Highlights
-
•
A conditional Col12a1 deletion mouse model was created and characterized.
-
•
The conditional model was used to target the Col12a1 deletion to tendons.
-
•
Targeted tendons had near baseline Col12a1 and collagen XII expression.
-
•
Col12a1 deficiency in tendons alters tendon function.
-
•
Intrinsic roles for collagen XII determining tendon function were suggested.
Keywords: Collagen XII, Conditional mouse model, Tendon, Transgenic, Col12a1, Biomechanics
Abstract
Collagen XII is a fibril-associated collagen with interrupted triple helices (FACIT). This non-fibrillar collagen is a homotrimer composed of three α1(XII) chains assembled into a collagenous molecule with a C terminal collagenous domain and a large N terminal non-collagenous domain. During tendon development and growth, collagen XII is broadly expressed throughout the extracellular matrix and enriched pericellularly around tenocytes. Tendons in a global Col12a1-/- knockout model demonstrated disrupted fibril and fiber structure and disordered tenocyte organization, highlighting the critical regulatory roles of collagen XII in determining tendon structure and function. However, muscle and bone also are affected in the collagen XII knockout model. Therefore, secondary effects on tendon due to involvement of bone and muscle may occur in the global knockout. The global knockout does not allow the definition of intrinsic mechanisms involving collagen XII in tendon versus extrinsic roles involving muscle and bone. To address this limitation, we created and characterized a conditional Col12a1-null mouse model to permit the spatial and temporal manipulation of Col12a1 expression. Collagen XII knockout was targeted to tendons by breeding conditional Col12a1flox/flox mice with Scleraxis-Cre (Scx-Cre) mice to yield a tendon-specific Col12a1-null mouse line, Col12a1Δten/Δten. Both mRNA and protein expression in Col12a1Δten/Δten mice decreased to near baseline levels in flexor digitorum longus tendons (FDL). Collagen XII immuno-localization revealed an absence of reactivity in the tendon proper, but there was reactivity in the cells of the surrounding peritenon. This supports a targeted knockout in tenocytes while peritenon cells from a non-tendon lineage were not targeted and retained collagen XII expression. The tendon-targeted, Col12a1Δten/Δten mice had significantly reduced forelimb grip strength, altered gait and a significant decrease in biomechanical properties. While the observed decrease in tendon modulus suggests that differences in tendon material properties in the absence of Col12a1 expression underlie the functional deficiencies. Together, these findings suggest an intrinsic role for collagen XII critical for development of a functional tendon.
Introduction
The unique hierarchical structure of tendon is critical for efficient force transmission and directly influences mechanical function [1], [2]. The highly aligned tendon extracellular matrix is composed of hierarchically organized components: collagen molecules assemble into fibrils, fibrils bundle to form fibers, and fibers together with tendon cells organize into fascicles [1], [2], [3], [4], [5]. However, the mechanisms underlying the development of tendon hierarchical structure and how it dictates overall tendon function are not fully understood.
Collagen XII, a member of the Fibril-Associated Collagens with Interrupted Triple Helices (FACIT) family, is a quantitatively minor component in tendons and ligaments [6], [7], [8], [9], [10], [11]. It is a non-fibrillar collagen that associates with both collagen I fibrils and cell interfaces [12], [13]. The molecular structure and localization to flexible bridges between neighboring collagen fibrils suggest a critical role for collagen XII in regulating collagen I fibrillogenesis [6], [7], [14], [15], fibril organization, and interactions with other extracellular matrix components [14], [16]. Additionally, collagen XII has been shown to control cell organization and assembly into communicating networks [10], [11], [17], [18], suggesting dual regulatory roles for collagen XII.
Collagen XII is expressed in tendons, as well as bones and muscles [10]. Human mutations in COL12A1 result in myopathic Ehlers-Danlos Syndrome (mEDS), and a number of COL12A1 dominant and recessive mutations have been identified. In these patients, there is an overlapping phenotype with involvement of muscle and connective tissues [19], [20], [21], [22], [23], [24]. COL12A1 mutations result in excessive weakness at birth, strikingly hypermobile distal joints, and an absence of deep tendon reflexes. These clinical manifestations indicate a critical role(s) of collagen XII in tendon and ligament as well as muscle and bone development and function.
Previous work in flexor digitorum longus (FDL) tendons from global Col12a1-/- knockout mice support the dual roles of collagen XII in driving tendon structure and function [11]. In addition to altered fibril packing and fiber assembly, collagen XII deficiency altered the formation of interacting cellular processes and tenocyte shape, resulting in impaired cell–cell communication possibly via connexin 43. However, these studies using global knockout models were not able to distinguish between intrinsic roles for collagen XII in the tendon versus extrinsic roles due to altered muscle, and bone or other alterations external to the tendon.
The objectives of this work were threefold. One, to create and characterized a novel conditional Col12a1 mouse model. This mouse model allows targeting of the Col12a1 deletion to specific tissues as well as temporal control of collagen XII knockdown. Second, to demonstrate that the deletion can be targeted to tendons using Scleraxis-Cre (Scx-Cre) resulting in a deletion of Col12a1 and collagen XII expression in cells of the tendon lineage. This would be critical to isolating the roles of collagen XII in tendons. Third, to evaluate the effects of a tendon targeted collagen XII knockout on gait and tendon biomechanics. Targeting the collagen XII knockout to the tendon allows an analysis of its intrinsic regulatory roles separate from extrinsic effects due to it roles in muscle and other tissues that exert indirect effects on tendons. This work provides a foundation for future studies to define the intrinsic and extrinsic regulatory mechanisms whereby collagen XII influences the development and maintenance of tendon structure and function.
Results
Generation of a tendon-targeted Col12a1 conditional knockout mouse model
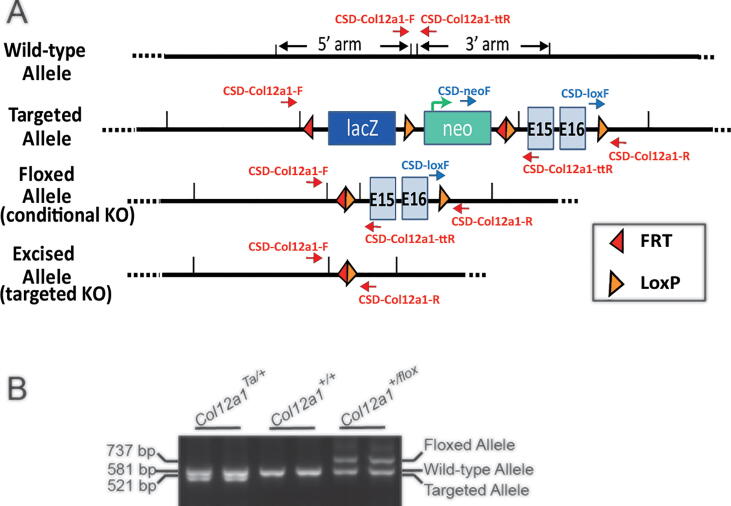
The strategy presented in Fig. 1A was used to generate a novel conditional Col12a1 mouse line (Col12a1flox/flox). In this line, exons 15 and 16 in the Col12a1 allele were flanked by loxP elements. Deletion of these exons results in a skip from exon 14 to 17 where the resulting transcript is out of frame. The sequence coded by exons up stream of exon 14 is susceptible to nonsense mediated decay. Therefore, there is no expression of either the long or short Col12a1 transcript. Since collagen XII is a homotrimer of Col12a1 chains, a knockout of Col12a1 results in a knockout of all possible collagen XII isoforms. The presence of the wild type, targeted and floxed alleles were followed at different stages of model development using PCR analyses (Fig. 1B).
Fig. 1.
Strategy for creation of a tendon targeted Col12a1 conditional knockout mouse model. A targeted Col12a1 ES cell line, Col12a1tm2a(KOMP)Wtsi, was obtained from the KOMP Repository (project ID: CSD29388). (A) This schematic diagram illustrates the wild-type Col12a1 allele; the targeted allele where exons 15–16 are flanked with LoxP sites and the Neo cassette is flanked with FRT sites; the floxed allele after removal of the FRT flanked Neo cassette; and the excised allele after Cre recombination of the Col12a1 gene. Location of primers for LacZ, as well as 3′ and 5′ primers used to determine insert orientation are shown (red arrows). (B) Genotyping of the targeted allele and floxed alleles in different stages of creating the conditional Col12a1 knock-out mice. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Tendon-targeted bitransgenic collagen XII knockout models were obtained after breeding conditional mice with scleraxis Cre (Scx-Cre) mice. Using reporter mice, Cre excision was only observed in tendons and ligaments with other limb tissue showing no excision [25]. These mice were subject to genotyping analysis using Cre primers and specific primers at the junction of the 3′ arm and targeting sequence. Excision of the Col12a1 exons was identified in tendon-targeted Col12a1 conditional knockout Col12a1+/Δten and Col12a1Δten/Δten mice, but as expected, not in parental Scx-Cre mice or Col12a1flox/flox mice (Fig. 2). The results confirmed Cre-mediated recombination of the Col12a1 allele in the FDL tendon.
Fig. 2.
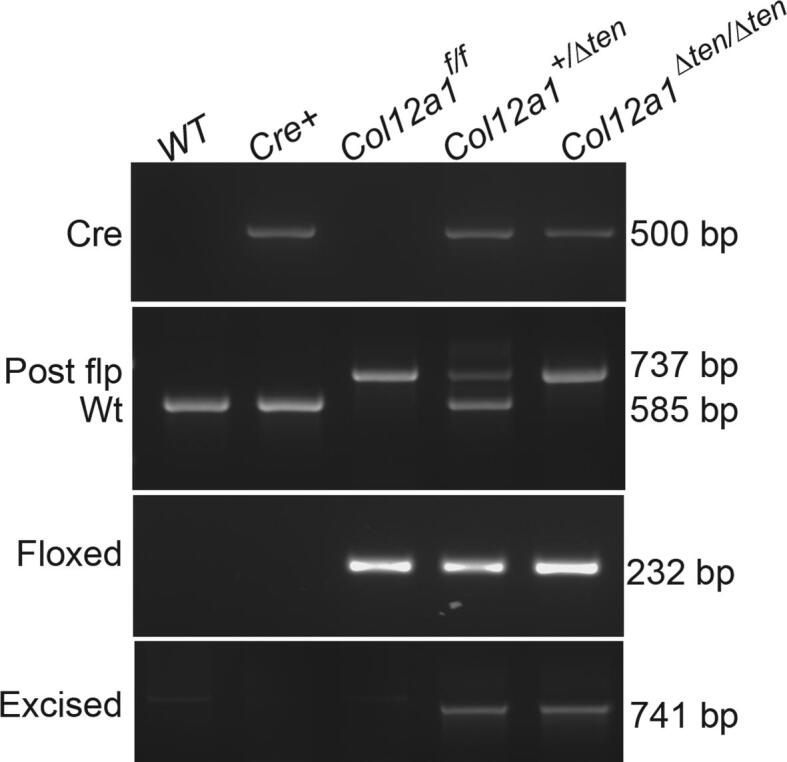
Genotyping analysis of tendon targeted Col12a1 knockout mice. Genotyping of the control mice; wild type (WT), Scleraxis-Cre (Scx-Cre), floxed (Col12a1f/f) as well as targeted heterozygous (Col12a1+/Δten) and homozygous (Col12a1Δten/Δten) mice was used to characterize the targeted models.
Col12a1 gene and protein expression is knocked out in FDLs of Col12a1Δten /Δten mice
Scx-Cre-mediated excision of exons 15–16 resulted in targeting of the Col12a1 null mutation to tendons. FDLs from day 10 mice were analyzed using qPCR (Fig. 3). Expression of Col12a1 mRNA in wild type control mice was compared to heterozygous (Col12a1+ /Δten) and homozygous (Col12a1Δten /Δten) mice. Col12a1 had a significant 76% reduction in Col12a1Δten/Δten mice compared to wild type control mice. In contrast, expression of Col12a1 mRNA was reduced by 24% in Col12a1+/Δten mice compared to wild type control mice. Baseline expression was determined using traditional collagen XII knockout mice [11]. Expression of Col12a1 was not significantly different in the 3 control groups (data not shown).
Fig. 3.
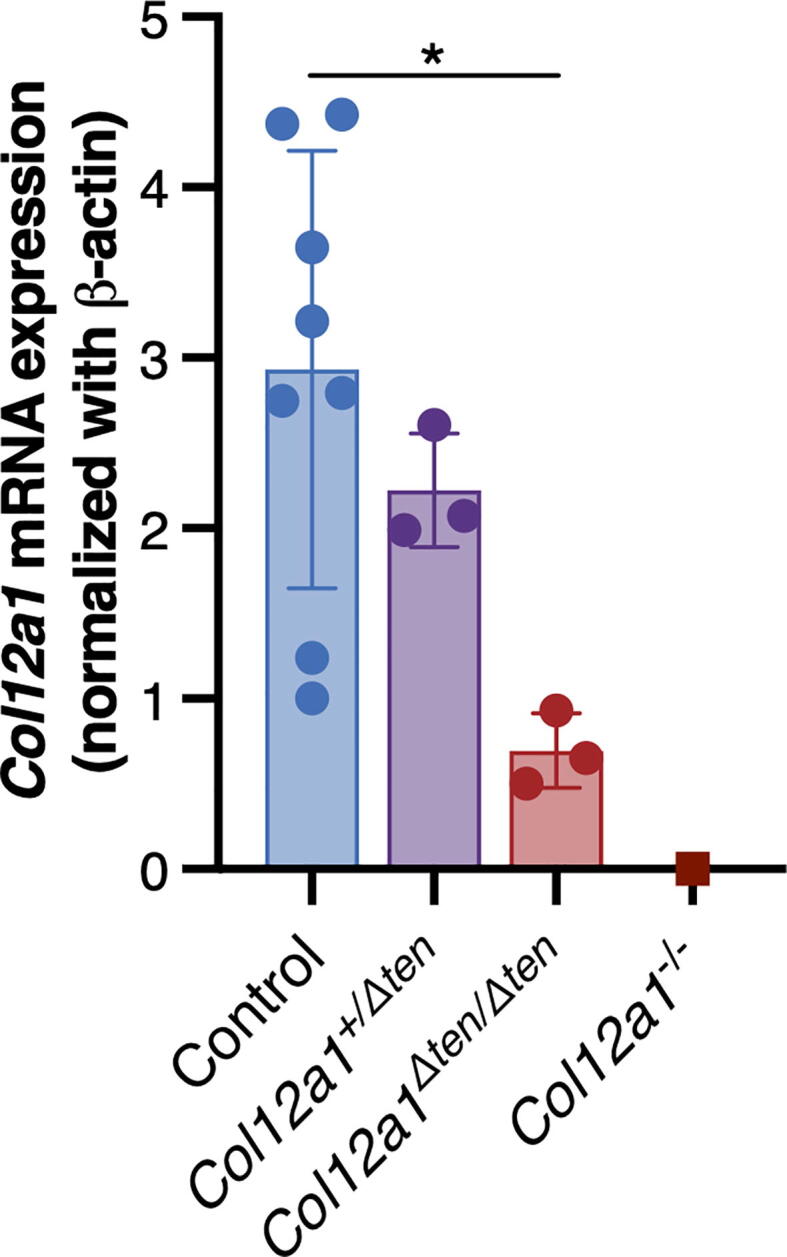
Knockout of Col12a1 mRNA expression in tendon targeted conditional mice. Quantitative real-time PCR analyses of Col12a1 mRNA expression in Col12a1Δten/Δten FDLs is reduced to baseline while expression in the Col12a1+/Δten FDLs was ∼ 25% of control values. Day 10 Control (n = 8, including WT n = 4, Scx-Cre n = 2, Col12a1+/flox n = 2), Col12a1+/Δten (n = 3), Col12a1Δten/Δten (n = 3), and Col12a1-/- (n = 1) mice (*p < 0.05).
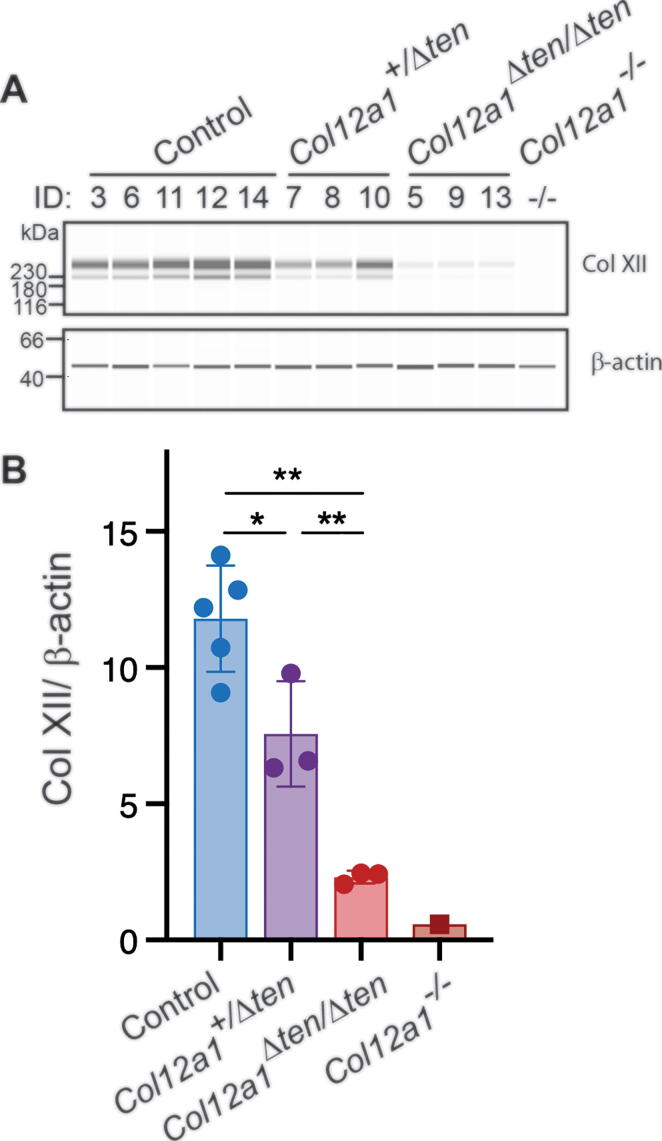
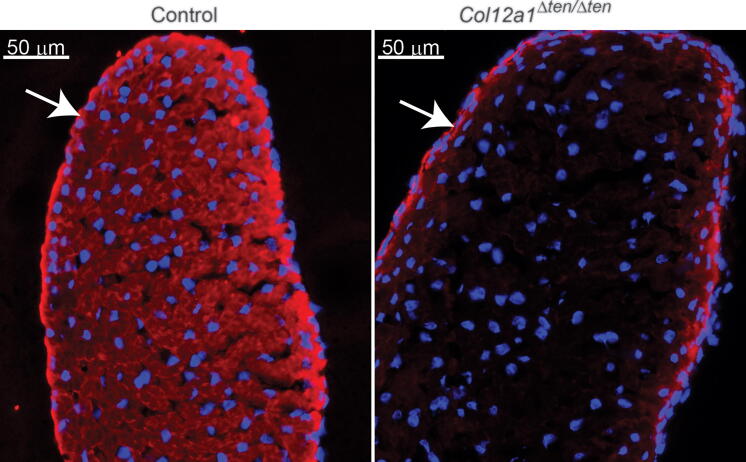
The α1(XII) chain of collagen XII was analyzed immuno-chemically in tendon-targeted Col12a1 knockout mice. The α1(XII) chain was analyzed in day 10 FDL tendons using a WES simple western blotting system. The α1(XII) chain was present at comparable levels in the control group: wild type, Scx-Cre and Col12a1flox/flox mice (data not shown). In contrast, the α1(XII) chain was present at lower levels in Col12a1+/Δten mice and just above background in Col12a1Δten/Δten mice (Fig. 4). Representative samples are shown in Fig. 4A, and the total results are quantitated in Fig. 4B. The Col12a1Δten/Δten and Col12a1+/Δten were reduced 80% and 36%, respectively relative to controls. As seen for mRNA expression, the protein expression in the Col12a1Δten/Δten mice did not reach the baseline established using traditional collagen XII knockout mice. This suggests that there are collagen XII expressing cells from a non-tendon lineage that would not be targeted in these samples. To address this, immuno-localization studies of collagen XII in wild type and Col12a1Δten/Δten FDLs were done. In these analyses, there was an absence of reactivity in the tendon proper in Col12a1Δten/Δten mice compared to wild type controls. However, cells of the surrounding peritenon show reactivity for collagen XII in both genotypes (Fig. 5). In areas where the tendon is not in perfect cross section there is some reactivity in the adjacent matrix that we believe to be artifact. However, collagen XII is enriched in a variety of interfacial matrices and can interact with collagen fibrils. Therefore, we cannot exclude functional interactions in this narrow zone that require further study. Overall, the data support a targeted knockout in tenocytes while peritenon cells from a non-tendon lineage retained collagen XII expression. Taken together, the gene and protein expression data show a knockout of the α1(XII) chain in the Col12a1Δten/Δten mice resulting in the absence of tenocyte produced collagen XII.
Fig. 4.
Knockout of collagen XII expression in the Col12a1 tendon targeted conditional mice. Analysis of Col12a1 protein (α1(XII)) content in ; Control (n = 5, including WT n = 3, Scx-Cre n = 1, Col12a1+/flox n = 1), Col12a1+/Δten (n = 3), Col12a1Δten/Δten (n = 3) mice, as well as global knock out mice, Col12a1-/- (n = 1) was done using Wes automated western blotting. The Col12a1+/Δten and Col12a1Δten/Δten FDLs in day 10 mice contained reduced and virtually no α1(XII) reactivity relative to controls. (A) shows a representative image and (B) presents the quantitation of results from all mice (**p < 0.01, *p < 0.05).
Fig. 5.
Collagen XII in wild type and Col12a1Δten/Δten FDLs. Immuno-localization analyses of collagen XII demonstrated an absence of reactivity in the tendon proper of Col12a1Δten/Δten compared to wild type FDLs. In contrast, cells of the surrounding peritenon (white arrows) show reactivity for collagen XII in both genotypes. Day 14 Control (Col12a1flox/flox, n = 2) and Col12a1Δten/Δten (n = 2) FDLs.
Gross phenotype of tendon-targeted collagen XII null mouse models
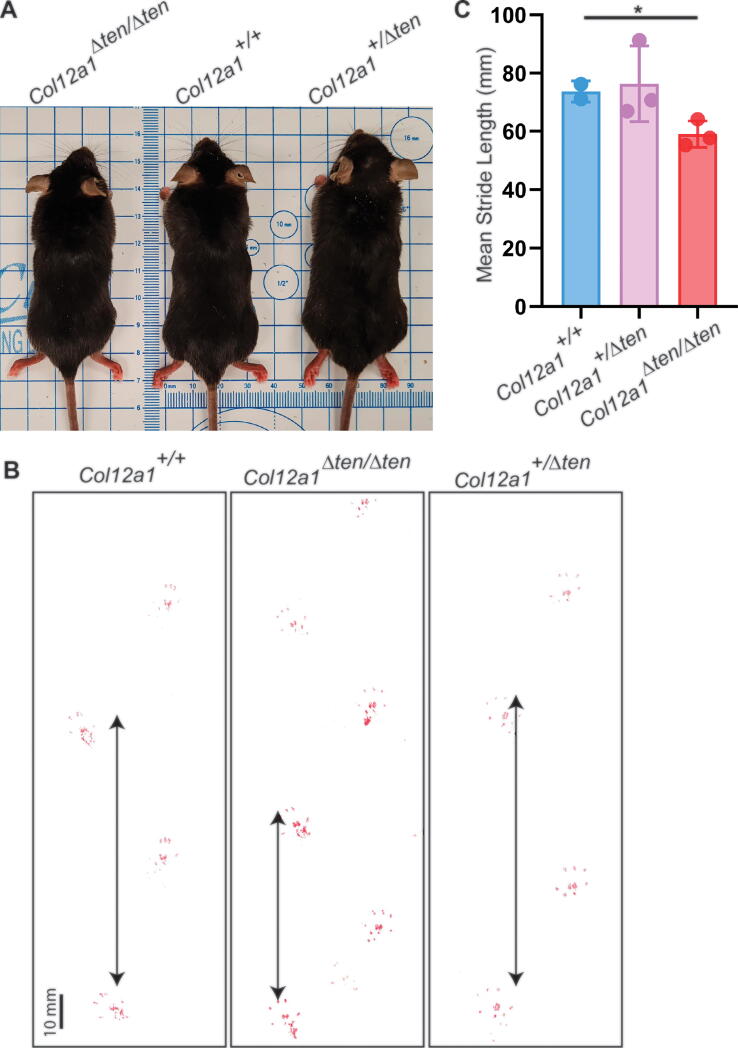
The Col12a1Δten/Δten mice were fertile. Grossly, homozygous Col12a1Δten/Δten male mice were smaller than both heterozygous Col12a1+/Δten and wild type controls. The wild type and heterozygous mice were comparable in size (Fig. 6A). The mean body weights of mature male Col12a1+/Δten and Col12a1Δten/Δten mice were significantly less (p < 0.05) than that of wild type control male mice. The mean body weights were 26.6 ± 1.6 g (n = 5), 24.2 ± 1.7 g (n = 5) and 23.8 ± 1.8 g (n = 8) for male wild type control, Col12a1+/Δten and Col12a1Δten/Δten mice, respectively. Comparable to the male mice, grossly female homozygous Col12a1Δten/Δten mice were smaller than both heterozygous Col12a1+/Δten and wild type controls with the wild type and heterozygous mice comparable in size (data not shown). Female mice had decreased body weights compared to male mice. The mean body weights of female homozygous Col12a1Δten/Δten mice were significantly less than than both heterozygous Col12a1+/Δten and wild type controls with no significant difference between wild type and heterozygous mice. The mean body weights were 21.8 ± 1.0 g (n = 2), 21.5 ± 1.5 g (n = 8), and 19.5 ± 0.9 g (n = 7) for female wild type control, Col12a1+/Δten and Col12a1Δten/Δten mice. Data from both male and female mice were comparable with Col12a1+/Δten mice smaller than wild type controls without statistical significance, while Col12a1Δten/Δten mice were significantly smaller than wild type mice.
Fig. 6.
Smaller size and altered gait in Col12a1Δten/Δten mice. (A) Representative gross images showing that Col12a1Δten/Δten mice are smaller than control Col12a1+/+ and heterozygous Col12a1+/Δten mice, with no obvious body size difference between control and heterozygous mice. All mice are male litter mates at 60 days. (B) Representative gait analysis showed shorter stride length in Col12a1Δten/Δten mice compared to wild type controls. However, all mice walk with regular, even steps, and there is no obvious change in the width of toe spread. The arrowed vertical lines indicate the stride lengths from the 3rd toe to adjacent 3rd toe of the same hind foot. Male 60 day mice. (C) Quantitative presentation of the stride length data from (B). There was a significant decrease in stride length in Col12a1Δten/Δten mice compared to heterozygous and wild type mice. There was no difference in the latter two groups. (*p < 0.05).
An analysis of gait was done, and mice from all genotype groups walked with regular, even steps with no obvious change in the width of toe spread irrespective of genotype. However, there was a decrease in stride length in the Col12a1Δten/Δten mice compared to wild type controls (Fig. 6B). The mean stride lengths in wild type and Col12a1+/Δten mice were comparable. In contrast, there was a significant decrease (p < 0.05) in the stride length of Col12a1Δten/Δten mice compared to wild type controls (Fig. 6C). The mean stride lengths for wild type, Col12a1+/Δten and Col12a1Δten/Δten mice were 73.7 +/- 3.6 mm (n = 2), 76.3 +/- 13.1 mm (n = 3), 59.1 +/- 4.6 mm (n = 3), respectively. Additionally, gross observation of the knee joint upon dissection indicated joint laxity in Col12a1Δten/Δten mice, with greater varus and valgus rotation and occasional patella dislocation.
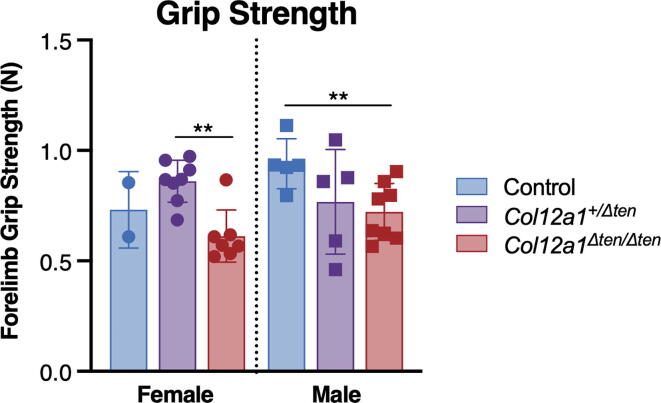
To evaluate musculoskeletal and motor function in the tendon-targeted heterozygous and homozygous mice, grip strength tests were conducted on the forelimbs of mature female and male mice and compared to wild type controls (Fig. 7). Significant impairment of function was observed with the Col12a1Δten/Δten male mice being weaker than the control mice. Heterozygous mice also showed reduced strength, but the results were not statistically significant. The mean grip strengths were significantly different (p < 0.01). The value for male control mice was 0.94 ± 0.10 N (n = 5) while the heterozygous and homozygous mice were 0.76 ± 0.24 N (n = 5) and 0.72 ± 0.13 N (n = 8), respectively. For females, the value for control mice was 0.73 ± 0.17 N (n = 2) while the heterozygous and homozygous mice were 0.86 ± 0.09 N (n = 5) and 0.61 ± 0.11 N (n = 8), respectively. There was no statistical significance in female heterozygous or homozygous mice vs wild type control mice due to small sample size.
Fig. 7.
Tendon-targeted Col12a1 knockout mice are weaker. Fore-limb grip strength is significantly decreased in Col12a1Δten/Δten male mice (n = 8) compared to Col12a1flox/flox control mice (n = 5). Mature male and female mice were tested at day 65 to 90, n = 5–8 mice per genotype (**p < 0.01).
Reduction in biomechanical properties of FDL tendons in Col12a1Δten/Δten mice
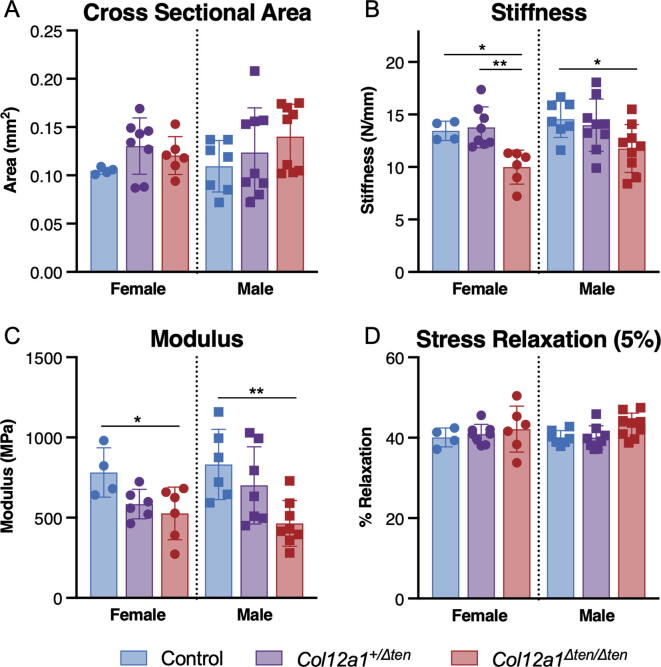
FDL tendons from day 60 male and female Col12a1Δten/Δten mice exhibited a reduction in biomechanical properties. There was no difference in cross-sectional area (Fig. 8A). However, stiffness and modulus were both significantly decreased in the Col12a1Δten/Δten FDLs compared to controls (Fig. 8B,C). In female mice, stiffness of Col12a1Δten/Δten FDLs was also significantly less compared to heterozygous Col12a1 Col12a1+/Δten, and there were no significant biomechanical changes due to partial Col12a1 knockdown in Col12a1+/Δten mice. These results suggest that an absence of Col12a1 expression significantly reduces the biomechanical properties of these FDL tendons. In contrast, there were no differences stress relaxation (Fig. 8D), indicating a comparable viscoelastic response between Col12a1Δten/Δten, Col12a1+/Δten, and control Col12a1flox/ flox tendons.
Fig. 8.
Altered biomechanical properties in FDLs in tendon targeted Col12a1 knockout mice. FDL tendons from tendon-targeted Col12a1Δten/Δten null mice demonstrated significant alterations in the biomechanical properties. No differences in (A) cross-sectional area were observed between genotypes. However, (B) stiffness and (C) modulus were significantly reduced in Col12a1Δten/Δten mice compared to controls, while there were no differences in (D) stress relaxation at 5% strain. Decreases in modulus suggest that fundamental differences in the material properties of Col12a1Δten/Δten and control FDL tendons underlie the mechanical deficiencies. Mature day 60 male Col12a1flox/flox (n = 7), Col12a1+/Δten (n = 9) and Col12a1Δten/Δten (n = 9), and female Col12a1flox/flox (n = 4), Col12a1+/Δten (n = 8), and Col12a1Δten/Δten (n = 6) mice were tested (**p < 0.01, *p < 0.05).
Discussion
We created a conditional Col12a1-null mouse model and specifically targeted collagen XII knockout to tendons using a scleraxis (Scx) Cre driver. Collagen XII is a fibril-associated collagen with interrupted triple helices. Although collagen XII is considered a quantitatively minor component in collagen I-containing tissues, it is widely expressed in embryonic tissues and throughout tendon development [11]. Collagen XII localizes to the fascicular ECM, interfacial regions, and near cell–cell junctions within tendons [6]. Collagen XII also has been shown to interact with collagen I [12], influencing fibril assembly [13], [26].
In humans, mutations in COL12A1 result in myopathic Ehlers-Danlos syndrome (mEDS), leading to a broad range of connective tissue defects. Patients classified with mEDS present with myopathy as well as symptoms similar to other EDS types, such as joint laxity and hypermobility, joint contractures, and abnormal wound healing. These symptoms clearly implicate alterations in tendon and ligament, and a global Col12a1-/- knockout murine model has been shown to recapitulate many features of mEDS [21]. Our previous work using the Col12a1-/- model demonstrated that collagen XII affects tenocyte organization, cell–cell communication, and collagen fibril interactions, thereby regulating tendon structure and function [11]. However, a broad range of musculoskeletal tissues including muscle and bone are affected in this model, and the intrinsic regulatory roles of collagen XII on tendon structure and function remain unelucidated. Our conditional Col12a1 knockout model overcomes this limitation by permitting spatial and temporal manipulation of collagen XII expression. Using this model to target the collagen XII deficiency specifically to cells of the tendon lineage allowed the analysis of the intrinsic roles of collagen XII in the tendon as opposed to extrinsic effects due to alterations in bone and muscle and their interactions with tendon. The current results support intrinsic roles for collagen XII in determining tendon function.
In FDLs of tendon-targeted Col12a1Δten/Δten mice, both mRNA and protein expression levels were near baseline, but did not reach the baseline established using global collagen XII knockout mice. This suggests that cells from a non-tendon lineage are not targeted, as expected. Immunofluorescence localization of collagen XII demonstrated efficient knockdown in the tendon proper, but not in the surrounding peritenon, indicating that this population contributes to above baseline expression in whole tendons. This finding is not unexpected as peritenon cells express significantly lower levels of scleraxis [27], [28], [29], and collagen XII also is expressed in tissue sheaths and basement membrane zones [30], [31].
In the absence of Col12a1 expression and therefore collagen XII, Col12a1Δten/Δten mice have impaired joint and tendon function, as evidenced by reduced forelimb grip strength and FDL tendon mechanical properties. Reduced grip strength in this tendon-targeted model is consistent with joint function in the global Col12a1-/- knockout model [21]. This indicates that altered collagen XII interactions in the tendon proper are critical determinants of joint function. Grip strength in the Col12a1Δten/Δten mice is reduced, but not to the same magnitude as in the global knockout [21]. This suggests that involvement of extrinsic influences such as from muscle and bone are important. However, the deletion in tendon contributes substantially to the observed effect supporting intrinsic roles for collagen XII in the tendon. Interestingly, FDL tendon mechanical properties in the targeted mice were altered compared to control tendons, but contrasted with previous findings in the global knockout model. In the global Col12a1-/- knockout model, FDLs had larger cross-sectional area and greater stiffness with no difference in tendon material properties [11]. In this study, however, there were no differences in FDL cross-sectional area in Col12a1Δten/Δten mice, but stiffness was significantly lower, resulting in inferior tendon elastic modulus. Differences in mechanical properties suggest that collagen XII is a critical regulator of tendon structure–function, and the discrepancy from the global knockout model may be a result of extrinsic effects, such as those due to altered muscle and bone. Therefore, joint and tendon functional changes observed in the tendon-targeted Col12a1Δten/Δten model support significant contributions intrinsic to tendon and ligament in altered mechanical properties and in the reduction in grip strength.
Tendon-targeted Col12a1Δten/Δten mice allowed the spatial targeting of the mutation to tendons, circumventing any potential secondary influences such as those due to alterations in cartilage, bone, and muscle. The conditional model also can be utilized in its heterozygous and/or homozygous state to isolate dose dependent effects, the contributions of different tissues, define the temporal requirements for Col12a1 expression, and to probe the mechanisms underlying pathobiology. In conclusion, we created a conditional Col12a1 mouse model that permits the spatial and temporal targeting of the deletion. Utilizing these mice, intrinsic roles for Col12a1 in tendon function were supported.
Experimental procedures
This work was approved by the University of South Florida and the University of Pennsylvania Institutional Animal Care and Use Committees. These experimental studies utilized male and female mice. The tendon-targeted (Scx-Cre) collagen XII knockout mouse model (Col12a1Δten/Δten) is in a C57/BL6 Charles River background. Control mice were wild type, Scx-Cre, Col12a1+/flox and Col12a1flox/flox, all in a C57/BL6 Charles River background.
Development of tendon-targeted collagen XII knockout mouse model
A novel conditional Col12a1 mouse line (Col12a1flox/flox) was created using a strategy previously described [41–44]. The strategy is presented in (Fig. 1A). A targeted Col12a1 ES cell line, Col12a1tm2a(KOMP)Wtsi was obtained from the KOMP Repository (University of California at Davis, project ID: CSD29388). The line was validated, and the ES cell were microinjected into blastocysts and subsequently implanted. We obtained chimeric mice that were crossed with C57BL/6 mice and targeted Col12a1Ta/+ mice were selected (Fig. 1B). The targeted mice were crossed with a germline-specific FLPe transgenic mouse (B6;SJL-Tg(ACTFLPe)9205Dym/J, Jackson Labs) to remove the FRT flanked neo cassette and Lac Z sequences to yield heterozygous floxed Col12a1+/flox mice (Fig. 1A). The resulting offspring were cross bred with C57BL/6 (Charles River) mice for 6 generations and then inter-crossed resulting in the conditional knockout mouse model, Col12a1flox/flox. Mice at each different stage were characterized using PCR that amplified specific element sequences.
The conditional mice were bred with Scleraxis Cre (Scx-Cre) mice to produce bitransgenic tendon-targeted collagen XII knockout models (Fig. 2) as previously described [32], [33]. The Scx-Cre mice were a gift from Dr. Ronen Schweitzer, Oregon Health and Science University. Genotyping analysis of tendon-targeted Col12a1 conditional knockout mouse was carried out using Cre primers and specific primers at the junction of the 3′ arm and targeting sequence. The primers for the genotyping and characterization of these mice are listed in Table 1.
Table 1.
Primers for selection and characterization of Col12a1 targeted and conditional mice.
 |
RT-PCR
FDL tendons were removed from wild type, Scx-Cre, Col12a1flox/flox, Col12a1+/Δten, and Col12a1Δten/Δten mice (gender not determined) at day 10. The tissue samples were cut into small pieces and lysed in QIAzol reagent (Qiagen, Germantown, MD), and the crude total RNA was processed using the RNeasy MinElute Cleanup Kit (Qiagen, Germantown, MD). The resulting RNA underwent reverse transcription with the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). This was followed by real time PCR using SYBR Green PCR master mix (Thermo Fisher Scientific) in a StepOnePlus Real Time PCR system (Applied Biosystems). The resulting Col12a1 mRNA expression levels were normalized with β-actin. The primers for the real time PCR reactions are Col12a1 FW: 5′-CCCTACAACAGATGGGCCTAC-3′, Col12a1 RV: 5′-TCTTCTCCCCTGGCTTTGTA-3′; β-actin FW: 5′-AGATGACCCAGATCATGTTTGAGA-3′, β-actin RV: 5′-CACAGCCTGGATGGCTACGT-3′. A one-way ANOVA with Tukey post-hoc tests was conducted to compare genotypes. Significance was set at p ≤ 0.05.
Immuno-blots
Collagen XII content was analyzed immuno-chemically using a WesTM automated western blotting system (ProteinSimple, San Jose, CA). FDL samples were dissected from wild type, Scx-Cre, Col12a1flox/flox, Col12a1+/Δten, and Col12a1Δten/Δten mice at day 10. Individual mice (n = 3–8) were used for each genotype. The FDLs from each mouse were cut into small pieces, and protein was extracted using RIPA buffer (EMD Millipore) with proteinase inhibitor cocktail (ThermoFisher Scientific). Denatured protein samples (0.375 μg) were loaded into single designated wells of Wes Separation 12–230 kDa 25 Capillary Cartridges, 1:50 diluted rabbit anti-mouse Col XII antibody (KR33, gift from Dr. Manuel Koch, University of Cologne, Cologne, Germany), and the Wes anti-rabbit detection module was used for collagen XII detection. Quantification by densitometry was performed using the area of the targeted protein (collagen XII) and normalized to the area of β-actin. The loading of denatured protein samples β -actin was 1.5 μg and the monoclonal anti- β -actin antibody produced in mice (AC-15) was ordered from Sigma. All Wes reagents (separation module and detection modules) were purchased from ProteinSimple and the Wes assay was carried out following the manufacturer’s instructions. Data analyses were performed using the Compass Software (ProteinSimple). A one-way ANOVA with Tukey post-hoc tests was conducted to compare genotypes. Significance was set at p ≤ 0.05.
Immunofluorescence microscopy
FDL tendons were dissected from wild type and Col12a1Δten/Δten mice at day 14, fixed in 4% paraformaldehyde, embedded in optimal cutting temperature (OCT) compound, and frozen at −80 °C. Frozen sections (5 µm were cut using a HM 525 NX cryostat (ThermoFisher Scientific). Immunofluorescence localization was performed to analyze spatial expression of collagen XII using a rabbit anti-mouse Col XII antibody (KR33, 1:500 dilution) with a donkey anti-rabbit IgG-Alexa Fluor 568 (ThermoFisher Scientific, 1:200 dilution) secondary antibody. Sections were mounted using Fluoromount-G® clear mounting solution (SouthernBiotech, Birmingham, AL) with DAPI was used as a nuclear marker. Images were captured with a Leika DMi8 Microsystem (Wetzlar, Germany) and Leica DMC6200 digital camera. Antibody incubations and image acquisition were done concurrently for WT and Col12a1Δten/Δten sections using identical procedures and settings to facilitate comparison.
Gait analysis
For gait analysis footprint recording was done for individual mice. The hind feet were dipped in nontoxic paint, and the mouse was allowed to walk freely on a piece of white paper in a walkway consisting of two Plexiglas walls, spaced 8 cm apart. Stride length was the distance between adjacent prints made by the same hind limb from 3rd toe to 3rd toe. A mixed sample of male and female mice at day 60 was analyzed. The footprint recording was repeated at least twice for each mouse.
Grip strength
Grip strength was evaluated in mature Col12a1flox/flox control, Col12a1+/Δten and Col12a1Δten/Δten mature male and female mice at day 65 to 90. A grip strength meter (San Diego Instrument, San Diego, CA) was used to record the peak force each mouse exerts in grasping a grip placed at their forelimb. The mouse was held by the tail and lowered toward the grip strength platform until it grasped the grip with its forepaws. The mouse was then pulled steadily by the tail away from the rod until the grip was broken. The force applied to the grip just before the animal loses its grip was recorded as the peak tension. Mice were tested for each genotype (n = 5–8). Ten measurements from each mouse were recorded, and the average force was used to represent the grip strength for individual mice. For female and male mice, separate one-way ANOVAs with Tukey post-hoc tests were conducted to compare genotypes. Significance was set at p ≤ 0.05.
Biomechanical analyses
FDL tendons (n = 4–9/group) were dissected from the mouse foot, cleaned free of excess soft tissue, and mechanically evaluated as previously described [32], [33], [34], [35]. Tendon cross-sectional area was measured using a custom laser device [36], and each end of the FDL tendon was adhered to sandpaper using cyanoacrylate glue at a gauge length of approximately 5 mm. Tendons were then secured in custom made fixtures, placed in a phosphate buffered saline bath at 37 °C, and loaded in a mechanical testing system (model 5542, Instron, Norwood, MA). To determine biomechanical properties, mechanical tensile testing was performed with the following protocol: a preload to 0.05 N; 10 cycles of preconditioning (0.1–0.2 N); rest for 300 s; stress relaxation at 5 % strain (ramp rate of 5 %/s) followed by a 600 s hold; a return to zero-displacement, 60 s hold, and ramp to failure at a rate of 0.5 %/s. A 10 N load cell was used with a resolution of 0.01 N. During testing, images were captured (Basler, Exton, PA) every 5 s for optical strain analysis. A custom Matlab program (Matlab R2019b, Natick, MA) was used to optically track strain lines to quantify elastic modulus. For female and male mice, separate one-way ANOVAs with Tukey post-hoc tests were conducted to compare genotypes. Significance was set at p ≤ 0.05.
Funding
This study was supported by NIH/NIAMS grant AR078790, and grant AR069619 supporting the Penn Center for Musculoskeletal Disorders.
Author contributions
A.F. and M.S were involved in investigation, methodology, validation, visualization, and writing the original draft. All authors were involved with data curation, and formal analysis of the data. L.J.S. and D.E.B. were involved in project conceptualization, funding acquisition, supervision, administration, and review and editing of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.Birk D.E., Trelstad R.L. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J. Cell Biol. 1986;103(1):231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang G., Young B.B., Ezura Y., Favata M., Soslowsky L.J., Chakravarti S., Birk D.E. Development of tendon structure and function: regulation of collagen fibrillogenesis. J. Musculoskelet. Neuronal Interact. 2005;5(1):5–21. [PubMed] [Google Scholar]
- 3.Birk D.E., Zycband E. Assembly of the tendon extracellular matrix during development. J. Anat. 1994;184(Pt 3):457–463. [PMC free article] [PubMed] [Google Scholar]
- 4.Graham H.K., Holmes D.F., Watson R.B., Kadler K.E. Identification of collagen fibril fusion during vertebrate tendon morphogenesis. The process relies on unipolar fibrils and is regulated by collagen-proteoglycan interaction. J. Mol. Biol. 2000;295(4):891–902. doi: 10.1006/jmbi.1999.3384. [DOI] [PubMed] [Google Scholar]
- 5.Canty E.G., Kadler K.E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 2005;118(Pt 7):1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- 6.Zhang G., Young B.B., Birk D.E. Differential expression of type XII collagen in developing chicken metatarsal tendons. J. Anat. 2003;202(5):411–420. doi: 10.1046/j.1469-7580.2003.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh S.P., Griffith C.M., Hay E.D., Olsen B.R. Tissue-specific expression of type XII collagen during mouse embryonic development. Dev. Dyn. 1993;196(1):37–46. doi: 10.1002/aja.1001960105. [DOI] [PubMed] [Google Scholar]
- 8.Walchli C., Koch M., Chiquet M., Odermatt B.F., Trueb B. Tissue-specific expression of the fibril-associated collagens XII and XIV. J. Cell Sci. 1994;107(Pt 2):669–681. doi: 10.1242/jcs.107.2.669. [DOI] [PubMed] [Google Scholar]
- 9.Bohme K., Li Y., Oh P.S., Olsen B.R. Primary structure of the long and short splice variants of mouse collagen XII and their tissue-specific expression during embryonic development. Dev. Dyn. 1995;204(4):432–445. doi: 10.1002/aja.1002040409. [DOI] [PubMed] [Google Scholar]
- 10.Izu Y., Sun M., Zwolanek D., Veit G., Williams V., Cha B., Jepsen K.J., Koch M., Birk D.E. Type XII collagen regulates osteoblast polarity and communication during bone formation. J. Cell Biol. 2011;193(6):1115–1130. doi: 10.1083/jcb.201010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izu Y., Adams S.M., Connizzo B.K., Beason D.P., Soslowsky L.J., Koch M., Birk D.E. Collagen XII mediated cellular and extracellular mechanisms regulate establishment of tendon structure and function. Matrix Biol. 2021;95:52–67. doi: 10.1016/j.matbio.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keene D.R., Lunstrum G.P., Morris N.P., Stoddard D.W., Burgeson R.E. Two type XII-like collagens localize to the surface of banded collagen fibrils. J. Cell Biol. 1991;113(4):971–978. doi: 10.1083/jcb.113.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch M., Bohrmann B., Matthison M., Hagios C., Trueb B., Chiquet M. Large and small splice variants of collagen XII: differential expression and ligand binding. J. Cell Biol. 1995;130(4):1005–1014. doi: 10.1083/jcb.130.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal P., Zwolanek D., Keene D.R., Schulz J.N., Blumbach K., Heinegard D., Zaucke F., Paulsson M., Krieg T., Koch M., Eckes B. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J. Biol. Chem. 2012;287(27):22549–22559. doi: 10.1074/jbc.M111.335935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw L.M., Olsen B.R. FACIT collagens: diverse molecular bridges in extracellular matrices. Trends Biochem. Sci. 1991;16(5):191–194. doi: 10.1016/0968-0004(91)90074-6. [DOI] [PubMed] [Google Scholar]
- 16.Font B., Eichenberger D., Rosenberg L.M., van der Rest M. Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin. Matrix Biol. 1996;15(5):341–348. doi: 10.1016/s0945-053x(96)90137-7. [DOI] [PubMed] [Google Scholar]
- 17.Hemmavanh C., Koch M., Birk D.E., Espana E.M. Abnormal corneal endothelial maturation in collagen XII and XIV null mice. Invest. Ophthalmol. Vis. Sci. 2013;54(5):3297–3308. doi: 10.1167/iovs.12-11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun M., Zafrullah N., Devaux F., Hemmavanh C., Adams S., Ziebarth N.M., Koch M., Birk D.E., Espana E.M. Collagen XII Is a Regulator of Corneal Stroma Structure and Function. Invest. Ophthalmol. Vis. Sci. 2020;61(5):61. doi: 10.1167/iovs.61.5.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohassel P., Liewluck T., Hu Y., Ezzo D., Ogata T., Saade D., Neuhaus S., Bolduc V., Zou Y., Donkervoort S., Medne L., Sumner C.J., Dyck P.J.B., Wierenga K.J., Tennekoon G., Finkel R.S., Chen J., Winder T.L., Staff N.P., Foley A.R., Koch M., Bonnemann C.G. Dominant collagen XII mutations cause a distal myopathy. Ann. Clin. Transl. Neurol. 2019;6(10):1980–1988. doi: 10.1002/acn3.50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Punetha J., Kesari A., Hoffman E.P., Gos M., Kaminska A., Kostera-Pruszczyk A., Hausmanowa-Petrusewicz I., Hu Y., Zou Y., Bonnemann C.G. Novel Col12A1 variant expands the clinical picture of congenital myopathies with extracellular matrix defects. Muscle Nerve. 2017;55(2):277–281. doi: 10.1002/mus.25232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou Y., Zwolanek D., Izu Y., Gandhy S., Schreiber G., Brockmann K., Devoto M., Tian Z., Hu Y., Veit G., Meier M., Stetefeld J., Hicks D., Straub V., Voermans N.C., Birk D.E., Barton E.R., Koch M., Bonnemann C.G. Recessive and dominant mutations in COL12A1 cause a novel EDS/myopathy overlap syndrome in humans and mice. Hum. Mol. Genet. 2014;23(9):2339–2352. doi: 10.1093/hmg/ddt627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks D., Farsani G.T., Laval S., Collins J., Sarkozy A., Martoni E., Shah A., Zou Y., Koch M., Bonnemann C.G., Roberts M., Lochmuller H., Bushby K., Straub V. Mutations in the collagen XII gene define a new form of extracellular matrix-related myopathy. Hum. Mol. Genet. 2014;23(9):2353–2363. doi: 10.1093/hmg/ddt637. [DOI] [PubMed] [Google Scholar]
- 23.Delbaere S., Dhooge T., Syx D., Petit F., Goemans N., Destree A., Vanakker O., De Rycke R., Symoens S., Malfait F. Novel defects in collagen XII and VI expand the mixed myopathy/Ehlers-Danlos syndrome spectrum and lead to variant-specific alterations in the extracellular matrix. Genet. Med. 2020;22(1):112–123. doi: 10.1038/s41436-019-0599-6. [DOI] [PubMed] [Google Scholar]
- 24.Malfait F., Francomano C., Byers P., Belmont J., Berglund B., Black J., Bloom L., Bowen J.M., Brady A.F., Burrows N.P., Castori M., Cohen H., Colombi M., Demirdas S., De Backer J., De Paepe A., Fournel-Gigleux S., Frank M., Ghali N., Giunta C., Grahame R., Hakim A., Jeunemaitre X., Johnson D., Juul-Kristensen B., Kapferer-Seebacher I., Kazkaz H., Kosho T., Lavallee M.E., Levy H., Mendoza-Londono R., Pepin M., Pope F.M., Reinstein E., Robert L., Rohrbach M., Sanders L., Sobey G.J., Van Damme T., Vandersteen A., van Mourik C., Voermans N., Wheeldon N., Zschocke J., Tinkle B. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017;175(1):8–26. doi: 10.1002/ajmg.c.31552. [DOI] [PubMed] [Google Scholar]
- 25.Sun M., Chen S., Adams S.M., Florer J.B., Liu H., Kao W.W., Wenstrup R.J., Birk D.E. Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model. J. Cell Sci. 2011;124(Pt 23):4096–4105. doi: 10.1242/jcs.091363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishiyama T., McDonough A.M., Bruns R.R., Burgeson R.E. Type XII and XIV collagens mediate interactions between banded collagen fibers in vitro and may modulate extracellular matrix deformability. J. Biol. Chem. 1994;269(45):28193–28199. [PubMed] [Google Scholar]
- 27.Mienaltowski M.J., Adams S.M., Birk D.E. Tendon proper- and peritenon-derived progenitor cells have unique tenogenic properties. Stem Cell Res. Ther. 2014;5(4):86. doi: 10.1186/scrt475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C.F., Aschbacher-Smith L., Barthelery N.J., Dyment N., Butler D., Wylie C. Spatial and temporal expression of molecular markers and cell signals during normal development of the mouse patellar tendon. Tissue Eng. Part A. 2012;18(5–6):598–608. doi: 10.1089/ten.tea.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cadby J.A., Buehler E., Godbout C., van Weeren P.R., Snedeker J.G. Differences between the cell populations from the peritenon and the tendon core with regard to their potential implication in tendon repair. PLoS ONE. 2014;9(3):e92474. doi: 10.1371/journal.pone.0092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bader H.L., Keene D.R., Charvet B., Veit G., Driever W., Koch M., Ruggiero F. Zebrafish collagen XII is present in embryonic connective tissue sheaths (fascia) and basement membranes. Matrix Biol. 2009;28(1):32–43. doi: 10.1016/j.matbio.2008.09.580. [DOI] [PubMed] [Google Scholar]
- 31.Wessel H., Anderson S., Fite D., Halvas E., Hempel J., SundarRaj N. Type XII collagen contributes to diversities in human corneal and limbal extracellular matrices. Invest. Ophthalmol. Vis. Sci. 1997;38(11):2408–2422. [PubMed] [Google Scholar]
- 32.Sun M., Connizzo B.K., Adams S.M., Freedman B.R., Wenstrup R.J., Soslowsky L.J., Birk D.E. Targeted deletion of collagen V in tendons and ligaments results in a classic Ehlers-Danlos syndrome joint phenotype. Am. J. Pathol. 2015;185(5):1436–1447. doi: 10.1016/j.ajpath.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun M., Luo E.Y., Adams S.M., Adams T., Ye Y., Shetye S.S., Soslowsky L.J., Birk D.E. Collagen XI regulates the acquisition of collagen fibril structure, organization and functional properties in tendon. Matrix Biol. 2020;94:77–94. doi: 10.1016/j.matbio.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connizzo B.K., Freedman B.R., Fried J.H., Sun M., Birk D.E., Soslowsky L.J. Regulatory role of collagen V in establishing mechanical properties of tendons and ligaments is tissue dependent. J. Orthop. Res. 2015;33(6):882–888. doi: 10.1002/jor.22893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izu Y., Ansorge H.L., Zhang G., Soslowsky L.J., Bonaldo P., Chu M.L., Birk D.E. Dysfunctional tendon collagen fibrillogenesis in collagen VI null mice. Matrix Biol. 2011;30(1):53–61. doi: 10.1016/j.matbio.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Favata M. University of Pennsylvania; 2006. Scarless Healing in the Fetus: Implications and Strategies for Postnatal Tendon Repair. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.