Abstract
Background
Chlamydia trachomatis is an obligate intracellular gram-negative pathogen, responsible for diverse affections, mainly trachoma and sexually transmitted diseases. Antibiotics are the commonly used drugs to tackle chlamydiae infections. However, when overused or wrongly used this may lead to strains’ resistance to antibiotics, this phenomenon represents a real health problem worldwide. Numerous studies showed the association of Chlamydia trachomatis resistance with mutations in different genes; these mutations could have a deleterious or neutral impacts on the encoded proteins. The aim of this study is to perform an in silico analysis of C. trachomatis rpoB-encoded proteins using numerous bioinformatics tools and to identify the functional and structural-related effects of the mutations and consequently their impact on the bacteria sensitivity to antibiotics.
Results
The analysis revealed that the prediction of the damaging impact related to the mutations in rpoB-encoded proteins showed eight mutations: V136F, Q458K, V466A, A467T, H471N, H471Y, H471L, and I517M with big deleterious effects. Among them, six mutations, V136F, Q458K, V466A, A467T, H471N, and I517M, are located in a highly conserved regions decreasing the protein’s stability. Furthermore, the structures analysis showed that the mutations A467T, H471N, I517M, and V136F models had a high deviation compared to the wild type. Moreover, the prediction of protein-protein network indicated that rpoB wild type interacts strongly with 10 proteins of C. trachomatis, which are playing different roles at different levels.
Conclusion
As conclusion, the present study revealed that the changes observed in the encoded proteins can affect their functions and structures, in addition to their interactions with other proteins which impact the bacteria sensitivity to antibiotics. Consequently, the information revealed through this in silico analysis would be useful for deeper exploration to understand the mechanisms of C. trachomatis resistance and enable managing the infection to avoid its complications. We recommend further investigations and perform deeper experimental analysis with collaboration between bioinformaticians, physicians, biologists, pharmacists, and chemistry and biochemistry scientists.
Keywords: Chlamydia trachomatis, rpoB gene, Mutations, In silico analysis, Antibiotic, Resistance
Background
The family Chlamydiaceae comprises a group of obligate intracellular Gram-negative bacteria that are responsible for a broad range of infections in mammals, birds, and humans [1]. Current classification within this family recognizes a single genus within this family, Chlamydia, 14 species [2–4] and four Candidatus (Ca.) species (Ca. Chlamydia ibidis, Ca. Chlamydia corallus, Ca. Chlamydia sanzinia, and Ca. Chlamydia testudinis) [5–8]. Humans are only Chlamydia trachomatis (C. trachomatis) natural hosts [9]; when C. trachomatis bacteria is untreated, it may lead to severe complications. In females, pelvic inflammatory disease leads to tubal infertility [10–13], ectopic pregnancy [13], and chronic pelvic pain [14]. Furthermore, C. trachomatis can be transferred to the newborn from the infected mothers; they can develop ocular, respiratory, and gastrointestinal infections [15]. In men, it would be urethritis, epididymitis, prostatitis, and proctitis [16, 17]. In addition, lymphogranuloma venereum [18] and reactive arthritis [19] are the less common diseases caused by C. trachomatis in both men and women infected with C. trachomatis. In another hand, C. trachomatis produces chronic ocular infections that can lead to trachoma, which is one of the leading causes of blindness worldwide [20, 21].
The recommended treatment for chlamydia infections is antibiotics. However, the misuse or the overuse of these antibiotics may lead to antibiotic resistance. The acquired resistance occurs when the bacterium that has been sensitive to antibiotics develops resistance via mutation or via acquisition of new DNA [22].
Different studies showed that C. trachomatis may develop resistance to macrolides via mutations in the 23S rRNA gene, to fluoroquinolones via mutations in the gyrA gene, and to rifamycins via mutations in the rpoB gene [20, 21, 23–27]. The resistance to rifamycins was shown to be associated with a nucleotide substitution in rpoB gene, impacting the inhibition of bacterial transcription related to interacting with beta-subunit of bacterial DNA-dependent RNA polymerase [28].
All mutations within the genomic sequence can lead to alterations in the sequence of the encoded protein, which could have a deleterious or neutral impacts on the protein. Furthermore, it may ultimately affect alteration of protein charge, geometry, hydrophobicity dynamics, translation, and inter- or intra-protein interaction set cells in danger [29].
The aim of the present study is to perform an in silico analysis of the retrieved amino acid variations in C. trachomatis rpoB gene-encoded protein and identify the functional and structural-related effects of the protein’s variations, which consequently may impacting the bacteria sensitivity to antibiotics.
Methods
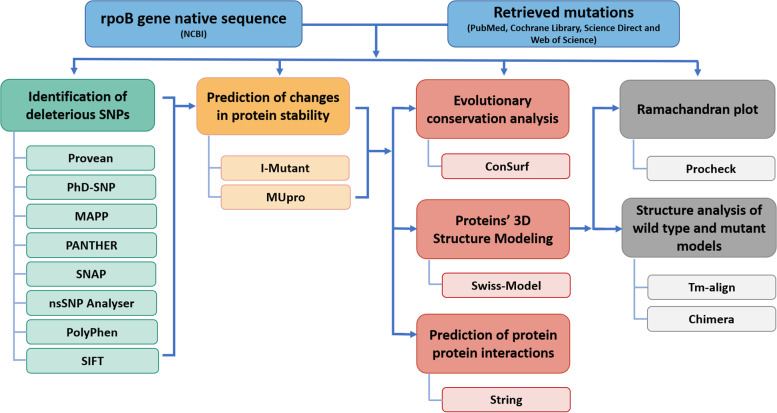
In the present study, we performed an in silico analysis using different machine learning algorithms, following the various steps described below and illustrated in the flowchart (Fig. 1).
Fig. 1.
Study steps flowchart
Mutations collection
To find the rpoB gene mutations linked to C. trachomatis resistance to antibiotics, we proceeded to extract all the rpoB mutations from various resources, which were gathered in our precedent investigations [22].
Prediction of rpoB mutations’ deleterious effects
The damaging effects of the mutations on the protein were predicted using PredictSNP1.0 (http://loschmidt.chemi.muni.cz/predictsnp1/) [30]; this tool includes nine different Bioinformatics’ tools: SIFT [31], PolyPhen-1 [32], PolyPhen-2 [33], MAPP [34], PhD-SNP [35], SNAP [36], PANTHER [37], PredictSNP [38], and nsSNPAnalyzer [39]. Most of these tools are designed to predict whether a particular substitution is neutral or deleterious, based on various parameters derived from the evolutionary, physicochemical, or structural characteristics.
PredictSNP1.0 displays the confidence scores generated by each tool and a consensus prediction as percentages by using their observed accuracy values to simplify comparisons [38]. We classified the mutations as deleterious if the results of five among the nine tools is identified as damaging.
Prediction of changes on the protein stability
To predict the change on the protein stability, we performed the Sanavia et al. protocol, where the effects of the variants on the protein stability are quantified in terms of the Gibbs free energy of unfolding (ΔG), and the measure of interest is the difference of the unfolding free energy between the mutant and wild type proteins (ΔΔGu); the sign of ΔΔG indicates if the mutation decreases (ΔΔGu < 0) or increases (ΔΔGu > 0) the protein stability [40].
We analyzed the different approaches available and selected I-Mutant 3.0 and MUpro to perform this analysis. I-Mutant 3.0 (http://gpcr2.biocomp.unibo.it/cgi/predictors/I-Mutant3.0/I-Mutant3.0.cgi) is a support vector machine (SVM) and a web-based tool that provides the predicted change in Gibbs free energy (ΔΔG) [41]. MUpro server (http://mupro.proteomics.ics.uci.edu/) is based on SVM [38]. We submitted the input data of the retrieved mutations of rpoB in FASTA format.
Evolutionary conservation analysis
The evolutionary conservation analysis consists in estimating the degree of the amino acid conservation based on multiple sequence alignment using the ConSurf server (https://consurf.tau.ac.il) [42]. The degree to which an amino acid position is evolutionarily conserved is strongly dependent on its structural and functional importance [42, 43]. In ConSurf, the evolutionary rate is estimated based on the evolutionary relatedness between the protein and its homologs and considering the similarity between amino acids as reflected in the substitutions matrix [44, 45]. In the present study, the homologous sequences were collected using BLAST (or PSI-BLAST) search against a selected database.
The grade ranges from 1 to 9 estimates the extent of conservation of the amino acid throughout evolution. Therefore, grade 9 represents the most highly conserved residue, and the numbers descend to 1 representing the least conserved region.
Proteins’ 3D structure modeling and validation
To understand the effect of each mutation on the protein structure, the 3D models of rpoB-encoded protein and its selected mutants are designed with SWISS-MODEL (https://swissmodel.expasy.org/) [46]. According to QMEAN score, and sequence identity, the best quality model was selected. Furthermore, to confirm the models, the Ramachandran plots were generated with PROCHECK [47].
Structure analysis of wild type and mutant models
To compare the native and mutated protein structures, structural similarities were calculated using TM-align tool (https://zhanglab.ccmb.med.umich.edu/TM-align/), based on template modeling score (TM-score) and the root mean square deviation (RMSD) scores [48]. Tm-align produces a result between 0 and 1. TM-score equal to 1 means that there is no difference between wild type and the mutant structure; however, TM-score closer to 0 means higher deviation. Furthermore, the RMSD score is proportional to the deviation between the wild type and the mutated structures. Finally, the visualization of the wild type and mutants’ structures were performed by Chimera tool [49].
Structural effect of point mutation rpoB-encoded protein
The energy minimization is essential to determine the proper molecular arrangement in space; it used to eliminate high energies in the predicted model and achieve local minima that is closer to native structure [50]. For this reason, the energy minimization was performed for the wild type and mutants’ structure; in addition, the structural consequences of mutations were visualized by Chimera tool [49].
Prediction of protein-protein interactions
Protein-protein interaction plays key role in predicting the protein function of target protein and drug ability of molecules. The majority of genes and proteins realize resulting phenotype functions as a set of interactions [51]. To investigate the interaction of rpoB-encoded protein with various proteins, the STRING database was used (https://string-db.org) [52]. This database aims to integrate all known and predicted associations between proteins, including both physical interactions and functional associations [53].
Results
rpoB gene’s reference sequence and mutations’ datasets
In order to identify the rpoB gene mutations associated with C. trachomatis resistance to antibiotics, we performed a literature search pertaining to the topic exhaustively, and a total of nine mutations were retrieved (Table 1). Furthermore, the rpoB gene sequence of C. trachomatis reference strain BU-434/L2 was extracted from NCBI database using the published accession number AY623623.1 [24].
Table 1.
Associated rpoB mutations with C. trachomatis resistance to antibiotics
| Author name | Study date | Reference | C. trachomatis strain | Antibiotic | Gene | Mutation | Accession number |
|---|---|---|---|---|---|---|---|
| Rupp et al. | 2007 | [20] | BU-434/L2 | Rifampin | rpoB gene |
Q458K H471Y H471N |
NC |
| Suchland et al. | 2005 | [25] | BU-434/L2 | Rifampin | rpoB gene |
I517M V466A H471L |
NC |
| Suchland et al. | 2005 | [25] | BU-434/L2 | Rifalazil | rpoB gene |
I517M V466A H471L |
NC |
| Kutlin et al. | 2005 | [24] | UW-3/Cx/D | Rifampin and rifalazil | rpoB gene | H471Y | AY623623 |
| Kutlin et al. | 2005 | [24] | BU-434/L2 | Rifalazil | rpoB gene | V136F | AY623623 |
| Dresses-Werringloer et al. | 2003 | [21] | Serovar K | Rifampin | rpoB gene |
A467T H471Y |
NC |
NC not cited
Prediction of rpoB mutations’ deleterious effects
According to the rules and recommendation of predictSNP, the results revealed that all the mutations are deleterious. Indeed, the variations V136F, Q458K, A467T, H471Y, H471L, and I517M were shown to be deleterious with a high confidence score 87%, whereas the mutations V466A and H471N were shown to be deleterious with 61% confidence score (Table 2).
Table 2.
Prediction of mutations’ deleterious effects
| SNP | PredictSNP | MAPP | PhD-SNP | PolyPhen-1 | PolyPhen-2 | SIFT | SNAP | nsSNP-Analyzer | PANTHER |
|---|---|---|---|---|---|---|---|---|---|
| V136F | D | D | D | D | D | D | D | D | D |
| Q458K | D | D | D | D | D | D | D | D | - |
| V466A | D | D | D | N | N | D | D | D | N |
| A467T | D | D | D | D | D | D | D | D | N |
| H471N | D | N | D | N | D | D | D | N | N |
| H471Y | D | D | D | D | D | D | D | D | D |
| H471L | D | D | D | D | D | D | D | D | D |
| I517M | D | D | D | D | D | D | D | D | D |
N refers to the neutral/stable effect of variant, while D refers to its deleterious/nonstable effect
Prediction of changes on the protein stability
The eight mutations predicted as deleterious from the previous step were analyzed with both I-Mutant3.0 and MUpro tools; the results showed that the six mutations, V136F, Q458K, V466A, A467T, H471N, and I517M, were predicted to decrease the rpoB-encoded protein’s stability (Table 3). Furthermore, the most of the mutations that predicted to decrease the stability of the protein were be shown in C. trachomatis strains serovar L2.
Table 3.
Prediction of changes on the protein stability
| SNP | I-Mutant3 | MUpro | ||
|---|---|---|---|---|
| ΔΔG | Prediction | ΔΔG | Prediction | |
| V136F | − 1.37 | DECREASE | − 0.68 | Decrease |
| Q458K | − 0.56 | DECREASE | − 1.62 | Decrease |
| V466A | − 1.51 | DECREASE | − 0.93 | Decrease |
| A467T | − 0.84 | DECREASE | − 0.9 | Decrease |
| H471N | − 0.62 | DECREASE | − 0.82 | Decrease |
| H471Y | 0.27 | INCREASE | − 0.36 | Decrease |
| H471L | 0.52 | INCREASE | − 0.05 | Decrease |
| I517M | − 1.68 | DECREASE | − 0.68 | Decrease |
Evolutionary conservation analysis
The six mutations, which were shown decreasing the rpoB-encoded protein stability, were analyzed by the ConSurf web server, and the results revealed that these mutations had a high conservation score and located in the highly conserved regions (Table 4); three mutations were predicted as functional and exposed (on protein surface), whereas the rest were predicted to be structural and buried (inside protein core).
Table 4.
Evolutionary conservancy of amino acids in rpoB
| Residue and position | Conservation score | Prediction |
|---|---|---|
| V136 | 9 | Highly conserved and buried (S) |
| Q458 | 9 | Highly conserved and exposed (F) |
| V466 | 9 | Highly conserved and buried (S) |
| A467 | 8 | Highly conserved and exposed (F) |
| H471 | 9 | Highly conserved and exposed (F) |
| I517 | 9 | Highly conserved and buried (S) |
Proteins’ 3D structure modeling and validation
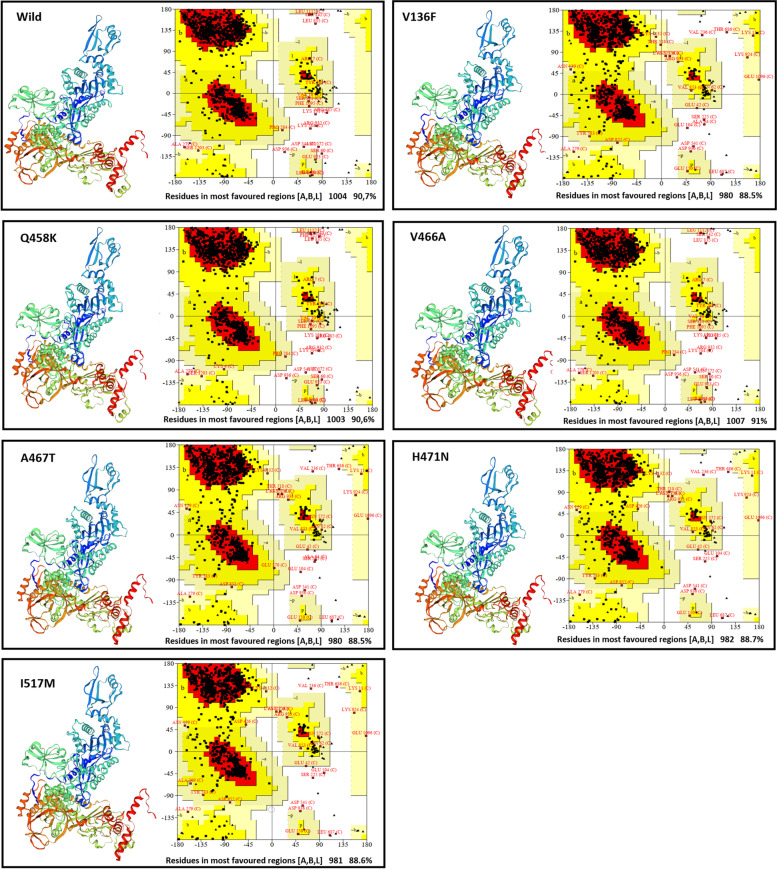
The tertiary structures of rpoB wild type and mutants’ proteins were subjected to a modeling process using the Swiss-Model (Table 5). Furthermore, the Ramachandran plot of each model shows that the residues in most favored regions are greater than 80%, which explain the accurate of the modeling results (Fig. 2).
Table 5.
Structural assessment scores
| Models | QMEAN score | Sequence identity | Ramachandran favorable region (%) |
|---|---|---|---|
| Wild | − 1.69 | 51.90 % | 90.7 % |
| V136F | − 2.34 | 51.82 % | 88.5 % |
| Q458K | − 1.89 | 51.82 % | 90.6 % |
| V466A | − 1.80 | 51.90 % | 91 % |
| A467T | − 2.19 | 51.82 % | 88.5 % |
| H471N | − 2.23 | 51.82 % | 88.6 % |
| I517M | − 2.15 | 51.82 % | 88.7 % |
Fig. 2.
Proteins’ 3D models and Ramachandran plots
Structure analysis of the wild type and mutant models
The comparison between mutants and wild type structural 3D models was performed using TM-align; the results showed that the models, A467T, H471N, I517M, and V136F, had the highest root mean square deviation (RMSD = 3.14); furthermore, all the models had template modeling score (TM-score) near to 1; these results signify that the status of protein folding is identical. In addition, the structural 3D models had high RMSD score which signify a high deviation between mutants and wild type (Table 6). Consequently, the 3D mutants’ models, V136F, A467T, H471N, and I517M, were considered for more explorations and further analysis.
Table 6.
TM-align analysis
| SNP | RMSD | Tm score |
|---|---|---|
| V136F | 3.14 | 0.93293 |
| Q458K | 0.54 | 0.99830 |
| V466A | 0.44 | 0.99887 |
| A467T | 3.14 | 0.93296 |
| H471N | 3.14 | 0.93293 |
| I517M | 3.14 | 0.93294 |
Structural effect of point mutation in rpoB-encoded protein
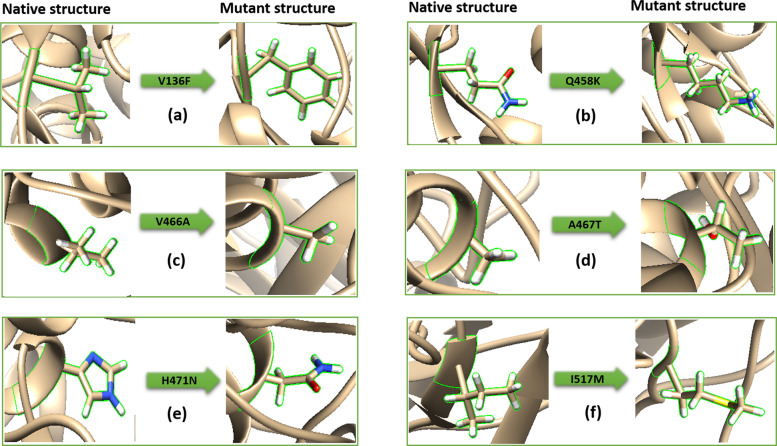
The energy minimization results showed a wide variance between the energy of wild type and the energy of each mutant’s model (Table 7); in addition, the mutants’ structures have more hydrogen bonds (H-Bond) interactions with the adjacent molecules which signify the dispute between wild type and models energies (Fig. 3).
Table 7.
Energy minimization
| Models | H-Bond | Total energy before energy minimization | Total energy after energy minimization |
|---|---|---|---|
| Wild | 1054 | − 82498.723359 | − 103016.025122 |
| V136F | 1149 | − 92419.636836 | − 111911.006170 |
| A467T | 1128 | − 90960.932537 | − 110710.926310 |
| 471N | 1143 | − 93179.859414 | − 112836.201011 |
| I517M | 1137 | − 92718.651461 | − 112342.326497 |
Fig. 3.
Comparison between native and mutant rpoB-encoded proteins’ tridimensional structures. a Wild type V and mutant F residues at 136th position (V136F). b Wild type Q and mutant K residues at 458th position (Q458K). c Wild type V and mutant A residues at 466th position (V466A). d Wild type A and mutant T residues at 476th position (A476T). e Wild type H and the mutant N residues at 471th position (H471N). f Wild type I and mutant M residues at 517th position (I517M)
Prediction of protein-protein interactions
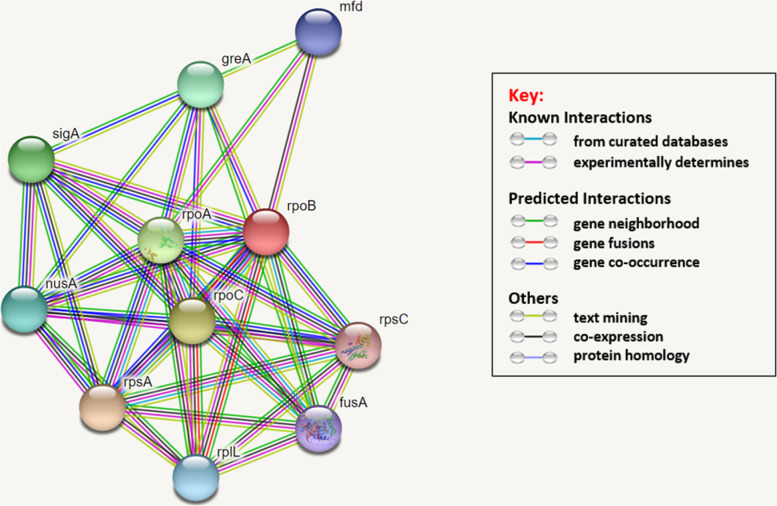
The prediction of protein-protein interactions using STRING indicated that rpoB-encoded protein interacts with 10 proteins from C. trachomatis bacteria, including rpsA, rpoC, rpoA, sigA, greA, nusA, rplL, mfd, fusA, and rpsC (Fig. 4).
Fig. 4.
rpoB protein-protein interaction network
It is known that any occurred changes in the protein can affect the protein network interaction. Therefore, the results revealed that rpoB network have high confidence interaction scores; moreover, the molecular action of rpoB-encoded protein with other proteins could be modified (Table 8).
Table 8.
Prediction of molecular interaction of rpoB with other proteins
| Protein | Information | Score |
|---|---|---|
| rpsA | Binds mRNA; thus facilitating recognition of the initiation point. It is needed to translate mRNA with a short Shine-Dalgarno (SD) purine-rich sequence (By similarity) | 0.999 |
| rpoC | DNA-dependent RNA polymerase catalyzes the transcription of DNA into RNA using the four ribonucleoside triphosphates as substrates | 0.999 |
| rpoA | DNA-dependent RNA polymerase catalyzes the transcription of DNA into RNA using the four ribonucleoside triphosphates as substrates | 0.999 |
| sigA | Sigma factors are initiation factors that promote the attachment of RNA polymerase to specific initiation sites and are then released. This sigma factor is the primary sigma factor during exponential growth | 0.998 |
| greA | Necessary for efficient RNA polymerase transcription elongation past template-encoded arresting sites. The arresting sites in DNA have the property of trapping a certain fraction of elongating RNA polymerases that pass through, resulting in locked ternary complexes. Cleavage of the nascent transcript by cleavage factors such as GreA or GreB allows the resumption of elongation from the new 3′ terminus. GreA releases sequences of 2 to 3 nucleotides (By similarity) | 0.997 |
| nusA | Participates in both transcription termination and antitermination DNA-directed RN polymerase subunit beta; DNA-dependent RNA polymerase catalyzes the transcription of DNA into RNA using the four ribonucleoside triphosphates as substrates | 0.995 |
| rplL | Forms part of the ribosomal stalk which helps the ribosome interact with GTP-bound translation factors. Is thus essential for accurate translation | 0.993 |
| mfd | Couples transcription and DNA repair by recognizing RNA polymerase (RNAP) stalled at DNA lesions. Mediates ATP-dependent release of RNAP and its truncated transcript from the DNA, and recruitment of nucleotide excision repair machinery to the damaged site | 0.993 |
| fusA | Catalyzes the GTP-dependent ribosomal translocation step during translation elongation. During this step, the ribosome changes from the pre-translocational (PRE) to the post-translocational (POST) state as the newly formed A-site-bound peptidyl-tRNA and P-site-bound deacylated tRNA move to the P and E sites, respectively. Catalyzes the coordinated movement of the two tRNA molecules, the mRNA and conformational changes in the ribosome (By similarity) | 0.988 |
| rpsC | Binds the lower part of the 30S subunit head. Binds mRNA in the 70S ribosome, positioning it for translation | 0.987 |
Discussion
The treatments adopted commonly against C. trachomatis infections are macrolides, tetracyclines, rifamycins, and quinolones [54]. However, the bacteria can acquire resistant to different antibiotics family via a range of mechanisms. The main causes of resistance to antibiotics are: the abusive usage of antibiotics, the spread of resistant strains, or the spread of genes bearing information able to induce resistance [55].
Different studies revealed that the resistance to rifamycin was associated with mutations in the C. trachomatis RNA polymerase β-subunit gene (rpoB) [20, 21, 24, 25, 56]; these mutations could have a deleterious or neutral impacts on the encoded proteins; moreover, to understand the impact of these mutations on the proteins’ biological functions, stability, and structure, an in silico analysis was performed.
In genomics and proteomics, the in silico analysis plays a significant role to predict the impact of the mutations on the proteins’ function and structure; this analysis can be performed using different bioinformatics tools. However, using these tools could have strengths and weaknesses in the predictions, because every algorithm uses different parameters for prediction [57, 58]. Consequently, to screen and prioritize the candidate functional SNPs requires the implementation of algorithms with different parameters and aspects to combine their advantages, enhance the accuracy and reliability of the predictions, and minimize the errors [59–61]. Basically, to perform the SNP prediction, it is recommended to use at least five tools to obtain an agreement on the effect of the variations on the structure and function of the studied proteins [58]. In our approach, nine tools were used to predict the SNP deleterious effects on function and structure of the rpoB-encoded protein (Fig. 1); this protocol was adopted by different investigators [62–64]. Our results revealed that the nine used tools showed that all the SNPs (n = 8) have a deleterious effect on the encoded protein (Table 2); indeed, almost the same results of prediction were found by the different used tools; this explains the prediction accuracy and result validity.
It is known that the protein structure governs its stability and determines its function [65]; in our study, the prediction of changes on the proteins’ stability showed differences in the predictions’ results (Table 3). Indeed, the stability prediction using I-Mutant3.0 and MUpro showed discrepant results for the mutations H471Y and H471L, which consist in decreased proteins’ stability by MUpro, and increased proteins’ stability by I-Mutant3.0. In addition, according to the retrieved results, the six mutations, V136F, Q458K, V466A, A467T, H471N, and I517M, were shown to be destabilizing the proteins’ structure by indicating a negative score for the Gibbs free energy. Hence, these mutations can cause misfolding, degradation, or aberrant conglomeration of the rpoB-encoded proteins. The results discrepancies’ can be considered as a negative aspect of the analysis; for that, we suggest performing deeper prediction using new tools.
On the other hand, the ConSurf results revealed that the studied mutations had high conservation scores and located in the highly conserved regions (Table 4). The mutation position can directly affect the proteins’ function and structure; consequently, the mutations position could affect the drug accessibility to the bacteria and so its level of antibiotics sensitivity.
The wild protein 3D structures are essential to more understand the functional and structural effect of mutations; the rpoB protein structure was not available in the Protein Data Bank (PDB). Thus, we predict the 3D structure of wild type and mutants, by changing the six mutations into the native sequence. In addition, Ramachandran plot analysis was performed to validate these protein structures; all the structures had the residues in most favored regions more than 80%, which means that all the structures are valid (Fig. 2). Hence, no negative aspect was notified using the Swiss-Model Server.
Additionally, the structural changes in the encoded proteins were analyzed using TM-Align to compute the RMSD and the TM-score by superimposing models of native and mutant proteins. The results showed that the models with the mutations V136F, A467T, H471N, and I517M had high RMSD values and their TM-score near to 1 (Table 6). Hence, these results indicate a quite large structural dissimilarity between the native and mutant models. Indeed, the structural changes of mutants’ proteins indicate potential alterations in the binding affinity of mutants’ structures of rpoB-encoded proteins with their receptors which may lead to resistance to antibiotics. Regarding the predictions of the mutations Q458K and V466A, the results showed a lowest RMSD score and their TM-score near to 1; these results signify that the status of the protein folding is identical.
In another hand of the analysis, the results of the energy minimization revealed that the total energy of the mutants: V136F, A467T, H471N, and I517M were low compared to that of the wild type protein. Moreover, the mutants present the higher number of H-bonds. In opposition to the high total energy, the wild type structure had the lower number of H-bonds (Table 7). The discrepancy of results found between wild type and mutant structures signifies the dispute between wild type and mutants’ models, which can explain the observed resistance to antibiotics mechanisms.
At the final step of our in silico analysis, the prediction of the protein-protein interaction revealed that rpoB interacts with 10 different bacteria’s proteins (Fig. 4), which are showed playing a role in the bacteria cycle progression and DNA replication events (Table 8). In addition, STRING predicts these protein-protein interactions with high confidence scores; therefore, it can be suggested that any change in the rpoB-encoded protein structure and function might affect the bacteria sensitivity to antibiotics.
Conclusion
This study compiled the mutations in rpoB gene which were revealed to be associated with C. trachomatis resistance to rifamycin and predicts their effects using various bioinformatics tools. The results revealed that the mutations, V136F, A467T, H471N, and I517M, had the most impact on both stability and RMSD, in addition to their location in the highly conserved regions; therefore, they can affect the protein’s function, structure, and their interaction with other proteins. All these changes can explain the observed resistance to antibiotics. Moreover, the study revealed that all mutations are not necessarily translated to strong phenotypic expression. Consequently, the revealed information through this in silico analysis would be useful for deeper exploration to understand the mechanisms of C. trachomatis resistance; this could enable managing the infection and avoid its complications. We recommend further investigations to perform deeper experimental analysis and explore alternative therapies and new drug design by molecular docking; this can be done in collaboration between bioinformaticians, physicians, biologists, pharmacists, and chemistry and biochemistry scientists.
Acknowledgements
We acknowledge the H3ABionet network (Pan African Bioinformatics Network for the consortium Human Heredity and Health in Africa “H3Africa”) for the provided support.
Abbreviations
- C. trachomatis
Chlamydia trachomatis
- Ca
Candidatus
- SNP
Single nucleotide polymorphism
- ΔG
Gibbs free energy of unfolding
- ΔΔG
Change in Gibbs free energy
- SVM
Support vector machine
- TM-score
Template modeling score
- RMSD
Root means square deviation
- BLAST
Basic Local Alignment Search Tool
- PSI-BLAST
Position-Specific Iterative Basic Local Alignment Search Tool
- QMEAN
Qualitative Model Energy Analysis
- NCBI
National Center for Biotechnology Information
- H-Bond
Hydrogen bond
Authors’ contributions
IB: data extraction, data curation, data analysis, designed the study, drafting the paper. MA: data curation. AM: reviewing and editing. FR: project conception, following the process, validated the data, reviewing the paper. All the authors read and approved the manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ichrak Benamri, Email: Ichrakbenamri@gmail.com, Email: ichrak.benamri@etu.uae.ac.ma.
Maryame Azzouzi, Email: maryame.azz@gmail.com.
Ahmed Moussa, Email: amoussa@uae.ac.ma.
Fouzia Radouani, Email: fouzia.radouani@pasteur.ma, Email: radouani@gmail.com.
References
- 1.Longbottom D, Coulter LJ. Animal chlamydioses and zoonotic implications. J Comp Pathol. 2003;128:217–244. doi: 10.1053/jcpa.2002.0629. [DOI] [PubMed] [Google Scholar]
- 2.Sachse K, Bavoil PM, Kaltenboeck B, Stephens RS, Kuo CC, Rossello-Mora R, et al. Emendation of the family Chlamydiaceae: proposal of a single genus, Chlamydia, to include all currently recognized species. Syst Appl Microbiol. 2015;38(2):99–103. doi: 10.1016/j.syapm.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Laroucau K, Vorimore F, Aaziz R, Solmonson L, Hsia RC, Bavoil PM, et al. Chlamydia buteonis, a new Chlamydia species isolated from a red-shouldered hawk. Syst Appl Microbiol. 2019;42(5):125997. doi: 10.1016/j.syapm.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Staub E, Marti H, Biondi R, Levi A, Donati M, Leonard CA, et al. Novel Chlamydia species isolated from snakes are temperature-sensitive and exhibit decreased susceptibility to azithromycin. Sci Rep. 2018;8(1):5660. doi: 10.1038/s41598-018-23897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vorimore F, Hsia RC, Huot-Creasy H, Bastian S, Deruyter L, Passet A, et al. Isolation of a new Chlamydia species from the feral sacred Ibis (Threskiornis aethiopicus): Chlamydia ibidis. PLoS One. 2013;8(9):e74823. doi: 10.1371/journal.pone.0074823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor-Brown A, Bachmann NL, Borel N, Polkinghorne A. Culture-independent genomic characterisation of Candidatus chlamydia sanzinia, a novel uncultivated bacterium infecting snakes. BMC Genomics. 2016;17:710. doi: 10.1186/s12864-016-3055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor-Brown A, Spang L, Borel N, Polkinghorne A. Culture-independent metagenomics supports discovery of uncultivable bacteria within the genus Chlamydia. Sci Rep. 2017;7(1):10661. doi: 10.1038/s41598-017-10757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laroucau K, Ortega N, Vorimore F, Aaziz R, Mitura A, Szymanska-Czerwinska M, et al. Detection of a novel Chlamydia species in captive spur-thighed tortoises (Testudo graeca) in southeastern Spain and proposal of Candidatus chlamydia testudinis. Syst Appl Microbiol. 2020;43(2):126071. doi: 10.1016/j.syapm.2020.126071. [DOI] [PubMed] [Google Scholar]
- 9.Witkin SS, Minis E, Athanasiou A, Leizer J, Linhares IM. Chlamydia trachomatis: the persistent pathogen. Clin Vaccine Immunol. 2017;24(10):e00203–e00217. doi: 10.1128/CVI.00203-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakken IJ, Ghaderi S. Incidence of pelvic inflammatory disease in a large cohort of women tested for Chlamydia trachomatis: a historical follow-up study. BMC Infect Dis. 2009;9:130. doi: 10.1186/1471-2334-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender N, Herrmann B, Andersen B, Hocking JS, van Bergen J, Morgan J, et al. Chlamydia infection, pelvic inflammatory disease, ectopic pregnancy and infertility: cross-national study. Sex Transm Infect. 2011;87(7):601–608. doi: 10.1136/sextrans-2011-050205. [DOI] [PubMed] [Google Scholar]
- 12.Price MJ, Ades AE, De Angelis D, Welton NJ, Macleod J, Soldan K, et al. Risk of pelvic inflammatory disease following Chlamydia trachomatis infection: analysis of prospective studies with a multistate model. Am J Epidemiol. 2013;178(3):484–492. doi: 10.1093/aje/kws583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price MJ, Ades AE, Welton NJ, Simms I, Macleod J, Horner PJ. Proportion of pelvic inflammatory disease cases caused by Chlamydia trachomatis: consistent picture from different methods. J Infect Dis. 2016;214(4):617–624. doi: 10.1093/infdis/jiw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis. 2010;201(Suppl 2):S134–S155. doi: 10.1086/652395. [DOI] [PubMed] [Google Scholar]
- 15.Adachi K N, Nielsen-Saines K, Klausner J D (2021) Chlamydia trachomatis Screening and Treatment in Pregnancy to Reduce Adverse Pregnancy and Neonatal Outcomes: A Review. Front Public Health 9:531073. 10.3389/fpubh.2021.531073 [DOI] [PMC free article] [PubMed]
- 16.Malhotra M, Sood S, Mukherjee A, Muralidhar S, Bala M (2013) Genital Chlamydia trachomatis: an update. Indian J Med Res 138(3):303–316 [PMC free article] [PubMed]
- 17.Mohseni M, Sung S, Takov V. Chlamydia. (2021) In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
- 18.Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect. 2002;78(2):90–92. doi: 10.1136/sti.78.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villareal C, Whittum-Hudson JA, Hudson AP. Persistent Chlamydiae and chronic arthritis. Arthritis Res Ther. 2002;4(1):1–5. doi: 10.1186/ar376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rupp J, Solbach W, Gieffers J. Variation in the mutation frequency determining quinolone resistance in Chlamydia trachomatis serovars L2 and D. J Antimicrob Chemother. 2008;61(1):91–94. doi: 10.1093/jac/dkm447. [DOI] [PubMed] [Google Scholar]
- 21.Dreses-Werringloer U, Padubrin I, Köhler L, Hudson AP. Detection of nucleotide variability in rpoB in both rifampin-sensitive and rifampin-resistant strains of Chlamydia trachomatis. Antimicrob Agents Chemother. 2003;47(7):2316–2318. doi: 10.1128/AAC.47.7.2316-2318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkey PM. The origins and molecular basis of antibiotic resistance. BMJ. 1998;317(7159):657–660. doi: 10.1136/bmj.317.7159.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benamri I, Azzouzi M, Sanak K, Moussa A, Radouani F. An overview of genes and mutations associated with Chlamydiae species’ resistance to antibiotics. Ann Clin Microbiol Antimicrob. 2021;20(1):59. doi: 10.1186/s12941-021-00465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutlin A, Kohlhoff S, Roblin P, Hammerschlag MR, Riska P. Emergence of resistance to rifampin and rifalazil in Chlamydophila pneumoniae and Chlamydia trachomatis. Antimicrob Agents Chemother. 2005;49(3):903–907. doi: 10.1128/AAC.49.3.903-907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suchland RJ, Bourillon A, Denamur E, Stamm WE, Rothstein DM. Rifampin-resistant RNA polymerase mutants of Chlamydia trachomatis remain susceptible to the ansamycin rifalazil. Antimicrob Agents Chemother. 2005;49(3):1120–1126. doi: 10.1128/AAC.49.3.1120-1126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Y, Zhu H, Yang LN, Liu YJ, Hou SP, Qi ML, et al. Differences in 23S ribosomal RNA mutations between wild-type and mutant macrolide-resistant Chlamydia trachomatis isolates. Exp Ther Med. 2015;10(3):1189–1193. doi: 10.3892/etm.2015.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deguchi T, Hatazaki K, Ito S, Kondo H, Horie K, Nakane K, et al. Macrolide and fluoroquinolone resistance is uncommon in clinical strains of Chlamydia trachomatis. J Infect Chemother. 2018;24(8):610–614. doi: 10.1016/j.jiac.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Chen LF, Kaye D. Current use for old antibacterial agents: polymyxins, rifamycins, and aminoglycosides. Med Clin North Am. 2011;95(4):819–8ix. doi: 10.1016/j.mcna.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Kucukkal TG, Petukh M, Li L, Alexov E. Structural and physico-chemical effects of disease and non-disease nsSNPs on proteins. Curr Opin Struct Biol. 2015;32:18–24. doi: 10.1016/j.sbi.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bendl J, Stourac J, Salanda O, Pavelka A, Wieben ED, Zendulka J, et al. PredictSNP: robust and accurate consensus classifier for prediction of disease-related mutations. PLoS Comput Biol. 2014;10(1):1003440. doi: 10.1371/journal.pcbi.1003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31(13):3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30(17):3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;76(1):7–20. doi: 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stone EA, Sidow A. Physicochemical constraint violation by missense substitutions mediates impairment of protein function and disease severity. Genome Res. 2005;15(7):978–986. doi: 10.1101/gr.3804205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capriotti E, Calabrese R, Casadio R. Predicting the insurgence of human genetic diseases associated to single point protein mutations with support vector machines and evolutionary information. Bioinformatics. 2006;22(22):2729–2734. doi: 10.1093/bioinformatics/btl423. [DOI] [PubMed] [Google Scholar]
- 36.Bromberg Y, Rost B. SNAP: predict effect of non-synonymous polymorphisms on function. Nucleic Acids Res. 2007;35(11):3823–3835. doi: 10.1093/nar/gkm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang H, Thomas PD. PANTHER-PSEP: predicting disease-causing genetic variants using position-specific evolutionary preservation. Bioinformatics. 2016;32(14):2230–2232. doi: 10.1093/bioinformatics/btw222. [DOI] [PubMed] [Google Scholar]
- 38.Cheng J, Randall A, Baldi P. Prediction of protein stability changes for single-site mutations using support vector machines. Proteins. 2006;62(4):1125–1132. doi: 10.1002/prot.20810. [DOI] [PubMed] [Google Scholar]
- 39.Bao L, Zhou M, Cui Y. nsSNPAnalyzer: identifying disease-associated nonsynonymous single nucleotide polymorphisms. Nucleic Acids Res. 2005;33(Web Server issue):W480–W482. doi: 10.1093/nar/gki372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanavia T, Birolo G, Montanucci L, Turina P, Capriotti E, Fariselli P. Limitations and challenges in protein stability prediction upon genome variations: towards future applications in precision medicine. Comput Struct Biotechnol J. 2020;18:1968–1979. doi: 10.1016/j.csbj.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.AddCapriotti E, Fariselli P, Casadio R. I-Mutant2.0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005;33(Suppl. 2):306–310. doi: 10.1093/nar/gki375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashkenazy H, Abadi S, Martz E, Chay O, Mayrose I, Pupko T, Ben-Tal N. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016;44(W1):W344–W350. doi: 10.1093/nar/gkw408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010;38:W529–W533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pupko T, Bell RE, Mayrose I, Glaser F, Ben-Tal N. Rate4Site: an algorithmic tool for the identification of functional regions in proteins by surface mapping of evolutionary determinants within their homologues. Bioinformatics. 2002;18(Suppl. 1):S71–S77. doi: 10.1093/bioinformatics/18.suppl_1.S71. [DOI] [PubMed] [Google Scholar]
- 45.Mayrose I, Graur D, Ben-Tal N, Pupko T. Comparison of site-specific rate-inference methods for protein sequences: empirical Bayesian methods are superior. Mol Biol Evol. 2004;21:1781–1791. doi: 10.1093/molbev/msh194. [DOI] [PubMed] [Google Scholar]
- 46.Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46(W1):W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26(2):283–291. doi: 10.1107/S0021889892009944. [DOI] [Google Scholar]
- 48.Zhang Y, Skolnick J. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005;33(7):2302–2309. doi: 10.1093/nar/gki524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 50.Kini RM, Evans HJ. Molecular modeling of proteins: a strategy for energy minimization by molecular mechanics in the AMBER force field. J Biomol Struct Dyn. 1991;9(3):475–488. doi: 10.1080/07391102.1991.10507930. [DOI] [PubMed] [Google Scholar]
- 51.Rao VS, Srinivas K, Sujini GN, Kumar GN. Protein-protein interaction detection: methods and analysis. Int J Proteomics. 2014;2014:147648. doi: 10.1155/2014/147648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(Database issue):D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(D1):D605–D612. doi: 10.1093/nar/gkaa1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohlhoff SA, Hammerschlag MR. Treatment of Chlamydial infections: 2014 update. Expert Opin Pharmacother. 2015;16(2):205–212. doi: 10.1517/14656566.2015.999041. [DOI] [PubMed] [Google Scholar]
- 55.Laura V M, Vladi M H, Vasile S C (2017) Determining the Antibiotic Resistance of Bacterial Pathogens in Sexually Transmitted Diseases. In: Kumavath R N (ed) Antibacterial Agents. IntechOpen. London. 10.5772/67871
- 56.Borel N, Leonard C, Slade J, Schoborg RV. Chlamydial antibiotic resistance and treatment failure in veterinary and human medicine. Curr Clin Microbiol Rep. 2016;3:10–18. doi: 10.1007/s40588-016-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen S, Huo X, Lin Y, Ban H, Lin Y, Li W, et al. Association of MDR1 and ERCC1 polymorphisms with response and toxicity to cisplatin-based chemotherapy in non-small-cell lung cancer patients. Int J Hyg Environ Health. 2010;213(2):140–145. doi: 10.1016/j.ijheh.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Brown DK, Bishop ÖT. Role of structural bioinformatics in drug discovery by computational SNP analysis: analyzing variation at the protein level. Glob Heart. 2017;12(2):151–161. doi: 10.1016/j.gheart.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doss CGP, Rajith B. A new insight into structural and functional impact of single-nucleotide polymorphisms in PTEN gene. Cell Biochem Biophys. 2013;66(2):249–263. doi: 10.1007/s12013-012-9472-9. [DOI] [PubMed] [Google Scholar]
- 60.Bhatnager R, Dang AS. Comprehensive in-silico prediction of damage associated SNPs in human Prolidase gene. Sci Rep. 2018;8(1):9430. doi: 10.1038/s41598-018-27789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pires AS, Porto WF, Franco OL, Alencar SA. In silico analyses of deleterious missense SNPs of human apolipoprotein E3. Sci Rep. 2017;7(1):2509. doi: 10.1038/s41598-017-01737-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pshennikova VG, Barashkov NA, Romanov GP, Teryutin FM, Solov'ev AV, Gotovtsev NN et al (2019) Comparison of predictive in silico tools on missense variants in GJB2, GJB6, and GJB3 genes associated with autosomal recessive deafness 1A (DFNB1A). ScientificWorldJournal:5198931. 10.1155/2019/5198931 [DOI] [PMC free article] [PubMed]
- 63.Periwal N, Rathod SB, Pal R, Sharma P, Nebhnani L, Barnwal RP et al (2021) In silico characterization of mutations circulating in SARS-CoV-2 structural proteins. J Biomol Struct Dyn:1–16. 10.1080/07391102.2021.1908170 [DOI] [PMC free article] [PubMed]
- 64.Remali J, Aizat WM, Ng CL, Lim YC, Mohamed-Hussein ZA, Fazry S. In silico analysis on the functional and structural impact of Rad50 mutations involved in DNA strand break repair. PeerJ. 2020;8:e9197. doi: 10.7717/peerj.9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panja AS, Maiti S, Bandyopadhyay B. Protein stability governed by its structural plasticity is inferred by physicochemical factors and salt bridges. Sci Rep. 2020;10(1):1822. doi: 10.1038/s41598-020-58825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.