Abstract
Background
Ofatumumab is a high-efficacy disease-modifying therapy (DMT) approved for first-line treatment of relapsing-remitting multiple sclerosis (RRMS) in Canada.
Objective
The aim of this study was to evaluate the cost effectiveness of ofatumumab from a Canadian healthcare system perspective.
Methods
A Markov cohort model was run over 65 years using annual cycles, 1.5% annual discount rate, and 100% treatment discontinuation at 10 years. The British Columbia database informed natural history transition probabilities. Treatment efficacy for DMTs were sourced from a network meta-analysis. Clinical trial data were used to estimate probabilities for treatment-related adverse events. Health utilities and costs were obtained from Canadian sources (if available) and the literature.
Results
Among first-line indicated therapies for RRMS, ofatumumab was dominant (more effective, lower costs) over teriflunomide, interferons, dimethyl fumarate, and ocrelizumab. Compared with glatiramer acetate and best supportive care, ofatumumab resulted in incremental cost-effectiveness ratios (ICERs) of $24,189 Canadian dollars per quality-adjusted life-year (QALY) and $28,014/QALY, respectively. At a willingness-to-pay threshold of $50,000/QALY, ofatumumab had a 64.3% probability of being cost effective. Among second-line therapies (scenario analysis), ofatumumab dominated natalizumab and fingolimod and resulted in an ICER of $50,969 versus cladribine.
Conclusions
Ofatumumab is cost effective against all comparators and dominant against all currently approved and reimbursed first-line DMTs for RRMS, except glatiramer acetate.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41669-022-00363-1.
Key Points for Decision Makers
| From a Canadian public healthcare system perspective, ofatumumab is cost effective against all currently approved and reimbursed disease-modifying therapies for the treatment of relapsing-remitting multiple sclerosis (RRMS), and dominant compared with all disease-modifying therapies with a first-line indication, except glatiramer acetate. |
| Ofatumumab’s cost effectiveness, alongside its high efficacy and favorable safety profile, demonstrate its value as an early treatment option in RRMS. |
Introduction
Multiple sclerosis (MS) is a chronic, autoimmune disorder of the central nervous system characterized by inflammation, demyelination, and eventual axonal degeneration resulting in irreversible neurological impairments and disability [1]. About 85% of patients initially experience periodic relapses followed by complete or partial remission, known as relapsing-remitting MS (RRMS) [1]. There is a substantial economic burden of MS to the healthcare system, patients, caregivers, and society because of the chronic progressive disease course and a broad range of symptoms. In addition to high healthcare resource use, MS significantly impacts patient health-related quality of life leading to decreased independence, and productivity loss or unemployment [2].
Management of RRMS involves disease-modifying therapies (DMTs), which aim to reduce relapse frequency and severity, and prevent or decrease disability [1, 3]. In Canada, 16 DMTs are approved for patients with RRMS. Treatment selection is informed by level of disease activity, treatment efficacy, safety and tolerability, and likelihood of adherence [4]. Base therapies (DMTs approved for use as starting treatments) include injectables (glatiramer acetate and interferons) and orals (teriflunomide and dimethyl fumarate) [3]. They are generally considered to have mild to moderate efficacy (average relapse reduction of 30–50%) [4, 5].
In Canada, five DMTs have higher efficacy (average relapse reduction > 50%); the oral fingolimod and cladribine and the injectable monoclonal antibodies, natalizumab, ocrelizumab, and alemtuzumab. Evidence suggests that patients have better disease outcomes (i.e., slower disease progression and reduced long-term disability) when higher efficacy treatments are initiated earlier in the disease course [6]. However, most higher efficacy DMTs are associated with increased risk of adverse events (AEs), limiting treatment to patients with persistent or high initial disease activity due to a suboptimal response to other DMTs [7].
Therapies that selectively deplete B cells have demonstrated improved treatment outcomes in MS [8]. Ocrelizumab is approved in Canada for initial therapy but is often reserved for patients with more aggressive disease [3]. As with other monoclonal antibodies, ocrelizumab is given intravenously; however, its administration is associated with risk of infusion reactions, inconvenience of travelling to infusion clinics, time and resource use required for administration, and added financial burden to patients and the healthcare system. Therefore, there is a high unmet need for an easy-to-administer DMT with high efficacy and a favorable benefit–risk profile that can be used early in the treatment pathway.
Ofatumumab, the first fully human monoclonal anti-CD20 antibody, is approved in Canada for first-line therapy in the treatment of adult patients with RRMS with active disease. A network meta-analysis (NMA) ranked ofatumumab among the most efficacious DMTs with respect to disease progression and reduction in relapse rates [5]. Ofatumumab also has a favorable safety profile as observed in the ASCLEPIOS trials1 [9]; adverse events (AEs) were balanced between ofatumumab and teriflunomide (a widely used first-line DMT), with no unexpected safety findings. In contrast to other monoclonal antibodies which are given intravenously, ofatumumab is a subcutaneous injection that can be self-administered at home, allowing greater patient independence, reduced healthcare resource utilization, and treatment access in areas outside of infusion facilities.
Given the increasing number of available DMTs, alongside the substantial economic burden associated with MS, it is critical to identify which treatment options are cost effective. The current study evaluated the cost effectiveness of ofatumumab against other DMTs and best supportive care (BSC; i.e., no active therapy) for the treatment of patients with RRMS with active disease from a Canadian public healthcare system perspective. A societal perspective was conducted as a scenario analysis.
Methods
Model Structure
A cohort multi-state Markov transition model was developed in Microsoft® Excel® for Microsoft 365 MSO (16.0.14326.20936) 64-bit Version 2018 (Build 14326.20962 Click-to-Run) to predict the costs and effectiveness of treatments with a first-line indication in adults with RRMS; a scenario analysis was conducted to compare against DMTs indicated for second-line use. A global deterministic model was adapted for Canada to include probabilistic sequential analyses [10, 11]. Table 1 presents the probabilistic distributions used for each parameter. The base case probabilistic analysis was conducted from the Canadian public healthcare system perspective and run over a lifetime time horizon (i.e., 65 years) using an annual cycle length and 1.5% annual discount rate for costs and effects [12]. A sensitivity analysis examined the impact of a 25-year time horizon. A half-cycle correction was applied to the calculation of costs (excluding drug costs), life-years, and quality-adjusted life-years (QALYs). Drug costs were excluded from half-cycle corrections as a conservative estimate, as drugs were given at different intervals throughout the year (e.g., 4-weekly or monthly vs annually at the start of a cycle), noting that costs for drugs provided on a 4-weekly or monthly basis may in fact be lower than estimated in the model. The primary outcome was the difference in costs and QALYs gained between ofatumumab and each individual comparator arm, presented as the incremental cost-effectiveness ratio (ICER). A willingness to pay (WTP) threshold of $50,000 Canadian dollars (CAD) per QALY gained was used to assess cost effectiveness [13, 14].
Table 1.
Distribution used for classes of parameters in probabilistic base-case analysis
| Input | Probabilistic distribution |
|---|---|
| Patient proportions per EDSS state | Dirichlet |
| Percentage of males (%) | Beta |
| Age of cohort (years) | Gamma |
| Years since diagnosis | Gamma |
| Efficacy source for disease progression | Log-normal |
| Efficacy source for relapse rate | Log-normal |
| Relapse severity | Dirichlet |
| Discontinuation probabilities | Beta |
| RRMS relapse rate | Log-normal |
| Mortality multiplier for MS | Log-normal |
| Relapse rate (EDSS dependent) | Beta |
| AE probabilities | Beta |
| EDSS health state costs (direct) | Gamma |
| Relapse costs | Gamma |
| AE costs | Gamma |
| EDSS health state utilities | Beta |
| Disutilities for relapse | Beta |
| Disutilities for AEs | Beta |
AE adverse event, EDSS expanded disability status scale, MS multiple sclerosis
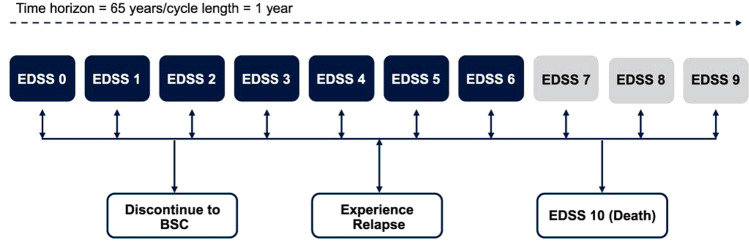
At model entry, patients were assigned to baseline Expanded Disability Status Scale (EDSS) state distributions informed by a pooled analysis of the ASCLEPIOS trials for patients with RRMS with active disease and in line with the Health Canada product monograph indication [9, 15]. A detailed description of baseline patient characteristics has previously been published elsewhere [9]. This study population is representative of Canadian patients treated for RRMS, with respect to the average starting age of treatment (37.8 years) and sex distribution (31.8% males). In the model, patients transitioned between EDSS states 0 through 9, and the death state (EDSS 10) (Fig. 1). Each year, patients could transition to a higher or lower EDSS state (i.e., worsen or improve, respectively), remain at their current state, or die. Patients could also discontinue therapy and switch to health states associated with BSC, with transition probabilities between EDSS states for BSC informed by natural history data from the British Columbia MS (BCMS) database (Table S1 in the electronic supplementary material [ESM]) [16]. This population-based dataset measured changes in EDSS from 1980 to 1995 and was used instead of the London Ontario cohort, which may not reflect present-day MS patients as data were collected from the 1970s to the 1980s [16, 17]. The London dataset also censored any improvement in EDSS states (i.e., backward transitions), resulting in faster progressions through states and underestimations of the cumulative QALYs gained [18]. As such, the BCMS dataset may be more reflective of the current treatment landscape [19], was suggested as the preferred source of natural history transitions [18], and was supported by clinical expert opinion. Furthermore, patients who reached an EDSS score of ≥ 7 while on DMTs would discontinue treatment and receive BSC, in line with Canadian clinical practice and current recommendations from the Association of British Neurologists [7].
Fig. 1.
Model structure. Rounded rectangles on the top row represent health states and rounded rectangles on the bottom row represent events that patients could experience at any time. Patients who reached an EDSS score of ≥ 7 while on treatment would discontinue treatment and receive BSC. BSC best supportive care, EDSS expanded disability status scale
In line with previous economic models, EDSS-dependent natural history annualized relapse rates (ARR) were informed by Patzold and Pocklington, and Orme and colleagues [20–22]. Relapse severity was sourced from a prospective cohort of patients with RRMS [23]; second event relapse severity data were used (mild, 47%; moderate, 35%; severe, 18%) as relapses could occur continually throughout the model.
Age- and sex-specific all-cause mortality rates for the Canadian general population [24] were adjusted for the MS population by using an EDSS-dependent MS-specific hazard ratio (HR) (Table S2 in the ESM) [25].
Treatment-Specific Inputs
For patients receiving DMTs, natural history data were adjusted by a treatment effect derived from an NMA by Samjoo et al. [5], which estimated the comparative efficacy of DMTs versus placebo for 6-month confirmed disability progression (CDP-6) and ARR (Table 2). An analysis for CDP-6 was conducted with the individual patient data from ASCLEPIOS aligned with the definition from OPERA [5, 8]. The base-case analysis assumed no waning of treatment efficacy, as data are limited and the inclusion of waning may be considered as double counting of discontinuations [26]. However, treatment waning was considered in a sensitivity analysis and assumed to be associated with time on treatment; in alignment with previous models, efficacy was reduced to 75% after 3 years and 50% after 5 years [27].
Table 2.
Treatment efficacy and discontinuation probabilities for ofatumumab and comparators versus BSC from the network meta-analysis [5]
| Treatment | 6-Month confirmed disability progression, HR (SE) | Annualized relapse rate, RR (SE) | Discontinuation probabilities, % Risk (SE) |
|---|---|---|---|
| Base-case analysis (first-line therapies) | |||
| Ofatumumab | 0.43 (0.12) | 0.30 (0.05) | 10.64 (0.76) |
| Ocrelizumab | 0.47 (0.16) | 0.33 (0.05) | 10.95 (2.93) |
| Teriflunomide | 0.80 (0.15) | 0.66 (0.06) | 13.12 (1.33) |
| Dimethyl fumarate | 0.68 (0.12) | 0.50 (0.06) | 12.24 (2.28) |
| Glatiramer acetate | 0.78 (0.15) | 0.65 (0.10) | 11.54 (2.23) |
| Avonex | 0.71 (0.12) | 0.79 (0.06) | 13.22 (2.07) |
| Rebif 22 | 0.71 (0.14) | 0.69 (0.09) | 15.44 (2.66) |
| Rebif 44 | 0.77 (0.21) | 0.62 (0.05) | 15.06 (1.69) |
| Betaseron | 0.79 (0.14) | 0.68 (0.07) | 10.54 (2.75) |
| Extavia | 0.79 (0.14) | 0.68 (0.07) | 10.54 (2.75) |
| BSC | 1.00 (0.00) | 1.00 (0.00) | 0.00 (0.00) |
| Scenario-analysis (second-line therapies) | |||
| Cladribine | 0.54 (0.13) | 0.42 (0.07) | 9.02 (3.63) |
| Natalizumab | 0.46 (0.10) | 0.31 (0.05) | 11.25 (3.20) |
| Fingolimod | 0.67 (0.11) | 0.46 (0.05) | 11.44 (2.33) |
BSC best supportive care, HR hazard ratio, RR relative risk, SE standard error
Discontinuation rates for each treatment were calculated using the relative effect estimates from the NMA [5], using the annualized all-cause discontinuation probability of ofatumumab from ASCLEPIOS [9] as a reference arm (Table 2). To capture real-world management of patients with RRMS, the base-case analysis assumed constant discontinuation for 9 years, followed by 100% discontinuation for all treatments at 10 years according to clinician opinion (see Sect. 2.5). A sensitivity analysis examined constant discontinuation for all treatments over the full 65-year time horizon. Given the limited data and high level of uncertainty regarding treatment discontinuation rates for induction therapies, the all-cause discontinuation probability for cladribine (scenario analysis) was adjusted to 16% after 2 years, as previously suggested for modeling other induction therapies [28].
AE probabilities (Table 3) were modeled as non-serious AEs and serious AEs (SAEs). The percent of patients with at least one AE or SAE was sourced from each of the treatments’ pivotal trials, or from a pivotal trial where the treatment was a comparator.
Table 3.
Adverse event probabilities for ofatumumab and comparatorsa
| Treatment | Non-serious AE probabilities (%) (SE) Y1+ | Serious AE probabilities (%) (SE) Y1+ |
|---|---|---|
| Base-case analysis (first-line therapies) | ||
| Ofatumumab | 33.86 (6.77) | 3.68 (0.74) |
| Ocrelizumab | 45.33 (9.07) | 3.79 (0.76) |
| Teriflunomide | 32.39 (6.48) | 3.04 (0.61) |
| Dimethyl fumarate | 55.95 (11.19) | 4.52 (0.90) |
| Glatiramer acetate | 47.14 (9.43) | 4.15 (0.83) |
| Avonex | 45.35 (9.07)b | 4.29 (0.86) |
| Rebif 22 | 45.35 (9.07)b | 4.74 (0.95)b |
| Rebif 44 | 45.35 (9.07) | 4.74 (0.95) |
| Betaseron | 45.35 (9.07)b | 4.10 (0.82) |
| Extavia | 45.35 (9.07)b | 4.10 (0.82) |
| BSC | 0.00 (0.00) | 0.00 (0.00) |
| Scenario analysis (second-line therapies) | ||
| Cladribine | 46.37 (9.27) | 4.81 (0.96) |
| Natalizumab | 41.42 (8.28) | 5.66 (1.13) |
| Fingolimod | 48.88 (9.78) | 7.40 (1.48) |
AE adverse event, BSC best supportive care, SE standard error, Y1+ year 1 onward
aWhen more than one trial for a treatment was available, a weighted average of the AEs and SAEs probabilities were calculated. Annualized rates were calculated by adjusting the probability reported in the respective clinical trial by the total trial length or mean duration of treatment. When the reported probability included MS relapse as an event, the probability was adjusted to avoid double counting of relapses in the model
bData not available; probabilities assumed to be equivalent to those reported for interferon β-1a 44 mg
Cost Inputs
The annual, per-patient direct medical costs (Table 4) for management of EDSS 1–6 were sourced from Grima and colleagues [2]. The remaining EDSS state costs were calculated based on exponential extrapolation from Patwardhan and colleagues [29]. Professional care costs (i.e., costs associated with professional assistance by a nurse, personal assistance, and/or help at home) [30] were added to the total health state costs, as previously suggested [31]. A societal perspective that included MS-associated indirect costs (i.e., informal care, sick leave, and retirement due to MS; Table 4) was conducted as a scenario analysis [30].
Table 4.
Costs by health state
| Health state (EDSS) | Direct medical cost [2, 30] (SE), CAD | Indirect costs [30] (SE), CAD |
|---|---|---|
| 0 | 1268 (254) | 12,110 (2422) |
| 1 | 1908 (382) | 12,110 (2422) |
| 2 | 2590 (518) | 12,110 (2422) |
| 3 | 4218 (844) | 12,110 (2422) |
| 4 | 5837 (1167) | 31,660 (6332) |
| 5 | 9746 (1949) | 31,660 (6332) |
| 6 | 15,143 (3029) | 31,660 (6332) |
| 7 | 36,461 (7292) | 50,153 (10,031) |
| 8 | 43,217 (8643) | 50,153 (10,031) |
| 9 | 52,936 (10,587) | 50,153 (10,031) |
CAD Canadian dollars, EDSS expanded disability status scale, SE standard error
Mild/moderate relapse costs ($7275) were informed by Karampampa and colleagues [30]. Severe relapse costs ($17,459) were extrapolated based on a systematic review [29] that suggested a severe relapse in RRMS costs 240% more than a mild or moderate relapse.
Treatment-related costs included drug acquisition (Table 5), administration and monitoring (Table 6), and AEs (Table 6), with the accrual of costs assumed to apply for the entire time patients received treatment in the model. Drug acquisition costs were sourced from Ontario formularies [32, 33] and the manufacturer anticipated list price for ofatumumab using doses from Canadian product monographs. If there were multiple cost options for a comparator drug, the lowest option was used to represent the most conservative estimate. Administration and monitoring costs were calculated by multiplying the annual resource use (informed by Health Canada product monographs) by the respective unit cost (Table S3 and Table S4 in the ESM). Unit costs were sourced from the Ontario Schedule of Benefits, Ontario Case Costing Initiative, formularies, published literature, and clinician opinion [32–38]. Non-serious AE costs ($84) were assumed to be a physician visit [35], while serious AE costs ($363) were assumed to be an MS Day Case admission (ambulatory care, 18–69 years, Most Responsible Diagnosis Code G35) [34]. All costs were converted to 2021 CAD, using a Canadian Cost Inflation Tool which provided Canadian price index estimates for the health care component of the Consumer Price Index. The tool provided Canadian price index estimates for complete years, as reported by table 18-10-0004-01 of the Consumer Price Index (Health care; monthly, not seasonally adjusted) presented by Statistics Canada [39].
Table 5.
Drug acquisition costsa
| Treatment | Route | Unit cost (CAD) | Recommended dosage | Total cost Y1 (CAD)b | Total cost Y2+ (CAD)b | References |
|---|---|---|---|---|---|---|
| Base-case analysis (first-line therapies) | ||||||
| Ofatumumab | Injection | 2208.33 | 20 mg s.c. at Weeks 0, 1, and 2, then monthly beginning at week 4 | 30,917 | 26,500 | Manufacturer’s submitted price |
| Ocrelizumab | Infusion | 8150.00 | 600 mg i.v. every 6 monthsc | 32,600 | 32,600 | OEAP Formulary [33] |
| Teriflunomide | Oral | 60.25 | 14 mg QD | 22,005 | 22,005 | OEAP Formulary [33] |
| Dimethyl fumarate | Oral |
18.39/120 mg 36.77/240 mg |
120 mg BID; after 7 days increase to 240 mg BID | 26,606 | 26,863 | OEAP Formulary[33] |
| Glatiramer acetate | Injection | 32.40 | 20 mg s.c. QD | 11,834 | 11,834 | ODB Formulary [32] |
| Avonex | Injection | 476.94 | 300 μg i.m. once weekly | 24,886 | 24,886 | OEAP Formulary [33] |
| Rebif 22 | Injection | 150.83 | 22 μg 3 times weekly | 23,610 | 23,610 | OEAP Formulary [33] |
| Rebif 44 | Injection | 183.62 | 44 μg 3 times weekly | 28,743 | 28,743 | OEAP Formulary [33] |
| Betaseron | Injection | 110.00 | 0.25 mg (8 MIU) s.c. every other day | 20,089 | 20,089 | OEAP Formulary[33] |
| Extavia | Injection | 104.69 | 0.25 mg (8 MIU) s.c. every other day | 19,119 | 19,119 | OEAP Formulary [33] |
| BSC | Injection | 0.00 | 0 | 0 | ||
| Scenario analysis (second-line therapies) | ||||||
| Cladribine | Oral | 3212.00 | 1.75 mg/kg body weight per year taken over 2 weeks, for 2 yearsd | 44,968e | 44,968e,f | OEAP Formulary [33] |
| Natalizumab | Infusion | 3596.17 | 300 mg i.v. every 4 weeks | 46,911 | 46,911 | OEAP Formulary [33] |
| Fingolimod | Oral | 73.91 | 0.5 mg QD | 26,996 | 26,996 | ODB Formulary [32] |
BID twice daily, BSC best supportive care, CAD Canadian dollars, i.m. intramuscular, i.v. intravenous, MIU million international units, ODB Ontario Drug Benefit, OEAP Ontario Exceptional Access Program, QD daily, s.c. subcutaneous, Y1 year 1, Y2+ years 2 onward
aDrug dispensing fees or markups were excluded
bAnnual period assumes 52.18 weeks or 365.25 days for all comparators
cThe initial 600-mg dose is administered as two separate i.v. infusions: a 300-mg infusion, followed 2 weeks later by a second 300-mg infusion. Subsequent doses thereafter are administered as single 600-mg i.v. infusions every 6 months
dThe total annual dose per patient is divided into two treatment courses, one at the beginning of the first month and the next at the beginning of the second month of the respective year. Each treatment week consists of 10–20 mg as a single daily dose. For example, a patient weighing 70 kg would take 7 tablets in treatment Weeks 1 and 2 for both year 1 and 2 of the treatment course (14 tablets annually)
eThe cost for cladribine was calculated using the annual cost from the CADTH Canadian Drug Expert Committee Recommendation report for cladribine. The reported annual cost was adjusted to reflect a change in the unit price of cladribine: the submitted unit price was $3082.70; however, the unit price has since been updated to $3212.00 effective May 6, 2021.
fThe cost for cladribine was only applied in year 2 unless the patient was treated with a third dose (25.6% of patients) [19], in which case the cost of cladribine would also be applied to year 3
Table 6.
Drug administration, monitoring, and adverse event costs
| Treatment | Total cost Y1 (CAD) | Total cost Y2+ (CAD) | AE annual costsa (CAD) | References |
|---|---|---|---|---|
| Base-case analysis (first-line therapies) | ||||
| Ofatumumab | 1136 | 38 | 42 | OCCI, Physician and Laboratory Services, nurse time [34–36, 44] |
| Ocrelizumab | 3374 | 1581 | 52 | Drug formulary, OCCI, Physician and Laboratory Services, Tam et al., nurse time [32, 34–38, 44] |
| Teriflunomide | 1196 | 38 | 38 | OCCI, Physician and Laboratory Services, nurse time [34–36, 44] |
| Dimethyl fumarate | 1141 | 74 | 64 | OCCI, Physician and Laboratory Services, nurse time [34–36, 44] |
| Glatiramer acetate | 1125 | 38 | 55 | OCCI, Physician and Laboratory Services, nurse time [34–36, 44] |
| Avonex | 1261 | 84 | 54 | OCCI, Physician and Laboratory Services, nurse time [34–36, 44] |
| Rebif 22 | 1261 | 84 | 56 | OCCI, Physician and Laboratory Services, nurse time [34–36, 44] |
| Rebif 44 | 1261 | 84 | 56 | OCCI, Physician and Laboratory Services, nurse time [34–36, 44] |
| Betaseron | 1631 | 70 | 53 | OCCI, Physician and Laboratory Services, nurse time [34–36, 44] |
| Extavia | 1631 | 70 | 53 | OCCI, Physician and Laboratory Services, nurse time [34–36, 44] |
| BSC | 0 | 0 | 0 | |
| Scenario analysis (second-line therapies) | ||||
| Cladribine | 1158 | 82 | 57 | OCCI, Physician and Laboratory Services, nurse time [34–36, 44] |
| Natalizumab | 6397 | 3681 | 56 | OCCI, Physician and Laboratory Services, Tam et al., nurse time [34–37, 44] |
| Fingolimod | 1682 | 84 | 68 | OCCI, Physician and Laboratory Services, nurse time [34–36, 44] |
AE adverse event, BSC best supportive care, CAD Canadian dollars, OCCI Ontario Case Costing Initiative, Y1 year 1, Y2+ years 2 onward
aAE costs were calculated as total weighted costs based on probability of non-serious and serious events for each comparator
Utilities and Disutilities
Utility data are provided in Table 7. Mean utility values for EDSS health states were derived from normative utility data for the Canadian population (EDSS 0) [40] and the functional form modeled Health Utilities Index Mark 3 (HUI3) utility data (using a parametric function with least squares minimization technique) from a Canadian study of MS patients from Nova Scotia (EDSS 1–9) [41]. Informal caregiver disutilities [42] were examined alongside indirect costs in a scenario analysis for the societal perspective. Relapse disutilities distinguished between mild or moderate and severe relapses, as used in previous economic models [19, 31, 43]. Given the limited literature, AE disutilities were derived based on assumptions and aligned with previous MS economic models [20].
Table 7.
Summary of (dis)utilities
| (Dis)utilities (SE) | References | |
|---|---|---|
| Patient health state utility | ||
| EDSS | ||
| 0 | 0.86 (0.00) | Guertin et al.a [40] |
| 1 | 0.73 (0.15) | Tappenden et al.b [41] |
| 2 | 0.62 (0.12) | Tappenden et al.b [41] |
| 3 | 0.54 (0.11) | Tappenden et al.b [41] |
| 4 | 0.47 (0.09) | Tappenden et al.b [41] |
| 5 | 0.40 (0.08) | Tappenden et al.b [41] |
| 6 | 0.30 (0.06) | Tappenden et al.b [41] |
| 7 | 0.17 (0.03) | Tappenden et al.b [41] |
| 8 | − 0.03 (− 0.01) | Tappenden et al.b [41] |
| 9 | − 0.31 (− 0.06) | Tappenden et al.b [41] |
| Caregiver disutility | ||
| EDSS | ||
| 0 | 0.00 (0.11) | Acaster et al. [42] |
| 1 | 0.00 (0.11) | Acaster et al. [42] |
| 2 | 0.04 (0.11) | Acaster et al. [42] |
| 3 | 0.04(0.11) | Acaster et al. [42] |
| 4 | 0.14 (0.12) | Acaster et al. [42] |
| 5 | 0.16 (0.11) | Acaster et al. [42] |
| 6 | 0.17 (0.11) | Acaster et al. [42] |
| 7 | 0.03 (0.08) | Acaster et al. [42] |
| 8 | 0.09 (0.15) | Acaster et al. [42] |
| 9 | 0.09 (0.15) | Acaster et al. [42] |
| Relapse disutility | ||
| Mild | 0.09 (0.01) | Prosser et al. [43] |
| Moderate | 0.09 (0.01) | Prosser et al. [43] |
| Severe | 0.30 (0.03) | Prosser et al. [43] |
| AE disutility | ||
| Non-serious | 0.00 (0.00) | Assumptionc |
| Serious | 0.04 (0.01) | Assumptiond |
aGiven that the modeled data for EDSS 0 resulted in a utility value that was not clinically plausible, the mean utility value for EDSS 0 was based on normative utility data for the Canadian population
bMean utility values for EDSS health states 1–9 were obtained from the functional form modeled Health Utilities Index Mark 3 (HUI3) utility data in Fig. 1 of Tappenden et al. [41] using PlotDigitizer software
cAssumption that a non-serious AE disutility of 0.25 was experienced for 7 days
dAssumption that a serious AE disutility of 0.5 was applied for 1 month
AE adverse event, EDSS expanded disability status scale, SE standard error
Model Validation
In order to ensure the predictions obtained from this model were accurate to the MS population, the model underwent a validation assessment by an external vendor not involved in the original programming. This assessment included a review of the model scope, structure, assumptions, and calculations. The model also underwent internal validation through a review of all calculations and data inputs. As the model structure is aligned with previous submissions to Canadian Health Technology Assessment agencies, it was assumed that the model structure and approach is valid within the Canadian landscape. Additionally, the appropriateness of Canadian-specific data sources and various clinical assumptions were validated by Canadian clinical experts. Clinician opinion was informed by three clinical experts from varying regions across Canada (Nova Scotia, Ontario, and British Columbia) during three rounds of advisory meetings where model inputs were reviewed and discussed until consensus was reached.
Results
Base-Case Results
Considering DMTs with a first-line indication, ofatumumab dominated (more effective, lower costs) teriflunomide, interferons, dimethyl fumarate, and ocrelizumab; and resulted in ICERs of $24,189 CAD per QALY gained versus glatiramer acetate and $28,014/QALY versus BSC (Table 8, Table S5 and Table S6 in the ESM). The base-case sequential analysis is presented in Table S7 and the efficiency frontier in Fig. S1 of the ESM. Ofatumumab and BSC make up the efficiency frontier; ofatumumab resulted in an ICER of $28,014 versus BSC, which falls below the $50,000/QALY WTP threshold. All other treatments, except glatiramer acetate, are dominated (less effective, higher costs), while glatiramer acetate is extendedly dominated (less effective, lower costs) by ofatumumab. The cost-effectiveness accessibility curve is presented in Fig. S2 in the ESM. At a WTP threshold of $50,000/QALY gained, ofatumumab had the highest probability of being cost effective at 64.3%.
Table 8.
Results of the base-case (first-line therapies) and scenario (second-line therapies) probabilistic analyses
| Treatment | Total cost (CAD) | LY | QALY | Incremental cost of ofatumumab vs comparator (CAD) | Incremental QALY of ofatumumab vs comparator | ICER ($ per QALY) vs ofatumumab |
|---|---|---|---|---|---|---|
| Base case analysis (first-line therapies) | ||||||
| Ofatumumab | 743,015 (739,331–746,700) | 28.406 (28.392–28.420) | 9.277 (9.215–9.339) | |||
| Ocrelizumab | 784,832 (780,987–788,678) | 28.383 (28.369–28.398) | 9.145 (9.077–9.212) | − 41,817 (− 43,364 to − 40,270) | 0.132 (0.091–0.173) | Ofatumumab dominant |
| Teriflunomide | 761,998 (757,775–766,221) | 28.170 (28.155–28.184) | 7.950 (7.894–8.006) | − 18,983 (− 20,410 to − 17,555) | 1.327 (1.293–1.361) | Ofatumumab dominant |
| Dimethyl fumarate | 771,029 (766,988–775,070) | 28.238 (28.224–28.253) | 8.341 (8.284–8.397) | − 28,014 (− 29,340 to − 26,688) | 0.936 (0.903–0.969) | Ofatumumab dominant |
| Glatiramer acetate | 713,474 (709,330–717,619) | 28.190 (28.175–28.205) | 8.056 (7.997–8.115) | 29,541 (28,116 to 30,966) | 1.221 (1.187–1.256) | $24,189 |
| Avonex | 770,188 ($766,116–774,259) | 28.216 (28.202–28.231) | 8.118 (8.062–8.175) | − 27,173 (− 28,451 to − 25,894) | 1.158 (1.127–1.189) | Ofatumumab dominant |
| Rebif 22 | 756,048 (751,998–760,098) | 28.202 (28.187–28.217) | 8.085 (8.028–8.142) | − 13,033 (− 14,393 to − 11,672) | 1.192 (1.158–1.226) | Ofatumumab dominant |
| Rebif 44 | 781,810 (777,612–786,008) | 28.178 (28.163–28.193) | 7.994 (7.936–8.052) | − 38,795 (− 40,362 to − 37,227) | 1.283 (1.244–1.321) | Ofatumumab dominant |
| Betaseron | 759,927 (755,753–764,100) | 28.189 (28.175–28.204) | 8.041 (7.982–8.099) | − 16,911 (− 18,335 to − 15,487) | 1.236 (1.202–1.270) | Ofatumumab dominant |
| Extavia | 755,037 (750,843–759,232) | 28.189 (28.174–28.204) | 8.032 (7.977–8.088) | − 12,022 (− 13,518 to − 10,527) | 1.245 (1.209–1.280) | Ofatumumab dominant |
| BSC | 689,506 (685,203–693,810) | 28.073 (28.059–28.088) | 7.367 (7.317–7.416) | 53,509 (52,339 to 54,679) | 1.910 (1.883–1.937) | $28,014 |
| Scenario analysis (second-line therapies) | ||||||
| Cladribine | 715,734 (711,796–719,671) | 28.311 (28.297–28.325) | 8.742 (8.683–8.800) | 27,282 (25,948 to 28,615) | 0.535 (0.502–0.568) | $50,969 |
| Natalizumab | 869,833 (865,872–873,793) | 28.382 (28.368–28.396) | 9.138 (9.078–9.198) | − 126,818 (− 128,603 to − 125,032) | 0.139 (0.103–0.174) | Ofatumumab dominant |
| Fingolimod | 772,790 (768,805–776,776) | 28.251 (28.236–28.265) | 8.422 (8.364–8.479) | − 29,775 (− 31,093 to − 28,457) | 0.855 (0.822–0.888) | Ofatumumab dominant |
Data are presented as mean (95% confidence interval).
BSC best supportive care, CAD Canadian dollars, ICER incremental cost-effectiveness ratio, LY life-years, QALY quality-adjusted life-year
Scenario Analyses Results
Considering therapies indicated for second-line use, ofatumumab dominated natalizumab and fingolimod, and resulted in an ICER of $50,969 versus cladribine (Table 8). Ofatumumab had a probability of being cost effective of 40.8%. From a societal perspective, ofatumumab was dominant over all DMTs with a first-line indication (Table S8 in the ESM) and had the highest probability of being cost effective at 65.7%. The inclusion of indirect costs increased total costs relative to the base-case analysis, whereas the inclusion of caregiver disutilities reduced net QALYs relative to the base-case analysis.
Sensitivity Analyses Results
Using a 25-year time horizon, results aligned with the base case; ofatumumab resulted in ICERs versus glatiramer acetate ($30,826) and BSC ($34,957) and dominated all other DMTs with a first-line indication (Table S9 in the ESM). Similar to the base case, ofatumumab had the highest probability of being cost effective (59.3%). Constant treatment discontinuation also resulted in ICERs for ofatumumab versus glatiramer acetate ($26,971) and BSC ($29,681) and ofatumumab dominating all other DMTs with a first-line indication (Table S10 in the ESM); ofatumumab had the highest probability of being cost effective at 64.2%. When including efficacy waning (Table S11 in the ESM), ofatumumab resulted in ICERs versus Rebif 22 ($2324), Extavia ($2610), glatiramer acetate ($49,754), and BSC ($55,148) and dominated all remaining DMTs with a first-line indication. Ofatumumab had the third-highest probability of being cost effective (29.5%) behind BSC (37.1%) and glatiramer acetate (30.40%).
Discussion
This analysis is the first to show that ofatumumab is cost effective, and often dominant (more effective, lower costs), compared with currently approved and reimbursed DMTs for RRMS by the Canadian public healthcare system. When considering the broader societal perspective, ofatumumab dominated all DMTs with a first-line indication (i.e., ofatumumab provided more QALYs at a lower cost). Taken together, these findings may help guide treatment decision making for clinicians and their patients and highlight the cost effectiveness of using a high efficacy treatment such as ofatumumab early in the treatment pathway (i.e., as a first-line option).
Considering first-line indicated treatments, the cost effectiveness of ofatumumab is driven not only by the high treatment efficacy, reflected by the largest total QALYs, but also by the reduction in total costs against all comparators except glatiramer acetate and BSC. With the exception of BSC and glatiramer acetate, ofatumumab resulted in predicted cost savings over the lifetime model horizon ranging from $12,022 (vs Extavia) to $41,817 (vs ocrelizumab). The increase in costs associated with ocrelizumab, another high-efficacy B-cell therapy indicated for first-line use, is in part related to the greater healthcare resource utilization associated with intravenous administration in an infusion center. Ofatumumab also resulted in greater incremental QALYs, ranging from 1.327 (vs teriflunomide) to 0.132 (vs ocrelizumab), which could translate into reduced disease burden for the patient. Together, these results suggest that early initiation of treatment with ofatumumab benefits both the patient (i.e., greater QALYs gained) and the healthcare system (i.e., lower total costs). Considering DMTs indicated for second-line use, ofatumumab resulted in predicted cost savings of $29,775 versus fingolimod and $126,818 versus natalizumab, but did not result in cost savings versus cladribine. Like ocrelizumab, the increased costs associated with natalizumab are partly related to requirements for frequent intravenous infusion.
Two approved treatments for RRMS were not considered in this analysis: peginterferon β-1a and alemtuzumab. Comparators for our analysis were selected based on data available in the NMA by Samjoo et al. [5] and relevant treatment sequencing in clinical practice. Peginterferon β-1a was excluded from the NMA based on results indicating greater efficacy than other β-interferons and highly efficacious monoclonal antibodies [5], contrary to clinical experience, as suggested by the NICE technology appraisal committee [26]. Alemtuzumab was excluded due to increased risk of serious and life-threatening safety concerns and is indicated as a third-line DMT limited to patients with highly active disease and inadequate response to at least two other DMTs [7]. Given ofatumumab’s favorable safety profile and first-line indication, comparing ofatumumab and alemtuzumab was deemed irrelevant in the context of treatment sequencing in clinical practice.
The current analysis has limitations common to economic models. Treatment efficacy was modeled based on clinical trial outcomes extrapolated over a lifetime horizon, which introduces uncertainties surrounding the long-term effectiveness of DMTs in RRMS [18]. As such, the base-case analysis set treatment discontinuation to 100% by 10 years to reflect the clinical opinion that the majority of patients are no longer on the same treatment after this time. Although treatment switching often occurs in clinical practice [3], this feature was not included in the present analysis due to a lack of clinical data to inform treatment efficacy for subsequent lines of therapy, an approach that is consistent with previous economic analyses [18, 19]. Alternative scenarios were provided to investigate constant discontinuation over a lifetime horizon, and the impact of a shorter time horizon (25 years). Our analyses also investigated the inclusion of a waning effect that gradually reduced treatment efficacy beyond 2 years, consistent with assumptions used in previous models and HTA recommendations [18, 27]; however, inclusion of waning may be considered as double counting of discontinuations, as previously suggested [26]. Lastly, while some references used to interpret and inform inputs into the model were dated (due to a lack of alternative Canadian-specific date sources), all data used to inform inputs in the model are from commonly used references, represent Canadian data and were appropriately inflated. Results of the sensitivity analyses demonstrated that the cost effectiveness of ofatumumab was generally robust to the various assumptions made, with the inclusion of efficacy waning having the largest impact on the results.
Cross-trial comparisons of discontinuation rates and AE-related outcomes are also subject to limitations [5]. Induction therapies like cladribine tend to have low discontinuation rates due to the predetermined treatment administration schedule. In most cases, patients can no longer discontinue treatment once the induction phase of treatment occurs. As a result, given the small amount of data available and the high level of uncertainty regarding discontinuation rates of cladribine, the NMA-derived all-cause discontinuation probability was applied for years 1 and 2, with a withdrawal rate of 16% thereafter, as suggested in the Canadian Agency for Drugs and Technologies in Health (CADTH) submission for alemtuzumab [28], another 2-year induction therapy. Modeling of treatment-specific AE-related outcomes also poses challenges, including (i) inadequate power to assess safety outcomes, (ii) patients were not blinded to treatment in some pivotal open-label trials, (iii) lack of meaningful understanding of long-term safety profiles, (iv) dissimilar safety profiles between drugs, and (v) infrequent reporting of definitions for AE-related outcomes in clinical trial publications [5]. Accordingly, the present model distinguishes between non-serious and serious AEs in an attempt to capture the majority of AEs observed in clinical practice.
Conclusion
Ofatumumab is cost effective compared with currently approved and reimbursed DMTs for the treatment of patients with RRMS in Canada at a threshold of $50,000 per QALY gained. Given ofatumumab’s high efficacy, favorable risk–benefit profile, easy and accessible route of administration, and demonstrated cost effectiveness, it should be considered a valuable early treatment option for patients with RRMS.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This work was supported by Novartis Pharmaceutical Canada Inc.
Conflict of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SM and FB are employees of Novartis Pharmaceutical Canada Inc. KT, BPP and DG are employees of CRG-EVERSANA Canada Inc., which received funding from Novartis Pharmaceutical Canada Inc. to conduct this analysis. NA is an employee of Novartis International AG. PC is an employee of Novartis Ireland Limited. VB has received compensation for activity with Biogen, BMS, Celgene, EMD Serono, Genzyme, Novartis, Roche, Sanofi, and Teva. MB has received compensation for advisory board/consulting services to Alexion, Biogen, BMS, EMD Serono, Novartis, Pendopharm, Genzyme, Teva Neuroscience, Roche, and Xfacto communications. FC has received compensation for activity with Biogen, BMS, Celgene, EMD Serono, Genzyme, Novartis, Roche, Sanofi, and Teva.
Availability of data and material
The economic model used for analyses is not publicly available at this time.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Author contributions
SM, FB, KT, and DG contributed to the study conception and design. KT, BPP, and DG contributed to data analysis. MB, VB, and FC contributed to clinical interpretation of the data. The first draft of the manuscript was written by KT and BPP and all authors reviewed the manuscript, made substantial contributions to the content, and approved the final draft.
Footnotes
The ASCLEPIOS trials were two double-blind, double-dummy, phase III trials comparing subcutaneous ofatumumab (20 mg every 4 weeks after 20-mg loading doses at days 1, 7, and 14) with oral teriflunomide (14 mg daily) for up to 30 months. In both trials, patients receiving ofatumumab experienced significantly fewer relapses (primary endpoint; annualized relapse rate) relative to patients treated with teriflunomide (p < 0.001 for both). Additional details of the ASCLEPIOS trials have been published elsewhere [9].
Co-first authors: Moogeh Baharnoori, Virender Bhan and Fraser Clift.
References
- 1.Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96–120. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 2.Grima DT, Torrance GW, Francis G, Rice G, Rosner AJ, Lafortune L. Cost and health related quality of life consequences of multiple sclerosis. Mult Scler. 2000;6(2):91–98. doi: 10.1177/135245850000600207. [DOI] [PubMed] [Google Scholar]
- 3.Freedman MS, Devonshire V, Duquette P, Giacomini PS, Giuliani F, Levin MC, et al. Treatment optimization in multiple sclerosis: Canadian MS working group recommendations. Can J Neurol Sci. 2020;47(4):437–455. doi: 10.1017/cjn.2020.66. [DOI] [PubMed] [Google Scholar]
- 4.Samjoo IA, Worthington E, Drudge C, Zhao M, Cameron C, Haring DA, et al. Efficacy classification of modern therapies in multiple sclerosis. J Comp Eff Res. 2021;10(6):495–507. doi: 10.2217/cer-2020-0267. [DOI] [PubMed] [Google Scholar]
- 5.Samjoo IA, Worthington E, Drudge C, Zhao M, Cameron C, Haring DA, et al. Comparison of ofatumumab and other disease-modifying therapies for relapsing multiple sclerosis: a network meta-analysis. J Comp Eff Res. 2020;9(18):1255–1274. doi: 10.2217/cer-2020-0122. [DOI] [PubMed] [Google Scholar]
- 6.He A, Merkel B, Brown JWL, Zhovits Ryerson L, Kister I, Malpas CB, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19(4):307–316. doi: 10.1016/S1474-4422(20)30067-3. [DOI] [PubMed] [Google Scholar]
- 7.Scolding N, Barnes D, Cader S, Chataway J, Chaudhuri A, Coles A, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol. 2015;15(4):273–279. doi: 10.1136/practneurol-2015-001139. [DOI] [PubMed] [Google Scholar]
- 8.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 9.Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383(6):546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 10.Canadian Agency for Drugs and Technologies in Health. Procedures for CADTH reimbursement reviews. https://www.cadth.ca/sites/default/files/Drug_Review_Process/CADTH_Drug_Reimbursement_Review_Procedures.pdf. Accessed 15 June 2022. [PubMed]
- 11.Canadian Agency for Drugs and Technologies in Health Methods and Guidelines. Guidelines for the economic evaluation of health technologies: Canada: 4th edition. https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf. Accessed 15 June 2022.
- 12.Canadian Agency for Drugs and Technologies in Health. Procedures for CADTH reimbursement reviews. 2021. https://cadth.ca/sites/default/files/Drug_Review_Process/CADTH_Drug_Reimbursement_Review_Procedures.pdf. Accessed 20 Apr 2021.
- 13.Black C. Assessing the potential impact of recommendations made through the CADTH Common Drug Review (CDR) program. CADTH Symposium. https://www.cadth.ca/sites/default/files/symp-2019/presentations/april15-2019/B5-presentation-cblack.pdf. Accessed 15 June 2022.
- 14.Griffiths EA, Vadlamudi NK. CADTH’s $50,000 cost-effectiveness threshold: fact or fiction? Value Health. 2016;19:A347–A766. doi: 10.1016/j.jval.2016.09.821. [DOI] [Google Scholar]
- 15.Health Canada. KESIMPTA (ofatumumab injection) Product Monograph. Health Canada Drug Product Database. 2021. https://pdf.hres.ca/dpd_pm/00059817.PDF. Accessed 20 Apr 2021.
- 16.Palace J, Bregenzer T, Tremlett H, Oger J, Zhu F, Boggild M, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open. 2014;4(1):e004073. doi: 10.1136/bmjopen-2013-004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112(Pt 1):133–146. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- 18.Hettle R, Harty G, Wong SL. Cost-effectiveness of cladribine tablets, alemtuzumab, and natalizumab in the treatment of relapsing-remitting multiple sclerosis with high disease activity in England. J Med Econ. 2018;21(7):676–686. doi: 10.1080/13696998.2018.1461630. [DOI] [PubMed] [Google Scholar]
- 19.Chirikov V, Ma I, Joshi N, Patel D, Smith A, Giambrone C, et al. Cost-effectiveness of alemtuzumab in the treatment of relapsing forms of multiple sclerosis in the United States. Value Health. 2019;22(2):168–176. doi: 10.1016/j.jval.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Mauskopf J, Fay M, Iyer R, Sarda S, Livingston T. Cost-effectiveness of delayed-release dimethyl fumarate for the treatment of relapsing forms of multiple sclerosis in the United States. J Med Econ. 2016;19(4):432–442. doi: 10.3111/13696998.2015.1135805. [DOI] [PubMed] [Google Scholar]
- 21.Patzold U, Pocklington PR. Course of multiple sclerosis. First results of a prospective study carried out of 102 MS patients from 1976–1980. Acta Neurol Scand. 1982;65(4):248–66. [DOI] [PubMed]
- 22.Orme M, Kerrigan J, Tyas D, Russell N, Nixon R. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health. 2007;10(1):54–60. doi: 10.1111/j.1524-4733.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- 23.Mowry EM, Carey RF, Blasco MR, Pelletier J, Duquette P, Villoslada P, et al. Multiple sclerosis susceptibility genes: associations with relapse severity and recovery. PLoS ONE. 2013;8(10):e75416. doi: 10.1371/journal.pone.0075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Statistics Canada. Life tables, Canada, provinces and territories, Table 13-10-0114-01 (2017-2019, qx column). 2019. https://www150.statcan.gc.ca/n1/en/catalogue/84-537-X. Accessed 20 Feb 2021.
- 25.Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insur Med. 1997;29(2):101–106. [PubMed] [Google Scholar]
- 26.National Institute for Health and Care Excellence. Ocrelizumab for treating relapsing–remitting multiple sclerosis Technology appraisal guidance [TA533]. 2018 https://www.nice.org.uk/guidance/ta533/resources/ocrelizumab-for-treating-relapsingremitting-multiple-sclerosis-pdf-82606899260869. Accessed 7 Sept 2021.
- 27.Canadian Agency for Drugs and Technologies in Health. CDR pharmacoeconomic review report for Mavenclad. 2018.
- 28.Canadian Agency for Drugs and Technologies in Health. CDR pharmacoeconomic review report for Lemtrada. 2015. https://www.cadth.ca/sites/default/files/cdr/pharmacoeconomic/SR0405_Lemtrada_RRMS_PE_Report.pdf. Accessed 20 May 2020.
- 29.Patwardhan MB, Matchar DB, Samsa GP, McCrory DC, Williams RG, Li TT. Cost of multiple sclerosis by level of disability: a review of literature. Mult Scler. 2005;11(2):232–239. doi: 10.1191/1352458505ms1137oa. [DOI] [PubMed] [Google Scholar]
- 30.Karampampa K, Gustavsson A, Miltenburger C, Kindundu CM, Selchen DH. Treatment experience, burden, and unmet needs (TRIBUNE) in multiple sclerosis: the costs and utilities of MS patients in Canada. J Popul Ther Clin Pharmacol. 2012;19(1):e11–25. [PubMed] [Google Scholar]
- 31.Canadian Agency for Drugs and Technologies in Health. CADTH therapeutic review. Comparative clinical and cost-effectiveness of drug therapies for relapsing-remitting multiple sclerosis [Internet]/. 2013. http://www.cadth.ca/media/pdf/TR0004_RRMS_ScienceReport_e.pdf. Accessed 20 May 2020. [PubMed]
- 32.Government of Ontario. Ontario Drug Benefit Formuarly. 2021. https://www.formulary.health.gov.on.ca/formulary/. Accessed 6 May 2021.
- 33.Government of Ontario. Ontario Exceptional Access Program Formulary. 2021. http://www.health.gov.on.ca/en/pro/programs/drugs/odbf/odbf_except_access.aspx. Accessed 6 May 2021.
- 34.Ontario Case Costing Initiative. Costing analysis tool. 2018. https://hsimi.ca/occp/occpreports/. Accessed 20 Apr 2021.
- 35.Ontario Ministry of Health. Schedule of benefits for laboratory services (effective July 1, 2020). http://www.health.gov.on.ca/en/pro/programs/ohip/sob/lab/lab_mn2020.pdf. Accessed 20 Apr 2021.
- 36.Ontario Ministry of Health. Schedule of benefits, physician services under the health insurance act (effective march 14, 2021). http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20200306.pdf. Accessed 20 Apr 2021.
- 37.Tam VC, Ko YJ, Mittmann N, Cheung MC, Kumar K, Hassan S, et al. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Curr Oncol. 2013;20(2):e90–e106. doi: 10.3747/co.20.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Government of Alberta. Alberta interactive drug benefit list. 2021. https://idbl.ab.bluecross.ca/idbl/load.do. Accessed 20 Apr 2021.
- 39.Statistics Canada. Consumer Price Index [Health care], monthly, not seasonally adjusted; Table 18-10-0004-01. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000401. Accessed 13 Mar 2021.
- 40.Guertin JR, Feeny D, Tarride JE. Age- and sex-specific Canadian utility norms, based on the 2013–2014 Canadian Community Health Survey. CMAJ. 2018;190(6):E155–E161. doi: 10.1503/cmaj.170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tappenden P, McCabe C, Chilcott J, Simpson E, Nixon R, Madan J, et al. Cost-effectiveness of disease-modifying therapies in the management of multiple sclerosis for the Medicare population. Value Health. 2009;12(5):657–665. doi: 10.1111/j.1524-4733.2008.00485.x. [DOI] [PubMed] [Google Scholar]
- 42.Acaster S, Perard R, Chauhan D, Lloyd AJ. A forgotten aspect of the NICE reference case: an observational study of the health related quality of life impact on caregivers of people with multiple sclerosis. BMC Health Serv Res. 2013;13:346. doi: 10.1186/1472-6963-13-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prosser LA, Kuntz KM, Bar-Or A, Weinstein MC. Cost-effectiveness of interferon beta-1a, interferon beta-1b, and glatiramer acetate in newly diagnosed non-primary progressive multiple sclerosis. Value Health. 2004;7(5):554–568. doi: 10.1111/j.1524-4733.2004.75007.x. [DOI] [PubMed] [Google Scholar]
- 44.Government of Canada. Job Bank: Wages for registered nurses and registered psychiatric nurses. 2021. https://www.jobbank.gc.ca/wagereport/occupation/993. Accessed 20 Apr 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.