Abstract
Background & Aims
Among people living with HBV, only a subset of individuals with chronic hepatitis is in need of treatment, and this proportion varies according to the population, region, and setting. No estimates of the proportion of people who are infected with HBV and meet the treatment eligibility criteria in France are available.
Methods
552 treatment-naïve individuals with chronic HBV infection referred for the first time to a hepatology reference centre between 2008 and 2012 were prospectively included. Demographic, clinical, and laboratory data were analysed.
Results
In total, 61.1% of patients were males, with a median age of 37.5 years. Moreover, 64% were born in an intermediate- or high-HBV endemicity country, and 90% were HBeAg-negative. At referral, median HBV DNA and HBsAg levels were 3.3 and 3.6 log IU/ml, respectively; 37.8% of patients had alanine aminotransferase >40 U/L, and 29.0% had moderate or severe fibrosis (≥F2), including 9.4% with cirrhosis. The most prevalent genotypes were D (34.7%), E (27.4%), and A (25.7%). Coinfections were rare: 2.4% were HIV-positive, 4.0% were HCV-positive, and 6.0% were HDV-positive. According to the 2017 EASL Clinical Practice Guidelines, using a single time point analysis, 2.7% of patients were classified as HBeAg-positive chronic infection, 6.1% as HBeAg-positive chronic hepatitis B, 26.5% as HBeAg-negative chronic hepatitis B, and 61.1% as HBeAg-negative chronic infection, whereas 3.6% patients could not be classified. The performance of HBsAg level quantification to identify individuals with HBeAg-negative chronic hepatitis B was poor. A total of 29.1% met the criteria for initiation of antiviral treatment, whereas 66.5% remained under routine clinical surveillance. Most eligible patients initiated recommended first-line therapies, including tenofovir (45.3%), entecavir (36.8%), or pegylated interferon alpha (11.6%).
Conclusions
Of all cases, 9.4% had cirrhosis at presentation and 29.1% met the 2017 EASL Clinical Practice Guidelines treatment criteria. HBsAg levels failed to accurately identify individuals with HBeAg-negative chronic infection.
Lay summary
Among French adults chronically infected with HBV referred for the first time to hepatology reference centres, about one-third had a significant liver disease. Approximately one-third of individuals met criteria for initiation of antiviral treatment based on entecavir or tenofovir or, occasionally, pegylated interferon alpha.
Keywords: Hepatitis B, Chronic HBV infection, Eligibility to antiviral treatment, Natural history, Phase of chronic infection, Hepatitis delta
Abbreviations: ALT, alanine aminotransferase; AUROC, area under the ROC curve; BMI, Body mass index; CAP/CTM, COBAS AmpliPrep/COBAS TaqMan; CHB, Chronic hepatitis B; EASL, European Association for the Study of the Liver; EIA, enzyme immunoassay; HBcrAg, hepatitis B core-related antigen; HBeAg, hepatitis B e antigen; HBsAg, Hepatitis B surface antigen; HBV, Hepatitis B virus; HCC, hepatocellular carcinoma; HCV, Hepatitis C virus; HDV, hepatitis D (delta) virus; HIV, Human immunodeficiency virus; LLoD, lower limit of detection; ROC, receiver operating characteristic; ULN, upper limit of normal; WHO, World Health Organization
Graphical abstract
Highlights
-
•
In French adults with chronic hepatitis B infection, the most prevalent genotypes were D, E, and A.
-
•
Patients were predominantly HBeAg-negative (90.0%).
-
•
The seroprevalence of delta hepatitis was 6%.
-
•
HBsAg quantification is not useful in identifying patients with HBeAg-negative chronic hepatitis B.
-
•
A total of 29.1% of patients were eligible for antiviral treatment.
Introduction
Chronic HBV infection is a global public health concern, with an estimated 296 million people infected worldwide.1 Chronic hepatitis B may lead to decompensation of cirrhosis and/or hepatocellular carcinoma (HCC), which both carry significant morbidity and mortality, with an estimated annual death rate of 820,000.2 Currently, HBV is the number 1 cause for HCC worldwide, accountable for an estimated >50% of all primary liver cancers.3 The incidence rate of liver cancer caused by viral hepatitis is, however, heterogeneous across the world, with the highest incidence observed in Eastern Asia (mainly China) and Western sub-Saharan Africa, where HBV is highly endemic.4 Only a small proportion of the people living with chronic HBV infection has been diagnosed and treated.5 In 2016, The World Health Organization (WHO) estimated that 27 million (10.5%) of HBsAg-positive carriers had been diagnosed and, among them, 4.5 million (16.7%) were receiving antiviral treatment.6
In most cases, hepatitis B therapy is based on lifelong administration of a nucleoside or a nucleotide analogue with a high barrier to resistance (i.e. entecavir or tenofovir, respectively). This treatment maintains undetectable HBV DNA levels in the long term and substantially improves the prognosis of HBV-related liver disease, while decreasing the incidence of their complications, including HCC.[7], [8], [9] Another option is pegylated interferon alpha-2a administered for 48 weeks.10,11 International liver societies have published clinical practice guidelines for the treatment of chronic hepatitis B.2,[10], [11], [12] Generally, treatment is recommended in individuals with moderate or severe liver inflammation and/or fibrosis and ongoing viral replication.
In its 2017 Clinical Practice Guidelines, EASL indicates that only a subset of people with chronic HBV infection is eligible for treatment, and this subset varies according to the population, region, and setting. However, no estimates of the proportion of people who are infected with HBV and meet these treatment eligibility criteria in France are available.
This cross-sectional study aimed to characterise chronic HBV infection among treatment-naïve patients referred for the first time to reference centres in France and examine their eligibility to antiviral treatment according to the criteria from the 2017 EASL Clinical Practice Guidelines.
Patients and methods
Study design and participants
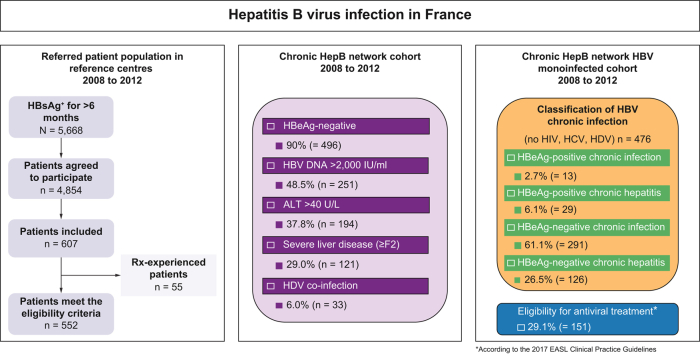
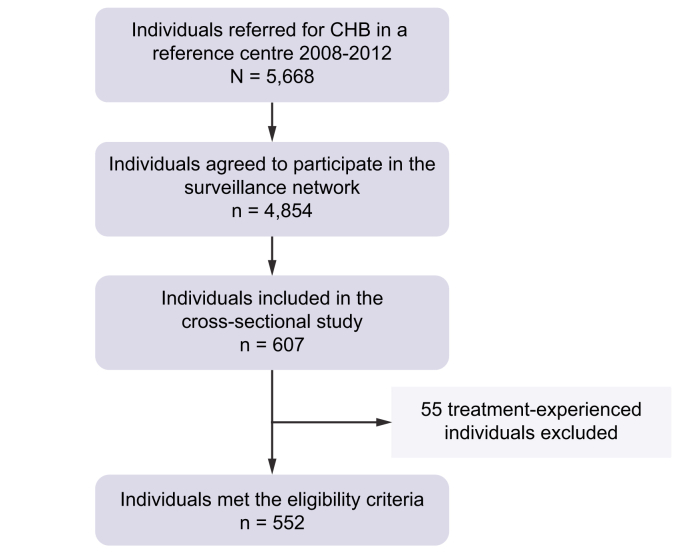
The French national public health agency conducted a cohort study that included all individuals with a diagnosis of chronic HBV carriage (defined as the presence of hepatitis B surface antigen [HBsAg] for more than 6 months) referred for the first time to 1 of the French reference hepatology centres. Among 5,668 consecutive individuals aged ≥18 years with chronic HBV carriage referred to 1 of the 33 French reference centres between 2008 and 2012, a total of 4,854 agreed to participate and underwent further evaluation, including clinical review and blood tests (https://www.santepubliquefrance.fr/maladies-et-traumatismes/hepatites-virales/hepatites-b-et-d/articles/donnees-epidemiologiques-2008-2012). During the medical visits (at referral and 6 months later), standardised reporting forms were filled in, and the information was entered into a web-based electronic data collection system. Demographical (sex, age, country of birth, height and weight, excessive alcohol intake, and history of drug use), biochemical (alanine aminotransferase [ALT] activity), virological (HCV and HIV status), histological (severity of liver disease), and treatment (previously exposed to antiviral drugs or not) data were collected. The first consecutive 552 treatment-naïve patients included were selected for the present study. As shown in Fig. 1, out of 607 patients, 552 finally met the inclusion criteria. The principal reason for non-inclusion was the existence of a prior antiviral treatment against HBV infection.
Fig. 1.
Patient disposition.
The study was conducted in accordance with the Declaration of Helsinki and followed the principles of Good Clinical Practice. It was approved by the appropriate ethics committee (Comité Consultatif sur le traitement de l’information en matière de recherche dans le domaine de la santé, CCTIRS; No. 2015-A01252-47). All patients gave written informed consent.
Assessment of liver fibrosis
The fibrosis stage was assessed by means of liver biopsy, and/or transient elastography (FibroScan®, Echosens™, Paris, France), and/or FibroTest® (BioPredictive, Paris, France) in 169, 347, and 193 patients, respectively. Cirrhosis was defined as METAVIR score F4 on liver biopsy, FibroScan score ≥11.7 kPa or FibroTest score ≥0.85.[13], [14], [15], [16]
Laboratory measurements
HBV DNA levels were measured by means of a real-time PCR assay (COBAS AmpliPrep/COBAS TaqMan [CAP/CTM] version 2, Roche Molecular Systems, Pleasanton, CA, USA), according to the manufacturer’s instructions. The dynamic range of quantification is 20 to 1.7 × 108 IU/ml (1.3–8.2 log IU/ml). The lower limit of detection (LLoD) is 20 IU/ml. HBsAg levels were quantified by means of the Architect® HBsAg assay on an Architect automated device (Abbott Diagnostics, Chicago, IL, USA), according to the manufacturer’s instructions. The dynamic range of quantification is 0.05 to 250.0 IU/ml (-1.3 to 2.4 log IU/ml). The hepatitis B e (HBe) status was determined by means of a commercial enzyme immunoassay (EIA; VIDAS™ HBe/Anti-HBe, Biomérieux, Marcy-l’Etoile, France). The HBV genotype was determined by means of sequencing followed by phylogenetic analysis of a portion of the overlapping genes encoding HBsAg and the B and C subdomains of the HBV reverse transcriptase, as previously described.17
HDV antibodies were sought with an EIA (ETI-AB-DELTAK-2, Bio-Rad Laboratories, Hercules, CA, USA). Serum and plasma HDV RNA levels were measured by means of a homebrew consensus quantitative real-time PCR assay, as previously described.18,19 The dynamic range of quantification is 103 to 109 copies/ml (3.0–9.0 log copies/ml), with an LLoD of 100 copies/ml (2.0 log copies/ml). The HDV genotype was determined by means of sequencing followed by phylogenetic analysis of an amplicon of a portion of the so-called R0 region of the viral genome (positions 920 to 1,289), as previously described.20,21
Statistical analysis
Results are presented as numbers and percentages for categorical data and median with IQR for continuous variables. Comparisons used Fisher’s exact test or the Mann–Whitney U test for categorical or continuous variables, respectively. The Kruskall–Wallis test was used when categorical variables had more than 2 classes, and the correction of Bonferroni was used for multiple comparisons. Area under the receiver operating characteristic (ROC) curve (AUROC) was assessed to determine the capacity of HBsAg level to identify chronic infection or chronic hepatitis. Statistical analysis was performed using Stata® 10.0 (StataCorp LP, College Station, TX, USA). Values of p <0.05 were considered statistically significant.
Results
Characteristics of the study population at referral
Table 1 summarises the characteristics of the 552 treatment-naïve individuals with chronic HBsAg carriage included. Patients were predominantly males (61.1%). The median age at referral was 37 years (range 18–83 years). No significant age difference was found between males and females (38 [30–52] vs. 37 [28–49] years, respectively; p = 0.234). A total of 63.9% of patients were born in an intermediate- (n = 115; 20.8%) or high- (n = 238; 43.1%) endemicity country. Indeed, more than a third of patients were born in sub-Saharan Africa (n = 210; 38.7%), followed by one-third in Europe (n = 180; 32.5%), including 22.1% in France, and a minority in Asia, North Africa, the West Indies, the Middle East, and Pacific regions (Table 1). Patients born in sub-Saharan Africa were significantly younger at referral (31.0 [26–39] years) when compared with those originating from all other regions (45.0 [38–50] years; p <0.001). Among the 552 patients, 496 (90.0%) were HBeAg-negative. HBeAg-negative patients were significantly older (38 [29–51] vs. 28 [23–38] years; p <0.001) and had significantly lower HBV DNA levels (3.1 [2.4–4.1] vs. 7.5 [5.6–8.0] log IU/ml; p <0.001) and HBsAg levels (3.6 [3.0–4.0] vs. 4.2 [3.7–4.6] log IU/ml; p <0.001) than HBeAg-positive patients.
Table 1.
Baseline characteristics of the study population (N = 552).
| Characteristics | |
|---|---|
| Age, years (median [IQR]) (range) | 37.5 [29–50] (18–83) |
| Sex, male [n (%)] | 337 (61.1) |
| BMI (kg/m2), (median [IQR]) (range)∗ | 24 [22–27] (15–49) |
| History of alcohol abuse [n (%)]† | 30 (5.4) |
| History of intravenous drug use [n (%)]‡ | 10 (2.3) |
| Country of birth [n (%)]§ | |
| France | 120 (22.1) |
| Europe, excluding France | 59 (10.9) |
| North Africa | 38 (7.0) |
| Middle East | 24 (4.4) |
| Asia | 56 (10.3) |
| West Indies | 34 (6.3) |
| Pacific | 1 (0.2) |
| Sub-Saharan Africa | 210 (38.7) |
| Coinfections [n (%)] | |
| HIV¶ | 13 (2.4) |
| HCV∗∗ | 22 (4.0) |
| HDV | 33 (6.0) |
| HBeAg-negative [n (%)] | 496 (90.0) |
| Detectable HBV DNA [n (%)] | 520 (94.2) |
| HBV DNA level (log IU/ml), (median [IQR]) (range) | 3.3 [2.4–4.4] (1.0–9.1) |
| HBV DNA >2,000 IU/ml [n (%)] | 251 (48.5) |
| HBsAg level (log IU/ml), median [IQR]) (range) | 3.6 [3.0–4.1] (-0.8 to 5.4) |
| HBV genotype [n (%)]†† | |
| A | 109 (25.7) |
| B | 22 (5.2) |
| C | 27 (6.4) |
| D | 147 (34.7) |
| E | 116 (27.4) |
| F | 1 (0.25) |
| G | 2 (0.50) |
| Fibrosis stage distribution [n (%)]‡‡ | |
| F0–F1 | 296 (71.0) |
| F2 | 56 (13.4) |
| F3 | 26 (6.2) |
| F4 | 39 (9.4) |
| ALT level (U/L) (median [IQR]) (range)§§ | 33 [22–52] (5–4,043) |
| ALT >40 U/L [n (%)] | 194 (37.8) |
| Treatment eligibility [n (%)]¶¶ | 151 (29.1) |
BMI is missing in 159 patients.
History of alcohol abuse is missing in 71 patients.
History of intravenous drug use is missing in 109 patients.
Country of birth is missing in 10 patients.
HIV status is missing in 20 patients.
HCV status is missing in 7 patients.
HBV genotype was not determined in 129 patients owing to HBV DNA level <2.5 log IU/ml or undetectable HBV DNA.
Fibrosis stage is missing in 135 patients.
ALT level is missing in 39 patients.
According to the 2017 EASL Clinical Practice Guidelines,10 only HBV-monoinfected patients were considered. Inconclusive findings in 23 patients.
When detectable, the median HBV DNA level was 3.3 [2.4–4.4] log IU/ml (range 1.0–9.1 log IU/ml); 32 patients had undetectable HBV DNA, whereas 268 (48.5%) patients had an HBV DNA level >2,000 IU/ml. At referral, the median HBsAg level was 3.6 [3.0–4.1] log IU/ml (range -0.8–5.4 log IU/ml). The median ALT level was 33 [22–53] U/L. ALT levels were significantly higher in males (median 40 U/L) than in females (median 24 U/L; p <0.001). A total of 194 patients (37.8%) had ALT levels above 40 U/L. At referral, 121 patients (29.0%) had fibrosis ≥F2, including 39 (9.4%) patients who had cirrhosis (Table 1). The proportion of individuals with ALT >40 U/L and fibrosis ≥F2 was significantly higher in HBeAg-positive than in HBeAg-negative patients (62.5 vs. 34.3%, p <0.001, and 42.9 vs. 26.5%, p = 0.003, respectively).
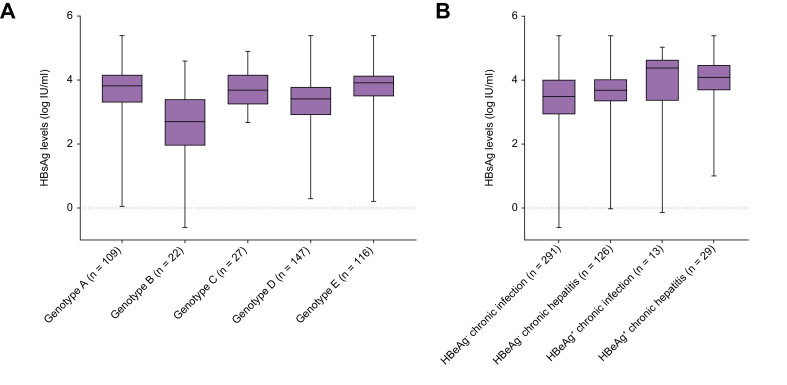
Hepatitis B genotype determination was performed in all individuals with HBV DNA >2.5 log IU/ml. The most prevalent genotypes were D (34.7%), E (27.4%), and A (25.7%). A higher HBV DNA level was observed in genotype C-infected patients than in patients infected with genotypes A, D, or E (5.2 [2.9–7.5] log IU/ml vs. 3.4 [2.7–4.5] log IU/ml, p = 0.015; 3.4 [2.7–4.5] log IU/ml, p = 0.018; and 3.6 [2.7–5.0] log IU/ml, p = 0.022, respectively). A lower HBsAg level was found in genotype B-infected patients than in those infected with genotypes A, C, D, or E (2.7 [1.9–3.3] log IU/ml vs. 3.8 [3.3–4.2] log IU/ml, p <0.001; 3.7 [3.2–4.2] log IU/ml, p <0.0001, 3.4 [2.9–3.8] log IU/ml, p = 0.0003; and 3.9 [3.5–4.2] log IU/ml, p <0.001, respectively), whereas a higher HBsAg level was observed in genotype A- and E-infected patients than in those infected with genotype D (3.8 [3.3–4.2] log IU/ml and 3.9 [3.5–4.2] log IU/ml vs. 3.4 [2.9–3.8] log IU/ml; p = 0.0049 and p <0.0001, respectively) (Fig. 2A). HBeAg positivity was more frequent in patients infected with genotype C (37.0%) than in those with genotype E (16.2%), genotype D (9.5%), genotype A (7.3%), or genotype B (4.5%) (p = 0.001).
Fig. 2.
Box plot representation of the HBsAg level. (A) According to HBV genotypes. (B) Phases of chronic hepatitis B infection.
The midline and the lower and upper edges of boxes represent the median value, 25th percentile, and 75th percentile, respectively. The lower and upper error bars represent the minimum and maximum values, respectively.
Coinfections were rare: 13 patients (2.4%) were HIV antibody-positive, 22 (4.0%) were HCV antibody-positive with HCV RNA detectable in 9 (42.9%) patients, and 33 (6.0%) were HDV antibody-positive. The proportion of patients coinfected with HCV was significantly higher in HBeAg-negative patients and in patients seropositive for HDV than in HBeAg-positive patients (4.1 and 18.8% vs. 0%, p = 0.002). Ten individuals with HIV, HCV, and/or HDV coinfection had cirrhosis, representing a substantial proportion of the 39 patients diagnosed with cirrhosis in this cohort.
Prevalence of HDV infection
Among 552 HBsAg-positive patients, 33 (6.0%) had HDV antibodies. Most of these patients were males (78.8%), and their median age was 43 [32–52] years. HDV-infected patients were older than individuals without HDV antibodies (43 vs. 37 [29–50] years, p <0.001) (Table S2). Nine patients (27.3%) were born in high-HDV endemicity countries.22 HDV replication was assessed in 16 of the 33 patients, and 10 of them (62.5%) had detectable HDV RNA, with a median HDV RNA level of 6.4 [4.4–6.9] log copies/ml (range 3.8–7.3 log copies/ml).
HDV genotypes were determined only in HDV RNA-positive patients. HDV-1 was predominant (n = 7), followed by genotypes almost exclusively found in sub-Saharan Africa, including HDV-5 (n = 2) and HDV-8 (n = 1). The proportion of individuals with detectable HBV replication was lower in individuals with than in those without HDV infection (60.6% vs. 87.9%, p <0.001). At referral, the median HBV DNA and HBsAg levels were 2.7 [1.8–4.0] log IU/ml and 3.3 [1.2–3.9] log IU/ml, respectively, significantly lower than those in HDV-negative individuals (3.3 [2.5–4.5] log IU/ml, p = 0.004, and 3.6 [3.0–4.1] log IU/ml, p = 0.034, respectively). The ALT level was significantly higher in HDV-positive than in HDV-negative HBV-infected patients (49.5 vs. 32.0 U/L, p = 0.027). Seventeen (56.7%) patients had ALT above 40 U/L, vs. 173 (34.5%) patients in the HDV-negative group (p <0.001). Thirteen of the 24 patients (54.2%) whose fibrosis score was measured had significant or severe fibrosis, whereas 5 patients (20.8%) had cirrhosis. This proportion was significantly higher than that observed in HDV-negative patients (8.8%, p = 0.003).
HBV parameters and phase of chronic infection
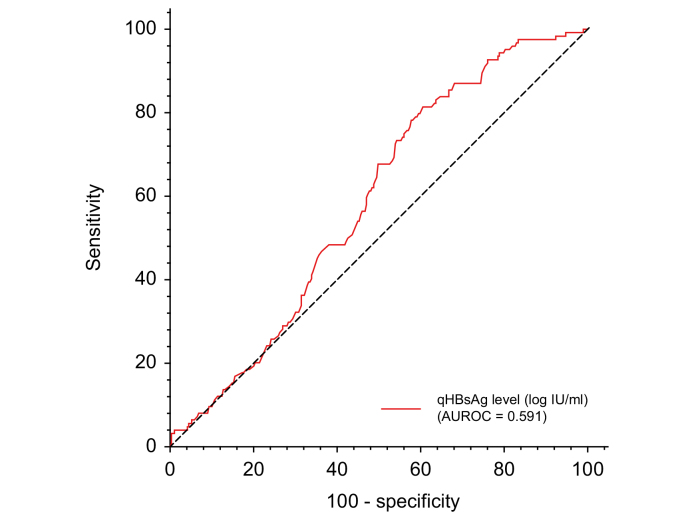
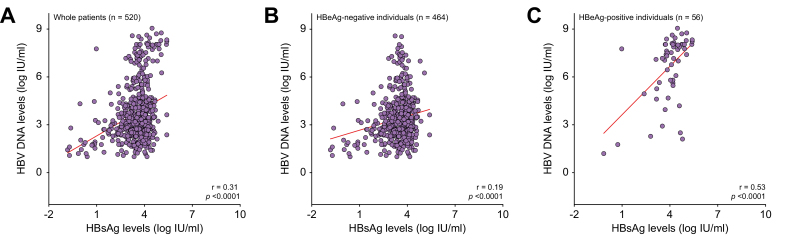
The 76 out of 552 individuals with viral coinfection or unknown coinfection status were excluded from subsequent analysis. Using a single time point analysis in the remaining 476 patients and the classification from the 2017 EASL Clinical Practice Guidelines,10 13 (2.7%) patients were classified as HBeAg-positive chronic HBV infection (immune tolerant), 29 (6.1%) as HBeAg-positive chronic hepatitis B, 126 (26.5%) as HBeAg-negative chronic hepatitis B, and 291 (61.1%) as HBeAg-negative chronic HBV infection (inactive carrier). Finally, 17 (3.6%) patients could not be classified because of missing ALT values, HBV DNA, and/or discrepant assessment of fibrosis score. The HBsAg levels were slightly higher in individuals with HBeAg-negative chronic hepatitis B than in those with HBeAg-negative chronic infection (3.7 [3.3–4.1] vs. 3.5 [2.9–4.0] log IU/ml; p <0.001) (Fig. 2B). The performance of HBsAg level measurement for identifying individuals with HBeAg-negative chronic hepatitis B was poor, with an AUROC at 0.591 (0.538–0.644) (Fig. 3). Based on ROC curve analysis, the optimal cut-off was established at 3.5 log IU/ml and was associated with a sensitivity of 65.9% (95% CI 57.2–73.9%) and a specificity of 50.0% (95% CI 44.5–55.5%). In addition, HBsAg levels correlated with HBV DNA levels, regardless of the HBeAg status (r = 0.31; p <0.001) (Fig. 4A). This correlation was stronger in HBeAg-positive than in HBeAg-negative patients (r = 0.53 vs. r = 0.19; p <0.001, respectively) (Fig. 4B and C).
Fig. 3.
ROC curve and diagnostic performance of HBsAg level for differentiating individuals with HBeAg-negative chronic hepatitis from those with HBeAg-negative chronic infection (inactive carriers).
Fig. 4.
Correlation of HBsAg and HBV DNA levels. (A) The entire study population (n = 520), (B) HBeAg-negative individuals (n = 464), (C) HBeAg-positive individuals (n = 56).
Hepatitis B treatment eligibility
Using the 2017 EASL Clinical Practice Guidelines criteria for treatment initiation eligibility (age >30 years, HBeAg status, HBV DNA >2,000 or >20,000 IU/ml, ALT >1–2 × upper limit of normal (ULN), and/or significant fibrosis and/or necroinflammation; Table S1), 151 (29.1%) patients were found eligible. Twenty-three (4.4%) patients could not be classified because of inconclusive ALT level or liver fibrosis and necroinflammation evaluations. The remaining 345 (66.5%) patients, who did not meet treatment eligibility criteria, remained under routine clinical surveillance. Among patients eligible to treatment, 95 (62.9%) initiated therapy with pegylated interferon (11/95 [11.6%]), entecavir (35/95 [36.8%]), tenofovir (43/95 [45.3%]), or adefovir (3/95 [3.2%]). Three patients (2.8%) were included in clinical trials.
Among the patients meeting treatment initiation criteria, 133 (88.1%) and 121 (80.1%) had HBV DNA levels above 2,000 and 20,000 IU/ml, respectively. Eighteen (11.9%) patients had HBV DNA levels below 2,000 IU/ml, but most of them has severe liver disease. Finally, 104 (68.9%) patients had ALT levels above the ULN.
Discussion
In this large cohort of individuals with chronic HBsAg carriage living in France, one-third of cases were eligible for antiviral treatment. Among the HBsAg-positive participants, 9.4% had cirrhosis, 48.5% had an HBV DNA level exceeding 2,000 IU/ml, and roughly a third had elevated ALT levels on at least 1 occasion. The main strength of this prospective study was the sample size, as a result of the inclusion of 1 of the largest cohorts of treatment-naïve individuals with chronic hepatitis B ever generated in France. All patients underwent a panel of tests at baseline, including liver function tests, virological markers (viral load, HBeAg status, HBsAg level, and HDV status) and fibrosis assessment.
To the best of our knowledge, this is the first study analysing the proportion of patients requiring antiviral therapy in patients infected with HBV referred to reference centres for viral hepatitis in France. Studies on HBV infection in France are mainly sero-surveillance studies assessing the prevalence of HBsAg in the general population or in specific groups or virological studies describing the characteristics of HBV in infected individuals.23,24 None of these studies described the severity of liver disease in individuals with chronic HBV infection never exposed to antiviral treatment.
Our findings were in keeping with the results of a recent meta-analysis including more than 145,000 participants.25 However, the proportion of patients eligible for antiviral treatment according to the 2017 EASL Clinical Practice Guidelines10 in our population was higher than that reported in other studies, owing to differences in the ALT and HBV DNA thresholds used for decision. This was because the 2017 guidelines expanded the criteria for eligibility compared with previous guidelines published in 2012.
The proportion of HBeAg-negative patients has substantially increased in France over the past 20 years: 22.1% in 1994, 74.6% in 2003, and 90.0% in the present study.26,27 Several factors may explain this increasing proportion of HBeAg-negative patients, including longer duration of infection in an ageing population and an older age at the time of referral. As observed in other studies, HBsAg levels poorly correlated with HBV DNA levels, especially in HBeAg-negative patients.28 In contrast with what has been suggested by previous studies conducted in Europe, HBsAg levels failed to accurately identify HBeAg-negative individuals with chronic hepatitis from those who were inactive carriers.29,30 However, our results are in keeping with a recent study conducted in HBV-infected African patients mainly infected with genotype E, which showed a poor clinical utility of quantifying HBsAg levels to identify inactive carriers and HBeAg-negative individuals with chronic hepatitis B eligible for antiviral therapy.31
HDV infection was estimated to be present in approximately 6.0% of chronic HBsAg carriers referred to healthcare facilities, among which approximately one-third was viremic. The prevalence of HDV infection in our study was higher than in a survey investigating French blood donors, owing to a higher proportion of patients originating from sub-Saharan Africa in our cohort.32 It was of the same order as that reported in a study conducted in HBsAg-positive patients in 2008.33 More than 80% of HDV-infected patients were migrants originating from sub-Saharan Africa and Eastern and Southern Europe, a proportion similar to that reported in other European countries.[34], [35], [36] At referral, more than 20% of HDV-infected patients had cirrhosis, a proportion in keeping with studies from other European countries.[36], [37], [38], [39] The proportion of individuals with advanced liver disease was higher in HDV-coinfected than in non-HDV-infected patients.
Our study has limitations. First, it was cross-sectional in design, and patients were assessed on a single time point. We used laboratory results from baseline evaluation instead of repeated measurements, which may not reflect the dynamic nature of the disease. Thus, changes in ALT and HBV DNA levels over time were not captured in this analysis. Second, we used the 2017 EASL Clinical Practice Guidelines eligibility criteria. Using criteria from other guidelines could have yielded slightly different results. Third, the fibrosis stage was not assessed using the same method in all patients. Transient elastography has its inherent limitations, as both ALT flares, congestive heart failure, extrahepatic cholestasis, and recent food intake may induce falsely elevated FibroScan values.40 Fourth, the treatment eligibility criterion pertaining to the presence of HCC or cirrhosis in close family members was not available. This could have led to underestimating the proportion of patients eligible for treatment, according to the 2017 EASL Guidelines. Fifth, the present study was carried out at reference centres located in university hospitals, where individuals with more advanced liver disease might be overrepresented. Finally, virological, biochemical, and histological parameters were missing in a few participants; in particular, HDV RNA level was measured in only 16 of 33 HDV-seropositive patients, owing to an insufficient volume of serum or plasma available. Fibrosis stage was not assessed in 99 HBV-monoinfected patients, but their characteristics at referral did not differ from those in patients who underwent fibrosis assessment (Table S3). Finally, new virological parameters of potential interest, including hepatitis B core-related antigen (HBcrAg) or circulating HBV RNA, were not assessed.
In conclusion, this article reports on 1 of the largest cohorts evaluating the proportion of patients requiring antiviral treatment, as well as HDV prevalence, in a low-endemicity country. In HBV-infected patients referred for the first time to reference centres in France (including about 10% with cirrhosis at presentation), one-third met the 2017 EASL Clinical Practice Guidelines criteria for treatment initiation. Most treatment-eligible patients received 1 of the recommended first-line therapies. Although HBsAg quantification is simple and inexpensive, its clinical utility at a single time point is limited to characterise the phase of chronic HBV infection, especially in individuals with a low risk of liver disease progression. The prevalence of HDV infection was relatively high in individuals with chronic hepatitis B, with a predominance of genotype 1.
Financial support
This work was supported by Santé publique France. It was partly funded by the Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS).
Authors’ contributions
Concept and design: SC, FRT, CB. Experiments and procedures: CP, CB, SC, EG, FZ, SB, VB. Writing – original draft preparation: SC, FRT. Writing – review and editing: SC, FRT, CB, JMP, VL.
Data availability statement
Data presented in this study are available from the authors on request.
Conflicts of interest
The authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank the patients and nurses for their involvement in the study, as well as the French Society of Hepatology (Association Française pour l’Etude du Foie), the Fédération Nationale des Pôles de Référence et Réseau Hépatites, the National Agency for Research on AIDS and Viral Hepatitis (ANRS), and all clinicians and virologists from the reference hepatology centres and laboratories (see list in the Supplementary information).
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100593.
Supplementary data
The following are the supplementary data to this article:
References
- 1.World Health Organization. Hepatitis B. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b Accessed 27 July 2021.
- 2.Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. World Health Organization; 2015. Geneva. [PubMed] [Google Scholar]
- 3.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl. 1):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z., Jiang Y., Yuan H., Fang Q., Cai N., Suo C., et al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70:674–683. doi: 10.1016/j.jhep.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Polaris Observatory Collaborators Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 6.Hutin Y., Nasrullah M., Easterbrook P., Nguimfack B.D., Burrone E., Averhoff F., et al. Access to treatment for hepatitis B virus infection – worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:773–777. doi: 10.15585/mmwr.mm6728a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang T.-T., Liaw Y.-F., Wu S.-S., Schiff E., Han K.-H., Lai C.-L., et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 8.Marcellin P., Gane E., Buti M., Afdhal N., Sievert W., Jacobson I.M., et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 9.Papatheodoridis G.V., Idilman R., Dalekos G.N., Buti M., Chi H., van Boemmel F., et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology. 2017;66:1444–1453. doi: 10.1002/hep.29320. [DOI] [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Terrault N.A., Lok A.S.F., McMahon B.J., Chang K.-M., Hwang J.P., Jonas M.M., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarin S.K., Kumar M., Lau G.K., Abbas Z., Chan H.L., Chen C.J., et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardoso A.-C., Carvalho-Filho R.J., Stern C., Dipumpo A., Giuily N., Ripault M.-P., et al. Direct comparison of diagnostic performance of transient elastography in patients with chronic hepatitis B and chronic hepatitis C. Liver Int. 2012;32:612–621. doi: 10.1111/j.1478-3231.2011.02660.x. [DOI] [PubMed] [Google Scholar]
- 14.Chon Y.E., Choi E.H., Song K.J., Park J.Y., Kim D.Y., Han K.-H., et al. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Association for Study of Liver and Asociacion Latinoamericana para el Estudio del. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Leroy V., Sturm N., Faure P., Trocme C., Marlu A., Hilleret M.-N., et al. Prospective evaluation of FibroTest®, FibroMeter®, and HepaScore® for staging liver fibrosis in chronic hepatitis B: comparison with hepatitis C. J Hepatol. 2014;61:28–34. doi: 10.1016/j.jhep.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Chevaliez S., Hézode C., Bahrami S., Grare M., Pawlotsky J.M. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58:676–683. doi: 10.1016/j.jhep.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 18.Le Gal F., Dziri S., Gerber A., Alloui C., Ben Abdesselam Z., Roulot D., et al. Performance characteristics of a new consensus commercial kit for hepatitis D virus RNA viral load quantification. J Clin Microbiol. 2017;55:431–441. doi: 10.1128/JCM.02027-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Gal F., Gordien E., Affolabi D., Hanslik T., Alloui C., Dény P., et al. Quantification of hepatitis delta virus RNA in serum by consensus real-time PCR indicates different patterns of virological response to interferon therapy in chronically infected patients. J Clin Microbiol. 2005;43:2363–2369. doi: 10.1128/JCM.43.5.2363-2369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivaniushina V., Radjef N., Alexeeva M., Gault E., Semenov S., Salhi M., et al. Hepatitis delta virus genotypes I and II cocirculate in an endemic area of Yakutia, Russia. J Gen Virol. 2001;82:2709–2718. doi: 10.1099/0022-1317-82-11-2709. [DOI] [PubMed] [Google Scholar]
- 21.Le Gal F., Brichler S., Drugan T., Alloui C., Roulot D., Pawlotsky J.-M., et al. Genetic diversity and worldwide distribution of the deltavirus genus: a study of 2,152 clinical strains. Hepatology. 2017;66:1826–1841. doi: 10.1002/hep.29574. [DOI] [PubMed] [Google Scholar]
- 22.Da B.L., Rahman F., Lai W.C., Kleiner D.E., Heller T., Koh C. Risk factors for delta hepatitis in a North American cohort: who should be screened? Am J Gastroenterol. 2021;116:206–209. doi: 10.14309/ajg.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meffre C., Le Strat Y., Delarocque-Astagneau E., Dubois F., Antona D., Lemasson J.-M., et al. Prevalence of hepatitis B and hepatitis C virus infections in France in 2004: social factors are important predictors after adjusting for known risk factors. J Med Virol. 2010;82:546–555. doi: 10.1002/jmv.21734. [DOI] [PubMed] [Google Scholar]
- 24.Moussa S., Brah S., Parola P., Gerolami R., Gamerre M., Boubli L., et al. Epidemiological, clinical, virological features of hepatitis B newly diagnosed in 2011 in Marseille University hospitals, southeastern France. J Med Virol. 2016;88:828–836. doi: 10.1002/jmv.24398. [DOI] [PubMed] [Google Scholar]
- 25.Tan M., Bhadoria A.S., Cui F., Tan A., Van Holten J., Easterbrook P., et al. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:106–119. doi: 10.1016/S2468-1253(20)30307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarski J.P., Marcellin P., Cohard M., Lutz J.M., Bouche C., Rais A. Comparison of anti-HBe-positive and HBe-antigen-positive chronic hepatitis B in France. French multicentre group. J Hepatol. 1994;20:636–640. doi: 10.1016/s0168-8278(05)80352-6. [DOI] [PubMed] [Google Scholar]
- 27.Zarski J.-P., Marcellin P., Leroy V., Trepo C., Samuel D., Ganne-Carrie N., et al. Characteristics of patients with chronic hepatitis B in France: predominant frequency of HBe antigen negative cases. J Hepatol. 2006;45:355–360. doi: 10.1016/j.jhep.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Thompson A.J., Nguyen T., Iser D., Ayres A., Jackson K., Littlejohn M., et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933–1944. doi: 10.1002/hep.23571. [DOI] [PubMed] [Google Scholar]
- 29.Brunetto M.R., Oliveri F., Colombatto P., Moriconi F., Ciccorossi P., Coco B., et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010;139:483–490. doi: 10.1053/j.gastro.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 30.Martinot-Peignoux M., Lapalus M., Asselah T., Marcellin P. The role of HBsAg quantification for monitoring natural history and treatment outcome. Liver Int. 2013;33(Suppl. 1):125–132. doi: 10.1111/liv.12075. [DOI] [PubMed] [Google Scholar]
- 31.Post G., Howell J., Sow A., Ndow G., Chemin I., Lo G., et al. Clinical utility of quantifying hepatitis B surface antigen in African patients with chronic hepatitis B. J Viral Hepat. 2021;28:1003–1010. doi: 10.1111/jvh.13499. [DOI] [PubMed] [Google Scholar]
- 32.Servant-Delmas A., Le Gal F., Gallian P., Gordien E., Laperche S. Increasing prevalence of HDV/HBV infection over 15 years in France. J Clin Virol. 2014;59:126–128. doi: 10.1016/j.jcv.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Piroth L., Pol S., Lacombe K., Miailhes P., Rami A., Rey D., et al. Management and treatment of chronic hepatitis B virus infection in HIV positive and negative patients: the EPIB 2008 study. J Hepatol. 2010;53:1006–1012. doi: 10.1016/j.jhep.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 34.Cross T.J., Rizzi P., Horner M., Jolly A., Hussain M.J., Smith H.M., et al. The increasing prevalence of hepatitis delta virus (HDV) infection in South London. J Med Virol. 2008;80:277–282. doi: 10.1002/jmv.21078. [DOI] [PubMed] [Google Scholar]
- 35.Reinheimer C., Doerr H.W., Berger A. Hepatitis delta: on soft paws across Germany. Infection. 2012;40:621–625. doi: 10.1007/s15010-012-0287-9. [DOI] [PubMed] [Google Scholar]
- 36.Roulot D., Brichler S., Layese R., BenAbdesselam Z., Zoulim F., Thibault V., et al. Origin, HDV genotype and persistent viremia determine outcome and treatment response in patients with chronic hepatitis delta. J Hepatol. 2020;73:1046–1062. doi: 10.1016/j.jhep.2020.06.038. [DOI] [PubMed] [Google Scholar]
- 37.Buti M., Homs M., Rodriguez-Frias F., Funalleras G., Jardí R., Sauleda S., et al. Clinical outcome of acute and chronic hepatitis delta over time: a long-term follow-up study. J Viral Hepat. 2011;18:434–442. doi: 10.1111/j.1365-2893.2010.01324.x. [DOI] [PubMed] [Google Scholar]
- 38.Niro G.A., Smedile A., Ippolito A.M., Ciancio A., Fontana R., Olivero A., et al. Outcome of chronic delta hepatitis in Italy: a long-term cohort study. J Hepatol. 2010;53:834–840. doi: 10.1016/j.jhep.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Wranke A., Pinheiro Borzacov L.M., Parana R., Lobato C., Hamid S., Ceausu E., et al. Clinical and virological heterogeneity of hepatitis delta in different regions world-wide: the Hepatitis Delta International Network (HDIN) Liver Int. 2018;38:842–850. doi: 10.1111/liv.13604. [DOI] [PubMed] [Google Scholar]
- 40.Wong G.L.-H. Transient elastography: kill two birds with one stone? World J Hepatol. 2013;5:64–74. doi: 10.4254/wjh.v5.i5.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data presented in this study are available from the authors on request.