Abstract
Introduction:
The role of bevacizumab combined with paclitaxel and carboplatin in the first-line treatment of advanced non-squamous non-small-cell lung cancer (NSCLC) has been supported by a large number of data. However, whether bevacizumab biosimilars have the same efficacy and safety as the original drug is still controversial. This meta-analysis was designed to evaluate whether bevacizumab biosimilars have the same clinical efficacy and safety as the original drug in patients with advanced non-squamous NSCLC.
Methods:
Electronic databases (PubMed, Embase, Cochrane, CNKI, Wanfang, and VIP) and the ClinicalTrail.gov website were extensively searched using relevant search criteria. We included phase III randomized controlled trials (RCTs) to compare the efficacy and safety of marketed biosimilars and Avastin in the first-line treatment of patients with advanced NSCLC. The risk of bias of the included studies was assessed using the RoB 2 assessment scale, and the RevMan 5.4 statistical software was used for meta-analysis.
Results:
A total of 6360 patients were included in 11 RCTs. There was no statistical difference between the experimental group and the control group in terms of effectiveness [objective response rate (at week 18), disease control rate (at week 18), median duration of response, median progression-free survival, median overall survival (OS), and OS after 12 months]. In terms of safety [treatment-emergent adverse events (grade ⩾3) and treatment-related adverse events (grade ⩾3)], there was also no significant difference between biosimilars and Avastin.
Conclusions:
The efficacy and safety of bevacizumab biosimilars in the treatment of advanced non-squamous NSCLC are highly similar to those of the original drug combined with paclitaxel and carboplatin, respectively.
Keywords: Avastin, bevacizumab, biosimilar, efficacy, meta-analysis, non-small-cell lung cancer, safety
Introduction
Lung cancer (LC) is the malignant tumor with the highest mortality rate in the world, and non-small-cell lung cancer (NSCLC) accounts for more than 80% of newly diagnosed LC patients every year.1 Therefore, in recent years, scientists all over the world are exploring safe and effective treatment methods for NSCLC. In addition to traditional chemotherapy, there are new methods such as precision targeted drugs and immunotherapy. Bevacizumab, a monoclonal antibody that inhibits the biological function of vascular endothelial growth factor (VEGF), is widely used in the treatment of advanced or metastatic non-squamous NSCLC. The original drug (Avastin) was launched in 2004. According to IQVIATM(a cross-country sales and medical database), global sales revenue in 2019 was $7.5 billion, but as biosimilars are launched one after another, global sales revenue in 2020 will drop to $5.4 billion. A previous meta-analysis found that the combination of bevacizumab with paclitaxel and carboplatin did increase objective response rate (ORR), overall survival (OS), and progression-free survival (PFS) in advanced non-squamous NSCLC compared with paclitaxel and carboplatin.2–4
Biosimilars refer to therapeutic biological products that are similar to the approved reference drug in terms of quality, safety, and efficacy, and the reference drug is usually the original brand drug.5 Biosimilars have entered a period of rapid development as Avastin’s patent protection has gradually expired. Compared with other countries, the Chinese market is more competitive. As of December 2021, there are as many as seven domestic bevacizumab biosimilars on the market in China, and five of them were approved for marketing in 2021. However, biological products have the characteristics of large molecular weight, complex structure, strong dependence of biological activity on their structural integrity, and complex production process, and are late on the market leading to a short clinical use time. Therefore, there are still some potential risks in clinical use, and it is necessary to further verify its efficacy and safety.
Methods
Search strategy
Search PubMed, Embase, Cochrane Library, CNKI, VIP, Wanfang database, and ClinicalTrials.gov website, and the search time is from the establishment of each database to 31 December 2021 (Supplemental Table A). Pub Med search strategy: (((‘Bevacizumab’[Mesh]) OR (Bevacizumab[Title/Abstract])) OR (Avastin [Title/Abstract])) AND (((((‘Biosimilar Pharmaceuticals’[Mesh]) OR (Biosimilar*[Title/Abstract])) OR (Generic*[Title/Abstract])) OR (Analog*[Title/Abstract]))). The search strategies followed the Cochrane Handbook for Systematic Reviews of Interventions (Version 6.2), and were adapted to the specific database. All search strategies were determined after multiple pre-searches.
Study eligibility
Double-blind, phase III randomized controlled trials (RCTs) were included, and the languages were limited to Chinese and English. The population was limited to (1) pathologically or histologically confirmed non-squamous NSCLC; (2) metastatic or recurrent stage IIIB-IV patients over the age of 18; (3) the Eastern Cooperative Oncology Group (ECOG) score was 0–2; and (4) any race, nationality, and gender. The intervention was first-line treatment with ‘bevacizumab biosimilar + paclitaxel + carboplatin’ versus ‘Avastin + paclitaxel + carboplatin’. Efficacy was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) (complete response, partial response, stable disease, progressive disease, and not evaluated). The main outcome was ORR. The secondary outcomes were as follows: median PFS (mPFS), median OS (mOS), median duration of response (mDOR), and disease control rate (DCR). Adverse events (AEs) included treatment-emergent AEs (TEAEs) and treatment-related AEs (TRAEs), assessed according to the Common Terminology Criteria for Adverse Events version 4.0. Exclusions: (1) review, (2) republished literature, (3) unavailable full-text literature, (4) case report, (5) single-arm trial, (6) non-target drug, (7) non-target population, (8) non-target outcomes, and (9) the trial drug has not been approved for marketing.
Data collection process
The results of the included studies were cross-checked by two investigators. The extracted information mainly included the following: (1) general information: title, author’s name, publication date and literature source, etc.; (2) study characteristics: code of trial drug, general information of research subjects, baseline comparability of patients in each group, and intervention measures; and (3) outcome indicators. Disagreements should be resolved through discussion or consultation according to the opinion of the third researcher.
Risk of bias assessment
According to the Cochrane Handbook for Systematic Reviews of Interventions (Version 6.2), the methodological quality of the included studies was evaluated using a uniform evaluation scale (RoB 2).6 The quality evaluation was carried out by two researchers independently and cross-checked. In case of disagreement, a third researcher was invited to help resolve the disagreement.
Data synthesis and statistical analysis
Meta-analysis of the included RCTs was performed using RevMan 5.4 statistical software. For enumeration data (ORR, DCR, OS rate, TEAEs, and TRAEs), relative risk (RR) was used as analysis statistic, and for survival data (DOR, mPFS, and mOS), hazard ratio (HR) was used as analysis statistic. Each effect size was expressed as 95% confidence intervals (CIs). When available, we relied on outcomes in intention-to-treat populations; otherwise, we used results in the full analysis set. The heterogeneity among the results of the included studies was analyzed by the chi-square test. If p > 0.05 and I2 ⩽ 50%, it means that the heterogeneity could be ignored, and a fixed effect model will be adopted for analysis. If there was statistical heterogeneity (p ⩽ 0.05 or I2 > 50%), the source of heterogeneity would be analyzed to determine whether the random effects model could be used. If there was significant clinical heterogeneity between studies, only descriptive analyses would be performed. Finally, a sensitivity analysis was used to test the stability of the results.
Results
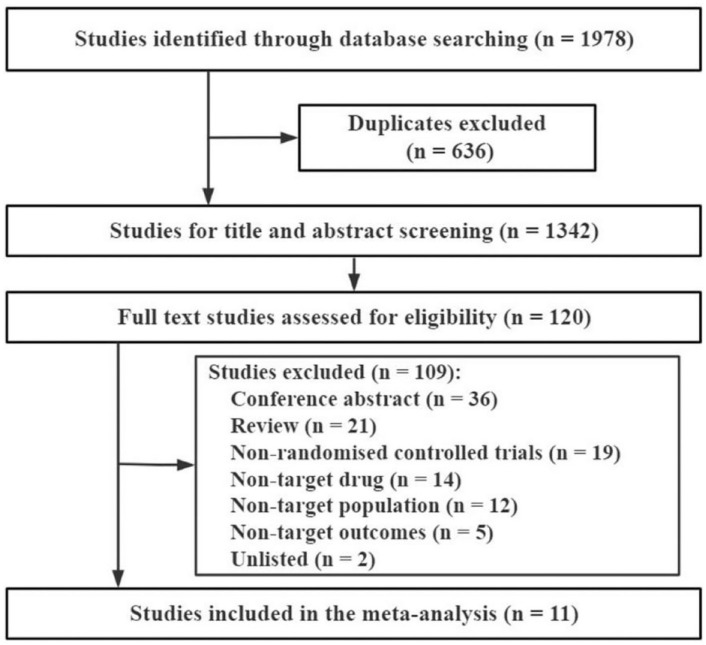
A total of 1677 related articles and 301 clinical trial records were collected, and 11 phase III RCTs were finally included (Figure 1; In 2015, 2019, 2020, and 2021, 1, 3, 1, and 6 articles were published, respectively). A total of 6360 patients were enrolled, and the biosimilars and Avastin groups were comparable in patient characteristics (Table 1): cases, 3185 versus 3175; males, 63.39% versus 62.65%; mean age, 60.16 versus 60.15 years, respectively.
Figure 1.
CONSORT diagram outlining study selection.
Table 1.
Study characteristics and summary of findings.
| Trial | Population | Number of patients | Median age | Number of male | EGFR mutation | ALK mutation | Treatment arms | Reported outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | C | E | C | E | C | E | C | E | C | E | C | |||
| ABP 215, 20197 | Stage IV or recurrent metastatic | 328 | 314 | Mean (SD): 61.6 (9.09) | Mean (SD): 61.6 (8.88) | 196 | 188 | NG | NG | NG | NG | ABP 215 (15 mg/kg, Q21d, IV) + paclitaxel (Q21d, IV) + carboplatin (Q21d, IV) for 4–6 cycles | Avastin (15 mg/kg, Q21d, IV) + paclitaxel (Q21d, IV) + carboplatin (Q21d, IV) for 4–6 cycles | ①③④⑤⑥ |
| BCD-021, 20158,9 | Newly diagnosed stage IIIB-IV | 68 | 66 | Mean (SD): 57.79 (8.88) | Mean (SD): 58.67 (8.33) | 43 | 42 | NG | NG | NG | NG | BCD-021 (15 mg/kg, Q21d, IV) + paclitaxel (175 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21, IV) for 6 cycles | Avastin (15 mg/kg, Q21d, IV) + paclitaxel (175 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21, IV) for 6 cycles | ① |
| FKB238, 202110 | Newly diagnosed stage IV or recurrent | 364 | 367 | Mean (SD): 60.8 (8.79) | Mean (SD): 61.1 (9.42) | 245 | 238 | NG | NG | NG | NG | FKB238 (15 mg/kg, Q21 ± 3d, IV) + paclitaxel (200 mg/m2, Q21 ± 3d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21 ± 3d, IV) for 4–6 cycles | Avastin (15 mg/kg, Q21 ± 3d, IV) + paclitaxel (200 mg/m2, Q21 ± 3d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21 ± 3d, IV) for 4–6 cycles | ①④⑤⑥ |
| IBI305, 201911 | Unresectable locally advanced (stage IIIB), metastatic (stage IV), or recurrent | 221 | 220 | Mean (SD): 57.6 (8.69) | Mean (SD): 57.2 (9.28) | 142 | 137 | 0 | 0 | NG | NG | IBI305 (15 mg/kg, Q21d, IV) + paclitaxel (175 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21d, IV) for a maximum of 6 cycles; maintenance therapy: IBI305 (15 mg/kg, Q21d, IV) | Avastin (15 mg/kg, Q21d, IV) + paclitaxel (175 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21d, IV) for a maximum of 6 cycles; maintenance therapy: Avastin (15 mg/kg, Q21d, IV). | ①②③④⑤⑥ |
| LY01008, 202112 | Unresectable, untreated, metastatic, or recurrent stage IIIB-IV | 293 | 296 | 58 | 59 | 177 | 175 | 123 | 123 | NG | NG | LY01008 (15 mg/kg, Q21d, IV) + paclitaxel (175 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21d, IV) for 4–6 cycles; maintenance therapy: LY01008 (15 mg/kg, Q21d, IV). | Avastin (15 mg/kg, Q21d, IV) + paclitaxel (175 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21d, IV) for 4–6 cycles; maintenance therapy: LY01008 (15 mg/kg, Q21d, IV) | ①②③④⑤⑥ |
| MB02, 202113 | Newly diagnosed or recurrent stage IIIB-IV | 315 | 312 | 61 | 61 | 193 | 190 | 0/UK | 0/UK | 0/UK | 0/UK | MB02 (15 mg/kg, Q21d, IV) + paclitaxel (200 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21 ± 3d, IV) for 6 cycles; maintenance therapy: MB02 (15 mg/kg, Q21d, IV) | Avastin (15 mg/kg, Q21d, IV) + paclitaxel (200 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21 ± 3d, IV) for 6 cycles; maintenance therapy: Avastin (15 mg/kg, Q21d, IV). | ①④⑤⑥ |
| MIL60, 202114 | Stage IV or recurrent metastatic | 253 | 255 | 61 | 61 | 163 | 162 | 54 | 57 | 0/UK | 0/UK | MIL60 (15 mg/kg, Q21d, IV) + paclitaxel (175 mg/m2, Q21d, IV) + carboplatin (AUC 5.0 mg/mL/min, Q21d, IV) for 4–6 cycles; maintenance therapy: MIL60 (7.5 mg/kg, Q21d, IV) | Avastin (15 mg/kg, Q21d, IV) + paclitaxel (175 mg/m2, Q21d, IV) + carboplatin (AUC 5.0 mg/mL/min, Q21d, IV) for 4–6 cycles; maintenance therapy: MIL60 (7.5 mg/kg, Q21d, IV) | ①②③④⑤⑥ |
| MYL-1402O, 202115 | Stage IV unresectable, recurrent or metastatic | 337 | 334 | 60 | 59 | 213 | 211 | 0/UK | 0/UK | 0/UK | 0/UK | MYL-1402O (15 mg/kg, Q21 ± 3d, IV) + paclitaxel (200 mg/m2 or 175 mg/m2, Q21 ± 3d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21 ± 3d, IV) for 6 cycles; maintenance therapy: MYL-1402O | Avastin (15 mg/kg, Q21 ± 3d, IV) + paclitaxel (200 mg/m2 or 175 mg/m2, Q21 ± 3d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21 ± 3d, IV) for 6 cycles; maintenance therapy: Avastin | ①②③④⑤⑥ |
| PF-06439535, 201916 | Newly diagnosed stage IIIB-IV, or recurrent | 358 | 361 | 62 | 61 | 237 | 230 | 0/UK | 0/UK | 0/UK | 0/UK | PF-06439535 (15 mg/kg, Q21d, IV) + paclitaxel (200 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21d, IV) for 4–6 cycles; maintenance therapy: PF-06439535 (15 mg/kg, Q21d, IV) | Avastin (15 mg/kg, Q21d, IV) + paclitaxel (200 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21d, IV) for 4–6 cycles; maintenance therapy: Avastin (15 mg/kg, Q21d, IV) | ①③④⑤⑥ |
| QL1101, 202117 | Untreated local stage IIIB-IV | 269 | 266 | 59 | 58 | 158 | 160 | 104 | 107 | 0/UK | 0/UK | QL1101 (15 mg/kg, Q21d, IV) + paclitaxel (175 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21d, IV) for 4–6 cycles; maintenance therapy: QL1101 (15 mg/kg, Q21d, IV) | Avastin (15 mg/kg, Q21d, IV) + paclitaxel (175 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21d, IV) for 4–6 cycles; maintenance therapy: QL1101 (15 mg/kg, Q21d, IV) | ①②④⑤⑥ |
| SB8, 202018 | Advanced or recurrent metastatic | 379 | 384 | mean (SD): 60.2 (8.95) | mean (SD): 60.0 (9.18) | 252 | 256 | 0 | 0 | 0/UK | 0/UK | SB8 (15 mg/kg, Q21d, IV) + paclitaxel (200 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21d, IV) for 4–6 cycles; maintenance therapy: SB8 (15 mg/kg, Q21d, IV) | Avastin (15 mg/kg, Q21d, IV) + paclitaxel (200 mg/m2, Q21d, IV) + carboplatin (AUC 6.0 mg/mL/min, Q21d, IV) for 4–6 cycles; maintenance therapy: Avastin (15 mg/kg, Q21d, IV) | ①③④⑤⑥ |
ALK, anaplastic lymphoma kinase; AUC, area under the curve; C, control group; E, experiment group; EGFR, epidermal growth factor receptor; IV, intravenous injection; NG, not given; SD, standard deviation; UK, unknown; ①, ORR; ②, DCR; ③, DOR; ④, PFS; ⑤, OS; ⑥, adverse events (grade ⩾ 3).
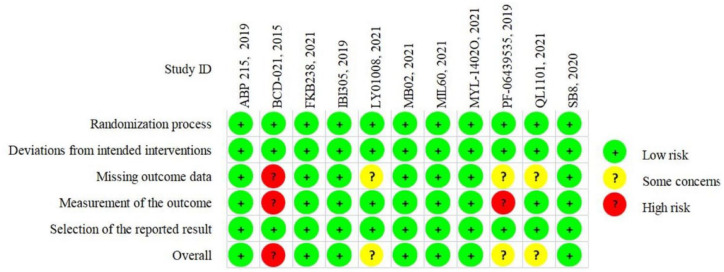
The quality of included literatures was relatively good. Among the 11 articles, 7 were low risk, 3 were medium risk, and 1 was high risk (Figure 2; Supplemental Figure S1). The three medium risk articles were all caused by ⩾5% follow-up loss. One of the studies (PF-06439535, 2019) was not blinded to the evaluators of the results and should have been considered high risk. However, considering that the US Food and Drug Administration (FDA) approved the investigative drug in this study (commercial: Zirabev) in 2019 after a rigorous approval process, so this study was assessed as medium risk. One study (BCD-021, 2015) was considered high risk due to excessive missing data and failure to state whether assessors were blinded.
Figure 2.
Risk of bias assessment.
ORR: week 18
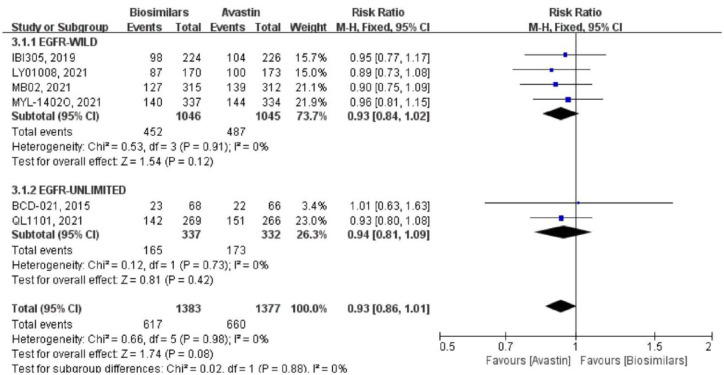
Six studies reported the ORR at the 18th week after randomization (Figure 3). Four of them were targeted at the epidermal growth factor receptor (EGFR) wild-type population, with a total of 2091 patients (biosimilars group = 1046 patients, Avastin group = 1045 patients; Table 1). There was no heterogeneity among the studies (p = 0.91, I2 = 0%). Using the fixed effect model, the result showed that there was no significant difference between the two groups (RR = 0.93, 95% CI: 0.84–1.02, p = 0.12). Two study did not limit EGFR genotype, which included a total of 669 patients (biosimilars group = 337 patients, Avastin group = 332 patients; Table 1). Using a fixed effect model, the result also showed that there was no significant difference between the two groups (RR = 0.94, 95% CI: 0.81–1.09, p = 0.42). Heterogeneity between the two studies was negligible. And there was no heterogeneity in the six studies (p = 0.98, I2 = 0%) and between the two subgroups (p = 0.88, I2 = 0%) after combining the subgroups. At the same time, there was no significant difference in the overall ORR between the biosimilars group and the Avastin group (RR = 0.93, 95% CI: 0.86–1.01, p = 0.08).
Figure 3.
Meta-analysis of ORR (week 18).
ORR, objective response rate.
DCR: week 18
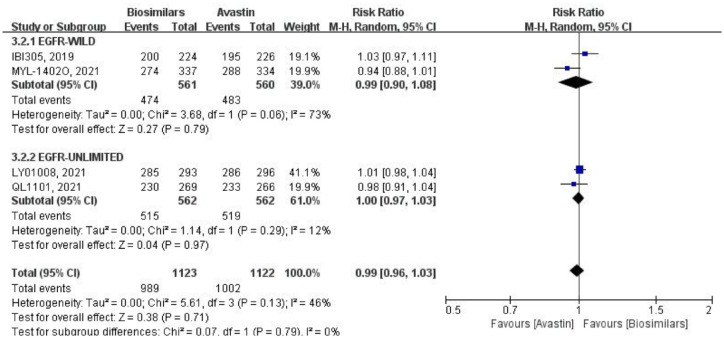
Four studies reported the DCR at the 18th week after randomization (Figure 4), which were analyzed using a random-effects model. Two of them only focused on the EGFR wild-type population, with a total of 1121 patients (biosimilars group = 561 patients, Avastin group = 560 patients; Table 1). Although the heterogeneity (p = 0.06, I2 = 73%) among studies was obvious, but the result was no significant difference between the two groups (RR = 0.99, 95% CI: 0.90–1.08, p = 0.79). The other two articles did not limit the genotype, with a total of 1124 patients (biosimilars group = 562 patients, Avastin group = 562 patients; Table 1). There was little heterogeneity among studies (p = 0.29, I2 = 12%), and the results showed that there was no significant difference between the two groups (RR = 1.00, 95% CI: 0.97–1.03, p = 0.97). After merging two subgroups, heterogeneity emerged among the four studies (p = 0.13, I2 = 46%). While there was no heterogeneity between the two subgroups (p = 0.79, I2 = 0%), and the DCR of the biosimilars group and the Avastin group was not statistically different (RR = 0.99, 95% CI: 0.96–1.03, p = 0.71).
Figure 4.
Meta-analysis of DCR (week 18).
DCR, disease control rate.
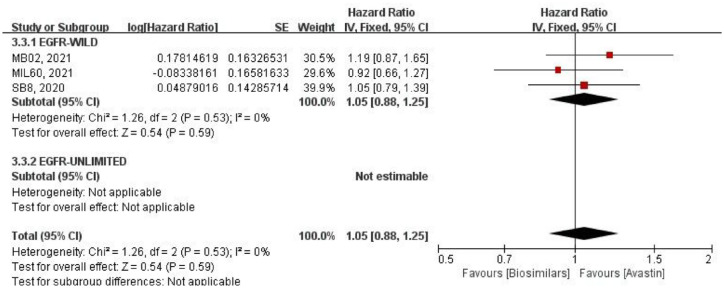
Median duration of response
The mDOR was reported in three studies (Figure 5), and all of them were only aimed at EGFR wild-type population. There was no heterogeneity among these studies (p = 0.53, I2 = 0%), so the fixed-effect model was used. Compared with HR, there was no significant difference in mDOR between the two groups (HR = 1.05, 95% CI: 0.88–1.25, p = 0.59).
Figure 5.
Meta-analysis of DOR.
DOR, duration of response.
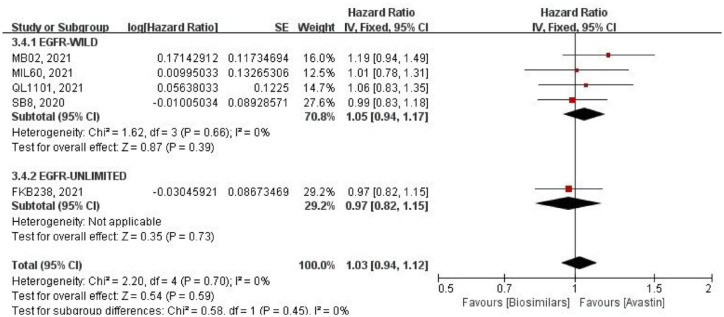
Mean progression-free survival
Five studies reported the mPFS (Figure 6). Using HR for analysis by a fixed-effects model. Four of them were conducted on the EGFR wild-type population, with no heterogeneity among these studies (p = 0.66, I2 = 0%), and no significant difference in two groups (HR = 1.05, 95% CI: 0.94–1.17, p = 0.39). One study did not limit EGFR genotype, and the difference between two groups was also not statistically significant (HR = 0.97, 95% CI: 0.82–1.15, p = 0.73). After combining subgroups, there was no heterogeneity among all studies (p = 0.70, I2 = 0%), and also the two subgroups (p = 0.45, I2 = 0%). At the same time, there was no significant difference between the biosimilars and Avastin groups (HR = 1.03, 95% CI: 0.94–1.12, p = 0.59).
Figure 6.
Meta-analysis of mPFS.
mPFS, mean progression-free survival.
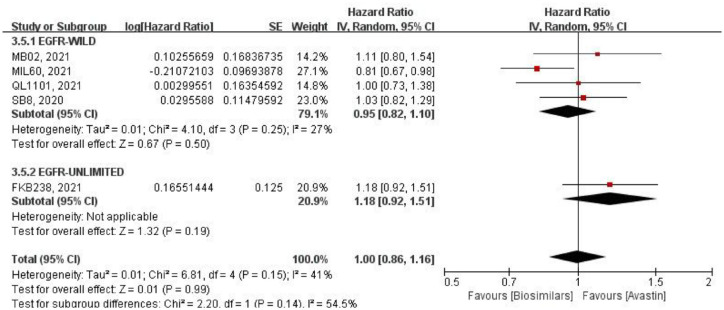
Overall survival
Five studies reported the mOS (Figure 7). Using HR for analysis by a random effects model. Four studies included only the EGFR wild-type population, with little heterogeneity among them (p = 0.25, I2 = 27%), and no significant difference in mOS (HR = 0.95, 95% CI: 0.82–1.10, p = 0.50). One study did not limit EGFR genotype, and the result between two groups was also not statistically significant (HR = 1.18, 95% CI: 0.92–1.51, p = 0.19). After merging two subgroups, the mOS was highly similar between the biosimilars and Avastin groups (HR = 1.00, 95% CI: 0.86–1.16, p = 0.99). While there was some degree of heterogeneity among all studies (p = 0.15, I2 = 41%), and the heterogeneity between the two subgroups was large (p = 0.14, I2 = 54.5%).
Figure 7.
Meta-analysis of mOS.
mOS, mean overall survival.
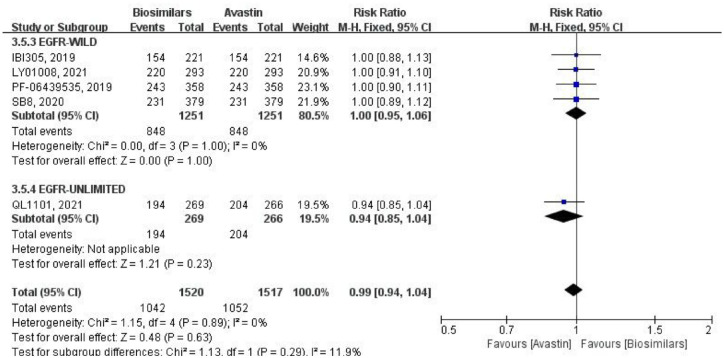
Five studies reported the 1-year OS rate, and comparison was made by RR, using a fixed-effects model (Figure 8). Four of them were aimed at the EGFR wild-type population, with a total of 2502 patients (biosimilars group = 1251 patients, Avastin group = 1251 patients; Table 1). No heterogeneity within this subgroup (p = 1.00, I2 = 0%), and the difference of result was not statistically significant (RR = 1.00, 95% CI: 0.95–1.06, p = 1.00). One study did not limit EGFR genotype, with a total of 535 patients (biosimilars group = 269 patients, Avastin group = 266 patients; Table 1). The difference in 1-year OS was also not statistically significant in this study (RR = 0.94, 95% CI: 0.85–1.04, p = 0.23). After combining subgroups, there was no heterogeneity between all studies (p = 0.89, I2 = 0%), and no statistical difference in 1-year OS between the two groups (RR = 0.99, 95% CI: 0.94–1.04, p = 0.63). Moreover, the heterogeneity between two subgroups was low (p = 0.29, I2 = 11.9%).
Figure 8.
Meta-analysis of 1-year OS rate.
OS, overall survival.
AEs (grade ⩾3)
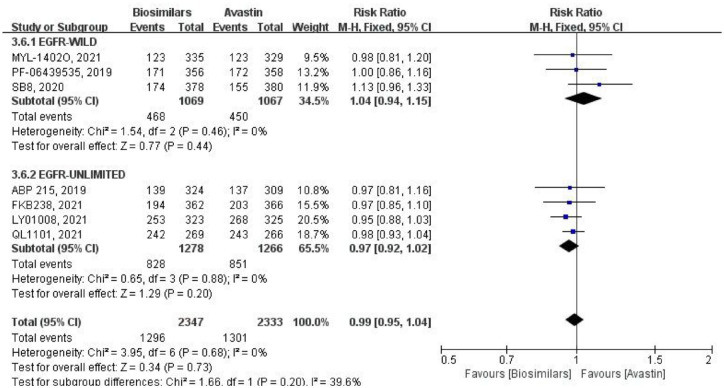
Seven studies reported TEAEs of grade ⩾3, using a fixed-effects model for comparative analysis (Figure 9). Three of them were targeted at the EGFR wild-type population, with a total of 2136 patients (biosimilars group = 1069 patients, Avastin group = 1067 patients; Table 1). There was no heterogeneity among these studies (p = 0.46, I2 = 0%), and the result was not statistically different (RR = 1.04, 95% CI: 0.94–1.15, p = 0.44). Other studies did not limit the EGFR genotype, with a total of 2544 patients (biosimilars group = 1278 patients, Avastin group = 1266 patients; Table 1). There was also no heterogeneity within this subgroup (p = 0.88, I2 = 0%), and no statistically significant difference in the outcome (RR = 0.97, 95% CI: 0.92–1.02, p = 0.20). After combining subgroups, the heterogeneity among all studies was negligible (p = 0.68, I2 = 0%). However, there was some heterogeneity between the two subgroups (p = 0.20, I2 = 39.6%). As for the result, there was also no statistical difference (RR = 0.99, 95% CI: 0.95–1.04, p = 0.73).
Figure 9.
Meta-analysis of TEAEs (grade ⩾3).
TEAEs, treatment-emergent adverse events.
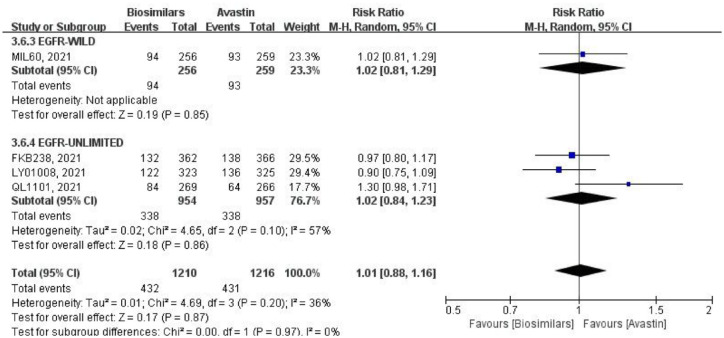
Four studies reported TRAEs of grade ⩾3, using the fixed-effects model for comparative analysis (Figure 10). One of them was for the EGFR wild-type population, with a total of 515 patients (biosimilars group = 256 patients, Avastin group = 259 patients; Table 1). The study showed no statistical difference in the result (RR = 1.02, 95% CI: 0.81–1.29, p = 0.85). Three studies did not limit EGFR genotype, with a total of 1911 patients (biosimilars group = 954 patients, Avastin group = 957 patients; Table 1). The difference in result was also not statistically significant (RR = 1.02, 95% CI: 0.84–1.23, p = 0.86). However, there was considerable heterogeneity among these studies (p = 0.10, I2 = 57%). After merging subgroups, the heterogeneity of all studies decreased (p = 0.20, I2 = 36%), while there was also no significant difference between the biosimilars and Avastin groups (RR = 1.01, 95% CI: 0.88–1.16, p = 0.87). Heterogeneity between the two subgroups was not present (p = 0.97, I2 = 0%).
Figure 10.
Meta-analysis of TRAEs (grade ⩾3).
TRAEs, treatment-related adverse events.
Sensitivity analysis
One study was sequentially excluded to investigate the effect of this single study on heterogeneity. When the MYL-1402O study was excluded from the DCR, the heterogeneity was significantly reduced after combining subgroups (p = 0.49, I2 = 0%), and the RR changed from 0.99 (95% CI: 0.96–1.03, p = 0.71) to 1.00 (95% CI: 0.97–1.04; p = 0.79). Since there were only two studies in this subgroup, within-subgroup heterogeneity disappeared after removing this study. After excluding the MIL60 study in mOS, the heterogeneity between subgroups decreased significantly (p = 0.40, I2 = 0%), and the HR after merging subgroups changed from 1.00 (95% CI: 0.86–1.16, p = 0.99) to 1.08 (95% CI: 0.95–1.24; p = 0.26). If FKB238 was removed, only the EGFR wild-type subgroup was left, with a slight decrease in integrated heterogeneity (p = 0.25, I2 = 27%) and little change in HR. After excluding the QL1101 study from TRAEs, within-subgroup heterogeneity was significantly reduced (p = 0.25, I2 = 27%), and RR changed from 1.02 (95% CI: 0.84–1.23, p = 0.86) to 0.94 (95% CI: 0.82–1.07, p = 0.33). It can be seen that excluding studies with significant heterogeneity has no effect on the results. The causes of heterogeneity in each study need to be further explored, and the hypothesis of this paper needs to be confirmed.
Sensitivity analysis of each outcome index showed that the results of this meta-analysis after removing each study one by one showed little change compared with those before the elimination, and the results of this meta-analysis after model transformation showed little change. The results of this study were confirmed to be robust.
Immunogenicity
In all, 10 studies assessed antidrug antibodies (ADAs) and all showed low and similar immunogenicity between the two treatment groups, with no clinically meaningful differences (Table 2).
Table 2.
Immunogenicity of included studies.
| Studies | ADAs, % | Original description | |
|---|---|---|---|
| Biosimilar | Avastin | ||
| ABP 215, 20197 | 1.4 | 2.5 | Four (1.4%) and 7 (2.5%) patients in the biosimilar and Avastin groups, respectively, developed binding ADAs during the study, of which three patients (1.0% and 1.1%) in each arm had transient binding ADAs, that is, they were negative result at the patient’s last time point tested within the study period |
| BCD-021, 20158 | 1.5 | 1.5 | The incidence of ADAs was 1.5% in both groups |
| FKB238, 202110 | 3.0 | 3.0 | In both treatment arms, 3.0% (nine patients) tested positive for ADAs at any visit, whereas the incidence of treatment-emergent ADAs was 2.3% (seven patients) in each treatment arm |
| IBI305, 201911 | 0.9 | 0.9 | In the biosimilar group, 2 (0.9%) patients tested positive for ADAs, 1 at baseline, and the other at the end of treatment. In the Avastin group, 2 (0.9%) patients tested positive for ADAs at baseline |
| LY01008, 202112 | 2.2 | 1.8 | Of 648 patients in the safety set, 13 (2.0%) were positive for ADAs before the first cycle of treatment [7 (2.2%) in the biosimilar group versus 6 (1.8%) in the Avastin group]. Six (0.9%) patients (three in each group) showed at least once ADAs positivity during the study of combined treatment, which were all transient. One patient showed transient post-treatment ADAs-positivity |
| MB02, 202113 | 17 | 16.1 | After 52 weeks of study, most subjects tested negative for ADAs at all time points. The treatment-induced ADAs were observed in 53 subjects (17%) in the biosimilar group and 50 subjects (16.1%) in the Avastin group |
| MIL60, 202114 | 0 | 0 | A total of 515 patients were analyzed for ADAs. No patient had positive result during treatment |
| MYL-1402O, 202115 | 6.5 | 4.8 | The incidence of treatment-emergent ADAs (treatment-induced plus treatment-boosted) was similar for both treatment arms (biosimilar, 6.5%; Avastin, 4.8%) |
| PF-06439535, 201916 | 1.5 | 1.4 | In the overall post-treatment assessment, five (1.5%) of 339 patients in the biosimilar group and five (1.4%) of 350 patients in the Avastin group were reported ADAs positive |
| QL1101, 202117 | – | – | – |
| SB8, 202018 | 13.5 | 10.1 | The incidences of an overall positive ADAs result were comparable between the biosimilar and Avastin groups in the safety set throughout the study, including up to cycle 7 [biosimilar, n = 46/341 (13.5%); Avastin, n = 34/337 (10.1%)]. |
ADAs, antidrug antibodies.
Discussion
Bevacizumab for NSCLC
NSCLC is the most common type of LC, which includes squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma. Compared with small-cell carcinoma, its cancer cells grow and divide more slowly and spread and metastasize later. NSCLC accounts for about 80–85% of newly diagnosed LC cases each year. And according to the Tumor Node Metastasis classification criteria (version 8th), about 75% of patients are found in the middle and advanced stages.19 Bevacizumab is a monoclonal antibody against VEGF, which binds VEGF and inhibits its binding to VEGF receptor-2, thereby inhibiting the biological effects of VEGF.20–22 It is the first antitumor vascular endothelial drug approved for sale in the United States and Europe and the first drug to extend OS at active levels.21 It has been approved in combination with standard platinum-based chemotherapy to treat patients with advanced NSCLC who do not have driving mutations or as maintenance therapy after chemotherapy,20,23–26 such as the guidelines for NSCLC published in 2022 by National Comprehensive Cancer Network (NCCN, version 1).
Characteristics of biosimilars
It is important to clarify that, unlike small molecule chemicals, biologics cannot be ‘consistent’ themselves due to the complexity of their structure and production. Even if it is the same kind of medicine produced by the same manufacturer, different origins, different batches, and even the same batch of products will also have certain differences. The difference between the biosimilar and the original drug is strictly limited to the difference between the original drug themselves to ensure the same clinical effect of the two. The development of biosimilars aims to improve the availability of biologics, meet the needs of patients, and reduce medical expenditures. The guarantee of their quality, efficacy, and safety is the most basic premise. Therefore, the drug regulatory authorities in various countries have extremely strict requirements for their approval process. Although the United States, the European Union, the China, and the World Health Organization have different definitions of the biosimilar, they all refer to a biological product that is highly similar to the reference drug (original drug) in terms of quality, safety, and efficacy, and there is no clinical difference between the two. For example, the US. FDA proposed the principle of ‘totality-of-the-evidence’,27 which requires biosimilars to provide progressive and comprehensive research results in pharmaceutical, pre-clinical, and clinical aspects. It must demonstrate ‘similarity’ to a biological product already approved for marketing by the FDA. And strictly define ‘similarity’ as: although there are minor differences in inactive ingredients, there are no clinically meaningful differences between this product and the reference product in terms of safety, purity, and efficacy. The China National Medical Products Administration (NMPA) has similar principle requirements for the similarity evaluation of biosimilars.
Biosimilars provide a new choice for clinical treatment because of their outstanding economic advantages.28,29 However, due to the special nature of biosimilars, such as immunogenicity, short time of use and limited clinical evidence, there are potential risks in the effectiveness and safety of biosimilars in clinical treatment in the real world. Therefore, more high-quality studies are needed as an evidence-based basis. The development of biosimilars in China is relatively late. The first drug was launched in 2019, and the development has gradually matured since then. High-quality clinical trials can be conducted according to the ‘totality-of-the-evidence’ principle issued by the FDA, the Technical Guidelines for Biosimilars Development and Evaluation (2015) and the Technical Guidelines for Similarity Evaluation and Indication Extrapolation of Biosimilars (2021) issued by NMPA,27,30,31 or the biosimilars development principles issued by other countries.
Summary of evidence
In this study, we used a meta-analysis to conduct evidence-based analysis and evaluation of the evidence related to the efficacy and safety of existing bevacizumab biosimilars in clinical treatment, so as to provide evidence-based reference for their rational clinical application. A total of 11 RCT studies were included. It should be noted that drugs that have not been marketed after phase III clinical trials or have not yet been marketed were excluded. Because if the drug is not on the market, it means that the drug does not meet the requirements of policy documents related to drug marketing, and fails to meet the corresponding evaluation technical standards in terms of quality, efficacy, and safety. These drugs cannot represent the efficacy and safety of bevacizumab biosimilars on the market, and have no significance for analysis. Instead, they may affect the analysis results of this study and cause erroneous conclusions.
Of the 11 RCTs, 7 were international multicenter studies, and 4 were multicenter studies conducted in China. The age of the included population was relatively high, the median age of each study was around 60 years, and most of the patients were male (mean: 63%, range: 59%–67%). We suspect that this may be related to smoking, which needs to be further explored.32,33 In terms of efficacy, the bevacizumab biosimilars have highly similar ORR (week 18), DCR (week 18), mDOR, mPFS, mOS and 1-year OS rate to the original drug. ORR is a direct measure of the efficacy of treatment, is approved by the FDA and the European Medicines Agency for comparing the antitumor activity of the putative biosimilar with that of the reference biologic, and has previously been used successfully for this purpose.7,16,34,35 On the other hand, because PFS/OS is not suitable for demonstrating biosimilarity,34 all included studies used ORR as the primary endpoint. Therefore, we used ORR as the primary outcome measure. Of the 11 included studies, 9 were independent imaging evaluation, 1 was investigator evaluation, and 1 had no relevant description (Supplemental Table B). At the same time, we found that all 10 studies were evaluated by RECIST version 1.1 except for one study that evaluated by RECIST version 1.0. In terms of safety, the bevacizumab biosimilars did not increase both TEAEs (grade ⩾3) and TRAEs (grade ⩾3) compared with the original one. Furthermore, all outcomes were observed to be highly similar in the EGFR wild-type subgroup and the EGFR undefined subgroup. Common AEs include the following: alopecia, anemia, nausea, neutropenia, thrombocytopenia, asthenia, arthralgia, fatigue, hypertension, leukopenia, peripheral neuropathy, and so on.
Efficacy and safety
Forest map analysis of DCR (week 18), mOS, and TRAEs (grade ⩾3) showed significant heterogeneity between various studies or subgroups, but the results did not change much after heterogeneity was reduced. And when one drug was removed in turn for sensitivity analysis of each outcome index, it was found that there was no statistically significant change in the results before and after elimination, which confirmed the robustness of the research results. However, when MYL-1402O was excluded from DCR, MIL60 excluded from mOS, and QL1101 excluded from TREAs, the integrated, between-subgroup, and within-subgroup heterogeneity were significantly reduced. We performed a comprehensive comparison of two EGFR wild-type studies in the DCR indicator. MYL-1402O was found to enroll only patients with negative or unknown EML4-ALK rearrangement. However, IBI305 did not describe the EML4-ALK genotype, which means they may not have excluded patients with known EML4-ALK rearrangement. In addition, large differences in the race of the populations they were included in were found (IBI305: Han 97.1%, Others 2.9%; MYL-1402O: White 68.3%, Asian 31.7%). We suspect that differences in race and genetics may lead to their greater heterogeneity. A comprehensive comparison was also performed on the four EGFR wild-type studies in the mOS indicator. We found different doses of maintenance therapy in the MIL60 study relative to the other three studies (MIL60: 7.5 mg/kg; MB02, QL1101, and SB8: 15 mg/kg). In addition, MIL60 was found to include patients with brain metastases, while the other three studies excluded patients with combined symptomatic brain metastases. We think this may be the main source of heterogeneity in MIL60 study. In contrast, when IBI305 or QL1101, MB02, QL1101 or SB8, MYL-1402O or PF-06439535 were excluded, the integrated heterogeneity of each index increased. Sources of heterogeneity across studies need to be further identified. Analyses of all outcome measures, both in the EGFR wild-type subgroup and in the EGFR-unlimited subgroup, showed that the bevacizumab biosimilars had a highly similar efficacy and safety profile to the innovator drug. And only mOS had heterogeneity between subgroups, so we speculate that the heterogeneity is more likely due to other unknown factors rather than EGFR gene status. In conclusion, we believe that EGFR gene status does not affect the efficacy and safety of bevacizumab.
Immunogenicity
Immunogenicity refers to the performance that can cause an immune response. That is, antigens can stimulate specific immune cells to activate, proliferate, differentiate, and finally produce immune effector antibodies and sensitized lymphocytes. Biological products all have certain immunogenicity due to their large molecular weight and complex structure. In all, 10 studies assessed ADAs, but due to incomplete data and lack of commonalities, they were not meta-analyzed, but were systematically reviewed. Results of the evaluation showed low and similar immunogenicity between the two treatment groups, with no clinically meaningful differences. And the experimental drug QL1101 was shown to have similar immunogenicity to the Avastin in a previous randomized, double-blind, single-dose study in healthy male subjects.36 Therefore, we believe that the immunogenicity of bevacizumab biosimilars is not different from that of Avastin. However, some differences were found in the incidence of immunogenicity among these studies. The immunogenicity of the IBI305 and MIL60 studies were less than 1%, the ABP 215, FKB238, LY01008, MYL-1402O, QL1101, and PF-06439535 studies were between 1% and 10%, while the SB8 and MB02 studies were in the range of 10%–20%. We speculate that it may be related to the time of antibody detection, and as for the specific reasons for the difference could not be known in this paper.
Implications for practice
In recent years, the sales of bevacizumab biosimilars have been increasing, and there is a trend of gradually replacing the original drug. However, these drugs all have the problem of short clinical use time. Our study adopted an evidence-based method to explore the efficacy and potential clinical risks of currently marketed bevacizumab biosimilars, which is conducive to promoting the use of bevacizumab biosimilars, thereby reducing the economic burden of social medicine.
Limitations
Of the 11 studies, 3 studies did not describe the EGFR mutation status, 5 studies only included patients with wild-type or unknown gene status, and the remaining 3 studies did not limit the genotype. As for anaplastic lymphoma kinase (ALK) gene, 5 studies did not describe it, and 6 studies only included wild-type or unknown patients. In addition, 3 of the 11 studies did not receive maintenance treatment, 3 studies used the biosimilar for maintenance treatment both in the experimental group and the control group, and the remaining 5 studies used the biosimilar and Avastin for maintenance treatment, respectively (Table 1). Because of the small number of articles, subgroup analyses by ALK mutation status or maintenance therapy were not performed. At the same time, since the number of articles of each outcome was less than 10, the funnel plot was not used to analyze the publication deviation. Finally, only marketed drugs were included in this study. We did this to avoid publication bias due to negative test results, but it also resulted in the exclusion of drugs that are not currently on the market but may be on the market in the future. This article showed the authorities made the right decision regarding their approval.
Conclusion
In the first-line treatment of advanced non-squamous NSCLC, there is no difference in efficacy, safety, and immunogenicity between the bevacizumab biosimilars and the original drug (Avastin). The above conclusion needs to be verified by more high-quality clinical studies.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221130501 for Efficacy and safety of adding bevacizumab biosimilar or original drug to platinum-based chemotherapy as first-line treatment in patients with advanced NSCLC: a systematic review and meta-analysis by Liu Yang, Maobai Liu, Na Li, Bin Zheng, Jianhao Deng, Hongfu Cai and Xueqiong Cao in Therapeutic Advances in Medical Oncology
Acknowledgments
We would like to express our appreciation to all authors of the primary studies included in the current systematic review and meta-analysis.
Footnotes
ORCID iD: Hongfu Cai  https://orcid.org/0000-0002-4923-4882
https://orcid.org/0000-0002-4923-4882
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Liu Yang, Fujian Medical University Union Hospital, Fuzhou, China; Fujian Medical University, Fuzhou, China.
Maobai Liu, Fujian Medical University Union Hospital, Fuzhou, China; Fujian Medical University, Fuzhou, China.
Xueqiong Cao, Fujian Medical University Union Hospital, Fuzhou, China; Fujian Medical University, Fuzhou, China.
Na Li, Fujian Medical University Union Hospital, Fuzhou, China; Fujian Medical University, Fuzhou, China.
Bin Zheng, Fujian Medical University Union Hospital, Fuzhou, China; Fujian Medical University, Fuzhou, China.
Jianhao Deng, The Second Hospital of Longyan, Longyan, Fujian, China.
Hongfu Cai, Fujian Medical University Union Hospital, Xinquan road 29, Fuzhou, Fujian 350001, China. Fujian Medical University, Fuzhou, China.
Declarations
Ethics approval and consent to participate: Not Applicable.
Consent for publication: All authors participated in this study and approved the final version.
Author contribution(s): Liu Yang: Writing – original draft.
Maobai Liu: Methodology.
Xueqiong Cao: Modification.
Na Li: Software.
Bin Zheng: Data curation.
Jianhao Deng: Software.
Hongfu Cai: Supervision.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Fujian Provincial Department of Science & Technology (grant no. 2018Y9037, 2020Y9070, and 2021R0053) of the People’s Republic of China.
The authors declare that there is no conflict of interest.
Availability of data and materials: All datasets generated for this study are included in the article/supplementary material.
References
- 1. Scott WJ, Howington J, American College of Chest Physicians, et al. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007; 132: 234S–242S. [DOI] [PubMed] [Google Scholar]
- 2. Botrel TE, Clark O, Clark L, et al. Efficacy of bevacizumab (Bev) plus chemotherapy (CT) compared to CT alone in previously untreated locally advanced or metastatic non-small cell lung cancer (NSCLC): systematic review and meta-analysis. Lung Cancer 2011; 74: 89–97. [DOI] [PubMed] [Google Scholar]
- 3. Lima AB, Macedo LT, Sasse AD. Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2011; 6: e22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA 2011; 305: 487–494. [DOI] [PubMed] [Google Scholar]
- 5. 本刊讯. 食品药品监督管理总局发布《生物类似药研发与评价技术指导原则(试行)》 %J 中国药房 2015; 26: 1318. [Google Scholar]
- 6. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 7. Thatcher N, Goldschmidt JH, Thomas M, et al. Efficacy and safety of the biosimilar ABP 215 compared with bevacizumab in patients with advanced nonsquamous non-small cell lung cancer (MAPLE): a randomized, double-blind, phase III study. Clin Cancer Res 2019; 25: 2088–2095. [DOI] [PubMed] [Google Scholar]
- 8. ClinicalTrials.gov. A safety and efficacy study of BCD-021 with paclitaxel and carboplatin compared to avastin with paclitaxel and carboplatin in non-small cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT01763645?term=BCD-021&draw=2&rank=3 (2018, accessed 1 September 2022).
- 9. Filon O, Orlov S, Burdaeva O, et al. Efficacy and safety of BCD-021, bevacizumab biosimilar candidate, compared to Avastin: results of international multicenter randomized double blind phase III study in patients with advanced non-squamous NSCLC. J Clin Oncol 2015; 33: 8057. [Google Scholar]
- 10. Syrigos K, Abert I, Andric Z, et al. Efficacy and safety of bevacizumab biosimilar FKB238 versus originator bevacizumab: results from AVANA, a phase III trial in patients with non-squamous non-small-cell lung cancer (non-sq-NSCLC). BioDrugs 2021; 35: 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang Y, Wu B, Huang L, et al. Biosimilar candidate IBI305 plus paclitaxel/carboplatin for the treatment of non-squamous non-small cell lung cancer. Transl Lung Cancer Res 2019; 8: 989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi Y, Lei K, Jia Y, et al. Bevacizumab biosimilar LY01008 compared with bevacizumab (Avastin) as first-line treatment for Chinese patients with unresectable, metastatic, or recurrent non-squamous non-small-cell lung cancer: a multicenter, randomized, double-blinded, phase III trial. Cancer Commun (Lond) 2021; 41: 889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trukhin D, Poddubskaya E, Andric Z, et al. Efficacy, safety and immunogenicity of MB02 (Bevacizumab Biosimilar) versus reference bevacizumab in advanced non-small cell lung cancer: a randomized, double-blind, phase III study (STELLA). BioDrugs 2021; 35: 429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wan R, Dong X, Chen Q, et al. Efficacy and safety of MIL60 compared with bevacizumab in advanced or recurrent non-squamous non-small cell lung cancer: a phase 3 randomized, double-blind study. EClinicalMedicine 2021; 42: 101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Socinski MA, Waller CF, Idris T, et al. Phase III double-blind study comparing the efficacy and safety of proposed biosimilar MYL-1402O and reference bevacizumab in stage IV non-small-cell lung cancer. Ther Adv Med Oncol 2021; 13: 17588359211045845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reinmuth N, Bryl M, Bondarenko I, et al. PF-06439535 (a Bevacizumab Biosimilar) compared with reference bevacizumab (Avastin((R))), both plus paclitaxel and carboplatin, as first-line treatment for advanced non-squamous non-small-cell lung cancer: a randomized, double-blind study. BioDrugs 2019; 33: 555–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chu T, Lu J, Bi M, et al. Equivalent efficacy study of QL1101 and bevacizumab on untreated advanced non-squamous non-small cell lung cancer patients: a phase 3 randomized, double-blind clinical trial. Cancer Biol Med 2021; 18: 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reck M, Luft A, Bondarenko I, et al. A phase III, randomized, double-blind, multicenter study to compare the efficacy, safety, pharmacokinetics, and immunogenicity between SB8 (proposed bevacizumab biosimilar) and reference bevacizumab in patients with metastatic or recurrent nonsquamous non-small cell lung cancer. Lung Cancer 2020; 146: 12–18. [DOI] [PubMed] [Google Scholar]
- 19. Iacono D, Chiari R, Metro G, et al. Future options for ALK-positive non-small cell lung cancer. Lung Cancer 2015; 87: 211–219. [DOI] [PubMed] [Google Scholar]
- 20. Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3: 391–400. [DOI] [PubMed] [Google Scholar]
- 21. Yi JS, Ready N, Healy P, et al. Immune activation in early-stage non-small cell lung cancer patients receiving neoadjuvant chemotherapy plus ipilimumab. Clin Cancer Res 2017; 23: 7474–7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. 周振兴, 宋军民, 陈姬华, 吴强. 贝伐珠单抗在肿瘤治疗中的应用研究进展 %J 药学进展. 2015; 39: 525–32. [Google Scholar]
- 23. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355: 2542–2550. [DOI] [PubMed] [Google Scholar]
- 24. Petrioli R, Francini E, Fiaschi AI, et al. Switch maintenance treatment with oral vinorelbine and bevacizumab after induction chemotherapy with cisplatin, gemcitabine and bevacizumab in patients with advanced non-squamous non-small cell lung cancer: a phase II study. Med Oncol 2015; 32: 134. [DOI] [PubMed] [Google Scholar]
- 25. Crino L, Dansin E, Garrido P, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol 2010; 11: 733–740. [DOI] [PubMed] [Google Scholar]
- 26. Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009; 27: 1227–1234. [DOI] [PubMed] [Google Scholar]
- 27. Tsong Y, Dong X, Shen M. Development of statistical methods for analytical similarity assessment. J Biopharm Stat 2017; 27: 197–205. [DOI] [PubMed] [Google Scholar]
- 28. Abraham I, Han L, Sun D, et al. Cost savings from anemia management with biosimilar epoetin alfa and increased access to targeted antineoplastic treatment: a simulation for the EU G5 countries. Future Oncol 2014; 10: 1599–1609. [DOI] [PubMed] [Google Scholar]
- 29. 梅丹, 都丽萍, 张钰宣. 多维度关注生物类似药的管理与临床应用 %J 中国药房 2020; 31: 373–378. [Google Scholar]
- 30. National Medical Products Administration. Circular of the state food and drug administration on issuing the technical guiding principles for the R&D and evaluation of biosimilars. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20150228155701114.html (2015, accessed 1 September 2022).
- 31. Center For Drug Evaluation, Nmpa. Notice of the center for drug evaluation of the state food and drug administration on issuing the “Technical guiding principles for similarity evaluation and indication extrapolation of biosimilars”. https://www.cde.org.cn/main/news/viewInfoCommon/d92c6507a57bee9ccfc5baa1ee87fda9 (2021, accessed 1 September 2022).
- 32. Harjes U. Non-small cell lung cancer: where there’s smoke. Nat Rev Cancer 2017; 17: 634–635. [DOI] [PubMed] [Google Scholar]
- 33. Vaz M, Hwang SY, Kagiampakis I, et al. Chronic cigarette smoke-induced epigenomic changes precede sensitization of bronchial epithelial cells to single-step transformation by KRAS mutations. Cancer Cell 2017; 32: 360–376 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. European Medicines Agency. Guideline on similar biological medicinal products. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf (2014, accessed 1 September 2022).
- 35. United States Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product. https://www.fda.gov/media/82647/download. (2015, accessed 1 September 2022).
- 36. Liu YN, Huang J, Guo C, et al. A randomized, double-blind, single-dose study to evaluate the biosimilarity of QL1101 with bevacizumab in healthy male subjects. Cancer Chemother Pharmacol 2020; 85: 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221130501 for Efficacy and safety of adding bevacizumab biosimilar or original drug to platinum-based chemotherapy as first-line treatment in patients with advanced NSCLC: a systematic review and meta-analysis by Liu Yang, Maobai Liu, Na Li, Bin Zheng, Jianhao Deng, Hongfu Cai and Xueqiong Cao in Therapeutic Advances in Medical Oncology