Abstract
Introduction
Survival following colorectal cancer (CRC) has improved in the US since 1975, but there is limited information on stage-specific survival trends among racial and ethnic subgroups.
Objectives
The purpose of this study was to estimate and compare trends in 1- and 5-year CRC cause-specific survival in the United States by both stage and race/ethnicity.
Methods
We performed a retrospective cohort study of individuals diagnosed with CRC using the 1992-2018 Surveillance, Epidemiology and End Results (SEER) database. We estimated and compared time trends in 1- and 5-year survival for CRC stage by race/ethnicity.
Results
Data from 399 220 individuals diagnosed with CRC were available. There were significant differences in stage-specific 1-year survival trends by race and ethnicity. Differences were most notable for distant stage CRC: survival probabilities increased most consistently for non-Hispanic American Indian/Alaska Native (AIAN) and Black (NHB) persons, but their trend lines were lower than those of Hispanic, and non-Hispanic Asian/Pacific Islander (API) and White (NHW) persons, whose initially greater gains appear to be slowing. Although the data do not support significant racial/ethnic differences in 5-year CRC survival trends by stage, AIAN and NHB persons have the lowest average survival probabilities for multiple CRC stages, and no racial/ethnic group has 5-year survival probabilities above 20% for distant-stage CRC.
Conclusion
Although there has been an overall improvement in adjusted CRC-specific survival probabilities since 1992, AIAN and NHB persons continue to experience worse prognosis than those of other races/ethnicities. This highlights the importance of reinvigorating efforts to understand the causes of mortality in CRC, including those which may differ according to an individual’s race or ethnicity.
Keywords: colorectal cancer, cause-specific survival, trends, minority health, disparities
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer deaths in the United States.1,2 It has been estimated that 151 030 people will be newly diagnosed with CRC and 52 580 people with die from CRC in 2022.2,3 Although CRC mortality rates have decreased significantly since 1975,1-5 available reports suggest that these declines vary substantially by the stage of the disease, and that disparities in mortality rates for racial and ethnically minoritized groups persist.4-15 For example, according to a report by the American Cancer Society, the 2-year relative survival rate for distant-stage CRC increased from 21% in mid-1990’s to 37% for those diagnosed during 2009-2015, with commensurate improvements for regional- and localized-stage CRC.2 This same report asserts that CRC mortality rates are highest for persons who are non-Hispanic Black, followed by those who are American Indian or Alaska Native, and lowest for those who are Asian or Pacific Islander.2
Several studies have compared trends in CRC mortality by the stage of the disease and race but have done so under broad classifications as follows: White and Black;4,7-10 White, Black, and Hispanic;11,16,17 White, Black, Asian, Hispanic, and other;18 or White, Black, and other.19 In particular, limited information is available on CRC survival trends in persons who are American Indian or Alaska Native.20-22 A 2010 study by Edwards et al,5 compared CRC mortality trends in persons belonging to five racial and ethnic groups, but more than 10 years have passed since that report.
To the best of our knowledge, no study has simultaneously compared recent trends in CRC-specific mortality by stage of diagnosis for individuals that are non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic Asian or Pacific Islander, and non-Hispanic American Indian or Alaska Native. To address this gap, we undertook an analysis of population-based CRC cause-specific survival data to understand the trends in 1- and 5-year CRC cause-specific survival probabilities by stage of diagnosis within five racial and ethnic groups for individuals who received a CRC diagnosis between 1992 and 2018. Understanding differences in cause-specific survival, both over time and across CRC stages, is critical for further investigations into the social, structural, and political determinants that contribute to the disparities noted in CRC outcomes among individuals from distinct racial and ethnic groups. Through our efforts, we aim to provide new impetus to refocus efforts on improving CRC detection and treatment among racial and ethnically minoritized populations.
Methods
Study Design and Data Source
This study was based on a retrospective cohort of CRC patients, ascertained on a population level, with data captured as part of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program. The SEER program collects cancer incidence and survival data from population-based cancer registries representing approximately 35% of the U.S. population.23 Data from the 12 SEER registries for the period 1992 to 2018 were used in this analysis. The 12 SEER registries cover Alaska, Connecticut, Atlanta, rural Georgia, San Francisco-Oakland, San Jose-Monterey, Hawaii, Iowa, Los Angeles, New Mexico, Seattle-Puget Sound and Utah.24 This project, Study ID: 21-102, was reviewed by the University of New Mexico Health Sciences Institutional Review Board, and was granted exemption on 31 March 2021 according to Category 4: Secondary research on data or specimens (no consent required). This report conforms to RECORD guidelines for SEER-based studies.25 This study was supported by funding from the National Cancer Institute; the funders played no other role in this work. Researchers desiring additional details about the data and programs used to carry out this work may obtain programming code from the corresponding author.
Study Population and Variables
We used SEER*Stat software (version 8.4.0)26 as the data source for this study. We used the “Incidence––SEER Research Plus Data, 12 Registries, Nov 2021 Sub (1992-2019)” database. We included a consecutive series of all individuals who received their first primary CRC diagnosis with malignant behavior from 1992 through 2018. We excluded “All Death Certificate Only and Autopsy Only” and “Alive with No Survival Time” via checkboxes in SEER*Stat. We also excluded CRC cases without a record for summary stage at diagnosis, as well as those with unknown age at diagnosis. We included all CRC cases that originated from the cecum, ascending colon, hepatic flexure of colon, transverse colon, splenic flexure of colon, descending colon, sigmoid colon, overlapping lesion of colon, colon not otherwise specified, rectosigmoid junction, and rectum. We classified CRC cases as localized, regional and distant stage using SEER’s “Combined Summary Stage (2004+)” classifications, supplemented with values from the “Historic Stage A (1973-2015)” variable when necessary. In-situ cancers and cases that were un-staged/unknown were excluded. These rules, as applied in SEER*Stat 8.0.4, defined the number of individuals included in this study.
The SEER program works closely with providers of cancer care in their population-based catchment areas to collect patient- and cancer-specific information. Because of its population-based nature, it is typically necessary to rely on medical records as the source of the demographic and other data. SEER-defined race and ethnic categories were utilized and labeled as follows: Non-Hispanic White (NHW), Non-Hispanic Black (NHB), Hispanic, Non-Hispanic American Indian/Alaska Native (AIAN), and Non-Hispanic Asian or Pacific Islander (API). Additional variables were extracted from SEER, and estimates of CRC-specific survival probabilities were obtained for combinations of these variables in the primary analyses: sex as assigned at birth (male or female), age at diagnosis (categorized into decades), year of diagnosis, grade (grades I-IV, or unknown), and Rural-Urban Continuum Code in 2003 (RUCC, coded as: Metro Counties, Non-Metro Counties [Metro-Adjacent], Non-Metro Counties [not Metro-Adjacent], and Alaska or Hawaii, or unknown).
The outcome of interest in this work was cause-specific survival for CRC, estimated at 1- and 5-years post diagnosis. We used SEER*Stat software (version 8.4.0)26 to calculate 1- and 5-year cause-specific survival probabilities of CRC using the “Incidence – SEER Research Plus Data, 12 Registries, Nov 2021 (1992-2019)” database while relying on the rules implemented in SEER*Stat for loss to follow-up. We obtained cause-specific survival probabilities and their standard errors within combinations of the characteristics of interest, ie, year of diagnosis, race and ethnicity, stage, sex, grade, and RUCC. No person-level data were used, nor were the SEER data linked to any other data sources. Using SEER*Stat 8.4.0, we formed tables of cause-specific survival probabilities and their standard errors according to all combinations of the factors of interest, with separate “Pages” defined for combinations of Year of diagnosis, age at diagnosis (in decades), and RUCC groups, and with rows defined by combinations of race/ethnicity, summary stage, grade, and sex. We used text processing approaches to form an analysis-ready data set which enumerated the numbers of individuals with a primary CRC diagnosis, and estimates of cause-specific survival probabilities and standard errors, within categories defined by all of the factors of interest in this study.
Statistical Analysis
We summarized the numbers and percentages of individuals with CRC diagnoses within the levels of the variables of interest. We used linear models to perform meta-regressions, weighting by the inverse of the squared standard errors, to estimate the degree to which the combinations of the variables of interest explained differences in the estimated cause-specific survival probabilities. When the SEER-estimated standard errors of survival probabilities were equal to zero, we approximated them with the square root of the variance of the binomial distribution, calculated after adding a value of .5 to both the numerator and denominator counts reported by SEER for the relevant group. We modelled the survival probabilities obtained at 1 year and 5 years post-diagnosis separately. The initial models included all possible interactions among race/ethnicity, stage, and year of diagnosis, while controlling for potential confounding by age at diagnosis (categorized into decades), sex, grade, and RUCC. We simplified these initial models subsequently modelling year-at-diagnosis trends with natural cubic spline basis functions, and selected the degree of smoothing that best explained the race by stage by year of diagnosis trends by minimizing Akaike’s Information Criterion (AIC) while adjusting for the other factors. We obtained smoothed trend estimates within groups defined by race and ethnicity, and by CRC stage at diagnosis, from a separate model for each of the two follow-up time periods. These final models were obtained by removing non-significant interactions in a hierarchical fashion. Analyses were performed using the tools available in SEER*Stat26 and SAS 9.4 (SAS Institute Inc, Cary, NC). Two-sided type I error was set at .05 for tests of significance.
Results
This study was based on the 1975-2019 SEER Research Plus Data (November 2021 Submission), which contains information on 425 520 CRC diagnoses from 1992 through 2018. Excluding those individuals without a declared race or ethnicity, and with unknown stage at diagnosis, removed 1372 (.3%) and 24 928 (5.9%) individuals, respectively. This report is based on analysed data from 399 220 individual CRC diagnoses with known race/ethnicity and stage at diagnosis from 1992 through 2018, or 93.8% of all CRC diagnoses from this time period. Table 1 contains tabulations of the numbers of individuals according to levels of the variables of interest, overall and within race and ethnic groups. There was at least 1 year of follow-up for 318 443 (79.8%) persons with CRC eligible at baseline.
Table 1.
Characteristics of Patients With Colorectal Cancer From 1992 to 2018, by Racial and Ethnic Groups.
| AIAN | API | Hispanic | NHB | NHW | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | n | % | n | % | n | % | ||
| Total | 3931 | 1.0 | 45 869 | 11.5 | 41 947 | 10.5 | 34 164 | 8.6 | 273 309 | 68.5 | 399 220 | 100.0 | |
| Sex | Male | 1979 | 50.3 | 24 400 | 53.2 | 22 216 | 53.0 | 16 674 | 48.8 | 139 897 | 51.2 | 205 166 | 51.4 |
| Female | 1952 | 49.7 | 21 469 | 46.8 | 19 731 | 47.0 | 17 490 | 51.2 | 133 412 | 48.8 | 194 054 | 48.6 | |
| Age | <40 years | 214 | 5.4 | 1572 | 3.4 | 2415 | 5.8 | 1217 | 3.6 | 5999 | 2.2 | 11 417 | 2.9 |
| 40-49 years | 459 | 11.7 | 4070 | 8.9 | 4553 | 10.9 | 3401 | 10.0 | 16 466 | 6.0 | 28 949 | 7.3 | |
| 50-59 years | 918 | 23.4 | 9241 | 20.1 | 9308 | 22.2 | 7813 | 22.9 | 41 018 | 15.0 | 68 298 | 17.1 | |
| 60-69 years | 1042 | 26.5 | 11 404 | 24.9 | 10 656 | 25.4 | 9161 | 26.8 | 60 839 | 22.3 | 93 102 | 23.3 | |
| 70-79 years | 865 | 22.0 | 11 407 | 24.9 | 9130 | 21.8 | 7772 | 22.7 | 78 108 | 28.6 | 107 282 | 26.9 | |
| 80+ years | 433 | 11.0 | 8175 | 17.8 | 5885 | 14.0 | 4800 | 14.0 | 70 879 | 25.9 | 90 172 | 22.6 | |
| Summary stage | Localized | 1520 | 38.7 | 19 182 | 41.8 | 16 575 | 39.5 | 13 491 | 39.5 | 115 254 | 42.2 | 166 022 | 41.6 |
| Regional | 1491 | 37.9 | 17 833 | 38.9 | 16 159 | 38.5 | 12 001 | 35.1 | 103 896 | 38.0 | 151 380 | 37.9 | |
| Distant | 920 | 23.4 | 8854 | 19.3 | 9213 | 22.0 | 8672 | 25.4 | 54 159 | 19.8 | 81 818 | 20.5 | |
| Year of diagnosis | 1992-95 | 348 | 8.9 | 4583 | 10.0 | 3623 | 8.6 | 4074 | 11.9 | 43 151 | 15.8 | 55 779 | 14.0 |
| 1996-99 | 454 | 11.6 | 5583 | 12.2 | 4514 | 10.8 | 4491 | 13.2 | 44 925 | 16.5 | 59 967 | 15.0 | |
| 2000-03 | 481 | 12.2 | 6710 | 14.6 | 5277 | 12.6 | 4959 | 14.5 | 44 343 | 16.2 | 61 770 | 15.4 | |
| 2004-07 | 587 | 14.9 | 7116 | 15.5 | 6368 | 15.2 | 5439 | 15.9 | 40 992 | 15.0 | 60 502 | 15.2 | |
| 2008-11 | 657 | 16.7 | 7811 | 17.0 | 7220 | 17.2 | 5607 | 16.4 | 38 192 | 13.9 | 59 487 | 14.9 | |
| 2012-15 | 778 | 19.8 | 7897 | 17.2 | 7997 | 19.0 | 5406 | 15.8 | 35 536 | 13.0 | 57 614 | 14.4 | |
| 2016-18 | 626 | 15.9 | 6169 | 13.4 | 6948 | 16.6 | 4188 | 12.3 | 26 170 | 9.5 | 44 101 | 11.0 | |
| Grade | I | 375 | 9.5 | 3497 | 7.6 | 4335 | 10.3 | 3153 | 9.2 | 23 936 | 8.8 | 35 296 | 8.8 |
| II | 2260 | 57.5 | 27 757 | 60.5 | 23 477 | 56.0 | 19 375 | 56.7 | 155 562 | 56.9 | 228 431 | 57.2 | |
| III | 448 | 11.4 | 6269 | 13.7 | 5753 | 13.7 | 4344 | 12.7 | 46 961 | 17.2 | 63 775 | 16.0 | |
| IV | 56 | 1.4 | 445 | 1.0 | 547 | 1.3 | 415 | 1.2 | 4394 | 1.6 | 5857 | 1.5 | |
| Unknown | 792 | 20.1 | 7901 | 17.2 | 7835 | 18.7 | 6877 | 20.1 | 42 456 | 15.5 | 65 861 | 16.5 | |
| Rural-urban continuum code | Metro counties | 1500 | 38.2 | 40 557 | 88.4 | 38 814 | 92.5 | 33 186 | 97.1 | 228 179 | 83.5 | 342 236 | 85.7 |
| Non-metro counties (metro-adjacent) | 383 | 9.7 | 112 | 0.2 | 1301 | 3.1 | 540 | 1.6 | 23 584 | 8.6 | 25 920 | 6.5 | |
| Non-metro counties (not metro-adjacent) | 396 | 10.1 | 1654 | 3.6 | 1691 | 4.0 | 410 | 1.2 | 20 426 | 7.5 | 24 577 | 6.2 | |
| Alaska or Hawaii | 1644 | 41.8 | 0 | 0.0 | 5 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1649 | 0.4 | |
| Unknown | 8 | 0.2 | 3546 | 7.7 | 136 | 0.3 | 28 | 0.1 | 1120 | 0.4 | 4838 | 1.2 | |
One- and Five-Year Survival: All Persons With CRC
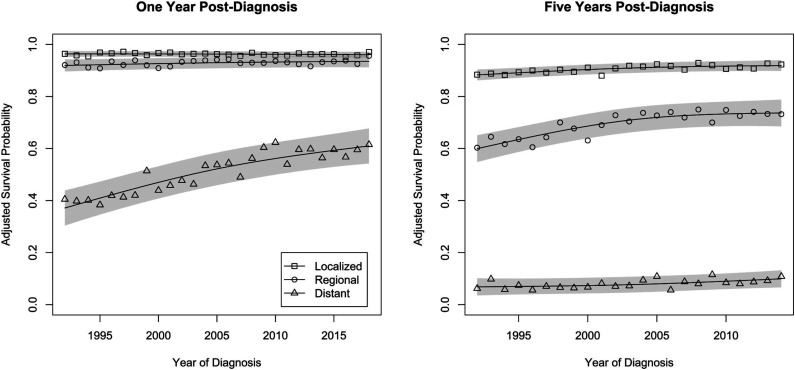
Adjusted 1- and 5-year cause-specific survival probabilities following CRC diagnosis by stage and year of diagnosis are shown in Figure 1. For those diagnosed with local stage CRC, the estimate of linear trend in 1-year survival probabilities was negligible, at −.01% ([95% Confidence Interval (CI)] −.03%-.01%) per year, with an average 1-year survival probability of 96.2% (93.8%-98.6%). Those diagnosed with regional stage CRC displayed a small improvement over time in 1-year survival probabilities, .06% (.001%-.12%) per year. Their average 1-year survival probability was 93.0% (90.5%-95.5%). For those diagnosed with distant stage CRC, there was a significant improvement in 1-year survival probabilities over time (P < .001), but there was evidence of departure from a linear trend (P = .04). The slope of the trend line suggested an improvement in survival probability of 1.0% (.8%-1.2%) per year in 2005, with instantaneous slopes suggesting greater gains, by .05% (.01%-.09%), for each year prior to 2005 and slower gains by that same amount for each year following 2005. Their average 1-year survival probability was 52.8% (48.1%-57.5%).
Figure 1.
Trends in 1- and 5-year adjusted cause-specific survival probabilities for individuals diagnosed at different stages of colorectal cancer. Shaded bands reflect 95% prediction intervals for the year-specific survival probabilities.
Those diagnosed with local stage CRC experienced improvements in 5-year cause-specific survival, with an average improvement of .16% (.10%-.21%) per year. Their average 5-year survival probability was 90.9% (87.9%-93.8%). Those diagnosed with regional stage CRC displayed significant improvement in 5-year survival probabilities over time (P < .001), but there was evidence of significant departure from a linear trend (P = .004), such that the slope of the trend line showed an improvement of .55% (.41%-.69%) per year in 2003, with instantaneous slopes suggested greater gains, by .08% (.04-.12%), for each year prior to 2003 and slower gains by that same amount for each year following 2003. Their average 5-year survival probability was 70.3% (66.5%-74.0%). For those diagnosed with distant stage CRC, there was a significant but small improvement over time in 5-year survival probabilities, .15% (.5%-.25%) per year. Their average 5-year survival probability was 8.1% (4.4%-11.8%).
One-Year Survival by Race and Ethnicity
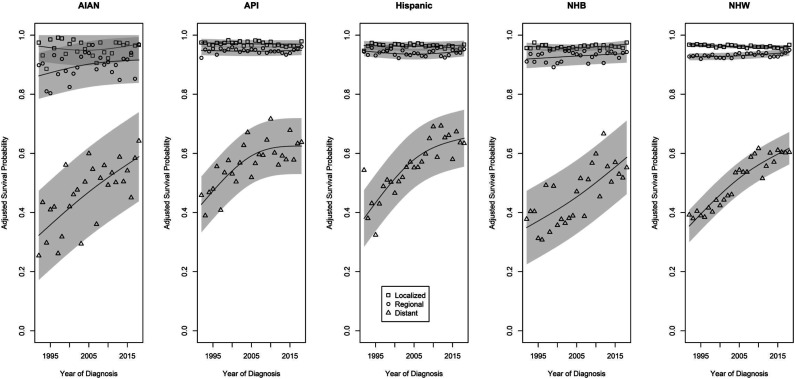
Estimates of adjusted 1-year cause-specific survival probabilities following diagnosis are shown for all race/ethnicity by stage combinations in Tables 2 and 3. Trends in 1-year survival for those of different races/ethnicities who were diagnosed at different stages of CRC differed significantly (P < .001, see Figure 2). For those diagnosed with localized stage CRC, 1-year survival probabilities were consistently high for individuals of all race and ethnic groups. AIAN persons experienced lower 1-year survival probabilities than those from other race and ethnic groups over the study period. Although due to lack of precision in the estimates, the difference was only significantly different when compared to API persons; average 1-year survival probabilities were 1.5 (.5-2.5) percentage points lower for AIAN than for API persons.
Table 2.
Unadjusted 1-Year Cause-Specific Survival Probabilities (%), by Race and Ethnicity, Stage, and Year of Diagnosis.
| Localized | Regional | Distant | Overall | ||
|---|---|---|---|---|---|
| Race/ethnicity | Year of Dx | Survival (95% CI) | Survival (95% CI) | Survival (95% CI) | Survival (95% CI) |
| AIAN | 1992 | 100 (84.6-100) | 91.6 (75.4-100) | 27.0 (5.6-48.4) | 72.9 (62.6-83.2) |
| AIAN | 1996 | 96.9 (81.8-100) | 95.7 (77.9-100) | 41.8 (23.5-60.1) | 78.1 (68.2-88.0) |
| AIAN | 2000 | 98.7 (83.6-100) | 83.2 (65.9-100) | 43.0 (20.0-66.0) | 75.0 (64.1-85.8) |
| AIAN | 2004 | 96.7 (85.6-100) | 96.4 (84.8-100) | 51.8 (35.8-67.7) | 81.6 (74.1-89.2) |
| AIAN | 2008 | 100 (86.4-100) | 95.5 (83.0-100) | 53.5 (35.5-71.5) | 83.0 (74.4-91.6) |
| AIAN | 2012 | 100 (88.9-100) | 91.6 (79.1-100) | 52.7 (38.0-67.4) | 81.4 (74.0-88.8) |
| AIAN | 2016 | 96.0 (85.5-100) | 95.9 (85.6-100) | 46.5 (33.4-59.6) | 79.5 (72.9-86.0) |
| AIAN | 2018 | 100 (90.7-100) | 99.3 (88.9-100) | 83.5 (67.2-99.8) | 94.3 (87.1-100) |
| API | 1992 | 99.5 (96.2-100) | 94.6 (90.8-98.3) | 46.5 (38.4-54.6) | 80.2 (77.0-83.3) |
| API | 1996 | 99.4 (97.1-100) | 95.8 (92.6-98.9) | 56.5 (48.7-64.3) | 83.9 (81.0-86.8) |
| API | 2000 | 99.2 (97.0-100) | 97.9 (95.8-99.9) | 52.8 (45.0-60.6) | 83.3 (80.5-86.1) |
| API | 2004 | 99.6 (97.9-100) | 97.3 (95.1-99.5) | 68.6 (62.7-74.4) | 88.5 (86.3-90.6) |
| API | 2008 | 99.7 (98.3-100) | 98.4 (96.5-100) | 59.5 (53.9-65.0) | 85.9 (83.9-87.9) |
| API | 2012 | 99.4 (97.9-100) | 96.9 (94.7-99.2) | 55.8 (49.4-62.3) | 84.1 (81.7-86.4) |
| API | 2016 | 99.1 (97.4-100) | 98.4 (96.7-100) | 57.6 (51.8-63.3) | 85.0 (82.9-87.1) |
| API | 2018 | 99.6 (98.5-100) | 97.8 (96.0-99.6) | 69.1 (62.7-75.5) | 88.8 (86.6-91.1) |
| Hispanic | 1992 | 97.0 (93.0-100) | 96.7 (93.3-100) | 55.6 (46.2-64.9) | 83.1 (79.5-86.7) |
| Hispanic | 1996 | 98.8 (95.8-100) | 96.1 (92.7-99.5) | 43.4 (35.7-51.1) | 79.4 (76.4-82.4) |
| Hispanic | 2000 | 99.2 (96.6-100) | 96.3 (93.3-99.4) | 47.0 (39.8-54.2) | 80.8 (78.1-83.6) |
| Hispanic | 2004 | 98.9 (96.5-100) | 95.8 (92.9-98.7) | 57.8 (50.8-64.8) | 84.2 (81.5-86.8) |
| Hispanic | 2008 | 99.1 (97.3-100) | 96.2 (93.5-98.8) | 60.7 (54.4-67.1) | 85.3 (83.0-87.7) |
| Hispanic | 2012 | 99.2 (97.4-100) | 95.8 (93.3-98.2) | 71.6 (66.6-76.7) | 88.9 (86.9-90.8) |
| Hispanic | 2016 | 98.9 (97.1-100) | 98.2 (96.6-99.7) | 69.0 (64.0-74.1) | 88.7 (86.9-90.6) |
| Hispanic | 2018 | 99.6 (98.6-100) | 97.8 (96.1-99.5) | 58.8 (52.5-65.0) | 85.4 (83.2-87.6) |
| NHB | 1992 | 98.0 (94.7-100) | 93.7 (89.6-97.8) | 38.5 (30.0-47.0) | 76.7 (73.4-80.1) |
| NHB | 1996 | 98.2 (94.7-100) | 96.3 (93-99.6) | 30.1 (23.1-37.2) | 74.9 (72.0-77.7) |
| NHB | 2000 | 97.3 (93.8-100) | 93.2 (89.4-97.0) | 34.5 (27.3-41.7) | 75.0 (72.0-77.9) |
| NHB | 2004 | 98.4 (95.6-100) | 97.0 (94.3-99.7) | 37.0 (30.7-43.3) | 77.5 (75.0-79.9) |
| NHB | 2008 | 98.6 (96.2-100) | 93.3 (89.4-97.2) | 51.4 (44.7-58.1) | 81.1 (78.4-83.8) |
| NHB | 2012 | 99.5 (97.2-100) | 93.6 (90.0-97.1) | 68.5 (62.1-74.8) | 87.2 (84.7-89.7) |
| NHB | 2016 | 99.5 (97.2-100) | 96.0 (93.0-99.0) | 53.1 (47.2-58.9) | 82.9 (80.5-85.2) |
| NHB | 2018 | 99.3 (97.5-100) | 97.0 (94.2-99.8) | 57.1 (49.6-64.6) | 84.5 (81.7-87.2) |
| NHW | 1992 | 98.6 (97.9-99.2) | 94.6 (93.5-95.7) | 38.0 (35.0-40.9) | 77.0 (76.0-78.1) |
| NHW | 1996 | 98.9 (98.3-99.5) | 96.1 (95.1-97.0) | 36.7 (33.8-39.5) | 77.2 (76.2-78.2) |
| NHW | 2000 | 99.0 (98.5-99.6) | 94.3 (93.2-95.4) | 40.9 (37.9-43.9) | 78.1 (77.0-79.1) |
| NHW | 2004 | 98.9 (98.3-99.5) | 96.1 (95.1-97.1) | 52.6 (49.7-55.4) | 82.5 (81.5-83.6) |
| NHW | 2008 | 99.2 (98.6-99.7) | 97.0 (96.1-98.0) | 58.6 (55.6-61.7) | 85.0 (83.9-86.0) |
| NHW | 2012 | 98.9 (98.2-99.6) | 97.2 (96.2-98.2) | 55.2 (52.3-58.0) | 83.8 (82.7-84.8) |
| NHW | 2016 | 99.1 (98.5-99.8) | 96.9 (95.9-97.9) | 60.7 (57.9-63.5) | 85.6 (84.6-86.6) |
| NHW | 2018 | 98.7 (98.0-99.4) | 97.5 (96.7-98.4) | 59.0 (55.7-62.3) | 85.1 (83.9-86.3) |
Table 3.
Adjusted 1-Year Cause-Specific Survival Probabilities (%), by Race and Ethnicity, Stage, and Year of Diagnosis.
| Localized | Regional | Distant | Overall | ||
|---|---|---|---|---|---|
| Race/ethnicity | Year of Dx | Survival (95% CI) | Survival (95% CI) | Survival (95% CI) | Survival (95% CI) |
| AIAN | 1992 | 97.5 (83.7-100) | 89.8 (75.3-100) | 25.4 (6.2-44.6) | 70.9 (61.6-80.2) |
| AIAN | 1996 | 95.6 (82.0-100) | 93.2 (77.3-100) | 41.9 (25.5-58.3) | 76.9 (68.0-85.8) |
| AIAN | 2000 | 97.0 (83.5-100) | 82.5 (67.0-97.9) | 42.0 (21.4-62.6) | 73.8 (64.1-83.5) |
| AIAN | 2004 | 95.3 (85.3-100) | 94.2 (83.8-100) | 50.4 (36.1-64.7) | 80.0 (73.2-86.8) |
| AIAN | 2008 | 97.5 (85.3-100) | 92.4 (81.2-100) | 51.6 (35.5-67.7) | 80.5 (72.8-88.2) |
| AIAN | 2012 | 97.5 (87.5-100) | 89.9 (78.7-100) | 50.2 (37.0-63.3) | 79.2 (72.5-85.9) |
| AIAN | 2016 | 93.4 (84.0-100) | 94.1 (84.8-100) | 45.0 (33.3-56.8) | 77.5 (71.6-83.4) |
| AIAN | 2018 | 96.7 (87.8-100) | 96.9 (87.6-100) | 64.2 (51.1-77.5) | 85.9 (79.7-91.7) |
| API | 1992 | 97.5 (94.5-100) | 92.3 (88.9-95.7) | 45.8 (38.6-53.1) | 78.6 (75.7-81.4) |
| API | 1996 | 97.4 (95.2-99.6) | 93.4 (90.5-96.3) | 55.6 (48.5-62.6) | 82.1 (79.4-84.8) |
| API | 2000 | 97.4 (95.3-99.5) | 95.9 (93.9-97.9) | 53.1 (46.1-60.1) | 82.1 (79.6-84.7) |
| API | 2004 | 97.8 (96.2-99.4) | 94.6 (92.5-96.7) | 67.1 (61.8-72.4) | 86.5 (84.5-88.5) |
| API | 2008 | 97.5 (96.0-98.9) | 95.6 (93.7-97.4) | 59.4 (54.4-64.4) | 84.2 (82.2-86.1) |
| API | 2012 | 96.8 (95.3-98.3) | 94.6 (92.5-96.7) | 56.1 (50.3-61.9) | 82.5 (80.3-84.7) |
| API | 2016 | 96.4 (94.8-98.0) | 95.0 (93.4-96.6) | 57.8 (52.6-63.0) | 83.1 (81.1-85.0) |
| API | 2018 | 97.9 (96.5-99.0) | 96.1 (94.5-97.9) | 63.8 (58.3-69.3) | 86.0 (83.9-88.0) |
| Hispanic | 1992 | 94.7 (91.1-98.4) | 94.4 (91.3-97.5) | 54.3 (45.9-62.7) | 81.2 (77.9-84.4) |
| Hispanic | 1996 | 96.8 (93.9-99.6) | 94.2 (91.0-97.3) | 42.9 (36.0-49.9) | 77.9 (75.2-80.7) |
| Hispanic | 2000 | 97.0 (94.6-99.4) | 93.4 (90.6-96.2) | 46.6 (40.1-53.0) | 79.0 (76.4-81.5) |
| Hispanic | 2004 | 97.2 (95.0-99.4) | 93.3 (90.6-96.0) | 57.1 (50.8-63.4) | 82.5 (80.1-85.0) |
| Hispanic | 2008 | 96.2 (94.5-97.9) | 92.8 (90.4-95.3) | 59.8 (54.1-65.5) | 83.0 (80.8-85.2) |
| Hispanic | 2012 | 96.0 (94.3-97.8) | 92.9 (90.6-95.2) | 69.3 (64.8-73.9) | 86.1 (84.2-88.0) |
| Hispanic | 2016 | 94.8 (93.0-96.5) | 94.1 (92.5-95.6) | 67.3 (62.8-71.9) | 85.4 (83.6-87.2) |
| Hispanic | 2018 | 96.8 (95.3-98.3) | 95.3 (93.7-96.9) | 63.4 (58.3-68.6) | 85.2 (83.2-87.2) |
| NHB | 1992 | 95.7 (92.6-98.7) | 91.1 (87.3-94.8) | 37.8 (30.2-45.4) | 74.8 (71.8-77.9) |
| NHB | 1996 | 96.6 (93.4-99.9) | 93.8 (90.7-96.8) | 30.8 (24.4-37.1) | 73.7 (71.1-76.3) |
| NHB | 2000 | 95.6 (92.5-98.8) | 90.5 (87.1-94.0) | 35.7 (29.2-42.2) | 74.0 (71.2-76.7) |
| NHB | 2004 | 95.9 (93.3-98.5) | 94.0 (91.4-96.5) | 38.9 (33.2-44.6) | 76.3 (73.9-78.6) |
| NHB | 2008 | 96.1 (93.9-98.3) | 90.3 (86.8-93.9) | 51.2 (45.1-57.2) | 79.2 (76.7-81.7) |
| NHB | 2012 | 96.6 (94.4-98.7) | 90.6 (87.4-93.8) | 66.7 (60.9-72.4) | 84.6 (82.2-87.0) |
| NHB | 2016 | 96.0 (93.9-98.2) | 92.3 (89.6-95.1) | 53.0 (47.7-58.3) | 80.5 (78.3-82.6) |
| NHB | 2018 | 97.5 (95.6-99.1) | 94.3 (91.7-97.1) | 55.2 (49.2-61.3) | 82.4 (80.0-84.8) |
| NHW | 1992 | 96.8 (95.9-97.7) | 92.8 (91.6-94.0) | 39.2 (36.5-42.0) | 76.3 (75.1-77.4) |
| NHW | 1996 | 96.7 (95.9-97.6) | 93.1 (92.1-94.2) | 38.4 (35.8-41.1) | 76.1 (75.0-77.2) |
| NHW | 2000 | 96.3 (95.5-97.2) | 92.4 (91.2-93.6) | 42.4 (39.6-45.1) | 77.0 (75.9-78.2) |
| NHW | 2004 | 96.5 (95.6-97.3) | 93.5 (92.4-94.7) | 53.5 (50.8-56.1) | 81.2 (80.0-82.3) |
| NHW | 2008 | 96.4 (95.6-97.3) | 93.8 (92.7-94.9) | 58.8 (56.0-61.6) | 83.0 (81.8-84.2) |
| NHW | 2012 | 95.9 (95.0-96.8) | 93.9 (92.8-95.0) | 55.7 (53.0-58.3) | 81.8 (80.7-83.0) |
| NHW | 2016 | 95.2 (94.3-96.0) | 93.5 (92.4-94.6) | 60.4 (57.8-63.0) | 83.0 (81.9-84.2) |
| NHW | 2018 | 96.7 (95.8-97.6) | 95.0 (93.95-96.1) | 60.4 (57.6-63.3) | 84.0 (82.9-85.3) |
Figure 2.
Trends in 1-year adjusted survival probabilities for individuals from racial and ethnic subgroups diagnosed at different stages of colorectal cancer from 1992 through 2018.
For those diagnosed with regional stage CRC, trends in adjusted 1-year cause-specific survival probabilities similarly reflect little change over time, with the possible exception of an imprecisely-estimated positive trend over time among AIAN persons. Although there was little evidence of significant trends over time, there was a clear gradient in the average survival probabilities among those from different race and ethnic groups. AIAN persons had the lowest average survival probability (90.2%, 88.2%-92.2), followed by NHB, NHW, Hispanic, and API persons (93.2%, 92.6%-93.8%; 93.3%, 93.1%-93.5%; 94.2%, 93.8%-94.6%; and 94.8%, 94.4%-95.2%, respectively). All of these estimates were significantly different (all P < .01), except for the comparison between NHB and NHW persons (P = .83).
Those diagnosed with distant stage CRC experienced the biggest improvements in 1-year cause-specific survival probabilities over time. An increasing trend in 1-year survival probabilities is apparent for persons of all race and ethnic groups. For AIAN and NHB persons, the two groups with lowest initial 1-year survival probabilities, improvements continued at a consistent rate over time. For API, Hispanic, and NHW persons, there appears to be a slowing of the rate of improvement in the second half of the study period. The adjusted time-averaged survival probabilities were low: 46.6% (42.3%-50.9%) for NHB, 48.3% (37.9%-58.7%) for AIAN, 50.5% (48.5%-52.5%) for NHW, 58.1% (54.2%-62.0%) for Hispanic, and 58.3% (54.2%-62.4%) for API persons. All pairwise comparisons among these groups were statistically significant (all P < .01), except for differences between the two groups with the lowest (AIAN vs NHB, P = .87), and between the two groups with the highest (API vs Hispanic, P = .69), average adjusted 1-year cause-specific survival probabilities.
Five-Year Survival by Race and Ethnicity
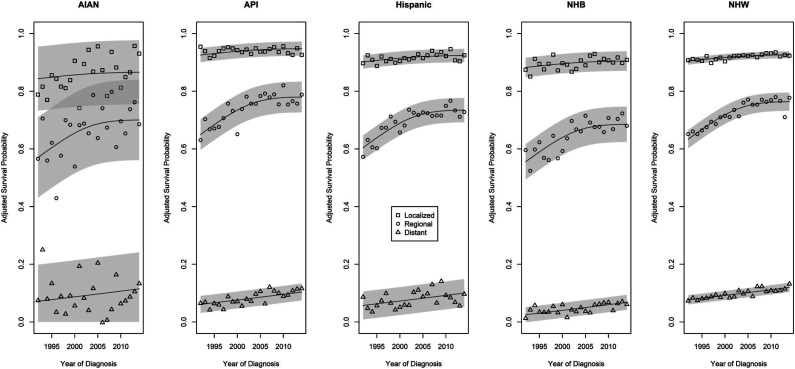
Estimates of adjusted 5-year cause-specific survival probabilities are shown for all race/ethnicity by stage combinations in Tables 4 and 5. Neither the race/ethnicity by stage by year of diagnosis three-way interaction (P = .90) nor the two-way interaction between the year of diagnosis and race/ethnicity (P = .59) were statistically significant; there is not sufficient evidence to conclude that there are race- or ethnic-specific differences in 5-year survival trends either within or across stages of CRC at diagnosis. There were statistically significant interactions between race/ethnicity and stage (P < .001), and between stage and the year of diagnosis trends (P < .001) with respect to differences in 5-year survival (see Figure 3).
Table 4.
Unadjusted 5-Year Cause-Specific Survival Probabilities (%), by Race and Ethnicity, Stage, and Year of Diagnosis.
| Localized | Regional | Distant | Overall | ||
|---|---|---|---|---|---|
| Race/ethnicity | Year of Dx | Survival (95% CI) | Survival (95% CI) | Survival (95% CI) | Survival (95% CI) |
| AIAN | 1992 | 83.2 (67.0-99.3) | 58.1 (40.1-76.0) | 8.5 (0-29.1) | 49.9 (39.3-60.5) |
| AIAN | 1995 | 87.5 (70.6-100) | 64.7 (47.7-81.7) | 13.4 (0-33.0) | 55.2 (44.9-65.5) |
| AIAN | 1998 | 85.4 (69.9-100) | 73.1 (57.2-89.0) | 1.6 (0-18.5) | 53.3 (44.0-62.6) |
| AIAN | 2002 | 91.8 (79.0-100) | 70.4 (56.5-84.3) | 10.8 (0-33.9) | 57.6 (47.7-67.6) |
| AIAN | 2005 | 97.1 (85.6-100) | 65.6 (52.5-78.6) | 23.0 (6.8-39.2) | 61.9 (54.0-69.8) |
| AIAN | 2008 | 97.5 (84.4-100) | 83.4 (70.7-96.1) | 5.9 (0-22.7) | 62.3 (54.0-70.6) |
| AIAN | 2011 | 89.3 (77.0-100) | 67.5 (55.3-79.6) | 9.5 (0.0-22.7) | 55.4 (48.2-62.7) |
| AIAN | 2014 | 96.5 (86.4-100) | 71.4 (60.1-82.6) | 15.1 (2.0-28.2) | 61.0 (54.3-67.6) |
| API | 1992 | 97.1 (93.4-100) | 63.9 (57.9-70.0) | 7.1 (.9-13.2) | 56.0 (52.9-59.1) |
| API | 1995 | 94.8 (91.1-98.4) | 67.5 (62.0-73.0) | 4.9 (0-10.7) | 55.7 (52.8-58.6) |
| API | 1998 | 96.1 (93.0-99.2) | 76.8 (71.8-81.8) | 7.9 (2.5-13.4) | 60.3 (57.6-62.9) |
| API | 2002 | 96.7 (94.3-99.1) | 79.8 (75.4-84.1) | 7.1 (2.5-11.8) | 61.2 (58.9-63.5) |
| API | 2005 | 96.5 (94.1-98.9) | 80.0 (75.9-84.1) | 9.8 (4.7-14.8) | 62.1 (59.8-64.4) |
| API | 2008 | 97.7 (95.7-99.7) | 80.6 (76.3-85) | 9.0 (4.1-13.9) | 62.4 (60.1-64.7) |
| API | 2011 | 96.7 (94.4-99.0) | 76.7 (72.5-81.0) | 8.4 (3.6-13.1) | 60.6 (58.3-62.9) |
| API | 2014 | 97.2 (95.0-99.4) | 81.5 (77.6-85.5) | 8.0 (3.8-12.1) | 62.2 (60.2-64.3) |
| Hispanic | 1992 | 92.6 (87.7-97.5) | 58.1 (51.7-64.4) | 6.7 (0-13.4) | 52.4 (48.9-55.9) |
| Hispanic | 1995 | 90.7 (85.5-95.8) | 61.0 (54.6-67.4) | 5.4 (0-11.4) | 52.4 (49.0-55.8) |
| Hispanic | 1998 | 94.3 (90.6-98.0) | 72.7 (67.0-78.3) | 5.5 (0-11.5) | 57.5 (54.4-60.5) |
| Hispanic | 2002 | 93.8 (90.1-97.4) | 75.5 (70.3-80.8) | 6.0 (.8-11.2) | 58.4 (55.7-61.2) |
| Hispanic | 2005 | 94.6 (91.6-97.6) | 74.1 (69.3-78.9) | 9.3 (4.1-14.5) | 59.3 (56.8-61.9) |
| Hispanic | 2008 | 96.0 (93.5-98.6) | 74.0 (69.1-78.9) | 6.3 (1.6-10.9) | 58.8 (56.4-61.2) |
| Hispanic | 2011 | 97.6 (95.5-99.6) | 79 (74.6-83.3) | 8.9 (4.6-13.1) | 61.8 (59.6-63.9) |
| Hispanic | 2014 | 96.9 (94.8-99) | 75.2 (70.9-79.4) | 9.4 (5.1-13.7) | 60.5 (58.4-62.6) |
| NHB | 1992 | 90.6 (85.7-95.4) | 60.9 (54.1-67.7) | 2.2 (0-6.8) | 51.2 (48.1-54.4) |
| NHB | 1995 | 91.3 (86.7-95.9) | 63.8 (57.0-70.6) | 4.3 (0-9.6) | 53.1 (49.8-56.4) |
| NHB | 1998 | 96.0 (92.5-99.4) | 66.4 (60.0-72.9) | 4.8 (0-9.8) | 55.7 (52.8-58.7) |
| NHB | 2002 | 89.9 (85.2-94.6) | 71.6 (65.9-77.3) | 4.5 (0-9.0) | 55.3 (52.4-58.2) |
| NHB | 2005 | 91.8 (87.9-95.7) | 73.5 (67.7-79.3) | 4.3 (.1-8.4) | 56.5 (53.8-59.2) |
| NHB | 2008 | 93.8 (90.4-97.1) | 69.3 (63.3-75.3) | 5.7 (1.4-10.1) | 56.3 (53.6-59.0) |
| NHB | 2011 | 93.7 (90.2-97.2) | 68.7 (62.4-75.0) | 5.4 (1.2-9.5) | 55.9 (53.2-58.7) |
| NHB | 2014 | 94.7 (91.4-98.0) | 69.2 (63.1-75.4) | 5.9 (1.7-10.1) | 56.6 (53.9-59.3) |
| NHW | 1992 | 92.4 (91.1-93.7) | 64.4 (62.2-66.5) | 4.8 (3.2-6.5) | 53.9 (52.9-54.9) |
| NHW | 1995 | 91.8 (90.5-93.2) | 65.6 (63.3-67.8) | 5.9 (4.2-7.6) | 54.4 (53.4-55.5) |
| NHW | 1998 | 93.2 (92-94.4) | 67.8 (65.7-69.9) | 4.2 (2.7-5.7) | 55.1 (54.1-56) |
| NHW | 2002 | 93.7 (92.6-94.9) | 73.4 (71.3-75.5) | 4.1 (2.7-5.5) | 57.1 (56.2-58) |
| NHW | 2005 | 95.3 (94.2-96.3) | 77.7 (75.7-79.7) | 5.1 (3.5-6.7) | 59.4 (58.4-60.3) |
| NHW | 2008 | 95.4 (94.4-96.4) | 77.6 (75.6-79.7) | 9.0 (7.0-11.1) | 60.7 (59.7-61.7) |
| NHW | 2011 | 96.1 (95.1-97.2) | 78.8 (76.7-80.9) | 4.8 (3.3-6.4) | 59.9 (59.0-60.9) |
| NHW | 2014 | 96.2 (95.1-97.3) | 79.3 (77.2-81.4) | 8.1 (6.2-10.1) | 61.2 (60.2-62.2) |
Table 5.
Adjusted 5-Year Cause-Specific Survival Probabilities (%), by Race and Ethnicity, Stage, and Year of Diagnosis.
| Localized | Regional | Distant | Overall | ||
|---|---|---|---|---|---|
| Race/ethnicity | Year of Dx | Survival (95% CI) | Survival (95% CI) | Survival (95% CI) | Survival (95% CI) |
| AIAN | 1992 | 78.8 (64.3-93.4) | 56.6 (40.4-72.7) | 7.5 (.0-26.0) | 47.6 (38.1-57.1) |
| AIAN | 1995 | 85.5 (70.3-100) | 62.1 (46.8-77.4) | 13.3 (.0-31.0) | 53.7 (44.4-63.0) |
| AIAN | 1998 | 81.1 (67.1-95.0) | 70.0 (55.6-84.3) | 2.8 (.0-18.0) | 51.3 (42.8-59.7) |
| AIAN | 2002 | 89.4 (77.9-100) | 68.8 (56.3-81.3) | 8.2 (.0-29.1) | 55.5 (46.5-64.5) |
| AIAN | 2005 | 95.6 (85.2-100) | 63.8 (52.0-75.6) | 20.4 (5.8-35.0) | 59.9 (52.7-67.1) |
| AIAN | 2008 | 93.6 (81.8-100) | 79.8 (68.3-91.2) | 4.3 (.0-19.5) | 59.2 (51.8-66.7) |
| AIAN | 2011 | 84.9 (73.8-96.0) | 65.4 (54.4-76.3) | 7.3 (.0-19.2) | 52.5 (46.0-59.1) |
| AIAN | 2014 | 93.0 (84.0-100) | 68.6 (58.4-78.7) | 13.3 (1.5-25.1) | 58.3 (52.3-64.3) |
| API | 1992 | 95.4 (92.1-98.8) | 63.1 (57.6-68.5) | 6.5 (.9-12.0) | 55.0 (52.1-57.9) |
| API | 1995 | 92.3 (88.9-95.7) | 67.1 (62.1-72.1) | 6.4 (1.1-11.6) | 55.3 (52.5-58.0) |
| API | 1998 | 95.2 (92.4-98.1) | 75.7 (71.2-80.2) | 8.8 (3.8-13.7) | 59.9 (57.4-62.4) |
| API | 2002 | 94.5 (92.2-96.7) | 78.1 (74.2-82.1) | 7.9 (3.7-12.1) | 60.2 (58.0-62.3) |
| API | 2005 | 93.7 (91.4-96.0) | 78.3 (74.6-82.1) | 10.6 (6.0-15.2) | 60.9 (58.6-63.1) |
| API | 2008 | 95.3 (93.3-97.2) | 78.9 (74.9-82.9) | 10.7 (6.2-15.2) | 61.6 (59.4-63.8) |
| API | 2011 | 93.2 (90.9-95.4) | 75.4 (71.5-79.3) | 9.3 (5.0-13.7) | 59.3 (57.1-61.5) |
| API | 2014 | 92.6 (90.5-94.7) | 78.9 (75.2-82.5) | 11.6 (7.8-15.4) | 61.0 (59.0-63.0) |
| Hispanic | 1992 | 89.7 (85.3-94.2) | 57.3 (51.5-63.0) | 8.6 (2.5-14.8) | 51.9 (48.6-55.1) |
| Hispanic | 1995 | 88.8 (84.1-93.5) | 60.2 (54.4-66.0) | 5.6 (.2-11.0) | 51.5 (48.4-54.7) |
| Hispanic | 1998 | 91.2 (87.8-94.6) | 71.2 (66.1-76.4) | 6.5 (1.1-12.0) | 56.3 (53.5-59.1) |
| Hispanic | 2002 | 91.0 (87.7-94.4) | 73.6 (68.8-78.4) | 5.8 (1.0-10.5) | 56.8 (54.2-59.4) |
| Hispanic | 2005 | 91.5 (88.7-94.4) | 72.7 (68.3-77.0) | 8.8 (4.0-13.5) | 57.7 (55.2-60.1) |
| Hispanic | 2008 | 92.6 (90.2-95.0) | 71.6 (67.2-76.1) | 6.6 (2.3-10.8) | 57.0 (54.7-59.2) |
| Hispanic | 2011 | 94.6 (92.6-96.6) | 76.7 (72.7-80.7) | 8.4 (4.5-12.3) | 59.9 (57.8-62.0) |
| Hispanic | 2014 | 92.5 (90.4-94.5) | 72.8 (68.9-76.7) | 9.6 (5.7-13.6) | 58.3 (56.2-60.4) |
| NHB | 1992 | 87.5 (83.0-91.9) | 59.6 (53.4-65.8) | 1.3 (.0-5.5) | 49.4 (46.5-52.4) |
| NHB | 1995 | 89.5 (85.2-93.7) | 62.3 (56.2-68.5) | 3.4 (.0-8.3) | 51.7 (48.7-54.8) |
| NHB | 1998 | 92.7 (89.5-95.9) | 64.6 (58.7-70.4) | 5.4 (.9-10.0) | 54.2 (51.5-57.0) |
| NHB | 2002 | 86.8 (82.5-91.0) | 69.7 (64.5-74.9) | 4.2 (.0-8.4) | 53.6 (50.8-56.3) |
| NHB | 2005 | 89.0 (85.4-92.6) | 71.5 (66.2-76.8) | 3.7 (.0-7.5) | 54.7 (52.2-57.3) |
| NHB | 2008 | 90.0 (86.9-93.2) | 67.7 (62.2-73.1) | 6.2 (2.1-10.2) | 54.6 (52.1-57.2) |
| NHB | 2011 | 90.0 (86.7-93.2) | 66.8 (61.1-72.6) | 4.0 (.1-7.8) | 53.6 (51.0-56.2) |
| NHB | 2014 | 90.9 (87.8-94.0) | 68.0 (62.4-73.6) | 6.1 (2.2-10.0) | 55.0 (52.4-57.6) |
| NHW | 1992 | 90.7 (89.3-92.1) | 65.1 (63.0-67.2) | 7.3 (5.7-9.0) | 54.4 (53.2-55.6) |
| NHW | 1995 | 90.5 (89.1-92.0) | 66.4 (64.3-68.6) | 8.0 (6.3-9.8) | 55.0 (53.8-56.2) |
| NHW | 1998 | 91.1 (89.8-92.4) | 68.6 (66.6-70.7) | 9.4 (7.8-10.9) | 56.4 (55.2-57.5) |
| NHW | 2002 | 92.1 (90.8-93.4) | 73.5 (71.5-75.6) | 8.7 (7.2-10.2) | 58.1 (57.0-59.3) |
| NHW | 2005 | 92.3 (91.0-93.5) | 77.1 (75.1-79.0) | 10.7 (9.0-12.3) | 60.0 (58.9-61.1) |
| NHW | 2008 | 92.7 (91.5-93.9) | 77.1 (75.0-79.1) | 12.3 (10.3-14.3) | 60.7 (59.5-61.9) |
| NHW | 2011 | 93.5 (92.2-94.7) | 78.0 (75.9-80.0) | 10.7 (9.1-12.3) | 60.7 (59.6-61.9) |
| NHW | 2014 | 92.3 (91.1-93.6) | 77.7 (75.7-79.8) | 13.2 (11.3-15.1) | 61.1 (59.9-62.3) |
Figure 3.
Trends in 5-year adjusted survival probabilities for individuals from racial and ethnic subgroups diagnosed at different stages of colorectal cancer from 1992 through 2014.
The trends reflect improvements in 5-year cause-specific survival probabilities over time for all CRC stages. The rate of improvement in 5-year survival probabilities over time was greatest for those with regional stage CRC, although the rate of improvement appears to be slowing in the second half of the study period. As there are no significantly different trends over time in adjusted 5-year survival probabilities by race or ethnicity, key differences among race and ethnic groups are best summarized by differences in their adjusted time-averaged survival probabilities. For those diagnosed at localized stage CRC, estimates of average adjusted 5-year survival probabilities were 86.9% (84.2%-89.6%) for AIAN, 90.2% (89.4%-91.0%) for NHB, 92.0% (91.8%-92.2%) for NHW, 92.1% (91.5%-92.7%) for Hispanic, and 94.1% (93.5%-94.7%) for API. These differed significantly between all pairs of groups (all P < .04), except for the Hispanic vs NHW comparison (P = .56). For those diagnosed at regional stage CRC, estimates of average adjusted 5-year survival probabilities were 65.1% (62.2%-68.0%) for NHB, 67.5% (61.0%-74.0%) for AIAN, 70.5% (68.1%-72.9%) for Hispanic, 72.5% (71.5%-73.5%) for NHW, and 75.1% (72.9%-77.3%) for API persons. These differed significantly between all pairs of groups (all P < .05), except for the AIAN vs NHB comparison (P = .22). For those diagnosed at distant stage CRC, estimates of average adjusted 5-year survival probabilities were 4.6% (3.6%-5.6%) for NHB, 7.8% (6.6%-9.0%) for Hispanic, 8.5% (7.3%-9.7%) for API, 9.0% (5.5%-12.5%) for AIAN, and 9.6% (9.2%-10.0%) for NHW. The AIAN average did not differ significantly from those of the API, Hispanic, or NHW groups, nor did the API vs Hispanic averages. All others differed significantly (all P < .01).
Discussion
The purpose of this study was to estimate and compare race- and ethnicity-specific 1- and 5-year CRC cause-specific survival trends, within the context of the stage at which the cancer was first detected. We utilized data from the SEER registries for persons diagnosed with CRC from 1992 to 2018 and estimated 1- and 5-year survival probabilities within groups of persons with CRC by race and ethnicity, stage of disease, and year of diagnosis. Study findings contribute to existing knowledge gaps regarding trends in survival by including estimates for an often-overlooked population: American Indian or Alaska Natives.
There are significant differences in the degree to which improvements in 1-year survival probabilities following stage-specific CRC are experienced according to a person’s race and ethnicity. The estimates that we report specifically for AIAN and API subgroups contribute towards the limited literature focused on identifying survival trend differences for these racially minoritized populations. Study findings suggest that AIAN individuals have the lowest 1-year survival probabilities compared to those of other races. Lower 1-year CRC-specific survival probabilities are also seen among NHB persons. In particular, these persons have the lowest average survival probabilities following the diagnosis of distant-stage CRC. Although differences among racial and ethnic groups have moderated somewhat over the study period, these disparities do not appear to have resolved completely.
There is established evidence that CRC screening can prevent or detect CRC early.27,28 For screening to be effective in improving outcomes, timely follow up of any abnormal test is necessary. Structural barriers (eg, lack of insurance or social support, racism and discrimination) to obtaining appropriate primary care services may play an important role in reducing the possibility for racially minoritized populations to receive guideline-compliant screening services.5,29-31 The fact that some studies conducted within the Veterans’ Health Administration found no differences between White and Black persons in diagnostic follow up testing,32,33 suggests that access to appropriate structures and services may play an important role in appropriate post-screening follow-up for minoritized populations. As expected, the greatest differences in survival probabilities are apparent among individuals diagnosed at different stages of CRC. Although these patterns are largely similar among persons from different race and ethnic groups disparities in survival persist. Enhanced follow-up of abnormal results, may help overcome at least some of the persistent disparities in CRC survival probabilities among persons from different race and ethnic groups.
Other differences in access to and utilization of quality health care may also contribute to the observed differences of survival trends by race.34-36 Some studies have suggested NHB persons are less likely to receive surgical treatment and adjuvant chemotherapy.35-38 Social, structural and political determinants also contribute towards the likelihood of NHB and AIAN persons to be diagnosed with advanced stage CRC compared to NHW persons.39,40 This may further contribute to the lower survival probabilities observed in NHB persons with distant stage CRC.41,42 Racial and ethnic minoritized populations also experience disparities in terms of post-treatment surveillance and distinct baseline comorbidities, which further contribute to lower survival rates.34,35 Several studies continue to highlight that transportation barriers, cultural beliefs, fear and stigma about screening, and concerns about privacy issues are contributing factors to survival outcomes43-47 for persons with CRC.
Lifestyle and biological factors may also play a role in CRC risk and outcomes. For instance, lifestyle factors such as obesity, smoking, alcohol consumption, and physical inactivity have a higher prevalence among Black populations.48 Also, genetic mutations and microsatellite instability can differ among racially and ethnically minoritized populations. All of these things can affect CRC development and prognosis,49-54 and may play into the disparities noted in this work.
There are several potential limitations in our analysis. First, we must acknowledge that this is a retrospective analysis of data from the SEER registries. However, SEER is a population-based resource which provides information about the most critical factors of interest, and we tried to control for these. Second, we cannot fully exclude the possibility that regional variations may contribute to the observed CRC survival probability estimates that average across SEER registries. Third, we chose to report on cause-specific survival, and this may be influenced by different practices in cause of death ascertainment. However, in sensitivity analyses performed using relative survival estimates we found that overall findings were largely concordant. This is similar to a report of data from Canada where differences in survival probabilities were noted, and that differences between First Nations and non-aboriginal persons were somewhat smaller for cause-specific survival when compared to relative survival estimates.55 Fourth, SEER data have relatively little information on comorbidities, access to care, and insurance status. Fifth, racial and ethnic classifications in medical records may reflect misclassification,56 which may bias our estimates. Finally, SEER registries represent a subset of AIAN persons spanning the United States, but do not capture data from some regions with large AIAN populations, including those in Oklahoma, Arizona, or the Northern Plains and Great Lakes.57 Even with these limitations, we are able to provide new data concerning trends in CRC survival over time for multiple racial and ethnic groups in the United States, according to their stage at diagnosis.
In conclusion, we present trends in 1- and 5-year CRC cause-specific survival probabilities for persons of five racial and ethnic groups. These estimates of, and trends in, survival probabilities for groups of minoritized races and ethnicities may enable the development of a more complete picture of CRC prognosis. We have identified significant differences in the race- and ethnic-specific trends in 1-year stage-specific CRC survival probabilities. In particular, AIAN persons have historically experienced poorer CRC prognosis, as have NHB individuals. Although these disparities appear to be lessening somewhat, the current differences in survival probabilities continue to call for further work in order to erase them completely. Trends in 1-year survival probabilities are significantly different among those diagnosed at different stages of CRC. This, coupled with the finding that the distribution of stages at diagnosis appears to differ among persons of different racial and ethnic groups, suggests that prioritizing specific stages of disease at diagnosis may provide 1 avenue that may help us to overcome race- and ethnic-specific disparities in CRC-specific survival probability. This also argues for a potential need to improve screening for CRC, as this may influence the distribution of stages at which it is diagnosed. Future research should strive to capture cancer incidence and survival information from all key racial and ethnic subgroups. Future efforts should also incorporate and evaluate multi-level interventions at the individual, structural, and policy levels to address the persistent disparities in CRC survival.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NIH/NCI (P30 CA118100, P30 CA118100-16S4, and R01 CA192967).
ORCID iDs
Vernon S. Pankratz https://orcid.org/0000-0002-3742-040X
Prajakta Adsul https://orcid.org/0000-0003-2860-4378
References
- 1.Siegel RL. Colorectal cancer statistics, 2020. Ca-Cancer J Clin. 2020;70:145-164. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . Colorectal Cancer Facts & Figures 2020-2022; 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf [Google Scholar]
- 3.National Cancer Institute . Cancer Stat Facts: Colorectal Cancer. https://seer.cancer.gov/statfacts/html/colorect.html [Google Scholar]
- 4.Chu KC, Tarone RE, Chow W-H, Alexander GA. Colorectal cancer trends by race and anatomic subsites, 1975 to 1991. Arch Fam Med. 1995;4:849. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000;88:2398-2424. [DOI] [PubMed] [Google Scholar]
- 7.Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012;30:401-405. [DOI] [PubMed] [Google Scholar]
- 8.Soneji S, Iyer SS, Armstrong K, Asch DA. Racial disparities in stage-specific colorectal cancer mortality: 1960–2005. Am J Publ Health. 2010;100:1912-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phipps AI, Scoggins J, Rossing MA, Li CI, Newcomb PA. Temporal trends in incidence and mortality rates for colorectal cancer by tumor location: 1975–2007. Am J Publ Health. 2012;102:1791-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troisi RJ, Freedman AN, Devesa SS. Incidence of colorectal carcinoma in the US: An update of trends by gender, race, age, subsite, and stage. Cancer Interdiscip Int J Am Cancer Soc. 1999;85:1670-1676. [PubMed] [Google Scholar]
- 11.Gomez SL, O’Malley CD, Stroup A, Shema SJ, Satariano WA. Longitudinal, population-based study of racial/ethnic differences in colorectal cancer survival: impact of neighborhood socioeconomic status, treatment and comorbidity. BMC Cancer. 2007;7:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kauh J, Brawley OW, Berger M. Racial disparities in colorectal cancer. Curr Probl Cancer. 2007;31:123-133. [DOI] [PubMed] [Google Scholar]
- 13.Enewold L, Horner M-J, Shriver CD, Zhu K. Socioeconomic disparities in colorectal cancer mortality in the United States, 1990–2007. J Community Health. 2014;39:760-766. [DOI] [PubMed] [Google Scholar]
- 14.Lai Y, Wang C, Civan JM, et al. Effects of cancer stage and treatment differences on racial disparities in survival from colon cancer: A United States population-based study. Gastroenterology. 2016;150:1135-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silber JH, Rosenbaum PR, Ross RN, et al. Racial disparities in colon cancer survival: A matched cohort study. Ann Intern Med. 2014;161:845-854. [DOI] [PubMed] [Google Scholar]
- 16.Hao Y, Jemal A, Zhang X, Ward EM. Trends in colorectal cancer incidence rates by age, race/ethnicity, and indices of access to medical care, 1995–2004 (United States). Cancer Causes Control. 2009;20:1855-1863. [DOI] [PubMed] [Google Scholar]
- 17.Tawk R, Abner A, Ashford A, Brown CP. Differences in colorectal cancer outcomes by race and insurance. Int J Environ Res Publ Health. 2016;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang PS, Mayer JD, Wakefield J, Ko CW. Temporal trends in geographic and sociodemographic disparities in colorectal cancer among medicare patients, 1973-2010. J Rural Health. 2017;33:361-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansa BE, Coughlin SS, Alema-Mensah E, Smith SA. Evaluation of colorectal cancer incidence trends in the United States (2000–2014). J Clin Med. 2018;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perdue DG, Haverkamp D, Perkins C, Daley CM, Provost E. Geographic variation in colorectal cancer incidence and mortality, age of onset, and stage at diagnosis among American Indian and Alaska Native people, 1990–2009. Am J Publ Health. 2014;104:S404-S414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao A, Gilliland FD, Hunt WC, et al. Increasing incidence of colon and rectal cancer among Hispanics and American Indians in New Mexico (United States), 1969-94. Cancer Causes Control. 1998;9:137-144. [DOI] [PubMed] [Google Scholar]
- 22.Simon MS, Thomson CA, Pettijohn E, et al. Racial differences in colorectal cancer incidence and mortality in the Women’s Health Initiative. Cancer Epidemiol Prev Biomark. 2011;20:1368-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Institute Surveillance Epidemiology and End Results Program . Overview of the SEER Program; 2018. https://seer.cancer.gov/about/overview.html [Google Scholar]
- 24.National Cancer Institute . Registry Groupings in SEER Data and Statistics; 2018. https://seer.cancer.gov/registries/terms.html [Google Scholar]
- 25.Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surveillance Research Program . National Cancer Institute SEER*Stat software. www.seer.cancer.gov/seerstat www.seer.cancer.gov/seerstat
- 27.Elmunzer BJ, Singal AG, Sussman JB, et al. Comparing the effectiveness of competing tests for reducing colorectal cancer mortality: a network meta-analysis. Gastrointest Endosc. 2015;81:700-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan J, Xin L, Ma Y-F, Hu L-H, Li Z-S. Colonoscopy reduces colorectal cancer incidence and mortality in patients with non-malignant findings: a meta-analysis. Am J Gastroenterol. 2016;111:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haverkamp D, English K, Jacobs-Wingo J, Tjemsland A, Espey D. Peer reviewed: Effectiveness of interventions to increase colorectal cancer screening among American Indians and Alaska Natives. Prev Chronic Dis. 2020;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: A randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics. CACancer J Clin. 2014;64:104-117. [DOI] [PubMed] [Google Scholar]
- 32.Etzioni DA, Yano EM, Rubenstein LA, et al. Measuring the quality of colorectal cancer screening: the importance of follow-up. Dis Colon Rectum. 2006;49:1002-1010. [DOI] [PubMed] [Google Scholar]
- 33.Partin MR, Gravely AA, Burgess J, et al. Contribution of patient, physician, and environmental factors to demographic and health variation in colonoscopy follow-up for abnormal colorectal cancer screening test results. Cancer. 2017;123:3502-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez SL, O’Malley CD, Stroup A, Shema SJ, Satariano WA. Longitudinal, population-based study of racial/ethnic differences in colorectal cancer survival: Impact of neighborhood socioeconomic status, treatment and comorbidity. BMC Cancer. 2007;7:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: Did the gap narrow between 1992 and 2002? Cancer. 2008;112:900-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potosky AL, Harlan LC, Kaplan RS, Johnson KA, Lynch CF. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol. 2002;20:1192-1202. [DOI] [PubMed] [Google Scholar]
- 37.Baldwin L-M. Explaining black–white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005;97:1211-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demissie K, Oluwole OO, Balasubramanian BA, et al. Racial differences in the treatment of colorectal cancer: A comparison of surgical and radiation therapy between Whites and Blacks. Ann Epidemiol. 2004;14:215-221. [DOI] [PubMed] [Google Scholar]
- 39.Chien C, Morimoto LM, Tom J, Li CI. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer Interdiscip Int J Am Cancer Soc. 2005;104:629-639. [DOI] [PubMed] [Google Scholar]
- 40.Wu XC, Chen VW, Steele B, et al. Subsite-specific incidence rate and stage of disease in colorectal cancer by race, gender, and age group in the United States, 1992–1997. Cancer Interdiscip Int J Am Cancer Soc. 2001;92:2547-2554. [DOI] [PubMed] [Google Scholar]
- 41.Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, et al. Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Cancer Epidemiol Prev Biomark. 2012;21:728-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valeri L. The role of stage at diagnosis in colorectal cancer black–white survival disparities: a counterfactual causal inference approach. Cancer Epidemiol Prev Biomark. 2016;25:83-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daley CM, James AS, Filippi M, et al. American Indian community leader and provider views of needs and barriers to colorectal cancer screening. J Health Disparities Res Pract. 2012;5:2. [PMC free article] [PubMed] [Google Scholar]
- 44.Filippi MK, James AS, Brokenleg S, et al. Views, barriers, and suggestions for colorectal cancer screening among American Indian women older than 50 years in the Midwest. J Prim Care Community Health. 2013;4:160-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.James AS, Filippi MK, Pacheco CM, et al. Barriers to colorectal cancer screening among American Indian men aged 50 or older, Kansas and Missouri, 2006–2008. Prev Chronic Dis. 2013;10:E170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandhi N, Guadagnolo BA, Kanekar S, Petereit DG, Smith MA. Cancer screening in native Americans from the Northern Plains. Am J Prev Med. 2010;38:389-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schumacher MC, Slattery ML, Lanier AP, et al. Prevalence and predictors of cancer screening among American Indian and Alaska native people: The EARTH study. Cancer Causes Control. 2008;19:725-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Cancer InstituteNIH, DHHS . Cancer Trends Progress Report; 2021. https://progressreport.cancer.gov/ [Google Scholar]
- 49.Fariña-Sarasqueta AV, van Lijnschoten G, Moerland E, et al. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann Oncol. 2010;21:2396-2402. [DOI] [PubMed] [Google Scholar]
- 50.Gavin PG, Colangelo LH, Fumagalli D, et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clin Cancer Res. 2012;18:6531-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindblom A. Different mechanisms in the tumorigenesis of proximal and distal colon cancers. Curr Opin Oncol. 2001;13:63-69. [DOI] [PubMed] [Google Scholar]
- 52.Nayani R, Ashktorab H, Brim H, Laiyemo AO. Genetic basis for colorectal cancer disparities. Curr Colorectal Cancer Rep. 2015;11:408-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitworth A. New research suggests access, genetic differences play role in high minority cancer death rate. J Natl Cancer Inst. 2006;98:669-669. [DOI] [PubMed] [Google Scholar]
- 54.Yoon HH, Shi Q, Alberts SR, et al. Racial differences in BRAF/KRAS mutation rates and survival in stage III colon cancer patients. JNCI J Natl Cancer Inst. 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Withrow DR, Pole JD, Nishri ED, Tjepkema M, Marrett LD. Choice of relative or cause-specific approach to cancer survival analysis impacts estimates differentially by cancer type, population, and application: Evidence from a Canadian population-based cohort study. Popul Health Metrics. 2017;15:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atekruse SF, Cosgrove C, Cronin K, Yu M. Comparing cancer registry abstracted and self-reported data on race and ethnicity. J Regist Manag. 2017;44:30-33. [PubMed] [Google Scholar]
- 57.Melkonian SC, Weir HK, Jim MA, et al. Incidence of and trends in the leading cancers with elevated incidence among American Indian and Alaska Native populations, 2012-2016. Am J Epidemiol. 2021;190:528-538. [DOI] [PMC free article] [PubMed] [Google Scholar]