Abstract
Objectives:
Comparison of continuous subcutaneous insulin infusion (CSII) with multiple daily injections (MDI) in achieving glycemic control in youths with type 1 diabetes mellitus (T1DM).
Methods:
Retrospective cohort study including 2 matched groups of youths with T1DM treated by CSII or MDI in a tertiary specialized children’s hospital in Saudi Arabia. Children and adolescents aged up to 18 years, diagnosed with T1DM and using CSII or MDI, from the period 2016 to 2018. Patients on MDI were newly-diagnosed patients with T1DM who had the disease for only 1 year duration; all CSII patients had at least 1 to 2 years of T1DM but who had just started on pumps in the past 3 months. We excluded patients with other autoimmune diseases, non-ambulatory patients and those admitted to hospital for non-diabetes reasons. Primary outcome was HbA1c at 1, 2, and 3 years, with weight gain as a secondary outcome. Ambulatory glycemic profile was analyzed from a subset of patients using intermittently scanned continuous glucose monitoring (isCGM).
Results:
A total of 168 youths with T1DM (n = 129 in the MDI group, n = 39 in the CSII group) were included. The CSII group consistently had lower HbA1c levels compared to the MDI group throughout a 3-year follow up period: 8.1% versus 10.1, P-value < .001 at 1 year, 7.5% versus 10.1% at 2 years, P-value < .001, 8.9% versus 10.3% at 3 years, P-value = .033. Body mass index significantly increased in both groups at 1 year, although greater in CSII group. In a subgroup using isCGM (n = 37 on MDI and n = 29 on CSII), the CSII group had a lower average blood glucose (194 mg/dL vs 228 mg/dL, P-value = .028) and a lower estimated HbA1c level (8.4% vs 9.6%, P-value = .022).
Conclusion:
Treatment with CSII resulted in lower HbA1c compared to MDI in our cohort, which was sustained over a 3-year period.
Keywords: CSII, glycemic, hemoglobin A1c, insulin, MDI, pump, type 1 diabetes mellitus
Introduction
Type 1 diabetes mellitus (T1D) occurs when the pancreas fails to produce insulin. Therefore, exogenous insulin therapy is critical to achieve glycemic control in patients with T1D and to prevent its complications.1-3 Treating diabetes mellitus is a costly effort to the patient, family and health systems due to nonexistence of a cure for the disease.4 The ultimate goal of management of T1D is to achieve adequate glycemic control in order to prevent or delay the development of diabetes-related complications. Therefore, understanding the different concepts underpinning the presence and persistence of vascular complications of diabetes is essential to guide an optimized treatment approach. The pathophysiology of a concept of metabolic memory to explain these complications even after optimizing glucose level and reaching the HbA1c target is still not well established.5,6 However, it was suggested that the persistent complications are due to the cumulative glycemic exposure,7 whether it was from a low exposure for a long period of time or a high exposure in a short period of time, mediated by an epigenetic alteration.8 While the incidence of diabetes complications was lower in those initially receiving intensive insulin therapy from the Diabetes Control and Complications Trial up to a decade after the end of the study,9-11 the year-to-year incidence of retinopathy was similar in both intensive insulin and conventional therapy groups after a longer period of follow up, supporting the notion that the memory effect of accumulation of glycemic exposure plays an important role in driving diabetes complications.12
Two major aspects in the management of T1D are monitoring of blood glucose levels and administering intensive insulin therapy to achieve glycemic control. Self-monitoring of blood glucose (SMBG) allows patients an accurate measurement of glycemic control that facilitates intelligent adjustment of insulin. The American Diabetes Association (ADA) recommends that SMBG should be performed multiple times daily for patients who are using MDI of insulin or CSII.13 However, the need for continuous glucose monitoring (CGM) has been increasingly emphasized over the years. From a patient/family perspective, there is resistance towards SMBG due to associated pain, the non-discreet nature of testing, competing priorities and the fear of repeated failures from the results .14,15 CGM, however, do not obviate the need for SMBG because they tend to be less accurate than direct measurements during times of rapid glucose fluctuation and they are also more expensive than using SMBG.16,17 Nonetheless, CGM may be useful in the treatment of patients with T1D in terms of compliance to their insulin regimen and in giving early warning alarms that could be beneficial in patients suffering from hypoglycemia unawareness.18
With regards to insulin administration, the standard treatment in most settings of clinical practice worldwide is administering multiple daily injections (MDI) of insulin analogs that have different pharmacokinetic properties.19 However, a continuous subcutaneous insulin infusion (CSII) or insulin pump trying to mimic the function of the pancreas may be more physiological.4,20 The results from previous studies comparing MDI versus CSII are conflicting.4,21-25 In some literature, it has been shown that CSII is superior to MDI in lowering hemoglobin A1c levels as well improving glycemic profile.21,23 However, others showed no difference between the 2 groups especially in the first year of treatment.4,25 Also, there was no significant difference between CSII and MDI regarding non-severe hypoglycemia as an adverse effect in previous studies.22,24 Additionally, whether the effect of CSII on glycemic control is sustained over prolonged periods is unclear. Most evidence regarding CSII is derived from clinical trials, and while evidence from clinical trials is invaluable, real-world data from clinical practice is essential to better understand the impact of CSII on diabetes care and glycemic control outside of controlled settings.
In this study, we aimed to compare the effectiveness of CSII versus MDI in children with T1D in our specialized children hospital in Riyadh, Saudi Arabia, using the glycemic control as an outcome measure. Our study provides real-life data pertaining to glycemic control for a prolonged follow up period of 3 years. With the increasing use of CGM and ongoing increase in the incidence of T1D among children, the analysis of such data would inform decision making and patients’ choice of management from clinical and cost effectiveness point of views in our population and in children with similar characteristics and psychosocial settings (eg, in other regions of Saudi Arabia and Gulf region).
Materials and Methods
Objectives
Primary objective: The main objective of our study was to compare insulin pump therapy, also known as continuous subcutaneous insulin infusion (CSII) with multiple daily insulin (MDI) in their efficacy of managing T1D in pediatric patients as reflected by HbA1c levels, at a tertiary specialized children hospital in Riyadh, Saudi Arabia.
Secondary objective: In a subgroup of patients who were using continuous flash glucose monitoring, the 2-week glycemic profile was used as a measure of efficacy of T1D treatment. We also evaluated the effect of insulin mode delivery on weight gain over a 1-year period.
Study area
This study was conducted in King Abdullah Specialized Children’s Hospital (KASCH) in Riyadh, Saudi Arabia that has the capacity of 600 beds and a pediatric intensive care unit. KASCH is the only specialized children’s hospital in Saudi Arabia that opened in early 2015 to provide tertiary care of children with acute and chronic problems. The endocrinology department provides inpatient and outpatient consultations, diagnostic evaluation, and management of children and adolescents with diabetes, mainly T1DM. The department provides inpatient and outpatient care for these children, including intensive education utilizing the latest technology for children with diabetes. On average, we treated 562 children with T1DM patients annually in our institute during the study period.
Patients in our center were offered CSII as per guidance from the national institute of clinical excellence (NICE), UK. NICE guidance mainly focuses on considering the difficulty in achieving glycemic control in older children and the appropriateness and practicality of using CSII in younger age groups. Adherence to the NICE guidance helps limit potential selection bias in offering pumps to the patients in our center. All patients receive the same level of education targeting intensification of insulin therapy with variations only based on the type of therapy they receive.
Participants
We retrieved the medical records of all children and adolescents (0-18 years), who were diagnosed with T1D and using CSII or MDI, from the period 2016 to 2018. All of the MDI patients selected in this study were newly diagnosed patients with T1DM who had the disease for only 1 year duration prior to the first point of data collection, and all CSII had at least 1 to 2 years of T1D but were just started on pumps 0 to 3 months at T0 time of the study. We excluded patients who were diagnosed with other autoimmune diseases to facilitate easy matching of patients in the 2 cohorts. Also, to avoid potential confounders that could affect the variations in glycemic control between the 2 groups, we also excluded those who were admitted to hospital due to non-diabetes reasons that could had affect their HbA1c level such as surgeries, infections, and when requiring a use of antibiotics. Patients who were bedridden or wheelchair bound patients were also excluded in this study.
Study design
Our study is a retrospective cohort study that compares MDI versus CSII in 2 groups of young children and adolescents with T1D who have follow up in our institute (January 2016-May 2019). Both groups were matched for age, male to female ratio, HbA1c at the time of initial data collection, total daily dose of insulin; there was no difference in mean age and mean HbA1c at the start of the comparison. We tried to maintain the proportions in each of the comparative groups and the p values were not different in all of the above matching points such as age, gender and initial mean HbA1c.
In addition to variables that described patients’ demographics, we collected data that pertained to past medical history and laboratory studies. The main outcome variable of the study was the glycemic control using HbA1c that we assessed after the first year of treatment and whether that effect was sustainable in the subsequent 2 years. HbA1c is measured using a standardized ion exchange high-performance liquid chromatography (HPLC) method and the target is to be performed in all children with T1D every 3 to 4 months in our center; however, that depends on patients’ attendance to outpatient clinic and commitment to do HbA1c in the laboratory. Point of care HbA1c service was not available at the time of the study; therefore, we were unable to check it in all patients that attended the clinic. Our study center is a tertiary hospital receiving patients from all over the country; hence, we had some patients who occasionally do their HbA1C test in other health facilities nearby their homes with no track in our records. This explains the missing at random of HbA1C results at different points of the study for patients in the matched 2 cohorts of MDI and CSII groups. We also assessed the effect of treatment modality on body mass index after a year of treatment.
We included a small subgroup of patients who used intermittently scanned continuous glucose monitoring isCGM (Free Style libre) for at least 3 months. Average BG, time in target as well as above and below target of BG readings were compared in patients on CSII versus MDI. The glycemic profile data in this subgroup were collected for a 2-week period during the last 3 months of the study period. Time in range was defined as blood glucose between 70 and 180 mg/dL, time above range was defined as blood glucose above 180 mg/dL and time below range was defined as blood glucose less than 70 mg/dL. During the period of the study, we did not have the smart pumps with a low glucose suspend function available to most of patients in our center and all of our patients were using conventional Medtronic Veo pumps or had limited supply of CGM sensors for 640G Medtronic pumps; hence, they were only using Free Style libre as isCGM.
Statistical analysis
Sample size, with alpha set at .05 to achieve a power of 90%, was estimated using Piface based on results of a previous similar study, where the HbA1c mean difference was 0.5% with standard deviation of 1%.23 A non-probability convenient sampling technique was employed by including all children who were diagnosed with T1D and using CSII with a selected matched group of MDI patients. Data was analyzed using SPSS version 24. Descriptive statistics contained 2 types of variables: categorical variables such as gender and mode of insulin delivery (MDI vs CSII), and continuous variables such as age, HbA1c and BMI. Categorical variables were analyzed by frequency and percentage. For continuous variables, we reported means and standard deviations. In the analysis of the subgroup using isCGM, we reported median and interquartile ranges as the number of patients in the subgroup was relatively small. Independent t-test was used to compare HbA1c levels between the 2 groups (MDI vs CSII). A test with a P-value less than .05 was considered statistically significant.
Ethics approval
This study was approved by the institutional review board (IRB) of King Abdullah International Medical Research Center, Riyadh, Saudi Arabia (RC19/125/R, May 2019). Given the retrospective nature of the study and the lack of active intervention, consent was waived.
Results
Our cohort included a total 168 children and adolescents, who were followed in the pediatric diabetes clinic over a 3-year period (2016-2018) including 129 participants (50% females) in the MDI group and 39 participants (n = 17, 43% females) in the CSII group (Table 1). At baseline, both groups were similar for age, male to female ratio, HbA1c, total daily dose of insulin; there was no difference in mean age and mean HbA1c at the start of the comparison (Table 1).
Table 1.
Characteristics of our patients.
| Characteristics | MDI | CSII | P value |
|---|---|---|---|
| n | 129 | 39 | |
| Age (years), (mean ± SD) | 12.40 ± 2.20 | 12.64 ± 2.69 | .575 |
| Gender | .475 | ||
| M | 65 (50.40%) | 17 (43.60%) | |
| F | 64 (49.60%) | 22 (56.40%) | |
| Total daily dose (units/kg/d), (mean ± SD) | 0.8 ± 0.4 | 0.8 ± 0.4 | 1 |
| HbA1c (%), (mean ± SD) | 9.5 ± 1.6 | 9.0 ± 1.9 | |
| M | 9.4 ± 1.4 | 8.6 ± 1.9 | .056 |
| F | 9.7 ± 1.8 | 9.4 ± 1.8 | .474 |
| BMI (kg/m2) | 18.0 | 19.3 | .093 |
| Number of available HbA1c resultsa | |||
| Baseline | 129 | 39 | |
| Year 1 | 235 | 73 | |
| Year 2 | 236 | 45 | |
| Year 3 | 213 | 27 | |
| isCGM Study groupb | 37 | 29 | |
Abbreviations: CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections; SD, standard deviation; isCGM, intermittently scanned continuous glucose monitoring.
Average number of HbA1c tests per patient per study period was 5.9 ± 2.6 tests.
Patients in this study group used Freestyle libre (continuous glucose monitoring) over 2 weeks period.
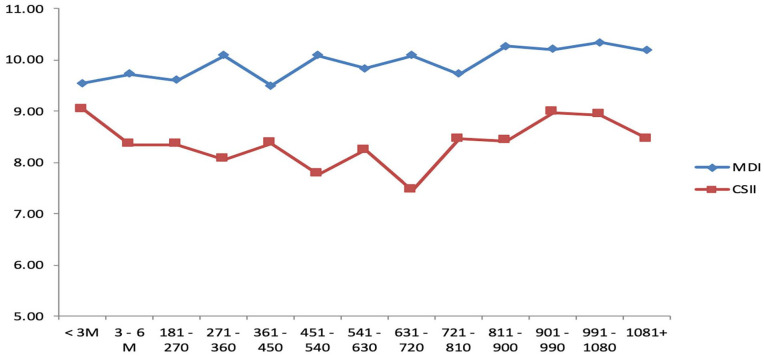
While both groups started out with similar HbA1c levels (9.5% (80 mmol/mmol) in MDI group versus 9.0% (75 mmol/mmol) in CSII group, P-value = .102), HbA1c in the CSII group was better compared to the MDI group at 1-year (8.1% (65 mmol/mmol) versus 10.1% (87 mmol/mmol), P-value < .001). The lower HbA1c level observed in the CSII group compared with the MDI group was sustainable and remained throughout the study (7.5% (58 mmol/mmol) versus 10.1% (87 mmol/mmol) at 2 years, P-value < .001; 8.9% (74 mmol/mmol) versus 10.3% (89 mmol/mmol) at 3 years, P-value = .033; 8.5% (mmol/mmol) versus 10.2% (88 mmol/mmol) at more than 3 years, P-value = .005) (Table 2).
Table 2.
Comparison between HbA1c between MDI and CSII.
| Insulin regimen |
MDI |
CSII |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| Years | Days | Number of HbA1c | Mean HbA1c | SD | N | Mean HbA1c | SD | |
| Year 1 | −30 to 90 | 129 | 9.5 | 1.6 | 39 | 9.0 | 1.9 | .102 |
| 91-180 | 61 | 9.7 | 2.1 | 22 | 8.4 | 1.0 | .005 | |
| 181-270 | 55 | 9.6 | 1.8 | 22 | 8.4 | 1.1 | <.001 | |
| 271-360 | 61 | 10.1 | 1.7 | 14 | 8.1 | 1.2 | <.001 | |
| 361-450 | 58 | 9.5 | 1.5 | 15 | 8.4 | 1.1 | .008 | |
| Year 2 | 451-540 | 58 | 10.1 | 1.6 | 11 | 7.8 | 1.4 | <.001 |
| 541-630 | 64 | 9.8 | 1.6 | 15 | 8.2 | 1.2 | <.001 | |
| 631-720 | 63 | 10.1 | 1.4 | 8 | 7.5 | 1.0 | <.001 | |
| 721-810 | 51 | 9.7 | 1.6 | 11 | 8.5 | 1.5 | .018 | |
| Year 3 | 811-900 | 57 | 10.3 | 1.7 | 8 | 8.4 | 1.2 | .004 |
| 901-990 | 40 | 10.2 | 1.8 | 4 | 9.0 | 2.3 | .2 | |
| 991-1080 | 51 | 10.3 | 1.6 | 7 | 8.9 | 1.2 | .033 | |
| 1080+ | 65 | 10.2 | 1.6 | 8 | 8.5 | 1.1 | .005 | |
In the CSII group, most of the improvement in HbA1c occurred in the first 3 months after treatment and was sustained achieving the best HbA1c level by 2 years of treatment (Table 2). However, patients returned to the same point of 1-year HbA1c level by 3 years of treatment (Table 2). Overall, we observed that HbA1c in the MDI group increased throughout the study period, starting at 9.5% (80 mmol/mmol) at the start of the study and increasing up to 10.2% (88 mmol/mmol) at the end of the observation period (Figure 1). There were no differences in HbA1c level between genders, except during the first 3 months where males had lower HbA1c level compared to females in the MDI group. However, this gender difference was no longer present beyond the first 3 months of the study. Additionally, to test the influence of puberty, a sub analysis based on age using a cut-off of 12 years was conducted and found no difference in HbA1c between both age groups.
Figure 1.
Fluctuations in A1c in MDI and CSII patients.
With regards to BMI, baseline BMI in both groups were similar (18.0 kg/m2 in the MDI group vs 19.3 kg/m2 in the CSII, P-value = .093); BMI at 1-year was more elevated in the CSII group compared to the MDI group (20.9 vs 18.6 kg/m2, P-value = .003) (Table 3). When comparing BMI changes within each group, BMI at 1-year increased significantly compared to baseline BMI in both groups, with the absolute change in BMI being greater in the CSII group. BMI at baseline was 18.1 kg/m2 in the MDI group and increased to 18.7 kg/m2 after 1 year (P-value = .001). In the CSII group, BMI was 19.1 kg/m2 at baseline and 20.9 kg/m2 after 1 year (P-value < .001).
Table 3.
Comparing BMI of MDI versus CSII patients at baseline and after 1 year of treatment.
| BMI | Current insulin regimen | N | Mean | SD | P-value |
|---|---|---|---|---|---|
| BMI baseline | MDI | 122 | 18.0 | 4.0 | .093 |
| CSII | 34 | 19.3 | 3.7 | ||
| BMI 1 year | MDI | 122 | 18.6 | 3.8 | .003 |
| CSII | 34 | 20.9 | 4.0 |
We included a subgroup of patients with continuous glucose monitoring data using the Freestyle Libre device. This subgroup consisted of 37 individuals in the MDI group and 29 individuals in the CSII group (Table 4). Individuals in the CSII group using Freestyle Libre were older than those in the MDI group (median age 14 vs 11 years, P-value < .001). Comparing the 2-week glycemic profile between both groups revealed that average glucose was lower in the CSII group (194 vs 228 mg/dL, P-value = .028) as well as HbA1c levels (8.4% (68 mmol/mmol) vs 9.6% (81 mmol/mmol), P-value = .022). The time in target was higher and time above target was lower in CSII patients compared to MDI patients; however, this did not reach statistical significance. Time in hypoglycemia and low glucose events were greater in the CSII group compared to the MDI group, but this was not statistically significance.
Table 4.
Comparison of patients’ characteristics and glycemic profile in MDI versus CSII patients who used Freestyle libre.
| Regimen | P-value | ||||||
|---|---|---|---|---|---|---|---|
| MDI (n = 37) | CSII (n = 29) | ||||||
| Median | Q1 | Q3 | Median | Q1 | Q3 | ||
| Age (years) | 11 | 8 | 14 | 14 | 12 | 16 | <.001 |
| BMI (kg/m2) | 20.1 | 16 | 25.1 | 21 | 18.7 | 24.4 | .208 |
| Average Glucose (mg/dL) | 228 | 176 | 273 | 194 | 172 | 211 | .028 |
| % above target (>180 mg/dL) | 66 | 44 | 79 | 52 | 42 | 65 | .119 |
| % in target (70-180 mg/dL) | 33 | 21 | 54 | 40 | 31 | 53 | .137 |
| % below target (<70 mg/dL) | 2 | 0 | 4 | 4 | 1 | 8 | .082 |
| Low glucose events | 5 | 1 | 10 | 6 | 2 | 12 | .183 |
| HbA1C (%) | 9.6 | 7.7 | 11.1 | 8.4 | 7.5 | 8.9 | .022 |
Discussion
The results of our study demonstrated that in our population of youth with T1D, those who used CSII as a mode of insulin delivery have improved hemoglobin A1c levels and glycemic profiles compared to those who are on an MDI regimen. Most of the improvement in HbA1c in the CSII group occurred during the first 3 months after initiation of pump therapy, with continued and sustained improvement during the first 2 years. After the second year, HbA1c levels increased slightly but remained better than at baseline suggesting a loss of intensification of treatment possibly due to reduced enthusiasm from either the patients/parents and/or the treating team. On the other hand, HbA1c levels continued to increase over the study period in the MDI group. These findings were also confirmed using isCGM in a smaller subgroup of our cohort.
Studies that compared CSII to MDI have shown that most of the improvement in HbA1c occurs in the first few months after initiation of CSII,26-28 which is in agreement with our observations. The initial prominent reduction in HbA1c after initiation of CSII may be related to intensification of insulin therapy during this time compared to the previous insulin regimens applied. However, further improvement in HbA1c does not typically occur after 6 to 12 months; and in some studies, this improvement is not necessarily sustained.26,28-31 This is likely due to an element of diabetes burnout32-34 or due to loss of enthusiasm for the new technology. This contrasts with our observations, where the improvement in HbA1c achieved by the CSII cohort was not only maintained past 1 year, but also continued to show a reduction in HbA1c until the 2-year mark. The reason behind this is not clear and needs to be further explored, as understanding factors that can positively influence motivation and compliance is essential to appropriately manage individuals with T1D. However, the longer sustainability of a positive effect of a pump could be reflected on the commitments of patients/family to intensification of treatment. A tight system of “When a patient should start on a pump?” and “When it should be withdrawn from a patient?” in a clear contract, between patients/family and the treating team, that is periodically reviewed with the patient might also contribute to this success.
The 3-year sustained and persistent reduction in HbA1c levels in the CSII group is an important finding, as it reflects a reduction in the cumulative glycemic exposures. Based on the concept of metabolic memory, this persistent improvement in glycemic control during the study period should translate into a reduction of diabetes related complications such as retinopathy and nephropathy,35 which in turn should lead to reduced healthcare costs related to diabetes complications. It would be interesting to explore cost-effectiveness in future studies, comparing the cost of CSII with the long-term reduction in healthcare costs related to decreased burden of diabetes complications.
The analysis of the glycemic profiles for the subgroup who were using flash glucose monitoring in the form of Freestyle Libre further confirmed the overall improvement of HbA1c, with lower estimated HbA1c levels and average blood glucose in the CSII group. Both time in range and time above range demonstrated improving trends, albeit without reaching statistical significance. This is likely related to the small sample of patients analyzed. Interestingly, we did note a trend, though not statistically significant, of increase in hypoglycemic events and in time spent in hypoglycemia in the CSII group, which is somewhat different from previous studies comparing CSII with MDI. However, this difference was not significant in our cohort. Most evidence supports either reduced hypoglycemia with CSII or improvement in HbA1c without a significant increase in hypoglycemia.22,23,28,36-38 The increased hypoglycemia we observed in the CSII may be related to inherent limitations in the Freestyle Libre sensor, which lacks accuracy in low blood glucose ranges,39,40 increasing the possibility that some of the hypoglycemic events recorded may not reflect true hypoglycemia. This could not be verified by fingerstick blood glucose testing in our retrospective data.
The improvement in HbA1c in the CSII group compared to the MDI group is related to many factors. Despite both groups having a similar total daily dose of insulin, the distribution of insulin administration in the CSII group matches carbohydrate intake better than in the MDI, with the majority of carbohydrate intake covered by rapid insulin. Additionally, compliance in the CSII group with prandial insulin is likely higher, with users administering rapid insulin for snacks especially since they do not require injections to be administered. With regards to education, patients in both groups in our study are taught about the elements of intensified insulin therapy (IIT). However, the CSII group have more frequent visits with specialized diabetes educators and dieticians in the clinic, particularly during the first few months after pump initiation. Diabetes education as well as frequent follow up have a positive impact on glycemic control.41-43 An additional factor to be considered is enthusiasm and motivation due to the novelty of the insulin pump and the use of new technology, which may contribute to better compliance to insulin administration. Understanding the factors that help drive patient motivation is essential to reduce rates of diabetes burn-out.32-34,44
An increase in BMI was noted in both the MDI and the CSII groups in our study, which is an expected finding given the anabolic function of insulin.45,46 The increase in BMI was more significant in the CSII group compared to those on MDI, which can be explained by the poorer glycemic control in the MDI group that may have negatively impacted weight gain. In concordance with our findings, observational studies have found an association between the use of CSII and increased weight gain.47,48 A study conducted in Kuwait had a similar result despite a reduction in insulin daily dose. Kuwait’s study stated that the significant increase in BMI in patients on CSII therapy may have been due to the liberty to eat without receiving extra injections of insulin.49 On the other hand, other studies including randomized controlled trials, large cohort-matched studies and systematic reviews, did not find a significant difference between CSII and MDI with regards to weight or BMI.4,22,26,28,38 The need of long-term studies is a necessity to determine the actual effect of both treatments on BMI.
One of the strengths of our study is that it provides real life data on the effect of CSII on lowering HbA1c outside of a controlled trial environment, which may be more reflective of the benefit of CSII in the clinical setting. We also present data from a 3-year follow up period, which is longer than typically reported by other studies that compared CSII to MDI. The analysis of glycemic profiles from isCGM in a subgroup of patients is another strength of our study, as it provides, to a certain degree, safety data regarding the risk of hypoglycemia that may occur with tighter glycemic control.
Among the limitations of our study is the small number of patients on CSII who stayed in the service for the whole duration of the study as well as for the small number of patients in the subgroup with isCGM data; hence, there was a limitation in generalizing the findings of this one center study. Additionally, because our center is a tertiary specialized center, some HbA1c values were missing because some patients occasionally perform some of their HbA1c tests closer to home. While the implementation of the NICE guidance in offering CSII helps limit selection bias, certain factors may have inadvertently affected the decision to start CSII, such as frequency of monitoring blood glucose levels, number of insulin injections per day, clinic attendance and history of severe hypoglycemia or DKA. Another question that could have been addressed by this paper, if numbers of patients in the subgroup analysis were sufficient, is whether isCGM added additional value to either the MDI or CSII patients.
Conclusion
The use of CSII was strongly associated with improved glycemic control in our patients and the gap between CSII and MDI patients increased with time. The analysis in a small subgroup using isCGM have supported the same findings. In the era of rapid advances in diabetes technology including the advent of autonomous artificial pancreas systems these data could be of use in future research and/or clinical practice. The addition of CGM and the development of closed-loop systems may offer further improvement of glycemic control without the risk of hypoglycemia. While costly management options, these advances may ultimately prove cost-effective as our data support the long-term benefit of CSII on glycemic control in real life uncontrolled settings, which may translate to lower diabetes complication rates.
Acknowledgments
The authors would like to thank our diabetes educators at KASCH for their assistance in collecting the isCGM data.
Footnotes
ORCID iD: Haifa Al Faraidi  https://orcid.org/0000-0001-5812-2658
https://orcid.org/0000-0001-5812-2658
Declarations
Ethics approval and consent to participate: This study was approved by the institutional review board (IRB) of King Abdullah International Medical Research Center, Riyadh, Saudi Arabia (RC19/125/R, May 2019). Given the retrospective nature of the study and the lack of active intervention, consent was waived.
Consent for publication: Not applicable.
Author contributions: Amir Babiker: Conceptualization; Formal analysis; Methodology; Supervision; Writing—original draft; Writing—review & editing. Nawaf Alammari: Conceptualization; Data curation; Methodology; Writing—review & editing. Abdulrahman Aljuraisi: Conceptualization; Data curation; Methodology; Writing—review & editing. Rakan Alharbi: Conceptualization; Data curation; Methodology; Writing—review & editing. Hamoud Alqarni: Conceptualization; Data curation; Methodology; Writing—review & editing. Emad Masuadi: Formal analysis; Writing—review & editing. Haifa Al Faraidi: Formal analysis; Writing—original draft; Writing—review & editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The datasets generated and analyzed during the current study are available the corresponding author on reasonable request.
References
- 1. Genuth S. Insights from the diabetes control and complications trial/epidemiology of diabetes interventions and complications study on the use of intensive glycemic treatment to reduce the risk of complications of type 1 diabetes. Endocr Pract. 2006;12:34-41. [DOI] [PubMed] [Google Scholar]
- 2. Lasker RD. The diabetes control and complications trial. Implications for policy and practice. N Engl J Med. 1993;329:1035-1036. [DOI] [PubMed] [Google Scholar]
- 3. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the epidemiology of diabetes interventions and complications (EDIC) study. JAMA. 2003;290:2159-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blair J, McKay A, Ridyard C, et al. Continuous subcutaneous insulin infusion versus multiple daily injections in children and young people at diagnosis of type 1 diabetes: the SCIPI RCT. Health Technol Assess. 2018;22:1-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berezin A. Metabolic memory phenomenon in diabetes mellitus: achieving and perspectives. Diabetes Metab Syndr. 2016;10:S176-S183. [DOI] [PubMed] [Google Scholar]
- 6. Ihnat MA, Thorpe JE, Ceriello A. Hypothesis: the 'metabolic memory', the new challenge of diabetes. Diabet Med. 2007;24:582-586. [DOI] [PubMed] [Google Scholar]
- 7. Miller RG, Orchard TJ. Understanding metabolic memory: a tale of two studies. Diabetes. 2020;69:291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Z, Miao F, Paterson AD, et al. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc Natl Acad Sci U S A. 2016;113:E3002-E3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albers JW, Herman WH, Pop-Busui R, et al. Effect of prior intensive insulin treatment during the diabetes control and complications trial (DCCT) on peripheral neuropathy in type 1 diabetes during the epidemiology of diabetes interventions and complications (EDIC) Study. Diabetes Care. 2010;33:1090-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pop-Busui R, Low PA, Waberski BH, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the diabetes control and complications trial/epidemiology of diabetes interventions and complications study (DCCT/EDIC). Circulation. 2009;119:2886-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. White NH, Sun W, Cleary PA, et al. Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the diabetes control and complications trial. Arch Ophthalmol. 2008;126:1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, Nathan DM. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 2015;64:631-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Diabetes Association. 13. Children and adolescents: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S180-S199. [DOI] [PubMed] [Google Scholar]
- 14. Ong WM, Chua SS, Ng CJ. Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: a qualitative study. Patient Prefer Adherence. 2014;8:237-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borus JS, Blood E, Volkening LK, Laffel L, Shrier LA. Momentary assessment of social context and glucose monitoring adherence in adolescents with type 1 diabetes. J Adolesc Health. 2013;52:578-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campos-Náñez E, Fortwaengler K, Breton MD. Clinical impact of blood glucose monitoring accuracy: an in-silico Study. J Diabetes Sci Technol. 2017;11:1187-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heinemann L. Continuous glucose monitoring (CGM) or blood glucose monitoring (BGM): interactions and implications. J Diabetes Sci Technol. 2018;12:873-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391:1367-1377. [DOI] [PubMed] [Google Scholar]
- 19. Danne T, Phillip M, Buckingham BA, et al. ISPAD Clinical Practice Consensus Guidelines 2018: insulin treatment in children and adolescents with diabetes. Pediatr Diabetes. 2018;19:115-135. [DOI] [PubMed] [Google Scholar]
- 20. Rys PM, Ludwig-Slomczynska AH, Cyganek K, Malecki MT. Continuous subcutaneous insulin infusion vs multiple daily injections in pregnant women with type 1 diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials and observational studies. Eur J Endocrinol. 2018;178:545-563. [DOI] [PubMed] [Google Scholar]
- 21. Petrovski G, Al Khalaf F, Hussain K, Campbell J, El Awwa A. Continuous subcutaneous insulin infusion characteristics in type 1 diabetes children and Adolescents in Qatar. Diabetes Ther. 2018;9:2091-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Misso ML, Egberts KJ, Page M, O'Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010;2010:CD005103. [DOI] [PubMed] [Google Scholar]
- 23. Blackman SM, Raghinaru D, Adi S, et al. Insulin pump use in young children in the T1D Exchange clinic registry is associated with lower hemoglobin A1c levels than injection therapy. Pediatr Diabetes. 2014;15:564-572. [DOI] [PubMed] [Google Scholar]
- 24. Alamoudi R, Alsubaiee M, Alqarni A, et al. Comparison of insulin pump therapy and multiple daily injections insulin regimen in patients with type 1 diabetes during Ramadan fasting. Diabetes Technol Ther. 2017;19:349-354. [DOI] [PubMed] [Google Scholar]
- 25. Blair JC, McKay A, Ridyard C, et al. Continuous subcutaneous insulin infusion versus multiple daily injection regimens in children and young people at diagnosis of type 1 diabetes: pragmatic randomised controlled trial and economic evaluation. BMJ. 2019;365:l1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nabhan ZM, Kreher NC, Greene DM, Eugster EA, Kronenberger W, DiMeglio LA. A randomized prospective study of insulin pump vs. Insulin injection therapy in very young children with type 1 diabetes: 12-month glycemic, BMI, and neurocognitive outcomes. Pediatr Diabetes. 2009;10:202-208. [DOI] [PubMed] [Google Scholar]
- 27. Korkmaz Ö, Demir G, Çetin H, et al. Effectiveness of continuous subcutaneous insulin infusion pump therapy during five years of treatment on metabolic control in children and adolescents with Type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. 2018;10:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jakisch BI, Wagner VM, Heidtmann B, et al. Comparison of continuous subcutaneous insulin infusion (CSII) and multiple daily injections (MDI) in paediatric Type 1 diabetes: a multicentre matched-pair cohort analysis over 3 years. Diabet Med. 2008;25:80-85. [DOI] [PubMed] [Google Scholar]
- 29. Qin Y, Yang LH, Huang XL, Chen XH, Yao H. Efficacy and safety of continuous subcutaneous insulin infusion vs. Multiple daily injections on type 1 diabetes children: a meta-analysis of randomized control trials. J Clin Res Pediatr Endocrinol. 2018;10:316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nuboer R, Borsboom GJ, Zoethout JA, Koot HM, Bruining J. Effects of insulin pump vs. Injection treatment on quality of life and impact of disease in children with type 1 diabetes mellitus in a randomized, prospective comparison. Pediatr Diabetes. 2008;9:291-296. [DOI] [PubMed] [Google Scholar]
- 31. Mueller-Godeffroy E, Vonthein R, Ludwig-Seibold C, et al. Psychosocial benefits of insulin pump therapy in children with diabetes type 1 and their families: the pumpkin multicenter randomized controlled trial. Pediatr Diabetes. 2018;19:1471-1480. [DOI] [PubMed] [Google Scholar]
- 32. Lindström C, Åman J, Norberg AL, Forssberg M, Anderzén-Carlsson A. Mission Impossible"; the mothering of a child with Type 1 diabetes - from the perspective of Mothers Experiencing Burnout. J Pediatr Nurs. 2017;36:149-156. [DOI] [PubMed] [Google Scholar]
- 33. Helgeson VS. Diabetes burnout among emerging adults with type 1 diabetes: a mixed methods investigation. J Behav Med. 2021;44:368-378. [DOI] [PubMed] [Google Scholar]
- 34. Abdoli S, Vora A, Smither B, Roach AD, Vora AC. I don’t have the choice to burnout; experiences of parents of children with type 1 diabetes. Appl Nurs Res. 2020;54:151317. [DOI] [PubMed] [Google Scholar]
- 35. Lachin JM, Nathan DM; Group DER. Understanding metabolic memory: the prolonged influence of glycemia during the diabetes control and complications trial (DCCT) on future risks of complications during the study of the epidemiology of diabetes interventions and complications (EDIC). Diabetes Care. 2021;44:2216-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fendler W, Baranowska AI, Mianowska B, Szadkowska A, Mlynarski W. Three-year comparison of subcutaneous insulin pump treatment with multi-daily injections on HbA1c, its variability and hospital burden of children with type 1 diabetes. Acta Diabetol. 2012;49:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case–control study. Diabetologia. 2013;56:2392-2400. [DOI] [PubMed] [Google Scholar]
- 38. Karges B, Schwandt A, Heidtmann B, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with Type 1 Diabetes. JAMA. 2017;318:1358-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moser O, Eckstein ML, McCarthy O, et al. Performance of the Freestyle Libre flash glucose monitoring (flash GM) system in individuals with type 1 diabetes: a secondary outcome analysis of a randomized crossover trial. Diabetes Obes Metab. 2019;21:2505-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Galindo RJ, Migdal AL, Davis GM, et al. Comparison of the FreeStyle Libre pro Flash continuous glucose monitoring (CGM) system and Point-of-Care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care. 2020;43:2730-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cooke D, Bond R, Lawton J, et al. Structured type 1 diabetes education delivered within routine care: impact on glycemic control and diabetes-specific quality of life. Diabetes Care. 2013;36:270-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mühlhauser I, Jörgens V, Berger M, et al. Bicentric evaluation of a teaching and treatment programme for type 1 (insulin-dependent) diabetic patients: improvement of metabolic control and other measures of diabetes care for up to 22 months. Diabetologia. 1983;25:470-476. [DOI] [PubMed] [Google Scholar]
- 43. Uddin I, Ahmad TJ, Kurkuman AR, Iftikhar R. Diabetes education: its effects on glycemic control. Ann Saudi Med. 2001;21:120-122. [DOI] [PubMed] [Google Scholar]
- 44. Abdoli S, Hessler D, Vora A, Smither B, Stuckey H. Descriptions of diabetes burnout from individuals with Type 1 diabetes: an analysis of YouTube videos. Diabet Med. 2020;37:1344-1351. [DOI] [PubMed] [Google Scholar]
- 45. Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 2011;93:S52-S59. [DOI] [PubMed] [Google Scholar]
- 46. Newfield RS, Cohen D, Capparelli EV, Shragg P. Rapid weight gain in children soon after diagnosis of type 1 diabetes: is there room for concern? Pediatr Diabetes. 2009;10:310-315. [DOI] [PubMed] [Google Scholar]
- 47. Nansel TR, Lipsky LM, Iannotti RJ. Cross-sectional and longitudinal relationships of body mass index with glycemic control in children and adolescents with type 1 diabetes mellitus. Diabetes Res Clin Pract. 2013;100:126-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Birkebaek NH, Kahlert J, Bjarnason R, et al. Body mass index standard deviation score and obesity in children with type 1 diabetes in the Nordic countries. HbA1c and other predictors of increasing BMISDS. Pediatr Diabetes. 2018;19:1198-1205. [DOI] [PubMed] [Google Scholar]
- 49. Mousa M, Al-Mahdi M, Al-Sanaa H, Al-Kandari H. A comparison of continuous subcutaneous insulin infusion vs. Multiple daily insulin injection in children with type I diabetes in Kuwait: glycemic control, insulin requirement, and BMI. Oman Med J. 2015;30:336-343. [DOI] [PMC free article] [PubMed] [Google Scholar]