Abstract
Introduction
Negative reinforcement models posit that relapse to cigarette smoking is driven in part by changes in affect and craving during the quit attempt. Varenicline may aid cessation by attenuating these changes; however, this mediational pathway has not been formally evaluated in placebo-controlled trials. Thus, trajectories of negative affect (NA), positive affect (PA), and craving were tested as mediators of the effect of varenicline on smoking cessation.
Aims and Methods
Secondary data analysis was conducted on 828 adults assigned to either varenicline or placebo in a randomized controlled trial for smoking cessation (NCT01314001). Self-reported NA, PA, and craving were assessed 1-week pre-quit, on the target quit day (TQD), and 1 and 4 weeks post-TQD.
Results
Across time, NA peaked 1-week post-quit, PA did not change, and craving declined. Less steep rises in NA (indirect effect 95% CI: .01 to .30) and lower mean craving at 1-week post-quit (CI: .06 to .50) were mediators of the relationship between varenicline and higher cessation rates at the end of treatment. PA was associated with cessation but was not a significant mediator.
Conclusions
These results partially support the hypothesis that varenicline improves smoking cessation rates by attenuating changes in specific psychological processes and supported NA and craving as plausible treatment mechanisms of varenicline.
Implications
The present research provides the first evidence from a placebo-controlled randomized clinical trial that varenicline’s efficacy is due, in part, to post-quit attenuation of NA and craving. Reducing NA across the quit attempt and craving early into the attempt may be important treatment mechanisms for effective interventions. Furthermore, post-quit NA, PA, and craving were all associated with relapse and represent treatment targets for future intervention development.
Introduction
Tobacco smoking remains a leading preventable cause of death,1 and although many cigarette users attempt to quit in any given year, most relapse within the first weeks of a quit attempt.2 Theory and empirical research suggest that changes in several key psychological processes, including affect and craving, contribute to relapse.3–5 Thus, these constructs are viable treatment targets. Consistent with this perspective, pharmacotherapies, including nicotine replacement therapy (NRT), sustained-release bupropion, and varenicline,6 reduce negative affect (NA) and craving compared with placebo in randomized clinical trials (RCTs).7,8 Of these treatments, varenicline is the most efficacious monotherapy for smoking cessation9 and was developed to facilitate greater craving reductions.10,11
Although numerous RCTs support the efficacy of varenicline for quitting smoking,8,12 little research has examined how varenicline works to promote cessation. Treatment mechanisms can be formally evaluated using mediation modeling. Path c in Figure 1 represents the focus of most RCTs: Does varenicline improve quit rates compared with a placebo control? Additionally, many clinical trials (and laboratory studies of varenicline) assess path a, which examines the effect of medication on posited mediators. Both the a and c paths explore treatment effects on potential mediators and outcomes, respectively. However, the relationship between changes in candidate mediators and clinical outcomes (path b) is typically not evaluated. Path b is critical, however, as the multiplicative effect of the path a and b (a × b) provides essential evidence for the proposed mechanistic link (the indirect effect) in the causal chain.13 Assessing indirect effects distinguishes treatment mediators as those processes that are both sensitive to treatment (path a) and are predictive of clinical outcome (path b) within a single study.
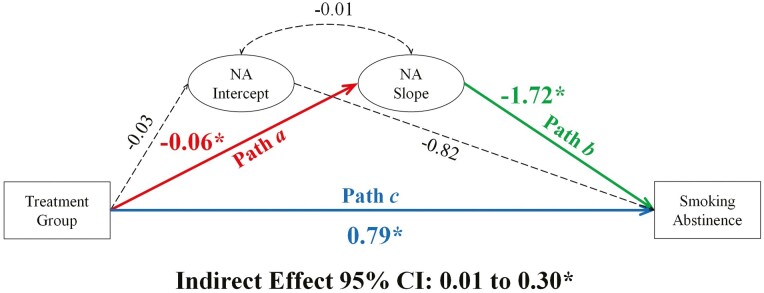
Figure 1.
Mediation model evaluating the relationship between treatment group, changes in negative affect, and smoking abstinence at end of treatment. Note. Negative affect (NA) is examined over four-time points: week −1 (intercept: pre-quit), week 0 (target quit day), week 1 (1-week post-quit), and week 4 (4 weeks post-quit). Coefficients are unstandardized. *statistically significant. Dashed lines, not statistically significant.
Although RCTs are well suited for the full assessment of the mediation chain and allow for repeated assessments of posited mediators during the quit attempt to evaluate treatment-associated changes in these processes, most research has examined treatment effects, rather than treatment mediators. Foulds et al.14 conducted a pooled analysis of RCT data and found NA peaked within the first few weeks of the quit attempt, while craving continuously declined. Varenicline attenuated the trajectories of NA and craving over time, compared to placebo.14 For NRT and bupropion, Piasecki et al.15 found withdrawal increased linearly over the first 8 weeks of the quit attempt and these trajectories were associated with both short-term lapse and longer-term relapse.15 Together these studies provided support for both the a and b paths in individual clinical trials. Less research has focused exclusively on varenicline and, in order to fully study posited treatment mechanisms, the multiplicative effect of these paths (the indirect effect) must be assessed in a single study.
Despite varenicline being approved in 2006, we could find no placebo-controlled study examining post-quit mediators of varenicline for smoking cessation. Lu et al.11 and Kim et al.16 examined mediators of pre-quit changes in smoking rate and short-term abstinence, respectively, but both studies compared varenicline with NRT; thus, these studies were insensitive to potential shared mechanisms that include craving and affect; see Ferguson et al.7 The present study seeks to fill that gap, providing valuable information about how varenicline works to promote smoking abstinence by testing the following hypotheses:
Hypothesis 1-Path a: Treatment with varenicline will attenuate changes in NA, positive affect (PA), and craving during the initial weeks of treatment, relative to placebo.
Hypothesis 2-Path b: Attenuated changes in NA, PA, and craving will predict smoking abstinence at end of treatment (EOT).
Hypothesis 3-indirect effect (Path a × Path b): Treatment-associated changes in each of the individual variables will uniquely mediate the relationship between treatment and smoking abstinence at EOT. Sleep problems, a common side effect of varenicline, were also examined as a mediator to determine if increases in sleep problems weakened the relationship between varenicline and smoking abstinence. Sleep problems were unrelated to abstinence, see Supplementary Materials Appendix 1.
Methods
Participants
Data were from 828 treatment-seeking, cigarette-using adults randomly assigned to 12 weeks of treatment with varenicline or placebo in a multi-site trial (clinicaltrials.gov: NCT01314001).17 See Table 1 for baseline participant characteristics. Primary inclusion criteria were typical of RCTs for smoking cessation (eg, age 18–65 years, smoking ≥ 10 cigarettes per day [CPD] for 6 + months, baseline carbon monoxide [CO] reading > 10 parts per million [ppm]). Participants were excluded for non-cigarette tobacco/nicotine use, frequent/problematic use of other substances, medical complications (eg, liver disease), other psychopathology, and use of psychiatric medication; see Lerman et al.17 for details.
Table 1.
Sample Demographic and Baseline Smoking Information
| Mean (SD) | Varenicline n = 420 | Placebo n = 408 | p-value |
|---|---|---|---|
| Age | 44.95 (11.71) | 45.73 (11.07) | .33 |
| Mean baseline CPD | 16.73 (6.00) | 17.31 (7.68) | .22 |
| FTND | 5.10 (2.01) | 5.36 (1.96) | .06 |
| Percent | |||
| Female | 44.52 | 42.65 | .59 |
| POC | 43.57 | 41.91 | .79 |
| Race n | .52 | ||
| White/Caucasian | 232 | 225 | |
| Black/African American | 160 | 151 | |
| Asian | 14 | 13 | |
| Other | 8 | 7 | |
| Multi-racial | 5 | 12 | |
| American Indian | 1 | 0 | |
| Ethnicity n | .31 | ||
| Non-Hispanic/Non-Latinx | 398 | 382 | |
| Hispanic/Latinx | 19 | 25 | |
| Missing/did not disclose | 3 | 1 | |
Differences assessed using one-way ANOVA and chi-square tests. CPD = cigarettes per day. FTND = Fagerstrom test for nicotine dependence. POC = people of color.
Procedures
Institutional review boards at each site approved the study protocol; all participants provided written informed consent. Participants were assessed for eligibility via phone screen and an in-person intake visit. Eligible participants were randomized to treatment and scheduled for an in-person pre-quit visit (week −1) to receive medication, attend group counseling, complete baseline measures for the posited mediators, and assess smoking rate via timeline follow-back (TLFB).18
Varenicline treatment followed standard titration during the week prior to the TQD (0.5 mg once daily for 3 days, 0.5 mg twice daily for 4 days), followed by 11 post-TQD weeks of treatment (1 mg twice daily). Phone sessions were conducted on the TQD (week 0) and at 1 and 4 weeks post-quit to collect TLFB, administer questionnaires, and conduct brief counseling. Study procedures can be found in Lerman et al.17
Measures
Consistent with the parent trial, the smoking outcome was 7-day point prevalence abstinence at end of treatment (EOT; 11 weeks post-TQD) using TLFB and bio-verified with a CO breath sample (≤8 ppm). At each time point (weeks −1, 0, 1, 4, and 11), at least 73% of the sample provided data. Participants with missing smoking data were assumed smoking.
The posited mediators were assessed using the following measures, informed by prior measurement modeling from this dataset.19 Current craving was assessed with the 10-item Questionnaire on Smoking Urges-Brief (eg, “I have a desire for a cigarette right now”).20 We used the widely used Positive and Negative Affect Scale, which is composed of two 10-item scales, to assess past week NA (eg, “distressed”) and PA (eg, “excited).21
Data Analysis
Analyses were conducted using structural equation modeling in Mplus: Version 8.22 Confirmatory factor analyses using the robust maximum likelihood estimator established a measurement model for the proposed mediators and measurement invariance procedures evaluated the consistency of the factor structure across weeks −1, 0, 1, and 4.23 Mean scores from these factors were used as indicators in unconditional latent growth models (LGMs) to determine the best fitting trajectory (slope) for each mediator (see Supplementary Appendix 2).
Following the approach of Feingold et al.24 for mediation with binary outcomes, a model was estimated for each mediator using the maximum likelihood estimator. Within these models (Figures 1, 2, and 3), the treatment group predicted smoking abstinence at EOT (path c) and the trajectory (slope) of each mediator (hypothesis 1: path a), and the trajectories of each mediator predicted smoking abstinence (hypothesis 2: path b). The indirect effect was assessed as the product of path a and path b using bias-corrected bootstrapped 95% confidence intervals (CIs; 10 000 draws) to evaluate the statistical significance of the mediated effect.13 Baseline levels (intercept) of each mediator were controlled for by regressing the intercept onto treatment group and abstinence. Effect sizes were estimated as the proportion of the total effect accounted for by the indirect effect and the proportion of variance (R2) in EOT smoking abstinence accounted for by the entire model.25 Consideration of both metrics provides information on the magnitude of the effect with the R2 value indicating the total explained variance in smoking abstinence and the indirect effect-total effect proportion indicating the contribution of the mediated effect within the explained variance.
Figure 2.
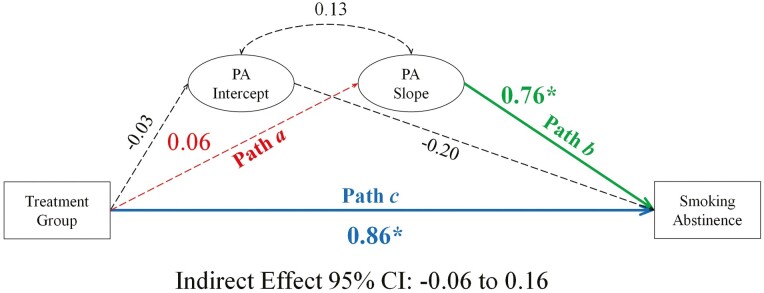
Mediation model evaluating the relationship between treatment group, changes in positive affect, and smoking abstinence at end of treatment. Note. Positive affect (PA) is examined over four-time points: week −1 (intercept: pre-quit), week 0 (target quit day), week 1 (1-week post-quit), and week 4 (4 weeks post-quit). Coefficients are unstandardized. *statistically significant. Dashed lines, not statistically significant.
Figure 3.
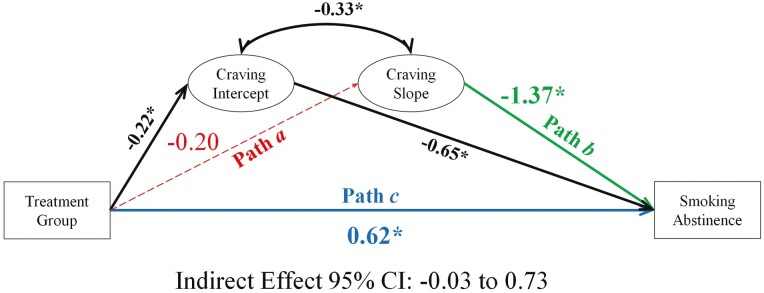
Mediation model evaluating the relationship between treatment group, changes in craving, and smoking abstinence at end of treatment. Note. Craving is examined over four-time points: week −1 (intercept: pre-quit), week 0 (target quit day), week 1 (1-week post-quit), and week 4 (4 weeks post-quit). Coefficients are unstandardized. *statistically significant. Dashed lines, not statistically significant.
After modeling each mediator individually, all significant mediators were included in a single, multiple-mediator model to examine the unique effect of each process, controlling for the other mediators. Since continued smoking may attenuate changes in the posited mediators, subgroup analyses contrasted participants who maintained (per TLFB) at least 1 day of abstinence versus those who did not (n = 212; 26%). This approach was selected over continuous abstinence due to unreliable model results related to low and unequal cell sizes for the placebo group (week 4 continuous: abstinent n = 57; smoking n = 351). Additionally, these analyses improve the generalizability of the mediation effects across individuals who experience smoking lapses and provide temporal precedence for the test of mediation. Sex, race/ethnicity, and NMR were explored as moderators of the mediational effects.17,26,27 No significant moderation effects were observed (see Supplementary Appendix 3).
Many factors influence the statistical power of LGMs for mediational analyses, including sample size, effect size, number of time points, and the proportion of total variance accounted for by the growth factor for the observed variables at each time point.28 A sample size > 600 is needed to detect small-to-medium mediation effects if the variance accounted for by the growth factors (R2) among the observed indicator variables is moderate in size (~.5).29 This suggests that the present work is adequately powered, however, low R2 from the LGMs would suggest that the possibility of Type II error.
Results
Path c: Treatment Group Effects on EOT Smoking Abstinence
As reported in Lerman et al.17, participants randomized to the varenicline group had a higher likelihood of abstinence at EOT than participants in the placebo group (34% vs. 18%; b = 0.87, p < .001; odds ratio [OR] = 2.39).
Negative Affect
On average, NA increased modestly from pre-quit to 1-week post-quit and then declined by 4 weeks post-quit (see Supplementary Figure S1, Panel A). Mediation model results for NA are presented in Figure 1; see Supplementary Table S14 for full model results.
Hypothesis 1: a Path
As predicted, the increase in NA was modestly steeper, on average, for the placebo group (b = 0.11, p < .001) compared to the varenicline group (b = 0.05, p = .06; ba path = −0.06, p = .055).
Hypothesis 2: b Path
NA trajectories were associated with EOT smoking abstinence (bb path = −1.72, p = .02); on average, less steep increases in NA were associated with a higher likelihood of abstinence (OR = 0.18, CI: .04 to .53). For participants with average increases in NA (Mslope = 0.08), the likelihood of abstinence was 24% (32% and 17% for varenicline and placebo groups, respectively). Among participants for whom NA declined over time (−1 SDslope = −0.11), the likelihood of abstinence increased to 31% (40% and 23% for varenicline and placebo groups). Conversely, among participants with above-average increases in NA (+1 SDslope = 0.27), the likelihood of abstinence was only 18% (25% and 13% for varenicline and placebo groups).
Hypothesis 3: Indirect Effect
NA trajectories over the quit attempt significantly mediated the relationship between the treatment group and EOT abstinence (indirect effect = 0.10, CI: .01 to .30). NA trajectories accounted for 11% of the total effect of varenicline treatment on smoking abstinence, and the model explained 12% of the variance in abstinence (R2 = 0.12, p = .02).
Positive Affect
The mean slope was non-significant indicating PA, on average, did not change across the quit attempt (see Supplementary Figure S1, Panel B). The variance of the PA slope was significant (p = .01), indicating PA trajectories varied between individuals. Mediation model results for PA are presented in Figure 2; see Supplementary Table S15 for full model results.
Hypothesis 1: a Path
Treatment group was not significantly associated with PA trajectories (ba path = 0.06, p = .38).
Hypothesis 2: b Path
PA trajectories were associated with EOT abstinence (bb path = 0.76, p = .01; OR = 2.15, CI: 1.25 to 4.17). The likelihood of abstinence for participants with average, non-significant change in PA (Mslope = 0.05) was 25% (33% and 17% for the varenicline and placebo groups, respectively). When PA declined over time (−1 SDslope = −0.31) the likelihood of abstinence decreased to 20% (27% and 14% for the varenicline and placebo groups). When PA increased over time (+1 SDslope = 0.41) the likelihood of abstinence increased to 30% (40% and 22% for the varenicline and placebo groups).
Hypothesis 3: Indirect Effect
PA trajectories did not significantly mediate the treatment effect on EOT abstinence (indirect effect = 0.04, CI: −.06 to .16).
Craving
On average, craving declined across the quit attempt (see Supplementary Figure S1, Panel D). Mediation model results for craving are presented in Figure 3; see Supplementary Table S16 for full model results.
Hypothesis 1: a Path
Although treatment was randomly assigned, participants in the placebo group reported, on average, greater baseline (pre-quit) craving (see Supplementary Figure S1, Panel D) than the varenicline group (b = −0.22, p = .02). However, the treatment group was not significantly associated with craving trajectories (ba path = −0.20, p = .08).
Hypothesis 2: b Path
On average, steeper declines in craving were associated with EOT abstinence (bb path = −1.37, p = .004; OR = 0.25; CI: .10 to .44). The likelihood of abstinence for participants with average declines in craving (Mslope = −1.10) was 20% (24% and 13% for the varenicline and placebo groups, respectively). For steeper than average declines in craving (−1 SDslope = −1.48) the likelihood of abstinence increased to 31% (60% and 24% for the varenicline and placebo groups). For less steep than average declines (+1 SDslope = −0.54) the likelihood of abstinence decreased to 10% (13% and 7% for the varenicline and placebo groups). Pretreatment craving was associated with smoking abstinence (b = −0.65, CI: −1.18 to −.18), suggesting that baseline craving levels predicted abstinence.
Hypothesis 3: Indirect Effect
Craving trajectories did not significantly mediate the treatment effect on EOT abstinence (indirect effect = 0.28, CI: −.03 to .73).
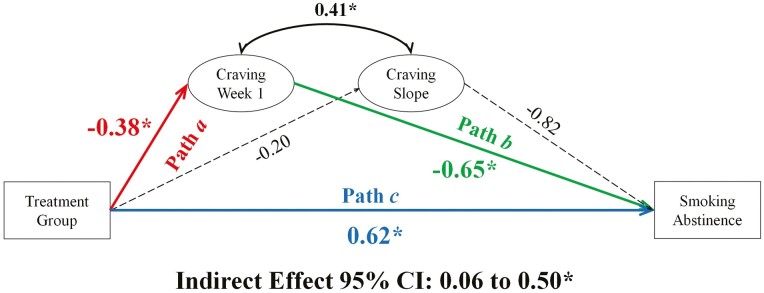
Exploratory Analysis: Craving 1-Week Post-TQD
The absence of a treatment effect on craving trajectories was surprising given prior work.10,14 The week following the TQD may be the most critical for relapse and craving;15,30,31 however, the present dataset only assessed the mediators once during this window. A post hoc model evaluated craving at week 1 post-TQD as a mediator. The intercept (0 point) of the LGM was shifted from craving at pre-quit (week −1) to 1-week post-quit (week 1). After re-assigning the intercept, treatment was used to predict the intercept (a path), which, in turn, was used to predict smoking abstinence at EOT (b path). Mediation was assessed by examining the significance of the indirect effect (a × b) using bias-corrected bootstrapped 95% CIs (10 000 draws).
Figure 4 shows model results for week 1 craving. As predicted (hypothesis 1: a Path), week 1 craving was, on average, greater among participants in the placebo group (M = 2.31, SE = 0.07) compared with the varenicline group (M = 1.90, SE = 0.09; ba path = −0.38, p < .001). Week 1 craving was associated with EOT abstinence (hypothesis 2: b Path; bb path = −0.65, p = .01); a 1-point increase in craving reduced the likelihood of abstinence by 34% (OR = 0.52). Week 1 craving significantly mediated the relationship between treatment group and abstinence (hypothesis 3: indirect effect = 0.25, CI: .06 to .50), accounting for 29% of the total effect of varenicline treatment on smoking abstinence. This model explained 31% of the variance in abstinence (R2 = 0.31, p = .001).
Figure 4.
Mediation model evaluating the relationship between treatment group, craving at 1-week post-quit, and smoking abstinence at end of treatment. Note. Coefficients are unstandardized. *statistically significant. Dashed lines, not statistically significant.
A parallel week 1 analysis was conducted for NA; no evidence for mediation was observed (see Supplementary Table S17).
Multiple-Mediator Model
The single-mediator models support NA trajectories (but not craving trajectories) and week 1 craving (but not week 1 NA) as mediators of the varenicline treatment effect. The correlations among intercepts (week −1), slopes, and week 1 scores for craving and NA (r = 0.12–0.39) suggest that the importance of running multiple-mediator models, due to potentially overlapping effects. Unfortunately, it was not possible to evaluate NA trajectories and week 1 craving in the same model. Therefore, we conducted two multiple-mediator models. The first examined the slopes/trajectories of both NA and craving (intercepts set at week −1); the second examined week 1 NA and craving (intercepts set at week 1) as mediators. Due to model complexity, 95% CIs were estimated using Monte Carlo simulation (20 000 repetitions). Models including PA were attempted, but they failed to estimate. See Supplementary Table S18 for results of the multiple-mediator models.
Mediation by NA and Craving Trajectories
Consistent with the single-mediator models, the increase in NA was steeper, on average, for the placebo group compared with the varenicline group and treatment did not predict craving trajectories (hypothesis 1: a Path). Steeper declines in craving continued to be associated with EOT smoking abstinence; however, NA trajectories were no longer associated with abstinence (hypothesis 2: b Path). Craving trajectories continued to show no evidence of mediation and, after controlling for the slope of craving, NA trajectories no longer significantly mediated the relationship between varenicline treatment and abstinence (hypothesis 3: indirect effect = −0.001, CI: −.12 to .12).
Mediation by Week 1 NA and Craving
Consistent with the single-mediator models, Week 1 craving and NA were, on average, greater among participants in the placebo group (MNA = 1.49, SENA = 0.03; MCraving = 2.30, SECraving = 0.05) compared to the varenicline group (MNA = 1.36, SENA = 0.04; MCravin = 1.92, SECraving = 0.08; hypothesis 1: a Path). Lower week 1 craving continued to be associated with EOT smoking abstinence, while week 1 NA was not associated with abstinence (hypothesis 2: b path). On average, a 1-point increase in Week 1 craving reduced the probability of abstinence by 36% (OR = 0.57). Finally, after controlling for week 1 NA, week 1 craving continued to mediate the relationship between varenicline treatment and abstinence (hypothesis 3: indirect effect = 0.22, CI: .06 to .42). Cumulatively, week 1 craving and NA accounted for 34% of the total effect between varenicline treatment and smoking abstinence; the majority of the explained variance was attributable to craving (23%), with NA accounting for a smaller and non-statistically significant proportion of the variance (10%; indirect effect = 0.10, CI: −.05 to .28). This model explained 32% of the variance in abstinence (p < .001).
Discussion
The present study used data from a placebo-controlled RCT to evaluate NA, PA, and craving as candidate mediators of the effect of varenicline versus placebo on smoking abstinence. Below, we discuss how the present findings contribute to understanding varenicline’s effect on smoking, as well as the future of other intervention efforts.32,33
Based on negative reinforcement models of addiction,3,5 theories regarding varenicline’s efficacy,10 and prior work examining the relationships between varenicline, affect, and craving with relapse, the present research tested three primary hypotheses: Hypothesis (1) Over the first month of the quit attempt, varenicline would attenuate trajectories of NA, PA, and craving compared to placebo, hypothesis (2) Attenuated trajectories in each process would be associated with smoking abstinence at EOT, and hypothesis (3) Each process would significantly mediate the relationship between varenicline and abstinence. There was partial support for these hypotheses.
The trajectory of NA across the first 4 weeks of the quit attempt and mean craving 1-week post-quit were attenuated by varenicline treatment (hypothesis 1), were associated with smoking abstinence at EOT (hypothesis 2), and partially mediated the relationship between treatment group and abstinence (hypothesis 3). In treatment settings, cessation barriers, including NA or craving, may be addressed through varenicline treatment. Additionally, new interventions can be developed to specifically target these processes to further improve cessation rates. NA and craving have been identified as treatment mediators across smoking pharmacotherapies (bupropion,34,35 NRT7,11,36); thus, these processes may be useful targets for screening new treatments37 including psychosocial interventions.38
The picture is less clear for PA. On average, PA did not change across the first month of the quit attempt, although there was significant between-person variability, which may explain the mixed literature on lowered PA during quit attempts.39 Although changes in PA were not sensitive to treatment, they were predictive of relapse; increases in PA were associated with smoking abstinence. These findings raise the possibility that enhancing PA, using interventions such as behavioral activation, may improve quit rates.40,41 Combining such an intervention with varenicline, which had no effect on PA, may be particularly efficacious because it targets multiple processes associated with abstinence.
Together, NA and craving explained approximately 35% of the relationship between treatment and smoking abstinence at EOT, leaving a sizeable, unexplained proportion of variance. Furthermore, the effect of NA was weak and only craving early in the quit attempt was a significant and independent mediator, indicating possible assessment limitations and the omission of other key mechanisms. Changes in craving are dynamic and primarily occur within the first few weeks of the quit attempt15,30 with craving peaking a few days after the TQD.31,42 This study only assessed craving twice within the first week of quitting. EMA work suggests that the relationship between craving and smoking lapse is a short-term process with higher momentary urges predicting lapse later that day or on the subsequent day.43,44 Additionally, the mediational effect of NA trajectories was not unique and overlapped with declines in craving during the quit attempt, indicating an interdependent relationship between changes in NA and craving. Both experimental and naturalistic research has found NA and craving predict one another during smoking abstinence.45–47 Reduced craving from varenicline treatment may also prevent rises in NA due to discomfort from smoking urges. However, to better understand and test these complex mediational pathways, daily, real-world assessments are needed. The addition of other posited mediators of varenicline including smoking expectancies,11 satisfaction,16 and reinforcement48 may also explain a substantial amount of the relationship between varenicline and smoking abstinence.
The present research provides a limited assessment of several key variables as an initial examination of treatment mechanisms for varenicline. However, even with this focus on a few candidate variables, numerous statistical tests were required, increasing the probability of type I error using conventional significance testing criteria. For example, the mediated effects of NA achieved the arbitrary cutoffs for statistical significance, but accounted for a small proportion of the total effect and NA trajectories showed little change over time. It remains questionable whether the small changes in NA observed between the treatment groups (eg, less than a 1-point difference on a self-report scale) actually represent a clinically significant effect.
Low sample diversity may have weakened effects due to restricted range. Individuals with a significant psychiatric history were ineligible for this study. Therefore, this sample likely reflects lower levels of NA than more typical cigarette users given the high comorbidity between smoking and psychopathology.49 Floor effects were evident for NA, allowing little room for downward growth or variability to predict between-person differences. Furthermore, all the mediators showed little change and this limited range has been observed in other clinical trials of varenicline.14 In addition to the highly selected samples that may restrict range,50 Foulds et al. (2013) suggested that the anchors of common self-report measures lead to a lower endorsement.14 Such limited variability may have contributed to small R2 lowering power within the mediation models.29 Low quit rates among the current sample may have further attenuated effects. By EOT, only 34% of participants in the varenicline group and 18% in the placebo group were bio-verified abstinent, which is lower than other varenicline trials (c.f., 7-day point prevalence rates at 11 weeks post-quit from Jorenby et al. (2006)8: Varenicline = 51%, Placebo = 21%. See Lerman et al. (2015) for discussion17).
Despite these limitations, the present research provides the first placebo-controlled evaluation of the post-quit psychological processes that mediate the effect of varenicline on smoking abstinence. Varenicline’s strong efficacy is partially explained by reductions in NA and craving, which accounted for 34%–36% of the effect of varenicline on abstinence at EOT. Although PA was associated with relapse, it did not mediate the relationship between varenicline and abstinence. These results inform future directions for understanding how varenicline and other effective interventions may work by attenuating changes in affect and craving. Using mediational modeling, the present research elucidates the processes underlying varenicline’s efficacy and relapse. This work informs intervention development by providing relapse prevention targets and allowing more personalized treatment options depending on an individual’s perceived cessation barriers.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
The authors acknowledge the contributions of Jennifer Adams, Constance Duerr, and the entire Pharmacogenetics of Nicotine Addiction Treatment group for their assistance in collecting these data. We also thank Drs. Stephen Tiffany and Paul Meyers for their helpful comments on this manuscript. Finally, we thank the community members who participated in this project.
Contributor Information
Sarah S Tonkin, Department of Psychology, University at Buffalo, State University of New York, Buffalo, NY, USA; Department of Psychiatry, University of Texas Health Science Center at Houston, Houston, TX, USA.
Craig Colder, Department of Psychology, University at Buffalo, State University of New York, Buffalo, NY, USA.
Martin C Mahoney, Departments of Internal Medicine and Health Behavior, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Gary E Swan, Department of Medicine, Stanford Prevention Research Center, Stanford University School of Medicine, Palo Alto, CA, USA.
Paul Cinciripini, Department of Behavioral Science MD Anderson Cancer Center, University of Texas, Houston, TX, USA.
Robert Schnoll, Department of Psychiatry and Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Tony P George, Departments of Psychiatry, Pharmacology & Toxicology, Centre for Addiction and Mental Health, University of Toronto, Toronto, ON, Canada.
Rachel F Tyndale, Departments of Psychiatry, Pharmacology & Toxicology, Centre for Addiction and Mental Health, University of Toronto, Toronto, ON, Canada.
Larry W Hawk, Jr, Department of Psychology, University at Buffalo, State University of New York, Buffalo, NY, USA.
Funding
Data collection was supported in part by the Pharmacogenomics Research Network, Pharmacogenetics of Nicotine Addiction Treatment Research Group, funded primarily through the National Institutes of Health (National Institute on Drug Abuse, National Cancer Institute, National Institute of General Medical Sciences, and National Human Genome Research Institute; U01-DA20830). This research was undertaken, in part, thanks to funding from the Canada Research Chairs program (Dr. Tyndale, the Canada Research Chair in Pharmacogenomic), and the Center for Addiction and Mental Health. The development of this article was supported in part by the National Institutes of Health (National Cancer Institute; R01CA206193) and the Association for Psychological Sciences Student Grant Competition. Pfizer provided varenicline and placebo for this study, but had no role in the study design, data analysis, or interpretation.
Declaration of Interest
Dr. Martin Mahoney has received medication from Pfizer in support of clinical trials, has served on the Speaker’s Bureau for Pfizer, and has served as the medical director of the New York State Smokers Quit Line. Paul Cinciripini has served on the scientific advisory board of Pfizer Pharmaceuticals, did educational talks sponsored by Pfizer, and has received grant and medication support from Pfizer. Dr. Robert Schnoll received medication and placebo free of charge from Pfizer for other clinical trials and has provided consultation to Pfizer, GlaxoSmithKline, and Palliatech. Dr. Tony George has been a consultant on smoking cessation pharmacotherapies for Novartis, Frutarom, and Sanford Burnham Prebys. Dr. Rachel Tyndale has acted as a consultant to Quinn Emanuel and Ethimos. Dr. Larry Hawk has received medication from Pfizer in support of clinical trials. The remaining authors declare no competing interests.
Data Availability
The data underlying this article were provided by Pharmacogenetics of Nicotine Addiction Treatment Research Group by permission. Data will be shared on request to the corresponding author with the permission of Pharmacogenetics of Nicotine Addiction Treatment Research Group.
References
- 1. Jamal A, Phillips E, Gentzke AS, et al. Current cigarette smoking among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(2):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. (CDC) CfDCaP. Quitting smoking among adults--United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- 3. Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC.. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. [DOI] [PubMed] [Google Scholar]
- 4. Hughes JR. Clinical significance of tobacco withdrawal. Nicotine Tob Res. 2006;8(2):153–156. [DOI] [PubMed] [Google Scholar]
- 5. Solomon RL. Addiction: an opponent-process theory of acquired motivation: the affective dynamics of addiction. New York, NY: W H Freeman/Times Books/Henry Holt & Co; 1977. [Google Scholar]
- 6. Cahill K, Stevens S, Lancaster T.. Pharmacological treatments for smoking cessation. JAMA. 2014;311(2):193–194. [DOI] [PubMed] [Google Scholar]
- 7. Ferguson SG, Shiffman S, Gwaltney CJ.. Does reducing withdrawal severity mediate nicotine patch efficacy? a randomized clinical trial. J Consult Clin Psychol. 2006;74(6):1153–1161. [DOI] [PubMed] [Google Scholar]
- 8. Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. [DOI] [PubMed] [Google Scholar]
- 9. Cahill K, Stevens S, Perera R, Lancaster T.. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 9329;2013(5):CD00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48(10):3474–3477. [DOI] [PubMed] [Google Scholar]
- 11. Kim N, McCarthy DE, Piper ME, Baker TB.. Comparative effects of varenicline or combination nicotine replacement therapy versus patch monotherapy on candidate mediators of early abstinence in a smoking cessation attempt. Addiction. 2020;116(4):926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166(15):1561–1568. [DOI] [PubMed] [Google Scholar]
- 13. MacKinnon DP, Fairchild AJ, Fritz MS.. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foulds J, Russ C, Yu CR, et al. Effect of varenicline on individual nicotine withdrawal symptoms: a combined analysis of eight randomized, placebo-controlled trials. Nicotine Tob Res. 2013;15(11):1849–1857. [DOI] [PubMed] [Google Scholar]
- 15. Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB.. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. J Abnorm Psychol. 2003;112(1):14–27. [PubMed] [Google Scholar]
- 16. Lu W, Chappell K, Walters JAE, et al. The effect of varenicline and nicotine patch on smoking rate and satisfaction with smoking: an examination of the mechanism of action of two pre-quit pharmacotherapies. Psychopharmacology (Berl). 2017;234(13):1969–1976. [DOI] [PubMed] [Google Scholar]
- 17. Lerman C, Schnoll RA, Hawk LW, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown RA, Burgess ES, Sales SD, et al. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12(2):101–112. [Google Scholar]
- 19. Tonkin SS, Williams TF, Simms LJ, et al. Withdrawal symptom, treatment mechanism, and/or side effect? developing an explicit measurement model for smoking cessation research. Nicotine Tob Res. 2020;22(4):482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cox LS, Tiffany ST, Christen AG.. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 21. Watson D, Clark LA, Tellegen A.. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 22. Mplus [computer program]. Version 8. Los Angeles, CA: Muthén & Muthén; 2017. [Google Scholar]
- 23. Cheung GW, Rensvold RB.. Evaluating goodness-of-fit indexes for testing measurement invariance. Struct Equ Modeling. 2002;9(2):233–255. [Google Scholar]
- 24. Feingold A, MacKinnon DP, Capaldi DM.. Mediation analysis with binary outcomes: direct and indirect effects of pro-alcohol influences on alcohol use disorders. Addict Behav. 2019;94:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee HR, Sung J, Lee S.. Point and interval estimators of an indirect effect for a binary outcome. Int. J. Assess. Eval. 2021;8(2):279–295. [Google Scholar]
- 26. Hawk LW, Jr., Ashare RL, Lohnes SF, et al. The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clin Pharmacol Ther. 2012;91(2):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH.. Sex differences in varenicline efficacy for smoking cessation: a meta-analysis. Nicotine Tob Res. 2016;18(5):1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diallo TM, Morin AJ, Parker PD.. Statistical power of latent growth curve models to detect quadratic growth. Behav Res Methods. 2014;46(2):357–371. [DOI] [PubMed] [Google Scholar]
- 29. Cheong J. Accuracy of estimates and statistical power for testing meditation in latent growth curve modeling. Struct Equ Modeling. 2011;18(2):195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shiffman SM, Jarvik ME.. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl). 1976;50(1):35–39. [DOI] [PubMed] [Google Scholar]
- 31. Van Zundert RM, Boogerd EA, Vermulst AA, Engels RC.. Nicotine withdrawal symptoms following a quit attempt: an ecological momentary assessment study among adolescents. Nicotine Tob Res. 2009;11(6):722–729. [DOI] [PubMed] [Google Scholar]
- 32. Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol. 2007;3:1–27. [DOI] [PubMed] [Google Scholar]
- 33. Kraemer HC, Wilson GT, Fairburn CG, Agras WS.. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–883. [DOI] [PubMed] [Google Scholar]
- 34. Lerman C, Roth D, Kaufmann V, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend. 2002;67(2):219–223. [DOI] [PubMed] [Google Scholar]
- 35. McCarthy DE, Piasecki TM, Lawrence DL, et al. Psychological mediators of bupropion sustained-release treatment for smoking cessation. Addiction. 2008;103(9):1521–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shiffman S. Effect of nicotine lozenges on affective smoking withdrawal symptoms: secondary analysis of a randomized, double-blind, placebo-controlled clinical trial. Clin Ther. 2008;30(8):1461–1475. [DOI] [PubMed] [Google Scholar]
- 37. Perkins KA, Stitzer M, Lerman C.. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology (Berl). 2006;184(3–4):628–636. [DOI] [PubMed] [Google Scholar]
- 38. Spears CA, Hedeker D, Li L, et al. Mechanisms underlying mindfulness-based addiction treatment versus cognitive behavioral therapy and usual care for smoking cessation. J Consult Clin Psychol. 2017;85(11):1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klemperer EM, Hughes JR, Peasley-Miklus CE, et al. Possible new symptoms of tobacco withdrawal III: reduced positive affect-a review and meta-analysis. Nicotine Tob Res. 2021;23(2):259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacPherson L, Tull MT, Matusiewicz AK, et al. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. J Consult Clin Psychol. 2010;78(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martinez-Vispo C, Rodriguez-Cano R, Lopez-Duran A, et al. Cognitive-behavioral treatment with behavioral activation for smoking cessation: randomized controlled trial. PLoS One. 2019;14(4):e0214252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doherty K, Kinnunen T, Militello FS, Garvey AJ.. Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology (Berl). 1995;119(2):171–178. [DOI] [PubMed] [Google Scholar]
- 43. Allen SS, Bade T, Hatsukami D, Center B.. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine Tob Res. 2008;10(1):35–45. [DOI] [PubMed] [Google Scholar]
- 44. Shiffman S, Engberg JB, Paty JA, et al. A day at a time: predicting smoking lapse from daily urge. J Abnorm Psychol. 1997;106(1):104–116. [DOI] [PubMed] [Google Scholar]
- 45. Bujarski S, Roche DJ, Sheets ES, et al. Modeling naturalistic craving, withdrawal, and affect during early nicotine abstinence: a pilot ecological momentary assessment study. Exp Clin Psychopharmacol. 2015;23(2):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lanza ST, Vasilenko SA, Liu X, Li R, Piper ME.. Advancing the understanding of craving during smoking cessation attempts: a demonstration of the time-varying effect model. Nicotine Tob Res. 2014;16(Suppl 2):S127–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leventhal AM, Greenberg JB, Trujillo MA, et al. Positive and negative affect as predictors of urge to smoke: temporal factors and mediational pathways. Psychol Addict Behav. 2013;27(1):262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lawson SC, Gass JC, CooperRK, Jr, et al. The impact of three weeks of pre-quit varenicline on reinforcing value and craving for cigarettes in a laboratory choice procedure. Psychopharmacology (Berl). 2021;238(2):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet. 1995;25(2):95–101. [DOI] [PubMed] [Google Scholar]
- 50. Motschman CA, Gass JC, Wray JM, et al. Selection criteria limit generalizability of smoking pharmacotherapy studies differentially across clinical trials and laboratory studies: a systematic review on varenicline. Drug Alcohol Depend. 2016;169:180–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by Pharmacogenetics of Nicotine Addiction Treatment Research Group by permission. Data will be shared on request to the corresponding author with the permission of Pharmacogenetics of Nicotine Addiction Treatment Research Group.