Abstract
BACKGROUND
Severe maternal morbidity refers to the most serious complications of pregnancy. Whether severe maternal morbidity is associated with post-traumatic stress disorder is currently under active investigation.
OBJECTIVE
This study aimed to examine the association between severe maternal morbidity and post-traumatic stress disorder at delivery.
STUDY DESIGN
This was a retrospective cohort study querying the Healthcare Cost and Utilization Project's National Inpatient Sample, which included 12,857,721 patients for national estimates who had vaginal or cesarean deliveries between January 2016 and December 2019. Patients with mental health conditions other than post-traumatic stress disorder and substance use disorder were excluded. Severe maternal morbidity was defined according to the Centers for Disease Control and Prevention definition (a total of 21 indicators). Main outcomes were trends and characteristics related to post-traumatic stress disorder, assessed with a multivariable binary logistic regression model. Sensitivity analysis included subcohort assessment restricted to patients per clinical and obstetrical demographics.
RESULTS
A total of 8880 patients had a diagnosis of post-traumatic stress disorder during the hospital admission for delivery (prevalence rate, 6.9 per 10,000). The prevalence rate of post-traumatic stress disorder increased from 5.0 to 8.8 per 10,000 deliveries between 2016 and 2019. This increasing trend remained independent in multivariable analysis. The adjusted odds ratio, compared with 2016, was 1.26 (95% confidence interval, 1.19–1.35) for 2017, 1.50 (95% confidence interval, 1.41–1.60) for 2018, and 1.73 (95% confidence interval, 1.63–1.84) for 2019. Severe maternal morbidity occurred in 210,605 (1.6%) patients. Patients who had severe maternal morbidity were more likely to have a diagnosis of post-traumatic stress disorder than those without severe maternal morbidity (12.8 vs 6.8 per 10,000 deliveries; adjusted odds ratio, 1.57; 95% confidence interval, 1.39–1.78) in multivariable analysis. This association remained robust in several subcohort analyses including (1) participants aged ≤35 years (adjusted odds ratio, 1.62; 95% confidence interval, 1.41–1.86), (2) those aged ≤35 years without medical comorbidity (adjusted odds ratio, 2.01; 95% confidence interval, 1.70–2.37), and (3) those aged <35 years without medical comorbidity, cesarean delivery, and preterm delivery (adjusted odds ratio, 4.52; 95% confidence interval, 3.56–5.74).
CONCLUSION
There has been a gradual increase in the number of patients with a diagnosis of post-traumatic stress disorder at delivery in recent years among those without other mental health or substance use conditions. These data suggest that there is a possible association between severe maternal morbidity and post-traumatic stress disorder.
Key words: pregnancy, post-traumatic stress disorder, severe maternal morbidity
AJOG Global Reports at a Glance.
Why was this study conducted?
The association between severe maternal morbidity and post-traumatic stress disorder (PTSD) is currently under active investigation.
Key findings
In an analysis of the National Inpatient Sample including 12.9 million pregnant patients without underlying mental health or substance use conditions, the prevalence of patients with a diagnosis of PTSD increased from 5.0 to 8.8 per 10,000 deliveries between 2016 and 2019. Patients who had severe maternal morbidity were more likely to have a diagnosis of PTSD than those who did not (12.8 vs 6.8 per 10,000 deliveries; adjusted odds ratio, 1.57; 95% confidence interval, 1.39–1.78).
What does this add to what is known?
The number of patients with a diagnosis of PTSD at delivery among those without other mental health or substance use conditions seems to be gradually increasing. These data suggest that there is an association between severe maternal morbidity and PTSD.
Introduction
Severe maternal morbidity (SMM) refers to serious unexpected labor and delivery outcomes that lead to significant acute or chronic maternal health consequences.1 In the United States, severe maternal morbidity occurs in approximately 1.5% of deliveries.2,3 The mortality rate among those who experience SMM is around 0.4% to 1.1%.2,3 Altogether, these statistics highlight that pregnancy, particularly when complicated by SMM, represents a serious life-threatening event to reproductive-aged pregnant individuals.
The interaction between maternal mental illness and SMM is an active area of interest.4,5 Post-traumatic stress disorder (PTSD) is characterized by intrusion symptoms, avoidance of specific stimuli, and negative alterations in mood and arousal for at least 1 month following the witness or experience of a traumatic event; the lifetime prevalence of PTSD among females is approximately 11.0% to 14.4%.6
Although not specific to SMM, childbirth is described as a traumatizing experience by one-third of mothers,7 and available data suggest that fewer patients will suffer from PTSD postpartum.5,7,8 There is also interest in understanding the interaction between maternal morbidity and PTSD.4,5,9, 10, 11 However, overall, the results of previous studies are heterogeneous or in some cases equivocal likely because of small sample sizes.4,9, 10, 11 Furthermore, although perinatal mental illness may contribute to worse obstetrical outcomes, less is known specifically about prenatal PTSD.12
This lack of knowledge is, in part, because of the scarcity of recent, national-level data for the United States on the PTSD trends and its association with SMM. Thus, this study aimed to examine the prevalence of PTSD at delivery and its temporal relationship with SMM.
Materials and Methods
Data source
The National Inpatient Sample (NIS) was queried for this study. The NIS program was developed as part of the Healthcare Cost and Utilization Project (HCUP), and the Agency for Healthcare Research and Quality supports the HCUP.13 The NIS program is a nationwide, all-payer database on hospital admissions that randomly selects 20% of inpatient records from each participating center. The weighted data for national estimates represent more than 90% of the US population.
Ethics statement
The current study was deemed exempt by the University of Southern California Institutional Review Board because of the use of publicly available, de-identified data. There is no public or patient involvement in this study.
Study population
This was a retrospective cohort study examining the NIS program database from January 2016 to December 2019. The study population included all patients who were recorded as having had vaginal or cesarean deliveries during hospital admission during the study period. This study period was selected because of the introduction of the World Health Organization's International Classification of Diseases, 10th Revision (ICD-10) codes in the NIS program. Case identification of vaginal and cesarean deliveries was based on the ICD-10 Clinical Modification and Procedure Classification System codes and the Diagnosis-Related Group codes. The current analysis followed the ICD-10 codes from a previous analysis.14
Patients with mental health conditions other than PTSD and patients with substance use disorders were excluded because these conditions are known to be associated with PTSD. Mental health conditions that were excluded were depressive disorder, bipolar spectrum disorder, schizophrenia spectrum disorder, anxiety disorder, acute stress reaction, and adjustment disorder.15 Excluded substance use disorders were tobacco use, alcohol use, and drug use.
Study variables
The diagnosis of PTSD was based on the ICD-10 code of F43.1. SMM was defined as per the Centers for Disease Control and Prevention definition with the following 21 indicators16: acute myocardial infarction, aneurysm, acute renal failure, adult respiratory distress syndrome, amniotic fluid embolism, cardiac arrest or ventricular fibrillation, conversion of cardiac rhythm, disseminated intravascular coagulation, eclampsia, heart failure or arrest during surgery or procedure, puerperal cerebrovascular disorders, pulmonary edema or acute heart failure, severe anesthesia complications, sepsis, shock, sickle cell disease with crisis, air and thrombotic embolism, blood products transfusion, hysterectomy, temporary tracheostomy, and ventilation.
Other study covariates assessed included patient demographics, medical comorbidities, hospital information, and pregnancy characteristics. Available patient demographics included age (<35 or ≥35 years), delivery year (2016, 2017, 2018, and 2019), race and ethnicity (White, Black, Hispanic, Asian including Pacific Islanders, and Native American) as determined by the NIS program, primary expected payer (Medicare, Medicaid, Private including Health Maintenance Organization (HMO), self-pay, and no charge), and census-level household income (every quarter).
Available hospital information included bed capacity (small, medium, and large), teaching and location setting (rural, urban noteaching, and urban teaching), and US region (Northeast, Midwest, South, and West). Medical comorbidities included obesity (yes or no), diabetes mellitus (no, preexisting, and gestational), and hypertensive disorder (no, preexisting, and gestational).
Pregnancy characteristics included gestational age at delivery (≥39, 37–38, 34–36, and <34 weeks’ gestation), delivery type (vaginal or cesarean delivery), previous uterine scar (yes or no), uterine myoma (yes or no), placenta previa (yes or no), placenta abruption (yes or no), placenta accreta spectrum (yes or no), multifetal gestation (yes or no), large for gestational age (yes or no), and intrauterine growth restriction (yes or no).
Statistical analysis
The prevalence rates of PTSD per 10,000 deliveries were computed for the study covariate. The temporal trend of PTSD prevalence rate was assessed for each study year using a linear segmented regression model with log transformation and using year-quarter time increments.17
A binary logistic regression model with a conditional step-forward selection method was fitted to identify the independent characteristics associated with PTSD in the multivariable analysis.18 Study variables with a P<.05 in the univariable analysis were considered in the initial model candidate. The analysis was executed with the stopping rule of P<.05 in the final model. The effect size was expressed as adjusted odds ratios (aOR) with the corresponding 95% confidence interval (CI).
In the sensitivity analyses, the association between SMM and PTSD was examined in several restricted cohorts. Sequential exclusion of a priori determined factors related to SMM was performed.1 In the first restriction, the cohort was restricted to patients aged <35 years (age-restricted cohort). In the second restriction, the cohort was restricted to patients aged <35 years without comorbidities (diabetes mellitus, hypertensive disorder, and obesity) (age and comorbidity factor–restricted cohort). In the third restriction, the cohort was restricted to patients aged <35 years without comorbidities and delivery factors (cesarean delivery and preterm delivery) (age, comorbidity, and delivery factor–restricted cohort).
In addition, the association between postpartum hemorrhage and PTSD and the association between preeclampsia and PTSD were assessed because these obstetrical complications have been examined previously.8 Last, a classification-tree was constructed to assess the extent of the effect of risk factors on PTSD prevalence rate. A recursive partitioning analysis with a chi-square automatic interaction detector method was used for the modeling (stopping rule, maximum 3 layers). In each determined pattern, the PTSD prevalence was computed.
The weighted values for national estimates provided by the NIS program were utilized for the analyses, and statistical interpretation was based on a 2-tailed hypothesis. A P value of <.05 was considered statistically significant. Missing variables were grouped as one category in each study variable. IBM SPSS Statistics (version 28.0; IBM Corp, Armonk, NY) and R statistics (version 3.5.3; R foundation for Statistical Computing, Vienna, Austria) were used for all analyses. The Strenghthening the Reporting of Observational Studies in Epidemiology reporting guidelines were followed to summarize the performance of the cohort study.19
Results
A total of 12,857,721 deliveries from 2016 to 2019 were examined for the study. Of those, 8880 patients had a diagnosis of PTSD during the index admission for delivery, equating to a PTSD prevalence rate of 6.9 per 10,000 deliveries.
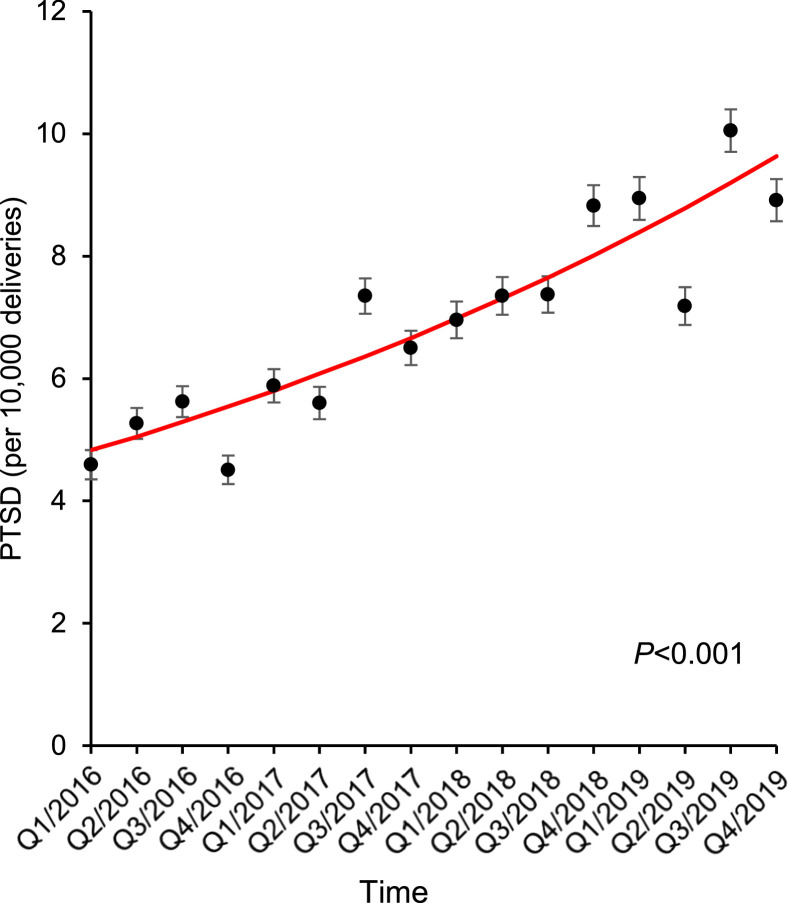
The prevalence of a PTSD diagnosis increased from 5.0 to 8.8 per 10,000 deliveries between 2016 and 2019 (1.8-fold increase; P<.001) (Figure and Table 1). The increasing trend of PTSD diagnoses over time remained independent in a multivariable analysis with an adjusted odds ratio, compared with 2016, of 1.26 (95% CI, 1.19–1.35) for 2017, 1.50 (95% CI, 1.41–1.60) for 2018, and 1.73 (95% CI, 1.63–1.84) for 2019 (Table 2).
Figure.
Trends in PTSD at delivery from 2016 to 2019
Temporal trends in PTSD prevalence among patients who had no other mental health condition or substance use disorders and delivered between 2016 and 2019 is shown. The dots represent the observed value, and the bars represent the standard error. The lines represent the modeled value.
PTSD, post-traumatic stress disorder.
Duval. Association of post-traumatic stress disorder and severe maternal morbidity. Am J Obstet Gynecol Glob Rep 2022.
Table 1.
Incidence of and risk factors for PTSD
| Characteristic | Number (%)a | PTSDb | P value |
|---|---|---|---|
| Number | 12,857,721 (100) | 6.9 | |
| Age (y) | .514 | ||
| <35 | 10,521,557 (81.8) | 6.9 | |
| ≥35 | 2,335,844 (18.2) | 6.7 | |
| Unknown | 320 (<0.1) | 0 | |
| Year | <.001 | ||
| 2016 | 3,344,286 (26.0) | 5.0 | |
| 2017 | 3,265,741 (25.4) | 6.4 | |
| 2018 | 3,167,799 (24.6) | 7.6 | |
| 2019 | 3,079,894 (24.0) | 8.8 | |
| Race and ethnicity | <.001 | ||
| White | 6,178,230 (48.1) | 8.3 | |
| Black | 1,847,780 (14.4) | 7.5 | |
| Hispanic | 2,746,298 (21.4) | 4.8 | |
| Asian, Pacific Islanders | 848,829 (6.6) | 2.8 | |
| Native American | 81,280 (0.6) | 15.4 | |
| Others | 604,600 (4.7) | 5.2 | |
| Unknown | 550,704 (4.3) | 6.7 | |
| Primary expected payer | <.001 | ||
| Medicare | 72,220 (0.6) | 41.5 | |
| Medicaid | 5,193,422 (40.4) | 8.6 | |
| Private including HMO | 6,862,710 (53.4) | 4.8 | |
| Self-pay | 350,710 (2.7) | 3.1 | |
| No charge | 8900 (0.1) | 0.0 | |
| Others | 354,695 (2.8) | 18.5 | |
| Unknown | 15,065 (0.1) | 26.6 | |
| Median household income | <.001 | ||
| QT1 (lowest) | 3,477,963 (27.0) | 7.1 | |
| QT2 | 3,160,812 (24.6) | 7.5 | |
| QT3 | 3,194,277 (24.8) | 7.1 | |
| QT4 (highest) | 2,906,753 (22.6) | 5.8 | |
| Unknown | 117,915 (0.9) | 6.8 | |
| Hospital bed capacity | <.001 | ||
| Small | 2,441,245 (19.0) | 6.2 | |
| Medium | 3,964,860 (30.8) | 6.2 | |
| Large | 6,451,616 (50.2) | 7.6 | |
| Hospital location and teaching status | <.001 | ||
| Rural | 1,099,406 (8.6) | 6.4 | |
| Urban nonteaching | 2,743,203 (21.3) | 5.2 | |
| Urban teaching | 9,015,112 (70.1) | 7.5 | |
| Hospital region | <.001 | ||
| Northeast | 2,043,750 (15.9) | 8.7 | |
| Midwest | 2,595,978 (20.2) | 6.9 | |
| South | 5,090,454 (39.6) | 5.0 | |
| West | 3,127,540 (24.3) | 9.0 | |
| Obesity | <.001 | ||
| No | 11,555,831 (89.9) | 6.2 | |
| Yes | 1,301,889 (10.1) | 12.8 | |
| Diabetes mellitus | <.001 | ||
| No | 11,729,887 (91.2) | 6.7 | |
| Preexisting | 128,260 (1.0) | 12.5 | |
| Gestational | 999,574 (7.8) | 8.2 | |
| Hypertensive disorder | <.001 | ||
| No | 11,706,581 (91.0) | 6.5 | |
| Preexisting | 438,875 (3.4) | 13.8 | |
| Gestational | 712,265 (5.5) | 9.6 | |
| Gestational age (wk) | <.001 | ||
| ≥39 | 8,119,054 (63.1) | 6.2 | |
| 37–38 | 3,378,773 (26.3) | 7.7 | |
| 34–36 | 834,924 (6.5) | 8.1 | |
| <34 | 391,720 (3.0) | 11.0 | |
| Unknown | 133,250 (1.0) | 9.4 | |
| Delivery type | <.001 | ||
| Vaginal delivery | 8,750,939 (99.3) | 6.6 | |
| Cesarean delivery | 4,106,782 (0.7) | 7.6 | |
| Previous uterine scar | .004 | ||
| No | 10,567,777 (82.2) | 6.8 | |
| Yes | 2,289,943 (17.8) | 7.4 | |
| Uterine myoma | .003 | ||
| No | 12,674,246 (98.6) | 6.9 | |
| Yes | 183,475 (1.4) | 8.7 | |
| Placenta previa | .201 | ||
| No | 12,793,991 (99.5) | 6.9 | |
| Yes | 63,730 (0.5) | 5.5 | |
| Placenta abruption | <.001 | ||
| No | 12,729,416 (99.0) | 6.9 | |
| Yes | 128,305 (1.0) | 10.1 | |
| Placenta accreta spectrum | .154 | ||
| No | 12,842,911 (99.9) | 6.9 | |
| Yes | 14,810 (0.1) | 10.1 | |
| Multiple gestation | .053 | ||
| No | 12,627,191 (98.2) | 6.9 | |
| Yes | 230,530 (1.8) | 5.9 | |
| IUGR | <.001 | ||
| No | 12,447,191 (96.8) | 6.9 | |
| Yes | 410,530 (3.2) | 8.3 | |
| LGA | .017 | ||
| No | 12,518,901 (97.4) | 6.9 | |
| Yes | 338,820 (2.6) | 8.0 | |
| Severe maternal morbidityc | <.001 | ||
| No | 12,647,116 (98.4) | 6.8 | |
| Yes | 210,605 (1.6) | 12.8 |
Chi-square tests were used to determine P values.
CDC, Centers for Disease Control and Prevention; IUGR, intrauterine growth restriction; LGA, large for gestational age; PTSD, post-traumatic stress disorder; QT, quartile.
Percentage per column (age included median with interquartile range). Total number may not be 12,857,721 because of the weighted model
A total of 8880 patients had a diagnosis of PTSD, and incidence rates are shown per row per 10,000 deliveries
Included any 1 of the 21 indicators for severe maternal morbidity according to the CDC definition.
Duval. Association of post-traumatic stress disorder and severe maternal morbidity. Am J Obstet Gynecol Glob Rep 2022.
Table 2.
Independent characteristics associated with post-traumatic stress disorder
| Characteristic | aOR (95% CI)a | P valuea |
|---|---|---|
| Year | <.001b | |
| 2016 | 1 | |
| 2017 | 1.26 (1.19–1.35) | <.001 |
| 2018 | 1.50 (1.41–1.60) | <.001 |
| 2019 | 1.73 (1.63–1.84) | <.001 |
| Race and ethnicity | <.001b | |
| White | 2.57 (2.40–2.74) | <.001 |
| Black | 1.74 (1.61–1.88) | <.001 |
| Hispanic | 1 | |
| Asian, Pacific Islanders | 0.76 (0.66–0.87) | <.001 |
| Native American | 3.06 (2.55–3.69) | <.001 |
| Others | 1.35 (1.19–1.53) | <.001 |
| Unknown | 1.96 (1.74–2.21) | <.001 |
| Primary expected payer | <.001b | |
| Medicare | 4.30 (3.82–4.84) | <.001 |
| Medicaid | 1 | |
| Private including HMO | 0.46 (0.44–0.49) | <.001 |
| Self-pay | 0.43 (0.35–0.52) | <.001 |
| No charge | — | .974 |
| Others | 1.89 (1.74–2.06) | <.001 |
| Unknown | 2.61 (1.91–3.57) | <.001 |
| Median household income | <.001b | |
| QT1 (lowest) | 1 | |
| QT2 | 1.06 (1.00–1.12) | .063 |
| QT3 | 1.01 (0.95–1.07) | .736 |
| QT4 (highest) | 0.92 (0.86–0.98) | .011 |
| Unknown | 1.00 (0.80–1.25) | .974 |
| Hospital bed capacity | <.001b | |
| Small | 0.94 (0.88–1.00) | .050 |
| Medium | 1 | |
| Large | 1.14 (1.08–1.19) | <.001 |
| Hospital location and teaching status | <.001b | |
| Rural | 1.18 (1.07–1.29) | |
| Urban nonteaching | 1 | <.001 |
| Urban teaching | 1.33 (1.25–1.41) | <.001 |
| Hospital region | <.001b | |
| Northeast | 1 | |
| Midwest | 0.73 (0.69–0.79) | <.001 |
| South | 0.53 (0.50–0.56) | <.001 |
| West | 1.19 (1.12–1.27) | <.001 |
| Obesity | ||
| No | 1 | |
| Yes | 1.70 (1.61–1.80) | <.001 |
| Diabetes mellitus | .039b | |
| No | 1 | |
| Preexisting | 1.14 (0.97–1.34) | .105 |
| Gestational | 1.08 (1.01–1.16) | .037 |
| Hypertensive disorder | <.001b | |
| No | 1 | |
| Preexisting | 1.54 (1.41–1.68) | <.001 |
| Gestational | 1.26 (1.16–1.36) | <.001 |
| Gestational age (wk) | <.001b | |
| ≥39 | 1 | |
| 37–38 | 1.19 (1.13–1.25) | <.001 |
| 34–36 | 1.16 (1.07–1.26) | <.001 |
| <34 | 1.47 (1.33–1.62) | <.001 |
| Unknown | 1.44 (1.21–1.72) | <.001 |
| Delivery type | ||
| Vaginal | 1 | |
| Cesarean | 1.08 (1.04–1.13) | <.001 |
| Severe maternal morbidityc | ||
| No | 1 | |
| Yes | 1.57 (1.39–1.78) | <.001 |
aOR, adjusted odds ratio; CI, confidence interval; PTSD, post-traumatic stress disorder; QT, quartile.
A binary logistic regression model for multivariable analysis. Conditional step-forward selection with the stopping rule of P<.05. Multicollinearity was assessed in the study covariates
Overall P values
Included any 1 of the 21 indicators for severe maternal morbidity per the Centers for Disease Control and Prevention definition.
Duval. Association of post-traumatic stress disorder and severe maternal morbidity. Am J Obstet Gynecol Glob Rep 2022.
SMM occurred in 210,605 (1.6%) deliveries. The prevalence rate of PTSD diagnoses was higher among those who had SMM when compared with those without SMM (12.8 vs 6.8 per 10,000 deliveries; P<.001) (Table 1). In a multivariable analysis (Table 2), this association remained robust, and SMM was associated with a 57% increased likeliness of having a PTSD diagnosis (aOR, 1.57; 95% CI, 1.39–1.78).
In addition, (1) patient characteristics such as being White, Black, or Native American, having obesity, and medical comorbidities, (2) hospital characteristics such as a large bed capacity, urban teaching setting, and being located in the Northeast or West regions, and (3) pregnancy characteristics, such as preterm birth and cesarean delivery, were independently associated with an increased likeliness of receiving a PTSD diagnosis (all P<.05) (Table 2). Conversely, patients who were Asian, privately insured, and had higher incomes were less likely to have a diagnosis of PTSD (all P<.05) (Table 2).
A classification tree model identified 26 patterns based on clinical and obstetrical demographics with 4 of these patterns having a greater than 3-fold higher prevalence of PTSD when compared with the whole cohort (6.9 per 10,000). These include Native American individuals without obesity in the South or West regions, patients with obesity in the Midwest region who had a late-preterm delivery, White or Black individuals with obesity in the West region, and patients with obesity in the Northeast region who had early- or extremely-preterm delivery (range, 22.1–26.3 per 10,000).
Sensitivity analyses were performed for 3 restricted cohorts, namely 10,521,557 patients in the age–restricted cohort (PTSD prevalence, 6.9/10,000 deliveries), 8,286,572 patients in the age and comorbidity factor-restricted cohort (PTSD prevalence, 5.9/10,000 deliveries), and 4,090,378 patients in the age, comorbidity, and delivery factor–restricted cohort (PTSD prevalence, 5.4/10,000 deliveries). In these 3 restricted cohorts, the prevalence of PTSD increased from 2016 to 2019 (5.0–8.9, 4.2–7.8, and 4.0–6.9 per 10,000 deliveries, respectively; all P<.001).
In these 3 subcohorts, the association of SMM and PTSD remained robust after multivariable analysis (all P<.001) (Table 3), and patients who had SMM were more likely to have a diagnosis of PTSD (aOR, 1.62, 2.01, and 4.52 for age-restricted cohort, age and comorbidity factor–restricted cohort, and age, comorbidity and delivery factor–restricted cohort, respectively).
Table 3.
Sensitivity analysis
| Cohort | PTSDa | OR (95% CI) | aOR (95% CI) |
|---|---|---|---|
| All cases | |||
| SMM (−) | 6.8 | 1 | 1 |
| SMM (+) | 12.8 | 1.88 (1.67–2.13) | 1.57 (1.39–1.78) |
| Aged <35 | |||
| SMM (−) | 6.8 | 1 | 1 |
| SMM (+) | 13.1 | 1.92 (1.68–2.20) | 1.62 (1.41–1.86) |
| Age <35 y without DM or HTN or obesity | |||
| SMM (−) | 5.8 | 1 | 1 |
| SMM (+) | 13.0 | 2.22 (1.89–2.62) | 2.01 (1.70–2.37) |
| Aged <35 y without DM or HTN or obesity or CD or PTD | |||
| SMM (−) | 5.3 | 1 | 1 |
| SMM (+) | 23.4 | 4.46 (3.52–5.67) | 4.52 (3.56–5.74) |
aOR, adjusted-odds ratio; CD, cesarean delivery; CI, confidence interval; DM, diabetes mellitus; HTN, hypertensive disorder; PTD, preterm delivery; PTSD, post-traumatic stress disorder; SMM, severe maternal morbidity.
PTSD prevalence rates are shown per row per 10,000 deliveries. Adjusting model were based on the independent factors as shown in Table 2.
Duval. Association of post-traumatic stress disorder and severe maternal morbidity. Am J Obstet Gynecol Glob Rep 2022.
Lastly, patients who had a diagnosis of preeclampsia (OR, 1.40; 95% CI, 1.29–1.53) or postpartum hemorrhage (OR, 1.60; 95% CI, 1.47–1.73) were both more likely to have a diagnosis of PTSD.
Discussion
Principal findings
Key results of this study are the following: first, the national prevalence of a PTSD diagnosis at time of delivery may significantly underestimate the true prevalence of the disease; second, among patients without other mental health or substance use conditions, there has been a gradual increase in the number of patients with a diagnosis of PTSD at delivery in recent years; third, these data suggest that there is a possible association between SMM and PTSD.
Results
Prevalence of post-traumatic stress disorder
Previously reported prevalence rates of antepartum and postpartum PTSD vary widely. Most estimates fall between 0.4% and 12.0% for antepartum PTSD20, 21, 22, 23 and between 0.8% and 16.6% for postpartum PTSD.5,7,8 The cause of this wide range is likely multifactorial, including discrepant study populations and diagnostic criteria. The prevalence of PTSD among patients with no other mental health and substance use conditions in this study, 6.9 per 10,000 deliveries, is lower than in most previous studies.
The lower prevalence seen in this study is likely multifactorial. Overall, this discrepancy is most likely because of the lack of information regarding the postpartum period after discharge from the hospital. Exclusion of other mental health conditions and substance use disorders in the current study is another explanation for the lower prevalence because these factors were not routinely excluded in previous studies.8 A comparison with the studies that excluded these factors were not applicable because of the different inclusion periods (eg, postpartum period).
This study also found the highest prevalence of PTSD among Native American individuals than among any other race or ethnicity groups examined. This observation was previously not well studied. Previous research demonstrated that adverse childhood experiences seem to be associated with an increased risk for PTSD symptoms among Native American adolescents and young adults.24 In addition, Native American populations often face extreme economic adversity and discrimination, leading to disproportionate rates of both chronic physical25 and mental health conditions.26 Given these acute challenges that are spread across their social network, it is understandable how Native Americans could be at risk for both PTSD and SMM. However, this study itself was unable to examine the past experiences of the group, which may have caused the higher prevalence. Nonetheless, these findings suggest that Native American mothers face distinct challenges. Further investigation is warranted to validate the results.
Trends in post-traumatic stress disorder
Despite the overall lower prevalence captured here, this study still reported a gradual increase in PTSD diagnoses among patients who reported no other mental health conditions or substance use disorders and who gave birth during recent years in the United States. The prevalence rate increased from approximately 1 in 1997 to 1136 patients during the 4-year study period. Logue et al15 recently demonstrated a 10-fold increase in delivery hospitalizations with 1 or more mental health diagnoses from 2000 to 2018. Although Logue et al15 did not assess PTSD diagnoses and this study excluded these conditions, the rise in other mental health disorders validates the findings here.
This study was not able to examine the causality of this increase in the prevalence of maternal PTSD at delivery. Because this study population was restricted to obstetrical delivery admissions, it is unknown if this rise mirrors a rise in the general population during recent years. It is also unknown if this captures an increasing trend in routine screening for PTSD rather than an increasing trend in the disease itself. Although national guidelines emphasize the importance of universal screening for anxiety and depression,27 universal screening for PTSD is not well studied.
Association between severe maternal morbidity and post-traumatic stress disorder
The results of the current analysis suggest that a temporal association between the diagnoses of SMM and PTSD exist. This relationship was sustained even among patient <35 years old who delivered vaginally and in the absence of medical comorbidities, endorsing that the observed relationship between the 2 is likely robust. Because we were not able to assess the causality between SMM and PTSD in this study, discussion in this section was based on a hypothesis-based speculation to equally review both directionalities, that is, (1) SMM at delivery could trigger the symptoms of PTSD and (2) patients with PTSD may be at greater risk for SMM at delivery.
It is possible that the experience of SMM triggers PTSD.28 For example, a recent study found that postpartum hemorrhage is associated with PTSD at 2 months postpartum.28 However, most studies assessing PTSD and SMM were either equivocal in their findings or underpowered to comment on an association.4,9, 10, 11 Furthermore, an important component of a PTSD diagnosis is the duration of symptoms for at least 1 month. Although patients in the SMM group, on average, had longer hospitalizations (data not shown), most patients were discharged before 1 month postpartum. Thus, a diagnosis of PTSD was either premature (before meeting the 1-month criterion) or the PTSD diagnosis predated SMM.
Chronic psychosocial stress may indeed portend worse pregnancy outcomes. Previous studies have demonstrated that PTSD was associated with an increased risk for spontaneous abortion, gestational diabetes, preeclampsia, preterm birth, and prolonged hospitalization.21,22,29 Although the mechanism of psychological stress causing worse obstetrical outcomes is unknown, there is evidence that antenatal depression, anxiety, and stress are associated with poorer outcomes such as preterm birth, low birthweight, and cesarean delivery.30,31 Previous literature has also demonstrated the increased risk for SMM among mothers with mental health disorders.32
Another analogous and important example is that worse obstetrical outcomes among Black mothers who experience the highest rates of SMM, maternal mortality, and infant mortality2,3,33, 34, 35 are hypothesized to, in part, be a consequence of the traumatic toll of racism in the United States.36 Thus, it is possible that if the psychological needs of patients were screened for and adequately addressed antepartum, the negative impact of maternal stress could be mitigated.
Overall, in the absence of chronology between the 2 diagnoses of SMM and PTSD in this study, a causal relationship cannot be clarified. Nonetheless, this finding merits further investigation.
Strengths and Limitations
The use of nationwide data, a large sample size, recent time period, various sensitivity analyses, and assessing the association between SMM and PTSD enriched the interpretation of the study findings.
Key limitations included unmeasured bias that is inherent to this type of study. For instance, details regarding the PTSD diagnosis (eg, diagnostic criteria, extent of symptoms, preexisting diagnoses, and treatment) were not available, but this could be key information required for the analyses. A history of PTSD is another possible confounder that is absent from the database. As above, details about the chronology between PTSD and SMM were not available in this study, limiting the causality assessment between the 2.
Lack of information on PTSD after discharge from the index admission for delivery is another limitation. The NIS program as an administrative type database solely relied on the ICD-10 codes, and accuracy of the diagnoses was not assessable because an actual medical record review was not performed in this study. Ascertainment bias caused by the data capturing schema may limit the interpretation of the study findings. Generalizability of our results to another population is unknown because this study only examined the United States population.
Conclusion
The gradual increase in the prevalence of PTSD among pregnant patients with no other mental health conditions and substance use disorders in the recent years in the United States is noteworthy. Extra care and evaluation may be needed particularly for the subgroup of pregnant patients with an increased risk for PTSD as suggested in this study. The observed association between SMM and PTSD, together with the increasing trends, is recognizable and highlights important issues in the arena of the pregnant individual's mental health. Additional research is needed to clarify the relationship between SMM and PTSD.
Footnotes
The authors report no conflict of interest.
K.M. reports receiving funding from the Ensign Endowment for Gynecologic Cancer Research. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
This study was deemed exempt by the University of Southern California Institutional Review Board (HS-16-00481).
Patient consent was not required because of the use of publicly available, de-identified data.
The data on which this study is based are publicly available on request at the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality https://www.hcup-us.ahrq.gov/nisoverview.jsp
K.M. affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. The National Inpatient Sample classified the race and ethnicity, and the current study used the default grouping for analysis; the program has not verified and is not responsible for the statistical validity of the data analysis or the conclusions derived by the study team.
Cite this article as: Duval CJ, Youssefzadeh AC, Sweeney HE, et al. Association of severe maternal morbidity and post-traumatic stress disorder. Am J Obstet Gynecol Glob Rep 2022;2:x.ex–x.ex.
References
- 1.Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. 2022. Available at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html. Accessed January 12, 2022.
- 2.Guglielminotti J, Wong CA, Friedman AM, Li G. Racial and ethnic disparities in death associated with severe maternal morbidity in the United States: failure to rescue. Obstet Gynecol. 2021;137:791–800. doi: 10.1097/AOG.0000000000004362. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo K, Mandelbaum RS, Matsuzaki S, et al. Decreasing failure-to-rescue from severe maternal morbidity at cesarean delivery: recent US trends. JAMA Surg. 2021;156:585–587. doi: 10.1001/jamasurg.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewkowitz AK, Rosenbloom JI, Keller M, et al. Association between severe maternal morbidity and psychiatric illness within 1 year of hospital discharge after delivery. Obstet Gynecol. 2019;134:695–707. doi: 10.1097/AOG.0000000000003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Small MJ, Gondwe KW, Brown HL. Post-traumatic stress disorder and severe maternal morbidity. Obstet Gynecol Clin North Am. 2020;47:453–461. doi: 10.1016/j.ogc.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26:537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayers S. Delivery as a traumatic event: prevalence, risk factors, and treatment for postnatal posttraumatic stress disorder. Clin Obstet Gynecol. 2004;47:552–567. doi: 10.1097/01.grf.0000129919.00756.9c. [DOI] [PubMed] [Google Scholar]
- 8.Verreault N, Da Costa D, Marchand A, et al. PTSD following childbirth: a prospective study of incidence and risk factors in Canadian women. J Psychosom Res. 2012;73:257–263. doi: 10.1016/j.jpsychores.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Furuta M, Sandall J, Bick D. A systematic review of the relationship between severe maternal morbidity and post-traumatic stress disorder. BMC Pregnancy Childbirth. 2012;12:125. doi: 10.1186/1471-2393-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angelini CR, Pacagnella RC, Parpinelli MA, et al. Post-Traumatic Stress Disorder and severe maternal morbidity: is there an association? Clinics (Sao Paulo) 2018;73:e309. doi: 10.6061/clinics/2018/e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wall-Wieler E, Carmichael SL, Urquia ML, Liu C, Hjern A. Severe maternal morbidity and postpartum mental health-related outcomes in Sweden: a population-based matched-cohort study. Arch Womens Ment Health. 2019;22:519–526. doi: 10.1007/s00737-018-0917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paschetta E, Berrisford G, Coccia F, et al. Perinatal psychiatric disorders: an overview. Am J Obstet Gynecol. 2014;210 doi: 10.1016/j.ajog.2013.10.009. 501–9.e6. [DOI] [PubMed] [Google Scholar]
- 13.Agency for Healthcare Research and Quality. Overview of the National (nationwide) Inpatient Sample (NIS). 2022. Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed January 12, 2022.
- 14.Matsuzaki S, Mandelbaum RS, Sangara RN, et al. Trends, characteristics, and outcomes of placenta accreta spectrum: a national study in the United States. Am J Obstet Gynecol. 2021;225:534. doi: 10.1016/j.ajog.2021.04.233. e1–38. [DOI] [PubMed] [Google Scholar]
- 15.Logue TC, Wen T, Monk C, et al. Trends in and complications associated with mental health condition diagnoses during delivery hospitalizations. Am J Obstet Gynecol. 2022;226:405. doi: 10.1016/j.ajog.2021.09.021. e1–16. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. How Does CDC identify severe maternal morbidity? 2019. Available at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm. Accessed January 28, 2022.
- 17.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Greenland S, Daniel R, Pearce N. Outcome modelling strategies in epidemiology: traditional methods and basic alternatives. Int J Epidemiol. 2016;45:565–575. doi: 10.1093/ije/dyw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaferi AA, Schwartz TA, Pawlik TM. STROBE reporting guidelines for observational studies. JAMA Surg. 2021;156:577–578. doi: 10.1001/jamasurg.2021.0528. [DOI] [PubMed] [Google Scholar]
- 20.Yildiz PD, Ayers S, Phillips L. The prevalence of posttraumatic stress disorder in pregnancy and after birth: a systematic review and meta-analysis. J Affect Disord. 2017;208:634–645. doi: 10.1016/j.jad.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Seng JS, Oakley DJ, Sampselle CM, Killion C, Graham-Bermann S, Liberzon I. Posttraumatic stress disorder and pregnancy complications. Obstet Gynecol. 2001;97:17–22. doi: 10.1016/s0029-7844(00)01097-8. [DOI] [PubMed] [Google Scholar]
- 22.Shaw JG, Asch SM, Kimerling R, Frayne SM, Shaw KA, Phibbs CS. Posttraumatic stress disorder and risk of spontaneous preterm birth. Obstet Gynecol. 2014;124:1111–1119. doi: 10.1097/AOG.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 23.Seng JS, Low LK, Sperlich M, Ronis DL, Liberzon I. Prevalence, trauma history, and risk for posttraumatic stress disorder among nulliparous women in maternity care. Obstet Gynecol. 2009;114:839–847. doi: 10.1097/AOG.0b013e3181b8f8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brockie TN, Dana-Sacco G, Wallen GR, Wilcox HC, Campbell JC. The relationship of adverse childhood experiences to PTSD, depression, poly-drug use and suicide attempt in reservation-based native American adolescents and young adults. Am J Community Psychol. 2015;55:411–421. doi: 10.1007/s10464-015-9721-3. [DOI] [PubMed] [Google Scholar]
- 25.US Department of Health and Human Services . 2022. Fact sheets: disparities. Indian health services.https://www.ihs.gov/newsroom/factsheets/disparities/ Available at. Accessed August 11, 2022. [Google Scholar]
- 26.US Department of Health and Human Services . Indian health services; 2022. Fact sheets: behavioral health.https://www.ihs.gov/newsroom/factsheets/behavioralhealth/ Available at. Accessed August 11, 2022. [Google Scholar]
- 27.ACOG Committee Opinion No. 757: screening for perinatal depression. Obstet Gynecol. 2018;132:e208–e212. doi: 10.1097/AOG.0000000000002927. [DOI] [PubMed] [Google Scholar]
- 28.Froeliger A, Deneux-Tharaux C, Seco A, Sentilhes L. TRAnexamic Acid for Preventing postpartum hemorrhage after vaginal delivery (TRAAP) Study Group*. Posttraumatic stress disorder symptoms 2 months after vaginal delivery. Obstet Gynecol. 2022;139:63–72. doi: 10.1097/AOG.0000000000004611. [DOI] [PubMed] [Google Scholar]
- 29.Shaw JG, Asch SM, Katon JG, et al. Post-traumatic stress disorder and antepartum complications: a novel risk factor for gestational diabetes and preeclampsia. Paediatr Perinat Epidemiol. 2017;31:185–194. doi: 10.1111/ppe.12349. [DOI] [PubMed] [Google Scholar]
- 30.Staneva A, Bogossian F, Pritchard M, Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: a systematic review. Women Birth. 2015;28:179–193. doi: 10.1016/j.wombi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Yedid Sion M, Harlev A, Weintraub AY, Sergienko R, Sheiner E. Is antenatal depression associated with adverse obstetric and perinatal outcomes? J Matern Fetal Neonatal Med. 2016;29:863–867. doi: 10.3109/14767058.2015.1023708. [DOI] [PubMed] [Google Scholar]
- 32.Brown CC, Adams CE, George KE, Moore JE. Mental health conditions increase severe maternal morbidity by 50 percent and cost $102 million yearly in the United States. Health Aff (Millwood) 2021;40:1575–1584. doi: 10.1377/hlthaff.2021.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonard SA, Main EK, Scott KA, Profit J, Carmichael SL. Racial and ethnic disparities in severe maternal morbidity prevalence and trends. Ann Epidemiol. 2019;33:30–36. doi: 10.1016/j.annepidem.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liese KL, Mogos M, Abboud S, Decocker K, Koch AR, Geller SE. Racial and ethnic disparities in severe maternal morbidity in the United States. J Racial Ethn Health Disparities. 2019;6:790–798. doi: 10.1007/s40615-019-00577-w. [DOI] [PubMed] [Google Scholar]
- 35.Mage DT, Maria Donner E, Holmes L., Jr Risk differences in disease-specific infant mortality between Black and White US children, 1968-2015: an epidemiologic investigation. J Racial Ethn Health Disparities. 2019;6:86–93. doi: 10.1007/s40615-018-0502-1. [DOI] [PubMed] [Google Scholar]
- 36.Dreyer BP. The toll of racism on African American mothers and their infants. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.38828. [DOI] [PubMed] [Google Scholar]