have been shown to be critical regulators of neural cell differentiation 15-18. Furthermore, transcriptional regulation has also been shown to be critical in neurogenesis 19, 20. Transcription factors are involved in the regulation of specific processes such as cell cycle exit, loss of progenitor properties and acquisition of neuronal features 21-25. Indeed, dysregulation of these mechanisms has been associated with neuropsychiatric, neurodevelopmental, and neurodegenerative disorders.
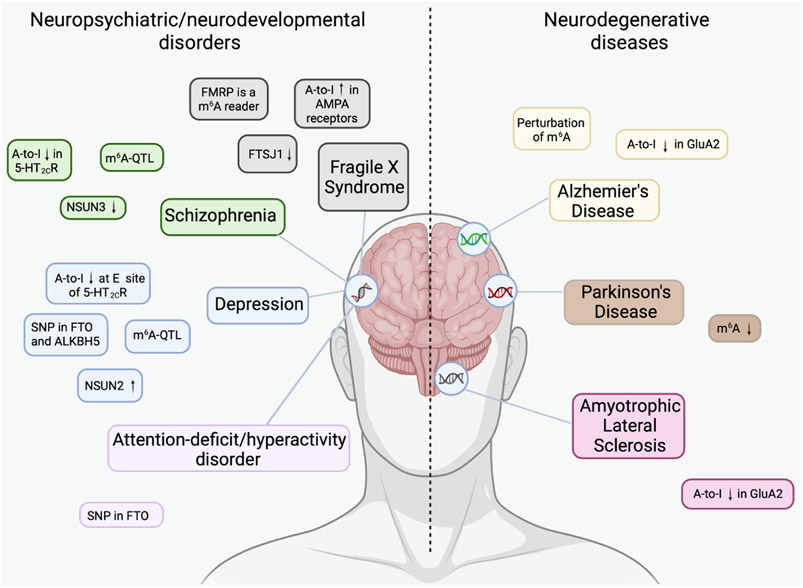
Neuropsychiatric/neurodevelopmental disorders reflect changes in brain function, but the underlying mechanisms are largely unknown. Neurodevelopmental disorders affect brain development and function; examples include attention deficit hyper-activity disorder (ADHD), autism and intellectual disability. Neuropsychiatric disorders affect brain function resulting in behavioral disorders such as seizures, addictions, depression and anxiety. However, neurodegenerative diseases are distinct and are characterized by the progressive degeneration of the central or peripheral nervous system. Recently, common variant risks for neuropsychiatric disorders were shown to correlate significantly with each other, especially among ADHD, bipolar disorder, major depressive disorder (MDD), and schizophrenia. Neurodegenerative disease such as Alzheimer’s (AD) and Parkinson’s diseases (PD), were more distinct from one another and from neuropsychiatric/neurodevelopmental disorders 26. The impact of RNA modifications on the regulation of neurobiological processes has only recently begun to emerge and is the focus of this review. Recent works have shown the N6-methyladenosine, 5-methylcytidine, pseudouridine, A-to-I RNA editing, 2’O-methylation, RNA modifications and/or the involved machinery to be associated with neuropsychiatric/neurodevelopmental and neurodegenerative disorders (Fig. 1). This review concentrates on these five modifications as they are the best studied modifications and are strongly implicated in affecting brain development and neurological disorders.
Figure 1: Perturbation of the epitranscriptome is associated with neuropsychiatric disorders and neurodegenerative diseases.
Neuropsychiatric disorders share common variant risks, whereas neurodegenerative diseases appear more distinct from one another and from the neuropsychiatric disorders. Summary of RNA modifications associated with neuropsychiatric disorders and neurodegenerative diseases.
N6-methyladenosine
N6-methyladenosine (m6A) is the most abundant internal epitranscriptomic mark in eukaryotic RNAs. The m6A modification is installed on mRNA by the m6A methyltransferases METTL3/14 27-29, in complex with RBM15/15B 30, ZC3H13 31, VIRMA 32, WTAP 33, 34. The methyltransferases are S-adenosyl methionine (SAM)-dependent methyltransferases that perform the methylation by transfer of a methyl group from the SAM cofactor to the adenosine target. The modification is dynamic and can be removed by the demethylases FTO which controls cellular homeostasis 35 and ALKBH5 36. N6-methyladenosine is recognized by m6A-binding proteins YTHDF1/2/3 10, 29, 37, YTHDC1/2, IGF2BP1/2/3 38 and HNRNPA2B1 39 which mediate m6A function in a specific context. N6-methyladenosine sites are identified within a consensus motif RRACH (in which R represents A or G, and H represents A, C or U). The modification has been identified in several thousands of human protein-coding genes and is preferentially present in long internal exons, locations upstream of stop codons, and the 3′-UTR of mRNA 40-42. N6-methyladenosine methylation has been implicated in the regulation of mRNA decay, translation, splicing and has critical roles in cellular pathways and processes such as cell differentiation, development and metabolism 43. N6-methyladenonise is established as a regulator of RNA stability and translation efficiency; however, evidence is beginning to emerge suggesting crosstalk between m6A methylation and epigenetic mechanisms. N6-methyladenosine has also been recently implicated to mark chromosome-associated regulatory RNAs (carRNAs) in mammalian cells and regulate gene regulation at the chromatin/transcriptional level. In this context, loss of m6A on these RNAs was associated with increased RNA expression and ultimately resulted in enhanced downstream gene transcription through increasing chromatin accessibility 44. N6-methyladenosine has also been shown to mark transcripts that encode histone-modifying proteins and affects the stability of those transcripts 45.
m6A in normal brain function
N6-methyladenosine is abundant in the mammalian brain and multiple studies have shown that m6A is an important regulator of normal neurodevelopment. More specifically, m6A has been implicated in postnatal brain development, neurogenesis, gliogenesis, synaptic function, learning and memory, and axon development.
Dynamic m6A during postnatal brain development
N6-methyladenosine has been profiled in different brain regions at different developmental time points in the mouse brain. Interestingly, these studies identify specific spatiotemporal m6A sites. One study showed that common m6A peaks were typically located at stop codons and in the 3’ UTR. However, cerebellum-specific peaks were distributed in the start codons and 3’ UTR, while cortex-specific peaks were mainly concentrated in the coding region 46. Another study noted that m6A peaks specific to postnatal day 7 (P7) in the mouse cerebellum were enriched at the stop codon, whilst at postnatal day 60 (P60) cerebellum specific m6A peaks were enriched at the start codon 47. More generally, the authors detected dynamic poly(A) RNA m6A methylation across cerebellar development, with the highest m6A level being present at P7. This observation correlated with the dynamic expression of METTL3 which was also highest at P7. Recently, we demonstrated that m6A has distinct mouse brain tissue-specific methylation profiles, most obvious in the hypothalamus 48. We showed that tissue-specific methylation was associated with an increase in mRNA expression and is associated with tissue-specific developmental processes. We also determined that differential m6A sites were enriched in alternative untranslated regions of genes that affect aging-related pathways. These m6A sites are associated with a strong negative effect on mRNA expression. Finally, we showed that the m6A modification is more prevalent in human cortex compared to mouse cortex. Altogether, these observations suggest m6A is a critical regulator of normal brain development, more so in humans compared to mouse.
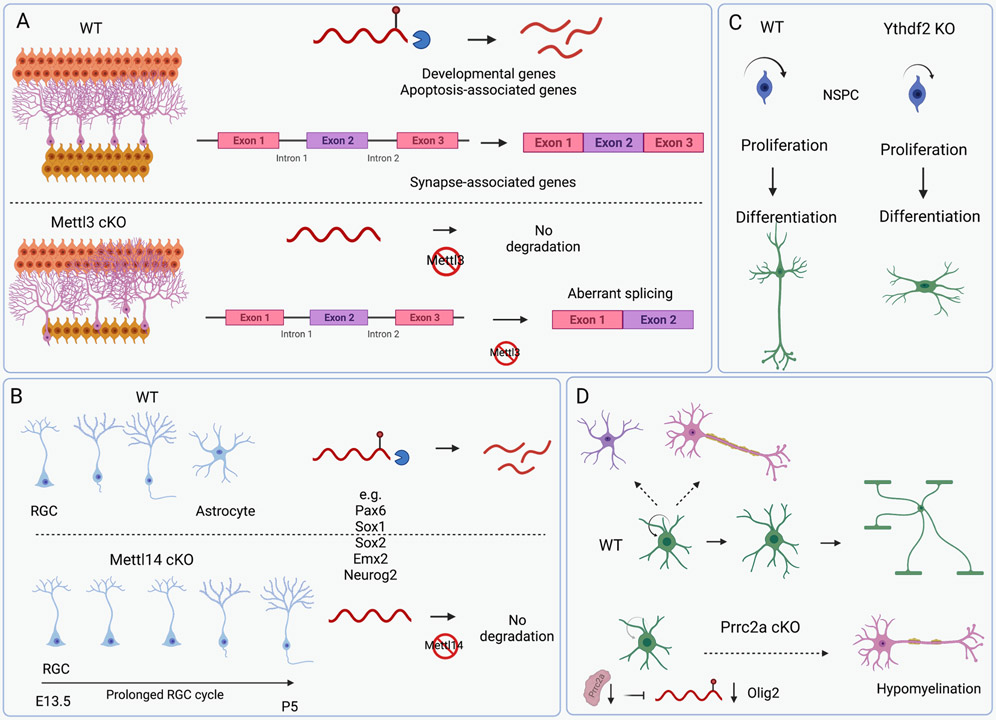
Mettl3 conditional knockout (cKO) mice are characterized by cerebellar hypoplasia resulting from significantly enhanced apoptosis of newborn cerebellar granule cells (CGCs) in the external granular layer 49. In this context, loss of m6A caused transcriptome-wide gene dysregulation and premature CGC death. For example, upon Mettl3 cKO, key developmental genes and apoptosis-associated genes, were upregulated as a result of extended mRNA half-lives, whilst aberrant splicing events on synapse-associated genes were also observed (Fig. 2A). Furthermore, another study demonstrated that knockdown of Mettl3 led to a severe alteration in Purkinje cell numbers, laminal structure, stunted dendrites and caused a reduction in astrocyte number in the developing cerebellum. The authors also showed that deletion of Alkbh5, under hypobaric hypoxia exposure, causes increased nuclear export of hypermethylated RNAs and abnormal proliferation and differentiation of granular neurons in the cerebellum 47.
Figure 2: Role of m6A in neural development.
(A) Mettl3 conditional knockout (cKO) mice are characterized by cerebellar hypoplasia resulting from significantly enhanced apoptosis of newborn cerebellar granule cells (CGCs) in the external granular layer. Upon Mettl3 cKO, key developmental genes, and apoptosis-associated genes, were upregulated as a result of extended mRNA half-lives, whilst aberrant splicing events on synapse-associated genes were also observed. (B) Mettl14 cKO in embryonic mouse brains results in loss of m6A and prolonged radial glial cell cycle. Transcription factors are marked with m6A, which results in their degradation. This is required to maintain proper cortical neurogenesis. (C) Loss of YTHDF2 in neural stem cells results in reduced proliferation and differentiation. Furthermore, neurons were shown to exhibit less neurite outgrowth and shorter neurites. (D) Prrc2a binds and stabilizes to the CDS of m6A-marked oligodendrocyte transcription factor 2 (Olig2) mRNA, a key oligodendroglial lineage-determining transcription factor. Prrc2a cKO resulted in significant hypomyelination.
Neurogenesis
A conditional Mettl14 knockout in embryonic mouse brains resulted in a loss of m6A modification, a prolonged radial glial cell (RGC) cycle extending the neurogenic phase into postnatal stages, suggesting a critical role for Mettl14 in neurogenesis and gliogenesis (Fig. 2B) 50. Mechanistically, many transcripts that encode transcription factors, such as Pax6, Sox1, Sox2, Emx2 and Neurog2/Neurogenin 2, are marked with m6A. These m6A marked transcripts were found to be enriched in cell cycle, stem cell and neuronal differentiation pathways. In this context, m6A was shown to mediate degradation, which is required to maintain proper cortical neurogenesis. Aberrant m6A during the process negatively affects temporal specification and cell cycle progression of neuronal progenitor cells 50. The authors further performed a comparison between human and mouse m6A profiles obtained during cortical neurogenesis. This revealed a more pervasive m6A landscape in humans than in mouse. Notably, they detected over 1100 transcripts that are expressed in both species but are only marked with m6A in human. Interestingly, gene ontology analysis revealed enrichment of genes related to mental disorders and mental retardation. N6-methyladenosine marked transcripts that are common to both human and mouse were found to be enriched for oncogenic processes.
It has been reported that the Mettl14 cKO resulted in a decrease in neural stem cell proliferation and loss of late neurons, further suggesting a role for METTL14 in cortical neurogenesis 51. These authors linked m6A with histone modifications. They showed that m6A affects the stability of histone modifiers and altered histone modifications results in repression of proliferation-related genes and activates differentiation-related genes which resulted in a loss of neural stem cell ground state. Similar to what was described in the Mettl14 cKO mice, Fmr1 KO mice exhibited an extended neuronal progenitor cell cycle, with neural progenitors still proliferating into postnatal stages 52. From these studies, it is clear that both FMRP and METTL14 are required for neural progenitor differentiation.
Another study showed that as a consequence of Mettl3 depletion, the proliferation and cell cycle progression of adult neural stem cells (aNSCs) and neuronal development are inhibited, and the maturation of newborn neurons was affected 53. The authors also showed that the loss of m6A results in the downregulation of the histone methyltransferase EZH2 at the protein level and a consequent reduction in H3K27me3 levels, which ultimately affected neuronal development. Overexpression of EZH2 was shown to rescue the defects observed as a result of Mettl3 knockout in neurogenesis and neuronal development.
Also, recently, m6A has been implicated as a critical regulator of direct lineage reprogramming into induced neuronal cells 54. The authors showed that loss of m6A results in less efficient induced neuronal cell formation. Furthermore, the m6A methylation of the transcription factor Btg2 was shown to be essential for the reprogramming of somatic cells to neuronal cells.
The m6A ‘reader’ YTHDF2 was also shown to have a role in cortical neurogenesis. Loss of Ythdf2 in neural stem cells resulted in reduced proliferation and differentiation. Furthermore, neurons were shown to exhibit less neurite outgrowth, shorter neurites and were susceptible to oxidative stress (Fig. 2C) 55. Given the fact that neurite outgrowth is essential for neuronal development and maturation, it is conceivable that the observed abnormal neurite branching upon loss of Ythdf2 could impact neurogenesis during neural development. An additional consequence of Ythdf2 deficiency is that neural stem progenitor cell self-renewal and spatiotemporal generation of neurons were significantly negatively impacted in the embryonic neocortex. Mechanistically, following the loss of Ythdf2, an increase in expression of m6A-marked transcripts involved with neural development and cortical neuron differentiation was observed. This suggests that the YTHDF2 mediated degradation is an essential mechanism for the regulation of cortical neurogenesis during embryonic neural development.
Gliogenesis
N6-methyladenosine is required in the regulation of oligodendrocyte lineage progression. Mettl14 deletion in developing oligodendrocyte lineage cells or in postmitotic oligodendrocytes results in a loss of mature oligodendrocytes and hypomyelination 56. Mechanistically, METTL14 is required for the canonical splicing of many mRNAs involved in the myelinating process, such as protein tyrosine phosphate receptor type Z1 and neurofascin 155. Another study identified that the novel m6A ‘reader’ proline rich coiled-coil 2A (Prrc2a) regulated oligodendrocyte progenitor cell proliferation and oligodendrocyte fate determination (Fig. 2D) 57. Specific oligodendroglial-lineage deletion of Prrc2a resulted in significant hypomyelination, as well as decreased lifespan and defects in locomotion and cognition in a mouse model. The authors also demonstrated that Prrc2a binds and stabilizes to the CDS of m6A-marked oligodendrocyte transcription factor 2 (Olig2) mRNA, a key oligodendroglial lineage-determining transcription factor, through a consensus GGACU motif. Moreover, FTO was shown to remove the m6A-mark from Olig2 resulting in its degradation. Altogether, these observations suggest a key role of Prrc2A functioning as a m6A ‘reader’ in oligodendrocyte specification.
Synaptic function
Furthermore, m6A has been implicated as a critical regulator of synaptic function. The m6A modification was detected in 2921 genes in synaptosomal RNAs comprising the ‘synaptic m6A epitranscriptome’ 58. They show that the YTHDF 1, 2 and 3 are present in hippocampal dendrites and loss of YTHDF1 or YTHDF3 here resulted in altered spine morphology, dampened excitatory synaptic transmission and altered cell-surface glutamate receptors. Indeed, the presence of m6A on synapses has been shown to critically contribute to synaptic function, and aberrant translation at synapses has been associated with autism, fragile X syndrome and other intellectual disorders. Additionally, a recent study determined the involvement of FMRP in m6A mechanisms at synapses. The authors determined that FMRP moderately colocalized with m6A modified RNAs at postsynaptic sites; however, colocalization significantly increased following activation with NMDA (which strengthens the synapses) 59. The authors suggest that FMRP is associated with m6A-regulatory mechanisms to influence short term plasticity at glutamatergic synapses. The authors also provide evidence that m6A demethylation mediated by ALKBH5 occurs at active synaptic ribosomes and at synapses during short term plasticity, however molecular underpinnings remain unknown. Furthermore, it should be noted that the authors identified translationally active ribosomes by puromycin binding which does not accurately localize translation at the subcellular level 60, and they used conventional fluorescent images which may be limited by imaging resolution.
The m6A modification was also shown to affect synaptic transmission in the mouse striatum and midbrain. Compared with wild type mice, Fto-deficient mice showed impaired synaptic dopamine release 61. Indeed, knockout of Fto in mice resulted in an increase in m6A methylation in a subset of mRNAs involved in neuronal signaling, including many dopaminergic signaling pathway genes in the striatum and midbrain; many of these proteins encoded by these mRNAs also had altered expression levels.
Learning and Memory
Cue-specific fear conditioning was shown to increase m6A levels in mouse medial prefrontal cortex, and Fto knockdown increased fear memory consolidation 62. Consistently, another study reported that behavioral training-induced memory, arising from contextual fear conditioning, was associated with a decrease in FTO levels, but only specifically near synapses. The authors show that loss of Fto in hippocampus enhanced contextual fear memory, suggesting that synaptic FTO could normally restrict memory formation 63. Both studies show that m6A and FTO may be key regulators in the memory formation process. Also, since a similar process was shown to occur in both the hippocampus and prefrontal cortex, it’s possible that a universal mechanism may be governing memory formation. Furthermore, METTL3 has also been implicated in memory formation. Specific deletion of METTL3 from the forebrain excitatory neurons resulted in a decrease of hippocampus-dependent long-term memory formation, whereas overexpression of METTL3 facilitated long-term memory consolidation 64. Mechanistically, the authors demonstrate that METTL3-mediated m6A modification promotes the translation efficiency of a set of immediate early genes which are essential for long-term memory consolidation. The m6A ‘reader’, YTHDF1, was also recently shown to be a regulator of learning and memory in the hippocampus 65. Ythdf1 KO mice displayed learning and memory defects, and impaired hippocampal synaptic transmission and long-term potentiation. These defects could be rescued by re-expressing YTHDF1 in the hippocampus of adult Ythdf1 KO mice. The authors showed that specifically in response to neuronal stimulation, YTHDF1 promotes the translation of m6A-methylated neuronal mRNAs, which facilitates learning and memory. Furthermore, another study profiled m6A in Mettl14 deleted striatum 66. The authors showed that mRNAs encoding neuron and synapse-specific proteins were downregulated in both striatonigral and striatopallidal neurons present, and therefore the loss of m6A in striatal neurons impaired learning and performance.
Axon Growth and Guidance
FTO is highly expressed in axons and can be locally translated. Interestingly, the axon-specific inhibition of Fto resulted in increased m6A levels and decreased local translation of axonal GAP-43 mRNA. This eventually resulted in repressed axon elongation 67. Another study showed that YTHDF1-mediated translation of the m6A-modified Robo3.1 mRNA was important for pre-crossing axon guidance in the spinal cord 68. More recently, YTHDF1 and YTHDF2 were both found to be expressed in cerebellar granule cells and their axons. The knockdown of either ‘reader’ protein in granule cell axons increased parallel fiber growth and promoted synapse formation in cerebellum in vivo. Mechanistically the authors provided evidence that translation of key components of the Wnt pathway, Dvl1 and Wnt5a, in axons is controlled by YTHDF1 and YTHDF2, respectively 69.
m6A in brain disorders
Neuropsychiatric disorders
Variants in two m6A demethylases, Fto and Alkbh5 have been associated with major depressive disorder (MDD). A positive association between the Fto rs9939609 A variant and MDD was observed 70. Interestingly though, this correlation was only observed in patients exhibiting atypical depression, which is a subtype of major depression that involves specific symptoms such as weight gain, excessive sleep, and fatigue. Compared with controls, the rs9939609 A variant was associated with atypical MDD, whereas typical and moderate subtypes were not associated with rs9939609. Furthermore, this association was found to be independent from body mass index. In contrast, another study found no allelic association of the same SNP rs9939609 in Fto with MDD 71. This study genotyped 23 SNPs in genes involved in the m6A mechanism including Mettl3, Mettl14, Wtap, Fto, Alkbh5 in 738 patients with MDD and 1098 controls. They found that of these SNPs, rs12936694 in the m6A demethylase, Alkbh5, was strongly associated with MDD. Another study found that depressed patients have altered levels of m6A and m6Am following glucocorticoid stimulation. This suggests that these modifications may play a role in stress-related psychiatric disorders. The authors also report no change in anxiety levels following cKO of Mettl3 or Fto 72. However, other studies report conflicting results. Following Fto−/− knockout, mice displayed an anxiety-like behavior and impairments in working memory 73, whereas Fto+/− mice exhibited decreased anxiety and depressive-like behaviors. These mice were also less susceptible to stress stimulation 74. A more recent study found that the expression of FTO is downregulated in the hippocampus of patients with MDD and in mouse models of depression. Indeed, knockdown or knockout of Fto in the hippocampus induced depression-like behaviors, whereas overexpression had the opposite effect. The authors suggest that adrenoceptor beta 2 (ADRB2) mRNA is modified by FTO. Fto knockdown accelerated the degradation of Adrb2 mRNA, whilst overexpression protected the mRNA. Finally, it was shown that ADRB2 activation rescued depressive-like behaviors and the spine loss caused by hippocampal Fto deficiency 75. Furthermore, another genetic variant in Fto, SNP rs8050136 has also been linked to modulating the risk of attention-deficit/hyperactivity disorder (ADHD) 76. The authors find the strongest relationship in children who were not exposed to maternal smoking during pregnancy. Altogether, these studies highlight the essential role of m6A and particular m6A machinery components in the pathogenesis of depression.
A recent study determined genetic variants that were responsible for controlling m6A level as a quantitative trait known as m6A quantitative trait loci (m6A-QTLs) 77. m6A-QTLs were found to mostly be independent of expression quantitative trait loci. In the brain, they found that m6A-QTLs are enriched in disease-associated loci. Indeed, several m6A-QTLs colocalize with genome wide association study (GWAS) loci. Interestingly, many brain m6A-QTLs are associated with psychiatric disorders. In total the authors observed 71 m6A-QTLs that colocalized with GWAS variants. Interestingly, they detected an m6A-QTL depression-associated SNP rs1827603 in the postsynaptic receptor GRM5. They also reported anxiety associated SNP rs1541627 as a m6A-QTL for the synaptic plasticity regulator ANKS1B. Further, they observed the schizophrenia-associated SNP rs7285557 as an m6A-QTL for a brain-enriched lincRNA, LINC00634, which has been shown to be downregulated in schizophrenia 78. Polymorphisms in ZC3H13, which plays a critical role in the m6A writer complex and facilitates m6A methylation, have also been associated with schizophrenia 79.
Neurodevelopmental disorders
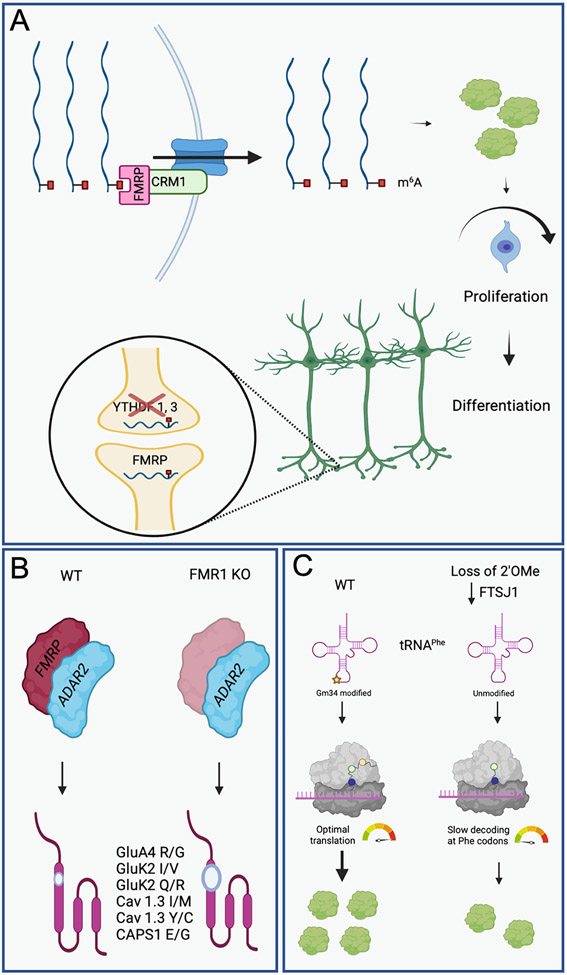
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability, but also is a leading genetic cause of autism spectrum disorders (ASD) 80, 81. Therefore, research on fragile X can provide invaluable insights into the development of autism, as well as other neurodevelopment disorders. FXS is caused by loss of FMR1 gene that encodes the fragile X mental retardation protein (FMRP) 82. Interestingly, FMRP was recently shown to preferentially bind an m6A-containing RNA probe 83, and, since FMRP is known to repress translation of a set of synaptic plasticity-related transcripts, the authors hypothesized that reduction of m6A or of Ythdf1 (a m6A ‘reader’ which enhances translation of particular m6A-marked mRNA targets), would alleviate FXS symptoms. However, further work is required to confirm this notion. Interestingly, a recent study found that the mRNA targets of FMRP are significantly enriched for m6A marks, and the loss of FMRP alters both transcriptome and epitranscriptome in cortex 84. Surprisingly, among FMRP targets, at least 92% of Fmr1 KO-downregulated mRNAs were marked by m6A, suggesting that FMRP could modulate mRNA stability through m6A. Furthermore, FMRP was shown to read m6A to promote nuclear export of methylated mRNA targets during neural differentiation 52 (Fig. 3A). The study provided evidence that reduced FMRP levels in neural stem cells resulted in decreased nuclear export of m6A-tagged RNAs and ultimately, slower production of the neurons that are essential for normal brain development, and defects in this process may contribute to fragile X syndrome. More recently, a study showed that in Drosophila, Ythdf (Drosophila has a single ortholog of YTHDF) and Fmr1 (the fly ortholog of FMRP) share common targets related to nervous system development. Mechanistically they were found to act in concert to inhibit the translation of positive regulators of axonal growth. This suggests that Fmr1’s function in axonal growth is modulated by its interaction with the m6A ‘reader’ Ythdf 85.
Figure 3: Mechanistic detail describing the association between RNA modifications with FMRP and potentially fragile X syndrome.
(A) FMRP is required to bind m6A to promote nuclear export of methylated mRNA targets during neural differentiation. Defects in this process may contribute to fragile X syndrome. m6A is present in synapses and is critical for synaptic function. Aberrant translation at synapses is associated with fragile X syndrome. Loss of m6A ‘readers’ YTHDF1 or 3 has been linked with reduced synaptic transmission. FMRP associates with m6A-marked RNAs at postsynaptic sites following NMDA activation. (B) FMRP interacts with ADAR2 in zebrafish and mouse. The absence of FMRP in these two organisms resulted in an increase in the editing levels of brain specific mRNAs. This may contribute to fragile X syndrome. (C) Loss of Ftsj results in a lack of 2’Omethylation modification at position 34 in tRNAPhe. This results in slow decoding at Phe codons, which corresponds with a significantly reduced translation efficiency in a subset of proteins involved in synaptic organization and function.
Other studies reported that individuals with autism spectrum disorder were determined to have rare and potentially damaging de novo missense variants in another m6A ‘reader’, Ythdc2 86, 87. Another study found another SNP downstream of the Ythdc2 gene (rs2170527, minor allele C) to associate with ASD in a case-control analysis of Japanese ASD cases 88. Altogether, there is evidence suggesting a link between m6A modification, m6A machinery, fragile X syndrome and possibly ASD, in general, but further work is required to explicitly implicate m6A in fragile X syndrome and autism, in general.
Neurodegenerative disorders
N6-methyladenosine has also been associated with neurodegenerative diseases. Early on, FTO (m6A demethylase) was linked with dementia-like Alzheimer’s disease risk 89. The authors suggested that FTO interacts with the known Alzheimer-risk factor gene, APOE. More recently, the dysregulation of m6A has been implicated in AD etiology. Changes in the RNA m6A methylome in APP/PS1 mice was reported whilst METTL3 and FTO expression increased and decreased, respectively, in the AD mouse model 90. However, another study documented a decrease in METTL3 at the protein level in the hippocampus of postmortem human brain samples 91. More recently, in the 5XFAD mouse model, we found METTL3 and FTO to be down and upregulated, respectively, in the cortex. In line with this, m6A was found to be downregulated in 5XFAD by both LC/MS-MS and m6A sequencing approaches. The reduction of m6A in 5XFAD was associated with a reduced expression of a subset of Alzheimer-related proteins 48. Furthermore, another recent study found decreased METTL3 and neuronal m6A levels in AD brains 92. They showed that these reductions are associated with deficits in memory, synaptic loss and neuronal death. These studies provide the first mechanistic link between m6A and Alzheimer’s disease progression. Also, the m6A ‘reader’, HNRNPA2/B1, has been linked to tau pathology. The authors demonstrate that under stress conditions, oligomeric tau binds to HNRNPA2/B1 and m6A-marked transcripts forming a complex. The formation of such complexes was found to be increased in human postmortem and mouse models of AD and is responsible for the formation of stress granules and reduced protein synthesis 93.
N6-methyladenosine has also been studied in Parkinson’s disease (PD). Levels of m6A were found to be reduced in the striatum of rats with 6-OHDA-induced PD 94. The authors indicate that the reduction of m6A may increase the expression of N-methyl-D-aspartate receptor 1 which resulted in an increase in oxidative stress. Interestingly, more recently, an extensive study was performed to determine if genomic variants of the m6A players are associated with Parkinson’s disease 95. The authors identified 214 rare variants in Mettl3, Mettl14, Wtap, Fto, Alkbh5, Ythdf1, Ythdf2, Ythdf3, Hnrnpc, and Elavl1. However, gene-wise association studies revealed that these m6A associated proteins are not strongly associated with Parkinson’s disease. Together these studies highlight the complex interplay amongst m6A, m6A players and neurodegenerative disease, suggesting that further research is required in order to fully understand the mechanistic details surrounding how m6A contributes to the progression of neurodegenerative diseases.
5-methylcytidine
RNA m5C methyltransferases belong to the superfamily of Rossman fold-containing enzymes that use the cofactor S-adenosyl-L-methionine (SAM). In mammals, the NSUN family includes seven enzymes or ‘m5C writers’ (NSUN1-NSUN7) that have known methyltransferase activity. NSUN2 is the most studied m5C methyltransferase and targets mainly tRNAs, non-coding RNAs, and has also been shown to target mRNAs 96-99. NSUN1 and NSUN5 target 28S rRNA 100, 101, NSUN4 specifically methylates 12S mitochondrial rRNA 102, NSUN3 and NSUN6 recognize tRNAs 103, 104, whilst NSUN7 has been shown to methylate enhancer RNAs 105. Recently, the first m5C ‘reader’ protein, ALYREF, was discovered 4. The authors showed that ALYREF promotes mRNA export of m5C-marked mRNAs. The YBX1 protein has also been shown to stabilize a subset of m5C-marked maternal mRNAs during the maternal to zygotic transition in zebrafish 6. The modification has also been shown to affect both mRNA stability and translation 9.
NSUN2 and m5C methylation have been implicated in brain disorders. Expression and activity of NSUN2 has an impact on synaptic transmission and can result in changes in complex behaviors. The overexpression of NSUN2 in the prefrontal cortex was shown to produce depressive-like behavior, whilst ablation of NSUN2 produces an anti-depressant phenotype 106. The authors showed mechanistically that neuronal Nsun2 deficiency caused a decrease in tRNA Gly m5C levels, resulting in a loss of Gly-rich proteins, which was associated with impaired synaptic signaling and complex behaviors, including cognition and depressive behaviors. Interestingly, another m5C methyltransferase, NSUN5, has been shown to be deleted in Williams–Beuren syndrome, which is a neurodevelopmental disorder characterized by social-cognitive deficits, and adult Nsun5 knockout mice show spatial cognitive defects 107. Furthermore, many mitochondrial-related genes, including NSUN3 displayed reduced expression in the cortical interneurons of schizophrenia postmortem brains 108. This observation suggested that a collective decrease in mitochondrial gene expression may contribute to a reduction in mitochondrial function observed in schizophrenia. Interestingly, lack of NSUN3 has also been associated with differentiation of mouse embryonic stem cells towards the neuroectoderm lineage. Since loss of NSUN3 ultimately negatively affects mitochondrial translation, this may be a factor in regulating the normal differentiation program 109.
In mice, the loss of NSUN2-mediated m5C tRNA methylation results in the accumulation of 5’ tRNA fragments. This has been linked to defects in neurogenesis leading to decreased production of upper-layer neurons and reduced brain development 110. Furthermore, individuals with a loss-of-function mutation in NSUN2 displayed intellectual disability (ID), microcephaly, behavioral deficits, speech delay, and growth retardation. A missense mutation at a conserved residue in Nsun2 causes a failure to localize to the nucleolus of Purkinje cells in the cerebellum, contributing to the ID phenotype 111. Another study found a homozygous c.1020delA variant in the Nsun2 gene in a patient displaying ID. This variant was shown to cause a frameshift and premature stop codon; this correlated with decreased levels of Nsun2 mRNA in the patient compared to normal 112. Another homozygous splice mutation in the Nsun2 gene was identified in ID patient cells, which lacked NSUN2 protein, compared to normal 113. The authors also identified loss of site-specific m5C methylation of tRNAAsp-GTC at C47 and C48, which are known NSUN2 targets. Whilst other homozygous Nsun2 variants (two nonsense mutations c.679C>T, c.1114C>T, and a splicing mutation g.6622224A>C) were associated with moderate to severe ID features and facial dysmorphism, suggesting that mutations may result in a syndromic form of ID 114. More recently, a novel homozygous nonsense variant in exon 9 of NSUN2 (c.1004T>A) was discovered and is also linked with ID 115. This variant was also consistent with novel phenotypes including feeding difficulties, slender hands and fingers and severely restricted finger mobility. NSUN2 has also been recently associated with ASD. Biallelic disruption in many recessive neurodevelopmental genes in ASD including a homozygous stop-gain mutation in Nsun2 has also been documented 116. Clearly, the aberrant regulation of m5C and its associated machinery have roles in brain disorders; however, further work is needed to determine specific mechanistic details.
Pseudouridine
Pseudouridylation can occur through two different mechanisms, in either an RNA-dependent, or RNA-independent manner, mediated by two classes of pseudouridine synthases (PUSs). The RNA-dependent PUS, dyskerin (DKC1) in humans, forms a ribonucleoprotein complex with BoxH/ACA snoRNAs. DKC1 primarily targets rRNA and does so through base-pairing between the guide snoRNA and substrate RNA. The RNA-independent PUS proteins (PUS1-PUS7 in humans) directly recognize sequence and structural elements and modify their targets. These enzymes have been shown to target tRNA, small nuclear RNA and rRNA. Interestingly, pseudouridine has been identified in hundreds of mRNA sequences 117-119. Moreover, mass spectrometry has revealed that the pseudouridine modification is comparable, in terms of abundance, to the m6A modification in human cell lines 120. Pseudouridine has been shown to change the fundamental properties of RNAs, including their secondary structures and base-pairing abilities 121-123. Indeed, pseudouridine containing stop codons have been shown to suppress translation termination in yeast 124 and E. coli 125.
Homozygous protein-truncating variants in the RNA-independent pseudouridine synthases Pus3 and Pus7 have been linked with an ID phenotype, including speech delay, a smaller physique, microcephaly, and aggressiveness. These homozygous protein-truncating mutations resulted in the loss of PUS3 and PUS7. Consequently, a significant decrease in pseudouridine modification at positions 38 and 39 in tRNAs of ID patient cells was observed, consistent with a loss of PUS3 in these patients 126, whilst the loss of pseudouridine on PUS7 tRNA and mRNA targets was also observed 127. These authors further confirmed their findings by determining the effects of Pus7 knockout in Drosophila. These knockout Pus7 flies displayed a number of behavioral defects, including increased activity, disorientation, and aggressiveness. This indicates that PUS7-mediated RNA pseudouridylation is critical to maintain proper neurodevelopment and function. Furthermore, another related study confirmed the association of PUS7 with ID 128. One missense and one frameshift mutation in PUS7 were identified which resulted in the abolishment of the enzyme. Consequently, a loss of pseudouridine at position 13 in PUS7 tRNA targets was identified. The loss of PUS7 and pseudouridine modification was correlated with a strong ID phenotype and progressive microcephaly. Moreover, another study reports another Pus7 homozygous mutation which resulted in a glycine to arginine translation 129. The ID patient presented similar but milder features to those previously described. This observation further confirms the role of PUS7 in ID.
A-to-I RNA editing
The A-to-I RNA-editing enzymes called ADARs (adenosine deaminases acting on RNA), are responsible for converting adenosine into inosine in double-stranded RNA. Inosine is recognized as guanosine by translational machinery and therefore has the potential to exchange amino acids. Depending on the genomic location, editing can also change splice sites, miRNA binding sites, pre- and mature miRNAs and modulate gene regulation. In humans, there are three ADARs (ADAR1, ADAR2, and ADAR3). The brain specific ADAR3 is enzymatically inactive and may even negatively regulate RNA editing 130. However, ADAR1 and ADAR2 are active editing enzymes and are highly expressed in the central nervous system even into adulthood in the forebrain of mice 131, suggesting proper maintenance of A-to-I editing is critical to neurodevelopment, and that dysregulation of RNA editing may result in neurological and neurodegenerative disorders. A-to-I RNA editing is prevalent in the mammalian brain, and aberrant editing has been implicated in psychiatric, neurological disorders and neurodegenerative diseases.
Neuropsychiatric Disorders
Serotonin receptors have been implicated in depression or depression-like behavior. On the 5HT2C pre-mRNA, which encodes the brain-specific serotonin receptor, five adenosine bases are subjected to A-to-I editing, and are known as A, B, C, D, E sites. A, B and C sites are modified specifically by ADAR1, whereas the D site was found to be targeted specifically by ADAR2 132, 133. As a result of the editing, 32 different mRNA variants and 24 protein isoforms are possible. Interestingly, as all editing sites in 5HT2C are present within the intracellular loop, this may suggest that alterations in editing efficiencies or patterns may result in altered intracellular signaling.
Early on, a study found no significant difference in editing levels of 5HT2C in the prefrontal cortex, with patients exhibiting depressive and schizophrenic behavior compared to control patients 134. Interestingly though, the authors found that editing levels increased specifically at the A site in suicide victims. In line with this observation, an increase in A site editing in a suicide victim with a history of major depression was reported 135, whilst they also observed a significant reduction in editing at the D site. Further studies report an increase in editing at A, B, C and D sites in the prefrontal cortex of suicide victims which occurred in the context of either bipolar disorder, schizophrenia or major depressive disorder; however, they found that those changes were independent of the comorbid psychiatric diagnoses 136, 137. The increased editing results in an overrepresentation of highly edited mRNA variants that encode hypoactive 5-HT2CR receptors in the brains of suicide victims. Another study reported brain region specific editing in 5-HT2CR in suicide victims 138. They determined that there were alterations in editing levels in both the anterior cingulate cortex and the dorsolateral prefrontal cortex. They confirmed previous findings showing that editing was generally upregulated in suicide victims. Compared with non-psychiatric control individuals, the authors report a major increase in editing in the anterior cingulate cortex compared to the dorsolateral prefrontal cortex in suicide victims. This suggests that region-specific changes in RNA editing of 5-HT2CR mRNA may contribute to the etiology of suicide 138. Furthermore, five isoforms of phosphodiesterase 8A (PDE8A) mRNA, resulting from A-to-I editing, were shown to be upregulated in depressed suicide decedents compared to non-psychiatric controls in the BA24 brain region 139. PDE8A is involved in inflammatory cell activation, memory and cognition. The authors, therefore, suggest that an altered pattern of PDE8A isoforms, as a consequence of A-to-I editing events, could serve as an immune response-related brain marker for suicide. Furthermore, a rat model of depression revealed an increase in editing in all sites with significance in the E site of 5-HT2CR 140. Whilst in schizophrenia patients, a reduction in editing in the frontal cortex of was observed 141. The authors showed that reduced RNA editing in 5-HT2CR was associated with decreased expression of the 5-HT2CR mRNA isoforms generated through editing in the schizophrenia group. In contrast to this, another study found no difference in editing efficiencies in the prefrontal cortex of elderly subjects with schizophrenia compared to matched controls 142.
Neurodevelopmental disorder
Recently FMRP has been demonstrated to interact with ADARs, suggesting that perturbation in A-to-I editing may be involved in fragile X syndrome. In Drosophila, the homolog of FMRP, dFMR1, was shown to interact with dADAR (Drosophila ADAR), regulating its activity. More specifically, loss or overexpression of dFMR1 modulated editing efficiency on a subset of dADAR targets involved in synaptic transmission 143. FMRP was also found to interact with ADAR2 in zebrafish 144 and mouse 145, affecting editing of RNA involved in neuronal and synaptic functions. The absence of FMRP in these two organisms resulted in an increase in the editing levels of brain specific mRNAs, suggesting FMRP may be an inhibitor of A-to-I editing on a specific set of mRNAs (Fig. 3B). More recently, a transcriptome-wide profiling of A-to-I editing events was performed in a large cohort of postmortem brains of people with ASD 146. The authors document hypoediting in ASD brains, which was observed across different brain regions and involved many synaptic genes. Furthermore, they observed convergent patterns of RNA-editing alterations in ASD and fragile X syndrome suggesting that there is a link between these related diseases. They also confirmed the previously identified interaction between FMRP and the ADAR proteins and its regulation of A-to-I editing.
Neurodegenerative disorders
RNA editing in the GluR2 mRNA (which encodes a subunit of AMPA receptor) at the glutamine/arginine (Q/R) site was decreased in the spinal motor neuron of a sporadic amyotrophic lateral sclerosis (ALS) patient. Interestingly, there was no significant differences in the expression profile of GluR2 mRNA to total GluR mRNAs between normal and ALS patients 147. GluR2 modification by A-to-I RNA editing is critical for neuronal survival, and its deficiency can cause neuronal death through AMPA receptor-mediated excitotoxicity 148. This is also in line with the observation that ADAR2 expression levels were significantly decreased in ALS spinal motor neurons 148. Furthermore, mutating the Q/R site to asparagine (N) in a mouse model led to a reduction of spinal motor neurons and late-onset motor dysfunction 149. There is also evidence to suggest that excitotoxicity mediated by dysregulation of editing in AMPA receptors is involved in AD. In the prefrontal cortex of AD patients, editing at the GluA2 Q/R site was decreased by approximately 1% 150. This observation was further supported by an additional study that showed a reduction from >99% to 95% in the AD hippocampus, which was correlated with progression of the disease and neuronal death 151. Later, another study found that editing levels were decreased in the hippocampus of Alzheimer patients in 35 target sites within 22 genes 152. More recently, an annotation database of A-to-I editing in AD was compiled in order to determine genes that could potentially be therapeutic targets 153. The authors identify 1,676,363 editing sites in 1524 samples across nine brain regions from ROSMAP, MayoRNAseq and MSBB datasets. Of those 108,010 and 26,168 sites were found to promote or inhibit AD progression, respectively. They also uncovered 5582 brain region specific editing sites that may have roles in AD. Altogether, these results suggest that reduction in RNA editing may be a general mechanism which results in neurodegenerative disease.
2’O-methylation
2’O-methylation is a highly abundant post-transcriptional modification that is found in both non-coding 154-156 and coding RNAs 157. A methyl group is added to the ribose sugar moiety of all four nucleotides. 2’O-methylation can be installed by independent methyltransferases 158 or by the enzyme fibrillarin guided by box C/D small nucleolar RNAs and are involved in the processing of precursor rRNAs 159, 160. FTSJ1 is an independent 2’O-methyltransferase, and targets the C32 and N34 positions in the anticodon loop of tRNAPhe and tRNATrp. 2’O-methylation on tRNAs has been associated with tRNA stability and/or proper translation 161-163. In rRNAs, the modification stabilizes the RNA scaffold, ensuring translation efficiency and accuracy 164. In mRNAs, 2’O-mehtylation is observed at the cap, but recently it has also been reported as an internal mRNA modification 157. Internal 2’O-methylation modifications of mRNA have been shown to regulate mRNA levels and tune protein translation, whilst 2’O-methylation of the mRNA cap protects RNAs from decapping and degradation 165.
Pathogenic mutations of 2’O-methyltransferase, Ftsj1, have been associated with X-linked ID 166-169. These mutations resulted in a loss of FTSJ1 in ID patients, and manifested in delayed speech, disabilities in reading and some patients exhibited behavior problems. Interestingly, though, patients did not show dysmorphism, suggesting that loss of FTSJ1 is specifically associated with nonsyndromic X-linked ID. FTSJ1 is responsible for 2′-O-methylation in the anticodon region of 11 species of cytosolic tRNAs. Defective 2’-O-methylation of the tRNA anticodon loop, specifically at Gm34 in tRNAPhe, was implicated in nonsyndromic X-linked ID as the critical modification 170. As a consequence of this lack of modification, a significant reduction in the steady-state levels of tRNAPhe was observed in the brain, while other tRNA species were not affected 171. This resulted in slow decoding at phenylalanine codons, which corresponded to a significantly reduced translation efficiency in a subset of genes that are involved in synaptic organization and function (Fig. 3C).
Future Perspectives
There is a need to map RNA modifications in discrete cell types and within specific brain regions to understand how they contribute to neurodevelopment and how dysregulation contributes to disease at the specific brain region and more so at the single cell and single molecule levels. Further challenges lie in understanding the precise function of how specific context dependent modification of RNA, or lack thereof, could contribute to disease. Determining the landscape of different RNA modifications on a single RNA molecule, and how they function in concert will be significant. Therefore, system level endeavors such as those by ENCODE and PsychENCODE are critical to our understanding of the interplay across different epitranscriptomic marks.
Without a complete fundamental understanding of epitranscriptomic changes in relevant biological samples, it would be difficult to determine causal relationships in humans. For example, possible therapeutic targets arising from mouse studies have largely proven unsuccessful in human clinical trials, suggesting that mouse models might not fully recapitulate human disease, highlighting the need for human cell-based models. One approach is to use human postmortem brain samples; however, these are not always well characterized and information regarding the individual’s experiences/environment are not usually available. Therefore, results may be confounded by other factors. Another approach is to use organoid models, which provide more robust outcomes and are a more accurate representation of human tissue. For example, recently a FXS organoid model was developed which identified human-specific mRNA targets of FMRP, with the potential to serve as human-specific druggable targets for FXS and autism, in general 172.
As highlighted throughout this review, RNA binding proteins (RBPs) have been linked to many brain disorders. For example, mutations in RBPs can disrupt the information flow from RNA to protein and have been associated with neurodegenerative disease. The interplay between RNA modifications and RBPs, though, is understated in the literature. There is very little information known regarding the mechanism by which RNA modifications recruit or repel RBPs. For example, what signals or factors control binding in particular biological processes? How does the location of the modification on the RNA affect binding? And do multiple modifications work in concert to control binding? And how does alteration in substrate recognition affect regulation? This information is essential to understanding how RNA modifications modulate RNA metabolic processes through m6A binding proteins. Also, further work to elucidate how specific cells/tissues affect RNA modifications levels, and how ‘writer’, ‘reader’ and ‘eraser’ proteins are regulated should be a focus. Indeed, further studies on cell-type specific ‘reader’ proteins will further expand our understanding of the dynamic epitranscriptome in brain development and disease. To this end, it is critically important to determine the epitranscriptome, expression profiles of effector proteins, binding specificity (and how alteration in substrate recognition could affect regulation), and the interplay between these aspects in specific cell types or specific tissues. For example, it has been shown that different brain tissues have different m6A-transcriptome profiles. It would be expected that the machinery associated with m6A is expressed at different levels in different biological contexts, and this would affect levels and function of m6A. Furthermore, how do different levels of ‘reader’ proteins affect m6A function in different cell types or tissues? Understanding mechanistic details regarding RNA modification and how they are mediated will inform us on how dysregulation of these mechanisms could be involved in brain disease. As a first approach, in silico studies could potentially reveal the specificity and regulatory functions of different ‘reader’ proteins by employing machine-based computational frameworks to predict targets. Studies are beginning to emerge postulating a redundant model of action amongst the YTHDF proteins 173-175. These studies suggest that YTHDF proteins bind equally to all m6A-containing transcripts and function together to promote the decay of these transcripts. Additional evidence is coming to light indicating that the three YTH proteins interact in a coordinated fashion. This is interesting, as both YTHDF1 and YTHDF2 have been shown to have important and distinct roles in the brain. Therefore, it is possible that differential expression patterns of YTHDF proteins across different cell types or developmental stages may control protein levels of m6A-modified transcripts through different mechanisms in a temporal-spatial fashion. Furthermore, even though the three YTHDF proteins are seemingly redundant, one of the proteins may have a dominant role that cannot be compensated for by the other two, due to differences in expression patterns across different cell types, both in quantity and spatial location. This could ultimately explain why there are seemingly distinct roles of the YTH proteins and again emphasizes the importance of accurately determining the m6A profile effector protein expression in different cell types and tissues.
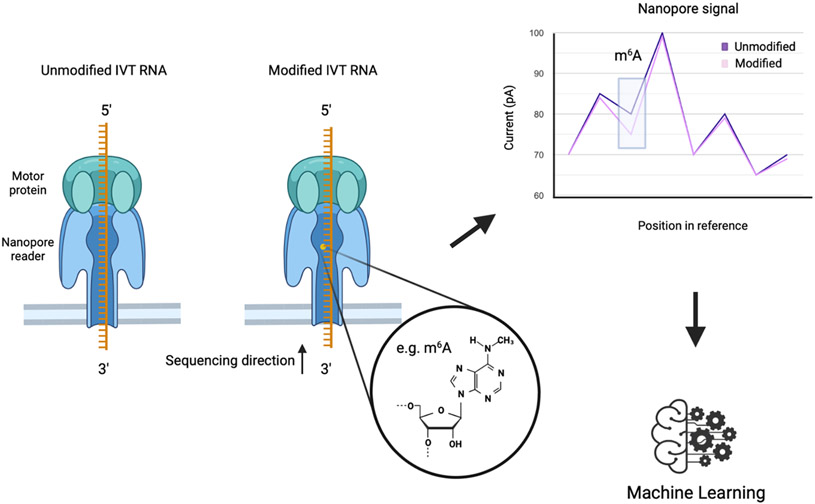
RNA undergoes processing steps such as splicing, editing, and chemical modifications. These modifications, which include over 170 known modified ribonucleotides, are known to affect RNA structure, function and have been implicated in many biological processes, including disease. However, the accurate transcriptome-wide mapping and quantification of the RNA modifications presents a pertinent challenge for the epitranscriptomics field. Many of the currently employed methods to detect RNA modifications rely on affinity-based approaches or reverse transcription signatures in cDNA, which are either naturally present in the RNA or are induced by an enzyme or chemical treatment. There are many disadvantages to these approaches. For example, modification calling by reverse transcription mediated methods typically relies on a large number of reads and specific mis-incorporations or terminations. Chemical treatments may inadvertently introduce artifacts. Also, the error rate of Illumina sequencing may also call artifacts, whilst the accurate detection of less abundant modifications or in less abundant sequences, may be challenging, and genomic SNPs may be incorrectly called as a modification. Furthermore, RNA enrichment approaches are susceptible to experiential artifacts, e.g., antibody specificity. Presently, no technology exists that can determine the identity and position all modifications simultaneously in full-length RNAs at single-molecule and transcriptome-wide scales. However, direct RNA sequencing technologies and bioinformatic tools for analysis are beginning to be developed that allows the sequencing of RNAs in a manner that preserves and reads the modifications. This technology has the potential to reveal information about the dynamics of modifications and may provide information regarding the role of modifications in biological processes and disease at the cellular and organismal levels. Studies are beginning to surface demonstrating the power of direct RNA Nanopore sequencing. N6-methyladenosine modifications were detected with high accuracy (~90%) after training the algorithm with m6A-modified and unmodified synthetic sequences 176. Another study revealed the complexity of mRNA processing and m6A modification in full-length single molecule reads by employing direct RNA sequencing in Arabidopsis 177. Other RNA modifications could be detected through a machine learning approach using Nanopore sequencing. For example, employing synthetic in vitro transcribed unmodified and modified RNA sequences, the algorithm could be ‘trained’ to identify differences in raw electrical signal corresponding to a particular modification of interest (Fig. 4). Concomitant to these new emerging technologies, new bioinformatic tools are required. This new type of data presents its own set of computational challenges and requires new software and dedicated analysis pipelines. Also, better bioinformatic tools are required for current data analysis pipelines (see for example 178). Indeed, gaining an accurate map of RNA modifications is necessary to understand function. Being able to accurately distinguish unmodified from modified nucleosides in a quantitative manner and mapping the distribution of modifications across RNA is critically important for understanding the effect of dynamic modification and how it relates to function. Significantly, direct RNA sequencing preserves the modification without the need for PCR enrichment steps, allowing the user to infer some RNA modifications in a quantitative fashion directly on the mRNA. Furthermore, simultaneous detection of modifications may be valuable in our understanding of how different RNA modifications on a single molecule may possibly work together. This could potentially reveal a ‘wide’ view into the complex function of RNA modifications and how they may influence each other.
Figure 4: Using Nanopore sequencing to detect RNA modifications.
Third generation sequencing, such as Oxford Nanopore Technologies, allows for direct sequencing of RNA molecules without prior fragmentation or amplification. ssRNA is sequenced in the 3’ to 5’ direction through the reader protein. The motor controls the translocation of the RNA strand through the nanopore reader. Using unmodified and modified synthetic in vitro transcribed (IVT) RNA sequences, the algorithm can be ‘trained’ to identify RNA modifications based on changes in raw electric signal output.
In many cases, direct causative links between m6A and brain function have not yet been elucidated. Single nucleotide resolution, quantitative m6A maps are beginning to emerge, using m6A-REF-seq, MAZTER-seq, DART-seq, and direct RNA sequencing (Nanopore) approaches, which have the potential to pinpoint exact changes in m6A on a given mRNA. Further, determining which effector proteins mediate m6A function in a specific context is important. Do effector proteins respond to a particular cellular cue? Or do the expression profiles of effector proteins dictate which protein targets the mRNA? These are important questions in understanding the mechanistic details and could allow us to infer the relationship between the dynamic nature of m6A levels in specific mRNAs, how that relates to changes in transcript/protein levels and ultimately how brain function is affected.
Concluding Remarks
RNA modifications are emerging as a pivotal regulator of RNA metabolism and can affect RNA splicing, stability, processing and translation. Although over 170 modifications are known, only a handful of these have been mapped on RNAs, and studies have begun to reveal functional information of these modifications in various biological processes and diseases. RNA modifications add another layer of gene expression regulation beyond what is coded from DNA. The dysregulation of five major RNA modifications and/or the RNA modification machinery described here is associated with neuropsychiatric disorders, neurodevelopmental disorders and neurodegenerative disorders. New emerging sequencing technologies are opening up avenues to directly determine the landscape of RNA modifications, even at the single molecule level. Elucidating the presence of multiple modifications on the same mRNA transcript will reveal more complex regulatory networks. Altogether, there is strong evidence indicating that the perturbation of RNA modifications or the involved machinery is a major contributor to brain disorders and diseases.
Funding information
This work was supported in part by National Institutes of Health (NS111602, HG008935 and MH116441 to P.J.)
Footnotes
Competing Interests
The authors declare no competing interests.
References
- 1.Boccaletto P, Machnicka MA, Purta E, Piatkowski P, Baginski B, Wirecki TK et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res 2018; 46(D1): D303–D307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang C, Klukovich R, Peng H, Wang Z, Yu T, Zhang Y et al. ALKBH5-dependent m6A demethylation controls splicing and stability of long 3'-UTR mRNAs in male germ cells. Proc Natl Acad Sci U S A 2018; 115(2): E325–E333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou KI, Shi H, Lyu R, Wylder AC, Matuszek Z, Pan JN et al. Regulation of Co-transcriptional Pre-mRNA Splicing by m(6)A through the Low-Complexity Protein hnRNPG. Mol Cell 2019; 76(1): 70–81 e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yang Y, Sun BF, Chen YS, Xu JW, Lai WY et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res 2017; 27(5): 606–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014; 505(7481): 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Wang L, Han X, Yang WL, Zhang M, Ma HL et al. RNA 5-Methylcytosine Facilitates the Maternal-to-Zygotic Transition by Preventing Maternal mRNA Decay. Mol Cell 2019; 75(6): 1188–1202 e1111. [DOI] [PubMed] [Google Scholar]
- 7.Liu F, Clark W, Luo G, Wang X, Fu Y, Wei J et al. ALKBH1-Mediated tRNA Demethylation Regulates Translation. Cell 2016; 167(3): 816–828 e816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao Y, Dong L, Liu XM, Guo J, Ma H, Shen B et al. m(6)A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat Commun 2019; 10(1): 5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumann U, Zhang HN, Sibbritt T, Pan A, Horvath A, Gross S et al. Multiple links between 5-methylcytosine content of mRNA and translation. BMC Biol 2020; 18(1): 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H et al. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015; 161(6): 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alarcon CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature 2015; 519(7544): 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J, Zhuang Y, Zhu C, Meng H, Lu B, Xie B et al. Differential roles of human PUS10 in miRNA processing and tRNA pseudouridylation. Nat Chem Biol 2020; 16(2): 160–169. [DOI] [PubMed] [Google Scholar]
- 13.Jonkhout N, Tran J, Smith MA, Schonrock N, Mattick JS, Novoa EM. The RNA modification landscape in human disease. RNA 2017; 23(12): 1754–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadumuri RV, Janga SC. Epitranscriptomic Code and Its Alterations in Human Disease. Trends Mol Med 2018; 24(10): 886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng J, Fouse S, Fan G. Epigenetic regulation of neural gene expression and neuronal function. Pediatr Res 2007; 61(5 Pt 2): 58R–63R. [DOI] [PubMed] [Google Scholar]
- 16.Hirabayashi Y, Gotoh Y. Epigenetic control of neural precursor cell fate during development. Nat Rev Neurosci 2010; 11(6): 377–388. [DOI] [PubMed] [Google Scholar]
- 17.Sun J, Sun J, Ming GL, Song H. Epigenetic regulation of neurogenesis in the adult mammalian brain. Eur J Neurosci 2011; 33(6): 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao B, Christian KM, He C, Jin P, Ming GL, Song H. Epigenetic mechanisms in neurogenesis. Nat Rev Neurosci 2016; 17(9): 537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A et al. Transcriptional landscape of the prenatal human brain. Nature 2014; 508(7495): 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nord AS, Pattabiraman K, Visel A, Rubenstein JLR. Genomic perspectives of transcriptional regulation in forebrain development. Neuron 2015; 85(1): 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egan CM, Nyman U, Skotte J, Streubel G, Turner S, O'Connell DJ et al. CHD5 is required for neurogenesis and has a dual role in facilitating gene expression and polycomb gene repression. Dev Cell 2013; 26(3): 223–236. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Taylor CA, Barnes KM, Shen A, Stewart EV, Chen A et al. A Myt1 family transcription factor defines neuronal fate by repressing non-neuronal genes. Elife 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mu L, Berti L, Masserdotti G, Covic M, Michaelidis TM, Doberauer K et al. SoxC transcription factors are required for neuronal differentiation in adult hippocampal neurogenesis. J Neurosci 2012; 32(9): 3067–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rraklli V, Sodersten E, Nyman U, Hagey DW, Holmberg J. Elevated levels of ZAC1 disrupt neurogenesis and promote rapid in vivo reprogramming. Stem Cell Res 2016; 16(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 25.Sansom SN, Griffiths DS, Faedo A, Kleinjan DJ, Ruan Y, Smith J et al. The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet 2009; 5(6): e1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brainstorm C, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J et al. Analysis of shared heritability in common disorders of the brain. Science 2018; 360(6395). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 1997; 3(11): 1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 2014; 10(2): 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol 2014; 16(2): 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 2016; 537(7620): 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen J, Lv R, Ma H, Shen H, He C, Wang J et al. Zc3h13 Regulates Nuclear RNA m(6)A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol Cell 2018; 69(6): 1028–1038 e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z et al. VIRMA mediates preferential m(6)A mRNA methylation in 3'UTR and near stop codon and associates with alternative polyadenylation. Cell Discov 2018; 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwala SD, Blitzblau HG, Hochwagen A, Fink GR. RNA methylation by the MIS complex regulates a cell fate decision in yeast. PLoS Genet 2012; 8(6): e1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horiuchi K, Kawamura T, Iwanari H, Ohashi R, Naito M, Kodama T et al. Identification of Wilms' tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem 2013; 288(46): 33292–33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 2011; 7(12): 885–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 2013; 49(1): 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res 2017; 27(3): 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang H, Weng H, Sun W, Qin X, Shi H, Wu H et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol 2018; 20(3): 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 2015; 162(6): 1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012; 485(7397): 201–206. [DOI] [PubMed] [Google Scholar]
- 41.Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A et al. A majority of m6A residues are in the last exons, allowing the potential for 3' UTR regulation. Genes Dev 2015; 29(19): 2037–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell 2012; 149(7): 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shafik AM, Allen EG, Jin P. Dynamic N6-methyladenosine RNA methylation in brain and diseases. Epigenomics 2020; 12(4): 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Dou X, Chen C, Chen C, Liu C, Xu MM et al. N (6)-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science 2020; 367(6477): 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Li Y, Yue M, Wang J, Kumar S, Wechsler-Reya RJ et al. N(6)-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat Neurosci 2018; 21(2): 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang M, Lv H, Zhang W, Ma C, He X, Zhao S et al. Region-specific RNA m(6)A methylation represents a new layer of control in the gene regulatory network in the mouse brain. Open Biol 2017; 7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma C, Chang M, Lv H, Zhang ZW, Zhang W, He X et al. RNA m(6)A methylation participates in regulation of postnatal development of the mouse cerebellum. Genome Biol 2018; 19(1): 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shafik AM, Zhang F, Guo Z, Dai Q, Pajdzik K, Li Y et al. N6-methyladenosine dynamics in neurodevelopment and aging, and its potential role in Alzheimer's disease. Genome Biol 2021; 22(1): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang C-X, Cui G-S, Liu X, Xu K, Wang M, Zhang X-X et al. METTL3-mediated m6A modification is required for cerebellar development. PLOS Biology 2018; 16(6): e2004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon K-J, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D et al. Temporal Control of Mammalian Cortical Neurogenesis by m(6)A Methylation. Cell 2017; 171(4): 877–889.e817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Li Y, Yue M, Wang J, Kumar S, Wechsler-Reya RJ et al. N6-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nature Neuroscience 2018; 21(2): 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edens BM, Vissers C, Su J, Arumugam S, Xu Z, Shi H et al. FMRP Modulates Neural Differentiation through m(6)A-Dependent mRNA Nuclear Export. Cell Rep 2019; 28(4): 845–854 e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J, Zhang Y-C, Huang C, Shen H, Sun B, Cheng X et al. m(6)A Regulates Neurogenesis and Neuronal Development by Modulating Histone Methyltransferase Ezh2. Genomics Proteomics Bioinformatics 2019; 17(2): 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi H, Baek S, Cho B, Kim S, Kim J, Chang Y et al. Epitranscriptomic N6-Methyladenosine Modification Is Required for Direct Lineage Reprogramming into Neurons. ACS Chemical Biology 2020; 15(8): 2087–2097. [DOI] [PubMed] [Google Scholar]
- 55.Li M, Zhao X, Wang W, Shi H, Pan Q, Lu Z et al. Ythdf2-mediated m6A mRNA clearance modulates neural development in mice. Genome Biology 2018; 19(1): 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu H, Dzhashiashvili Y, Shah A, Kunjamma RB, Weng Y-l, Elbaz B et al. m6A mRNA Methylation Is Essential for Oligodendrocyte Maturation and CNS Myelination. Neuron 2020; 105(2): 293–309.e295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu R, Li A, Sun B, Sun J-G, Zhang J, Zhang T et al. A novel m6A reader Prrc2a controls oligodendroglial specification and myelination. Cell Research 2019; 29(1): 23–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merkurjev D, Hong W-T, Iida K, Oomoto I, Goldie BJ, Yamaguti H et al. Synaptic N6-methyladenosine (m6A) epitranscriptome reveals functional partitioning of localized transcripts. Nat Neurosci 2018; 21(7): 1004–1014. [DOI] [PubMed] [Google Scholar]
- 59.Martinez De La Cruz B, Markus R, Malla S, Haig MI, Gell C, Sang F et al. Modifying the m6A brain methylome by ALKBH5-mediated demethylation: a new contender for synaptic tagging. Molecular Psychiatry 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Enam SU, Zinshteyn B, Goldman DH, Cassani M, Livingston NM, Seydoux G et al. Puromycin reactivity does not accurately localize translation at the subcellular level. Elife 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hess ME, Hess S, Meyer KD, Verhagen LA, Koch L, Brönneke HS et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci 2013; 16(8): 1042–1048. [DOI] [PubMed] [Google Scholar]
- 62.Widagdo J, Zhao QY, Kempen MJ, Tan MC, Ratnu VS, Wei W et al. Experience-Dependent Accumulation of N6-Methyladenosine in the Prefrontal Cortex Is Associated with Memory Processes in Mice. J Neurosci 2016; 36(25): 6771–6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walters BJ, Mercaldo V, Gillon CJ, Yip M, Neve RL, Boyce FM et al. The Role of The RNA Demethylase FTO (Fat Mass and Obesity-Associated) and mRNA Methylation in Hippocampal Memory Formation. Neuropsychopharmacology 2017; 42(7): 1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z, Wang M, Xie D, Huang Z, Zhang L, Yang Y et al. METTL3-mediated N6-methyladenosine mRNA modification enhances long-term memory consolidation. Cell Research 2018; 28(11): 1050–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi H, Zhang X, Weng YL, Lu Z, Liu Y, Lu Z et al. m(6)A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 2018; 563(7730): 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koranda JL, Dore L, Shi H, Patel MJ, Vaasjo LO, Rao MN et al. Mettl14 Is Essential for Epitranscriptomic Regulation of Striatal Function and Learning. Neuron 2018; 99(2): 283–292.e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu J, Chen M, Huang H, Zhu J, Song H, Zhu J et al. Dynamic m6A modification regulates local translation of mRNA in axons. Nucleic Acids Res 2018; 46(3): 1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhuang M, Li X, Zhu J, Zhang J, Niu F, Liang F et al. The m6A reader YTHDF1 regulates axon guidance through translational control of Robo3.1 expression. Nucleic Acids Research 2019; 47(9): 4765–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu J, She Y, Yang L, Zhuang M, Han P, Liu J et al. The m(6) A Readers YTHDF1 and YTHDF2 Synergistically Control Cerebellar Parallel Fiber Growth by Regulating Local Translation of the Key Wnt5a Signaling Components in Axons. Adv Sci (Weinh) 2021; 8(22): e2101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Milaneschi Y, Lamers F, Mbarek H, Hottenga JJ, Boomsma DI, Penninx BW. The effect of FTO rs9939609 on major depression differs across MDD subtypes. Mol Psychiatry 2014; 19(9): 960–962. [DOI] [PubMed] [Google Scholar]
- 71.Du T, Rao S, Wu L, Ye N, Liu Z, Hu H et al. An association study of the m6A genes with major depressive disorder in Chinese Han population. J Affect Disord 2015; 183: 279–286. [DOI] [PubMed] [Google Scholar]
- 72.Engel M, Eggert C, Kaplick PM, Eder M, Röh S, Tietze L et al. The Role of m(6)A/m-RNA Methylation in Stress Response Regulation. Neuron 2018; 99(2): 389–403.e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spychala A, Rüther U. FTO affects hippocampal function by regulation of BDNF processing. PLoS One 2019; 14(2): e0211937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun L, Ma L, Zhang H, Cao Y, Wang C, Hou N et al. Fto Deficiency Reduces Anxiety- and Depression-Like Behaviors in Mice via Alterations in Gut Microbiota. Theranostics 2019; 9(3): 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu S, Xiu J, Zhu C, Meng K, Li C, Han R et al. Fat mass and obesity-associated protein regulates RNA methylation associated with depression-like behavior in mice. Nat Commun 2021; 12(1): 6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choudhry Z, Sengupta SM, Grizenko N, Harvey WJ, Fortier ME, Schmitz N et al. Body weight and ADHD: examining the role of self-regulation. PLoS One 2013; 8(1): e55351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiong X, Hou L, Park YP, Molinie B, Consortium GT, Gregory RI et al. Genetic drivers of m(6)A methylation in human brain, lung, heart and muscle. Nat Genet 2021; 53(8): 1156–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 2018; 362(6420). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oldmeadow C, Mossman D, Evans TJ, Holliday EG, Tooney PA, Cairns MJ et al. Combined analysis of exon splicing and genome wide polymorphism data predict schizophrenia risk loci. J Psychiatr Res 2014; 52: 44–49. [DOI] [PubMed] [Google Scholar]
- 80.Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annual review of pathology 2012; 7: 219–245. [DOI] [PubMed] [Google Scholar]
- 81.Wang LW, Berry-Kravis E, Hagerman RJ. Fragile X: leading the way for targeted treatments in autism. Neurotherapeutics 2010; 7(3): 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991; 65(5): 905–914. [DOI] [PubMed] [Google Scholar]
- 83.Edupuganti RR, Geiger S, Lindeboom RGH, Shi H, Hsu PJ, Lu Z et al. N(6)-methyladenosine (m(6)A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol 2017; 24(10): 870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang F, Kang Y, Wang M, Li Y, Xu T, Yang W et al. Fragile X mental retardation protein modulates the stability of its m6A-marked messenger RNA targets. Hum Mol Genet 2018; 27(22): 3936–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Worpenberg L, Paolantoni C, Longhi S, Mulorz MM, Lence T, Wessels HH et al. Ythdf is a N6-methyladenosine reader that modulates Fmr1 target mRNA selection and restricts axonal growth in Drosophila. EMBO J 2021; 40(4): e104975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014; 515(7526): 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 2012; 485(7397): 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu X, Shimada T, Otowa T, Wu YY, Kawamura Y, Tochigi M et al. Genome-wide Association Study of Autism Spectrum Disorder in the East Asian Populations. Autism Res 2016; 9(3): 340–349. [DOI] [PubMed] [Google Scholar]
- 89.Keller L, Xu W, Wang HX, Winblad B, Fratiglioni L, Graff C. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer's disease risk: a prospective cohort study. J Alzheimers Dis 2011; 23(3): 461–469. [DOI] [PubMed] [Google Scholar]
- 90.Han M, Liu Z, Xu Y, Liu X, Wang D, Li F et al. Abnormality of m6A mRNA Methylation Is Involved in Alzheimer's Disease. Front Neurosci 2020; 14: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang H, Camats-Perna J, Medeiros R, Anggono V, Widagdo J. Altered Expression of the m6A Methyltransferase METTL3 in Alzheimer's Disease. eNeuro 2020; 7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao F, Xu Y, Gao S, Qin L, Austria Q, Siedlak SL et al. METTL3-dependent RNA m(6)A dysregulation contributes to neurodegeneration in Alzheimer's disease through aberrant cell cycle events. Mol Neurodegener 2021; 16(1): 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang L, Lin W, Zhang C, Ash PEA, Verma M, Kwan J et al. Interaction of tau with HNRNPA2B1 and N(6)-methyladenosine RNA mediates the progression of tauopathy. Mol Cell 2021; 81(20): 4209–4227 e4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen X, Yu C, Guo M, Zheng X, Ali S, Huang H et al. Down-Regulation of m6A mRNA Methylation Is Involved in Dopaminergic Neuronal Death. ACS Chem Neurosci 2019; 10(5): 2355–2363. [DOI] [PubMed] [Google Scholar]
- 95.Qin L, Min S, Shu L, Pan H, Zhong J, Guo J et al. Genetic analysis of N6-methyladenosine modification genes in Parkinson's disease. Neurobiol Aging 2020; 93: 143 e149–143 e113. [DOI] [PubMed] [Google Scholar]
- 96.Amort T, Rieder D, Wille A, Khokhlova-Cubberley D, Riml C, Trixl L et al. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol 2017; 18(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hussain S, Sajini AA, Blanco S, Dietmann S, Lombard P, Sugimoto Y et al. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep 2013; 4(2): 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khoddami V, Cairns BR. Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat Biotechnol 2013; 31(5): 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res 2012; 40(11): 5023–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heissenberger C, Liendl L, Nagelreiter F, Gonskikh Y, Yang G, Stelzer EM et al. Loss of the ribosomal RNA methyltransferase NSUN5 impairs global protein synthesis and normal growth. Nucleic Acids Res 2019; 47(22): 11807–11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharma S, Yang J, Watzinger P, Kotter P, Entian KD. Yeast Nop2 and Rcm1 methylate C2870 and C2278 of the 25S rRNA, respectively. Nucleic Acids Res 2013; 41(19): 9062–9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Metodiev MD, Spahr H, Loguercio Polosa P, Meharg C, Becker C, Altmueller J et al. NSUN4 is a dual function mitochondrial protein required for both methylation of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet 2014; 10(2): e1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haag S, Sloan KE, Ranjan N, Warda AS, Kretschmer J, Blessing C et al. NSUN3 and ABH1 modify the wobble position of mt-tRNAMet to expand codon recognition in mitochondrial translation. EMBO J 2016; 35(19): 2104–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haag S, Warda AS, Kretschmer J, Gunnigmann MA, Hobartner C, Bohnsack MT. NSUN6 is a human RNA methyltransferase that catalyzes formation of m5C72 in specific tRNAs. RNA 2015; 21(9): 1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aguilo F, Li S, Balasubramaniyan N, Sancho A, Benko S, Zhang F et al. Deposition of 5-Methylcytosine on Enhancer RNAs Enables the Coactivator Function of PGC-1alpha. Cell Rep 2016; 14(3): 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blaze J, Navickas A, Phillips HL, Heissel S, Plaza-Jennings A, Miglani S et al. Neuronal Nsun2 deficiency produces tRNA epitranscriptomic alterations and proteomic shifts impacting synaptic signaling and behavior. Nat Commun 2021; 12(1): 4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang T, Chen P, Li W, Sha S, Wang Y, Yuan Z et al. Cognitive deficits in mice lacking Nsun5, a cytosine-5 RNA methyltransferase, with impairment of oligodendrocyte precursor cells. Glia 2019; 67(4): 688–702. [DOI] [PubMed] [Google Scholar]
- 108.Ni P, Noh H, Park GH, Shao Z, Guan Y, Park JM et al. iPSC-derived homogeneous populations of developing schizophrenia cortical interneurons have compromised mitochondrial function. Mol Psychiatry 2020; 25(11): 2873–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Trixl L, Amort T, Wille A, Zinni M, Ebner S, Hechenberger C et al. RNA cytosine methyltransferase Nsun3 regulates embryonic stem cell differentiation by promoting mitochondrial activity. Cell Mol Life Sci 2018; 75(8): 1483–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Flores JV, Cordero-Espinoza L, Oeztuerk-Winder F, Andersson-Rolf A, Selmi T, Blanco S et al. Cytosine-5 RNA Methylation Regulates Neural Stem Cell Differentiation and Motility. Stem Cell Reports 2017; 8(1): 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khan MA, Rafiq MA, Noor A, Hussain S, Flores JV, Rupp V et al. Mutation in NSUN2, which encodes an RNA methyltransferase, causes autosomal-recessive intellectual disability. Am J Hum Genet 2012; 90(5): 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Komara M, Al-Shamsi AM, Ben-Salem S, Ali BR, Al-Gazali L. A Novel Single-Nucleotide Deletion (c.1020delA) in NSUN2 Causes Intellectual Disability in an Emirati Child. J Mol Neurosci 2015; 57(3): 393–399. [DOI] [PubMed] [Google Scholar]
- 113.Martinez FJ, Lee JH, Lee JE, Blanco S, Nickerson E, Gabriel S et al. Whole exome sequencing identifies a splicing mutation in NSUN2 as a cause of a Dubowitz-like syndrome. J Med Genet 2012; 49(6): 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abbasi-Moheb L, Mertel S, Gonsior M, Nouri-Vahid L, Kahrizi K, Cirak S et al. Mutations in NSUN2 cause autosomal-recessive intellectual disability. Am J Hum Genet 2012; 90(5): 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun S, Chen L, Wang Y, Wang J, Li N, Wang X. Further delineation of autosomal recessive intellectual disability syndrome caused by homozygous variant of the NSUN2 gene in a chinese pedigree. Mol Genet Genomic Med 2020; 8(12): e1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Doan RN, Lim ET, De Rubeis S, Betancur C, Cutler DJ, Chiocchetti AG et al. Recessive gene disruptions in autism spectrum disorder. Nat Genet 2019; 51(7): 1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carlile TM, Rojas-Duran MF, Zinshteyn B, Shin H, Bartoli KM, Gilbert WV. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 2014; 515(7525): 143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lovejoy AF, Riordan DP, Brown PO. Transcriptome-wide mapping of pseudouridines: pseudouridine synthases modify specific mRNAs in S. cerevisiae. PLoS One 2014; 9(10): e110799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, Leon-Ricardo BX et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 2014; 159(1): 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li X, Zhu P, Ma S, Song J, Bai J, Sun F et al. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat Chem Biol 2015; 11(8): 592–597. [DOI] [PubMed] [Google Scholar]
- 121.Davis DR. Stabilization of RNA stacking by pseudouridine. Nucleic Acids Res 1995; 23(24): 5020–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kierzek E, Malgowska M, Lisowiec J, Turner DH, Gdaniec Z, Kierzek R. The contribution of pseudouridine to stabilities and structure of RNAs. Nucleic Acids Res 2014; 42(5): 3492–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Newby MI, Greenbaum NL. Investigation of Overhauser effects between pseudouridine and water protons in RNA helices. Proc Natl Acad Sci U S A 2002; 99(20): 12697–12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karijolich J, Yu YT. Converting nonsense codons into sense codons by targeted pseudouridylation. Nature 2011; 474(7351): 395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fernandez IS, Ng CL, Kelley AC, Wu G, Yu YT, Ramakrishnan V. Unusual base pairing during the decoding of a stop codon by the ribosome. Nature 2013; 500(7460): 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shaheen R, Han L, Faqeih E, Ewida N, Alobeid E, Phizicky EM et al. A homozygous truncating mutation in PUS3 expands the role of tRNA modification in normal cognition. Hum Genet 2016; 135(7): 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Brouwer APM, Abou Jamra R, Kortel N, Soyris C, Polla DL, Safra M et al. Variants in PUS7 Cause Intellectual Disability with Speech Delay, Microcephaly, Short Stature, and Aggressive Behavior. Am J Hum Genet 2018; 103(6): 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shaheen R, Tasak M, Maddirevula S, Abdel-Salam GMH, Sayed ISM, Alazami AM et al. PUS7 mutations impair pseudouridylation in humans and cause intellectual disability and microcephaly. Hum Genet 2019; 138(3): 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Darvish H, Azcona LJ, Alehabib E, Jamali F, Tafakhori A, Ranji-Burachaloo S et al. A novel PUS7 mutation causes intellectual disability with autistic and aggressive behaviors. Neurol Genet 2019; 5(5): e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen CX, Cho DS, Wang Q, Lai F, Carter KC, Nishikura K. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA 2000; 6(5): 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jacobs MM, Fogg RL, Emeson RB, Stanwood GD. ADAR1 and ADAR2 expression and editing activity during forebrain development. Dev Neurosci 2009; 31(3): 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hartner JC, Schmittwolf C, Kispert A, Muller AM, Higuchi M, Seeburg PH. Liver disintegration in the mouse embryo caused by deficiency in the RNA-editing enzyme ADAR1. J Biol Chem 2004; 279(6): 4894–4902. [DOI] [PubMed] [Google Scholar]
- 133.Vitali P, Basyuk E, Le Meur E, Bertrand E, Muscatelli F, Cavaille J et al. ADAR2-mediated editing of RNA substrates in the nucleolus is inhibited by C/D small nucleolar RNAs. J Cell Biol 2005; 169(5): 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Niswender CM, Herrick-Davis K, Dilley GE, Meltzer HY, Overholser JC, Stockmeier CA et al. RNA editing of the human serotonin 5-HT2C receptor. alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology 2001; 24(5): 478–491. [DOI] [PubMed] [Google Scholar]
- 135.Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron 2002; 34(3): 349–356. [DOI] [PubMed] [Google Scholar]
- 136.Dracheva S, Patel N, Woo DA, Marcus SM, Siever LJ, Haroutunian V. Increased serotonin 2C receptor mRNA editing: a possible risk factor for suicide. Mol Psychiatry 2008; 13(11): 1001–1010. [DOI] [PubMed] [Google Scholar]
- 137.Lyddon R, Dwork AJ, Keddache M, Siever LJ, Dracheva S. Serotonin 2c receptor RNA editing in major depression and suicide. World J Biol Psychiatry 2013; 14(8): 590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weissmann D, van der Laan S, Underwood MD, Salvetat N, Cavarec L, Vincent L et al. Region-specific alterations of A-to-I RNA editing of serotonin 2c receptor in the cortex of suicides with major depression. Transl Psychiatry 2016; 6(8): e878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chimienti F, Cavarec L, Vincent L, Salvetat N, Arango V, Underwood MD et al. Brain region-specific alterations of RNA editing in PDE8A mRNA in suicide decedents. Transl Psychiatry 2019; 9(1): 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Iwamoto K, Nakatani N, Bundo M, Yoshikawa T, Kato T. Altered RNA editing of serotonin 2C receptor in a rat model of depression. Neurosci Res 2005; 53(1): 69–76. [DOI] [PubMed] [Google Scholar]
- 141.Sodhi MS, Burnet PW, Makoff AJ, Kerwin RW, Harrison PJ. RNA editing of the 5-HT(2C) receptor is reduced in schizophrenia. Mol Psychiatry 2001; 6(4): 373–379. [DOI] [PubMed] [Google Scholar]
- 142.Dracheva S, Elhakem SL, Marcus SM, Siever LJ, McGurk SR, Haroutunian V. RNA editing and alternative splicing of human serotonin 2C receptor in schizophrenia. J Neurochem 2003; 87(6): 1402–1412. [DOI] [PubMed] [Google Scholar]
- 143.Bhogal B, Jepson JE, Savva YA, Pepper AS, Reenan RA, Jongens TA. Modulation of dADAR-dependent RNA editing by the Drosophila fragile X mental retardation protein. Nat Neurosci 2011; 14(12): 1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shamay-Ramot A, Khermesh K, Porath HT, Barak M, Pinto Y, Wachtel C et al. Fmrp Interacts with Adar and Regulates RNA Editing, Synaptic Density and Locomotor Activity in Zebrafish. PLoS Genet 2015; 11(12): e1005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Filippini A, Bonini D, Lacoux C, Pacini L, Zingariello M, Sancillo L et al. Absence of the Fragile X Mental Retardation Protein results in defects of RNA editing of neuronal mRNAs in mouse. RNA Biol 2017; 14(11): 1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tran SS, Jun HI, Bahn JH, Azghadi A, Ramaswami G, Van Nostrand EL et al. Widespread RNA editing dysregulation in brains from autistic individuals. Nat Neurosci 2019; 22(1): 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kwak S, Hideyama T, Yamashita T, Aizawa H. AMPA receptor-mediated neuronal death in sporadic ALS. Neuropathology 2010; 30(2): 182–188. [DOI] [PubMed] [Google Scholar]
- 148.Hideyama T, Yamashita T, Suzuki T, Tsuji S, Higuchi M, Seeburg PH et al. Induced loss of ADAR2 engenders slow death of motor neurons from Q/R site-unedited GluR2. J Neurosci 2010; 30(36): 11917–11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kuner R, Groom AJ, Bresink I, Kornau HC, Stefovska V, Muller G et al. Late-onset motoneuron disease caused by a functionally modified AMPA receptor subunit. Proc Natl Acad Sci U S A 2005; 102(16): 5826–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Akbarian S, Smith MA, Jones EG. Editing for an AMPA receptor subunit RNA in prefrontal cortex and striatum in Alzheimer's disease, Huntington's disease and schizophrenia. Brain Res 1995; 699(2): 297–304. [DOI] [PubMed] [Google Scholar]
- 151.Gaisler-Salomon I, Kravitz E, Feiler Y, Safran M, Biegon A, Amariglio N et al. Hippocampus-specific deficiency in RNA editing of GluA2 in Alzheimer's disease. Neurobiol Aging 2014; 35(8): 1785–1791. [DOI] [PubMed] [Google Scholar]
- 152.Khermesh K, D'Erchia AM, Barak M, Annese A, Wachtel C, Levanon EY et al. Reduced levels of protein recoding by A-to-I RNA editing in Alzheimer's disease. RNA 2016; 22(2): 290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu S, Yang M, Kim P, Zhou X. ADeditome provides the genomic landscape of A-to-I RNA editing in Alzheimer's disease. Brief Bioinform 2021; 22(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krogh N, Kongsbak-Wismann M, Geisler C, Nielsen H. Substoichiometric ribose methylations in spliceosomal snRNAs. Org Biomol Chem 2017; 15(42): 8872–8876. [DOI] [PubMed] [Google Scholar]
- 155.Marchand V, Pichot F, Thuring K, Ayadi L, Freund I, Dalpke A et al. Next-Generation Sequencing-Based RiboMethSeq Protocol for Analysis of tRNA 2'-O-Methylation. Biomolecules 2017; 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Taoka M, Nobe Y, Yamaki Y, Sato K, Ishikawa H, Izumikawa K et al. Landscape of the complete RNA chemical modifications in the human 80S ribosome. Nucleic Acids Res 2018; 46(18): 9289–9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Elliott BA, Ho HT, Ranganathan SV, Vangaveti S, Ilkayeva O, Abou Assi H et al. Modification of messenger RNA by 2'-O-methylation regulates gene expression in vivo. Nat Commun 2019; 10(1): 3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Somme J, Van Laer B, Roovers M, Steyaert J, Versees W, Droogmans L. Characterization of two homologous 2'-O-methyltransferases showing different specificities for their tRNA substrates. RNA 2014; 20(8): 1257–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cavaille J, Nicoloso M, Bachellerie JP. Targeted ribose methylation of RNA in vivo directed by tailored antisense RNA guides. Nature 1996; 383(6602): 732–735. [DOI] [PubMed] [Google Scholar]
- 160.Kiss-Laszlo Z, Henry Y, Bachellerie JP, Caizergues-Ferrer M, Kiss T. Site-specific ribose methylation of preribosomal RNA: a novel function for small nucleolar RNAs. Cell 1996; 85(7): 1077–1088. [DOI] [PubMed] [Google Scholar]
- 161.Ashraf SS, Guenther RH, Ansari G, Malkiewicz A, Sochacka E, Agris PF. Role of modified nucleosides of yeast tRNA(Phe) in ribosomal binding. Cell Biochem Biophys 2000; 33(3): 241–252. [DOI] [PubMed] [Google Scholar]
- 162.Benitez-Paez A, Villarroya M, Douthwaite S, Gabaldon T, Armengod ME. YibK is the 2'-O-methyltransferase TrmL that modifies the wobble nucleotide in Escherichia coli tRNA(Leu) isoacceptors. RNA 2010; 16(11): 2131–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pintard L, Lecointe F, Bujnicki JM, Bonnerot C, Grosjean H, Lapeyre B. Trm7p catalyses the formation of two 2'-O-methylriboses in yeast tRNA anticodon loop. EMBO J 2002; 21(7): 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Polikanov YS, Melnikov SV, Soll D, Steitz TA. Structural insights into the role of rRNA modifications in protein synthesis and ribosome assembly. Nat Struct Mol Biol 2015; 22(4): 342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Picard-Jean F, Brand C, Tremblay-Letourneau M, Allaire A, Beaudoin MC, Boudreault S et al. 2'-O-methylation of the mRNA cap protects RNAs from decapping and degradation by DXO. PLoS One 2018; 13(3): e0193804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Freude K, Hoffmann K, Jensen LR, Delatycki MB, des Portes V, Moser B et al. Mutations in the FTSJ1 gene coding for a novel S-adenosylmethionine-binding protein cause nonsyndromic X-linked mental retardation. Am J Hum Genet 2004; 75(2): 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gong P, Li J, Dai L, Zhang K, Zheng Z, Gao X et al. Genetic variations in FTSJ1 influence cognitive ability in young males in the Chinese Han population. J Neurogenet 2008; 22(4): 277–287. [DOI] [PubMed] [Google Scholar]
- 168.Ramser J, Winnepenninckx B, Lenski C, Errijgers V, Platzer M, Schwartz CE et al. A splice site mutation in the methyltransferase gene FTSJ1 in Xp11.23 is associated with non-syndromic mental retardation in a large Belgian family (MRX9). J Med Genet 2004; 41(9): 679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Takano K, Nakagawa E, Inoue K, Kamada F, Kure S, Goto Y et al. A loss-of-function mutation in the FTSJ1 gene causes nonsyndromic X-linked mental retardation in a Japanese family. Am J Med Genet B Neuropsychiatr Genet 2008; 147B(4): 479–484. [DOI] [PubMed] [Google Scholar]
- 170.Guy MP, Shaw M, Weiner CL, Hobson L, Stark Z, Rose K et al. Defects in tRNA Anticodon Loop 2'-O-Methylation Are Implicated in Nonsyndromic X-Linked Intellectual Disability due to Mutations in FTSJ1. Hum Mutat 2015; 36(12): 1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nagayoshi Y, Chujo T, Hirata S, Nakatsuka H, Chen CW, Takakura M et al. Loss of Ftsj1 perturbs codon-specific translation efficiency in the brain and is associated with X-linked intellectual disability. Sci Adv 2021; 7(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kang Y, Zhou Y, Li Y, Han Y, Xu J, Niu W et al. A human forebrain organoid model of fragile X syndrome exhibits altered neurogenesis and highlights new treatment strategies. Nat Neurosci 2021; 24(10): 1377–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lasman L, Krupalnik V, Viukov S, Mor N, Aguilera-Castrejon A, Schneir D et al. Context-dependent functional compensation between Ythdf m(6)A reader proteins. Genes Dev 2020; 34(19-20): 1373–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li Y, Bedi RK, Moroz-Omori EV, Caflisch A. Structural and Dynamic Insights into Redundant Function of YTHDF Proteins. J Chem Inf Model 2020; 60(12): 5932–5935. [DOI] [PubMed] [Google Scholar]
- 175.Zaccara S, Jaffrey SR. A Unified Model for the Function of YTHDF Proteins in Regulating m(6)A-Modified mRNA. Cell 2020; 181(7): 1582–1595 e1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu H, Begik O, Lucas MC, Ramirez JM, Mason CE, Wiener D et al. Accurate detection of m(6)A RNA modifications in native RNA sequences. Nat Commun 2019; 10(1): 4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Parker MT, Knop K, Sherwood AV, Schurch NJ, Mackinnon K, Gould PD et al. Nanopore direct RNA sequencing maps the complexity of Arabidopsis mRNA processing and m(6)A modification. Elife 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guo Z, Shafik AM, Jin P, Wu Z, Wu H. Detecting m6A methylation regions from Methylated RNA Immunoprecipitation Sequencing. Bioinformatics 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]