Abstract
Background
Research from the International Cancer Benchmarking Partnership (ICBP) demonstrates that international variation in lung cancer survival persists, particularly within early stage disease. There is a lack of international consensus on the critical contributing components to variation in lung cancer outcomes and the steps needed to optimise lung cancer services. These are needed to improve the quality of options for and equitable access to treatment, and ultimately improve survival.
Methods
Semi-structured interviews were conducted with 9 key informants from ICBP countries. An international clinical network representing 6 ICBP countries (Australia, Canada, Denmark, England, Ireland, New Zealand, Northern Ireland, Scotland & Wales) was established to share local clinical insights and examples of best practice. Using a modified Delphi consensus model, network members suggested and rated recommendations to optimise the management of lung cancer. Calls to Action were developed via Delphi voting as the most crucial recommendations, with Good Practice Points included to support their implementation.
Results
Five Calls to Action and thirteen Good Practice Points applicable to high income, comparable countries were developed and achieved 100% consensus. Calls to Action include (1) Implement cost-effective, clinically efficacious, and equitable lung cancer screening initiatives; (2) Ensure diagnosis of lung cancer within 30 days of referral; (3) Develop Thoracic Centres of Excellence; (4) Undertake an international audit of lung cancer care; and (5) Recognise improvements in lung cancer care and outcomes as a priority in cancer policy.
Conclusion
The recommendations presented are the voice of an expert international lung cancer clinical network, and signpost key considerations for policymakers in countries within the ICBP but also in other comparable high-income countries. These define a roadmap to help align and focus efforts in improving outcomes and management of lung cancer patients globally.
Keywords: lung cancer, health policy, public health, epidemiology, survival
Introduction
Lung cancer accounts for nearly 1.8 million deaths annually – more than colorectal and breast cancers combined.1 Despite significant improvements in diagnosis and treatment, lung cancer is typically associated with low survival internationally.2 The International Cancer Benchmarking Partnership (ICBP) is a global collaboration of clinicians, policymakers, researchers, and cancer data experts. It seeks to benchmark and explain cancer survival, incidence, and mortality differences between high-income countries with comprehensive cancer registry coverage, similar budgetary spending on national health systems and universal access to healthcare. Recent ICBP research has demonstrated that international disparities in lung cancer survival persist, with 5-year survival ranging from 14.7% (UK) to 21.7% (Canada).2 Evidence shows that 3-year survival varies mostly in potentially curative lung cancer, by up to 20 percentage points for localised and regional disease (as per SEER summary staging)3 across the ICBP countries.4 Comparably, survival varied by just 3.4 percentage points in patients diagnosed with distant/metastatic disease across the same countries.4
Due to the inherent complexity of health systems, it is unlikely that one single component of the cancer care continuum can ultimately define why survival variation exists, yet the ability to diagnose and stage lung cancer patients as early as possible will likely influence their treatment options.5 Various work has been undertaken to articulate patient, healthcare professional and disease related factors that influence timeliness of diagnosis and ultimately, patient outcomes.6 This understanding has helped build initiatives across the pathway to address the poorer outcomes in lung cancer, but to differing levels internationally. This starts with preventative initiatives for lung cancer, including support for smoking cessation and lung cancer awareness campaigns. Both areas are of significance, with tobacco being a key factor in the aetiology of lung cancer, and some awareness campaigns influencing stage shift.7,8 Variation in primary care practitioner readiness to refer patients with potential symptoms and in service provision of key diagnostic and staging tools for lung cancer, such as PET-CT, have been demonstrated across ICBP countries with unknown quantifiable impacts upon survival.9,10 This is reflected in the variation seen in lung cancer stage distribution between ICBP countries, but not to the same extent as differences in survival by stage.4 In the diagnostic phase, it is clear that international differences exist for lung cancer patients, yet the evidence for how this affects outcomes is variable and complex.11 Variation within the diagnosis and staging of early stage lung cancer patients is important; unwarranted delays enabling tumour growth may shift patients away from being in a potentially curable stage towards more advanced stages with lower survival.12,13
Curative intent treatment options for lung cancer have increased and become significantly more sophisticated over recent years, with the development and adoption of more precise radiotherapy, and minimally invasive surgery.14 Less invasive options have opened up better opportunities for more complex patients or those with greater comorbidities. Guidance and uptake of these treatment options for early stage patients is likely to vary both within countries and internationally. This is particularly the case in patients deemed to be borderline candidates for curative therapy where there are more variables playing a role in decision-making for treatment.15 Variation in the use of curative intent therapies within early stage patients has been demonstrated in some countries.16 Disease stage, patient fitness/performance status, lung function, staffing skillset, socioeconomic status and comorbidities play key influential roles in the perceived operability of patients and subsequent treatment decisions.16
The organisation of each country’s healthcare system has been established to guide patients from presentation to the decision for treatment, with many informal and formal treatment pathways being established. However, gaps and biases within each system have been identified that undoubtedly affect the management of potentially curable patients.17 There is a current lack of internationally agreed recommendations for improving care of lung cancer patients, with the purpose of being interpreted at a policy level to align efforts to empower those in positions to instigate change.
Within this study, we have established an international lung cancer network to share local clinical insights, including locally defined best practices, mitigation of modifiable variables and opportunities for collaboration. We present a series of recommendations to improve lung cancer care, in order to improve survival, based on the consensus of our international clinical network. We hope that these recommendations serve to optimise lung cancer services so as to improve the quality of options for treatment, equitable access to treatment, and ultimately improve survival internationally.
Materials and Methods
Network Formation
Nine network members were recruited from nine ICBP jurisdictions: Australia, Canada, Denmark, England, Ireland, New Zealand, Northern Ireland, Scotland and Wales. Members were identified via purposive sampling based on them having roles in national, regional, and local tiers of health systems with expertise primarily being thoracic surgery, radiation oncology, clinical oncology, or respiratory medicine. Existing ICBP clinical networks and stakeholders were approached and asked for suggestions for the network, and desk-based research was conducted to assess suitability based on the criteria outlined in the previous sentence. Members were initially invited to join the network via email, and if interested, they were sent a Terms of Reference and invited to a telephone call to discuss the aims of the project and the ICBP in more detail. At that point, members were asked if they agreed to be part of the network and involved in the project.
Topic Guide Development and Initial Interviews
We initially employed a qualitative approach to understand potential contributing factors to international variation in lung cancer outcomes that could form the basis of the recommendations developed. The overarching focus of this was mostly on non-small cell lung cancer (NSCLC), and for early stage lung cancer. Semi-structured interviews were conducted with the network members to identify key themes and points of variation between the ICBP countries. Interview questions (Supplementary Materials) were developed by the lead study team (Finley, Lynch, Butler, Harrison), alongside an evidence scoping exercise and review of published literature. Interviews were recorded via Microsoft Teams and stored securely at Cancer Research UK. Qualitative data was transcribed by the lead study team and interview transcripts were analysed thematically, drawing on documents developed during processes undertaken to improve existing national guidelines and support for improving lung cancer care, largely the Pan-Canadian Standards for Thoracic Surgery.18 The analysis of these interviews helped generate the recommendations.
Roundtable Discussions and Recommendation Development
Four roundtable discussions were hosted by the lead study team with all network members. The first and second roundtables discussed the results from the interviews and shared the key themes emerging, which helped structure the discussion to begin generating the recommendations. Network members agreed on key topics for the recommendations to be based on (e.g. pre-diagnosis and screening), and the lead study team articulated these into written recommendations. The third and fourth roundtable meetings were hosted to receive feedback on the Delphi rating method (described below) and further refine the recommendations.
Modified Delphi Method
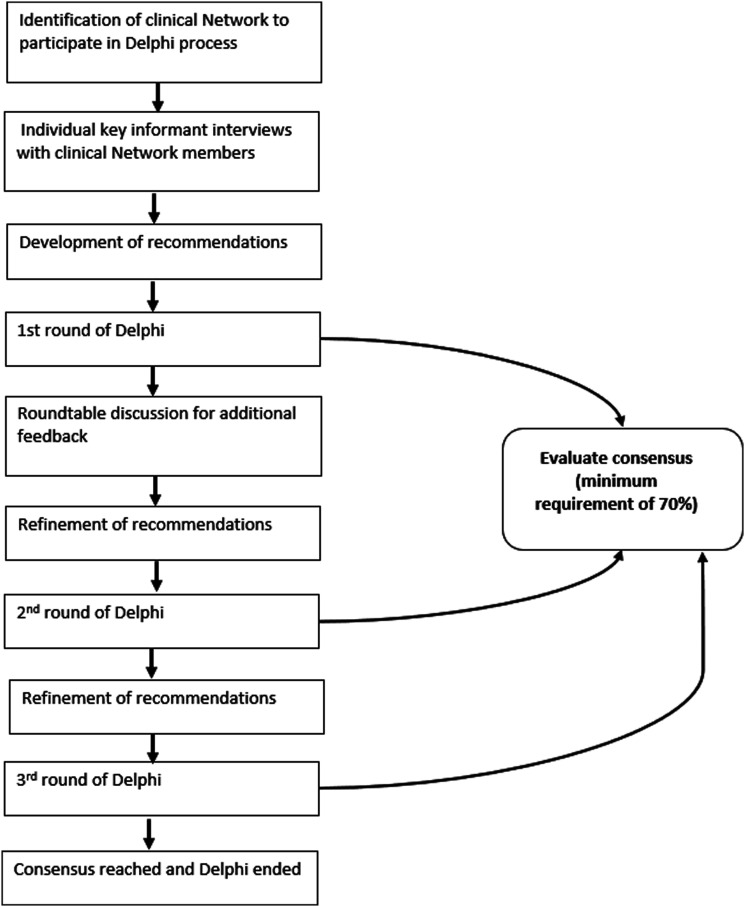
Network members were asked to rate recommendations using a modified Delphi consensus model (Figure 1).19 In the first round, interviewees were asked to rate 22 recommendations developed from the interview results and initial suggestions from the first and second roundtable discussions with the network members. Options to ‘agree’, ‘disagree’ and provide additional comments and/or suggestions within a free text box were available. A minimum satisfactory consensus of 70% was reached in the first round, with agreement on all recommendations. Subsequent roundtable discussions were hosted to obtain further feedback and consolidate agreement on any recommendations disagreed with or requiring modification. In the next round, 20 revised recommendations were shared with the same participants to rate agreement with the same format (agree, disagree, additional comments/suggestions). The recommendations were then further refined, with a final round of consensus voting resulting in 18 final recommendations.
Figure 1.
Stepwise modified Delphi Method.
Refinement of Recommendations
‘Calls to Action’ for each phase of care were identified by the network as the most crucial recommendations via Delphi consensus voting, requiring the most attention by and focus of policymakers, with ‘Good Practice Points’ being required to support the implementation of these Calls to Action. Good Practice points were further categorised broadly into phases of care (pre-diagnosis, diagnosis, therapeutic and all phases) for ease of implementation.
Results
Initial Interview Results
These are reported in the Supplementary Materials. Key themes in the differences reported between countries include: variation in data capture and auditing, composition and use of multidisciplinary teams, access to mitigating programmes for vulnerable populations and access to clinical research.
The remainder of Results section focuses on the recommendations developed during this study as these are our core output.
Recommendations
A total of 18 recommendations achieved 100% consensus. These were further grouped in to five Calls to Action (Box 1A) and thirteen Good Practice Points (Box 1B).
Calls to Action Summary
Calls to Action should be interpreted as key priorities to inform policy not only across the ICBP countries, but in similar high-income countries globally. It is recognised that different countries will have differing resource, capacity, funding, and population-based needs and should action these recommendations accordingly to their local settings.
Box 1A: Calls to Action
Implement cost-effective, clinically efficacious, and equitable lung cancer screening initiatives
Ensure diagnosis of lung cancer within 30 days of referral
Develop Thoracic Centres of Excellence
Undertake an international audit of lung cancer care
Recognise improvements in lung cancer care and outcomes as a priority in cancer policy
Good Practice Points Summary
The network agreed these points are important considerations for similar high-income countries in optimising management and treatment of lung cancer patients. They should be considered as key in supporting the development and implementation of the Calls to Action.
Box 1B: Good Practice Points
Pre-Diagnosis
• Timely and widespread availability for primary care referrals to cross sectional imaging should be prioritised in patients with a suspicion of lung cancer.
• National coordinated education materials and support should be available to public and health care providers on lung cancer and its management and outcomes.
Diagnosis
- • Where appropriate, lung malignancies should be discussed in a multidisciplinary format with essential components in place:
- • Protocols for incorporation into workplans, coordination and chairing of meetings
- • Appropriate attendance from core stakeholders
- • Means to record and report treatment decisions locally and centrally
- • Method to communicate treatment decisions to referring practitioners
• Molecular profiling of tumours should be prioritised where there is a clinical need, with pathology reports presented in a synoptic format and communicated within 2 weeks of biopsy or operation.
Therapeutic
• Incorporation of pre-habilitation programmes into routine care for patients undergoing surgical resection should be considered and implemented where appropriate.
• Curative intent therapy should be prioritised where possible, even in higher risk patients where the challenges in management of significant comorbidities and increased risk of adverse events is recognised.
• Integrated care teams should be in place and supported by surgeons with sufficient topical expertise to identify preventable adverse events in patients.
• It is the expectation that when adopting new technologies and techniques active tracking of adverse events and outcomes will be completed.
All Phases of Care
• Each country should have in place a minimum dataset for the evaluation of lung cancer patients’ diagnosis, treatment, and aftercare. Minimum datasets should have at least the following critical data elements: wait times, stage at diagnosis, risk adjusted resection and radiation rates, and significant adverse event rates.
• Commissioning of regular national clinical audits should be employed in all countries, with necessary analytical capacity for timely analysis and interrogation of data at local levels
• Emphasis on quality improvement should be placed in every part of the lung cancer pathway with regular review of audit data and processes in place to facilitate sharing and learning of best practice.
• Identification of patients at high-risk for negative outcomes should be part of routine care, with the development of appropriate pathways to mitigate poorer outcomes.
• System and investment planning for future advanced equipment should be undertaken to reflect emerging/new and innovative diagnostic methods and therapeutics.
Discussion
Rapid progress in areas of healthcare need can be achieved when international collaborative efforts, research and funding are aligned to a common goal, most recently illustrated by the development of the COVID vaccine.20 The international cancer community recognises the need to significantly improve lung cancer survival.21 The Calls to Action and Good Practice Points generated in this study provide a baseline for reflection, appropriate benchmarking, and identification of opportunities for enhancing lung cancer care. These can be interpreted from local, regional, national, and international perspectives. Many advances have been made in lung cancer prevention, screening, diagnosis, and treatment worldwide – from lower smoking rates, utilisation of CT scans for earlier detection, greater access to innovative medicines, improved operative techniques and more advanced radiation therapy. However, the potential improvements in survival have not yet been achieved. The international network formed, and recommendations developed within this study, are advocating for increased and better alignment of efforts within, and across, countries in order to improve lung cancer care and survival. We outline the importance and considerations to be had when addressing the Calls to Action below.
Implement Cost-Effective, Clinically Efficacious, and Equitable Lung Cancer Screening Initiatives
Due to variation seen in stage distribution between ICBP countries, it is important to consider where opportunities exist to optimise care and improve survival in the pre-treatment interval. Whilst the evidence is strong for a survival benefit of low dose CT screening in individuals with higher risk of lung cancer, implementation has been a complex issue for policymakers.22-24 Whilst low dose CT screening can support detection of early stage disease and increase the likelihood of curable treatment, several factors must be carefully considered to ensure clinical efficacy and cost effectiveness. Integration into existing infrastructure, target populations, recruitment of population at risk, reduction of harm, resource and cost, and engagement with the public and healthcare professionals need to be defined and implemented. Screening programmes need to be designed to reach a traditionally ‘hard to reach’ population, requiring their design to have greater engagement of those affected. There is no “one size fits all” approach, yet it is the collective opinion of this international clinical network that understanding the impact that screening could have for outcomes and considering all these factors within local contexts is a crucial activity for policymakers. Currently, no ICBP country has fully implemented a national lung cancer screening programme, though a number of countries are at different stages of exploration and implementation. Successful models of assessment and pilot trials have been underway internationally for several years and can serve as a baseline to inform the best implementation plan for screening in each jurisdiction.25-29
Ensure Diagnosis of Lung Cancer Within 30 days of Referral
Various temporal targets for key time points within the cancer patient journey exist for different countries, but there has previously been a lack of international consensus on a universal ‘best practice’ target. Unwarranted delays in diagnosis can rapidly shift lung cancer patients from being potentially curative to being difficult to treat, highlighting the importance of timely diagnosis to improve the possibility of treatment with curative intent.12 Additionally, differences in the diagnostic interval for lung cancer has been demonstrated across ICBP countries, supporting the need for greater alignment in international efforts to improve time to diagnosis and treatment parameters.30 Different countries take a more aggressive stance in their time targets with compulsory reporting, and the accountability for these (e.g. Denmark), whilst in others this can be less regimented.31 The network formed in this study have agreed that <30 days from point of referral for possible lung cancer diagnosis should be a minimum requirement to allow best opportunity for timely initiation of treatment. A crucial consideration within this target is the need to ensure timely access to diagnostic and staging tools, with strong coordination between diagnostic and treatment services – the need to improve this from a policy and practice perspective will differ, depending on jurisdictional context.
Develop Thoracic Centres of Excellence
Developing Thoracic Centres of Excellence and formally affiliated networks can help provide advanced and comprehensive patient-focused approaches to lung cancer treatment. Implementation of this aspiration in different regions may differ but essential components of Centres of Excellence for lung cancer have been researched and agreed by this international network. Expertise from multiple disciplines dedicated to lung cancer management (including thoracic surgery, oncology, anaesthesia, respiratory medicine, pathology, radiology, pulmonology rehabilitation) should be available within the Centre and its network. This enables seamless and multi-disciplinary care for patients. Critical to this is ensuring that robustly trained healthcare professionals and specialist experts relevant to these disciplines are deployed, with investment in their training and development to support continued improvement. Broader formal networks are required to provide contemporary access for patients, alongside streamlined processes across prevention, diagnosis, treatment, and survivorship care. It is important that Centres provide comprehensive care to all patients, including those first referred to local hospitals. There is some evidence in the UK that first referral to a local hospital is associated with fewer patients being offered curative treatment.32 The explanation for this is not clear but may relate to distances that patients need to travel.32 Centres should support availability of clinical trials to help deliver innovative research advances to treatments and to enhance patient outcomes. Ultimately, Thoracic Centres of Excellence and their networks should be viewed as the backbone of timely integrated care for lung cancer patients.18 Development of such Centres has been underway across ICBP countries, but relies heavily on available resource, funding, capacity, human resources, and coordination. When considering service improvement and expansion, Centres of Excellence should be in the forefront of policy plans and considerations.
Undertake an International Audit of Lung Cancer Care
High-quality data capture and timely feedback is crucial to improving care.33 The importance of this intelligence at local, regional, and national levels needs to be recognised across all countries. Embedding data capture and feedback loops into existing healthcare structures and processes in lung cancer care should be prioritised for self-evaluation and to drive service improvements. Whilst some work has been undertaken to better understand variation in delivery of lung cancer care between comparable high-income countries (as described in the Background), a truly comprehensive understanding of the key drivers of better outcomes in certain countries requires a detailed international audit. In order to deliver this vital intelligence, a number of considerations re required. A universal minimum dataset with key metrics (wait time, stage at diagnosis, risk adjusted resection and radiation rates, medical oncology treatments, significant adverse event rates) is required, alongside strong collaborative relationships between participating countries.34 Ensuring the minimum data metrics are collected as part of routine practice should be the first step to enable a successful audit within jurisdictions, in order to inform the delivery of an international audit.35 Few national audits have been undertaken for lung cancer care, but if more countries replicate this, the roadmap to commissioning an international audit will be clearer.36-38 This will allow clear identification of international best practice to facilitate sharing of optimal care and lessons learnt.
Recognise Improvements in Lung Cancer Care and Outcomes as a Priority in Cancer Policy
Continuous improvement in lung cancer care and outcomes should be recognised as an expectation by policymakers, requiring ongoing investment. The success of cancer site-specific policies in high-income countries, for example breast cancer, is reflected in enhanced policy focus, driving service development (screening programmes, diagnostic initiatives and treatment options) and underpinning improved survival internationally.39 However, even in higher performing countries, lung cancer survival is still low.2,4 Significant inequities in lung cancer risk, access to care and treatment outcomes have historically existed, driven largely by socioeconomic inequalities, and thus prohibiting improvements in lung cancer survival seen for other cancer sites.40 Recognising and addressing these inequities and inequalities in care collectively needs to be prioritised within cancer policy internationally, facilitating service improvement to optimise management and care of lung cancer patients. In order to drive this improvement, jurisdictions must consider the effectiveness of their leadership (within both clinical and political arenas) to instigate change, prioritise connection to and collaboration with appropriate stakeholders to ensure lung cancer is promoted on political agendas, and have the means to lobby for and disseminate improved lung cancer policy robustly.41 By calling for lung cancer care to be a priority internationally, we aim to encourage policymakers to prioritise the most pressing initiatives required within their respective countries to have the greatest impact upon lung cancer outcomes.
Limitations
It is acknowledged by the network and the authors that these recommendations were developed in the context of the ICBP country membership and may not fully represent the diversity of healthcare settings globally. We are aware the interviews undertaken to help inform the development of the recommendations were of a limited number, however we targeted these at stakeholders who would have a breadth of knowledge. Data saturation would therefore be unlikely to occur but we hope this format and level of questioning can now be replicated and built upon in other healthcare systems and countries to further develop our understanding of key factors in improving lung cancer care internationally. Both the network and authors hope for the recommendations developed to be applicable to other comparable high-income countries, who can interpret these in the context of their healthcare system structure and funding, cultures, socioeconomic situation, and other population-based needs. Additionally, the importance of rigorously evaluating new initiatives, policies, and the evidence base to support their implementation within local settings should not be underestimated – it is the aim of the network and the authors to bring these recommendations to the forefront of policy attention, in order for local assessment of the requirements needed to evaluate and implement.
Conclusion
The burden of lung cancer is significant and represents a global public health challenge. The Calls to Action and Good Practice Points presented here, reflecting the views of an international expert lung cancer clinical network, signpost the key considerations for policymakers within high-income comparable countries to effective change. They provide a roadmap to help align and focus efforts in improving outcomes and management of lung cancer patients. The Good Practice Points described are key to enable delivery of the Calls to Action, which should be considered the highest priority. Collaboration at local, regional, national, and international levels within the lung cancer community is imperative to empower policymakers to ensure policies are enacted that enhance lung cancer patient treatment and improve quality of life and patient outcomes.
Supplemental Material
Supplemental material for An International Consensus on Actions to Improve Lung Cancer Survival: A Modified Delphi Method Among Clinical Experts in the International Cancer Benchmarking Partnership by Charlotte Lynch, Samantha Harrison, John Butler, David R. Baldwin, Paul Dawkins, Joris van der Horst, Erik Jakobsen, Jonathan McAleese, Annette McWilliams, Karen Redmond, Anand Swaminath, and Christian J. Finley in Cancer Control
Acknowledgments
The authors would like to thank Gareth Collier of West Wales General Hospital for his participation in the key informant interviews and contribution to the development of the Calls to Action and Good Practice Points. We are grateful for Cynthia Morton, Craig Earle, Stephen Lam, Jennifer Chadder, Natalie Fitzgerald and Anubha Prashad of the Canadian Partnership Against Canada review and comments of the manuscript. We would also like to thank Mark Lawler, Rami Rahal, Diana Sarfati and Deirdre Murray of the ICBP Programme Board for providing reviews and advice of the study and contributions to the development of the manuscript.
Appendix.
Abbreviations
- CT
Computed Tomography
- ICBP
International Cancer Benchmarking Partnership
- NSCLC
Non-small cell lung cancer
- PET-CT
Positron Emission Tomography – Computed Tomography
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Charlotte Lynch https://orcid.org/0000-0002-8546-1310
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. American Cancer Society. 2018;68(6):394-424. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11):1493-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walters S, Maringe M, Butler J, Brierley JD, Rachet B, Coleman MP. Comparability of stage data in cancer registries in six countries: Lessons from the international cancer benchmarking partnership. Int J Cancer. 2012;132(3):676-685. [DOI] [PubMed] [Google Scholar]
- 4.Araghi M, Fidler-Benaoudia M, Arnold M, et al. International Differences in Lung Cancer Survival by Sex, Histological Type and Stage at Diagnosis: An ICBP SURVMARK-2 Study. Thorax. 2021;77:378-390. [DOI] [PubMed] [Google Scholar]
- 5.Morris M, Landon S, Reguilon I, Butler J, McKee M, Nolte E. Understanding the link between health systems and cancer survival: A novel methodological approach using a system-level conceptual model. Journal of Cancer Policy. 2020;25:100233. [Google Scholar]
- 6.Scott SE, Walter FM, Webster A, Sutton S, Emery J. The model of pathways to treatment: conceptualization and integration with existing theory. Br J Health Psychol. 2012;18(1):45-65. [DOI] [PubMed] [Google Scholar]
- 7.Vainio H, Weiderpass E, Kleihues P. Smoking cessation in cancer prevention. Toxicology. 2001;166(1-2):47-52. [DOI] [PubMed] [Google Scholar]
- 8.Martyn PT, Kennedy LC, Darby M, et al. Lung cancer stage-shift following a symptom awareness campaign. Thorax. 2018;73:1128-1136. [DOI] [PubMed] [Google Scholar]
- 9.Lynch C, Reguilon I, Langer DL, et al. A comparative analysis: international variation in PET-CT service provision in oncology—an international cancer benchmarking partnership study. Int J Qual Health Care. 2020;33:mzaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peter W, Rose GR, Perera-Salazar R, et al. Explaining variation in cancer survival between 11 jurisdictions in the international cancer benchmarking partnership: A primary care vignette survey. BMJ Open. 2015;5:e007212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobsen MM, Silverstein SC, Quinn M, et al. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer. 2017;112:156-164. [DOI] [PubMed] [Google Scholar]
- 12.Coalition UKLC . Millimetres Matter - Implementing the National Optimal Lung Cancer Pathway. 2018. https://www.uklcc.org.uk/sites/default/files/2021-06/UKLCC-Millimetres-Matter.pdf [Google Scholar]
- 13.Helen Hall AT, Burdett S, Fisher D, Ricketts WM, Robson J, Round T, et al. Neal Navani Association between Time-To-Treatment and Outcomes in Non-small Cell Lung Cancer: A Systematic Review. Lung Cancer; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones GS, Baldwin DR. Recent advances in the management of lung cancer. Clin Med. 2018;18(2):s41-s46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen NL, Jekunen A, Heinonen S, Dalton SO, Rasmussen TR. Lung cancer guidelines in Sweden, Denmark, Norway and Finland: a comparison. Acta Oncol. 2017;56(7):943-948. [DOI] [PubMed] [Google Scholar]
- 16.Lawrenson R, Lao C, Brown L, et al. Janice wong management of patients with early stage lung cancer – why do some patients not receive treatment with curative intent? BMC Cancer. 2020;20:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brice SN, Harper P, Crosby T, et al. Factors influencing the delivery of cancer pathways: a summary of the literature. J Health Organisat Manag. 2021;35:121-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer CPA . Pan-Canadian Standards for Thoracic Surgery. Toronto, Canada: Canadian Partnership Against Cancer (CPAC); 2018. [Google Scholar]
- 19.Okoli C, Pawlowski SD. The Delphi method as a research tool: An example, design considerations and applications. Inf Manag. 2004;42(1):15-29. [Google Scholar]
- 20.OECD . Resolving global challenges and crises through international collaboration. In: OECD Science, Technology and Innovation Outlook 2021: Times of Crisis and Opportunity. Paris, France: OECD Publishing; 2021. chap 5. [Google Scholar]
- 21.Scagliotti G. Improving survival in lung cancer: commitment of the lung ambition alliance. Evid base Oncol. 2019;25(12):SP387-SP389. [PubMed] [Google Scholar]
- 22.Stephen H, Bradley BS, Kennedy MP. What is the balance of benefits and harms for lung cancer screening with low-dose computed tomography? J R Soc Med. 2021;114(4):164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldwin DR, O'Dowd EL. Next steps and barriers to implementing lung cancer screening with low-dose CT. Br J Radiol. 2014;87(1044):20140416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darling GE, Tammemägi MC, Schmidt H, Buchanan DN, Leung Y, McGarry C, Rabeneck L. Organized lung cancer screening pilot: informing a province-wide program in Ontario, Canada. 2021. Ann Thorac Surg;111:6. [DOI] [PubMed] [Google Scholar]
- 25.Manners D, Dawkins P, Pascoe D, Crengle S, Bartholomew K, TracyLeong L, Leong TL. Lung cancer screening in Australia and New Zealand: the evidence and the challenge. Intern Med J. 2021;51(3):436-441. [DOI] [PubMed] [Google Scholar]
- 26.Crosbie PA, Balata B, Evison M, et al. Implementing lung cancer screening: baseline results from a community-based ‘Lung Health Check’ pilot in deprived areas of Manchester. Thorax. 2019;74:405-409. [DOI] [PubMed] [Google Scholar]
- 27.Australia C . Report on the Lung Cancer Screening Enquiry. New South Wales, Australia: Cancer Australia; 2020. [Google Scholar]
- 28.Toumazis I, Tsai EB, Erdogan SA, et al. Cost-effectiveness analysis of lung cancer screening accounting for the effect of indeterminate findings. JNCI Cancer Spectr. 2019;3(3):pkz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeva Sahakyan MK. Lung Cancer Screening with Low Dose Computed Tomography: Guidance for Business Case Development. Toronto, Canada: Canadian Partnership Against Cancer (CPAC); 2020. [Google Scholar]
- 30.Menon U, Vedsted P, Falborg AZ, et al. Time intervals and routes to diagnosis for lung cancer in 10 jurisdictions: cross-sectional study findings from the International Cancer Benchmarking Partnership (ICBP). BMJ Open. 2019;9:e025895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakobsen E, Green A, Oesterlind K, Rasmussen TR, Iachina M, Palshof T, Palshof T. Nationwide quality improvement in lung cancer care: the role of the danish lung cancer group and registry. J Thorac Oncol. 2013;8(10):1238-1247. [DOI] [PubMed] [Google Scholar]
- 32.Khakwani A, Rich AL, Powell HA, et al. The impact of the ‘hub and spoke’ model of care fo lung cancer and equitable access to surgery. Thorax. 2015;70(2):146-151. [DOI] [PubMed] [Google Scholar]
- 33.Spinks T, Ganz PA, Sledge GW, et al. Delivering high-quality cancer care: the critical role of quality measurement. Healthcare (Amst). 2014;2(1):53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ICfHO M . Lung Cancer; Data Collection Reference Guide. Boston, USA: International Consortium for Health Outcomes Measurement; 2015. [Google Scholar]
- 35.Rich A, Baldwin D, Alfageme I, et al. Achieving thoracic oncology data collection in Europe: A precursor study in 35 Countries. BMC Cancer. 2018;18:1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.RCo P. National Lung Cancer Audit Annual Report (For the Audit Period 2018). 2021. [Google Scholar]
- 37.Torsten G, Blum AR, Baldwin D, et al. The European initative for quality management in lung cancer care. Eur Respir J. 2014;43(5):1254-1277. [DOI] [PubMed] [Google Scholar]
- 38.Jakobsen E, Rasmussen TR. The danish lung cancer registry. Clin Epidemiol. 2016;8:537-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walters S, Benitez-Majano S, Muller P, et al. Is England closing the international gap in cancer survival? Br J Cancer. 2015;113:848-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forrest LF, Adams J, Wareham H, Rubin G, White M. Socioeconomic inequalities in lung cancer treatment: systematic review and meta-analysis. PLoS Med. 2013;10(2):e1001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris M, Seguin M, Landon S, McKee M, Notle E. Exploring the role of leadership in facilitating change to improve cancer survival: An analysis of experiences in seven high income countries in the international cancer benchmarking partnership (ICBP). Int J Health Pol Manag 2021. 10.34172/ijhpm.2021.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for An International Consensus on Actions to Improve Lung Cancer Survival: A Modified Delphi Method Among Clinical Experts in the International Cancer Benchmarking Partnership by Charlotte Lynch, Samantha Harrison, John Butler, David R. Baldwin, Paul Dawkins, Joris van der Horst, Erik Jakobsen, Jonathan McAleese, Annette McWilliams, Karen Redmond, Anand Swaminath, and Christian J. Finley in Cancer Control