Abstract
Application of organic acids via feed or drinking water is under discussion as a possible intervention strategy to reduce Campylobacter (C.) load in primary poultry production. A previous in vitro study showed that reduced concentrations of sorbic acid, benzoic acid, propionic acid, and acetic acid were required for antibacterial activity against Campylobacter when using a mixture of these 4 acids compared to when using the single acids. The present study aimed at determining the antibacterial efficiency of this combination in vivo as a drinking water additive for reducing shedding and intestinal C. jejuni colonization in broilers. Furthermore, we assessed whether the inoculated C. jejuni strain BfR-CA-14430 adapted in vivo to the applied organic acids. Results of this study showed that adding the organic acids consistently reduced Campylobacter loads in cloacal swabs. While significant reductions were observed within the entire study period, a maximum 2 log reduction occurred at an age of 18 d. However, after dissection at the end of the trial, no significant differences were detected in Campylobacter loads of cecal and colon contents compared to the control group. Susceptibility testing of re-isolates from cloacal swabs and cecal content revealed equal minimum inhibitory concentration (MIC) values compared to the inoculated test strain, suggesting that C. jejuni remained susceptible throughout the trial.
Key words: mitigation, colonization, resistance, adaptation, in vivo
INTRODUCTION
Campylobacteriosis was the most frequently reported foodborne gastrointestinal infection in the European Union (EU) in 2020 (EFSA, 2021) and poses a serious health risk to humans (Zautner et al., 2014). Broiler meat is considered to be the most important source for human infection, Campylobacter (C.) jejuni being the most frequently reported causative species (EFSA, 2021). Both intestinal colonization and external contamination of feathers and skin with Campylobacter have been shown to be sources for Campylobacter contaminating broiler carcasses during slaughter (Smith et al., 2007; Seliwiorstow et al., 2015a; Seliwiorstow et al., 2016). Recently, the European Food Safety Authority (EFSA) updated its previously published opinion on control options for Campylobacter. Interventions targeting Campylobacter at farm level that reduce intestinal Campylobacter concentrations by 2 log units (log10) colony-forming units (cfu) were estimated to reduce the public health risk by 42% compared to an estimated risk reduction of 76 to 98 % in a previous opinion from 2011 (EFSA, 2020). However, strategies applied at the beginning of the food chain offer the important advantage that their use in primary production can be easily combined with other measures during subsequent steps of the food production chain. Accordingly, combinations of control options targeting different stages of the food chain in a multiple-hurdle approach have been proposed to be more promising than the use of single measures (Klein et al., 2015; Alter and Klein, 2017; Kittler et al., 2021a). In the past years, research has focused on different Campylobacter mitigation strategies, such as bacteriophages, bacteriocins, or vaccines, with promising results in some studies (Stern et al., 2005; Neal-McKinney et al., 2014; Robyn et al., 2015; Meunier et al., 2017; Richards et al., 2019; Kittler et al., 2021b). A previous study conducted by Neal-McKinney et al. (2014) investigated the protective effect of Campylobacter antibodies after vaccination based on recombinant surface-exposed proteins. The authors observed a 2 log10 reduction in Campylobacter counts (Neal-McKinney et al., 2014). Wagle et al. (2017) tested the use of the phytochemical b-resorcyclic acid in an in vivo study. In this former study, the application significantly reduced cecal Campylobacter concentrations by ∼2.5 and 1.7 log10 cfu/g. However, there are as yet no approved products authorized for industrial use in poultry flocks (EFSA, 2020). In contrast, organic acids, such as sorbic acid or propionic acid are directly applicable, as they have already been approved as feed and drinking water additives in animal production (Jansen et al., 2014; Guyard-Nicodème et al., 2016; European Commission 2019). In addition, organic acid treatment is relatively inexpensive and can easily be administered via feed or drinking water (Mani-López et al., 2012; Meunier et al., 2016).
Previous in vivo studies investigated the antibacterial effect of organic acid supplements for feed or water on Campylobacter reduction, but results are contradictory (Solis de los Santos et al., 2008; Van Deun et al., 2008; Skånseng et al., 2010; Metcalf et al., 2011; Hermans et al., 2012). There is some evidence that the use of combined organic acids might be advantageous compared to the treatment with single organic acids. In fact, previous in vitro studies showed that combined organic acids exhibited synergistic activities against Campylobacter and Escherichia coli (Chaveerach et al., 2002; Kim and Rhee, 2013; Peh et al., 2020). Furthermore, adding a combination of formic acid and potassium sorbate to feed was shown to prevent C. jejuni colonization in broilers, whereas treatment with a single organic acid failed (Skånseng et al., 2010). Nonetheless, to our knowledge, in vivo studies investigating the antibacterial effect of systematically developed combinations of organic acids have not yet been published. Moreover, there are no published data on in vivo adaptive responses of Campylobacter although the ability to develop enhanced tolerances to organic acids has been shown in in vitro studies (Birk et al., 2012; Peh et al., 2021). New insights into the occurrence and development of decreased susceptibility of Campylobacter to organic acids might contribute to improved application schemes.
Therefore, the aim of the present study was to investigate the suitability of organic acids as a future component in a multiple-hurdle approach to reduce Campylobacter in broiler flocks. The present study focused 1) on the in vivo antibacterial effect of a systematically developed drinking water additive against Campylobacter colonization in broilers, and 2) on the monitoring of adaptive responses of Campylobacter during the in vivo animal experiment.
MATERIALS AND METHODS
Ethics
This study was carried out in accordance with the National Animal Protection Guidelines. The protocol was reviewed and approved by the German Animal Ethics Committee for the Protection of Animals of the Regional Office for Health and Social Affairs Berlin (“Landesamt für Gesundheit und Soziales”, LAGeSo, registration number G 0098/18). All applicable national and institutional guidelines of the Freie Universität Berlin for the care and use of animals were followed. Animal treatments approved by LAGeSO were classified as being of minor distress (minor pain with short duration).
Study Design
The animal trials were performed in the experimental facility of the Center for Infection Medicine of the Department for Veterinary Medicine of the Freie Universität Berlin, Germany. In total, 180 broiler hatching eggs (aerosol disinfected with formalin) of breed Ross 308 were received from a commercial hatchery in Germany. Immediately after arrival, the eggs were disinfected again using WESSOCLEAN K 50 Gold Line containing 2.37% hydrogen peroxide and 0.015% peracetic acid (Wesso AG, Hersbruck, Germany). Afterwards, the eggs were incubated in a hatching incubator (Easy 250; J. Hemel Brutgeräte GmbH & Co. KG, Verl, Germany) for 21 d until hatching. Facilities for animal keeping were cleaned, disinfected using evaporated H2O2, and tested for the absence of Campylobacter as described by Szott et al. (2020). Hatched broilers of both sexes (n = 180) were randomly selected and housed in 2 separate experimental rooms. Each group consisted of 90 chickens: a positive control group (challenged with C. jejuni, receiving drinking water without supplementation) and an experimental organic acid group (challenged with C. jejuni, receiving drinking water supplemented with a drinking water additive comprising 4 organic acids). Within these 2 groups, the 90 broiler chickens were randomly assigned to one of the following categories: 1) seeder (n = 18), 2) sentinels (n = 36), and 3) stocking density broilers (n = 36). The affiliation of the chickens to the respective category was ensured by attaching an individual sequential number (individual tagging). Each pen in the experimental facility was supplied with filtered air using an HEPA filter and equipped with a temperature control maintained by an electronic thermometer sensor, and a programmable light regimen. Aiming to imitate a commercial broiler husbandry environment, broilers were placed in the barn with fresh litter at a stocking density of 39 kg/m². Commercial broiler feed and filtered water from the municipal water supply were provided ad libitum during the entire study period. Water samples were routinely obtained every 4 to 8 wk to check the water quality. The results of the external testing laboratory showed that the water was of drinking water quality. Feed was offered in commercially available poultry troughs, and filtered water was given via nipple drinkers and changed twice a day. The organic acids were added to the drinking water of the experimental organic acid group as described below. The feed comprised a commercial standard 3-phase feeding program for broilers as shown in Table 1.
Table 1.
Ingredients and nutrient contents of the experimental diets.
| Components per kg | Starter diet (0–8 days) |
Grower diet (9–26 days) |
Finisher diet (27–33 days) |
|---|---|---|---|
| Crude protein (%) | 21.5 | 21.0 | 20.0 |
| Crude lipids (%) | 4.9 | 6.4 | 5.5 |
| Crude fiber (%) | 2.9 | 3.4 | 3.3 |
| Crude ash (%) | 5.3 | 5.1 | 4.9 |
| ME, kcal/kg | 2,961.7 | 2,961.7 | 2,961.7 |
| Calcium (%) | 0.9 | 0.8 | 0.8 |
| Phosphorous (%) | 0.6 | 0.6 | 0.5 |
| Sodium (%) | 0.1 | 0.1 | 0.1 |
| Methionine (%) | 0.6 | 0.5 | 0.5 |
| Lysine (%) | 1.3 | 1.2 | 1.1 |
Animal health parameters and weight gain were monitored daily. At the end of the trial, broilers were euthanized, and samples from cecal and colonic contents of the sentinels were collected for enumeration of Campylobacter.
Bacterial Strain and Broiler Challenge
The C. jejuni strain BfR-CA-14430, originally isolated from chicken breast, was provided by the Federal Institute for Risk Assessment (BfR) and used for experimental inoculation. The strain was stored and prepared for experimental challenge as described by Szott et al. (2020). Each seeder was orally inoculated with 500 µL containing approximately 2.2 × 104 cfu of the C. jejuni strain BfR-CA-14430 10 d post hatch. For control purposes, the concentration of the inoculum was determined before and immediately after oral inoculation of the seeders. For this, 10-fold dilutions were plated on modified Campylobacter-selective charcoal cefoperazone deoxycholate agar (mCCDA) plates prepared from Campylobacter blood-free selective agar base (CM0739; Oxoid Deutschland GmbH, Wesel, Germany) and CCDA selective supplement (SR0155; Oxoid Deutschland GmbH). After a 48-h incubation period at 37°C under microaerobic conditions (85% N2, 10% CO2, 5% O2), colonies on plates containing 30 to 300 colonies were counted for Campylobacter enumeration.
Combination of Organic Acids Used as a Water Additive
Based on the results of a previous in vitro study (Peh et al., 2020), a combination of sorbic acid, benzoic acid, propionic acid (Carl Roth GmbH + Co. KG, Karlsruhe, Germany), and acetic acid (E. Merck KG, Darmstadt, Germany) was selected for this study. All organic acids included are listed as authorized feed additives in the European Union (European Commission, 2019). A stock solution was prepared in autoclaved tap water at a total organic acid concentration of 480 mmol/L. The combination of organic acids was stored for up to 3 wk during the experiments. During storage and application, regular macroscopic checks were made to ensure that no precipitation of the organic acids occurred. Previous experiments showed that the MIC values of the organic acid mixture were constant during 4 wk of storage (data not shown), suggesting a stable antibacterial activity for the storage period of the present in vivo experiment. In order to achieve constant concentrations of organic acids in the drinking water, the application procedure was standardized. The water was freshly prepared and changed every 12 h. Before dosing, the stock solution was shaken vigorously before being added to the water and the required volumes were measured precisely using volumetric flasks. The organic acids were administered at a dilution of 1:30 via drinking water to achieve final concentrations of 6.4 mmol/L for sorbic acid, 4.8 mmol/L for benzoic acid, 3.2 mmol/L for propionic acid, and 1.6 mmol/L for acetic acid. Adding the acids to the drinking water adjusted the water to pH 6.0.
For susceptibility testing of re-isolates, Campylobacter colonies were isolated from cloacal swabs or cecal content during the animal experiment; 2 stock solutions of the organic acids in combination were prepared in cation-adjusted Mueller Hinton broth (CAMH, Carl Roth GmbH + Co. KG). The total organic acid concentration was 64 mmol/L, comprising 25.6 mmol/L sorbic acid, 19.2 mmol/L benzoic acid, 12.8 mmol/L propionic acid, and 6.4 mmol/L acetic acid. Stock solutions were adjusted to pH 7.3 or pH 6.0 using 2 mol/L and 8 mol/L sodium hydroxide, and a total of 11 two-fold serial dilutions were prepared in CAMH broth.
Sampling Design and Sample Analysis
Prior to oral inoculation of the seeders, at the fourth day post hatch, absence of Campylobacter was confirmed by cloacal swabbing (Sarstedt AG & Co. KG, Nümbrecht, Germany) of all 180 broilers. Seeders were verified to excrete C. jejuni 2 days postinoculation (dpi) (12 d post hatch) by qualitative analysis of cloacal swabs.
Throughout the study, Campylobacter colonization of the sentinels was determined by semiquantitative analysis of cloacal swabs. At the end of the trial, Campylobacter load in cecal and colonic contents of the broiler chickens was determined by semiquantitative analysis.
Semiquantitative analysis of Campylobacter was conducted using cloacal swabs which were taken at defined time points: 3 and 4 dpi, and subsequently twice a week (equivalent to 8, 11, 16, and 18 dpi) until necropsy. To ensure comparability of results, the same 36 sentinels (noninoculated, but naturally colonized with C. jejuni through contact with the seeders) were sampled in both groups. Cloacal swabs were analyzed semiquantitatively in accordance with DIN EN ISO 10272-3. Briefly, the standardized sampling procedure was as follows: cloacal swabs were inserted in the cloaca, rotated 5 times, removed, and immediately transferred to 3.0 mL Preston broth. Thereafter, cloacal swabs were homogenized for 3 s using a vortex shaker (VWR International GmbH, Darmstadt, Germany), allowing the fecal material to detach and evenly disperse in the medium. Afterward, cloacal swabs were serially diluted 10-fold in Preston broth (up to 10−8), incubated 24 h at 37°C under microaerophilic conditions, and then streaked out on quartered mCCDA plates using 10 µL inoculation loops (Sarstedt AG & Co. KG). Plates were incubated for another 48 h at 37°C under microaerophilic conditions and examined for C. jejuni growth. Presumptive colonies were examined microscopically for morphology and motility. Additionally, colonies were subcultured onto 5% sheep Columbia blood agar (Fisher Scientific, Germany) and then incubated for 24 h at 37°C under microaerophilic conditions. Afterward, colonies were analyzed using a Bruker Microflex system for matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). The highest evaluable dilution on mCCDA plates with confirmed Campylobacter growth was then used to calculate the MPN (most probable number) value using an MPN table modified according to ISO/TS 10272-3:2010/Cor.1:2011(E).
At the end of the growth period (as defined by an average bird's weight of 2.0 kg) at d 23 postinfection, all 36 sentinels per group were euthanized using ZKS poultry pliers (Corstechnology UG, Neerstedt, the Netherlands) after confirming deep anesthesia as indicated by muscle relaxation, absence of the corneal reflex, and absence of the eyelash reflex. The animals were dissected and cecal and colonic contents were collected for subsequent C. jejuni enumeration. For semiquantitative analysis, the intestinal contents were removed aseptically, diluted 1:8 in Preston broth, homogenized, and 10-fold serially diluted in Preston broth (up to 10−9). For enrichment, dilutions were incubated for 24 h at 37°C under microaerobic conditions. Approximately 2 µL of each dilution was streaked out on quartered mCCDA plates using an inoculation loop. After incubation for 48 h at 37°C under microaerobic atmosphere, the highest dilution showing bacterial growth was used for calculating the most probable number of bacterial counts.
Susceptibility Testing of Re-isolates In Vitro
A total of 90 Campylobacter re-isolates were collected during the animal trial to determine their susceptibility to the previously administered organic acids. Briefly, the re-isolates were selected from the mCCDA plates used for Campylobacter quantification as follows: 1) 18 presumptive Campylobacter colonies were isolated from cloacal swabs of each seeder bird (sampled 2 dpi), 1) 36 Campylobacter colonies were isolated from cloacal swabs of each sentinel bird (sampled 11 dpi), and 3) 36 colonies from the cecal content of each sentinel bird were collected during necropsy (sampled 23 dpi). Colonies were transferred to tubes containing 1 mL of skimmed milk and stored at −80°C as described earlier (Kittler et al., 2013, 2014). Prior to susceptibility testing, re-isolates were plated out on Columbia agar supplemented with sheep blood and incubated for 48 h at 42 ± 1°C under microaerobic conditions.
The minimum inhibitory concentration (MIC) values were determined as described earlier (Peh et al., 2020). In brief, susceptibility tests were performed using U-shaped bottom 96-well microtiter plates (Sarstedt AG & Co. KG). Fifty µL of the bacterial inocula at concentrations of 1 × 106 cfu/mL were dispensed into wells containing 50 µL of the double concentrated organic acid mixture to achieve final bacterial concentrations of 5×105 cfu/mL. After 48 h of incubation at 42 ± 1°C under microaerobic conditions, the lowest concentration that inhibited visible growth of bacteria was assessed. The susceptibility of all 90 re-isolates was tested at pH 7.3. Additionally, MIC values of 18 randomly selected re-isolates collected 20 d post-hatch and isolated from cecal content during dissection were determined at pH 6.0.
Statistical Analysis
The experimental data were analyzed using SPSS software version 25.0 for Windows (SPSS, Inc., Chicago, IL). Data were analyzed for normal distribution using the Shapirow-Wilk Test. As data were not normally distributed, we used the non-parametric Mann-Whitney U test. Campylobacter counts were logarithmically transformed (log10) and then analyzed for significant differences using the non-parametric Mann-Whitney U test. P-values below 0.05 were regarded as statistically significant. To ensure an alpha error of 0.05, a beta error of 0.18, and power of 0.80, a total of 90 animals per group were included in the present study. In order to determine statistically significant differences, 36 animals were sampled during the experiment, and the differences calculated by using a biologically relevant difference of delta = 1 log unit between Campylobacter counts of the groups and assuming a standard deviation of 1 log unit.
RESULTS
In Vivo Effect of Organic Acids on Campylobacter Colonization
Four days post hatch, broilers were confirmed to be Campylobacter free by microbial analysis of swabs. Eight days postinoculation, Campylobacter was detected in samples of all 72 sentinels.
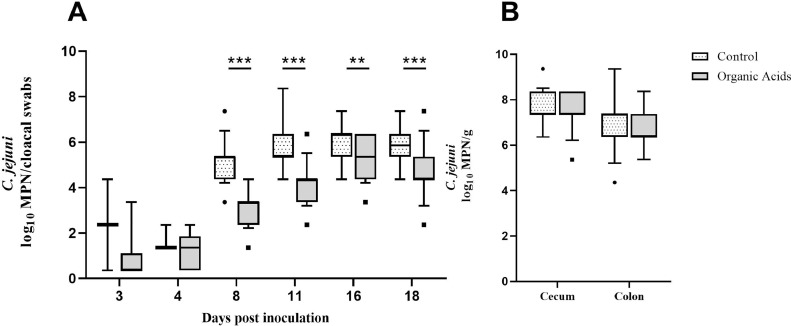
Significantly reduced Campylobacter counts were detected in cloacal swabs of the experimental group receiving the organic acids compared to the control group at d 8, 11, 16, and 18 postinoculation (P ≤ 0.003; Figure 1A). A maximum 2.0-log (P < 0.0001; r = 0.81) and 1.0-log reduction (P < 0.0001; r = 0.7) in C. jejuni counts were detected in the experimental group (Md = 3.36 and 4.36 log10 MPN/cloacal swabs) 8 and 11 dpi in comparison to the control group (Md = 5.36 and 5.36 log10 MPN/cloacal swabs). Slightly lower 1.0 and 1.5-log reductions (16 dpi p = 0.003; r = 0.35 and 18 dpi P < 0.0001; r = 0.65) were observed 16 and 18 dpi in the experimental group (Md = 5.36 and 4.36 log10 MPN/cloacal swabs) compared to the control group (Md = 6.36 and 5.86 log10 MPN/cloacal swabs).
Figure 1.
Campylobacter (C.) jejuni colonization of 36 sentinels per group determined by semiquantitative analysis (min to max). (A) C. jejuni counts in log10 most probable number (MPN) in cloacal swabs derived from sentinels confirmed to shed C. jejuni at distinct time points after oral inoculation of the seeders at d 10. Three and four days postinoculation (dpi), 2 (3 dpi) and 3 (4 dpi) sentinels of the control group and 6 (3 dpi) and 21 (4 dpi) sentinels of the experimental group shed C. jejuni. From eight dpi onwards, samples of all 36 sentinels were Campylobacter-positive. (B) C. jejuni counts in log10 MPN per gram in intestinal content of 36 sentinels per group upon necropsy (23 dpi). White dotted boxes represent the control group (broilers challenged with C. jejuni and not treated with a combination of organic acids); gray boxes represent the experimental group challenged with C. jejuni and treated with a combination of organic acids. Medians (bold line) and significance levels (P values) determined by the Mann-Whitney U test are indicated. * (P < 0.05), ** (P < 0.01), *** (P < 0.001).
No reduction in Campylobacter counts was observed in cecal content sampled 23 dpi (P > 0.05; Figure 1B).
Campylobacter Re-isolates Exhibited Minimum Inhibitory Concentration Values Equal to the Inoculated Test Strain
A total of 90 Campylobacter re-isolates collected at 3 different time points from samples of the animal experiment were tested for their susceptibility to the drinking water additive and its individual components. All tested re-isolates showed MIC values equal to those of the initially inoculated C. jejuni strain BfR-CA-14430 both at pH 7.3 and pH 6.0. At pH 7.3, MIC values of 1.6 mmol/L for sorbic acid, 1.2 mmol/L for benzoic acid, 0.8 mmol/L for propionic acid, and 0.4 mmol/L for acetic acid were determined. At pH 6.0, both re-isolates and the test strain exhibited MIC values of 0.4 mmol/L for sorbic acid, 0.3 mmol/L for benzoic acid, 0.2 mmol/L for proprionic acid, and 0.1 mmol/L for acetic acid.
Application of Organic Acids Showed No Adverse Effects on Broiler Growth Performance
At the end of the trial, no significant difference in the mean final body weight of sentinel birds of the experimental (1.84 kg) and the control group (1.87 kg) were observed (P > 0.05).
DISCUSSION
In this study, we examined the in vivo efficacy of a drinking water additive to reduce the intestinal Campylobacter colonization in broilers. The mixture consisting of sorbic acid, benzoic acid, propionic acid, and acetic acid was supplied during the entire growth period until slaughter age. By using a seeder bird model, we aimed to use an experimental set-up that imitated the natural spread of Campylobacter colonization in conventional broiler flocks as far as possible. Moreover, we included investigations on adaptive responses of Campylobacter to the applied organic acids. For this purpose, we assessed the MIC values of the administered organic acids in C. jejuni re-isolates after an intestinal passage in broilers.
The selection and proportions of organic acids as well as their final concentrations in the broilers’ drinking water were chosen based on results of a systematic approach from a previous in vitro study (Peh et al., 2020). When tested on a panel of 20 C. jejuni isolates, the selected combination of organic acids showed synergistic activities against 5 isolates, including the C. jejuni strain BfR-CA-14430 used in the present in vivo study. Furthermore, the MIC values of the organic acids decreased 2.5- (sorbic acid) to 160-fold (acetic acid) compared to those determined at pH 7.3 for the individual substances on the test strain (Peh et al., 2020). In this in vivo study, we decided to administer a drinking water additive containing the organic acids at final concentrations four-fold higher than the MIC values determined for C. jejuni strain BfR-CA-14430 at pH 7.3 (16-fold higher than the MIC values at pH 6.0). All of the organic acids were applied at concentrations lower than those indicated to cause adverse effects on broiler performance (Metcalf et al., 2011). Similar to the present study, organic acids and botanicals were administered at concentrations 2- to 8-fold higher than their MIC values for testing their antifungal and antibacterial effects in other in vivo studies (Chami et al., 2005; Grilli et al., 2013; Mousavi et al., 2020).
The results of the present in vivo study are encouraging, since the Campylobacter shedding was consistently reduced during the third and fourth fattening week, reaching a maximum 2-log reduction at d 8 postinoculation. However, the drinking water additive failed to diminish Campylobacter colonization in the intestinal colonic and cecal contents at the end of the trial. The reason for this finding remains unclear.
One possible explanation for the limited effectiveness of the organic acids might be due to decreased concentrations of the organic acids in the course of the intestinal tract. Similar effects have been shown in previous studies that were caused by different absorption and metabolization processes (Hume et al. 1993; Thompson and Hinton 1997; Hermans et al. 2012). In contrast to these results, several studies detected reduced Campylobacter concentrations in the cecum after administering organic acids (Solis de Los Santos et al. 2008; Skånseng et al. 2010; Jansen et al. 2014). If we assume that in our experiment only low or very low concentrations of the supplemented acids reach the cecum, the indirect effects on Campylobacter load might be an interesting factor. While we did not include any analysis on the immune status of the chickens, in other studies, organic acids induced the formation of immunoglobulin Y (IgY) (Park et al. 2009), which was reported to induce inhibition of Campylobacter colonization (Vandeputte et al. 2019; Nothaft et al. 2021).
Another explanation for the results is that the in vivo efficacy of organic acids might have decreased over an extended period of time. This would be in agreement with results of a previous in vivo study where different commercially available feed additives were administered during the entire rearing period (Guyard-Nicodème et al., 2016). Three dpi, the authors of the aforementioned study observed that 4 of 5 organic acids blends significantly decreased cecal Campylobacter counts, whereas after 24 and 31 dpi, only one mixture remained significantly efficient (Guyard-Nicodème et al., 2016). Similarly, Ren et al. (2021) found no sustained reduction in Campylobacter counts after fortifying the drinking water of broiler chickens with malic acid for three weeks during rearing. This observation might be explained by the development of an increased tolerance in the test strain to the administered organic acids over time, similar to the emergence of resistant Campylobacter during treatment with antibiotics (McDermott et al., 2002; Luo et al., 2005; Ladely et al., 2007; Lin et al., 2007). Similarly, a previous in vitro study demonstrated a stepwise adaptation to propionic acid and sorbic acid for 2 C. jejuni field isolates, resulting in 2-fold higher MIC values compared to the wild-type isolates (Peh et al., 2021). However, susceptibility testing of 90 re-isolates collected during the animal trial showed no evidence of an organic acid-tolerant Campylobacter population. It is, therefore, rather unlikely that the missing efficacy in cecal and colonic contents was due to adaptive responses in Campylobacter.
A previous study showed that Campylobacter counts may differ significantly between sample types (Seliwiorstow et al., 2015b). While quantitative analysis of intestinal content or droppings are the gold standard, selective sampling of sentinels is required for analysis of natural colonization models as used in our study. Fecal or cloacal sampling cannot be conducted in certain animals unless dissection or isolation of animals is used. This would require huge animal numbers or remove the desired practical conditions. Isolating seeder and sentinel broilers for a considerable time would have interfered with the experimental seeder bird model, aiming at a natural intestinal colonization and keeping conditions close to commercial poultry farming. Due to these considerations, we chose to use cloacal swabs for sampling and a semiquantitative approach for the enumeration of Campylobacter. This type of sampling ensured the sampling of “naturally” colonized sentinels and thus the examination of the individual course of each of the 36 sentinels (by assigning the samples to the tag-number). Furthermore, it allowed us to include a large sample size in our study. To overcome the issue of varying amounts of feces adhering to the swab, a standardized sampling and processing procedure was used to obtain comparable and reproducible data. The reproducibility and precision of the presented data are satisfactory, as in the control group, the Campylobacter counts in cloacal swabs were consistently homogeneous regardless of the sampling time (11, 16, and 18 days after inoculation). In agreement, no statistical difference was observed between enumeration by the semi-quantitative and quantitative technique for comparable concentrations of thermotolerant Campylobacter (P = 0.104) (Rosenquist et al., 2007). Perdoncini et al. (2022) also observed no significant differences (P > 0.05) in the detection and quantification of Campylobacter for either the source of isolation (cloacal swabs, carcasses, water) or the technique used (direct plating, MPN technique, and qPCR). Similarly, another research group found no significant differences (P > 0.05) between results obtained by direct plating of carcass rinse samples and an MPN procedure (Line et al., 2001). Likewise, Scherer et al. (2006) found a highly positive correlation coefficient of 0.9 between direct plating and the MPN technique.
Regarding the risk of foodborne infections, Campylobacter load in the intestinal segments of cecum/colon, and cloaca need to be considered, as previous studies could show that both colonized fecal shedding and/or intestinal content can result in Campylobacter-contaminated broiler carcasses. For example, Rosenquist et al. (2006) and Reich et al. (2008) found that Campylobacter counts on broiler carcasses correlated significantly with bacterial concentrations in cecal contents. Russell (2003) observed that a cecal cut occurred in 0 to 8% of 200 investigated broiler carcasses in one processing plant. Leakage of intestinal contents usually takes place when processing machines are not programmed to detect carcass size deviations among broiler batches. In contrast, other studies did not observe a significant correlation between cecal Campylobacter counts and carcass contamination (Seliwiorstow et al., 2015a, 2016), whereas the external contamination of feathers and skin with Campylobacter was shown to be an important source of carcass contamination during the slaughter process (Smith et al., 2007; Seliwiorstow et al., 2015a, 2016). Furthermore, a previous study demonstrated that the shedding of Campylobacter in feces from the cloaca during slaughter led to contamination of broiler carcasses during defeathering (Berrang et al., 2001). Thus, although there was no effect on colonic and cecal concentrations in our study, reduced Campylobacter levels in the feces might contribute to lower contamination levels of broiler carcasses during the slaughter process. However, a strict implementation of biosecurity measures and HACCP during slaughter and processing are necessary to avoid cross contamination between different slaughter batches.
In conclusion, the present study showed that a combination of sorbic acid, benzoic acid, propionic acid, and acetic acid applied via drinking water significantly reduced cloacal Campylobacter concentration in broilers in vivo, which might contribute to reduced entry of Campylobacter into the food chain. However, the drinking water additive failed to reduce Campylobacter concentrations in the cecum and colon of 33-day-old broilers at the end of the trial. Susceptibility testing of re-isolates collected at different stages of the animal experiment revealed no evidence of an organic-acid tolerant Campylobacter population during the long-term treatment with organic acids. Further research is needed to evaluate the effect of the organic acids in large-scale field studies and multiple-hurdle approaches.
ACKNOWLEDGMENTS
This study was supported by the German Federal Ministry of Education and Research (BMBF) within the framework of the consortium “PAC-Campy” (IP5/01Kl1725C and IP4/01KI1725A). This Open Access publication was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) within the program LE 824/10-1 “Open Access Publication Costs” and University of Veterinary Medicine Hannover, Foundation, Hannover, Germany.
We gratefully acknowledge the German Federal Institute for Risk Assessment, Berlin, Germany for providing the C. jejuni isolate for the study. We would also like to thank the colleagues at the Institute for Animal Hygiene and Environmental Health, Freie Universität Berlin, Germany, and the Institute for Food Quality and Food Safety, University of Veterinary Medicine Hannover, Foundation, Hannover, Germany for their excellent technical support. Our thanks go to Dr. Roswitha Merle and Dr. Racem Romdhane at the Institute for Veterinary Epidemiology and Biostatistics Center, Freie Universität Berlin, Berlin, Germany for their help with statistical animal experiments group scheduling.
Authors’ contributions: VS and EP performed the experiments, collected, analyzed, interpreted the data, and drafted the manuscript and figures with critical evaluation and support from all authors. SK, AF, UR, CK, and MP conceived and designed the experimental study as well as critically revising the manuscript. All authors approved the final version to be published.
DISCLOSURES
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alter, T. 2017. Prevention and mitigation strategies for Campylobacter with focus on poultry production. In: Klein G, editor. Page 111–129 in Cambridge, MA: Academic Press.
- Berrang M.E., Buhr R.J., Cason J.A., Dickens J.A. Broiler carcass contamination with Campylobacter from feces during defeathering. J. Food Prot. 2001;12:2063–2066. doi: 10.4315/0362-028x-64.12.2063. [DOI] [PubMed] [Google Scholar]
- Birk T., Wik M.T., Lametsch R., Knochel S. Acid stress response and protein induction in Campylobacter jejuni isolates with different acid tolerance. BMC Microbiol. 2012;12:174. doi: 10.1186/1471-2180-12-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chami N., Bennis S., Chami F., Aboussekhra A., Remmal A. Study of anticandidal activity of carvacrol and eugenol in vitro and in vivo. Oral Microbiol. Immunol. 2005;2:106–111. doi: 10.1111/j.1399-302X.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- Chaveerach P., Keuzenkamp D.A., Urlings H.A., Lipman L.J., van Knapen F. In vitro study on the effect of organic acids on Campylobacter jejuni/coli populations in mixtures of water and feed. Poult. Sci. 2002;5:621–628. doi: 10.1093/ps/81.5.621. [DOI] [PubMed] [Google Scholar]
- EFSA. 2020. Update and review of control options for Campylobacter in broilers at primary production. EFSA J. 4:89. [DOI] [PMC free article] [PubMed]
- EFSA. 2021. The European Union One Health 2020 Zoonoses Report. EFSA J. 19:6971, 324 pp. [DOI] [PMC free article] [PubMed]
- European Commission. Regulation (EC) No 1831/2003. European Union register of feed additives. Edition 7/2019 (273). Annex I - 07.08.2019.
- Grilli E., Vitari F., Domeneghini C., Palmonari A., Tosi G., Fantinati P., Massi P., Piva A. Development of a feed additive to reduce caecal Campylobacter jejuni in broilers at slaughter age: from in vitro to in vivo, a proof of concept. J. Appl. Microbiol. 2013;2:308–317. doi: 10.1111/jam.12053. [DOI] [PubMed] [Google Scholar]
- Guyard-Nicodème M., Keita A., Quesne S., Amelot M., Poezevara T., Berre B.Le, Sánchez J., Vesseur P., Pedel Á.M.P., Chemaly M. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period. Poult. Sci. 2016;2:298–305. doi: 10.3382/ps/pev303. [DOI] [PubMed] [Google Scholar]
- Hermans D., Martel A., Garmyn A., Verlinden M., Heyndrickx M., Gantois I., Haesebrouck F., Pasmans F. Application of medium-chain fatty acids in drinking water increases Campylobacter jejuni colonization threshold in broiler chicks. Poult. Sci. 2012;91:1733–1738. doi: 10.3382/ps.2011-02106. [DOI] [PubMed] [Google Scholar]
- Hume M.E., Corrier D.E., Ivie G.W., Deloach J.R. Metabolism of [14C]propionic acid in broiler chicks. Poult. Sci. 1993;5:786–793. doi: 10.3382/ps.0720786. [DOI] [PubMed] [Google Scholar]
- Jansen W., Reich F., Klein G. Large-scale feasibility of organic acids as a permanent preharvest intervention in drinking water of broilers and their effect on foodborne Campylobacter spp. before processing. J. Appl. Microbiol. 2014;6:1676–1687. doi: 10.1111/jam.12490. [DOI] [PubMed] [Google Scholar]
- Kim S.A., Rhee M.S. Marked synergistic bactericidal effects and mode of action of medium-chain fatty acids in combination with organic acids against Escherichia coli O157:H7. Appl. Environ. Microbiol. 2013;21:6552–6560. doi: 10.1128/AEM.02164-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler S., Fischer S., Abdulmawjood A., Glunder G., Klein G. Effect of bacteriophage application on Campylobacter jejuni loads in commercial broiler flocks. Appl. Environ. Microbiol. 2013;79:7525–7533. doi: 10.1128/AEM.02703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler S., Fischer S., Abdulmawjood A., Glünder G., Klein G. Colonisation of a phage susceptible Campylobacter jejuni population in two phage positive broiler flocks. PloS one. 2014;9 doi: 10.1371/journal.pone.0094782. e94782-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler S., Shakeri G., Peh E., Plötz M. A One Health perspective on a multi-hurdle approach to combat Campylobacter spp. in broiler meat. Curr. Clin. Microbiol Rep. 2021;2:49–61. [Google Scholar]
- Kittler S., Steffan S., Peh E., Plötz M. Phage biocontrol of Campylobacter: a One Health approach. Curr. Top. Microbiol. Immunol. 2021;431:127–168. doi: 10.1007/978-3-030-65481-8_6. [DOI] [PubMed] [Google Scholar]
- Klein G., Jansen W., Kittler S., Reich F. Mitigation strategies for Campylobacter spp. in broiler at pre-harvest and harvest level. Berl. Munch Tierarztl. Wochenschr. 2015;3-4:132–140. [PubMed] [Google Scholar]
- Ladely S.R., Harrison M.A., Fedorka-Cray P.J., Berrang M.E., Englen M.D., Meinersmann R.J. Development of macrolide-resistant Campylobacter in broilers administered subtherapeutic or therapeutic concentrations of tylosin. J. Food Prot. 2007;8:1945–1951. doi: 10.4315/0362-028x-70.8.1945. [DOI] [PubMed] [Google Scholar]
- Lin J., Yan M., Sahin O., Pereira S., Chang Y.J., Zhang Q. Effect of macrolide usage on emergence of erythromycin-resistant Campylobacter isolates in chickens. Antimicrob. Agents Chemother. 2007;5:1678–1686. doi: 10.1128/AAC.01411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Line J.E., Stern N.J., Lattuada C.P., Benson S.T. Comparison of methods for recovery and enumeration of Campylobacter from freshly processed broilers. J. Food Prot. 2001;64:982–986. doi: 10.4315/0362-028x-64.7.982. [DOI] [PubMed] [Google Scholar]
- Luo N., Pereira S., Sahin O., Lin J., Huang S., Michel L., Zhang Q. Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proc. Natl. Acad. Sci. U. S. A. 2005;3:541–546. doi: 10.1073/pnas.0408966102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani-López E., García H.S., López-Malo A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012;45:713–721. [Google Scholar]
- McDermott P.F., Bodeis S.M., English L.L., White D.G., Walker R.D., Zhao S., Simjee S., Wagner D.D. Ciprofloxacin resistance in Campylobacter jejuni evolves rapidly in chickens treated with fluoroquinolones. J. Infect. Dis. 2002;6:837–840. doi: 10.1086/339195. [DOI] [PubMed] [Google Scholar]
- Metcalf J.H., Donoghue A.M., Venkitanarayanan K., Reyes-Herrera I., Aguiar V.F., Blore P.J., Donoghue D.J. Water administration of the medium-chain fatty acid caprylic acid produced variable efficacy against enteric Campylobacter colonization in broilers. Poult. Sci. 2011;90:494–497. doi: 10.3382/ps.2010-00891. [DOI] [PubMed] [Google Scholar]
- Meunier M., Guyard-Nicodeme M., Dory D., Chemaly M. Control strategies against Campylobacter at the poultry production level: biosecurity measures, feed additives and vaccination. J. Appl. Microbiol. 2016;120:1139–1173. doi: 10.1111/jam.12986. [DOI] [PubMed] [Google Scholar]
- Meunier M., Guyard-Nicodème M., Vigouroux E., Poezevara T., Beven V., Quesne S., Bigault L., Amelot M., Dory D., Chemaly M. Promising new vaccine candidates against Campylobacter in broilers. PLoS One. 2017;11:14. doi: 10.1371/journal.pone.0188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi S., Schmidt A.M., Escher U., Kittler S., Kehrenberg C., Thunhorst E., Bereswill S., Heimesaat M.M. Carvacrol ameliorates acute campylobacteriosis in a clinical murine infection model. Gut Pathog. 2020;12:2. doi: 10.1186/s13099-019-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal-McKinney J.M., Samuelson D.R., Eucker T.P., Nissen M.S., Crespo R., Konkel M.E. Reducing Campylobacter jejuni colonization of poultry via vaccination. PLoS One. 2014;12:19. doi: 10.1371/journal.pone.0114254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothaft H., Perez-Muñoz M.E., Yang T., Murugan A.V.M., Miller M., Kolarich D., Plastow G.S., Walter J., Szymanski C.M. Improving chicken responses to glycoconjugate vaccination against Campylobacter jejuni. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.734526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K., Rhee A., Um J., Paik I.K. Effect of dietary available phosphorus and organic acids on the performance and egg quality of laying hens. J. Appl. Poult. Res. 2009;15:13. [Google Scholar]
- Peh E., Kittler S., Reich F., Kehrenberg C. Antimicrobial activity of organic acids against Campylobacter spp. and development of combinations—a synergistic effect? PLoS One. 2020;9:13. doi: 10.1371/journal.pone.0239312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peh E., Kittler S., Seinige D., Valero A., Kehrenberg C. Adaptation of Campylobacter field isolates to propionic acid and sorbic acid is associated with fitness costs. J. Appl. Microbiol. 2021;4:1749–1761. doi: 10.1111/jam.15057. [DOI] [PubMed] [Google Scholar]
- Perdoncini G., Sierra Arguello Y.M., Moreira Lima L., Quedi Furian T., Apellanis Borges K., Beatriz Rodrigues L., Ruschel Dos Santos L., Borsoi A., Werlang Isolan L., Pereira Gomes M.J., Pippi Salle C.T., de Souza Moraes H.L., Pinheiro do Nascimento V. Detection and Quantification of Campylobacter in poultry slaughterhouses using conventional microbiological technique, most probable number, and real-time PCR. Foodborne Pathog. Dis. 2022;19:143–150. doi: 10.1089/fpd.2021.0071. [DOI] [PubMed] [Google Scholar]
- Reich F., Atanassova V., Haunhorst E., Klein G. The effects of Campylobacter numbers in caeca on the contamination of broiler carcasses with Campylobacter. Int. J. Food Microbiol. 2008;1-2:116–120. doi: 10.1016/j.ijfoodmicro.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Ren, F., W. Yang, J. Hu, P. Huang, X.-A. Jiao, and J. Huang. 2021. Feeding malic acid to chickens at slaughter age improves microbial safety with regard to Campylobacter. 11: 1999. [DOI] [PMC free article] [PubMed]
- Richards P.J., Connerton P.L., Connerton I.F. Phage biocontrol of Campylobacter jejuni in chickens does not produce collateral effects on the gut microbiota. Front. Microbiol. 2019;10:476. doi: 10.3389/fmicb.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robyn J., Rasschaert G., Pasmans F., Heyndrickx M. Thermotolerant Campylobacter during broiler rearing: risk factors and intervention. Compr. Rev. Food Sci. Food Saf. 2015;14:81–105. doi: 10.1111/1541-4337.12124. [DOI] [PubMed] [Google Scholar]
- Rosenquist H., Sommer H.M., Nielsen N.L., Christensen B.B. The effect of slaughter operations on the contamination of chicken carcasses with thermotolerant Campylobacter. Int. J. Food Microbiol. 2006;2:226–232. doi: 10.1016/j.ijfoodmicro.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Rosenquist H., Bengtsson A., Hansen T.B. A collaborative study on a Nordic standard protocol for detection and enumeration of thermotolerant Campylobacter in food. Int. J. Food Microbiol. 2007;2:201–213. doi: 10.1016/j.ijfoodmicro.2007.07.037. [DOI] [PubMed] [Google Scholar]
- Russell S.M. The effect of airsacculitis on bird weights, uniformity, fecal contamination, processing errors, and populations of Campylobacter spp. and Escherichia coli. Poult. Sci. 2003;8:1326–1331. doi: 10.1093/ps/82.8.1326. [DOI] [PubMed] [Google Scholar]
- Scherer K., Bartelt E., Sommerfeld C., Hildebrandt G. Comparison of different sampling techniques and enumeration methods for the isolation and quantification of Campylobacter spp. in raw retail chicken legs. Int J Food Microbiol. 2006;108:115–119. doi: 10.1016/j.ijfoodmicro.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Seliwiorstow T., Baré J., Van Damme I., Uyttendaele M., De Zutter L. Campylobacter carcass contamination throughout the slaughter process of Campylobacter-positive broiler batches. Int. J. Food Microbiol. 2015;194:25–31. doi: 10.1016/j.ijfoodmicro.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Seliwiorstow T., Duarte A., Baré J., Botteldoorn N., Dierick K., Uyttendaele M., De Zutter L. Comparison of sample types and analytical methods for the detection of highly campylobacter-colonized broiler flocks at different stages in the poultry meat production chain. Foodborne Pathog. Dis. 2015;12:399–405. doi: 10.1089/fpd.2014.1894. [DOI] [PubMed] [Google Scholar]
- Seliwiorstow T., Baré J., Berkvens D., Van Damme I., Uyttendaele M., De Zutter L. Identification of risk factors for Campylobacter contamination levels on broiler carcasses during the slaughter process. Int. J. Food Microbiol. 2016;226:26–32. doi: 10.1016/j.ijfoodmicro.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Skånseng B., Kaldhusdal M., Moen B., Gjevre A.-G., Johannessen G.S., Sekelja M., Trosvik P P., Rudi K. Prevention of intestinal Campylobacter jejuni colonization in broilers by combinations of in-feed organic acids. J. Appl. Microbiol. 2010;109:1265–1273. doi: 10.1111/j.1365-2672.2010.04766.x. [DOI] [PubMed] [Google Scholar]
- Smith D.P., Northcutt J.K., Cason J.A., Hinton A., Buhr R.J., Ingram K.D. Effect of external or internal fecal contamination on numbers of bacteria on prechilled broiler carcasses. Poult. Sci. 2007;6:1241–1244. doi: 10.1093/ps/86.6.1241. [DOI] [PubMed] [Google Scholar]
- Solis de los Santos F., Donoghue A.M., Venkitanarayanan K., Dirain M.L., Reyes-Herrera I., Blore P.J., Donoghue D.J. Caprylic acid supplemented in feed reduces enteric Campylobacter jejuni colonization in ten-day-old broiler chickens. Poult. Sci. 2008;87:800–804. doi: 10.3382/ps.2007-00280. [DOI] [PubMed] [Google Scholar]
- Stern N.J., Svetoch E.A., Eruslanov B.V., Kovalev Y.N., Volodina L.I., Perelygin V.V., Mitsevich E.V., Mitsevich I.P., Levchuk V.P. Paenibacillus polymyxa purified bacteriocin to control Campylobacter jejuni in chickens. J. Food Prot. 2005;7:1450–1453. doi: 10.4315/0362-028x-68.7.1450. [DOI] [PubMed] [Google Scholar]
- Szott V., Reichelt B., Alter T., Friese A., Roesler U. In vivo efficacy of carvacrol on Campylobacter jejuni prevalence in broiler chickens during an entire fattening period. Eur. J. Microbiol. Immunol. 2020;3:131–138. doi: 10.1556/1886.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.L., Hinton M. Antibacterial activity of formic and propionic acids in the diet of hens on salmonellas in the crop. Br. Poult. Sci. 1997;38:59–65. doi: 10.1080/00071669708417941. [DOI] [PubMed] [Google Scholar]
- Vandeputte J., Martel A., Canessa S., v. Rysselberghe N., De Zutter L., Heyndrickx M., Haesebrouck F., Pasmans F., Garmyn A. Reducing Campylobacter jejuni colonization in broiler chickens by in-feed supplementation with hyperimmune egg yolk antibodies. Sci. Rep. 2019;9:8931. doi: 10.1038/s41598-019-45380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deun K., Haesebrouck F., Van Immerseel F., Ducatelle R., Pasmans F. Short-chain fatty acids and L-lactate as feed additives to control Campylobacter jejuni infections in broilers. Avian Pathol. 2008;37:379–383. doi: 10.1080/03079450802216603. [DOI] [PubMed] [Google Scholar]
- Wagle B.R., Upadhyay A., Arsi K., Shrestha S., Venkitanarayanan K., Donoghue A.M., Donoghue D.J. Application of β-resorcylic acid as potential antimicrobial feed additive to reduce Campylobacter colonization in broiler chickens. Front. Microbiol. 2017;8:599. doi: 10.3389/fmicb.2017.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautner A.E., Johann C., Strubel A., Busse C., Tareen A.M., Masanta W.O., Lugert R., Schmidt-Ott R.R., Groß U. Seroprevalence of campylobacteriosis and relevant post-infectious sequelae. Eur. J. Clin. Microbiol. Infect. Dis. 2014;6:1019–1027. doi: 10.1007/s10096-013-2040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]