Abstract
Mucopolysaccharidoses (MPS) are a group of lysosomal storage diseases caused by defects in genes coding for proteins involved in degradation of glycosaminoglycans (GAGs). These complex carbohydrates accumulate in cells causing their serious dysfunctions. Apart from the physical GAG storage, secondary and tertiary changes may contribute significantly to the pathomechanism of the disease. Among processes which were not systematically investigated in MPS cells to date there is the cell cycle. Here, we studied perturbances in this crucial cellular process in majority of MPS types. Transcriptomic analyses indicated that expression of many genes coding for proteins involved in the cell cycle is dysregulated in all tested MPS cells. Importantly, levels of transcripts of particular genes were changed in the same manner (i.e. either up- or down-regulated) in most or all types of the disease, indicating a common mechanism of the dysregulation. Flow cytometric studies demonstrated that the cell cycle is disturbed in all MPS types, with increased fractions of cells in the G0/G1 phase in most types and decreased fractions of cells in the G2/M phase in all types. We found that increased levels of cyclin D1 and disturbed timing of its appearance during the cell cycle may contribute to the mechanism of dysregulation of this process in MPS. Reduction of GAG levels by either a specific enzyme or genistein-mediated inhibition of synthesis of these compounds improved, but not fully corrected, the cell cycle in MPS fibroblasts. Therefore, it is suggested that combination of the therapeutic approaches devoted to reduction of GAG levels with cyclin D1 inhibitors might be considered in further works on developing effective treatment procedures for MPS.
Keywords: Mucopolysaccharidosis, cell cycle, cyclins, pathomechanism, enzyme replacement therapy, substrate reduction therapy
Impact Statement
The cell cycle abnormalities were not systematically studied previously in the group of lysosomal storage diseases called mucopolysaccharidoses (MPS). In this report, we demonstrate that this crucial cellular process is significantly disturbed in all tested MPS types (11 out of 13 described to date). Both expression of many genes related to the cell cycle and the process itself are dysregulated in MPS fibroblasts, as indicated by transcriptomic and flow cytometry studies, respectively. The mechanism of this dysregulation of the cell cycle in MPS cells may be related to disturbed levels and timing of appearance of cyclin D. On the other hand, reduction of GAG storage could partially revert the abnormalities of the cell cycle in MPS, though no complete correction could be observed. These studies suggest that a novel approach of a combined treatment of MPS with a GAG-reducing agents and a cyclin D inhibitor might be considered.
Introduction
Mucopolysaccharidoses (MPS) is a group of inherited metabolic diseases defined as disorders of degradation of glycosaminoglycans (GAGs) leading to accumulation and storage of these compounds, primarily in lysosomes, and then also in the cytoplasm and outside cells.1,2 Among GAGs stored in MPS cells, following are predominant: dermatan sulfate (DS), heparan sulfate (HS), keratan sulfate (KS), chondroitin sulfate (CS), and hyaluronate. Several enzymes are involved in degradation of GAGs, thus, deficiency or a lack of activity of a specific enzyme from this group (due to the presence of pathogenic variant(s) of the corresponding gene) causes impaired decay of these compounds, leading to their accumulation which is considered the primary biochemical defect of MPS. Depending on the kind(s) of accumulated GAG(s) and the defective enzyme, MPS are divided into 13 types and subtypes, and MPS-like diseases (caused by defects in genes coding for proteins other than lysosomal enzymes but resulting in GAG storage due to other metabolic disturbances). 3
MPS are rare diseases, inherited predominantly in an autosomal recessive manner (with exception of MPS II which is X-linked), with cumulative prevalence estimated to about 3 per 100,000 live births. 4 However, some types are extremely rare, with only several cases reported worldwide to date, and MPS IIIE has been identified until now only in an animal model.3,4 Nevertheless, these diseases cause serious medical and social problems as their course is very severe, with average life span of 1–2 decades. 2 Despite introduction of some therapeutical procedures into the clinical practice, including bone marrow or hematopoietic stem cell transplantation and enzyme replacement therapy, and ongoing studies on other therapies, like substrate reduction therapy and gene therapy, we are still far from curing MPS or even stop the disease progressions at least if the treatment is not introduced very early (before appearance of specific symptoms), 5 – 13 which is further complicated due to diagnostic problems and frequent misdiagnosis. 14 Moreover, already approved therapies are available only for some MPS types. 9 In fact, a lack of success in abolition of most, if not all, symptoms of MPS after treatment which theoretically should dispose primary biochemical cause of the disease (like application of the active form of the missing enzyme that should allow degradation of stored GAGs) suggested that molecular mechanisms of MPS are more complex than just physical accumulation of GAGs.
In this light, it is now clear that GAG storage is only the first, but definitely not the only cellular problem occurring in MPS. Recent works indicated that secondary and tertiary effects of such a storage may significantly influence the course of the disease, and some of pathogenic processes may cause the specific defects unreversible if a therapeutic treatment is initiated too late. 15 – 17 On the other hand, discovery of details of molecular mechanisms of pathological changes appearing in MPS cells may lead to identify new therapeutic targets and development of novel, effective medical procedures. 13 In fact, recent studies identified various cellular processes which are specifically affected in MPS cells, including impaired regulations of cell activation, 18 composition and activities of proteasomes, 19 apoptosis, 20 and ion homeostasis, 21 as well as dysfunctions of various cellular organelles. 22 Interestingly, very recent report suggested that many of these changes may result from considerable changes in the levels of factors involved in the gene expression regulation, thus, levels of hundreds of transcripts are disturbed in MPS cells relative to control ones. 23
One of crucial processes which are not systematically investigated in MPS is the cell cycle. Only a few reports addressed this issue, but just a few MPS types were studied to find more or less significant disturbances in the cell cycle in human MPS II fibroblasts, 24 mouse MPS VII chondrocytes, 25 and murine MPS I fobroblasts. 26 Therefore, in this study we aimed to systematically investigate changes in the cell cycle in models of human fibroblasts derived from patients suffering from 11 types of MPS.
Materials and methods
Cell cultures
Lines of MPS and control fibroblasts (HDFa) were purchased from the Coriell Institute for Medical Research (Camden, NJ, USA). Following types of MPS were investigated (pathogenic variants of defective genes and/or specific notes are indicated in parentheses): MPS I (IDUA, p.Trp402Ter/p.Trp402Ter); MPS II (IDS, p.His70ProfsTer29); MPS IIIA (SGSH, p.Glu447Lys/p.Arg245His); MPS IIIB (NAGLU, p.Arg626Ter/p.Arg626Ter); MPS IIIC (HGSNAT, p.Gly262Arg/pArg509Asp); MPS IIID (GNS, p. Arg355Ter/p.Arg355Ter); MPS IVA (GALNS, p.Arg386Cys/p.Phe285Ter); MPS IVB (GLB1, p.Trp273Leu/p.Trp509Cys); MPS VI, ARSB, mutations not determined, diagnosis made on the basis of increased urinary GAG level and drastically decreased activity of N-acetylgalactosamine-4-sulfatase); MPS VII (GUSB, p.Trp627Cys/p.Arg356Ter); MPS IX (HYAL1, mutations not determined, diagnosis made on the basis of increased urinary GAG level and drastically decreased activity of hyaluronidase).
Cells were cultured in the DMEM medium, supplemented with 10% fetal bovine serum (FBS) and standard mixture of antibiotics, at 37°C and 95% humidity, and saturation with 5% CO2.
Transcriptomic studies
Transcriptomic analyses were conducted according to previously described procedures.18,20 Briefly, 5 x 105 cells were cultured overnight, and then lysed and homogenized using the QIAshredder columns (Qiagen, Hilden, Germany). RNA extraction was conducted with the RNeasy Mini kit (Qiagen, Hilden, Germany). DNA contamination was removed with Turbo DNase (Life Technologies, Carlsbad, CA, USA). Quality of RNA was then tested with Nano Chips RNA kit (Agilent Technologies, Santa Clara, CA, USA) and the Agilent 2100 Bioanalyzer System. Isolation of RNA was performed from 4 independent cultures of each cell line.
Libraries of mRNAs were constructed using the Illumina TruSeq Stranded mRNA Library Prep Kit. Reverse transcription was conducted to obtain cDNA libraries, and sequencing was performed using HiSeq4000 (Illumina, San Diego, CA, USA) (PE150; 150 bp paired-end). In this procedure, at least 4 x 107 raw reads and 12 Gb of raw data per sample were obtained in each experiment. Data quality assessment was performed using FastQC (version v0.11.7). The mapping of raw readings was conducted with the Ensembl database and the GRCh38 human reference genome, employing the Hisat2 v. 2.1.0 software. Cuffquant and Cuffmerge programs (version 2.2.1) were used to calculate levels of transcripts, with the GTF Homo_sapiens.GRCh38.94.gtf file from the Ensembl database and the FPKM algorithm (https://www.ensembl.org/index.html; December 5, 2021). The raw results are deposited under accession no. PRJNA562649, in the NCBI Sequence Read Archive (SRA).
The R software v3.4.3 was used for statistical analyses of transcriptomic data. One-way ANOVA and post hoc Student’s t-test with Bonferroni correction was employed to determine statistical significance between two groups with normal continuous distribution, with log2(1 + x) values. To determine false discovery rate (FDR), the Benjamini–Hochberg method was used. Classification of transcripts was performed using the Ensembl gene database (the BioMart interface; https://www.ensembl.org/info/data/biomart/index.html; December 5, 2021).
Cell cycle analysis
The cell cycle course was measured using The Muse® Cell Cycle Kit (Luminex Corporation, Austin, TX, USA). A total of 1.5 x 105 cells were passed into 60 mm plates and incubated overnight. Then, the medium was exchanged for the FBS-free one, and after 24 h, it was exchanged again for that supplemented with 10% FBS. The cultivation was prolonged for 14–24 h. When indicated, cells were treated with genistein (final concentration 50 µM), Aldurazyme (recombinant human α-L-iduronidase; Genzyme Ireland Ltd., Waterford, Ireland; final concentration 0.58 µg/ml) or Elaprase (recombinant human iduronate sulfatase; Shire Human Genetic Therapies AB, Danderyd, Sweden; final concentration 0.5 µg/ml). DMSO (final concentration 0.05%) was used as a control (solvent). After the indicated incubation time, cells were harvested and processed according to the manufacturer’s protocol and analyzed using Muse™ Cell Analyzer (Millipore Corporation, Hayward, CA, USA). Each experiment was repeated 3 times (3 independent cultures were analyzed).
Western-blotting
Fibroblasts (4 x 105 cells) were passed into 100 mm plates and grown overnight. Then, the medium was replaced with the FBS-free one, and the incubation was continued for 24 h. The medium was exchanged for DMEM with 10% FBS and the cultures were incubated for 14–24 h. At indicated times, cells were harvested and lysed with the lysis solution (50 mM Tris-HCl pH 7.5, 1% Triton X-100, 150 mM NaCl, 0.5 mM EDTA, and protease and phosphatase inhibitor cocktails (Roche Applied Science, Penzberg, Germany)). Proteins were separated and detected using the WES system (WES—Automated Western Blots with Simple Western; ProteinSimple, San Jose, CA, USA) for automatic Western blotting. The 12–230 kDa Separation Module with 8 × 25 capillary cartridges (#SM-W004; ProteinSimple, San Jose, CA, USA) were used for separation. Following antibodies against cyclins were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA): rabbit anti-cyclin D1 (#2922), mouse anti-cyclin E1 (#4129), mouse anti-cyclin A2 (#4656), mouse anti-cyclin B1 (#4135). For the detection of specific signals, secondary anti-rabbit antibodies and Anti-Rabbit Detection Module (#DM-001, ProteinSimple) or anti-mouse antibodies and Anti-Mouse Detection Module (#DM-002, ProteinSimple) were used. Protein levels were determined with the WES system, using the mouse anti-GAPDH−peroxidase antibody (#G9295, Sigma-Aldrich) to assess levels of GAPDH as a loading control. Analysis of proteins’ levels was performed using the ImageJ software (https://imagej.nih.gov/ij/). Each experiment was repeated 3 times (protein samples from 3 independent cultures were analyzed).
Results
Dysregulation of genes coding for proteins involved in cell cycle in MPS cells
Using the RNA-seq technology, we have analyzed expressions of genes coding for proteins involved in the cell cycle in MPS cells relative to control (HDFa) fibroblasts. Four independent experiments were performed for each cell line, and although each MPS type was represented by one line, such an experimental approach was demonstrated previously to ensure obtaining reliable results of global analyses of gene expression, especially when assessing several MPS types simultaneously. 18 – 23 In such analyses, genes involved in the cell cycle, grouped in the Gene Ontology (https://www.ebi.ac.uk/QuickGO) term GO:0007049 (named “cell cycle”) were taken into consideration.
We found that expression of relatively high number of genes coding for proteins involved in the cell cycle is changed in each tested MPS type, relative to control cells (Figure 1). Both up- and down-regulated transcripts were detected in each MPS cell line, while in some types the dysregulation was especially pronounced. Namely, the highest numbers of the cell cycle-related transcripts with levels significantly different relative to control cells were observed in MPS IX (137 transcripts), MPS IIID (116 transcripts), and MPS VII (105 transcripts). On the other hand, the least pronounced (though still considerable) changes were detected in MPS VI (25 transcripts), MPS II (36 transcripts) and MPS IVA (37 transcripts) (Figure 1).
Figure 1.
Cell cycle-related (GO:0007049, “cell cycle”) transcripts with significantly changed (at FDR < 0.1; p < 0.1) levels in MPS cell lines related to the control (HDFa) cell line. Numbers of up- and down-regulated transcripts in fibroblasts of specific MPS types are indicated.
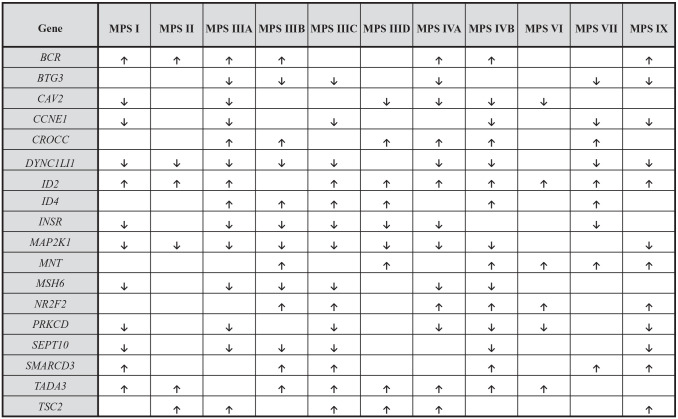
To answer the question if changes in expression of genes related to the cell cycle reflect a general feature of MPS, we assessed transcripts which were dysregulated in at least 6 (out of 11 tested) different MPS types. We found that this criterium was fulfilled by 18 genes (Figure 2). This indicates that expression of certain genes coding for proteins involved in the cell cycle is disturbed in most MPS types. Importantly, when analyzing every single gene from the list of these 18 genetic elements, it was either up- or down-regulated in each tested MPS type; in other words, there was no gene with expression up-regulated in some MPS types and down-regulated in other types (Figure 2). Such a pattern of gene expression dysregulation strongly suggests that there are common mechanisms operating in MPS cell, irrespective of the types of this disease, which lead to changes in transcription of cell cycle-related genes.
Figure 2.
Genes included into “cell cycle” (GO:0007049) term with expression significantly changed (at FDR < 0.1; p < 0.1) in most (6 or more) MPS types relative to control cells (HDFa). Up-regulation and down-regulation are marked by arrows, ↑ and ↓, respectively. Non-marked boxes indicate results in which no statistically significant differences between MPS and HDFa cells were determined.
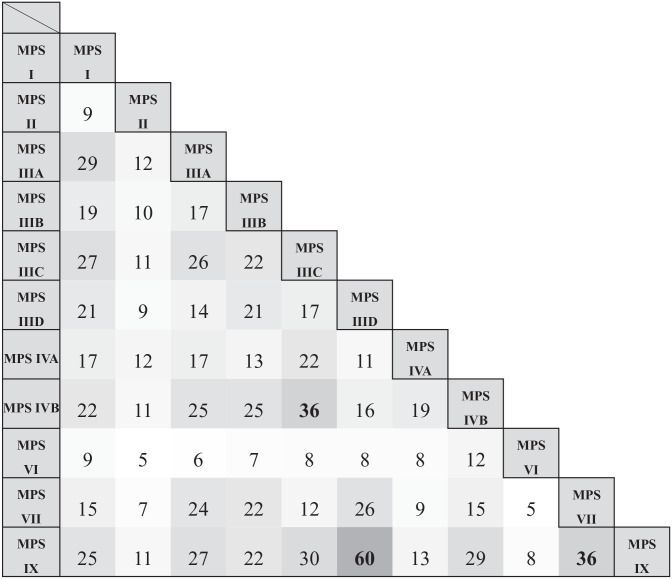
Considering this common feature of MPS diseases, we have compared particular types of MPS for number of shared genes which expression is changed relative to control fibroblasts. As expected, there were many genes with changed expression in various pairs of MPS types, with no single pair without any commonly dysregulated genes (Figure 3). Considering this feature, the biggest similarities were found between MPS IIID and MPS IX (60 common genes), MPS IIIC and MPS IVB (36 common genes), and MPS VII and MPS IX (36 common genes). These analyses corroborated the conclusion about the common feature of all MPS types, that is, changed regulation of expression of many genes related to the cell cycle. It was also possible to identify 16 genes from this group which are similarly regulated in 3 different MPS types which revealed the highest levels of similarity in the analysis presented in Figure 3. Namely, these specific 16 genes were dysregulated in similar manners in MPS IIID, MPS VII and MPS IX cells (Figure 4). Again, the indicated genes were always up- or down-regulated in the same way in these MPS types, confirming the common features of MPS cells in this process.
Figure 3.
Number of common cell cycle-related (GO:0007049, “cell cycle”) transcripts between different types of MPS which expression is significantly changed (at FDR < 0.1; p < 0.1) relative to control cells (HDFa).
Figure 4.

Common genes included into “cell cycle” (GO:0007049) term between MPS IIID, VII and IX which expression is significantly changed (at FDR < 0.1; p < 0.1) relative to control cells (HDFa), with indicated log2 of the fold change (log2FC).
We were also able to identify genes which expression is especially strongly changed in MPS cells. The transcripts which levels were at least 4-time higher or lower in MPS cells than in control fibroblasts (log2 fold change (FC) > 2 or <-2) are listed in Figure 5. Expression of some genes was highly dysregulated in several MPS types, and this group is exemplified with both up-regulated (e.g. ID2, ID4, LMNA, NR2 F2) and down-regulated (e.g. CDKN1A, CXCL8, PCLAF, RRM2) genes. Once more, there was no single gene up-regulated in one MPS type and down-regulated in another type.
Figure 5.
Genes included into “cell cycle” (GO:0007049) term with changed transcript levels at log2FC > 2.0 or log2FC < –2.0 in MPS types relative to control cells (HDFa).
To determine what specific processes related to the cell cycle could be affected in MPS cells, the genes dysregulated in these cells were divided into groups on the basis of child terms in the QuickGO database. As demonstrated in Figure 6, this analysis allowed as to identify following processes likely affected in MPS cells due to changes in the expression of corresponding genes: cell cycle process (GO:0022402), mitotic cell cycle (GO:0000278), regulation of cell cycle (GO:0051726) (including negative regulation of cell cycle (GO:0045786) and positive regulation of cell cycle (GO:0045787)), cell cycle DNA replication (GO:0044786), cell cycle checkpoint signaling (GO:0000075), regulation of cell cycle checkpoint (GO:1901976), cell cycle phase transition (GO:0044770), cell cycle G1/S phase transition (GO:0044843), and cell cycle G2/M phase transition (GO:0044839).
Figure 6.
Number of transcripts with significantly changed (at FDR < 0.1; p < 0.1) levels in MPS cell lines relative to control cells (HDFa) included into the cell cycle-related subprocesses, identified in the QuickGO database (https://www.ebi.ac.uk/QuickGO/).
In summary, transcriptomic analyses indicated global changes in expression of genes related to the cell cycle in MPS cells. These changes occurred in all MPS types, and their direction was common for all these disease types, that is, particular genes were either up- or down-regulated in all MPS types, strongly suggesting common mechanism(s) of these abnormalities.
Disturbances of the cell cycle in MPS fibroblasts
Since transcriptomic analyses indicated changes in expressions of genes coding for proteins involved in the cell cycle in all MPS types, we asked if this process is disturbed in all tested MPS types relative to control cells. To test this, cell cultures were synchronized by starvation, and after re-addition of FBS to the culture, incubation was continued for 14–24 h. Samples were withdrawn at 2 h time intervals and subjected to analyses by cytometry (using the Muse™ Cell Analyzer). Therefore, fractions of cells in particular phases of the cell cycle (G0/G1, S, G2/M) were determined.
Raw results of all experiments are demonstrated in Supplementary Figure S1. The summary of these results is shown in Figure 7, where significant changes in fractions of cells being in particular phases of the cell cycle between MPS and control cells are indicated as moderate (occurring in 1–3, out of 6, time points) or severe (occurring in 4–6, out of 6, time points). We found that the cell cycle was dysregulated in all types of MPS. Generally, in comparison to the control cell line, fractions of cells in the G0/G1 phase were increased in almost all MPS types (except types IVB and VII) and those in the G2/M phase were decreased in all MPS types. Fractions of cells in the S phase were either moderately increased (MPS types I, IIIA, IIIC, IVA, IVB, VI), unaffected (types II, IIIB, VII, IX) or moderately decreased (type IIID). These results indicate that in all MPS types the cell cycle was delayed relative to the control cells. Importantly, as in the case of transcriptomic analyses, a generally uniform effect on the cell cycle was observed in virtually all types of the disease.
Figure 7.
Changes in the progression of the cell cycle in MPS vs HDFa cells. The arrows demonstrate the significant (p < 0.05 in one-way ANOVA) changes in fractions of cells in indicated phases of the cell cycle. Down-headed arrows show decreases, and up-headed arrows show increases in fractions of MPS cells relative to HDFa. Thin and short arrows (↑ and ↓) denote moderate changes (1-3 time-points with significant differences), and thick and long arrows (↑ and ↓) denote severe changes (4 or more time-points with significant differences). Minus (-) indicates no statistically significant changes between MPS and HDFa cells. Panels A, B, and C show results of experiments with untreated cells, cells treated with genistein (at final concentration 50 µM), and cells treated with appropriate enzyme (α-L-iduronidase at final concentration 0.58 µg/ml or iduronate sulfatase at final concentration 0.5 µg/ml), respectively.
Since GAG storage is the primary biochemical defect in all MPS cells, we asked if reduction of levels of GAGs can restore the proper course of the cell cycle in these cells. Accumulation of GAGs in MPS cells can be effectively decreased in vitro by either providing an active form of the deficient enzyme or reduction of the rate of synthesis of these compounds. Since recombinant enzymes are available only for some MPS types, in our experiments we used two commercially sold products, aldurazyme (α-L-iduronidase) and elaprese (iduronate sulfatase) which are clinically used in treatment of patients suffering from MPS I and MPS II, respectively. 27 However, to decrease the levels of GAGs in all MPS types by employing a single method, we used genistein, an isoflavone which has been demonstrated previously to be effective in reducing the rate of GAG synthesis through inhibition of the epidermal growth factor-dependent pathway of signal transduction which is otherwise necessary to stimulate expression of genes coding for enzymes involved in GAG production. 28 We found that treatment with genistein considerably improved, or even corrected, the course of the cell cycle in most MPS types. In genistein-treated MPS cells, only moderate differences relative to control cells occurred in MPS types IIIA, IIIB, IIID, VII, and IX; however, even in these cases, considerable improvement could be observed relative to untreated MPS cells (Figure 7). Raw results of experiments with cells of all MPS types treated with genistein are demonstrated in Supplementary Figure S2. Treatment of MPS I and MPS II cells with specific enzymes, α-L-iduronidase and iduronate sulfatase, respectively, gave similar results (Supplementary Figure S3), with almost complete correction of the cell cycle (the only moderate changes relative to control fibroblasts were observed in the S phase of MPS I cells) (Figure 7).
In summary, the cell cycle is disturbed in all MPS types, however, it can be improved by either enzyme replacement therapy of substrate reduction therapy, at least in vitro.
Changes in levels of the cyclin D1 might contribute to disturbances of the cell cycle in MPS cells
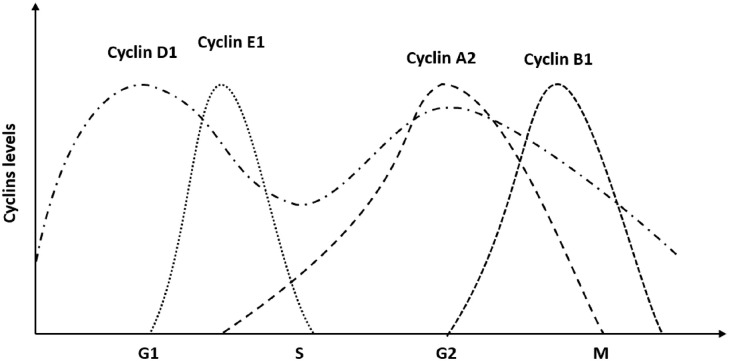
To learn about the molecular mechanism of cell cycle disturbances in MPS, we were looking for factors involved in the regulation of this process which might be dysregulated in affected cells. The cell cycle is regulated by various molecules, however, the crucial roles are played by cyclins. 29 These proteins appear in specific phases of the cell cycle and regulate its progression in cooperation with cyclin-dependent kinases. The distribution of various cyclins in cells throughout the cell cycle is presented schematically in Figure 8, and the regulatory process has been reviewed recently. 30
Figure 8.
A scheme demonstrating levels of certain cyclins during the cell cycle.
In this light, and because of identified dysregulation of the cell cycle in MPS cells, we have estimated levels of certain cyclins after starvation-mediated synchronization of the cultures of MPS cells and control fibroblasts. Cyclins D1, E1, A2, and B1 were investigated by Western-blotting in all tested types of MPS at various times after re-starting the cell growth, in comparison to HDFa cell line. Representative blots and quantification of the results (the average values of densitometric analyses from 3 independent experiments were calculated) are demonstrated in Supplementary Figure S4. The summary of the analysis of these results is presented in Figure 9. These experiments indicated that levels of cyclin D1 are significantly changed (predominantly increased) in most MPS types (except types I, IIIB, IVB, and IX) relative to the control cell line, and levels of cyclin B1 are increased in MPS IIIA and IVA. Moreover, the timing of appearance of elevated levels of cyclin D1 differs in most MPS cells from that observed in control ones (Supplementary Figure S4). Therefore, we suggest that changes in the levels of cyclin D1, and to some extent perhaps also cyclin B1, might considerably contribute to disturbances of the cell cycle in MPS cells.
Figure 9.
Changes in the levels of cyclins in MPS cells relative to control (HDFa) fibroblasts. The arrows ↓/↑ indicate changes in relative protein level. Down-headed arrows show decreases, and up-headed arrows show increases in levels of cyclins in MPS cells relative to HDFa. Thin and short arrows (↑ and ↓) denote moderate changes (1–3 time-points with significant differences), and thick and long arrows (↑ and ↓) denote severe changes (4 or more time-points with significant differences). Minus (-) indicates no statistically significant changes between MPS and HDFa cells.
Discussion
The primary cause of the diseases from the group of MPS are genetic defects in genes coding for enzymes involved in GAG degradation, and the storage of these compounds is the major biochemical defect in affected cells, responsible for specific symptoms. 1 – 3 However, the primary GAG storage is the only first step in the cascade of cellular changes which significantly contribute to disorders characteristic for MPS. This modified view on the pathomechanism of MPS has been discussed and summarized recently.13,31 It appears that various secondary and tertiary disturbances in cellular process can induce even more pronounced disorders in cell physiology (and further on functions of tissues and organs) than physical accumulation of GAGs itself. Moreover, it seems likely that some cellular defects cannot be effectively corrected even if GAG levels are subsequently normalized, potentially indicating the cause of the failure of abolishing all MPS symptoms by therapies focused solely on the reduction of GAG storge. 5 – 23 Therefore, it is crucial to understand complex pathomechanisms of MPS, in order to develop more effective therapeutic approaches. In fact, combinations of various therapies were already proposed as potential effective options, 32 – 34 however, it is unclear whether a mixture of already existing methods might be sufficient or introduction of additional treatments solving specific cellular problems should be considered together with reduction of the primary GAG storage.
One of crucial cellular processes which were not intensively studied in MPS to date is the cell cycle. To date, only a few reports were published in this field, and only a few MPS types (I, II, and VII) were investigated using either human cells or mouse models. 24 – 26 Those studies suggested that the cell cycle can be affected in MPS, therefore, we aimed to investigate this problem systematically in most of MPS types. Only very recently discovered types (MPS X and MPSPS), for which the biological material was not available at the time of starting our project, were not included in our experiments.
Our transcriptomic analysis indicated that expression of many genes coding for proteins involved in the cell cycle is dysregulated in fibroblasts of all tested MPS types (Figures 1 to 6). Importantly, when specific genes were taken into consideration, a strict rule was observed that each particular gene was dysregulated in the same manner (i.e. up-regulated or down-regulated) in all or almost all MPS types (Figures 2 and 4). These results indicate that there is a common mechanism of impaired control of transcription or post-transcriptional processes in virtually all MPS types. This conclusion was corroborated by results of our flow cytometric studies showing that the cell cycle is disturbed in all MPS types in a similar manner; namely, fractions of cells in the G0/G1 phase were increased in vast majority of MPS cell lines, and those of cells in the G2/M phase were decreased in all MPS types (Figure 7, Supplementary Figure S1). Interestingly, by estimating levels of crucial cyclins in tested cells, we found that cyclin D1, which normally accumulates in G1 and G2 phases (Figure 8), is more abundant in most MPS types than in control cells (Figure 9). Furthermore, not only the level but also the timing of appearance of high levels of this cyclin was improper in MPS cells (Supplementary Figure S4). Therefore, we propose that disturbed levels of cyclin D1 can significantly contribute to the pathomechanism of cell cycle impairment observed in MPS. Intriguingly, our transcriptomic analysis indicated that the levels of transcripts of the CCND1 gene, coding for cyclin D1, were not significantly affected in MPS cells. Therefore, other stages of the expression of this gene are likely dysregulated. In fact, regulation of the CCND1 gene expression is complex and involves various processes and mechanisms. 35
Since both degradation of already accumulated GAGs (in MPS I and MPS II fibroblasts) by relevant recombinant enzymes and reduction of efficiency of GAG synthesis (in all MPS types) by genistein caused a significant improvement in the cell cycle regulation in virtually all tested MPS cell lines (Figure 7, Supplementary Figures S2 and S3), we suggest that GAG storage has a crucial role in provoking the disturbance in the cell cycle. Such a pethomechanism appears reversible as the defect could be corrected by the enzymes or genistein. Nevertheless, some perturbations were still evident even after the above-mentioned treatments, especially in MPS I cells treated with α-L-iduronidase and MPS IIIA, IIIB, IIID, VII, and IX treated with genistein. Therefore, one might consider testing a combined therapy with the use of an agent decreasing the GAG accumulation together with a negative regulator of the cyclin D1. In fact, various inhibitors of this cyclin have already been developed, mainly as potential anti-cancer drugs, 35 which could nevertheless be considered when developing novel therapeutical strategies for MPS.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221114872 for Cell cycle disturbances in mucopolysaccharidoses: Transcriptomic and experimental studies on cellular models by Joanna Brokowska, Lidia Gaffke, Karolina Pierzynowska, Zuzanna Cyske and Grzegorz Węgrzyn in Experimental Biology and Medicine
Footnotes
Authors’ Contributions: J.B. conducted all experiments with testing the cell cycle and levels of cyclins, participated in specific analyses of transcriptomic data, and visualized all results. LG, KP, and ZC conducted the experimental part of the transcriptomic studies, and participated in basic analyses of transcriptomic results. GW was the principal investigator of the project, supervised the work, performed final analysis of the results, and drafted the manuscript. All authors contributed to work on the concept of the study and participated in reviewing and preparing the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Science Center, Poland (grant no. 2017/25/B/NZ2/00414).
Data Availability: The RNA-seq raw results have been deposited in the NCBI Sequence Read Archive (SRA) (https://www.ncbi.nlm.nih.gov/sra), and they are available at the accession no. PRJNA562649. Other raw results are available at request from the authors.
ORCID iD: Grzegorz Węgrzyn  https://orcid.org/0000-0003-4042-7466
https://orcid.org/0000-0003-4042-7466
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA. (eds) The online metabolic and molecular bases of inherited disease. New York: McGraw-Hill Education, 2019. DOI: 10.1036/ommbid.165 [Google Scholar]
- 2. Tomatsu S, Lavery C, Giugliani R, Hermatz P, Scarpa M, Węgrzyn G, Orii T. Mucopolysaccharidoses update, 2 volume set. New York: Nova Science Publishers, 2018. [Google Scholar]
- 3. Węgrzyn G, Pierzynowska K, Pavone LM. Editorial: molecular aspects of mucopolysaccharidoses. Front Mol Biosci 2022;9:874267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Çelik B, Tomatsu SC, Tomatsu S, Khan SA. Epidemiology of mucopolysaccharidoses update. Diagnostics 2021;11:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaffke L, Pierzynowska K, Piotrowska E, Węgrzyn G. How close are we to therapies for Sanfilippo disease. Metab Brain Dis 2018;33:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou J, Lin J, Leung WT, Wang L. A basic understanding of mucopolysaccharidosis: incidence, clinical features, diagnosis, and management. Intractable Rare Dis Res 2020;9:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Castro MJ, Del Toro M, Giugliani R, Couce ML. Gene therapy for neuronopathic mucopolysaccharidoses: state of the art. Int J Mol Sci 2021;22:9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pierzynowska K, Gaffke L, Cyske Z, Węgrzyn G, Buttari B, Profumo E, Saso L. Oxidative stress in mucopolysaccharidoses: pharmacological implications. Molecules 2021;26:5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McBride KL, Flanigan KM. Update in the mucopolysaccharidoses. Semin Pediatr Neurol 2021;37:100874. [DOI] [PubMed] [Google Scholar]
- 10. De Pasquale V, Sarogni P, Pistorio V, Cerulo G, Paladino S, Pavone LM. Targeting heparan sulfate proteoglycans as a novel therapeutic strategy for mucopolysaccharidoses. Mol Ther Methods Clin Dev 2018; 10:8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Pasquale V, Scerra G, Scarcella M, D’Agostino M, Pavone LM. Competitive binding of extracellular accumulated heparan sulfate reduces lysosomal storage defects and triggers neuronal differentiation in a model of mucopolysaccharidosis IIIB. Biochim Biophys Acta: Mol Cell Res 2021;1868:119113. [DOI] [PubMed] [Google Scholar]
- 12. Katrekar D, Yen J, Xiang Y, Saha A, Meluzzi D, Savva Y, Mali P. Efficient in vitro and in vivo RNA editing via recruitment of endogenous ADARs using circular guide RNAs. Nat Biotechnol 2022;40:938–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fecarotta S, Tarallo A, Damiano C, Minopoli N, Parenti G. Pathogenesis of mucopolysaccharidoses, an update. Int J Mol Sci 2020;21:2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wiśniewska K, Wolski J, Gaffke L, Cyske Z, Pierzynowska K, Węgrzyn G. Misdiagnosis in mucopolysaccharidoses. J Appl Genet. Epub ahead of print 13 May 2022. DOI: 10.1007/s13353-022-00703-1. [DOI] [PubMed] [Google Scholar]
- 15. Rappaport J, Manthe RL, Solomon M, Garnacho C, Muro S. A comparative study on the alterations of endocytic pathways in multiple lysosomal storage disorders. Mol Pharm 2016;13:357–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Pasquale V, Pavone LM. Heparan sulfate proteoglycans: the sweet side of development turns sour in mucopolysaccharidoses. Biochim Biophys Acta: Mol Basis Dis 2019;1865:165539. [DOI] [PubMed] [Google Scholar]
- 17. Pan C, Nelson MS, Reyes M, Koodie L, Brazil JJ, Stephenson EJ, Zhao RC, Peters C, Selleck SB, Stringer SE, et al. Functional abnormalities of heparan sulfate in mucopolysaccharidosis-I are associated with defective biologic activity of FGF-2 on human multipotent progenitor cells. Blood 2005;106:1956–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rintz E, Gaffke L, Podlacha M, Brokowska J, Cyske Z, Węgrzyn G, Pierzynowska K. Transcriptomic changes related to cellular processes with particular emphasis on cell activation in lysosomal storage diseases from the group of mucopolysaccharidoses. Int J Mol Sci 2020;21:3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pierzynowska K, Gaffke L, Jankowska E, Rintz E, Witkowska J, Wieczerzak E, Podlacha M, Węgrzyn G. Proteasome composition and activity changes in cultured fibroblasts derived from mucopolysaccharidoses patients and their modulation by genistein. Front Cell Dev Biol 2020;8:540726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brokowska J, Pierzynowska K, Gaffke L, Rintz E, Węgrzyn G. Expression of genes involved in apoptosis is dysregulated in mucopolysaccharidoses as revealed by pilot transcriptomic analyses. Cell Biol Int 2021;45:549–57 [DOI] [PubMed] [Google Scholar]
- 21. Gaffke L, Szczudło Z, Podlacha M, Cyske Z, Rintz E, Mantej J, Krzelowska K, Węgrzyn G, Pierzynowska K. Impaired ion homeostasis as a possible associate factor in mucopolysaccharidosis pathogenesis: transcriptomic, cellular and animal studies. Metab Brain Dis 2022;37:299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaffke L, Pierzynowska K, Rintz E, Cyske Z, Giecewicz I, Węgrzyn G. Gene expression-related changes in morphologies of organelles and cellular component organization in mucopolysaccharidoses. Int J Mol Sci 2021;22:2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cyske Z, Gaffke L, Pierzynowska K, Węgrzyn G. Complex changes in the efficiency of the expression of many genes in monogenic diseases, mucopolysaccharidoses, may arise from significant disturbances in the levels of factors involved in the gene expression regulation processes. Genes 2022;13:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moskot M, Gabig-Cimińska M, Jakóbkiewicz-Banecka J, Węsierska M, Bocheńska K, Węgrzyn G. Cell cycle is disturbed in mucopolysaccharidosis type II fibroblasts, and can be improved by genistein. Gene 2016;585:100–3 [DOI] [PubMed] [Google Scholar]
- 25. Jiang Z, Derrick-Roberts ALK, Reichstein C, Byers S. Cell cycle progression is disrupted in murine MPS VII growth plate leading to reduced chondrocyte proliferation and transition to hypertrophy. Bone 2020;132:115195. [DOI] [PubMed] [Google Scholar]
- 26. Węsierska M, Kloska A, Medina DL, Jakóbkiewicz-Banecka J, Gabig-Cimińska M, Radzińska M, Moskot M, Malinowska M. Cellular and gene expression response to the combination of genistein and kaempferol in the treatment of mucopolysaccharidosis type I. Int J Mol Sci 2022;23:1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parini R, Deodato F. Intravenous enzyme replacement therapy in mucopolysaccharidoses: clinical effectiveness and limitations. Int J Mol Sci 2020;21:2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jakóbkiewicz-Banecka J, Piotrowska E, Narajczyk M, Barańska S, Węgrzyn G. Genistein-mediated inhibition of glycosaminoglycan synthesis, which corrects storage in cells of patients suffering from mucopolysaccharidoses, acts by influencing an epidermal growth factor-dependent pathway. J Biomed Sci 2009;16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uzbekov R, Prigent C. A journey through time on the discovery of cell cycle regulation. Cells 2022;11:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Z. Regulation of cell cycle progression by growth factor-induced cell signaling. Cells 2021;10:3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaffke L, Pierzynowska K, Podlacha M, Brokowska J, Węgrzyn G. Changes in cellular processes occurring in mucopolysaccharidoses as underestimated pathomechanisms of these diseases. Cell Biol Int 2021; 45:498–506 [DOI] [PubMed] [Google Scholar]
- 32. Gabig-Cimińska M, Jakóbkiewicz-Banecka J, Malinowska M, Kloska A, Piotrowska E, Chmielarz I, Moskot M, Węgrzyn A, Węgrzyn G. Combined therapies for lysosomal storage diseases. Curr Mol Med 2015;15:746–71 [DOI] [PubMed] [Google Scholar]
- 33. Pierzynowska K, Gaffke L, Podlacha M, Brokowska J, Węgrzyn G. Mucopolysaccharidosis and autophagy: controversies on the contribution of the process to the pathogenesis and possible therapeutic applications. Neuromolecular Med 2020;22:25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Safary A, Moghaddas-Sani H, Akbarzadeh-Khiavi M, Khabbazzi A, Rafi MA, Omidi Y. Enzyme replacement combinational therapy: effective treatments for mucopolysaccharidoses. Expert Opin Biol Ther 2021;21:1181–97 [DOI] [PubMed] [Google Scholar]
- 35. González-Ruiz L, González-Moles MÁ, González-Ruiz I, Ruiz-Ávila I, Ayén Á, Ramos-García P. An update on the implications of cyclin D1 in melanomas. Pigment Cell Melanoma Res 2020;33:788–805 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221114872 for Cell cycle disturbances in mucopolysaccharidoses: Transcriptomic and experimental studies on cellular models by Joanna Brokowska, Lidia Gaffke, Karolina Pierzynowska, Zuzanna Cyske and Grzegorz Węgrzyn in Experimental Biology and Medicine