Abstract
Purpose
We aim to explore if there are any other candidate genetic variants in patients with a history of at least one hydatidiform mole (HM) besides the well-known variants in NLRP7 and KHDC3L.
Methods
The diagnosis of HM type was based on histopathology, and available HM tissues were collected for short tandem repeat (STR) genotyping to verify the diagnosis. DNA extracted from blood samples or decidual tissues of the 78 patients was subjected to whole-exome sequencing (WES).
Results
We identified five novel variants in NLRP7, two novel variants in KHDC3L, and a chromosome abnormality covering the KHDC3L locus among patients with HM. We found that patients with HM who carried heterozygous variants in KHDC3L had a chance of normal pregnancy. We also detected four novel genetic variants in candidate genes that may be associated with HM.
Conclusion
Our study enriched the spectrum of variants in NLRP7 and KHDC3L in Chinese HM patients and provided a new outlook on the effects of heterozygous variants in KHDC3L. The novel candidate genetic variants associated with HMs reported in this study will also contribute to further research on HMs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02592-z.
Keywords: Hydatidiform mole, Whole-exome sequencing, NLRP7, KHDC3L
Introduction
Hydatidiform mole (HM) is an obstetric disease characterized by villous edema and abnormal trophoblastic proliferation, manifesting as a “snowstorm” pattern in ultrasound images and extremely high β‐human chorionic gonadotrophin (β-hCG) levels. The risk of developing a second HM is 1–2% and the chance of a third HM is 15–20%. The occurrence of HM at least twice in the same patient is referred to as recurrent hydatidiform mole (RHM). HMs have been classified histopathologically as complete HMs (CHMs) and partial HMs (PHMs) [1, 2].
Previously, the diagnosis of HM relied majorly on histopathology. The villi in CHM are bulbous, whereas the villi in PHM tend to be large and irregular or small and immature. This kind of classification is very subjective and depends on the experience of clinicians. Recent studies have shown that CHM and PHM can be distinguished by the presence of a maternal contribution, and immunohistochemistry was found to be a feasible and economic way to distinguish them [3]. The cyclin-dependent kinase inhibitor 1C gene (CDKN1C) is located at chromosome 11p5.5 in humans, and its expression pattern is paternally imprinted and maternally expressed [3, 4]. CDKN1C encodes the p57 protein (also known as Kip2), which contains 316 amino acids. p57 is not expressed in CHMs because of the absence of maternal genetic material, whereas p57 is expressed in PHMs in inconsistent expression intensity. Therefore, immunostaining for p57 cannot identify PHMs from nonmolar villi, whereas short tandem repeat (STR) genotyping is able to distinguish them [3, 4]. STR genotyping can distinguish maternal from paternal haploids precisely through STR loci, which are repetitive DNA sequences of about 2–6 bp located in noncoding regions [5]. The earliest application of STR genotyping to HM diagnosis on PubMed was in 1999 [6]; however, although this method is now the golden standard for HM classification, it is still not widely used in clinical practice.
Genetically, most CHMs are caused by fertilization of a haploid sperm or two sperms without maternal haploidy (shown to be diploid), whereas most PHMs are caused by fertilization of a haploid sperm or two sperms with maternal haploidy (shown to be triploid) [2]. A subclassification of CHM named biparental HM (BiHM; also known as family biparental hydatidiform mole) is biparental diploid caused by genetic variation. The causative genes for HM are far from clear. In the OMIM database (https://omim.org), HM-related genes are NLRP7 (nucleotide oligomerization domain (NOD)-like receptor, pyrin containing 7; OMIM 609,661), KHDC3L (KH domain containing 3-like; OMIM 611,687), MEI1 (meiotic double-stranded break formation protein 1; OMIM 608,797), and C11orf80 (chromosome 11 open reading frame 80; OMIM 616,109). The most well-known genes so far are NLRP7 and KHDC3L, which encode proteins that are located on the oocyte cytoskeleton and cortical region and perform functions in human oocytes and preimplantation embryos [7]. KHDC3L and NLRP7, as a component and candidate member, respectively, of the subcortical maternal complex (SCMC), are related to imprinting disorders such as multi-locus imprinting disturbance and RHM [8]. It has been reported that 48–80% of patients with BiHM carry NLRP7 variants and 10–14% of patients with BiHM without NLRP7 variants carry KHDC3L variants [9]. There are 47 NLRP7 variants detected among patients with two defective alleles and 19 NLRP7 variants among patients with a single allele including missense variants, splice variants, stop codons, deletions, and insertions and complex rearrangements [2]. Seven KHDC3L variants have been detected among patients with two defective alleles, including missense variants, splice variants, deletions, and frameshift variants [10, 11], but heterozygous KHDC3L variants have rarely been reported. Other genes that may be related to the pathogenesis of HM have been reported, such as MEI1, C11orf80 [12], and peptidyl arginine deiminase 6 (PADI6) [13], although their roles are far from clear.
Genetic screening in Chinese patients with HM is limited. The spectrum of variants in NLRP7 and KHDC3L among Chinese HM patients is scarce. Two studies of Chinese patients with recurrent and sporadic HMs did not find any pathogenic KHDC3L variants [14, 15], whereas heterozygous KHDC3L variants have been detected in patients with recurrent pregnancy loss [16]. Furthermore, variants in NLRP7 and KHDC3L have been detected only in some patients with HM, implying that more candidate genes are still to be discovered. Two single nucleotide polymorphisms (SNPs) in ERC1 and KCNG4 that may be related to CHM were found in a Chinese population [17], but further studies are required to clarify the association. The genetic causes of HM need to be explored further to guide clinicians in providing reproductive advice that will be effective in reducing the incidence of HM.
Unlike Sanger sequencing, which generates long read sequences, next-generation sequencing can rapidly generate short read sequences, which has advantages in efficiency and cost. Whole-exome sequencing (WES) produces exome sequences that can be used to detect genetic variants in protein-coding sequences [18], which can facilitate genetic screening for diseases and provide input for subsequent reproductive guidance. Therefore, considering the obscure genetic factors associated with HM, we explored candidate genetic variants associated with HM by WES in this study.
We collected 78 patients with a history of at least one HM, of which 47 patients underwent STR genotyping at least once. We discovered novel NLRP7 and KHDC3L variants and a chromosome abnormality that covered the KHDC3L locus. To our knowledge, this is the first report that a chromosome abnormality covering the KHDC3L locus may be associated with HM. We also detected heterozygous KHDC3L variants in patients with HM and a history of normal pregnancy, implying that patients with heterozygous KHDC3L variants can have live births. Moreover, we screened four novel genetic variants in other candidate genes that may be associated with HM.
Materials and methods
Patient enrollment
Our research has been approved by the Peking University Third Hospital Medical Science Research Ethics Committee (2022SZ-004). All the patients and their families signed informed consents after fully informed. Patients who had HM at least once were enrolled in the research. The diagnosis of HM was based on the pathological characters of the products of conceptions (POCs) and clinical manifestations. We also collected the information on the patients’ medical history and family history. Then the blood sample and available POCs were collected. Patients who provided neither a valid blood sample nor available HM POCs for further study were excluded.
In total, 78 patients who had HM at least once were enrolled in this study. Most of them were diagnosed by histopathology at a local hospital. Forty-seven patients were collected based on the diagnosis of POCs at the Peking University Third Hospital.
STR genotyping and immunostaining for p57
For precise diagnosis, we performed STR genotyping of the available HM POCs. Sections from paraffin blocks of HM POCs were stained with hematoxylin and eosin (HE) and evaluated to distinguish between villous and decidual tissues. Immunostaining for p57 was performed using an anti-p57 antibody (Zhongshanjinqiao, China) following the supplier’s protocol to determine the expression levels of p57 in the samples. DNA was extracted separately from 10 serial 5-μm unstained villous and decidual sections using a QIAamp DNA FFPE Tissue Kit (Qiagen, Germany). For some of the patients, maternal DNA was extracted from their blood sample when there was a lack of decidual tissues. The maternal and villous DNA samples were performed STR genotyping using a PowerPlex 16 HS System (Promega, USA) and 3500 DNA Analyzer (Applied Biosystems, USA). The reaction system included polymerase chain reaction (PCR) amplification and capillary electrophoresis of 16 STR loci.
Whole-exome sequencing
The maternal DNA extracted from HM patients was required for whole-exome sequencing and extracted from 1–2 mL peripheral blood samples treated with EDTA anticoagulant. If peripheral blood samples were not available, maternal DNA was separated from decidual tissues from 10 serial 5-μm unstained sections. Blood samples were also collected from available family members for DNA extraction. Then, 1–3 µg of the extracted genomic DNA from the various samples was fragmented to around 300 bp using a Covaris-S220 ultrasonicator (Covaris Inc., USA). DNA fragments were validated with a Nanodrop 2000 spectrophotometer (Thermo Fisher, USA) and Agilent Bioanalyzer 2100 (Agilent, USA) and were then used to construct paired-end sequencing libraries with a Library Preparation Kit (MyGenostics Inc., China). The exon sequences were captured using a GenCap WES Capture Kit (MyGenostics Inc., China) and sequenced on an Illumina NovaSeq 6000 platform (Illumina, USA). After quality control, the sequencing data were aligned to the human reference genome (hg19). SNPs and insertions or deletions (indels) with minor allele frequencies (MAF) < 2% were validated or screened out by searches against the genomAD, 1000 Genome, ESP6500, ExAC_ALL, and ExAC_EAS databases.
Data analysis
The pathogenicity of the validated candidate SNPs and indels in NLRP7 and KHDC3L was analyzed using SIFT, MT, REVEL, PolyPhen-2, and GERP + + . All predicted pathogenic (P), likely pathogenic (LP), and uncertain significance (VUS) variants were annotated according to the American College of Medical Genetics and Genomics (ACMG) standards and guidelines, and the suspicious P, LP, and VUS variants were verified by Sanger sequencing.
All the gene variants identified in the patients with HM were screened using the criteria of variant count ≥ 4 and variant ratio ≥ 0.3. The data obtained were compared with a control group (data of 632 normal females from MyGenostics Inc.), and differential gene variants were screened using the criteria of OR ≥ 1 or ACME = inf, p ≤ 0.05, control = 0, case ≥ 2. All the gene variants were checked according to sequencing reads in IGV, and the suspicious P, LP, VUS variants were selected according to the ACMG standards and guidelines. The selected gene variants were annotated for functional enrichment analysis with specific KEGG pathways and gene ontology (GO) terms that were associated with the occurrence of HM (Table 1).
Table 1.
Selected KEGG pathway and gene ontology (GO) terms for functional enrichment analysis. The genes were selected according to two websites, http://geneontology.org/ and https://www.kegg.jp/. * recurrent genes in different terms were excluded
| KEGG pathway and GO term | Gene numbers |
|---|---|
| Pathway-oocyte meiosis | 122 |
| GO term-meiosis | 51 |
| GO term-imprint | 26 |
| GO term-fertilization | 116 |
| GO term-zona pellucida | 44 |
| GO term-DNA methylation | 64 |
| GO term-histone acetylation | 71 |
| GO term-spermatogenesis | 407 |
| GO term-capacitation | 28 |
| Total numbers | 799* |
Results
Fertility histories of patients with HM and STR genotyping of available HM tissues
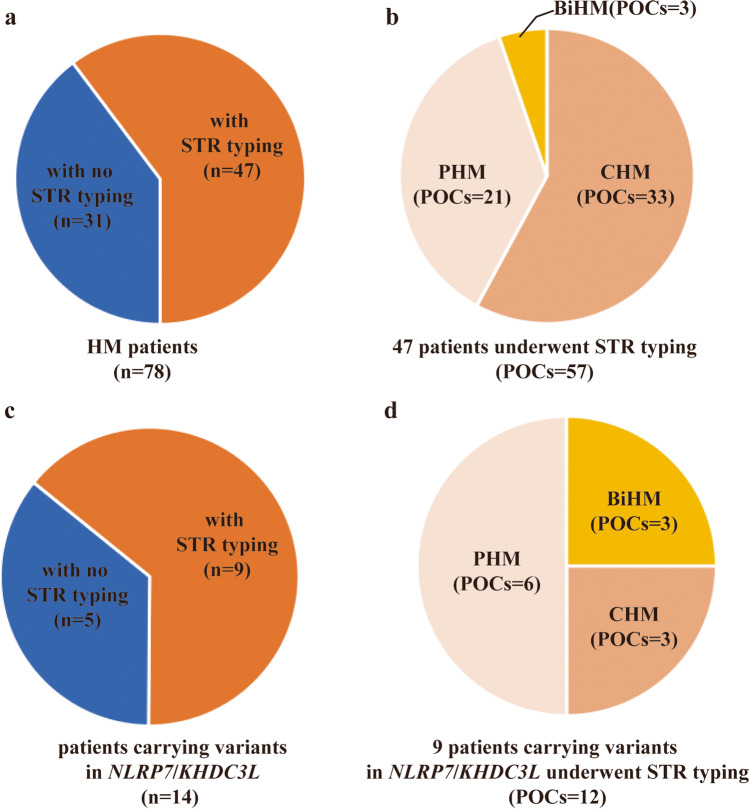
The fertility histories of the 78 unrelated patients in our study with at least one HM are shown in Supplementary Table 1. Among them, 15 patients had HM at least twice, 37 patients had HM only once with no normal pregnancies, and 26 patients had HM at least once and a history of at least one normal pregnancy. Fifty-seven POC tissues from 47 of the patients with HM were STR genotyped (Fig. 1a). Among them, 57.9% (33) of the POCs were identified as CHM, 36.8% (21) were identified as PHM, and 5.3% (3) were identified as BiHM (Fig. 1b).
Fig. 1.
Short tandem repeat (STR) genotyping of products of conception (POC) tissues from patients with hydatidiform mole (HM). a HM patients for which STR genotyping of the POC tissue was performed. b Classification of POCs that were STR genotyped. c HM patients with NLRP7 or KHDC3L variants for which STR genotyping of the POC tissue was performed. d Classification of the POCs of HM patients with NLRP7 or KHDC3L variants
Eleven of the patients carried variants in NLRP7, two patients carried heterozygous variants in KHDC3L, and one patient had a chromosome abnormality that covered the KHDC3L locus. Twelve POC tissues from nine of the patients carrying variants in NLRP7 or KHDC3L are shown in Fig. 1c and d. Among the available POCs of the patients carrying NLRP7 or KHDC3L variants, three were identified as CHM, six were identified as PHM, and three were identified as BiHM. The NLRP7 and KHDC3L variants are shown in Fig. 2, and the pedigree structures of patients with HM with available family members are shown in Fig. 3.
Fig. 2.
Pattern diagrams of the NLRP7 (a) and KHDC3L (b) proteins showing the positions of the variants. Black indicates variants that have been reported previously, and red indicates variants that are reported here for the first time
Fig. 3.
Pedigree structures of four Chinese patients with HM (HM27, HM8, HM28, and HM77) with variants or a chromosome structure abnormality. The genotypes of family members who underwent genetic testing are listed. “ = ” indicates the variant was not detected
Eleven of the patients with HM carried NLRP7 variants
Several NLRP7 variants were detected in the patients with HM in this study. For patient HM27 who twice had arrested intrauterine pregnancies and two BiHMs, the STR genotyping results of one of the HM tissues are shown in Fig. 4a, and the p57 immunostaining was negative in villous cytotrophoblast cells and interstitial cells but positive in extravillous intermediate trophoblast cells (Fig. 4b). Both these results confirmed that the HM tissue was BiHM. We identified a compound heterozygous variant c.1294C > T and c.2471 + 1G > A in NLRP7 (Fig. 4c), and the three-dimensional structure of the protein encoded by this NLRP7 variant was predicted to be disrupted (Fig. 4d). Sanger sequencing verified that both variants were from the parents of patient HM27. One of the variants was a classic splicing variant, and the other was a nonsense variant, and both were classified as pathogenic according to the ACMG guidelines. The other family members had no history of HM apart from one of her sisters, who carried the same genotype as patient HM27 (Fig. 3a). This sister had one HM occurrence after delivery of a healthy boy. Another patient, HM78, carried a homozygous variant c.2161C > T in NLRP7 and had a history of four HMs, one of which was identified as BiHM. She did not have a normal pregnancy, although the pathogenicity was classified as VUS according to the ACMG guidelines.
Fig. 4.
The description of morphology in available POCs of patient HM27 and variant analysis of patient HM27. a Short tandem repeat (STR) genotyping of the HM tissues. b Hematoxylin and eosin (HE) staining and p57 immunostaining of the HM tissues of patient HM27. c Sanger sequences of the NLRP7 variants. d Protein pattern diagram of wild type (WT) and NLRP7 variants of c.2471 + 1G > A and c.1294C > T
We also detected six heterozygous variants in nine patients, including c.376 T > G in patient HM65; c.1137G > C in patients HM8, HM41, and HM62; c.1148C > T in patient HM40; c.1792C > G in patients H49 and HM54; c.2324dupC in patient HM15; and c.2891A > T in patient HM77. The pathogenicity of these heterozygous variants was classified as VUS, except c.2324dupC in patient HM15 was classified as LP. The variants of patient HM8 and patient HM77 were inherited from their father (Fig. 3b and Fig. 3d).
Overall, we identified 11 patients who carried variants in NLRP7; four had a history of RHM and no normal pregnancy, five had a history of only one HM and no normal pregnancy, and two had HM at least once and a history of at least one normal pregnancy. Eleven POCs were available from eight patients carrying variants in NLRP7. STR genotyping of these POCs showed that five were PHM, three were CHM, and only the three POCs from patients HM27 and HM78 were identified as BiHM, both of whom carried biallelic variants of NLRP7.
Three of the patients with HM carried KHDC3L variants
Patient HM28 carried a copy number duplication of about 0.138 Mb on chromosome 6q13 that was inherited from her father (Fig. 3c). The duplication covered five genes, including KHDC3L, and had unknown pathogenicity. This patient had two HM occurrences with no normal pregnancy, and the first HM was followed by gestational trophoblastic neoplasia, although no abnormal pregnancies were recorded among her family members.
Patient HM6 had a history of RHM and was found to carry a heterozygous variant c.456_524del (p.152_175delREAGTQRSVEVQEVGTQGSPVEVQinsR) in KHDC3L that was classified as VUS. This patient conceived naturally and successfully delivered a healthy baby. A heterozygous variant c.460G > C in KHDC3L was found in patient HM37 and classified as VUS. This patient had two normal births before being diagnosed with PHM.
These results show that patients HM6 and HM37, who carried heterozygous variants in KHDC3L, both had normal deliveries. Furthermore, patient HM28, who had a history of two HMs, carried a copy number variant. Among these three patients who carried KHDC3L variants, POCs were available only for patient HM37, and STR genotyping identified the HM as PHM.
Candidate gene variants associated with HM
The 43 differential gene variants between patients with HM and controls are shown in Supplementary Table 2. The selected gene variants were annotated with specific KEGG pathways and GO terms for functional enrichment analysis (Table 1). After excluding synonymous variants and variants in noncoding regions of the genome, we identified four genetic variants that were enriched in the processes of fertilization, spermatogenesis, and histone acetylation (Table 2).
Table 2.
Gene variants enriched in KEGG pathways and gene ontology (GO) terms
| Gene name | Gene ID | Pathway and GO term | Nucleotide changes | Effect |
|---|---|---|---|---|
| SYCP2 | 10,388 | Fertilization | c.439G > A | Nonsynonymous |
| PCDHGA4 | 56,111 | Spermatogenesis | c.1187delT | Frameshift |
| UMODL1 | 89,766 | Fertilization | c.3385_3386insCCAT | Frameshift |
| NOS1 | 4842 | Histone acetylation | c.2557_2559delins TCA | Nonframeshift |
Discussion
In this study, we performed WES on 78 patients with HM, and 57 POCs from 47 of these patients were STR genotyped as CHM, PHM, or BiHM. Among them, 14 patients carried variants of NLRP7 or KHDC3L, and the STR genotyping varied between BiHM, CHM, and PHM, which corresponds to previous studies [19]. BiHM was identified in two patients, HM27 and HM78, both of whom carried biallelic variants of NLRP7 and had no history of normal pregnancy.
Variants in NLRP7 and KHDC3L were detected among the 14 HM patients. Patient HM27 carried a compound heterozygous variant, c.1294C > T and c.2471 + 1G > A in NLRP7, which was reported in 2009 [20]. The patient was predicted to have very little childbearing ability, based on the pathogenicity of the variants, and we inferred that the function of the encoded protein would be severely affected because of the altered protein structure of the variants, as shown in Fig. 4d. Notably, a sister of patient HM27 who had the same genotype delivered a healthy boy from her first pregnancy but was diagnosed with HM in her second pregnancy. We wondered if there was any possibility that the remaining protein of variant c.2471 + 1G > A in NLRP7 retained some function and caused the difference in reproductive outcomes between the two sisters with the same genotype. In contrast, patient HM78, who had a homozygous variant c.2161C > T in NLRP7, had four HM occurrences and no normal pregnancy; this variant was identified as VUS according to the ACMG guidelines, and the variant was reported in 2009 and 2018 [21, 22]. Loss of heterozygosity also needs to be considered. A study that summarized the records of 131 patients with NLRP7 biallelic variants found that only six live births were recorded among 612 pregnancies, giving a live birth rate of about 1% [23]. This finding suggests that even with two defective alleles in NLRP7, live births are possible but rare.
We also found nine HM patients who carried heterozygous variants in NLRP7, and two of them had a history of normal pregnancy. To note, c.376 T > G in patient HM65, c.1148C > T in patient HM40, c.1792C > G in patients HM49 and HM54, c.2324dupC in patient HM15, and c.2891A > T in patient HM77 were first reported in the current study, while c.1137G > C in patients HM8, HM41, and HM62 has been reported previously [20, 24, 25]. The differences between patients with or without a history of normal pregnancy may be explained by the unclear pathogenicity of the variants.
We also detected two novel variants in KHDC3L, namely c.456_524del and c.460G > C. In previous studies, almost all the reported KHDC3L variants were homozygous, and no live births were recorded in HM patients with homozygous KHDC3L variants. Previous reports on heterozygous variants in KHDC3L in HM patients are scarce. We identified two heterozygous variants in KHDC3L in two separate families, and both HM patients with the heterozygous variants conceived naturally and delivered healthy babies. We speculated that although heterozygous variants in KHDC3L can affect female fertility and cause abnormal pregnancies, live births are still possible. Two heterozygous variants in KHDC3L (c.448_480del and c.448_516del) have been reported in patients with recurrent pregnancy loss [16]. These variants are similar to variant c.456_524del in KHDC3L detected in patient HM6 in our study; however, patient HM6 delivered a healthy baby after two HM occurrences. The previous study found that KHDC3L may be associated with homologous recombination-mediated DNA repair and PARP1 activation and indicated that heterozygous KHDC3L variants may have a dominant-negative effect and that damage to embryos caused by these variants may follow an all-or-nothing pattern [16]. This idea is consistent with our finding that patients with heterozygous variants in KHDC3L can have the chance of a live birth. However, the hypothesis needs further verification.
Patient HM28, who had two HM occurrences and no normal pregnancy, carried a duplication of 6q13 (74,072,378–74,210,478) × 3, which covered the KHDC3L locus. This VUS was inherited from her father, and her family members had had no abnormal pregnancies. This duplication covered KHDC3L, OOEP, DDX43, CGAS, and MTO1, of which both KHDC3L and OOEP belong to the SCMC, which exerts function during the development of human embryos [7]. There are no reports on HM occurrences caused by chromosomal duplication, and the mechanism by which chromosomal duplication causes HM is still unclear and needs further investigation.
Some of the HM patients in this study carried no candidate variants in NLRP7 or KHDC3L, which indicated that other genetic factors may contribute to the incidence of HM. We analyzed the WES data of the HM patients in two steps. First, we found that 43 gene variants existed in HM patients through screening the WES data. Second, we selected four variants in SYCP2, NOS1, UMODL1, and PCDHGA4 that were enriched in associated biological processes, such as spermatogenesis, fertilization, or histone acetylation. SYCP2 is part of the synaptonemal complex and has been reported to be associated with male infertility in humans and mice. A previous study reported heterozygous SYCP2 variants in male patients with cryptozoospermia and azoospermia [26]. A study on a mouse model suggested that SYCP2 homozygous variants may cause an abnormality of chromosome synapsis in the process of spermatogenesis and lead to male infertility and showed that female mice with SYCP2 homozygous variants were subfertile with a decreased litter size [27]. NOS1 (also known as nNOS) is an isoform of nitric oxide synthase (NOS) enzyme and can participate in nitric oxide (NO) generation in the nerve system. Meanwhile, NO can impact the state of histone deacetylase 2 (HDAC2) to alter gene expression at an epigenetic level in neurons [28]. UMODL1 is located at chromosome 21q22.3 in humans, and overexpression of UMODL1 led to follicle depletion and ovarian senescence in a mouse model [29]. The GO term indicates that UMODL1 is associated with fertilization, and we speculated that whether it is the function of UMODL1 on the follicle that indirectly impacts fertilization by sperm. PCDHGA4 belongs to the protocadherin gamma gene clusters, and variants have been found in patients with atrial septal defect [30]. The status of PCDHGA4 methylation in the offspring of mothers with type 2 diabetes has been reported to change during pregnancy [31]. The GO term indicated that PCDHGA4 is associated with spermatogenesis; however, related reports are scarce. Because HM is linked to abnormalities in spermatogenesis, fertilization, and epigenetics, it is likely that variants of these four genes have important roles in HM. However, the effects of these gene variants on HM are unclear and require functional verification and elucidation of the molecular mechanisms.
In summary, we detected eight novel variants in NLRP7 and KHDC3L and showed for the first time that patients carrying heterozygous variants in KHDC3L have a chance of normal pregnancy. We also detected novel chromosome abnormalities in the KHDC3L locus that may increase the incidence of HM. Novel genetic variants in four candidate genes have been identified that may be associated with the incidence of HM and deserve further investigation. Our study will enrich the spectrum of variants in HM patients and provides new aspects to the understanding of the etiology of HM.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 The fertility histories of the 78 unrelatedpatients in our study with at least one HM. (DOCX 31.1 KB)
Supplementary file2 The 43 differential gene variants betweenpatients with HM and controls. (DOCX 47.1 KB)
Acknowledgements
We thank all the patients who participated in this study and the clinicians and embryologists of the Center for Reproductive Medicine, Peking University Third Hospital for supporting.
Funding
This work was supported by the National Natural Science Foundation of China (81971440, 81671458), the National Key Research and Development Program of China (2018YFC1002302), and the Leading Academic Discipline Project of Beijing Education Bureau (BMU20110254).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yan Liu, Email: laylaly@126.com.
Xu Zhi, Email: zhixujp@163.com.
References
- 1.Khawajkie Y, et al. Comprehensive analysis of 204 sporadic hydatidiform moles: revisiting risk factors and their correlations with the molar genotypes. Mod Pathol. 2020;33(5):880–892. doi: 10.1038/s41379-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 2.Kalogiannidis I, et al. Recurrent complete hydatidiform mole: where we are, is there a safe gestational horizon? Opinion and mini-review. J Assist Reprod Genet. 2018;35(6):967–973. doi: 10.1007/s10815-018-1202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronnett BM. Hydatidiform moles: ancillary techniques to refine diagnosis. Arch Pathol Lab Med. 2018;142(12):1485–1502. doi: 10.5858/arpa.2018-0226-RA. [DOI] [PubMed] [Google Scholar]
- 4.Stampone E, et al. Genetic and epigenetic control of CDKN1C expression: importance in cell commitment and differentiation, tissue homeostasis and human diseases. Int J Mol Sci. 2018;19(4):1055. [DOI] [PMC free article] [PubMed]
- 5.Wyner N, et al. Forensic autosomal short tandem repeats and their potential association with phenotype. Front Genet. 2020;11:884. [DOI] [PMC free article] [PubMed]
- 6.Bell KA, et al. Molecular genetic testing from paraffin-embedded tissue distinguishes nonmolar hydropic abortion from hydatidiform mole. Mol Diagn. 1999;4(1):11–19. doi: 10.1016/S1084-8592(99)80045-9. [DOI] [PubMed] [Google Scholar]
- 7.Amoushahi M, et al. The pivotal roles of the NOD-like receptors with a PYD domain, NLRPs, in oocytes and early embryo development. Biol Reprod. 2019;101(2):284–296. doi: 10.1093/biolre/ioz098. [DOI] [PubMed] [Google Scholar]
- 8.Bebbere D, et al. The subcortical maternal complex: emerging roles and novel perspectives. Mol Hum Reprod. 2021;27(7):gaab043. [DOI] [PubMed]
- 9.Moein-Vaziri N, et al. Clinical and genetic-epigenetic aspects of recurrent hydatidiform mole: a review of literature. Taiwan J Obstet Gynecol. 2018;57(1):1–6. doi: 10.1016/j.tjog.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Fallahi J, et al. Founder effect of KHDC3L, p.M1V mutation, on Iranian patients with recurrent hydatidiform moles. Iran J Med Sci. 2020;45(2):118–124. [DOI] [PMC free article] [PubMed]
- 11.Wang X, et al. Novel mutations in genes encoding subcortical maternal complex proteins may cause human embryonic developmental arrest. Reprod Biomed Online. 2018;36(6):698–704. doi: 10.1016/j.rbmo.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen NMP, et al. Causative mutations and mechanism of androgenetic hydatidiform moles. Am J Hum Genet. 2018;103(5):740–751. doi: 10.1016/j.ajhg.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian J, et al. Biallelic PADI6 variants linking infertility, miscarriages, and hydatidiform moles. Eur J Hum Genet. 2018;26(7):1007–1013. doi: 10.1038/s41431-018-0141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao W, et al. Absence of KHDC3L mutations in Chinese patients with recurrent and sporadic hydatidiform moles. Cancer Genet. 2013;206(9–10):327–329. doi: 10.1016/j.cancergen.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Ji M, et al. NLRP7 and KHDC3L variants in Chinese patients with recurrent hydatidiform moles. Jpn J Clin Oncol. 2019;49(7):620–627. doi: 10.1093/jjco/hyz036. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, et al. KHDC3L mutation causes recurrent pregnancy loss by inducing genomic instability of human early embryonic cells. PLoS Biol. 2019;17(10):e3000468. doi: 10.1371/journal.pbio.3000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Y, et al. Whole-exome sequencing reveals genetic variants in ERC1 and KCNG4 associated with complete hydatidiform mole in Chinese Han women. Oncotarget. 2017;8(43):75264–75271. doi: 10.18632/oncotarget.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanzi AM, et al. Next generation sequencing and bioinformatics analysis of family genetic inheritance. Front Genet. 2020;11:544162. [DOI] [PMC free article] [PubMed]
- 19.Nguyen NMP, et al. The genetics of recurrent hydatidiform moles: new insights and lessons from a comprehensive analysis of 113 patients. Mod Pathol. 2018;31(7):1116–1130. doi: 10.1038/s41379-018-0031-9. [DOI] [PubMed] [Google Scholar]
- 20.Deveault C, et al. NLRP7 mutations in women with diploid androgenetic and triploid moles: a proposed mechanism for mole formation. Hum Mol Genet. 2009;18(5):888–897. doi: 10.1093/hmg/ddn418. [DOI] [PubMed] [Google Scholar]
- 21.Wang CM, et al. Identification of 13 novel NLRP7 mutations in 20 families with recurrent hydatidiform mole; missense mutations cluster in the leucine-rich region. J Med Genet. 2009;46(8):569–575. doi: 10.1136/jmg.2008.064196. [DOI] [PubMed] [Google Scholar]
- 22.Begemann M, et al. Maternal variants in NLRP and other maternal effect proteins are associated with multilocus imprinting disturbance in offspring. J Med Genet. 2018;55(7):497–504. doi: 10.1136/jmedgenet-2017-105190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akoury E, et al. Live births in women with recurrent hydatidiform mole and two NLRP7 mutations. Reprod Biomed Online. 2015;31(1):120–124. doi: 10.1016/j.rbmo.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Andreasen L, et al. NLRP7 or KHDC3L genes and the etiology of molar pregnancies and recurrent miscarriage. Mol Hum Reprod. 2013;19(11):773–781. doi: 10.1093/molehr/gat056. [DOI] [PubMed] [Google Scholar]
- 25.Messaed C, et al. NLRP7 in the spectrum of reproductive wastage: rare non-synonymous variants confer genetic susceptibility to recurrent reproductive wastage. J Med Genet. 2011;48(8):540–548. doi: 10.1136/jmg.2011.089144. [DOI] [PubMed] [Google Scholar]
- 26.Schilit SLP, et al. SYCP2 translocation-mediated dysregulation and frameshift variants cause human male infertility. Am J Hum Genet. 2020;106(1):41–57. doi: 10.1016/j.ajhg.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang F, et al. Mouse SYCP2 is required for synaptonemal complex assembly and chromosomal synapsis during male meiosis. J Cell Biol. 2006;173(4):497–507. doi: 10.1083/jcb.200603063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nott A, et al. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455(7211):411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, et al. Overexpression of Uromodulin-like1 accelerates follicle depletion and subsequent ovarian degeneration. Cell Death Dis. 2012;3(11):e433. [DOI] [PMC free article] [PubMed]
- 30.Su W, et al. Identification of two mutations in PCDHGA4 and SLFN14 genes in an atrial septal defect family. Curr Med Sci. 2018;38(6):989–996. doi: 10.1007/s11596-018-1974-2. [DOI] [PubMed] [Google Scholar]
- 31.Chen P, et al. Differential methylation of genes in individuals exposed to maternal diabetes in utero. Diabetologia. 2017;60(4):645–655. doi: 10.1007/s00125-016-4203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 The fertility histories of the 78 unrelatedpatients in our study with at least one HM. (DOCX 31.1 KB)
Supplementary file2 The 43 differential gene variants betweenpatients with HM and controls. (DOCX 47.1 KB)