Abstract
Background
With increasing survival after cancer diagnoses, second primary cancers (SPCs) are becoming more prevalent. We investigated the incidence and site of non-breast SPC risks following male breast cancer (BC).
Methods
PubMed, Embase and Web of Science were systematically searched for studies reporting standardised incidence ratios (SIRs) for SPCs published by March 2022. Meta-analyses used the generic inverse-variance method, assuming a random-effects model. We evaluated SIRs for overall SPCs, site-specific risks, by age at BC onset, time since BC onset and geographic region. We assessed study quality using routine techniques.
Results
Eight population-based retrospective cohort studies were identified. SIRs ranged from 1.05 to 2.17. The summary SIR estimate was 1.27 (95% CI: 1.03–1.56, I2: 86%), and there were increased colorectal (SIR: 1.29, 95% CI: 1.03–1.61), pancreatic (SIR: 1.64, 95% CI: 1.05–2.55) and thyroid (SIR: 5.58, 95% CI: 1.04–30.05) SPC risks. When an outlying study was excluded, the summary SIR for men diagnosed with BC before age 50 was 1.50 (95% CI: 1.21–1.85), significantly higher than men diagnosed at older ages (SIR: 1.14, 95% CI: 0.98–1.33).
Conclusions
Male BC survivors are at elevated risks of developing second primary colorectal, pancreatic and thyroid cancers. The estimates may assist their clinical management and guide decisions on genetic testing.
Subject terms: Cancer epidemiology, Epidemiology, Risk factors, Breast cancer
Background
Male breast cancer (BC) is rare, accounting for less than 1% of all BC cases [1, 2]. As a result, few studies have investigated the risks of second primary cancers (SPCs) following male BC [3–12]. SPCs in male BC survivors are a growing health problem. The age-standardised incidence rate of male BC rose by 40% between 1975 and 2015 [13], whereas the age-standardised male BC specific mortality rate decreased by 22.5% and 12.4% between 2002 and 2016 in the European Union and the USA, respectively [14]. Most clinical management guidelines for male BC are extrapolated from information on BC in postmenopausal women [2], so this review could better inform clinical management decisions regarding SPC prevention measures following male BC.
No systematic review of SPC risks following male BC has been performed since 2008 [15]. No meta-analysis of SPC risks following male BC has been carried out to date. We therefore aimed to conduct a significantly updated systematic review (SR) and a novel meta-analysis of SPC risks in male BC survivors. Our objective was to review the latest evidence regarding the risks of developing SPCs following a first invasive primary male BC. A further objective was to assess site-specific second cancer risks among studies that also investigated the overall SPCs risks. Our final objective was to evaluate the variability in non-breast SPC risks by confounding variables, such as patient characteristics.
Methods
Exposure, outcome and measures of association
The exposure was defined as a previous first primary invasive male BC, with no prior cancer history. The outcome was defined as a non-breast SPC.
To minimise misclassification of recurrences or metastases of the first BC as second primaries, SPCs were determined using one of two possible sets of guidelines: those given by the Surveillance, Epidemiology and End Results (SEER) programme [16], primarily used in North America [17] and those given by the International Association of Cancer Registries (IACR)/International Agency for Research on Cancer (IARC) [18, 19], used in all other regions [17]. An explicit statement that SPCs had been confirmed by a physician, with efforts made to differentiate SPCs from recurrences or metastases, was accepted if the guidelines used were unstated.
Second primary BC counts following a first BC are not comparable under the SEER and IACR/IARC guidelines, as observed in a 2014 study of SPC counts [17], as the different guidelines take different approaches to coding SPCs in paired organs [20]. However, the same study [17] found non-breast SPC counts to be almost identical under either set of guidelines. Therefore, only non-breast cancers were considered as SPCs in this review.
The chosen measure of association was the standardised incidence ratio (SIR), which compares the incidence of non-breast SPCs among men with a prior first primary BC to the corresponding expected incidence of non-breast primaries in the general male population.
Data sources and search strategy
PubMed, Embase and Web of Science were each searched for relevant studies on March 11, 2022, using queries described in the Supplementary Material.
Inclusion and exclusion criteria
Studies were considered for inclusion if a SIR and associated standard error could be extracted that assessed the combined risk of non-breast SPCs in male BC survivors, they focussed primarily on adults and they were written in English. A final inclusion criterion was that a study must use IARC/IACR or SEER rules to identify SPCs, or if this was unstated, must state that diagnoses of any SPCs had been confirmed by a physician, with efforts made to differentiate from recurrences or metastases. Studies were excluded if they reported solely on SPC risks following a specific treatment (or lack thereof) of the first male BC, they reported solely on SPC risks following a non-invasive first male BC, or they had a cohort of fewer than 100 male BC survivors.
Studies with data overlapping entirely with another study were also excluded. Partially overlapping studies were included in the SR, although only the larger study was included in any meta-analyses. Data from the Swedish Family Cancer Database were considered to overlap with data from the Swedish national cancer registry due to close links between these resources [21].
Data extraction
Titles and abstracts were screened independently by two authors, with a third author resolving any conflicts. For each study, the first author, publication date, country and centre of data derivation, design, time period, follow-up and definitions of the cohort and of SPCs were extracted, together with additional fields such as stratification details and sample sizes. One author was contacted for clarification. The extracted data were input into a Microsoft Excel table.
Statistical analysis
All statistical analyses were performed in R version 4.1.2 [22]. For each eligible study, the SIR of developing any non-breast SPC following an invasive first primary male BC was extracted as the principal summary measure. Meta-analyses were performed using the random-effects generic inverse-variance method, with DerSimonian–Laird estimators [23, 24]. Standard errors were extracted by dividing the square root of observed non-breast SPC counts by the corresponding expected counts and were converted to the natural logarithm scale by dividing the result by the corresponding SIR [25]. When unreported, expected SPC counts were estimated by dividing observed SPC counts by SIRs. Unreported confidence intervals (CIs) were estimated using Byar’s approximation, assuming observed SPC counts followed a Poisson distribution [25].
We performed unstratified meta-analyses and also stratified by age and time elapsed since the onset of the first BC. The stratification point for age was set at 50 years, although data on men aged up to 60 at BC onset were added into the younger group if no stratification at 50 was provided. We also performed two separate meta-analyses, respectively stratifying at 5 years and 10 years post diagnosis of the first BC. We considered reported SIRs stratified at 9 years equivalent to reported SIRs stratified at 10 years.
We also performed sixteen further meta-analyses, respectively evaluating second cancer risks at the following specific sites: bladder, blood (leukaemia, myeloma and non-Hodgkins lymphoma), brain and central nervous system (CNS), colorectum, head and neck, kidney, liver, lung, oesophagus, pancreas, prostate, skin (melanoma), stomach and thyroid. These are the male-specific subset of the 20 most common cancer sites in the UK from 2016 to 2018, after excluding BC and cancer of unknown primaries [26]. Since the purpose was to examine the distribution of the sites of any observed combined SPC risks, a study providing a SIR and associated standard error of developing cancer at a specific site was included in the corresponding site-specific meta-analysis only if it was also included in the meta-analysis that was unrestricted by SPC site.
We assessed between-study heterogeneity using Cochran’s Q [27] and the I2 statistic [28, 29]. Publication bias was assessed using funnel plots and Egger’s test [30]. A study was regarded as an outlier if there was no overlap between the study-specific and pooled (unstratified) meta-analysis confidence intervals [31]. All meta-analyses were performed first, including, then excluding, outlier studies. The results of all meta-analyses were visually represented as forest plots. Further sensitivity analyses took the form of subgroup analyses testing the effect of the geographical region (continent) of data derivation. Differences between summary SIRs based on multiple different datasets, such as different age groups, were assessed by treating each set of data as a subgroup and comparing the resulting Cochran’s Q to a chi-squared distribution with the degrees of freedom being the number of subgroups minus one [31]. P values of less than 0.05 were deemed significant.
Study quality was assessed using the Newcastle–Ottawa scale (NOS) [32] (details in Supplementary Material).
Results
Results of literature search
The database searches yielded 2011 studies following deduplication, 46 of which were deemed suitable for full-text screening as well as bibliography sweeping. To ensure the capture of all relevant studies, we also swept the bibliographies of 26 studies deemed unsuitable for full-text screening solely due to their focus on female BC survivors. Overall, the bibliography sweeps yielded 33 additional studies for full-text screening. In total, eight studies were included in the SR (Fig. 1).
Fig. 1. Search process.
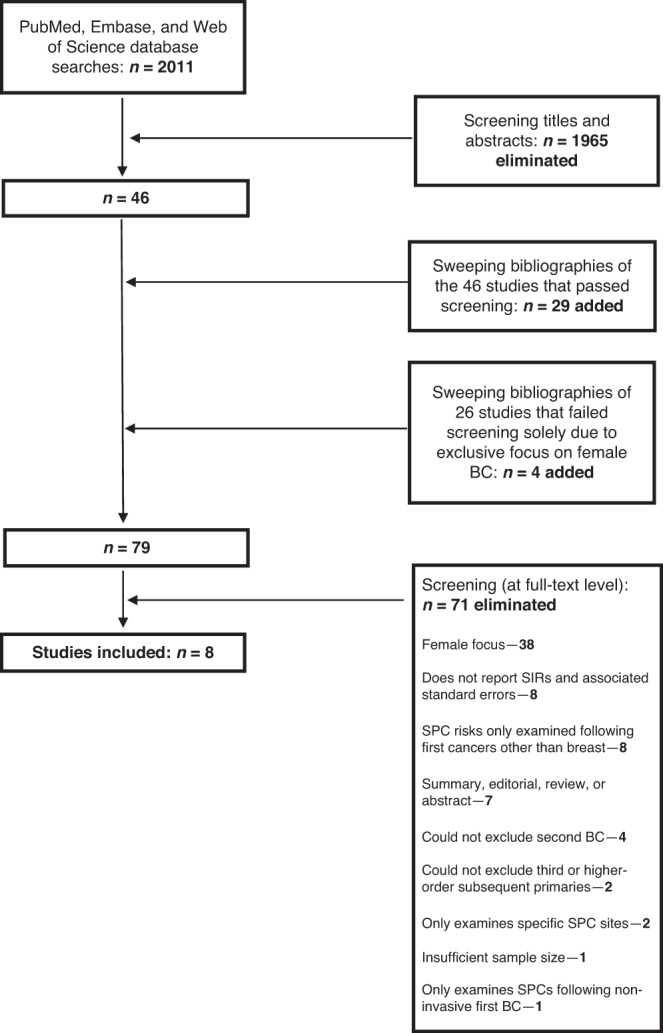
The search process used to identify the studies in this review, as described in the Methods section.
The total number of male BC survivors among the six studies [5–9, 12] which reported sample sizes was 10,038. All studies reported the number of SPCs which developed following male BC, yielding a total of 1451. Six studies [4, 6–9, 12] reported the total follow-up time contributed by their cohort, totalling 36,315 person-years.
All studies were population-based and followed a retrospective cohort design, with follow-up periods lasting between 63 [5] and 13 years [3]. The reported SIRs ranged from 1.05 [8] to 2.17 [7], with the majority lying between 1.05 and 1.34 [3–6, 8, 9, 12].
Further study characteristics are described in Tables 1 and 2. NOS scores may be seen in the Supplementary Material.
Table 1.
Study characteristics.
| Author and publication year | Period of first BC dxa for cohort/end of follow-up (if different) | Study design | Country and centre of data derivation | Definition of cohort | Definition of SPCsb |
|---|---|---|---|---|---|
| AIRTUM Working Group, 2013 [6] | Dx 1976–2010 | Retrospective cohort | Italy (multiple cancer registries covering up to 48% of the population) | All patients dx with a first cancer, although melanoma skin cancer cases, cases based on death certificate only, cases based on autopsy only, and cases with follow-up time equal to zero were excluded. Cohort was stratified by first cancer site, allowing analysis for first BC. | According to IARC/IACRc rules |
| Chen, 2015 [3] | Dx 1997–2010 | Retrospective cohort | Sweden (FCDd) and Germany (12 German cancer registries covering 33% of the population) | Patients aged 15 ye or over at dx of a first primary malignant tumour. Patients with only death certificate/autopsy information were excluded. Cohort was stratified by first cancer site, allowing analysis for first BC. | Germany: According to IARC/IACR rules, not including non-melanoma skin cancer. Sweden: SPC coding rules unstated, but Swedish FCD is linked to the national registry, which uses IARC/IACR rules. Malignancies had to be “clearly separated” to be registered as multiple primaries. |
| Dong, 2001 [9] | Dx 1958–1996 | Retrospective cohort | Sweden—FCD | All patients dx with an invasive cancer as a first primary malignancy that was reported to the Swedish FCD. Cohort was stratified by first cancer site, allowing analysis for first BC. | SPC coding rules unstated, but Swedish FCD is linked to Swedish national cancer registry, which uses IARC/AICR rules. |
| Hemminki, 2005 [5] | All Dx; Australia, New South Wales: 1972–1997, Canada, British Colombia: 1970–1998, Canada, Manitoba: 1970–1998, Canada, Saskatchewan: 1967–1998, Denmark: 1943–1997, Finland: 1953–1998, Iceland: 1955–2000, Norway: 1953–1999, Singapore, Chinese: 1968–1992, Slovenia: 1961–1998, Spain, Zaragoza: 1978–1998, Sweden: 1961–1998, UK, Scotland: 1975–1996 | Retrospective cohort | 13 large cancer registries. Canada (British Columbia, Manitoba and Saskatchewan), Singapore, Slovenia, Norway, Denmark, Scotland, Australia (New South Wales), Sweden, Finland, Iceland, Spain (Zaragoza) | Men dx with a first BC. | According to IARC/IACR rules. Tumours recorded according to the practice of the participating centres. |
| Hung, 2016 [7] | Dx 1997–2010, follow-up until 2011 | Retrospective cohort | Taiwan (Registry of Catastrophic Illness) | Patients dx with a first BC. | SPC coding rules unstated, but the registry histologically confirms cancer cases, and oncologists are required to give evidence of the diagnosis for review by commissioned expert panels. This evidence could include cytology reports, pathology reports, laboratory studies, and imaging studies. |
| Jégu, 2014 [4] | Dx 1989–2004, follow-up until 2007 | Retrospective cohort | France (10 registries covering the Bas-Rhin, Calvados, Doubs, Hérault, Isère, Manche, Somme and Tarn administrative regions) | Patients dx with a first cancer. Cohort was stratified by first cancer site, allowing analysis for first BC. | According to IARC/IACR rules, with second primary cancers occurring at least 2 mf (≥61 days) after a first cancer. |
| Satram-Hoang, 2007 [8] | Dx 1988–2003 | Retrospective cohort | USA—California Cancer Registry | Men aged under 85 dx with first primary BC, registered at California Cancer Registry. | According to SEERg rules. Accepted SPCs had to be malignant, metachronous, and develop at least 2 m post-BC dx. Synchronous SPCs developing before this were excluded. |
| Sung, 2020 [12] | Dx 1992–2011, follow-up until 2017 | Retrospective cohort | USA—12 large cancer registries covering 13% of the USA population (Atlanta (Metropolitan), Connecticut, Detroit (Metropolitan), Hawaii, Iowa, Los Angeles, New Mexico, Rural Georgia, San Francisco (Oakland), San Jose (Monterey), Seattle (Puget Sound), Utah) | Patients aged 20–84 dx with a first primary malignant cancer, who had survived at least 5 years since dx. Cohort was stratified by the first cancer site, allowing analysis for first BC. | According to SEER rules. |
BC breast cancer.
aDiagnosis/diagnoses/diagnosed.
bSecond primary cancer.
cInternational Association of Cancer Registries/International Agency for Research on Cancer.
dFamily cancer database.
eYear/years.
fMonth/months.
gSurveillance, epidemiology and end results.
Table 2.
Further study characteristics and standardised incidence ratio estimates.
| Author and publication year | Total person-years | Follow-up time strata (since first breast cancer diagnosis) | Age strata (at first breast cancer diagnosis) | Specific second primary cancers for which standardised incidence ratios were reported | Number with first breast cancer/number with second primary cancer | Standardised incidence ratio (95% confidence interval) for combined risk of non-breast second primary cancers |
|---|---|---|---|---|---|---|
| AIRTUM Working Group, 2013 [6] | 9402 | 0–1 ma, 2–11 m, 12–59 m, 60–119 m, >120 m | 0–19, 20–29, 30–39, 40–49, 50–69, >70 | Bladder and urinary tract, bone, brain and central nervous system, colon, colon rectum, gallbladder, head and neck, Hodgkin lymphoma, Kaposi sarcoma, kidney and renal pelvis, larynx, leukaemias, liver, lung, lymphoid leukaemia, mesothylioma, multiple myeloma, myeloid leukaemia, non-Hodgkin lymphomas, oesophagus, oral cavity, other leukaemias, other sites, pancreas, pharynx, prostate, rectum, skin melanoma, soft tissue, stomach, testis, thyroid, urinary bladder, urinary tract | 1904/221 | 1.11 (0.97–1.27) |
| Chen, 2015 [3] |
Germany: unreported Sweden: unreported |
Germany: unreported Sweden: unreported |
Germany: unreported Sweden: unreported |
Germany: unreported Sweden: unreported |
Germany: unreported/104 Sweden: unreported/52 |
Germany: 1.1 (0.9–1.3) Sweden: 1.1 (0.8–1.5) |
| Dong, 2001 [9] | 3105 | 0–9 yb, 10–38 y | Unreported | Unreported | 457/50 | 1.22 (0.91–1.61)c |
| Hemminki, 2005 [5] | Unreported | <1 y, 1–9 y, >9 y |
<56, 56–65, 66–74, >75 |
Oral cavity and pharynx, stomach, small intestine, colorectal, colon, rectum, liver (both alone and including gallbladder and bile ducts), pancreas, larynx, lung, melanoma of skin, other neoplasm of skin, prostate, bladder, kidney, lymphohaematopoietic (all lymphomas combined, non-Hodgkins lymphoma, multiple myeloma, and leukaemias (lymphoid leukaemia, myeloid leukaemia)) | 3409/426 | 1.34 (1.22–1.47) |
| Hung, 2016 [7] | 2773 | 0–1 y, 1–5 y, >=4 y | 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, >80 | Head and neck, oesophagus, stomach, colon and rectum and anus, liver and biliary tract, liver, lung and mediastinum, bone and soft tissue, skin, prostate, bladder, kidney, thyroid, haematologic malignancies, all others | 578/73 | 2.17 (1.70–2.73) |
| Jégu, 2014 [4] | 2282 | Unreported | Unreported | Unreported | Unreported/52 | 1.14 (0.85–1.50)c |
| Satram-Hoang, 2007 [8] | 8529 | <1 y, 1–5 y, >5 y | <60 y, 60–69 y, >69 y | Prostate, colorectal, lung and bronchus, bladder, melanoma, stomach | 1986/201 | 1.05 (0.91–1.20) |
| Sung, 2020 [12] | 10224 | Unreported | Unreported | Unreported | 1704/272 | 1.14 (1–1.3) |
aMonth/months.
bYear/years.
cConfidence interval generated using Byar’s approximation.
Results of meta-analyses
To aid the interpretation of the results of the meta-analyses, it should be noted that one study reported two sets of SIRs, including and excluding data from the first 2 months of follow-up [6]. The latter results were described by the study as the more reliable and hence were used in the meta-analyses. In addition, one study pooled their data from multiple centres across four continents [5]. This study was regarded as European for any meta-analyses stratified by geographic region, since the bulk of their data was drawn from European registries.
Unstratified results
The unstratified meta-analysis included six studies [3–8]. Only the German subset of the data used by Chen et al. [3] was included due to the rest of the data partially overlapping with a much larger study [5]. All studies reported an increase in SPC risks following a first primary male BC. Some variation in the reported SIRs was present, with the largest studies reporting estimates between 1.05 and 1.34 [3–6, 8]. The only Asian study was an outlier, reporting a SIR of 2.17 [7]. There was no significant evidence for publication bias (Supplementary Material).
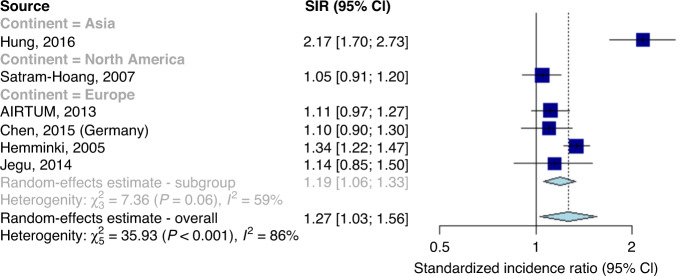
The summary SIR was estimated as 1.27 (95% CI: 1.03–1.56, Fig. 2). Significant heterogeneity was observed (Q: 35.93, I2: 86%, P < 0.001). Significant evidence was found for geographical location affecting summary SIRs (SIR: 2.17, 95% CI: 1.70–2.73 for the Asian study vs 1.19 (1.06–1.33) for European studies vs 1.05 (0.91–1.20) for the North American study, P for difference <0.001).
Fig. 2. Forest plot showing standardised incidence ratios and a pooled estimate of second primary cancer risks.
Association between a first primary male breast cancer and the onset of a non-breast second primary cancer, in comparison to the general male population, including the outlying study by Hung et al.
The study by Hung et al. reported a lower 95% CI bound of 1.70, which was greater than the upper 95% CI bound of 1.56 estimated in the above meta-analysis. Therefore, Hung et al. was regarded as an outlier, and thus all meta-analyses were performed twice: once including, and once excluding, Hung et al. No other outlier studies were present.
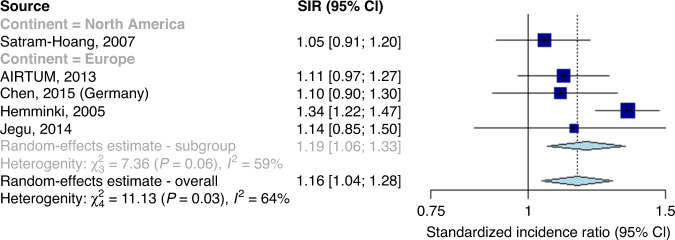
After excluding Hung et al., the summary SIR was estimated as 1.16 (95% CI: 1.04–1.28, Fig. 3). Heterogeneity decreased, but remained significant (Q: 11.13, I2: 64%, P: 0.025). There was no longer significant evidence for a difference in summary SIR by geographical location (Supplementary Material).
Fig. 3. Forest plot showing standardised incidence ratios and a pooled estimate of second primary cancer risks.
Association between a first primary male breast cancer and the onset of a non-breast second primary cancer, in comparison to the general male population, excluding the outlying study by Hung et al.
Whether including or excluding Hung et al., no significant evidence of heterogeneity was found within the continent-specific subgroups (Supplementary Material).
Effects of age at BC onset
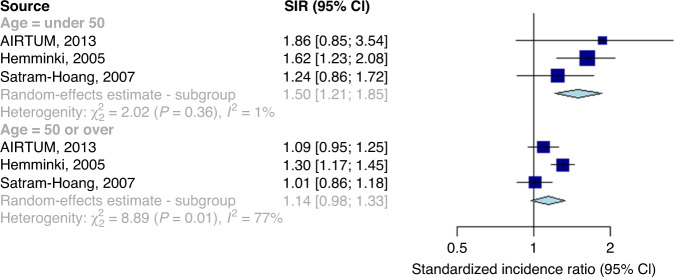
The age-stratified meta-analyses consisted of 4 studies [5–8]. When including Hung et al., we found no significant evidence for a difference between the summary SIR of men aged under 50 at first BC onset and the summary SIR of men aged over 50 at first BC onset (Supplementary Material).
Results when excluding Hung et al. are shown in Fig. 4. There was significant evidence for a difference in summary SIR between the age groups (SIR: 1.50, 95% CI: 1.21–1.85) for those aged under 50 at first BC onset vs. 1.14 (95% CI: 0.98–1.33) for those aged over 50 at first BC onset, P for difference: 0.040).
Fig. 4. Forest plot showing standardised incidence ratios and a pooled estimate of second primary cancer risks, stratified by age group at breast cancer onset.
Association between a first primary male breast cancer and the onset of a non-breast second primary cancer, in comparison to the general male population, stratified by age group at breast cancer onset, excluding the outlying study by Hung et al.
Effects of follow-up time elapsed since BC onset
We found no significant evidence for the length of time elapsed since the onset of the first BC affecting SPC risks (Supplementary Material).
Site-specific associations
Hung et al. provided sufficient data for inclusion in the meta-analyses assessing the risks of SPCs at ten of the examined sites —the bladder, colorectum, head and neck, kidney, liver, lung, pancreas, prostate, stomach and thyroid. Summary SIRs from these meta-analyses ranged from 1.09 to 5.58. Among these sites, the risks of second primaries were significantly higher than the risks for first primaries for colorectal cancer (SIR: 1.29, 95% CI: 1.03–1.61), pancreatic cancer (SIR: 1.64, 95% CI: 1.05–2.55) and thyroid cancer (5.58, 95% CI: 1.04–30.05). Following the exclusion of Hung et al., there was no significant evidence of elevated cancer risks following male BC for any of these ten sites other than the colorectum (SIR: 1.21, 95% CI: 1.00–1.46)), although all associated point estimates were greater than 1. Hung et al. did not provide sufficient data for inclusion in the meta-analyses of SPC risks at the remaining examined sites—the blood (leukaemia, myeloma and non-Hodgkins lymphoma), the brain and CNS, the oesophagus, and the skin (melanoma). The summary SIRs generated for these sites ranged from 1.00 for the blood (non-Hodgkins lymphoma) to 1.65 for the skin (melanoma), with no significant evidence for an increased risk of second primaries at any of these six sites.
Full results may be seen in Supplementary Material.
Discussion
Most published studies reporting SPC risks following male BC draw their data from European [3, 4, 6, 9] or North American [8, 10, 12] population-based cancer registries. The majority reported elevated risks [3–9, 12]. Male BC survivors have often been found to be at greater risk of primary cancers of the prostate [5–7, 10], skin [5, 7, 8, 10] and digestive system [5–8], although with varying magnitudes.
This systematic review and meta-analysis of such studies confirm the combined risks of non-breast SPCs to be significantly elevated following a first primary male BC. When excluding the outlier study by Hung et al., male BC survivors aged under 50 at the initial BC diagnosis were found to be at significantly higher risk than those over 50. This difference in risks may even have been slightly underestimated, due to our decision to include data on men aged under 60 at first BC diagnosis in the younger stratum when no direct stratification at 50 was provided [33]. We also found significant differences between risks reported by studies from Asia, North America and Europe, although larger studies from a wider range of countries are needed to clarify the extent of any risk differences between geographic regions. Finally, we found that male BC survivors are at increased risk of second colorectal, pancreatic and thyroid cancer.
The results of the age-stratified, continent-stratified and site-specific meta-analyses differed depending on whether Hung et al. were included. We therefore discuss the robustness of these results here. Firstly, we found significant evidence for SPC risks varying by geographical region only when including Hung et al. Since Hung et al. was the sole Asian study, this indicates that the difference was driven by this study rather than differences between the European and North American studies. Hung et al. is a well-designed study, using a complete and accurate database [34]. It therefore seems this finding may reflect a higher SPC risk in Asian (specifically Taiwanese) male BC survivors than a flaw in study design.
Although when the Hung et al. study was included in the meta-analysis, there was no significant difference in the SIRs by age at diagnosis, Hung et al. themselves found that men aged under 50 at the first BC diagnosis were at substantially greater SPC risk than men aged over 50 (SIR: 5.68, 95% CI: 1.83–13.26) for those under 50 vs 2.08 (1.61–2.63) for those over 50, p for difference: 0.030). Therefore, the patterns of younger male BC survivors being at greater SPC risk than older male BC survivors, which were seen when Hung et al. was excluded, are consistent.
Significant evidence for increased risks of second primary pancreatic and thyroid cancers was only found when Hung et al. was included in the relevant meta-analyses. The largest study in this review also found the risks of pancreatic SPCs to be significantly elevated [5]. There is also evidence of shared risk factors for male BC and pancreatic cancers. For example, pathogenic BRCA1 [35, 36], BRCA2 [35–37] and PALB2 [37, 38] variants are associated with both male BC and pancreatic cancer. Therefore, the finding that male BC survivors are at increased pancreatic cancer risk seems plausible. In contrast, the finding of increased second primary thyroid cancer risks was mainly driven by data from Hung et al. and was based on a total of just 4 observed cases. Although previous BC has been linked to elevated thyroid cancer risks in women [39], larger studies are needed to clarify this association in men. Finally, it should be noted that Hung et al. reported combined risks of colorectal and anal cancers and of lung and mediastinum cancers. Hence, the point estimates estimated for second colorectal and lung cancers when data from Hung et al. were included may be distorted slightly, although the fact that second colorectal cancer risks remained significant even following the exclusion of these data indicates that second colorectal primary risk is likely to be elevated in male BC survivors.
The strengths of this SR include the number of studies with large sample sizes, considering the rarity of male BC [5, 6, 8, 12]. There was also no significant evidence of publication bias (see Supplementary Material). This SR was built on studies of high methodological quality, with all studies being assigned NOS scores of 6 or higher. Finally, there was limited heterogeneity among European studies, which was the largest continent-specific subset of studies available.
It is known that BC treatments such as chemotherapy, radiotherapy, or hormonal therapy increase SPC risks in women [40–42]. Treatment effects could also partly explain our findings in men. Other non-genetic risk factors which may influence risks for the first primary male BC, such as hormonal imbalances or a family history of male BC [1], may also contribute to the observed elevated SIRs. However, this information was not available in the studies. Notably, in addition to pancreatic cancer, some cancers found to be at greater risk following male BC are also associated with pathogenic variants in genes linked to BC susceptibility in men. For example, both male BC susceptibility [37, 43] and colorectal cancer susceptibility [44, 45] are associated with pathogenic variants in the CHEK2 gene. We also found some evidence of elevated second stomach and prostate cancer risks when including Hung et al., although the associations were not significant (Prostate cancer: SIR: 1.32, 95% CI: 1.00–1.76, P: 0.050. Stomach cancer: SIR: 1.35, 95% CI: 0.99–1.84, P: 0.058). Both cancers are also associated with pathogenic variants in male BC susceptibility genes: prostate cancer with the BRCA1/2 [35, 36, 46] and CHEK2 [47–49] genes and stomach cancer with the BRCA1/2 [35, 36, 50] and CHEK2 [51] genes.
This evidence suggests that SPC risks for BC survivors with a genetic predisposition to BC may be increased in comparison to BC survivors without such a predisposition. Research in this area has been undertaken for contralateral BC in women [52], but is otherwise very scarce. There is some evidence that a higher proportion of male than female BC cases are due to pathogenic variants in BC susceptibility genes [53, 54], with the largest study of germline susceptibility in male BC cases finding 13.7% of male BC survivors to carry such variants [37]. Pathogenic germline variants in BC susceptibility genes could account for a sizeable proportion of second primaries following male BC, with a recent large study confirming non-breast primaries to be 58% more common among male carriers of deleterious BRCA1/2 variants than among male relatives of carriers who were either untested for, or confirmed not to carry, such a variant [35]. Further research in this area may thus be particularly relevant for male BC survivors. Genetic susceptibility could also account for part of the observed association between early-onset male BC and raised SPC risks, since pathogenic variants in such genes are associated with an earlier age at BC diagnosis [55–57]. An additional explanation for this relationship is that more aggressive treatment regimens tend to be offered to younger BC patients [58, 59], but these treatments can confer a higher risk of developing SPCs [40–42].
The study has some limitations. The estimated SIRs may have been affected by surveillance bias, whereby cancers are detected in BC survivors that would have gone unnoticed in individuals without any cancer history due to increased surveillance [6, 60]. However, this was likely reduced by the inclusion of data from four studies [4, 6, 8, 12] excluding SPCs occurring within a time period of at least 2 months immediately following the initial BC diagnosis, where surveillance bias is likely to be most intensive [6]. The paucity of studies reporting effects of treatments of the first male BC [7, 8] and the lack of studies reporting the influence of hormonal imbalances and family histories of male BC also meant that we could not adjust for several potential confounders. The rarity of second cancers at certain sites may also mean some analyses were underpowered, as evidenced by the wide confidence intervals. Therefore, it cannot be concluded that other associations do not exist. It also cannot be ruled out that some relevant published studies were missed, although the double-screening process and the sweeps of reference lists should minimise the likelihood of this.
To our knowledge, this is the first meta-analysis of SPC risks in male BC survivors to have been performed and the first systematic review since 2008. This study provided site-specific SIRs and assessed the variability in the estimates by age at first BC diagnosis, follow-up time and geographical region (continent). Future large cohort studies might consider the effects of BC treatment, family history, or hormonal imbalances, as they receive relatively little focus in the current literature. There is also a clear need for further research on the influence of pathogenic variants in BC susceptibility genes on SPC risks following male BC.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This study was undertaken as part of the CanGene-CanVar research project. The authors thank the associated researchers for their valuable input. MT was supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014).
Author contributions
IA screened the studies as part of the double-screening process, extracted the data, conducted the statistical analyses and wrote the manuscript. HH screened the studies as part of the double-screening process. ES resolved all conflicts arising from the double-screening process. MT, PP and AA all supervised the project and directly edited the manuscript. All authors provided critical feedback to inform the research and analysis.
Funding
This work was funded by the CRUK Catalyst Award CanGene-CanVar (C61296/A27223).
Data availability
The data from the study by the AIRTUM Working Group are publicly available at I tumori in Italia. Rapporto 2013: I tumori multipli | Epidemiologia&Prevenzione (epiprev.it). All data from the remaining studies were taken directly from their corresponding published, publicly available manuscripts or Supplementary Materials.
Code availability
All codes used to generate the results in this manuscript can be provided upon request.
Ethics approval and consent to participate
Not applicable; no human participants, human data or human tissue was used in the production of this article.
Consent to publish
Not applicable; no individual person’s data were used in the production of this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01940-1.
References
- 1.Abdelwahab Yousef AJ. Male breast cancer: epidemiology and risk factors. Semin Oncol. 2017;44:267–72. doi: 10.1053/j.seminoncol.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med. 2002;137:678–87. doi: 10.7326/0003-4819-137-8-200210150-00013. [DOI] [PubMed] [Google Scholar]
- 3.Chen T, Fallah M, Jansen L, Castro FA, Krilavicuite A, Katalinic A, et al. Distribution and risk of the second discordant primary cancers combined after a specific first primary cancer in German and Swedish cancer registries. Cancer Lett. 2015;369:152–66. doi: 10.1016/j.canlet.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Jégu J, Colonna M, Daubisse-Marliac L, Trétarre B, Ganry O, Guizard AV, et al. The effect of patient characteristics on second primary cancer risk in France. BMC Cancer. 2014;14:94. doi: 10.1186/1471-2407-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemminki K, Scélo G, Boffetta P, Mellemkjaer L, Tracey E, Andersen A, et al. Second primary malignancies in patients with male breast cancer. Br J Cancer. 2005;92:1288–92. doi: 10.1038/sj.bjc.6602505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AIRTUM Working Group. Italian cancer figures, report 2013: multiple tumours. Epidemiol Prev. 2013;37(4–5 Suppl 1):1–152. [PubMed]
- 7.Hung MH, Liu CJ, Teng CJ, Hu YW, Yeh CM, Chen SC, et al. Risk of second non-breast primary cancer in male and female breast cancer patients: a population-based cohort study. PLoS ONE. 2016;11:e0148597. doi: 10.1371/journal.pone.0148597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satram-Hoang S, Ziogas A, Anton-Culver H. Risk of second primary cancer in men with breast cancer. Breast Cancer Res. 2007;9:R10. doi: 10.1186/bcr1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong C, Hemminki K. Second primary neoplasms in 633,964 cancer patients in Sweden, 1958-1996. Int J Cancer. 2001;93:155–61. doi: 10.1002/ijc.1317. [DOI] [PubMed] [Google Scholar]
- 10.Auvinen A, Curtis RE, Ron E. Risk of subsequent cancer following breast cancer in men. J Natl Cancer Inst. 2002;94:1330–2. doi: 10.1093/jnci/94.17.1330. [DOI] [PubMed] [Google Scholar]
- 11.Mangone L, Ferrari F, Mancuso P, Carrozzi G, Michiara M, Falcini F, et al. Epidemiology and biological characteristics of male breast cancer in Italy. Breast Cancer. 2020;27:724–31. doi: 10.1007/s12282-020-01068-1. [DOI] [PubMed] [Google Scholar]
- 12.Sung H, Hyun N, Leach CR, Yabroff KR, Jemal A. Association of first primary cancer. with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. J Am Med Assoc. 2020;324:2521–35. doi: 10.1001/jama.2020.23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Shu X, Meszoely I, Pal T, Mayer IA, Yu Z, et al. Overall mortality after diagnosis of breast cancer in men vs women. JAMA Oncol. 2019;5:1589–96. doi: 10.1001/jamaoncol.2019.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pizzato M, Carioli G, Bertuccio P, Malvezzi M, Levi F, Boffetta P, et al. Trends in male breast cancer mortality: a global overview. Eur J Cancer Prev. 2021;30:472–9. doi: 10.1097/CEJ.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 15.Grenader T, Goldberg A, Shavit L. Second cancers in patients with male breast cancer: a literature review. J Cancer Surviv. 2008;2:73–8. doi: 10.1007/s11764-008-0042-5. [DOI] [PubMed] [Google Scholar]
- 16.Adamo M, Groves C, Dickie L, Ruhl J. SEER program coding and staging manual 2021. National Cancer Institute, Bethesda, MD 20892: U.S. Department of Health and Human Services National Institutes of Health National Cancer Institute; 2021.
- 17.Coyte A, Morrison DS, McLoone P. Second primary cancer risk—the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer. 2014;14:272. doi: 10.1186/1471-2407-14-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Working Group Report. International rules for multiple primary cancers (ICD-0 third edition) Eur J Cancer Prev. 2005;14:307–8. doi: 10.1097/00008469-200508000-00002. [DOI] [PubMed] [Google Scholar]
- 19.International Association of Cancer Registries. International rules for multiple primary cancers. Asian Pac J Cancer Prev. 2005;6:104–6. [PubMed]
- 20.Weir HK, Johnson CJ, Ward KC, Coleman MP. The effect of multiple primary rules on cancer incidence rates and trends. Cancer Causes Control. 2016;27:377–90. doi: 10.1007/s10552-016-0714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemminki K, Ji J, Brandt A, Mousavi SM, Sundquist J. The Swedish family-cancer database 2009: prospects for histology-specific and immigrant studies. Int J Cancer. 2010;126:2259–67. doi: 10.1002/ijc.24795. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2021. https://www.R-project.org/.
- 23.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Chichester (UK): Wiley; 2019.
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Breslow NE, Day NE. Statistical methods in cancer research. Volume II-The design and analysis of cohort studies. IARC Sci Publ. 1987;2:1–406. [PubMed]
- 26.Cancer Research UK. Cancer incidence for common cancers. https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/common-cancers-compared#heading-Zero, 2021.
- 27.Cooper H, Hedges LV. The handbook of research synthesis. New York: Russell Sage Foundation; 1994.
- 28.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis with R. Boca Raton: Chapman and Hall/CRC; 2021.
- 32.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, 2012.
- 33.Molina-Montes E, Requena M, Sánchez-Cantalejo E, Fernández MF, Arroyo-Morales M, Espín J, et al. Risk of second cancers cancer after a first primary breast cancer: a systematic review and meta-analysis. Gynecol Oncol. 2015;136:158–71. doi: 10.1016/j.ygyno.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Kao WH, Hong JH, See LC, Yu HP, Hsu JT, Chou IJ, et al. Validity of cancer diagnosis in the National Health Insurance database compared with the linked National Cancer Registry in Taiwan. Pharmacoepidemiol Drug Saf. 2018;27:1060–6. doi: 10.1002/pds.4267. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Silvestri V, Leslie G, Rebbeck TR, Neuhausen SL, Hopper JL, et al. Cancer risks associated with BRCA1 and BRCA2 pathogenic variants. J Clin Oncol. 2022;40:JCO2102112. [DOI] [PMC free article] [PubMed]
- 36.Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22:735–42. doi: 10.1200/JCO.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 37.Pritzlaff M, Summerour P, McFarland R, Li S, Reineke P, Dolinsky JS, et al. Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat. 2017;161:575–86. doi: 10.1007/s10549-016-4085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X, Leslie G, Doroszuk A, Schneider S, Allen J, Decker B, et al. Cancer risks associated with germline PALB2 pathogenic variants: an international study of 524 families. J Clin Oncol. 2020;38:674–85. doi: 10.1200/JCO.19.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen SM, White MG, Hong S, Aschebrook-Kilfoy B, Kaplan EL, Angelos P, et al. The breast-thyroid cancer link: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2016;25:231–8. doi: 10.1158/1055-9965.EPI-15-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bazire L, de Rycke Y, Asselain B, Fourquet A, Kirova YM. Risks of second malignancies after breast cancer treatment: Long-term results. Cancer Radiotherapie: J de la Soc francaise de radiotherapie oncologique. 2017;21:10–5. doi: 10.1016/j.canrad.2016.07.101. [DOI] [PubMed] [Google Scholar]
- 41.Kirova YM, de Rycke Y, Gambotti L, Pierga JY, Asselain B, Fourquet A, et al. Second malignancies after breast cancer: the impact of different treatment modalities. Br J Cancer. 2008;98:870–4. doi: 10.1038/sj.bjc.6604241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakami R, Hiyama T, Hanai A, Fujimoto I. Second primary cancers following female breast cancer in Osaka, Japan-a population-based cohort study. Jpn J Clin Oncol. 1987;17:293–302. [PubMed] [Google Scholar]
- 43.Liang M, Zhang Y, Sun C, Rizeq FK, Min M, Shi T, et al. Association between CHEK2*1100delC and breast cancer: a systematic review and meta-analysis. Mol Diagn Ther. 2018;22:397–407. doi: 10.1007/s40291-018-0344-x. [DOI] [PubMed] [Google Scholar]
- 44.de Jong MM, Nolte IM, te Meerman GJ, van der Graaf WTA, Mulder MJ, van der Steege G, et al. Colorectal cancer and the CHEK2 1100delC mutation. Genes Chromosomes Cancer. 2005;43:377–82. doi: 10.1002/gcc.20195. [DOI] [PubMed] [Google Scholar]
- 45.Cybulski C, Wokołorczyk D, Kładny J, Kurzawski G, Kurzwaski G, Suchy J, et al. Germline CHEK2 mutations and colorectal cancer risk: different effects of a missense and truncating mutations? Eur J Hum Genet. 2007;15:237–41. doi: 10.1038/sj.ejhg.5201734. [DOI] [PubMed] [Google Scholar]
- 46.Oh M, Alkhushaym N, Fallatah S, Althagafi A, Aljadeed R, Alsowaida Y, et al. The association of BRCA1 and BRCA2 mutations with prostate cancer risk, frequency, and mortality: a meta-analysis. Prostate. 2019;79:880–95. doi: 10.1002/pros.23795. [DOI] [PubMed] [Google Scholar]
- 47.Southey MC, Goldgar DE, Winqvist R, Pylkäs K, Couch F, Tischkowitz M, et al. PALB2, CHEK2 and ATM rare variants and cancer risk: data from COGS. J Med Genet. 2016;53:800–11. doi: 10.1136/jmedgenet-2016-103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seppälä EH, Ikonen T, Mononen N, Autio V, Rökman A, Matikainen MP, et al. CHEK2 variants associate with hereditary prostate cancer. Br J Cancer. 2003;89:1966–70. doi: 10.1038/sj.bjc.6601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong X, Wang L, Taniguchi K, Wang X, Cunningham JM, McDonnell SK, et al. Mutations in CHEK2 associated with prostate cancer risk. Am J Hum Genet. 2003;72:270–80. doi: 10.1086/346094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlebusch CM, Dreyer G, Sluiter MD, Yawitch TM, van den Berg HJ, van Rensburg EJ. Cancer prevalence in 129 breast-ovarian cancer families tested for BRCA1 and BRCA2 mutations. S Afr Med J. 2010;100:113–7. doi: 10.7196/SAMJ.3235. [DOI] [PubMed] [Google Scholar]
- 51.Teodorczyk U, Cybulski C, Wokołorczyk D, Jakubowska A, Starzyńska T, Lawniczak M, et al. The risk of gastric cancer in carriers of CHEK2 mutations. Fam Cancer. 2013;12:473–8. doi: 10.1007/s10689-012-9599-2. [DOI] [PubMed] [Google Scholar]
- 52.Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Am Med Assoc. 2017;317:2402–16. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 53.Silva SN, Gomes BC, André S, Félix A, Rodrigues AS, Rueff J. Male and female breast cancer: the two faces of the same genetic susceptibility coin. Breast Cancer Res Treat. 2021;188:295–305. doi: 10.1007/s10549-021-06159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bevier M, Sundquist K, Hemminki K. Risk of breast cancer in families of multiple affected women and men. Breast Cancer Res Treat. 2012;132:723–8. doi: 10.1007/s10549-011-1915-2. [DOI] [PubMed] [Google Scholar]
- 55.de Sanjosé S, Léoné M, Bérez V, Izquierdo A, Font R, Brunet JM, et al. Prevalence of BRCA1 and BRCA2 germline mutations in young breast cancer patients: a population-based study. Int J Cancer. 2003;106:588–93. doi: 10.1002/ijc.11271. [DOI] [PubMed] [Google Scholar]
- 56.Haffty BG, Choi DH, Goyal S, Silber A, Ranieri K, Matloff E, et al. Breast cancer in young women (YBC): prevalence of BRCA1/2 mutations and risk of secondary malignancies across diverse racial groups. Ann Oncol. 2009;20:1653–9. doi: 10.1093/annonc/mdp051. [DOI] [PubMed] [Google Scholar]
- 57.Cao AY, Huang J, Hu Z, Li WF, Ma ZL, Tang LL, et al. The prevalence of PALB2 germline mutations in BRCA1/BRCA2 negative Chinese women with early onset breast cancer or affected relatives. Breast Cancer Res Treat. 2009;114:457–62. doi: 10.1007/s10549-008-0036-z. [DOI] [PubMed] [Google Scholar]
- 58.Ademuyiwa FO, Cyr A, Ivanovich J, Thomas MA. Managing breast cancer in younger women: challenges and solutions breast. Cancer. 2016;8:1–12. doi: 10.2147/BCTT.S68848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radosa JC, Eaton A, Stempel M, Khander A, Liedtke C, Solomayer EF, et al. Evaluation of local and distant recurrence patterns in patients with triple-negative breast cancer according to age. Ann Surg Oncol. 2017;24:698–704. doi: 10.1245/s10434-016-5631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hemminki K, Hemminki O, Försti A, Sundquist K, Sundquist J, Li X. Surveillance bias in cancer risk after unrelated medical conditions: example urolithiasis. Sci Rep. 2017;7:8073. doi: 10.1038/s41598-017-08839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from the study by the AIRTUM Working Group are publicly available at I tumori in Italia. Rapporto 2013: I tumori multipli | Epidemiologia&Prevenzione (epiprev.it). All data from the remaining studies were taken directly from their corresponding published, publicly available manuscripts or Supplementary Materials.
All codes used to generate the results in this manuscript can be provided upon request.