Abstract
Aim
We assessed the impact of COVID-19 vaccination status and time elapsed since the last vaccine dose on morbidity and absenteeism among healthcare personnel (HCP) in the context of a mandatory vaccination policy.
Methods
We followed 7592 HCP from November 15, 2021 through April 17, 2022. Full COVID-19 vaccination was defined as a primary vaccination series plus a booster dose at least six months later.
Results
There were 6496 (85.6 %) fully vaccinated, 953 (12.5 %) not fully vaccinated, and 143 (1.9 %) unvaccinated HCP. A total of 2182 absenteeism episodes occurred. Of 2088 absenteeism episodes among vaccinated HCP with known vaccination status, 1971 (94.4 %) concerned fully vaccinated and 117 (5.6 %) not fully vaccinated. Fully vaccinated HCP had 1.6 fewer days of absence compared to those not fully vaccinated (8.1 versus 9.7; p-value < 0.001). Multivariable regression analyses showed that full vaccination was associated with shorter absenteeism compared to not full vaccination (OR: 0.56; 95 % CI: 0.36–0.87; p-value = 0.01). Compared to a history of ≤ 17.1 weeks since the last dose, a history of > 17.1 weeks since the last dose was associated with longer absenteeism (OR: 1.22, 95 % CI:1.02–1.46; p-value = 0.026) and increased risk for febrile episode (OR: 1.33; 95 % CI: 1.09–1.63; p-value = 0.004), influenza-like illness (OR: 1.53, 95 % CI: 1.02–2.30; p-value = 0.038), and COVID-19 (OR: 1.72; 95 % CI: 1.24–2.39; p-value = 0.001).
Conclusions
The COVID-19 pandemic continues to impose a considerable impact on HCP. The administration of a vaccine dose in less than four months before significantly protected against COVID-19 and absenteeism duration, irrespective of COVID-19 vaccination status. Defining the optimal timing of boosters is imperative.
Keywords: COVID-19 vaccination, Booster, Healthcare personnel, Morbidity, Absenteeism
1. Introduction
As we approach the fourth winter season since the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in late 2019, many healthcare systems globally are still facing consecutive surges of coronavirus disease 2019 (COVID-19)-associated healthcare demand and hospitalizations [1]. From the beginning of the COVID-19 pandemic, healthcare personnel (HCP) were recognized as a high-risk group for SARS-CoV-2 infection and adverse health outcomes including fatalities [2], [3], [4]. Beyond conferring direct protection to them, HCP were prioritized for COVID-19 vaccination to bolster the efficient provision of healthcare services and contain viral transmission, outbreaks, and staff absenteeism [5].
During the third pandemic wave in Greece, a multicenter study found that the effectiveness of two doses of BNT162b2 mRNA vaccine in HCP was 94.2 % against COVID-19, 83.6 % against SARS-CoV-2 infection, and 66.4 % against absenteeism [6]. Vaccination also significantly reduced the duration of absenteeism [6]. Starting from September 1, 2021, Greece was among the first countries globally to implement a mandatory COVID-19 vaccination policy for all employees in healthcare facilities as a prerequisite for employment [7]. In particular, according to law 1040/2921, HCP who refused COVID-19 vaccination were excluded from work with salary suspension [8]. Mandatory vaccination concerned all HCP in public and private healthcare facilities, and exemptions were granted based on strict medical criteria only, including a laboratory-confirmed SARS-CoV-2 infection over the past three months.
Despite the existing evidence on the effectiveness of COVID-19 vaccinations against COVID-19 associated morbidity and absenteeism [6], less is known about how time elapsed since the last COVID-19 vaccine dose is associated with this risk of SARS-CoV-2 infection. A study conducted in a French university hospital showed that the incidence of SARS-CoV-2 infection significantly increased with time since last dose among HCP who had received a primary vaccine series, with no differences according to vaccination scheme [9]. In this latter study the median time from vaccination completion to infection was 5.5 months [9]. To the best of our knowledge, there are no published data on the association between time elapsed since last COVID-19 vaccination, SARS-CoV-2 infections, overall morbidity, and absenteeism among HCP vaccinated with booster doses.
The aim of this study was to assess the association between COVID-19 vaccination status, time elapsed since the last vaccine dose, morbidity, and absenteeism in HCP in hospitals in Greece in the context of a mandatory vaccination policy. Our findings may guide COVID-19 vaccination policies for HCP and also contribute to the determination of the optimal seasonal timing for booster vaccinations.
2. Methods
2.1. Study setting
This was a prospective study conducted from November 15, 2021 (week 46/2021) through April 17, 2022 (week 15/2022), similar to our previous 2020–2021 work on COVID-19-associated absenteeism in Greece and in the same tertiary-care hospitals (3 in Athens, 1 in Thessaloniki, and 1 in Alexandroupoli) using the same methodology [6]. A total of 7772 HCP were employed in these hospitals. Of those, 180 (2.3 %) HCP were suspended from work due to COVID-19 vaccination refusal. Therefore, a total of 7592 (97.7 %) HCP (range: 1082-1827 HCP per hospital) were prospectively followed. In brief, HCP were actively surveyed for episodes of absenteeism through communication of the Infection Control Committee with the Heads of the Departments/Clinics and the Heads of Nurse Stations of each hospital. Absence for non-infectious causes, pregnancy leave, and annual leave was not recorded.
In Greece, the SARS-CoV-2 variant B.1.617.2 (Delta) dominated until week 50/2021 and co-circulated with B.1.1.529 (Omicron) during weeks 51/2021 and 52/2021, while Omicron dominated from week 01/2022 onwards [10]. Very low influenza activity was recorded from week 3/2022 through week 19/2022, which was attributed exclusively to influenza A/H3N2 [11].
2.2. Testing for SARS-CoV-2
HCP were tested for SARS-CoV-2 by real-time reverse transcriptase polymerase chain reaction (RT-PCR) and/or rapid antigen detection test (RADT) in the following cases: onset of symptoms, exposure to a COVID-19 case, investigation of nosocomial clusters, and return to work after annual leave. HCP were also tested every 7–14 days depending on the guidelines of the infection control committee of the hospital and the employee department/unit.
2.3. Data collection
The following data were prospectively collected per episode of absenteeism: demographic, professional, epidemiological, and clinical characteristics of HCP, history of COVID-19 vaccination (including number of doses and date of last dose), history of influenza vaccination, date and duration of absenteeism, and duration of presenteeism (if any). Data on absenteeism episodes were collected using one Excel sheet per week.
2.4. Definitions
HCP were defined as persons employed in healthcare facilities, regardless of direct contact with patients. HCP were grouped as physicians, nursing personnel, paramedical personnel, supportive personnel, and administrative personnel. There were no part-time employees.
Full COVID-19 vaccination was defined as a complete primary series with BNT162b2 (Comirnaty; 2 doses), mRNA-1273 (Spikevax; 2 doses), ChAdOx1-S (Vaxzevria; 2 doses), or AD26.COV2.S (Janssen; 1 dose), followed by a booster vaccine shot at least six months after the primary series. In our cohort, vaccinations were done as follows: BNT162b2: 94.7 %; Jahnsen: 3.1 %; mRNA-1273: 2.0 %; and ChAdOx1-S: 0.2 %. Morbidity was grouped by clinical presentation and/or by laboratory diagnosis. Clinical presentation was defined as febrile episode, acute respiratory illness (ARI), or influenza-like illness (ILI). Febrile episode was defined as fever only. ARI was defined as the onset of at least one respiratory symptom (e.g. cough, sore throat, dyspnea). ILI was defined as the sudden onset of symptoms and fever, malaise, myalgia or headache, and cough, sore throat or shortness of breath. SARS-CoV-2 infection was defined as a laboratory-confirmed SARS-CoV-2 infection by real-time RT-PCR and/or RADT regardless of symptoms. A COVID-19 case was defined as a patient with compatible symptoms and a positive SARS-CoV-2 RT-PCR and/or RADT. Influenza was defined as a case of laboratory-confirmed influenza by PCR and/or RADT. Absenteeism was defined as absence of a HCP from work duties due to the onset of symptoms, detection of SARS-CoV-2 infection or influenza, or for isolation purposes following exposure to COVID-19. The absenteeism ratio was defined as follows: total days of absence/number of HCP [12]. Presenteeism was defined as working while being ill [13].
2.5. Statistical analysis
We initially conducted a descriptive analysis of all HCP with absenteeism. Absolute numbers and percentages were used for categorical variables. Means and standard deviation (SD) were used for continuous variables. We then stratified HCP by COVID-19 vaccination status into fully and not fully vaccinated and compared them using the chi-square test for categorical variables and the two-tailed t-test or the Mann-Whitney U test for continuous variables depending on their distribution. Given that BNT162b2 mRNA vaccines were used in approximately 95 % of vaccinations, vaccine brands were not considered in data analysis. We similarly compared the incidences of ARI, febrile episode, ILI, asymptomatic SARS-CoV-2 infection, COVID-19, exposure to a COVID-19 case, and laboratory-confirmed influenza as well as the duration of absenteeism for each of these causes of absenteeism among fully vaccinated HCP compared with not fully vaccinated HCP. Finally, we used multivariable regressions to estimate the associations between multiple outcomes, COVID-19 vaccination status, and time since the last COVID-19 vaccine dose. The outcomes included days of absenteeism, ARI, febrile episode, ILI, asymptomatic SARS-CoV-2 infection, COVID-19, and influenza. Since the number of days of absenteeism was expected to be skewed, we transformed this variable using quartiles and conducted two ordered logistic regression models to estimate the association of duration of absenteeism with full or partial COVID-19 vaccination status, and time elapsed since the last COVID-19 vaccine (less than average versus more than average) in separate models. We also conducted logistic regressions to estimate similar associations among the six clinical outcomes. The odds ratio (OR) and 95 % confidence interval (CI) were estimated. P-values of ≤ 0.05 were considered statistically significant. All regression models controlled for age, gender, occupation, influenza vaccination status, and hospital fixed-effects, while standard errors were clustered at the hospital level. Statistical analyses were conducted using Stata version 17.0, StataCorp, College Station, TX, USA.
2.6. Ethical issues
The study was approved by The Ethics Committees of the participating hospitals (approval numbers: 30/8-1-2021, 2457/4-2-2021, 1/5-2-2021, ΥΣ 36/21-12-2020/251, 19814/11-2021). The study was conducted in accordance with the principles of the Declaration of Helsinki.
3. Results
In the full cohort of 7592 HCP, 6496 (85.6 %) HCP had received a complete COVID-19 primary vaccine series plus a booster dose the past six months (full vaccination), 953 (12.5 %) were not fully vaccinated, and 143 (1.9 %) were unvaccinated. Table 1 shows the overall morbidity recorded among the 7592 HCP during the 22-week study period. SARS-CoV-2 infection was diagnosed in 2130 (28.0 %) HCP, including 1727 (22.7 %) HCP with COVID-19 and 403 (5.3 %) HCP with asymptomatic infection. Influenza was diagnosed in 12 (0.2 %) cases.
Table 1.
Morbidity in the cohort of 7592 HCP, November 15, 2021 to April 17, 2022.
| Morbidity* | N = 7592 | (%) |
|---|---|---|
| Acute respiratory infection | 767 | (10.1) |
| Febrile episode | 166 | (2.2) |
| Influenza-like illness | 816 | (10.7) |
| Asymptomatic SARS-CoV-2 infection | 403 | (5.3) |
| COVID-19 | 1727 | (22) |
| Influenza | 12 | (0.2) |
HCP: healthcare personnel; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; COVID-19: coronavirus disease 2019.
Morbidity was defined by clinical presentation and/or by laboratory diagnosis.
A total of 2182 episodes of absenteeism occurred among the 7592 HCP during the study period, which corresponds to 28.7 episodes per 100 HCP. The characteristics of the HCP with absenteeism are shown in Table 2 . Their mean age was 41.3 (SD: 10.6) years, while 763 (35 %) were males and 1419 (65 %) females. The mean duration of absence from work was 8.2 (SD: 3.0) days per episode of absenteeism and the total duration of absenteeism during the study period was 17,815 days. The absenteeism ratio was estimated at 2.3 in our cohort. The episodes of absenteeism occurred at a mean of 17.0 (SD: 10.3) weeks after the last COVID-19 vaccine dose. Presenteeism was recorded in 405 (18.6 %) HCP for a mean duration of 1.1 (SD: 0.4) days before leaving work.
Table 2.
Characteristics of HCP with absenteeism, November 15, 2021 to April 17, 2022.
| Characteristic | N = 2182 | (%) |
|---|---|---|
| Mean age, years (SD) | 41.3 | (10.6) |
| Male gender | 763 | (35.0) |
| Profession | ||
| Physician | 561 | (25.7) |
| Nursing personnel | 874 | (40.1) |
| Paramedical personnel | 225 | (10.3) |
| Supportive personnel | 335 | (15.3) |
| Administrative personnel | 187 | (8.6) |
| Influenza vaccination | 554 | (25.4) |
| COVID-19 vaccination | ||
| Yes | 2144 | (98.3) |
| No* | 38 | (1.7) |
| Full COVID-19 vaccination | 1971 | (91.9) |
| Total days of absenteeism | 17,815 | |
| Mean duration of absenteeism, days (SD) | 8.2 | (3.0) |
| Presenteeism | 405 | (18.6) |
| Mean duration of presenteeism, days (SD) | 1.1 | (0.4) |
| Mean time from last COVID-19 vaccine dose, weeks (SD) | 17.0 | (10.3) |
*history of SARS-COV-2 infection the past 3 months: 17, medical exemption: 3, unspecified: 18.
HCP: healthcare personnel; SD: standard deviation; COVID-19: coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Of the 2182 episodes of absenteeism recorded during the study period, 2144 (98.3 %) episodes occurred among COVID-19 vaccinated HCP and 38 (1.7 %) among unvaccinated HCP. There were 2088 episodes of absenteeism among vaccinated HCP for whom the COVID-19 vaccination status was known. Of them, 1971 (94.4 %) HCP were fully vaccinated and 117 (5.6 %) were not fully vaccinated. Table 3 shows the characteristics of 2088 vaccinated HCP with absenteeism stratified by COVID-19 vaccination status while Table 4 summarizes the characteristics of absenteeism stratified by COVID-19 vaccination status. In our cohort, COVID-19 accounted for most episodes of absenteeism (79.2 % of episodes in the fully vaccinated group and 82.1 % of episodes in the not fully vaccinated group; p-value = 0.451) followed by asymptomatic SARS-CoV-2 infection (18.9 % and 15.4 %, respectively; p-value = 0.340). Similarly, the distribution of other causes of absence from work did not differ by COVID-19 vaccination status.
Table 3.
Characteristics of 2088* vaccinated HCP with absenteeism by COVID-19 vaccination status, November 15, 2021 to April 17, 2022.
| Fully vaccinated** | Not fully vaccinated** | p-value | |
|---|---|---|---|
| Characteristic | N = 1971 (%) | N = 117 (%) | |
| Mean age, years (SD) | 41.4 (10.5) | 40.9 (10.2) | 0.610 |
| Male gender | 677 (34.4) | 41 (35.0) | 0.878 |
| Profession | |||
| Physician | 531 (26.9) | 18 (15.4) | 0.008 |
| Nursing personnel | 777 (39.4) | 64 (54.7) | |
| Paramedical personnel | 203 (10.3) | 14 (12.0) | |
| Supportive personnel | 291 (14.8) | 14 (12.0) | |
| Administrative personnel | 169 (8.6) | 7 (5.9) | |
| Influenza vaccination | 524 (26.6) | 20 (17.1) | 0.023 |
| Total days of absenteeism | 15,897 | 1136 | |
| Mean days of absenteeism (SD) | 8.1 (2.8) | 9.7 (4.9) | <0.001 |
| Presenteeism | 377 (19.1) | 18 (15.4) | 0.315 |
| Mean days of presenteeism (SD) | 1.1 (0.4) | 1.1 (0.2) | 0.457 |
| Mean time from last COVID-19 vaccine dose, weeks (SD) | 15.7 (8.2) | 40.6 (13.2) | <0.001 |
HCP: healthcare personnel; COVID-19: coronavirus disease 2019; SD: standard deviation.
for whom the COVID-19 vaccination status was known.
against COVID-19.
Table 4.
Characteristics of absenteeism among 2088 vaccinated HCP by COVID-19 vaccination status, November 15, 2021 to April 17, 2022.
| Cause of absence | Fully vaccinated | Not fully vaccinated | p-value | |
|---|---|---|---|---|
| N = 1971 (%)a | N = 117 (%)a | |||
| ARI | 694 (35.2) | 37 (31.6) | 0.429 | |
| Mean days of absence due to ARI (SD) | 8.3 (2.4) | 9.2 (3.6) | 0.010 | |
| Febrile episode | 148 (7.5) | 11 (9.4) | 0.453 | |
| Mean days of absence due to febrile episode (SD) | 6.7 (2.4) | 8.5 (2.7) | 0.013 | |
| ILI | 736 (37.3) | 48 (41.0) | 0.424 | |
| Mean days of absence due to ILI (SD) | 8.7 (3.1) | 10.1 (5.7) | 0.001 | |
| Asymptomatic SARS-CoV-2 infection | 373 (18.9) | 18 (15.4) | 0.340 | |
| Mean days of absence due to asymptomatic SARS-CoV-2 infection (SD) | 7.0 (2.4) | 10.3 (6.1) | <0.001 | |
| COVID-19 | 1560 (79.2) | 96 (82.1) | 0.451 | |
| Mean days of absence due to COVID-19 (SD) | 8.4 (2.8) | 9.8 (4.6) | <0.001 | |
| Exposure to a COVID-19 case | 11 (0.6) | 2 (1.7) | 0.124 | |
| Mean days of absence due to exposure to a COVID-19 case (SD) | 13.1 (6.4) | 13.0 (12.7) | 0.506 | |
| Influenza | 11 (0.6) | 1 (0.9) | 0.680 | |
| Mean days of absence due to influenza (SD) | 5.0 (1.9) | 5.0 (0.0) | ||
HCP: healthcare personnel; COVID-19: coronavirus disease 2019; ARI: acute respiratory infection; SD: standard deviation; ILI: influenza-like illness; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Percentages add over 100 % since an episode of absenteeism could be recorded concomitantly by clinical manifestation (acute respiratory infection, febrile episode or ILI) and as COVID-19.
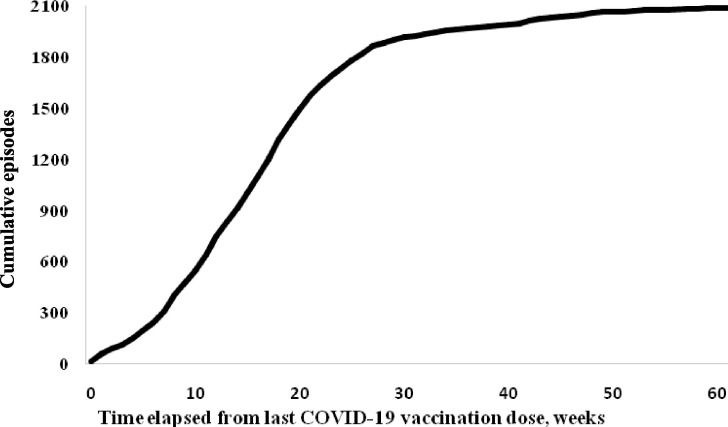
Overall, the episodes of absenteeism increased as the time-distance in weeks from the last COVID-19 vaccine dose increased (Fig. 1 ). The episodes of absenteeism occurred at a mean of 15.7 (SD: 8.2) weeks after the last COVID-19 vaccine dose in the fully vaccinated group compared to a mean of 40.6 (SD: 13.2) weeks after the last vaccine dose in the not fully vaccinated group (p-value < 0.001). Fully vaccinated HCP were absent from work for significantly shorter time periods than not fully vaccinated HCP [mean duration of absence per episode: 8.1 (SD: 2.8) days versus 9.7 (SD: 4.9) days, respectively; p-value < 0.001] (Table 3). Similarly, compared to not fully vaccinated HCP, fully vaccinated HCP with absenteeism, were excluded from work for a significant shorter mean time period for almost all causes of absence (Table 4). There was no difference between the two groups in terms of presenteeism characteristics.
Fig. 1.
Cumulative episodes of absenteeism among 2088 vaccinated HCP* by time elapsed since last COVID-19 vaccination dose. *with known COVID-19 vaccination status.
Table 5 presents the estimates of multivariable regression models. Compared to a not full COVID-19 vaccination status, full COVID-19 vaccination was significantly associated with fewer days of absence from work (OR: 0.56; 95 % CI: 0.36–0.87; p-value = 0.01) and a reduced risk for influenza (OR: 0.54; 95 % CI: 0.43–0.69; p-value < 0.001). In addition, compared with ≤ 17.1 weeks elapsing since the last COVID-19 vaccine dose, a history of > 17.1 weeks since the last vaccine dose was associated with increased duration of absence from work (OR: 1.22, 95 % CI: 1.02–1.46; p-value = 0.026) and increased risk for COVID-19 (OR: 1.72; 95 % CI: 1.24–2.39; p-value = 0.001), febrile episode (OR: 1,33; 95 % CI: 1.09–1.63; p-value = 0.004), and ILI (OR: 1.53; 95 % CI: 1.02–2.30; p-value = 0.038), while the opposite was observed for asymptomatic SARS-CoV-2 infection (OR: 0.60; 95 % CI: 0.39–0.92, p-value = 0.02). There was no significant association between not full COVID-19 vaccination status and several morbidity outcomes including SARS-CoV-2 infection.
Table 5.
Multivariable regressions’ estimates.
|
Days absent (quartiles) |
COVID-19 |
Asymptomatic SARS-CoV-2 infection |
ARI |
Febrile episode |
ILI |
Influenza |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95 % CI) | p-value | OR (95 % CI) | p-value | OR (95 % CI) | p-value | OR (95 % CI) | p-value | OR (95 % CI) | p-value | OR (95 % CI) | p-value | OR (95 % CI) | p-value | |
| COVID-19 vaccination | ||||||||||||||
| Partial | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||||
| Full | 0.56 (0.36–0.87) | 0.010 | 0.76 (0.51–1.14) | 0.187 | 1.44 (0.83–2.48) | 0.194 | 1.19 (0.78–1.81) | 0.416 | 0.77 (0.21–2.89) | 0.704 | 0.80 (0.51–1.24) | 0.322 | 0.54 (0.43–0.69) | <0.001 |
| Time since last vaccine dose (in weeks) | ||||||||||||||
| Less than average (≤17.1 weeks) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||||
| More than average (>17.1 weeks) | 1.22 (1.02–1.46) | 0.026 | 1.72 (1.24–2.39) | 0.001 | 0.60 (0.39–0.92) | 0.020 | 0.84 (0.66–1.08) | 0.172 | 1.33 (1.09–1.63) | 0.004 | 1.53 (1.02–2.30) | 0.038 | 0.69 (0.23–2.02) | 0.488 |
Notes: All regression models control for age, sex, occupation, influenza vaccination status, and hospital fixed-effects. Standard errors were clustered at the hospital level.
COVID-19: coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ARI: acute respiratory illness; ILI: influenza-like illness; OR: Adjusted Odds Ratio; CI: Confidence Intervals; Ref: reference.
4. Discussion
This prospective multicenter study of morbidity and absenteeism among 7592 HCP in the context of a mandatory COVID-19 vaccination policy showed that COVID-19 remains a major driver of morbidity and absenteeism among HCP. In our cohort, there were 2182 episodes of absenteeism in 2021–2022 compared to 910 episodes in the respective 2020–2021 period [6], which corresponds to 28.7 and 12.2 episodes per 100 HCP, respectively. Studies conducted before the deployment of COVID-19 vaccines found much higher rates and duration of COVID-19-associated absenteeism among HCP [2], [14], [15], [16], [17] than those recorded in pre-COVID-19 influenza seasons [12], [13], [18], [19], and excess absenteeism-associated costs [17], [20]. Moreover, in the current study the overall absenteeism ratio was estimated at 2.3 which far exceeds the absenteeism ratio of 1.3 recorded in the same cohort during the 2020–2021 season [6], as well as the absenteeism ratios recorded in pre-COVID-19 influenza seasons [12], [13], [18], [19]. For instance, in a United States (US) multicenter study conducted during three influenza seasons (2012–2015) with varying matching between circulating and vaccine strains, absenteeism ratio ranged from 0.58 to 0.62 among influenza vaccinated HCP compared with 0.66 to 1.03 among unvaccinated HCP [12].
In our cohort, COVID-19 or asymptomatic SARS-CoV-2 infections were detected in 1727 (22.7 %) and 403 (5.3 %) cases each, compared with 239 (3.2 %) and 96 (1.3 %) cases in 2020–2021 [6]. Overall, SARS-CoV-2 infection accounted for the overwhelming morbidity and absenteeism. The increased rate of SARS-CoV-2 infections in the current study compared with the 2020–2021 study is mainly attributed to the strict lockdown policies imposed in Greece during 2020–2021, as indicated by a COVID-19 stringency index ranging from 78.70 to 88.89 the last year [21], the ease of RADT testing during the current study in addition to RT-PCR only in 2020–2021, the dominance of highly transmissible Omicron subvariants during most of the current study period, but also to the waning vaccine-induced immunity [22], [23], [24]. Our findings indicate that COVID-19 continues to have a considerable impact on healthcare workforce morbidity and absenteeism, and therefore on healthcare services and costs. This may prove critical for healthcare systems in the upcoming seasons given the uncertainties about post-COVID influenza activity [25], [26]. In our study a very small number of HCP developed influenza. In Greece, as in other Northern Hemisphere countries, seasonal influenza showed unusually late and low activity in the first half of 2022, compared with pre-COVID-19 seasons [25]. Notably, approximately one out of five HCP continued working for a mean of 1.1 days despite the onset of symptoms, which indicates gaps in surveillance of HCP health and infection control procedures [27].
Another finding of our study is that fully COVID-19 vaccinated HCP were absent from work for significantly shorter periods of time compared to not fully vaccinated HCP. In practice, full COVID-19 vaccination prevented almost half of days of absence, which is consistent with a milder morbidity following full COVID-19 vaccination and which, in our opinion, justifies mandatory vaccination policies. The implementation of mandatory COVID-19 vaccination policies in the participating hospitals achieved vaccine uptake rates exceeding 95 %. Further studies are needed to investigate the cost-effectiveness of mandatory COVID-19 vaccination policies for HCP.
In our study, the administration of a COVID-19 vaccine dose in less than four months before conferred protection against several morbidity outcomes, including COVID-19, febrile episode, and ILI, as well as for shorter duration of absenteeism, independently of COVID-19 vaccination status. In practice, timing of last vaccine dose was a determinant of COVID-19-associated morbidity and absenteeism among HCP. The determination of the optimal seasonal timing for COVID-19 vaccine boosters for HCP is of outmost importance not only to maximize their protection, but also to reduce the impact of absenteeism on healthcare systems. Waning of antibody titers also occurs gradually over six months after influenza vaccination and is considered to achieve a satisfactory protection especially for high-risk groups [28], [29].
As reported by others [23], [24], [30], [31], our findings indicate that a booster vaccine dose confers protection against COVID-19-associated morbidity, yet protection wanes over time. A US case-control study found that following a primary mRNA vaccine series, three mRNA vaccine doses added a 50–56 % relative effectiveness during Omicron dominance and 78–96 % during Delta dominance, depending on vaccine brand [32]. They also found that booster protection remained largely stable for at least 16 weeks after vaccination [32]. Moreover, staff-to-staff and staff-to-patient transmission is a major driver of SARS-CoV-2 dissemination in healthcare facilities [33], [34], [35]. Real-life data found significantly lower SARS-CoV-2 viral loads in upper respiratory tract during the first five symptomatic days in boosted patients with Omicron breakthrough infections compared to unvaccinated infected patients [36]. Therefore, booster vaccines may reduce but not eliminate the risk of virus transmission and therefore infectiousness. The fact that current COVID-19 vaccines are less effective at controlling upper respiratory tract infection may account for that [24]. In addition, there is evidence that full COVID-19 vaccination of HCP reduces by more than half the hazard ratio for their household contacts to become infected with SARS-CoV-2 [37]. Therefore, full COVID-19 vaccination of HCP is justified to protect them but also their contacts.
Our study has several strengths. First, we prospectively followed a large cohort of fully vaccinated HCP throughout a 22-week period. Second, the study period spanned two SARS-CoV-2 variants, Delta followed by Omicron dominance, and low seasonal influenza activity in the second half of the study period [10], [11]. Third, we studied several clinical and laboratory-confirmed morbidity and absenteeism outcomes of importance for healthcare workforce. Fourth, we followed morbidity and absenteeism episodes by calendar week. Despite these strengths, an intrinsic limitation of our study is its observational design. We adjusted for hospital heterogeneity and clustering to address the possibility of variation of SARS-CoV-2 transmission dynamics across hospitals.
In conclusion, COVID-19 remains a major driver of morbidity and absenteeism among HCP despite the availability of COVID-19 vaccines. Full COVID-19 vaccination significantly reduced the duration of absenteeism during a period of high SARS-CoV-2 circulation in the community. Moreover, the administration of a COVID-19 vaccine dose in less than four months before conferred significant protection against COVID-19, several other morbidity outcomes, and duration of absenteeism among HCP, irrespective of full or partial COVID-19 vaccination status. Our findings may guide vaccination policies in healthcare facilities and other essential working settings. Lastly, our findings have implications in informing the optimal strategy for booster vaccinations. More studies are needed to elucidate the epidemiological characteristics towards SARS-CoV-2 endemicity including the optimal timing of booster vaccinations. The development of more efficient COVID-19 vaccines that will confer long-term protection not only against severe outcomes but also enhanced protective immunity against SARS-CoV-2 infection is imperative.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We are thankful to the Infection Control Committees of the participating hospitals for their assistance. We also thank Mrs Tentoma for technical assistance. The opinions presented in this article are those of the authors and do not necessarily represent those of their institutions.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data will be made available on request.
References
- 1.World Health Organization. Coronavirus disease (COVID-19) pandemic, available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (last accessed: October 9, 2022).
- 2.Maltezou H.C., Dedoukou X., Tseroni M., Tsonou P., Raftopoulos V., Papadima K., et al. SARS-CoV-2 infection in healthcare personnel with high-risk occupational exposure: evaluation of seven-day exclusion from work policy. Clin Infect Dis. 2020;71:3182–3187. doi: 10.1093/cid/ciaa888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Health Workforce Department. The impact of COVID-19 on health and care workers: a closer look at deaths, available at: https://www.who.int/publications/i/item/WHO-HWF-WorkingPaper-2021.1 (last accessed: October 9, 2022).
- 4.Gholami M., Fawad I., Shadan S., Rowaiee R., Ghanem H.A., Khamis A.H., et al. COVID-19 and healthcare workers: a systematic review and meta-analysis. Int J Infect Dis. 2021;104:335–346. doi: 10.1016/j.ijid.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. World Health Organization Strategic Advisory Group of Experts roadmap for prioritizing uses of COVID-19 vaccines. Version 21 January 2022, available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Prioritization-2022.1 (last accessed: October 9, 2022).
- 6.Maltezou H.C., Panagopoulos P., Sourri F., Giannoutsos T.V., Raftopoulos V., Gamaletsou M.N., et al. COVID-19 vaccination significantly reduces morbidity and absenteeism among healthcare personnel: a prospective multicenter study. Vaccine. 2021;39:7021–7027. doi: 10.1016/j.vaccine.2021.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Parliament Legal issues surrounding compulsory COVID-19 vaccination, available at: https://www.europarl.europa.eu/RegData/etudes/BRIE/2022/729309/EPRS_BRI(2022)729309_EN.pdf (last accessed: October 9, 2022).
- 8.Urgent regulations for the confrontation of COVID-19 (Law 4820/2021, Chapter C). Government Gazette 130/A/23-7-2021. Available at: https://www.e-nomothesia.gr/kat-dikasteria-dikaiosune/nomos-4820-2021-phek-130a-23-7-2021.html (last accessed: October 9, 2022) [in Greek].
- 9.Saade A., Cha L., Tadié E., Jurado B., Bihan A., Baron-Latouche P., et al. Delay between COVID-19 complete vaccination and SARS-CoV-2 infection among healthcare workers. Vaccine. 2022;40:3159–3164. doi: 10.1016/j.vaccine.2022.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Public Health Organization. Weekly Report of Epidemiological Surveillance of SARS-CoV-2 Infection, available at: https://eody.gov.gr/wp-content/uploads/2022/07/covid-gr-weekly-report-2022-28.pdf (last accessed: October 9, 2022) [in Greek].
- 11.National Public Health Organization. Weekly Report of Epidemiological Surveillance of Influenza, Week 20/2022 (16-22 May 2022), available at: https://eody.gov.gr/wp-content/uploads/2022/04/20.2022-FLU-WEEK.pdf (last accessed: October 9, 2022) [in Greek].
- 12.Frederick J., Brown A.C., Cummings D.A., Gaydos C.A., Gibert C.L., Gorse G.J., et al. Protecting healthcare personnel in outpatient settings: the influenza of mandatory versus nonmandatory influenza vaccination policies on workplace absenteeism during multiple respiratory virus seasons. Infect Control Hosp Epidemiol. 2018;39:452–461. doi: 10.1017/ice.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Challener DW, Breeher LE, Frain JE, Swift MD, Tosk PK, O’ Horo J. Healthcare personnel absenteeism, presenteeism, and staffing challenges during epidemics. Infect Control Hosp Epidemiol 2021;42:388-91. [DOI] [PMC free article] [PubMed]
- 14.Groenewold M.R., Burrer S.L., Ahmed F., Uzicanin A., Free H., Luckhaupt S.E. Increases in health-related workplace absenteeism among workers in essential critical infrastructure occupations during the COVID-19 pandemic – United States, March-April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:853–858. doi: 10.15585/mmwr.mm6927a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolton R, Bolton DG. People styles at work. Making bad relationships good and good relationships better. New York, NY: AMACON; 1996.
- 16.Kisiel M.A., Nordqvist T., Westman G., Svartengren M., Mainovschi A., Janols H. Patterns and predictors of sick leave among Swedish non-hospitalized healthcare and residential care workers with Covid-19 during the early phase of the pandemic. PLoS ONE. 2021;16:e0260652. doi: 10.1371/journal.pone.0260652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faramarzi A., Javan-Noughabi J., Tabatabaee S.S., Najafpoor A.A., Rezapour A. The lost productivity cost of absenteeism due to COVID-19 in health care workers in Iran: a case study in the hospitals of Mashhad University of Medical Sciences. BMC Health Serv Res. 2021;21:1169. doi: 10.1186/s12913-021-07200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Buynder P.G., Konrad S., Kersteins K.F., Preston E., Brown P.D., Keen D., et al. Healthcare worker influenza immunization vaccinate or mask policy: strategies for cost effective implementation and subsequent reductions in staff absenteeism due to illness. Vaccine. 2015;33:1625–1628. doi: 10.1016/j.vaccine.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 19.Gianino M.M., Politano G., Scarmozzino A., Stillo M., Amprino V., Di Carlo S., et al. Cost of sickness absenteeism during seasonal influenza outbreaks of medium intensity among health care workers. Int J Environ Res Public Health. 2019;16:747. doi: 10.3390/ijerph16050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maltezou H.C., Giannouchos T.V., Pavli A., Tsonou P., Dedoukou X., Tseroni M., et al. Costs associated with COVID-19 in healthcare personnel in Greece: a cost-of-illness analysis. J Hosp Infect. 2021;114:126–133. doi: 10.1016/j.jhin.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maltezou H.C., Krumbholz B., Mavrouli M., Tseroni M., Gamaletsou M.N., Botsa E., et al. A study of the evolution of the third COVID-19 pandemic wave in the Athens metropolitan area, Greece, through two cross-sectional seroepidemiological surveys: March, June 2021. J Med Virol. 2022;94:1465–1472. doi: 10.1002/jmv.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen N.N., Houhamdi L., Hoang V.T., Stoupan D., Fournier P.E., Raoult D., et al. High rate of reinfection with the SARS-CoV-2 omicron variant. J Infect. 2022;85:206–208. doi: 10.1016/j.jinf.2022.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrestha L.B., Foster C., Rawlinson W., Tedla N., Bull R.A. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: implications for immune escape and transmission. Rev Med Virol. 2022;32:e2381. doi: 10.1002/rmv.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burckhardt R.M., Dennehy J.J., Poon L.L.M., Saif L.J., Enquist L.W. Are COVID-19 vaccine boosters needed? the science behind boosters. J Virol. 2022;96:e0197321. doi: 10.1128/jvi.01973-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S.S., Viboud C., Petersen E. Understanding the rebound of influenza in the post COVID-19 pandemic period holds important clues for epidemiology and control. Int J Infect Dis. 2022;122:1002–1004. doi: 10.1016/j.ijid.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lofgren E, Naumova EN, Gorski J, Naumov Y, Fefferman NH. How drivers of seasonality in respiratory infections may impact vaccine strategy: a case study in how COVID-19 may help us solve one of influenza’s biggest challenges. Clin Infect Dis 2022;75 (Suppl 1):S121-9. [DOI] [PMC free article] [PubMed]
- 27.Maltezou H.C., Dounias G., Rapisarda V., Ledda C. Vaccination policies for healthcare personnel: current challenges and future perspectives. Vaccine X. 2022;11 doi: 10.1016/j.jvacx.2022.100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maltezou H.C., Rodolakis A. Vaccination of pregnant women against influenza: what is the optimal timing? Hum Vaccin Immunother. 2021;17:2723–2727. doi: 10.1080/21645515.2021.1889934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poland G.A. Influenza vaccine failure: failure to protect or failure to understand? Expert Rev Vaccines. 2018;17:495–502. doi: 10.1080/14760584.2018.1484284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu P., Faraone J., Evans J.P., Zou X., Zheng Y.M., Carlin C., et al. Neutralization of the SARS-CoV-2 omicron BA.4/5 and BA.2.12.1 subvariants. N Engl J Med. 2022;386:2526–2528. doi: 10.1056/NEJMc2206725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chemaitelly H., Ayoub H.H., Al Mukdad S., Coyle P., Tang P., Yassine H.M., et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022;13:3082. doi: 10.1038/s41467-022-30895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richterman A, Behrman A, Brennan PJ, O’ Donnell JA, Snider CK, Chaiyachati KH. Durability of SARS-CoV-2 mRNA booster vaccine protection against omicron among health care workers with a vaccine mandate. Clin Infect Dis 2022 Jun 6;ciac454. doi: 10.1093/cid/ciac454. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 33.Gordon C.L., Trubiano J.A., Holmes N.E., Chua K.Y.L., Feldman J., Young G., et al. Staff to staff transmission as a driver of healthcare worker infections with COVID-19. Infect Dis Health. 2021;26:276–283. doi: 10.1016/j.idh.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watt A.E., Sherry N.L., Andersson P., Lane C.R., Johnson S., Wilmot M., et al. State-wide genomic epidemiology investigations of COVID-19 in healthcare workers in 2020 Victoria, Australia: qualitative thematic analysis to provide insights for future pandemic preparedness. Lancet Reg Health West Pac. 2022;25 doi: 10.1016/j.lanwpc.2022.100487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jinadatha C., Jones L.D., Choi H., Chatterjee P., Hwang M., Redmond S.N., et al. Transmission of SARS-CoV-2 in inpatient and outpatient settings in a Veterans Affairs health care system. Open Forum. Infect Dis. 2021;8:ofab328. doi: 10.1093/ofid/ofab328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puhach O., Adea K., Hulo N., Sattonnet P., Genecand C., Iten A., et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat Med. 2022;28:1491–1500. doi: 10.1038/s41591-022-01816-0. [DOI] [PubMed] [Google Scholar]
- 37.Shah A.S.V., Gribben C., Bishop J., Hanlon P., Caldwell D., Wood R., et al. Effect of vaccination on transmission of SARS-CoV-2. N Engl J Med. 2021;385:1718–1720. doi: 10.1056/NEJMc2106757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.