Abstract
Background
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) has various similarities with AQP4-IgG-seropositive Neuromyelitis Optica Spectrum Disorder (AQP4-IgG + NMOSD) in terms of clinical presentations, magnetic resonance imaging (MRI) findings, and response to treatment. But unlike AQP4-IgG + NMOSD, which is known to coexist with various autoimmune diseases and cancers, an association of MOGAD with these conditions is less clear.
Methods
We conducted a systematic search in PubMed, Scopus, Web of Science, and Embase based on the preferred reporting items for systematic reviews and meta-analysis (PRISMA). Duplicates were removed using Mendeley 1.19.8 (USA production) and the citations were uploaded into Covidence systematic review platform for screening.
Results
The most common autoimmune disease overlapping with MOGAD was anti-N-Methyl-D-Aspartate receptor encephalitis (anti-NMDAR-EN), followed by autoimmune thyroid disorders, and the most common autoantibody was antinuclear antibody (ANA), followed by AQP4-IgG (double-positive MOG-IgG and AQP4-IgG). A few sporadic cases of cancers and MOG-IgG-associated paraneoplastic encephalomyelitis were found.
Conclusion
Unlike AQP4-IgG + NMOSD, MOGAD lacks clustering of autoimmune diseases and autoantibodies associated with systemic and organ-specific autoimmunity. Other than anti-NMDAR-EN and perhaps AQP4-IgG + NMOSD, the evidence thus far does not support the need for routine screening of overlapping autoimmunity and neoplasms in patients with MOGAD.
Keywords: Myelin oligodendrocyte glycoprotein, autoimmune disease, autoantibody, neoplasm, paraneoplastic, systematic review
Introduction
Myelin oligodendrocyte glycoprotein antibody disease (MOGAD) refers to a range of central nervous system (CNS) inflammatory conditions associated with the presence of autoantibodies against MOG. These conditions include acute disseminated encephalomyelitis (ADEM), optic neuritis (ON), or transverse myelitis (TM), as well as some cases of aquaporin-4 (AQP4)-IgG seronegative Neuromyelitis Optica Spectrum Disorder (NMOSD).1 Despite the diversity of clinical and pathologic manifestations and disease outcomes, seropositive MOGAD is now regarded as a distinct entity among CNS inflammatory disorders linked together by immunity to MOG. Preliminary studies indicate that MOGAD has a more benign course and relatively better recovery from relapses in most patients compared to AQP4-IgG + NMOSD.2
Identifying associations among autoimmune diseases is important in patients with CNS demyelinating disorders because it may change the therapeutic strategies, monitoring approaches and intervals, and prognosis of both CNS inflammatory diseases and the co-existing autoimmune entity.3 The underlying mechanisms of autoimmunity and autoantibody production in autoantibody-associated neurological syndromes are still unclear. One may postulate that a common immunologic defect may drive autoimmunity or autoantibody production in various organs, or that an existing autoimmune condition or malignancy may trigger additional defects in the immune system resulting in the development of new autoantibody or autoimmunity.4
One of the recognizable features of AQP4-IgG + NMOSD is the large overlap with other autoimmune conditions such as systemic lupus erythematosus (SLE), Sjogren's disease, autoimmune thyroid disorders (ATDs), and myasthenia gravis (MG).5,6 In contrast, there is less known about the association of immunological comorbidities with MOGAD.
Published reports of co-existing autoimmunity in a large cohort of pediatric and adult patients with MOGAD and AQP4-IgG + NMOSD highlight the point that concomitant autoimmunity is less common in MOGAD when compared to AQP4-IgG + NMOSD.3 Other comparative studies showed that antinuclear antibody (ANA) was positive in 43% of AQP4 + IgG NMOSD but only 7% of MOGAD patients.7 Similarly, concomitant autoimmune disorders were observed in 45% and 11% of AQP4-IgG + NMOSD, and MOGAD patients, respectively.8
Herein, we systematically reviewed the literature for cases of autoimmune diseases, autoantibodies, neoplasms, and paraneoplastic syndromes that have been reported to overlap with MOGAD.
Methods
We conducted this systematic review to investigate any overlapping of MOGAD/positive MOG-IgG and other autoimmune diseases, autoantibodies, neoplasms, and paraneoplastic syndromes, based on the preferred reporting items for systematic reviews and meta-analysis (PRISMA).
Search strategy
We designed our systematic search syntax based on PRISMA from inception to July 2022, which included all mesh terms of “MOGAD,” all autoimmune diseases, autoantibodies, neoplasms, and paraneoplastic syndromes (Supp. 1.) Our syntax was customized to match the four main systematic review databases including PubMed, Scopus, Web of Science, and Embase. The following results were imported to the desktop version of Mendeley 1.19.8 (USA production) to merge duplications and prepare for the screening. Two independent investigators (N.M. and G.B.) screened all publications to find probable appropriate studies by title and abstracts through Covidence systematic review software.9 Disagreements in screening were resolved by debating. In addition, the gray literature and reference lists of all articles included in the search were reviewed to ensure no information was left out.
Study selection and data extraction
The data extraction table advanced by the variables including author, year, the number of cases, sex, age, clinical presentation, antibody testing assay and source, and notable clinical features and MRI findings. The inclusion criteria consisted of all studies (case reports, case series, observational studies, etc.) which reported at least one autoimmune disease, positive autoantibody (with or without disease presentation), neoplasm, or paraneoplastic syndrome, overlapping with MOGAD or positive MOG-IgG serostatus (either simultaneously or with an interval between them). On the other hand, exclusion criteria comprised papers without English abstracts, and all types of reviews, letters, and commentaries. N.M and G.B. separately extracted the included articles and disagreements resolved by the expert opinion (M.L).
Results
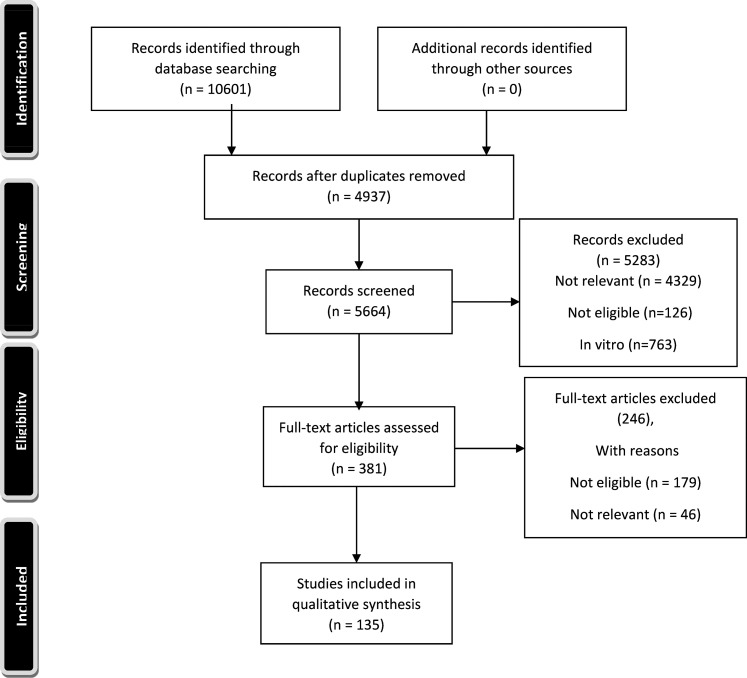
The PRISMA flow chart of this study is shown in Figure 1. Using the search strategy, 10,601 studies were identified however, 5664 studies remained after duplicate removes. After eliminating these duplicates, 296 and 343 studies assessed for full text eligibility by N.M. and I.L. independent screening. Our literature review showed that the most common autoimmune disease reported to overlap with MOGAD or positive MOG-IgG serostatus, is anti-NMDAR encephalitis (Anti-NMDAR-EN) (200 cases) (Table 1), followed by ATD (38cases), and SLE (35 cases) (Table 3). The most frequently reported autoantibodies overlapping with MOGAD were ANA (96 cases) (Table 3), and AQP4-IgG (70 cases of double-positive MOG-IgG and AQP4-IgG) (Table 2) respectively.
Figure 1.
PRISMA flow chart of the study.
Table 1.
Overlapping MOGAD/positive MOG-IgG and anti-NMDAR-encephalitis/positive anti-NMDAR-IgG.
| Reference | Number of cases | Sex/Age |
|
|
Notable clinical features, or MRI findings |
|---|---|---|---|---|---|
| Titulaer et al. (2014)10 | 12 | F/4 F/6 F/18 F/45 F/43 F/48 M/17 F/27 M/34 M/10 M/29 M/38 |
|
|
Presentation of encephalopathy symptoms with clinical and/or MRI features of demyelination (ON, myelitis, BS dysfunction), and/or T2-W/FLAIR multifocal, infratentorial, or extensive abnormalities, suggesting involvement of the WM associated with MOG-IgG, either simultaneously or separate in time. |
| Hacohen et al. (2014)11 | 3 | F/6 M/9 M/8 |
|
|
Presentation of BON and/or ADEM in patients with positive
MOG-IgG and anti-NMDAR-IgG (with or without
anti-NMDAR-EN). Brain MRIs were normal in patients with ON, and showed bilateral deep GM and WM, and further BS lesions in second relapse in the patient with ADEM. |
| Kaneko et al. (2014)12 | 1 | F/27 |
|
|
Presentation of anti-NMDAR-IgG positive limbic encephalitis followed by demyelinating lesions. |
| Yokoyama et al. (2016)13 | 1 | F/9 |
|
|
Presented with fever, somnolence, hemiplegia, dysphagia, and memory impairment with high signal intensity in the left temporal and parietal lobes on FLAIR MRI, started on steroids (IV followed by an oral taper), which was followed by recurrent ON with right optic nerve enhancing lesion on T1-W MRI, about 17 days after oral steroid cessation. |
| Kaneko et al. (2017)14 | 3 | NA/NA |
|
|
|
| Zhou et al. (2017)15 | 1 | M/31 |
|
|
Cortical encephalitis initially presented with fever, headache, and seizure, with FLAIR MRI showing high-intensity lesions of right temporal, parietal, and occipital cortex, followed by the development of MOG-IgG-EN, BS-EN, ON, and ADEM-like illness successively, indicating demyelination. MRI revealed ADEM-like multiple lesions in the right midbrain, pons, left brachium points and juxtacortical WM, and a focal serpentine lesion in the left parietal cortex. |
| Nagata, et al. (2018)16 | 1 | F/20 |
|
|
Presentation of seizures, disorientation, leg weakness with MRI lesions in the bilateral cingulate gyrus and the superior frontal gyrus with enhancement in the upper part of the corpus callosum, followed by newly developed ring-enhancing lesion on the left side of the cingulate gyrus, hyperintense lesion spreading in the subcallosal area and the brainstem, and BON on STIR images. |
| Zhou et al. (2018)17 | 1 | M/54 |
|
|
Progressive unsteadiness and narcoleptic attacks followed by behavioral change and psychosis, without visual disturbances or seizures, with FLAIR findings of multiple high-intensity lesions involving the cerebellum, brainstem, thalamus, and third ventricular peri-ependymal region, consistent with demyelination. |
| Dubey et al. (2018)18 | 1 | M/2 |
|
|
Recurrent encephalitis, seizures, ON, and multifocal MRI abnormalities in the context of double positive MOG-IgG and anti-NMDAR-IgG. |
| Fan et al. (2018)19 | 5 | F/3 M/23 M/6 M/25 M/9 |
|
|
Overlapping anti-NMDAR-EN occurred:
|
| Zhou et al. (2019)20 | 2 | NA/NA |
|
|
Initial presentation of CNS demyelinating syndrome, followed by acute mania and FLAIR MRI findings of extensive dorsal brainstem lesion. The second patient did not show encephalitic symptoms. |
| Taraschenko et al. (2019)21 | 1 | F/10 | • MOG-EN • CBA, serum | • Anti-NMDAR-EN • CBA, CSF | BON with enhancing lesions and perineural sheath swelling in bilateral optic nerves, followed by recurrent encephalitis with focal seizures. |
| Wang et al. (2019)22 | 6 | NA/NA |
|
|
Presentation of typical encephalitis symptoms including headache, decreased level of consciousness, lethargy, irritability, memory, or cognitive decline with the most frequent MRI findings of cortical lesions on T2-W and FLAIR images. |
| Kaneko et al. (2019)23 | 1 | F/28 |
|
|
Development of demyelinating MRI lesions, gait instability, and saccadic eye movements during exacerbation of previously diagnosed limbic encephalitis with positive anti-NMDAR-IgG. |
| Ren et al. (2019)24 | 4 | NA/31.5 (median age) |
|
|
Presentation of demyelinating symptoms associated with MOG-IgG in patients with previous diagnosis of anti-NMDAR-EN, or presentation of MOGAD with double positivity for MOG-IgG and anti-NMDAR-IgG, or development of psychiatric symptoms and limb weakness in double positive patient. |
| Kunchok et al. (2019)25 | 3 | NA/NA |
|
|
|
| Aoe et al. (2019)26 | 1 | F/36 |
|
|
Recurrent typical anti-NMDAR-EN coexisting with unusual symptoms such as balance disability, and MRI findings of bilateral medial frontal cortical lesions, and Broca's aphasia caused by involvement of the Broca's area and lower part of the precentral gyrus, further found to be MOG- IgG-positive. |
| Rojc et al. (2019)27 | 1 | M/47 |
|
|
Development of ON associated with MOG-IgG in a patient with history of recurrent ON who was diagnosed with anti-NMDAR-EN. (He was found to be MOG-IgG-positive in serum sample of previous ON attack when positive for anti-NMDAR-IgG, while having hyper-intense lesions in right frontoparietal WM on of T2-W and FLAIR MRI). |
| Kaneko et al. (2019)28 | 3 | NA/NA |
|
|
Anti-NMDAR-EN with overlapping MOG-IgG presenting with multifocal demyelination (N = 1) and meningoencephalitis (N = 2). |
| Sarigecili et al. (2019)29 | 1 | M/6 |
|
|
Clinical presentation of autoimmune encephalitis with simultaneous detection of antibodies against MOG and NMDAR. Cranial and spinal MRI showed no signs of encephalomyelitis. |
| Gao et al. (2020)30 | 2 | F/32 M/50 |
|
|
Presentation of cortical encephalitis and subsequent demyelination in patients positive for both NMDAR-IgG and MOG-IgG. The clinical manifestations were different from pure MOGAD or anti-NMDAR-EN. |
| Amano et al. (2020)31 | 1 | F/22 |
|
|
Initial presentation of headache, aphasia, and right hemiparesis associated with left frontal cortical lesions on MRI. Recurrent anti-NMDAR-EN episodes with dominant MRI finding of leptomeningeal enhancement, in which MOG-IgG was retrospectively identified in the CSF and in the archived serum samples drawn in previous EN attacks. |
| Sakamoto et al. (2020)32 | 1 | M/12 |
|
|
Presentation of psycho-behavioral symptoms in the first episode, followed by seizures, speech and writing difficulties, with abnormal MRI findings in the left frontal cortex, in the second episode. |
| Martinez-Hernandez et al. (2020)33 | 17 | F (N = 6) M (N = 11)/median age of symptoms onset: 38 (5–57) |
|
|
Sixteen patients presented with anti-NMDAR-EN symptoms (two of them with additional BS-cerebellar symptoms, one limb numbness and weakness, and one pyramidal signs); and one patient with BON. FLAIR MRI abnormalities included cortical lesions in six, subcortical WM lesions in three, and meningeal enhancement in one patient. |
| Ma et al. (2020)34 | 1 | M/12 |
|
|
Development of emotional lability, irritability, insomnia, dysphagia, and MRI abnormalities with positive anti-MOG and anti-NMDAR antibodies, about 40 days of an initial presentation of fever, headache, loss of consciousness, and seizure with abnormal T2-W and FLAIR MRI findings (hyperintense signals in the left frontotemporal parietal occipital lobes and right temporal-parietal lobes) which was diagnosed with viral encephalitis. |
| Du et al. (2020)35 | 4 | M/25 F/48 F/37 M/21 |
|
|
Overlapping occurred sequentially in three and simultaneously in one patient. |
| Gong et al. (2020)36 | 11 | F (N = 7) M (N = 4)/age of onset: 10.4 ± 2.3 |
|
|
From 29 episodes of 11 patients with overlapping MOGAD and anti-NMDAR-EN, the common symptoms were convulsions, psychosis, lethargy, and the most frequent MRI findings were cortical focus, subcortical WM lesions, and BS lesions. |
| Zheng et al. (2020)37 | 1 | NA/NA |
|
|
One pediatric patient with anti-NMDAR-EN and optic nerve damage combined with positive MOG-IgG. |
| Wegener-Panzer et al. (2020)38 | 2 | F/14 M/10 |
|
|
|
| Zhu et al. (2020)39 | 2 | NA/NA | • MOGAD • NA, NA | • Anti-NMDAR-EN • NA, NA | |
| Hou et al. (2020)40 | 7 | M/4 F/7 F/6 F/5 F/6 F/8 M/6 |
|
|
Overlapping anti-NMDAR-EN occurred:
The most frequent MRI findings were subcortical WM lesions in bilateral frontal and parietal lobes, and patchy abnormal signal in bilateral basal ganglia region. |
| Perez et al. (2020)41 | 1 | M/29 |
|
|
Development of BON with positive MOG-IgG, and non-enhancing T2-W hyperintense lesions in the left thalamus and left medulla, mild enhancement of the optic chiasm, left intra-orbital optic nerve, and the optic tracts bilaterally, in a patient with previous episode of anti-NMDAR-EN presented with headaches, blurry vision, urinary retention, gait instability, slurred speech, fever, hypertension, and confusion. |
| Cherian et al. (2020)42 | 1 | M/30 |
|
|
Presented with neuropsychiatric, cognitive symptoms, and long tract signs with a strong positivity of all three antibodies, and MRI findings of bilateral cingulate and hippocampal lesions. |
| Zhang et al. (2020)43 | 1 | NA |
|
|
Clinical and MRI findings not specified, but reported to be more in line with ADEM than with anti-NMDAR-EN. |
| VJ Lopez et al. (2021)44 | 1 | M/26 |
|
|
Development of behavioral changes (impaired executive function and memory, paranoia, and grandiose ideations), BON, nystagmus, pronator drift, and ataxia in the left arm, with MRI findings of multifocal, contrast-enhancing FLAIR hyperintensities involving the right pons, midbrain, thalami, and optic nerves. |
| Guzman et al. (2021)45 | 2 | NA/NA |
|
|
|
| Weiss et al. (2021)46 | 1 | F/20 |
|
|
Development of anti-NMDAR-EN and an overlapping demyelinating disorder with optic nerve involvement, gait disturbance, and MRI showing enhancing lesion in the dorsal column of the cervical spinal cord associated with anti- MOG-IgG, in a patient with a borderline personality disorder. |
| Cao et al. (2021)47 | 1 | M/37 |
|
|
Presentation of recurrent headaches with recent worsening, and seizures in a patient with a history of ON, and FLAIR MRI showing abnormal hyperintense signal in the bilateral frontal lobes, cingulate cortex, and enhancement in meninges the parts above the lesions. |
| Ren et al. (2021)48 | 1 | M/38 |
|
|
Epileptic seizures following recurrent episodes of cross-sensory disturbance and dizziness with a demyelinating lesion in the right brainstem on MRI, followed by further relapses, progression, and new serpentine lesions with extensive involvement of cerebral cortex. |
| Shen et al. (2021)49 | 18 | NA/NA |
|
|
Double-positive patients had more seizures, abnormal mental behavior, and visual impairment compared to MOG-IgG-positive group, and had more mental and behavioral abnormalities, and ataxia compared to anti-NMDAR-IgG positive group. |
| Fujimori et al. (2021)50 | 1 | M/3 |
|
|
Relapsing anti-NMDAR-EN with initial MRI findings of bilateral medial frontal cerebral cortical lesions, and positive MOG-IgG at the same time. |
| Chen et al. (2021)51 | 5 | F/63 M/41 F/18 M/19 M/31 |
|
|
Most frequent clinical presentations were headache, epileptic seizure, cognitive impairment, disturbance of consciousness, psychiatric disorders, motor deficit, and involuntary movement. The dominant MRI findings were subcortex and cortical lesions, followed by lesions in basal ganglia and brainstem. |
| Teng et al. (2021)52 | 6 | NA/NA |
|
|
The most common clinical attack of the first and all MOGAD in children was ADEM, and the most common clinical syndrome was NMOSD. The most frequently involved areas in MRI were subcortical WM, cortex, and periventricular WM. |
| Nan et al. (2021)53 | 1 | M/19 |
|
|
First episode of anti-NMDAR-EN with positive CSF and negative serum anti-NMDAR-IgG and normal brain MRI (MOG-Ab not tested at the time), followed by a demyelinating episode (lethargy, facial pruritus, and right hemiplegia) with T2 and FLAIR hyperintense lesions in the left frontal lobe, basal ganglia, thalamus, and pons, and positive CSF and negative serum anti-NMDAR-IgG. MOG-IgG was positive in serum and negative in CSF at this time. |
| Caparo-Zamalloa et al. (2021)54 | 1 | M/27 |
|
|
Presentation of speech disorder, irritability, aggressive behavior, and disorientation followed by psychomotor agitation, seizure, generalized rigidity associated with ataxic gait and right hemiparesis in one month. T2-W/FLAIR MRI showed faint lesions in both middle cerebellar peduncles, midbrain and some supratentorial lesions, with no enhancement. |
| Guang et al. (2021)55 | 27 | F (N = 16), M (N = 11)/Median age at disease onset: 8.25 (5.44–9.86) |
|
|
Overlapping cases showed milder conditions compared to patients with typical anti-NMDAR-EN and were more inclined to relapse. |
| Chen et al. (2021)56 | 3 | NA/NA |
|
|
|
| Li et al. (2021)57 | 3 | NA |
|
|
Two patients presented with ON, and one with myelitis. None met the diagnostic criteria for anti-NMDAR-EN. |
| Jia et al. (2021)58 | 1 | NA/NA |
|
|
Presented with epilepsy and encephalopathy, where epileptic seizures were the prominent feature. |
| Wang et al. (2021)59 | 3 | NA |
|
|
One patient presented with memory decline, seizures, and multifocal neurological symptoms; the other two presented with acute encephalitic symptoms without focal neurological signs. |
| Han et al. (2022)60 | 1 | M/3 |
|
|
Presented with drowsiness, generalized and focal seizures, abnormal behavior, aphasia, left arm weakness. Normal initial MRI, and development of diffuse brain atrophy in follow up MRI. MOG-IgG turned negative at month 37 of follow-up. |
| Wang et al. (2022)61 | 3 | NA |
|
|
|
| Lei et al. (2022)62 | 1 | M/24 |
|
|
Presented with recurrent encephalitis; also had ON during the first attack. |
| Guo et al. (2022)63 | 3 | M/25 |
|
|
A case of overlapping Anti-NMDAR-IgG and MOG-IgG associated refractory encephalitis mimicking the radiological characteristics of CLIPPERS. |
| Weihau et al. (2022)64 | 12 | F (N = 9), M (N = 3), median age at disease onset: 8.6 (6.7–11.4) |
|
|
Patients with overlapping Anti-NMDAR-EN and MOG-IgG were more prone to relapse through the disease course. |
| Yin et al. (2022)65 | 1 | M/29 |
|
|
Presentation of abnormal behavior and loss of consciousness, with MRI showing punctate abnormal signals in the left parietal lobe, followed by a demyelinating episode in one year, as of hoarseness and double vision, and MRI lesions in the medulla oblongata, left pons arm, left cerebellum and right midbrain, and thalamus. |
| Wang et al. (2022)66 | 1 | M/36 |
|
|
Initial presentation of left facial numbness and slurred speech with FLAIR MRI showing lesions in left pontine and cerebral peduncle (MOG-IgG positive, NMDAR-IgG negative) followed by an episode of behavioral changes and psychosis with MRI showing deep white matter demyelinating lesions in the left lateral ventricle frontal angle and right lateral ventricle occipital angle (positive for both antibodies). |
Abbreviations: MOGAD: myelin oligodendrocyte antibody associated disease; NMDAR: N-methyl-D-aspartate; MRI: magnetic resonance imaging; EN: encephalitis; CBA: cell-based-assay; IHC: immunohistochemistry; CSF: cerebrospinal fluid; ON: optic neuritis; BS: brainstem; T2-W: T2 weighted image; FLAIR: fluid-attenuated inversion recovery; WM: white matter; NA: not available, DS: demyelinating syndrome; BON: bilateral optic neuritis; ADEM: acute disseminated encephalomyelitis; GM: gray matter; T1-W: T1 weighted image; IIFT: indirect immunofluorescence test; STIR: short tau inversion recovery; FACS: fluorescence-activated cell sorting; EM: encephalomyelitis; IF: immunofluorescence; CASPR2: contactin-associated protein-like 2
Table 3.
Overlapping MOGAD/positive MOG-IgG and other autoimmune diseases and/or autoantibodies.
| References | Number of cases | Sex/age |
|
Overlapping autoimmunity and/or autoantibodies | Notable clinical features, or MRI findings | |
|---|---|---|---|---|---|---|
| Mader et al. (2011)67 | 2 | NA/NA |
|
SLE | ||
| Mader et al. (2018)89 | 3 | NA/NA |
|
Demyelinating or non-demyelinating NPSLE | All patients with demyelinating NPSLE met the ACR 1999 criteria for demyelination, and all had either TM occurring at 2 different time points or TM with WM demyelination or ON occurring at two different time points. | |
| Bilodeau et al. (2019)90 | 1 | F/32 |
|
SLE | Presented with severe TM (paraplegia) shortly after SLE onset in the post-partum period. FLAIR MRI showed lesions in medulla, pons, hypothalamus, subcortical WM, and T2-hyperintense lesions adjacent to the third and fourth ventricle. Spinal MRI showed LETM in the entire cervical and thoracic cord. | |
| Pröbstel et al. (2019)91 | 14 | M/34 F/33 F/61 F/35 F/63 F/49 F/51 F/34 M/54 F/26 F/35 F/31 F/25 M/40 |
|
NPSLE (N = 6) and
non-NPSLE (N = 8) |
Presentation of visual disturbance, bladder dysfunction, seizure, and psychosis. | |
| ANA Anti-ds-DNA Anti-Phospholipid Anti-Sm Anti SSA/SSB |
N = 14N = 9N = 8N = 3N = 2 | |||||
| Seth et al. (2020)92 | 11 | NA/NA |
|
NPSLE (N = 8) and
non-NPSLE (N = 3) |
MOG-IgG was positive in a significantly higher proportion of patients having mood disorder as compared to patients without it. | |
| Chawla et al. (2021)93 | 1 | F/33 |
|
Development of MOG-LETM in a patient with SLE, during active COVID-19 | Presents with numbness and weakness in bilateral lower extremities with associated diplopia, urinary retention, 10 days after diagnosis of COVID-19 infection.T2-FLAIR brain MRI showed hyperintense lesions in the superior and middle cerebellar peduncles. MRI of spine showed LETM involving C4-C7 and T11-T12. | |
| Kunchok et al. (2019)25 | 51 | NA/NA |
|
ANA Ds-DNA ENA Anti-CASPR2-IgG and LGI1-IgG antibodies GABA-A-R-IgG ATD RA Vitiligo Sjogren Pernicious anemia APS |
N = 17 N = 3 N = 7 N = 1 N = 1 N = 14 N = 1 N = 1 N = 1 N = 1 N = 1 |
|
| Kunchok et al. (2021)3 | 38 (from total of 170 MOGAD patients) | NA/NA | MOGAD Either flow cytometric assay or CBA | ANA ds-DNA Anti-Sm RNP RA SLE ATD Sjogren |
N = 13 N = 5 N = 1 N = 3 N = 2 N = 1 N = 12 N = 1 |
|
| Malli et al. (2021)94 | 17 | NA/NA |
|
ANA ATD/thyroid Autoantibody Atopic dermatitis |
N = 9 N = 5 N = 4 |
All autoantibodies: IIF, Presenting with NMOSD, recurrent TM, recurrent ON, and ADEM. |
| Jarius et al. (2016)95 | 19 | NA/NA |
|
ANA ANCA ACA or B2GP RF anti-TPO anti-TG Anti-TSHR (perinuclear) tTg-IgA RA ATD Atopic dermatitis Asthma bronchiole |
N = 14 N = 1 N = 2 N = 1 N = 2 N = 1 N = 1 N = 1 N = 2 N = 2 N = 1 N = 1 |
|
| Ramanathan et al. (2018)96 | 4 | NA/NA |
|
Type 1 DM and Hashimoto's thyroiditis Elevated thyroid Abs Henoch-Schönlein purpura Anti-LGI1 Abs |
N = 1 N = 1 N = 1 N = 1 |
|
| Sato et al. (2014)7 | 5 | M/29 F/15 M/49 M/70 M/38 |
|
ANA Anti-SSA Anti-TG Anti-TPO |
N = 1 N = 1 N = 1 N = 1 |
|
| Zhou et al. (2019)20 | 6 | NA/NA |
|
ANA Anti-TPO |
N = 5 N = 1 |
|
| Veselaj et al. (2021) 97 | 7 | F/25 F/21 M/66 F/40 F/58 F/17 M/55 |
|
ANA Autoimmune hepatitis Undifferentiated connective tissue disease with positive ANA Chronic juvenile arthritis ANA and P-ANCA |
N = 3 N = 1 N = 1 N = 1 N = 1 |
|
| Chen et al. (2021)56 | 8 | NA/NA |
|
Anti-TPO/TG ANA Anti-SSA Anti-SSB Anti-RO-52 |
N = 4 N = 1 N = 1 N = 1 N = 1 |
|
| Song et al. (2019)98 | 23 | NA/NA |
|
ANA Anti-centromere Abs Anti-SSA/SSB Anti-CL/β 2GPI Anti-perinuclear factor Abs Anti-TG/TPO |
N = 6 N = 2 N = 3 N = 6 N = 2 N = 4 |
|
| James et al. (2020)99 | 1 | F/39 |
|
ANA + anti-SSB Abs | ||
| Stathopoulos et al. (2019)100 | 9 | NA/NA |
|
RA Type 1 DM SLE |
N = 5 N = 2 N = 2 |
|
| Zhao et al. (2018)101 | 4 | NA/NA |
|
ANA Anti-SSA/SSB |
N = 3 N = 1 |
|
| Ciotti et al. (2020)102 | 6 | NA/NA |
|
ANA ENA |
N = 5 N = 1 |
|
| Papathanasiou et al. (2020)103 | 4 | NA/NA M/40 M/23 |
|
ANCA P-ANCA with PR3-ANCA GAD-Ab |
N = 3 N = 1 N = 1 |
|
| Soelberg et al. (2018)104 | 1 | NA/NA |
|
Anti-Sm antibody (weakly positive) | ||
| Cross et al. (2021)105 | 3 | M/39 F/33 M/40 |
|
ATD, psoriasis | ||
| Liu et al. (2021)106 | 2 | NA/NA |
|
ATD Psoriasis |
N = 1 N = 1 |
|
| Cobo-Calvo et al. (2016)107 | 1 | NA/NA |
|
MG | ||
| Chen et al. (2017)108 | 1 | F/10 |
|
Hashimoto thyroiditis encephalitis | Preceding autoimmune thyroid disease with elevated circulating
anti-thyroid Abs, followed by subacute onset of multifocal CNS
dysfunction. Brain MRI showed asymmetric, multifocal regions of T2-W and FLAIR signal hyperintensity in the subcortical white matter, left globus pallidus, thalami, midbrain, left pons, and cerebellum. |
|
| Nagahata et al. (2022)109 | 1 | F/63 |
|
|
Presentation of recurrent poor vision and hearing loss with MRI showing low contrast-enhanced right optic nerve sheath and contrast-enhanced cavernous sinusitis. | |
| Vural et al. (2017)80 | 2 | NA/NA |
|
Sjogren's syndrome | ||
| Jobling et al. (2019)110 | 1 | M/13 |
|
Sjogren's syndrome with positive anti-Ro and anti-La | Presentation of TM following a severe upper respiratory system infection. MRI showed a subtle TM of conus medullaris. | |
| Ling et al. (2020)111 | 1 | F/53 |
|
Sjogren's syndrome and positive SSA, ANA, RF | Acute ON with MRI finding of mild right optic nerve enhancing lesion and normal brain MRI, 8 years after diagnosis of Sjogren which presented with BON. | |
| Mittal et al. (2021)112 | 1 | F/32 |
|
Sjogren disease with positive ANA, anti-Ro, anti-RNP | Left optic neuritis with intraretinal and subretinal hemorrhages. MRI showed T2 hyperintensity and expansion of the left optic nerve and nerve sheath, along with flattening of the globe at the nerve insertion, and contrast enhancement of left optic nerve and sheath on T1. | |
| Stamenova et al. (2021)113 | 1 | F/31 |
|
Crohn's disease treated with TNF-alpha inhibitors (adalimumab) and azathioprine | Development of headache, fever, and left-sided focal motor seizures, which progressed to bilateral tonic-clonic seizures during pregnancy. MRI showed bilateral cortical FLAIR-hyperintense lesions. | |
| Philippart et al. (2019)114 | 1 | M/33 |
|
Crohn's disease treated with TNF-alpha inhibitors (adalimumab) and azathioprine | Recurrent myelitis and brainstem syndrome with non-enhancing T2 hyperintense lesion from T4 to T6, and a second T2 hyperintensity at the T2 level. Brain MRI unremarkable. | |
| Luo et al. (2021)115 | 1 | F/26 |
|
Ankylosing spondylitis treated with TNF-alpha inhibitors (adalimumab) | Left ON and TM in postpartum setting, in a patient with AS treated with TNF-alpha inhibitors (adalimumab). T2-W MRI showed high signal intensity within the left optic nerve and the spinal cord from C5 to T2. No lesions were found in the brain. | |
| Lommers et al. (2018)116 | 1 | M/40 |
|
Pustular psoriasis treated with TNF-alpha inhibitors | MRI showed LETM at the bulbo-medullar junction with gadolinium enhancement in first attack, and T2 hyperintense signal in the intra-orbital part of the left optic nerve with a discrete enhancement in second attack. | |
| Takei et al. (2017)117 | 1 | F/18 |
|
Atopic dermatitis | Development of left-side dominant diffuse muscle weakness and numbness with bilateral ankle pseudo clonus along with deterioration of atopic dermatitis, followed by left ON one month later. MRI showed no abnormal lesions except for slight enlargement of the central canal at T7. | |
| Adhikari et al. (2021)118 | 1 | F/26 |
|
Opsoclonus myoclonus syndrome | Progressive bilateral hand tremors, intermittent myoclonus, ataxia, vertigo, and opsoclonus for two weeks in postpartum setting, found to be MOG-IgG positive in the second relapse, four months later. MRI showed non-enhancing right periventricular and left globus pallidus T2 FLAIR hyperintensities in initial presentation, and two new bilateral subcortical hyperintensities, one with postcontrast enhancement in second relapse. | |
| Baptista et al. (2017)119 | 20 | NA/NA |
|
RA | Patients had significantly higher levels of anti-MOG-IgG (5.68 ± 1.34 vs 0.51 ± 0.49 ng/mL), than controls. | |
| Martinez-Hernandez et al. (2015)72 | 3 | M/27 F/27 M/11 |
|
|
||
| Hacohen et al. (2016)120 | 1 | NA/NA |
|
|
||
| Armangue et al. (2017)121 | 4 | NA/NA |
|
|
Children with acquired demyelinating syndrome with positive MOG-IgG had less frequent periventricular and tectum lesions, but more diffuse cerebellum involvement. | |
| Ding et al. (2020)122 | 1 | M/20 |
|
|
||
| Fang et al. (2021)123 | 4 | NA/NA |
|
|
||
| Ji et al. (2021)124 | 1 | 23/F |
|
|
Presentation of transient convulsions, a loss of consciousness, persistent fever, and vomiting, misdiagnosed as infectious meningoencephalitis. MRI showed asymmetric lesions of cerebellum, corona radiata, and enhancing white matter lesions. | |
| Zhao et al. (2020)125 | 1 | F/30 |
|
|
Normal brain and orbital imaging | |
| Martin et al. (2022)126 | 1 | M/53 |
|
|
Presentation of isolated meningitis and papillitis. | |
| Shimizu et al. (2019)127 | 10 | NA/NA |
|
|
The rate of GRP78 antibody positivity observed in acute MOG groups (10/15, 66%) was significantly higher than that in the disease control groups (3/27, 11%) or the healthy control groups (0/9, 0%). | |
| Liu et al. (2020)128 | 1 | F/48 |
|
|
Decreased vision in the right eye and subsequent episodes of neuropsychiatric disturbance including hypersomnia, agitation, apatheia, and memory impairment, with T2 and FLAIR hyperintense multiple lesions scattered in brain, brainstem, and cervical and thoracic spinal cord, with heterogenous patchy or ring-like enhancement in the majority of lesions. | |
| Rauer et al. (2006)129 | 15 | NA/NA |
|
|
There was no increased risk for developing definite MS in CIS patients with positive anti-MOG/MBP antibodies. | |
| Tomassini et al. (2007)130 | 13 | F (N = 11), M
(N = 2) Median age: 27 years (range 25–33) |
|
|
Presentation of MOG-IgG-associated ON (N = 4), BS-cerebellar syndrome (N = 3), myelitis (N = 3), and multifocal neurologic deficit (N = 3). Patients with double seropositivity had a higher risk of second relapse compared to seronegative or single seropositive patients. | |
| Rinaldi et al. (2021)131 | 4 | F/30 F/29 M/58 F/31 |
|
|
Coexistence of central and peripheral nervous system involvement in MOGAD patients. | |
| Li et al. (2022)132 | 1 | M/33 |
|
|
Presentation of FLAIR hyperintense lesions in MOG-IgG-associated encephalitis and seizures (FLAMES). | |
Abbreviations: SLE: systemic lupus erythematosus; NPSLE: neuro-psychiatric SLE; ANA: anti nuclear antibody; ds-DNA: double-strand DNA; Anti-Sm: Anti-smith; ENA: extractable nuclear antigen antibodies; GABA-A-R: gamma aminobutyric acid receptor; CASPR2: contactin-associated protein-like 2; LGI1: leucine-rich glioma-inactivated 1; ATD: autoimmune thyroid disease; RA: rheumatoid arthritis, APS: anti-phospholipid syndrome; RNP: ribonucleoprotein; ANCA: antineutrophil cytoplasmic antibodies; ACA: anticardiolipin; B2GP: beta(2) glycoprotein; RF: rheumatoid factor; TPO: thyroid peroxidase antibody; TG: thyroglobulin; TSHR: thyroid stimulating hormone receptor; tTg: tissue transglutaminase; Anti-CL: anti-cardiolipin; PR3: proteinase3; GAD: Glutamic acid decarboxylase-antibody; MG: Myasthenia gravis; MPO: Myeloperoxidase; AS: Ankylosing spondylitis; GlyRs-Ab: Glycine receptor antibody; GRP78: Glucose regulated protein 78; MBP: myelin basic protein; NF155: neurofascin155; GM1: ganglioside epitope.
Table 2.
Overlapping MOGAD/positive MOG-IgG and AQP4-IgG + NMOSD.
| References | Number of cases | Sex/age | Clinical phenotype or laboratory status | Antibody testing assay, source
(serum/CSF), notable clinical features, or MRI findings |
|---|---|---|---|---|
| Mader et al. (2011)67 | 1 | F/39 | NMOSD | AQP4-IgG: live CBA, NA MOG-IgG: live CBA, NA NMOSD with 4 relapses of ON, TM, LETM, and no MRI cerebral lesions. |
| Kezuka et al. (2012)68 | 6 | F/48 F/35 F/41 F/50 F/21 F/48 |
Unilateral or bilateral retrobulbar neuritis | AQP4-IgG: live CBA, serum MOG-IgG: ELISA, serum |
| Xu et al. (2012)69 | 1 | F/60 | NMOSD | AQP4-IgG: NA, serum MOG-IgG: NA, serum Multiple episodes of thoracic TM, ON with T2-hyperintense spinal cord lesion in T6-T9 and no typical MS lesions in the brain, initially misdiagnosed as MS and treated with IFNβ-1a, further found to be double positive for AQP-IgG and MOG-IgG. |
| Woodhall et al. (2013)70 | 1 | F/34 | NMOSD | AQP4-IgG: live CBA, serum MOG-IgG: live CBA, serum MOG-IgG and AQP4-IgG double-positive patients had significant disabilities. She had eight attacks over the disease duration of 12 years, EDSS = 9, progression index = 0.8, and no history of simultaneous ON + TM. |
| Cavus et al. (2013)71 | 1 | NA/NA | NMOSD | AQP4-IgG: CBA, serum MOG-IgG: CBA, serum GlyR-IgG: CBA, serum Patients with simultaneous ON and TM were more prone to have antibodies against the Glycine receptor. |
| Martinez-Hernandez et al. (2014)72 | 2 | NA/NA | NMOSD | AQP4-IgG: CBA, serum MOG-IgG: CBA, serum |
| Waters et al. (2015)73 | 11 | NA/NA | Non-MS CNS demyelinating disease | AQP4-IgG: CBA, serum MOG-IgG: CBA, serum, using either full-length (FL-MOG) or the short-length (SL-MOG) human MOG-Ab. The SL-MOG assay detected Abs in 1 patient (low positive SL-MOG), who was strongly positive for AQP4-IgG. |
| Sepúlveda et al. (2015)74 | 2 | NA/NA | NMOSD | AQP4-IgG: CBA, serum MOG-IgG: CBA, serum |
| Matsuda et al. (2015)75 | 2 | F/41 F/48 |
NMOSD | AQP4-IgG: CBA IIF, serum MOG-IgG: CBA, serum From MOG-IgG-positive patients, 2 were AQP4-IgG-positive BON (NMO), of which one had 4 relapses with vision improvement after treatment with residual visual field deficit. The other one had eight relapses, no vision improvement after treatment, and residual visual field deficit. |
| Hoftberger et al. (2015)76 | 2 | F/58 F/50 |
NMOSD | AQP4-IgG: CBA IIF, serum MOG-IgG: CBA, serum Patients with both antibodies presented with a classic NMO clinical picture of simultaneous BON and LETM. |
| Di Pauli et al. (2015)77 | 1 | M/71 | MOG-EMwith double positive AQP4-IgG and MOG-IgG | AQP4-IgG: Live CBA IIF, serum (AQP4 antibodies were absent at
disease onset but seroconverted to low-titer positive at week
nine with a titer of 1:40) MOG-IgG: Live CBA IIF, serum Presentation of MOG-EM with predominant optic and spinal involvement (acute visual and gait disturbance that dramatically worsened to bilateral amaurosis, tetraplegia, and respiratory insufficiency within a few days). MRI showed multiple supra- and infratentorial lesions with marked diffusion restriction, only slight hyperintensity on T2-W images, and intramedullary lesions (lesions marginally enhanced contrast). |
| Yan et al. (2016)78 | 10 | F (N = 10)/median age at first attack: 32 (age range 15–60) | NMOSD | AQP4-IgG: FACS-based cell-binding assay, serum MOG-IgG: FACS-based cell-binding assay, serum The double-positive patients had a multiphase disease course with a high annual relapse rate, and severe residual disability compared to patients with only MOG-IgG. Of the double-positive patients, 70% had MS-like brain lesions, more severe edematous, multifocal regions on spinal MRI, pronounced decreases of retinal nerve fiber layer thickness and atrophy of optic nerves. 80% of double-positive patients had clinical evidence of spinal cord involvement at the first attack of NMOSD. The conus was significantly more likely to be involved in the double-positive group and MOG-IgG-positive patients. |
| Idiman et al. (2017)79 | 5 | NA/NA | NMOSD with atypic aggressive demyelinating disease | AQP4-IgG: CBA, serum MOG-IgG: CBA, serum |
| Vural et al. (2017)80 | 2 | NA/NA | NMOSD with positive MOG-IgG and MOG-IgM | AQP-IgG: NA, NA MOG-IgG: CBA and flow cytometry, serum |
| Yang et al. (201881 | 1 | F/41 | GFAP astrocytopathy with double positive AQP4-IgG and MOG-IgG | AQP4-IgG: IIF, CSF MOG-IgG: IIF, serum Presented with SIADH and fever, vertigo, quadriplegia, dementia, and psychosis. Brain MRI showed lesions in bilateral thalamus, right hypothalamus and midbrain, left hippocampus, right parietal, frontal lobe, and T1-W “radial enhancing” lesions in the cerebellum. Spinal cord MRI was normal. |
| Kunchok et al. (2019)25 | 6 | NA/NA | CNS inflammatory demyelinating disorders | AQP4-IgG: live CBA, serum MOG-IgG: live CBA, serum All double-positive cases had high titer AQP4-IgG and low titer MOG-IgG. |
| Kunchok et al. (2020)82 | 10 | F (N = 9) M (N = 1)/median age: 47 (age range: 30–61 years) |
CNS inflammatory demyelinating disorders | AQP4-IgG: flow cytometric assay, serum MOG-IgG: flow cytometric assay, serum All 10 patients with dual positivity had high-titer AQP4-IgG (median, 1:10 000; range, 1:100 to 1:100 000) and low-titer MOG-IgG (median, 1:40; range, 1:20 to 1:100). |
| Ishikawa et al. (2019)83 | 1 | F/24 | Unilateral or bilateral optic neuritis | AQP4-IgG: IIF, serum MOG-IgG: CBA, serum Presented with right ON at age 13, followed by left ON at age 18 (positive AQP4-IgG), and left ON at age 22 (positive MOG-IgG). |
| Bates et al. (2020)84 | 1 | F/54 | Myasthenia gravis (MG) with double-positive AQP4-IgG and MOG-IgG | AQP4-IgG: CBA, NA MOG-IgG: CBA, NA Presentation of acute right-sided weakness in a patient with long-standing MG.MRI showed longitudinally extensive edema beginning at the level of T1-T2 and extending up into the obex and area postrema with associated contrast enhancement of levels C1-C4. |
| Mori et al. (2020)85 | 1 | F/NA | NMOSD | AQP4-IgG: NA MOG-IgG: NA Presentation of two episodes of right ON and two episodes of left ON resulting in severe visual function disability. |
| He et al. (2020)86 | 1 | NA | NMOSD | AQP4-IgG: ELISA or IIFT, NA MOG-IgG: IIFT, NA |
| Zhang et al. (2021)87 | 1 | F/79 months | NMOSD | AQP4-IgG: commercial kit, serum (assay not
specified) MOG-IgG: commercial kit, serum (assay not specified) |
| Mason et al. (2021)88 | 1 | F/66 | Isolated sequential bilateral optic neuritis | AQP4-IgG: NA, serum MOG-IgG: NA, serum MRI of the brain and orbits revealed enhancement of the left optic nerve with no cerebral WM plaques. |
Abbreviations: NMOSD: neuromyelitis optica spectrum disorder; CBA: cell-based assay; ON: optic neuritis, TM: transverse myelitis; LETM: longitudinally extensive transverse myelitis; MRI: magnetic resonance imaging; ELISA: enzyme-linked immunosorbent assay; MS: multiple sclerosis; EDSS: expanded disability status scale; GlyR-Ab: glycine receptor antibody; FL-MOG: full-length human MOG-Ab; SL-MOG: short-length human MOG-Ab; BON: bilateral optic neuritis; EM: encephalomyelitis; FACS: fluorescence-activated cell sorting; IIF: indirect immunofluorescence; MG: myasthenia gravis.
We found 9 cases of overlapping MOGAD and positive Glycine-Receptor (Gly-R)-antibody (Table 3), and one case of each of the following: double positive MOG-IgG and anti-NMDAR-EN with positive anti-contactin-associated protein-like 2 (CASPR2) antibody, glial fibrillary acidic protein (GFAP) astrocytopathy with double positive AQP4-IgG and MOG-IgG, MG with double positive AQP4-IgG and MOG-IgG, MOGAD with positive CASPR2-IgG and leucine-rich glioma-inactivated 1 (LGI1) IgG, MOGAD with atopic dermatitis and asthma bronchiole, and MOG-ON with Sjogren's syndrome with positive Sjogren's syndrome antigen A antibody (SSA), ANA, and rheumatoid factor (RF).
A review of neoplasms and paraneoplastic syndromes associated with MOGAD showed five sporadic cases of cancers (T-cell lymphoma, lung, and colon) and three cases of MOG-IgG-associated paraneoplastic encephalomyelitis in the setting of malignancies of the ovary, lungs, and breast. Results of overlap between MOGAD and cancers/paraneoplastic syndromes are summarized in Table 4.
Table 4.
Overlapping MOGAD/positive MOG-IgG and cancers or paraneoplastic syndromes
| Reference | Number of cases | Sex/age | MOG-IgG-associated clinical phenotype | Overlapping cancer/paraneoplastic syndrome |
|---|---|---|---|---|
| Kwon et al. (2020)133 | 1 | F/64 | MOG-EM | T-cell lymphoma |
| Li et al. (2020)134 | 1 | F/49 | MOG-EM | Lung adenocarcinoma |
| Cohen et al. (2020)135 | 1 | F/52 | MOGAD | Colon adenocarcinoma |
| Kwon et al. (2020)136 | NA | NA | MOG-EM | From 63 patients with MOG-EM, 11.3% had a history of cancer, and 6.5% had concurrent cancer. Types of cancers not specified. |
| Ajam et al. (2020)137 | 1 | M/25 | MOG-ON | Meningioma |
| Delgado et al. (2021)138 | 1 | F/37 | MOG-ON | Pituitary macroadenoma |
| Cirkel et al. (2021)139 | 1 | F/44 | MOG-EM | Paraneoplastic encephalomyeloradiculits with multiple
autoantibodies against ITPR-1, GFAP, and MOG Underlying malignancy: borderline tumor of the ovary |
| Cherian et al. (2021)140 | 1 | F/37 | MOG-LETM | MOG-LETM as paraneoplastic myelopathy Underlying malignancy: breast carcinoma |
| Rodenbeck et al. (2021)141 | 1 | F/64 | MOG-MY | MOG-MY as paraneoplastic myelopathy Underlying malignancy: lung adenocarcinoma |
Abbreviations: EM: encephalomyelitis; ON: optic neuritis; LETM: longitudinally extensive transverse myelitis; MY: myelitis
Discussion
To our knowledge, this is the first literature review of coexisting autoimmune diseases and cancers with MOGAD. Unlike AQP4-IgG + NMOSD, MOGAD lacks clustering of autoimmune diseases and autoantibodies associated with systemic and organ-specific autoimmunity.3 Kunchok et al. showed that the frequency of any autoimmune diseases was 33.5% in AQP4-IgG + NMOSD adult-onset patients, and 12.2% in MOGAD adult-onset patients. These frequencies were 14.3% and 3.6% in pediatric-onset AQP4-IgG + NMOSD, and MOGAD patients, respectively.3 This finding implies a difference in the immunopathogenic mechanisms involved in the development of each entity, such that AQP4-IgG + NMOSD is more attributable to a defect in immune tolerance, while MOGAD is more likely to be an aberrant autoimmune response to certain triggers, such as infection, vaccination, or malignancy.3,146
The major finding of our study was that the most frequent autoimmune disorder that has been reported to co-exist with MOGAD is anti-NMDAR-EN, which is a similar finding to previous studies.53 Some studies have referred to this co-occurrence as “overlapping syndrome of MOGAD and anti-NMDAR-EN, (MNOS).”19,51 In our literature review, we captured a total of 200 patients who either met the diagnostic criteria for autoimmune encephalitis and were simultaneously seropositive for both anti-NMDAR-IgG, and MOG-IgG or who met the diagnostic criteria for each of MOG-associated encephalitis (MOGAD-EN) or anti-NMDAR-EN and was seropositive for the antibody associated with the other one, at the same time.10 Given the rarity of both MOGAD and anti-NMDAR-EN, the co-occurrence of these two entities may happen in the context of an association or could be just a coincidence. This overlap could be categorized as one of the following chronological phenotypes: (1) cases that start with a presentation of anti-NMDAR-EN who develop demyelinating events consistent with MOGAD later; (2) cases with a known diagnosis of MOGAD who later present with anti-NMDAR-EN; and (3) cases of autoimmune encephalitis with concurrent positive MOG-IgG and anti-NMDAR-IgG, demonstrating that antibodies against NMDAR and MOG can be simultaneously detected in one patient.10,54 One possible explanation could be an immunological activation (e.g. an infection) driving the production of different subclasses of neuronal autoantibodies (anti-MOG-IgG, anti-NMDAR-IgG), resulting in the development of two different diseases.13,53 Another hypothesis is that since both MOG and NMDAR are expressed on oligodendrocytes, an immune attack against one may provoke targeting the other one by antigen spreading.22,53
Considering the different disease courses and the outcomes of the two diseases, the management of the co-existing MOGAD and anti-NMDAR-EN could be challenging. MOGAD was perceived primarily as a monophasic CNS inflammatory disease, but further observations showed that a relapsing course occur in 28–60% of patients, and in up to 83% of cases with long-term observation, especially those with persistent positive MOG-IgG status.99,143 Some evidence of subclinical disease activity and progression has been shown to be present in some of the patients with MOGAD.2 Patients with anti-NMDAR-EN tend to have more severe neurologic presentations, and poor long-term functional outcomes, and the mortality rate has been reported between 5% and 7%.144–146 In their study of 23 patients with overlapping demyelinating syndrome and anti-NMDAR-EN, Titaluer et al. demonstrate that the demyelinating episodes are more difficult to treat than anti-NMDAR-EN and often required more aggressive therapies, emphasizing the importance of its prompt diagnosis and treatment.10
We found 70 patients reported to be double-positive for MOG-IgG and AQP4-IgG, suggesting the co-existence of these two antibodies in the same patient is not as rare as previously thought.25 Patients with double positive AQP4-IgG and MOG-IgG have been reported to be more refractory to therapies, relapse frequently, and progress rapidly.68,75 Matsuda et al. reported one double seropositive case which had recurrent ON attacks with residual visual field deficit, resulting in no improvement in visual acuity despite courses of treatments. They concluded that regardless of the method of measurement, double-positive cases were found to have a significantly poorer visual outcome, suggesting that anti-AQP4 and anti-MOG antibodies may indicate the prognosis of visual function in ON.75 Kezuka et al. noted that 50% of double-positive patients did not respond to corticosteroid pulse therapy and plasmapheresis, and show no post-treatment improvement in visual function, whereas the visual acuity improved significantly in the double negative group following the same treatment.68–75 Overall, these findings suggest that MOG-AQP4-IgG double seropositivity might be a marker of a poor outcome in NMO spectrum disorders, possibly because of the involvement of both astrocytes and oligodendrocytes.68,75 Given the overall rarity of double positive cases based on the results of the larger studies, and considering the superior specificity of the AQP4-IgG test compared to the MOG-IgG test, and that a reliable cell-based-assay (CBA) for detecting MOG-IgG has been available just recently (2017), there is a high possibility that most of the double positive cases may likely be AQP4-IgG + NMOSD with false positive MOG-IgG test results, especially in reports made before this time with using enzyme-linked immunosorbent assay (ELISA).68,75–147 False positive AQP4-IgG test results may occur rarely as well.
We found 35 cases of coexisting MOGAD and SLE, or SLE patients harboring MOG-IgG, some of which had a presentation of neuropsychiatric SLE (NPSLE). It is worthy of mentioning that, since the denominator to get to 35 cases is unknown, therefore the causal connection between MOGAD and SLE is either unclear or not present.
The potential role of anti-tumor necrosis factor (TNF)-alpha inhibitors in the development of CNS demyelination has been postulated to explain the overlapping MOGAD and some cases of Crohn's disease, ankylosing spondylitis, and psoriasis.114–116 Other autoimmune disorders and autoantibodies that have been shown to overlap with MOGAD come from case reports and observational studies, which seem to be a coincidence and comparable with their frequency in the general population. Reports of MOGAD in patients with malignancies may represent the development of MOGAD as a paraneoplastic syndrome or could be an incidental co-occurrence, or false positive results of the MOG-IgG test.
Other than anti-NMDAR-EN and perhaps AQP4-IgG + NMOSD, the evidence thus far does not support the need for routine screening of overlapping autoimmunity and neoplasms in a patient with MOGAD. On the other hand, the antibody screening test for MOG-IgG may be helpful in cases of development of any atypical symptoms for encephalitis, or MRI findings attributable to demyelination, especially in patients with a diagnosis of anti-NMDAR-EN.
Most of our data come from case reports, case series, and observational studies, and only a few large studies looking at the association between MOGAD and autoimmune diseases are available, so we do not have a total denominator to report a fixed ratio or percentage of overlapping MOGAD and autoimmune disorders or cancers, and without the denominator, studies that found little or no association can make it appear like there is an association. The publication bias can inflate the potential connection between MOGAD and other autoimmune diseases or autoantibodies such as SLE or double AQP4-IgG and MOG-IgG positive cases, and this makes it hard to judge if there is a true connection between these two entities. As a result, we cannot draw a precise conclusion by only assessing and contrasting the total number of reported cases, which does not actually indicate the prevalence of concomitant autoimmune entities in MOGAD. In summary, our study suggests that the connection between autoimmune diseases and MOGAD is low or at least less than AQP4-IgG + NMOSD, and further studies are needed to establish the predisposing factors in the development of concurrent autoimmune diseases or autoantibodies in patients with MOGAD.
Supplemental Material
Supplemental material, sj-docx-1-mso-10.1177_20552173221128170 for Autoimmune diseases and cancers overlapping with myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): A systematic review by Negar Molazadeh, Gauruv Bose, Itay Lotan and Michael Levy in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Footnotes
The institution of Dr Molazadeh has received research support from Genentech, Inc. Dr Gauruv Bose has received an endMS Postdoctoral Fellowship award from the Multiple Sclerosis Society of Canada. Dr Lotan has nothing to disclose. Dr Levy received personal consulting fees from Genentech/Roche, Alexion, Horizon, Sanofi, and UCB. He has also received research support from Genentech/Roche, Alexion, Horizon, Sanofi, and UCB.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Negar Molazadeh https://orcid.org/0000-0003-0861-2178
Michael Levy https://orcid.org/0000-0002-7969-8346
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Negar Molazadeh, Department of Neurology, Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Gauruv Bose, Brigham MS Center, Department of Neurology, Brigham and Women’s Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA; Division of Neurology, Department of Medicine, The Ottawa Hospital and University of Ottawa; Ottawa Hospital Research Institute, ON, Canada.
References
- 1.Marignier R, Hacohen Y, Cobo-Calvo A, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol 2021; 20: 762–772. [DOI] [PubMed] [Google Scholar]
- 2.Molazadeh N, Filippatou AG, Vasileiou ES, et al. Evidence for and against subclinical disease activity and progressive disease in MOG antibody disease and neuromyelitis optica spectrum disorder. J Neuroimmunol 2021; 360: 577702. [DOI] [PubMed] [Google Scholar]
- 3.Kunchok A, Flanagan EP, Snyder M, et al. Coexisting systemic and organ-specific autoimmunity in MOG-IgG1-associated disorders versus AQP4-IgG + NMOSD. Mult Scler 2021; 27: 630–635. [DOI] [PubMed] [Google Scholar]
- 4.Molazadeh N, Ala S, Karaminia M, et al. Rituximab induced psoriasis in a patient with multiple sclerosis: a case report and literature review. Neuroimmunol Rep 2021; 1: 100027. [Google Scholar]
- 5.Shahmohammadi S, Doosti R, Shahmohammadi A, et al. Neuromyelitis optica spectrum disorder (NMOSD) associated with cancer: a systematic review. Mult Scler Relat Disord 2021; 56: 103227. [DOI] [PubMed] [Google Scholar]
- 6.Shahmohammadi S, Doosti R, Shahmohammadi A, et al. Autoimmune diseases associated with neuromyelitis optica spectrum disorders: a literature review. Mult Scler Relat Disord 2019; 27: 350–363. [DOI] [PubMed] [Google Scholar]
- 7.Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014; 82: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol 2014; 71: 276–283. [DOI] [PubMed] [Google Scholar]
- 9.Covidence systematic review software. Veritas Health Innovation, Melbourne, Australia.
- 10.Titulaer MJ, Hoftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2014; 75: 411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacohen Y, Absoud M, Hemingway C, et al. NMDA receptor antibodies associated with distinct white matter syndromes. Neurol Neuroimmunol Neuroinflamm 2014; 1: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneko S DK, Kurosawa K, Misu Tet al. et al. Case of autoantibodies against N-methyl-D-aspartate receptor + /antibodies against myelin-oligodendrocyte glycoprotein + multiphasic acute disseminated encephalomyelitis (ADEM). Clin Exp Neuroimmunol 2014; 5: 49–51. [Google Scholar]
- 13.Yokoyama K, Hori M, Yoshida A. Anti-myelin oligodendrocyte glycoprotein antibody neuritis optica following anti-NMDA receptor encephalitis. Pediatr Int 2016; 58: 953–954. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko J IT, Kanazawa N, Tominaga Net al. et al. Clinical investigations of 50 cases of autoimmune neurological disorders with antibodies against neuronal cell surface or synaptic proteins. Clin Exp Neuroimmunol 2017; 8: 362. [Google Scholar]
- 15.Zhou L, ZhangBao J, Li H, et al. Cerebral cortical encephalitis followed by recurrent CNS demyelination in a patient with concomitant anti-MOG and anti-NMDA receptor antibodies. Mult Scler Relat Disord 2017; 18: 90–92. [DOI] [PubMed] [Google Scholar]
- 16.Nagata S, Nishimura Y, Mitsuo K. [A case of anti-myelin oligodendrocyte glycoprotein (MOG) and anti-N-methyl-D-aspartate (NMDA) receptor antibody-positive encephalitis with optic neuritis]. Rinsho Shinkeigaku 2018; 58: 636–641. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Tan W, Tan SE, et al. An unusual case of anti-MOG CNS demyelination with concomitant mild anti-NMDAR encephalitis. J Neuroimmunol 2018; 320: 107–110. [DOI] [PubMed] [Google Scholar]
- 18.Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol 2018; 83: 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan S, Xu Y, Ren H, et al. Comparison of myelin oligodendrocyte glycoprotein (MOG)-antibody disease and AQP4-IgG-positive neuromyelitis optica spectrum disorder (NMOSD) when they co-exist with anti-NMDA (N-methyl-D-aspartate) receptor encephalitis. Mult Scler Relat Disord 2018; 20: 144–152. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Lu X, Zhang Y, et al. Follow-up study on Chinese children with relapsing MOG-IgG-associated central nervous system demyelination. Mult Scler Relat Disord 2019; 28: 4–10. [DOI] [PubMed] [Google Scholar]
- 21.Taraschenko O, Zabad R. Overlapping demyelinating syndrome and anti-N-methyl-d-aspartate receptor encephalitis with seizures. Epilepsy Behav Rep 2019; 12: 100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, ZhangBao J, Zhou L, et al. Encephalitis is an important clinical component of myelin oligodendrocyte glycoprotein antibody associated demyelination: a single-center cohort study in Shanghai, China. Eur J Neurol 2019; 26: 168–174. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko K SDK, Misu T, Kurosawa Ket al. et al. Pactrims invited lecture / ordinary submission. Mult Scler 2015; 21: 799–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren Y, Chen X, He Q, et al. Co-occurrence of anti-N-methyl-D-aspartate receptor encephalitis and anti-myelin oligodendrocyte glycoprotein inflammatory demyelinating diseases: a clinical phenomenon to be taken seriously. Front Neurol 2019; 10: 1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunchok A, Saadeh R, Jitprapaikulsan Jet al. Myelin oligodendrocyte glycoprotein-IgG associated disorders (MOGAD) co-existing autoimmunity study: sero-prevalence of coexisting gial (aquaporin-4), neuronal and non-organ specific autoantibodies. ECTRIMS 2019 – Oral Presentations. Mult Scler J 2019; 25(S2): 92–93. [Google Scholar]
- 26.Aoe S, Kokudo Y, Takata T, et al. Repeated anti-N-methyl-D-aspartate receptor encephalitis coexisting with anti-myelin oligodendrocyte glycoprotein antibody-associated diseases: a case report. Mult Scler Relat Disord 2019; 35: 182–184. [DOI] [PubMed] [Google Scholar]
- 27.Rojc B, Podnar B, Graus F. A case of recurrent MOG antibody positive bilateral optic neuritis and anti-NMDAR encephalitis: different biological evolution of the two associated antibodies. J Neuroimmunol 2019; 328: 86–88. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko A, Iizuka T, Suga H, et al. Atypical clinical manifestations and overlapping immunities associated with NMDA receptor antibodies (S11.006). Neurology 2019; 92: S11.006. [Google Scholar]
- 29.Sarigecili E, Cobanogullari MD, Komur M, et al. A rare concurrence: antibodies against myelin oligodendrocyte glycoprotein and N-methyl-d-aspartate receptor in a child. Mult Scler Relat Disord 2019; 28: 101–103. [DOI] [PubMed] [Google Scholar]
- 30.Gao MC, Yao XY, Ding J, et al. Cortical encephalitis with overlapping anti-N-methyl-D-aspartate receptor and anti-myelin oligodendrocyte glycoprotein antibodies: report of two cases. Chin Med J (Engl) 2020; 133: 1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amano E, Machida A, Kanazawa N, et al. Cerebrospinal fluid MOG-antibodies in anti-NMDA receptor encephalitis with leptomeningeal enhancement. Neurol Sci 2020; 41: 2635–2638. [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto O Y, Watanabe Y, Takeshita S. Overlapping anti-N-methyl-D-aspartate receptor encephalitis and anti-myelin oligodendrocyte glycoprotein cerebral cortical encephalitis. Jpn Soc Child Neurol 2020; 52: 47–48. [Google Scholar]
- 33.Martinez-Hernandez E, Guasp M, Garcia-Serra A, et al. Clinical significance of anti-NMDAR concurrent with glial or neuronal surface antibodies. Neurology 2020; 94: e2302–e2310. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Jiang L. Viral encephalitis followed by anti-NMDAR encephalitis with concomitant MOG antibody-positive central nervous system demyelination in a child. Neurol Sci 2020; 41: 2303–2305. [DOI] [PubMed] [Google Scholar]
- 35.Du L, Wang H, Zhou H, et al. Anti-NMDA receptor encephalitis concomitant with myelin oligodendrocyte glycoprotein antibody diseases: a retrospective observational study. Medicine (Baltimore) 2020; 99: e21238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong S, Zhang WH, Ren HT, et al. [Clinical observation on the overlapping syndrome of myelin oligodendrocyte glycoprotein antibody and anti-N-methyl-D aspartate receptor in children]. Zhonghua Er Ke Za Zhi 2020; 58: 581–585. [DOI] [PubMed] [Google Scholar]
- 37.Zheng J, Shen J, Wang A, et al. Clinical characteristics of anti-N-methyl-D-aspartate receptor encephalitis in children. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020; 45: 47–54. [DOI] [PubMed] [Google Scholar]
- 38.Wegener-Panzer A, Cleaveland R, Wendel EM, et al. Clinical and imaging features of children with autoimmune encephalitis and MOG antibodies. Neurol Neuroimmunol Neuroinflamm 2020; 7. DOI: 10.1212/NXI.0000000000000731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu HM, Sun D, Wu GF, et al. [Overlapping syndrome of myelin oligodendrocyte glycoprotein-antibody disease and anti-N-methyl-D-aspartate receptor encephalitis in two children]. Zhonghua Er Ke Za Zhi 2020; 58: 324–326. [DOI] [PubMed] [Google Scholar]
- 40.Hou C, Wu W, Tian Y, et al. Clinical analysis of anti-NMDAR encephalitis combined with MOG antibody in children. Mult Scler Relat Disord 2020; 42: 102018. [DOI] [PubMed] [Google Scholar]
- 41.Perez CA, Agyei P, Gogia B, et al. Overlapping autoimmune syndrome: a case of concomitant anti-NMDAR encephalitis and myelin oligodendrocyte glycoprotein (MOG) antibody disease. J Neuroimmunol 2020; 339: 577124. [DOI] [PubMed] [Google Scholar]
- 42.Cherian A, Divya KP, Shetty SC, et al. Coexistent MOG, NMDAR, CASPR2 antibody positivity: triumph over the triumvirate. Mult Scler Relat Disord 2020; 46: 102468. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Shen J, Zhou S, et al. Clinical and neuroimaging characteristics of pediatric acute disseminating encephalomyelitis with and without antibodies to myelin oligodendrocyte glycoprotein. Front Neurol 2020; 11: 593287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez VJ, Lawson E, Risman A, et al. Overlapping demyelinating syndrome associated with myelin oligodendrocyte glycoprotein antibody and anti-N-methyl-d-aspartate receptor encephalitis (4870). Neurology 2021; 96: 4870. [Google Scholar]
- 45.Guzman J, Vera F, Soler Bet al. Demographic and clinical profile of patients with myelin oligodendrocyte glycoprotein antibody-associated disease in Chile. ECTRIMS 2021 ePoster – Mult Scler J 2021; 27: 171. [Google Scholar]
- 46.Weiss D, Kertzscher L, Degering M, et al. Anti-NMDA receptor encephalitis and overlapping demyelinating disorder in a 20-year old female with borderline personality disorder: proposal of a diagnostic and therapeutic algorithm for autoimmune encephalitis in psychiatric patients “case report”. BMC Psychiatry 2021; 21: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao L, Ren L, Huang X. Clinical analysis of a patient simultaneously positive for antibodies of myelin oligodendrocyte glycoprotein and anti-N-methyl-D-aspartate receptor: a case report. Medicine (Baltimore) 2021; 100: e24234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren BY, Guo Y, Han J, et al. Case report: anti-NMDAR encephalitis with anti-MOG CNS demyelination after recurrent CNS demyelination. Front Neurol 2021; 12: 639265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen MY H, Liu X, Yang Wet al. et al. Analysis of clinical characteristics of patients with anti-myelin oligodendrocyte glycoprotein and anti-N-methyl-D-aspartate receptor antibody positive. Zhonghua J Neurol 2021; 54: 898–907. [Google Scholar]
- 50.Fujimori J, Takahashi T, Kaneko K, et al. Anti-NMDAR encephalitis may develop concurrently with anti-MOG antibody-associated bilateral medial frontal cerebral cortical encephalitis and relapse with elevated CSF IL-6 and CXCL13. Mult Scler Relat Disord 2021; 47: 102611. [DOI] [PubMed] [Google Scholar]
- 51.Chen W, Li Q, Wang T, et al. Overlapping syndrome of anti-N-methyl-D-aspartate receptor encephalitis and anti-myelin oligodendrocyte glycoprotein inflammatory demyelinating diseases: a distinct clinical entity? Mult Scler Relat Disord 2021; 52: 103020. [DOI] [PubMed] [Google Scholar]
- 52.Teng XL, Zhang J, Chang XT, et al. [Clinical follow-up study of myelin oligodendrocyte glycoprotein antibody-associated disease in children]. Zhonghua Er Ke Za Zhi 2021; 59: 1048–1054. [DOI] [PubMed] [Google Scholar]
- 53.Nan D, Zhang Y, Han J, et al. Clinical features and management of coexisting anti-N-methyl-D-aspartate receptor encephalitis and myelin oligodendrocyte glycoprotein antibody-associated encephalomyelitis: a case report and review of the literature. Neurol Sci 2021; 42: 847–855. [DOI] [PubMed] [Google Scholar]
- 54.Caparo-Zamalloa C, Alvarez-Toledo K, Yamunaque-Chunga C, et al. Autoimmune neurology: co-occurrence of anti-NMDAR encephalitis and anti-MOG associated disease, report of a case. J Neuroimmunol 2021; 358: 577663. [DOI] [PubMed] [Google Scholar]
- 55.Guang S, Ma J, Ren X, et al. Immunotherapies for anti-N-M-methyl-D-aspartate receptor encephalitis: multicenter retrospective pediatric cohort study in China. Front Pediatr 2021; 9: 691599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X, Ren Y, Zhang Y, et al. Comparative study of AQP4 antibody-related diseases and MOG antibody-related diseases among the population in Hunan, China. Acta Neurol Belg 2021; 121: 1649–1659. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Xie H, Zhang J, et al. Clinical and radiological characteristics of children and adults with first-attack myelin oligodendrocyte glycoprotein antibody disease and analysis of risk factors for predicting the severity at disease onset in central China. Front Immunol 2021; 12: 752557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia Y, Wang HF, Zhang MY, et al. Antibody prevalence and immunotherapy response in Chinese patients with epilepsy and encephalopathy scores for patients with different neuronal surface antibodies. Chin Med J (Engl) 2021; 134: 2985–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J, Qiu Z, Li D, et al. Clinical and imaging features of patients with encephalitic symptoms and myelin oligodendrocyte glycoprotein antibodies. Front Immunol 2021; 12: 722404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han JY, Kim SY, Kim H, et al. Clinico-radiological characteristics of anti-myelin oligodendrocyte glycoprotein antibody-associated autoimmune encephalitis in children. Dev Med Child Neurol 2022; 64: 998–1007. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Zhao R, Yang H, et al. Clinical analysis of myelin oligodendrocyte glycoprotein antibody-associated demyelination in children: a single-center cohort study in China. Mult Scler Relat Disord 2022; 58: 103526. [DOI] [PubMed] [Google Scholar]
- 62.Lei X, Guo S, Cui S, et al. Clinical profile and treatment outcome in MOGAD: a single-center case-series study in Guiyang, China. Front Neurol 2022; 13: 830488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo J, Bu Y, Liu W. Case report: a case with MOGAD and anti-NMDAR encephalitis overlapping syndrome mimicking radiological characteristics of CLIPPERS. Front Immunol 2022; 13: 832084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weihua Z, Shuai G, Changhong R, et al. Pediatric anti-N-methyl-D-aspartate receptor encephalitis with MOG-Ab co-existence: relapse propensity and treatability. Mult Scler Relat Disord 2022; 58: 103447. [DOI] [PubMed] [Google Scholar]
- 65.Yin XJ ZL, Bao LH, Feng ZCet al. et al. Overlapping syndrome of recurrent anti-N-methyl-D-aspartate receptor encephalitis and anti-myelin oligodendrocyte glycoprotein demyelinating diseases: a case report. World J Clin Cases 2022; 10: 6148–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang M, Tan J, Zhou Z, et al. Relapsing MOG-IgG-associated diseases coexisting with anti-NMDAR encephalitis: a case report and literature review. J Integr Neurosci 2022; 21: 82. [DOI] [PubMed] [Google Scholar]
- 67.Mader S, Gredler V, Schanda K, et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation 2011; 8: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kezuka T, Usui Y, Yamakawa N, et al. Relationship between NMO-antibody and anti-MOG antibody in optic neuritis. J Neuroophthalmol 2012; 32: 107–110. [DOI] [PubMed] [Google Scholar]
- 69.Xu Y, Zhang Y, Ye J, et al. Successful treatment of a woman with relapsing neuromyelitis optica by interferon beta. Neurol Sci 2012; 33: 911–913. [DOI] [PubMed] [Google Scholar]
- 70.Woodhall M, Coban A, Waters P, et al. Glycine receptor and myelin oligodendrocyte glycoprotein antibodies in Turkish patients with neuromyelitis optica. J Neurol Sci 2013; 335: 221–223. [DOI] [PubMed] [Google Scholar]
- 71.Cavus F, Ugurel E, Sehitoglu E, et al. Novel anti-neuronal antibodies in neuromyelitis optica patients with or without aquaporin-4 antibodies (P02.135). Neurology 2013; 80: P02.135. [Google Scholar]
- 72.Martinez-Hernandez E, Sepulveda M, Rostasy K, et al. Antibodies to aquaporin 4, myelin-oligodendrocyte glycoprotein, and the glycine receptor alpha1 subunit in patients with isolated optic neuritis. JAMA Neurol 2015; 72: 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waters P, Woodhall M, O’Connor KC, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm 2015; 2: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sepúlveda M, Armangué T, Solà-Valls Net al. et al. Neuromyelitis optica in Spain: a multicenter study of 178 patients. ECTRIMS 2015 - Mult Scler J 2015; 21: 82–83. [Google Scholar]
- 75.Matsuda R, Kezuka T, Umazume A, et al. Clinical profile of anti-myelin oligodendrocyte glycoprotein antibody seropositive cases of optic neuritis. Neuroophthalmology 2015; 39: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoftberger R, Sepulveda M, Armangue T, et al. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler 2015; 21: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di Pauli F, Hoftberger R, Reindl M, et al. Fulminant demyelinating encephalomyelitis: insights from antibody studies and neuropathology. Neurol Neuroimmunol Neuroinflamm 2015; 2: e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan Y, Li Y, Fu Y, et al. Autoantibody to MOG suggests two distinct clinical subtypes of NMOSD. Sci China Life Sci 2016; 59: 1270–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.İdiman E, İdiman F, Kaya Met al. et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies in Turkish population: a comparative study. ECTRIMS 2017 Mult Scler J 2017; 23(S3): P783. [Google Scholar]
- 80.Vural AT A, Spadaro M, Konuşkan Bet al. et al. Spectrum of autoantibodies against myelin oligodendrocyte glycoprotein. Eur J Neurol 2017; 24: 112–112.27699930 [Google Scholar]
- 81.Yang X, Xu H, Ding M, et al. Overlapping autoimmune syndromes in patients with glial fibrillary acidic protein antibodies. Front Neurol 2018; 9: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kunchok A, Chen JJ, McKeon A, et al. Coexistence of myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies in adult and pediatric patients. JAMA Neurology 2020; 77: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ishikawa H, Kezuka T, Shikishima K, et al. Epidemiologic and clinical characteristics of optic neuritis in Japan. Ophthalmology 2019; 126: 1385–1398. [DOI] [PubMed] [Google Scholar]
- 84.Bates M, Chisholm J, Miller E, et al. Anti-MOG and anti-AQP4 positive neuromyelitis optica spectrum disorder in a patient with myasthenia gravis. Mult Scler Relat Disord 2020; 44: 102205. [DOI] [PubMed] [Google Scholar]
- 85.Mori S, Kurimoto T, Murai Y, et al. Efficacy for the annual relapse rate after the immunosuppressive therapy in patients associated with anti-AQP4 or anti-MOG antibody-positive optic neuritis. J Ophthalmol 2020; 2020: 8871146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He M, Wu L, Huang D, et al. Application of the 2015 neuromyelitis optica spectrum disorders diagnostic criteria in a cohort of Chinese patients. Mult Scler Relat Disord 2020; 46: 102459. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Z, Zhou H, Liu X, et al. Identification of the clinical and neuroimaging characteristics in children with neuromyelitis optica spectrum disorders: a case series. Transl Pediatr 2021; 10: 2459–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mason MC, Marotta DA, Kesserwani H. Isolated double-positive optic neuritis: a case of aquaporin-4 and myelin oligodendrocyte glycoprotein antibody seropositivity. Cureus 2021; 13: e15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mader S, Jeganathan V, Arinuma Y, et al. Understanding the antibody repertoire in neuropsychiatric systemic lupus erythematosus and neuromyelitis optica spectrum disorder: do they share common targets? Arthritis Rheumatol 2018; 70: 277–286. [DOI] [PubMed] [Google Scholar]
- 90.Bilodeau PA, Kumar V, Rodriguez AE, et al. MOG-IgG myelitis coexisting with systemic lupus erythematosus in the post-partum setting. Mult Scler 2020; 26: 997–1000. [DOI] [PubMed] [Google Scholar]
- 91.Probstel AK, Thanei M, Erni B, et al. Association of antibodies against myelin and neuronal antigens with neuroinflammation in systemic lupus erythematosus. Rheumatology (Oxford) 2019; 58: 908–913. [DOI] [PubMed] [Google Scholar]
- 92.Seth G, Sundaresh A, Mariaselvam CM, et al. Immunological biomarkers in neuropsychiatric systemic lupus erythematosus: a comparative cross-sectional study from a tertiary care center in South India. Lupus 2020; 29: 413–420. [DOI] [PubMed] [Google Scholar]
- 93.Chawla. COVID-19 presenting as anti-MOG syndrome with longitudinally extensive transverse myelitis: a case report. In: 146th Annual Meeting American Neurological Association, Ann Neurol, 2021, pp. S1–S270. [DOI] [PubMed] [Google Scholar]
- 94.Malli C, Pandit L, D’Cunha MA, et al. Coexistence of autoantibodies and other autoimmune diseases with multiple sclerosis and related disorders - experience from the Mangalore demyelinating disease registry (MANDDIR). Ann Indian Acad Neurol 2021; 24: 740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 2016; 13: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramanathan S, Mohammad S, Tantsis E, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry 2018; 89: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Veselaj K, Kamber N, Briner M, et al. Evaluation of diagnostic criteria and red flags of myelin oligodendrocyte glycoprotein encephalomyelitis in a clinical routine cohort. CNS Neurosci Ther 2021; 27: 426–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song H, Zhou H, Yang M, et al. Clinical characteristics and outcomes of myelin oligodendrocyte glycoprotein antibody-seropositive optic neuritis in varying age groups: a cohort study in China. J Neurol Sci 2019; 400: 83–89. [DOI] [PubMed] [Google Scholar]
- 99.James J, Jose J, Gafoor VA, et al. Clinical course, imaging characteristics, and therapeutic response in myelin oligodendrocyte glycoprotein antibody disease: a case series. J Neurosci Rural Pract 2020; 11: 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stathopoulos P, Chastre A, Waters P, et al. Autoantibodies against neurologic antigens in nonneurologic autoimmunity. J Immunol 2019; 202: 2210–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao G, Chen Q, Huang Y, et al. Clinical characteristics of myelin oligodendrocyte glycoprotein seropositive optic neuritis: a cohort study in Shanghai, China. J Neurol 2018; 265: 33–40. [DOI] [PubMed] [Google Scholar]
- 102.Ciotti JR, Eby NS, Wu GF, et al. Clinical and laboratory features distinguishing MOG antibody disease from multiple sclerosis and AQP4 antibody-positive neuromyelitis optica. Mult Scler Relat Disord 2020; 45: 102399. [DOI] [PubMed] [Google Scholar]
- 103.Papathanasiou A, Tanasescu R, Davis J, et al. MOG-IgG-associated demyelination: focus on atypical features, brain histopathology and concomitant autoimmunity. J Neurol 2020; 267: 359–368. [DOI] [PubMed] [Google Scholar]
- 104.Soelberg K, Nilsson AC, Nielsen C, et al. Autoimmune and immunogenetic profile of patients with optic neuritis in a population-based cohort. Mult Scler Relat Disord 2018; 21: 97–102. [DOI] [PubMed] [Google Scholar]
- 105.Cross H, Sabiq F, Ackermans N, et al. Myelin oligodendrocyte glycoprotein (MOG) antibody positive patients in a multi-ethnic Canadian cohort. Front Neurol 2020; 11: 525933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu J, Mori M, Zimmermann H, et al. Anti-MOG antibody-associated disorders: differences in clinical profiles and prognosis in Japan and Germany. J Neurol Neurosurg Psychiatry 2020. DOI: 10.1136/jnnp-2020-324422 [DOI] [PubMed] [Google Scholar]
- 107.Cobo-Calvo A, Sepulveda M, Bernard-Valnet R, et al. Antibodies to myelin oligodendrocyte glycoprotein in aquaporin 4 antibody seronegative longitudinally extensive transverse myelitis: clinical and prognostic implications. Mult Scler 2016; 22: 312–319. [DOI] [PubMed] [Google Scholar]
- 108.Chen KA, Brilot F, Dale RC, et al. Hashimoto’s encephalopathy and anti-MOG antibody encephalitis: 50 years after lord brain’s description. Eur J Paediatr Neurol 2017; 21: 898–901. [DOI] [PubMed] [Google Scholar]
- 109.Nagahata K, Suzuki S, Yokochi R, et al. Recurrent optic perineuritis with myelin oligodendrocyte glycoprotein antibody-associated disease complicated with granulomatous polyangiitis. Cureus 2022; 14: e25239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jobling K, Ledingham D, Ng WF, et al. Positive anti-MOG antibodies in a patient with Sjogren’s syndrome and transverse myelitis. Eur J Rheumatol 2019; 6: 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ling J, Micieli JA. Recurrent MOG-IgG optic neuritis initially attributed to Sjogren’s syndrome. Can J Neurol Sci 2020; 47: 864–865. [DOI] [PubMed] [Google Scholar]
- 112.Mittal A, Baig IF, Merchant AG, et al. Sjogren disease and myelin oligodendrocyte glycoprotein antibody-associated optic neuritis. J Neuroophthalmol 2021; 41: e48–e50. [DOI] [PubMed] [Google Scholar]
- 113.Stamenova S, Redha I, Schmierer K, et al. FLAIR-hyperintense lesions in anti-MOG-associated encephalitis with seizures (FLAMES) unmasked by withdrawal of immunosuppression for Crohn's disease? Mult Scler Relat Disord 2021; 48: 102729. [DOI] [PubMed] [Google Scholar]
- 114.Philippart M, Fastre S, Rahier JF, et al. First report of coexistence of MOG-antibody-positive disease and Crohn’s disease. Mult Scler Relat Disord 2019; 28: –3. [DOI] [PubMed] [Google Scholar]
- 115.Luo W, Li R, Chang Y, et al. Myelin oligodendrocyte glycoprotein antibody-associated disorders coexisting with ankylosing spondylitis: potential association between demyelination and tumor necrosis factor inhibitors. Mult Scler Relat Disord 2021; 51: 102889. [DOI] [PubMed] [Google Scholar]
- 116.Lommers E, Depierreux F, Hansen I, et al. NMOSD with anti-MOG antibodies following anti-TNFalpha therapy: a case report. Mult Scler Relat Disord 2018; 26: 37–39. [DOI] [PubMed] [Google Scholar]
- 117.Takei K, Sato M, Nakamura Met al. et al. Case of anti-myelin oligodendrocyte glycoprotein antibody-associated demyelinating disease with atopic dermatitis. Clin Exp Neuroimmunol 2017; 8: 138–140. [Google Scholar]
- 118.Adhikari S, Thuringer A, Maali L, et al. Opsoclonus myoclonus syndrome in a postpartum period. Mult Scler Relat Disord 2021; 50: 102862. [DOI] [PubMed] [Google Scholar]
- 119.Baptista TSA, Petersen LE, Molina JK, et al. Autoantibodies against myelin sheath and S100beta are associated with cognitive dysfunction in patients with rheumatoid arthritis. Clin Rheumatol 2017; 36: 1959–1968. [DOI] [PubMed] [Google Scholar]
- 120.Hacohen Y, Nishimoto Y, Fukami Y, et al. Paediatric brainstem encephalitis associated with glial and neuronal autoantibodies. Dev Med Child Neurol 2016; 58: 836–841. [DOI] [PubMed] [Google Scholar]
- 121.Armangue T, Sepúlveda M, Auger Cet al. Clinical significance of anti-MOG antibodies in the evaluation of children with a first demyelinating episode: prospective Spanish national cohort. ECTRIMS 2017. Mult Scler J 2017; 23: 85–426. [Google Scholar]
- 122.Ding J, Ren K, Wu J, et al. Overlapping syndrome of MOG-IgG-associated disease and autoimmune GFAP astrocytopathy. J Neurol 2020; 267: 2589–2593. [DOI] [PubMed] [Google Scholar]
- 123.Fang H, Hu W, Jiang Z, et al. Autoimmune glial fibrillary acidic protein astrocytopathy in children: a retrospective analysis of 35 cases. Front Immunol 2021; 12: 761354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ji S, Liu C, Bi Z, et al. Overlapping syndrome mimicking infectious meningoencephalitis in a patient with MOG and GFAP IgG. BMC Neurol 2021; 21: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao C, Li A, Liu L, et al. Acute myelitis, recurrent optic neuritis, and seizures over 17 years. Front Neurol 2020; 11: 541146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martin AJ, Strathdee J, Wolfe N. Coexistent anti-GFAP and anti-MOG antibodies presenting with isolated meningitis and papillitis: more support for overlapping pathophysiology. BMJ Neurol Open 2022; 4: e000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shimizu F, Ogawa R, Hara Ket al. Blood-brain barrier-activation in anti-myelin oligodendrocyte glycoprotein antibody associated disorders. ECTRIMS 2019. Mult Scler J 2019; 25: 581–805. [Google Scholar]
- 128.Liu P, Bai M, Yan X, et al. Possible coexistence of MOG-IgG-associated disease and anti-Caspr2 antibody-associated autoimmune encephalitis: a first case report. Ther Adv Neurol Disord 2020; 13: 1756286420969462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rauer S, Euler B, Reindl M, et al. Antimyelin antibodies and the risk of relapse in patients with a primary demyelinating event. J Neurol Neurosurg Psychiatry 2006; 77: 739–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tomassini V, De Giglio L, Reindl M, et al. Anti-myelin antibodies predict the clinical outcome after a first episode suggestive of MS. Mult Scler 2007; 13: 1086–1094. [DOI] [PubMed] [Google Scholar]
- 131.Rinaldi S, Davies A, Fehmi J, et al. Overlapping central and peripheral nervous system syndromes in MOG antibody-associated disorders. Neurol Neuroimmunol Neuroinflamm 2021; 8. DOI: 10.1212/NXI.0000000000000924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li W, Du W, Jiang Y, et al. Rare autoimmune encephalitis presenting as fluid-attenuated inversion recovery-hyperintense lesions in anti-Myelin oligodendrocyte glycoprotein-associated encephalitis and seizures accompanied with anti-IgLON5 antibody. Quant Imaging Med Surg 2022; 12: 4007–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kwon YN, Koh J, Jeon YK, et al. A case of MOG encephalomyelitis with T- cell lymphoma. Mult Scler Relat Disord 2020; 41: 102038. [DOI] [PubMed] [Google Scholar]
- 134.Li K, Zhan Y, Shen X. Multiple intracranial lesions with lung adenocarcinoma: a rare case of MOG-IgG-associated encephalomyelitis. Mult Scler Relat Disord 2020; 42: 102064. [DOI] [PubMed] [Google Scholar]
- 135.Cohn SJ, Macaron G,, Mahajan KR, et al. MOG-antibody related disease presenting with a fulminant CNS demyelinating syndrome in an adult ACTRIMS Forum 2020. Mult Scler J 2020; 26: 90–159. [Google Scholar]
- 136.Kwon YN, Koh J, Waters Pet al. Malignancy in MOG encephalomyelitis: prevalence, description of case, and pathology report. PACTRIMS 2019 2020; 26: NP36–NP101. [Google Scholar]
- 137.Ajam Y, Li X. Anti-myelin oligodendrocyte glycoprotein (MOG) antibody-associated bilateral optic neuritis: to treat or not to treat? 145th Annual Meeting American Neurological Association. Ann Neurol 2020; 88: S59–S60. DOI: 10.1002/ana.25865 [DOI] [Google Scholar]
- 138.Delgado S, Dionísio J, Figueiredo C, et al. Anti-MOG bilateral optic neuritis and suspicion of a compressive pituitary macroadenoma: the relevance of semiology. Eur J Neurol 2021; 28(1): 529–530. DOI: 10.1111/ene.14975 [DOI] [Google Scholar]
- 139.Cirkel A, Wandinger K-P, Ditz C, et al. Paraneoplastic encephalomyeloradiculits with multiple autoantibodies against ITPR-1, GFAP and MOG: case report and literature review. Neurol Res Pract 2021; 3: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cherian A, Shetty SC, Divya KP, et al. MOG antibody-positive paraneoplastic myelopathy in breast carcinoma: the new culprit. Acta Neurol Belg 2021; 121. DOI: 10.1007/s13760-021-01602-8 [DOI] [PubMed] [Google Scholar]
- 141.Rodenbeck S, Crane P, Sitzmann A. Myelopathy in the setting of malignancy: the importance of a thoughtful approach to autoantibody testing based on clinical presentation (1295). Neurology 2021; 96. [Google Scholar]
- 142.Cotzomi E, Stathopoulos P, Lee CS, et al. Early B cell tolerance defects in neuromyelitis optica favour anti-AQP4 autoantibody production. Brain 2019; 142: 1598–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hennes EM, Baumann M, Lechner C, et al. MOG Spectrum disorders and role of MOG-antibodies in clinical practice. Neuropediatrics 2018; 49: 3–11. [DOI] [PubMed] [Google Scholar]
- 144.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang H, Xiao Z. Current progress on assessing the prognosis for anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis. Biomed Res Int 2020; 2020: 7506590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chi X, Wang W, Huang C, et al. Risk factors for mortality in patients with anti-NMDA receptor encephalitis. Acta Neurol Scand 2017; 136: 298–304. [DOI] [PubMed] [Google Scholar]
- 147.Sechi E, Buciuc M, Pittock SJ, et al. Positive predictive value of myelin oligodendrocyte glycoprotein autoantibody testing. JAMA Neurol 2021; 78: 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mso-10.1177_20552173221128170 for Autoimmune diseases and cancers overlapping with myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): A systematic review by Negar Molazadeh, Gauruv Bose, Itay Lotan and Michael Levy in Multiple Sclerosis Journal – Experimental, Translational and Clinical