Abstract
Electrochemical energy conversion and storage are central to developing future renewable energy systems. For efficient energy utilization, both the performance and stability of electrochemical systems should be optimized in terms of the electrochemical interface. To achieve this goal, it is imperative to understand how a tailored electrode structure and electrolyte speciation can modify the electrochemical interface structure to improve its properties. However, most approaches describe the electrochemical interface in a static or frozen state. Although a simple static model has long been adopted to describe the electrochemical interface, atomic and molecular level pictures of the interface structure should be represented more dynamically to understand the key interactions. From this perspective, we highlight the importance of understanding the dynamics within an electrochemical interface in the process of designing highly functional and robust energy conversion and storage systems. For this purpose, we explore three unique classes of dynamic electrochemical interfaces: self-healing, active-site-hosted, and redox-mediated interfaces. These three cases of dynamic electrochemical interfaces focusing on active site regeneration collectively suggest that our understanding of electrochemical systems should not be limited to static models but instead expanded toward dynamic ones with close interactions between the electrode surface, dissolved active sites, soluble species, and reactants in the electrolyte. Only when we begin to comprehend the fundamentals of these dynamics through operando analyses can electrochemical conversion and storage systems be advanced to their full potential.
Keywords: electrochemical interface, electrochemistry, energy conversion and storage, dynamics, reconstruction
1. Introduction
Electrochemical reactions occur at the electrode–electrolyte junctions, known as the electrochemical interface. Because both charge transfer and various types of chemical interactions are driven between the electrified electrode and electrolyte, the properties of the electrochemical interface determine the efficiency of electrochemical energy conversion and storage systems.1 Although significant advances in electrochemical performance have been achieved through material design on the electrode side, there is still a lack of both structural and dynamic information on atomic- and molecular-scale interactions between the solid electrode and liquid electrolyte.2 Thus, improving our understanding of the electrochemical interface is imperative for optimal electrochemical performance.
In electrocatalysis, electrochemically active sites are classically described as being in a frozen state (i.e., static active sites), an idea that is now outdated because dynamic phenomena have been observed in numerous systems, including phase transition, dissolution, and deposition during electrochemical reactions.3−12 Moreover, active sites continuously change because of vigorous interactions between the electrode and electrolyte. This highlights the importance of ‘dynamic electrochemical interfaces’ as the focus of our research because most studies have focused on either the electrode itself or the electrolyte separately. Therefore, the dynamic properties of electrochemical interfaces should be further investigated to design functional interface structures that can overcome the thermodynamic limitations of conventional electrode materials for producing advanced electrochemical energy conversion and storage systems.
Herein, we discuss three dynamic interfacial phenomena in electrocatalysis among various electrochemical environments in energy conversion and storage systems, with a focus on the regeneration of active sites by interaction between electrode and electrolyte and their mechanisms and impacts on applications (Figure 1). First, the self-healing concept allows catalyst regeneration through the redeposition of dissolved species, which enables stable oxygen evolution reactions. Second, “active site”–“stable host” pairs are covered as a new type of dynamic electrochemical interface. Unlike self-healing, active site regeneration in this dynamic interface requires a specific host (different elements with active sites) with a high affinity for the active sites. It can be extended to various reactions with diverse combinations of active sites and hosts. Finally, a redox mediator (or soluble catalyst) is discussed as an effective electron–hole transfer agent between the electrode and electrolyte to circumvent the limited number of active sites between the electrode and the reactant.
Figure 1.
Dynamic electrochemical interfaces during electrocatalysis (Rxn indicates where the catalytic reactions take place).
2. Dynamic Electrochemical Interfaces
2.1. Self-Healing Electrocatalysis
The electrocatalytic activity and stability can offset each other as a trade-off when the overall performance needs to be optimized. The most representative example is the oxygen evolution reaction (OER), where an inverse correlation is demonstrated between activity and stability.13 To leverage high OER performance with long-term stability, electrochemical systems can benchmark self-healing properties, which are commonly observed in biological systems, including photosynthesis systems.14,15 By definition, a self-healing system should recover from structural damage through a thermodynamically favorable, spontaneous regeneration reaction.16,17 In other words, the operating conditions of functional surfaces should also promote the structural regeneration process simultaneously. Because atomic dissolution is a major degradation mechanism in the OER, the self-healing strategy can effectively address OER stability problems by supplementing the active site loss.18,19
The working potential should be matched between the OER electrocatalysis and the electro (or chemical) deposition of active materials to fulfill the self-healing condition. The first case of a self-healing OER catalyst was reported by Kanan et al.11 An inert conductive surface (e.g., FTO glass) is polarized to the OER region in a Co2+-containing phosphate (Pi) buffer solution, and an amorphous Co-based film (i.e., Co-based oxygen-evolving catalyst, Co-OEC) is electrodeposited using Pi after oxidation of Co2+ to Co3+. This unique potential overlap between oxidative electrodeposition and OER electrocatalysis satisfies the thermodynamic requirements for the self-healing mechanism. A more detailed process for self-healing Co-OEC was further investigated using isotope-labeled 57Co because of its radioactive detectability (Figure 2a).5 After the 57Co-OEC film was electrodeposited, 57Co2+ was continuously dissolved into the electrolyte under open-circuit conditions. However, when the film was polarized to the OER region (i.e., 1.3 V vs NHE), the 57Co2+ concentration in the electrolyte decreased because of Co-OEC deposition onto the original film. This result indicates that the rate of Co-Pi assembly into Co-OEC is much faster than that of Co2+ dissolution during the OER. For Co-OEC electrodeposition, the Pi anion was found to be a key proton-accepting, pH buffer anion. When other nonbuffering anions (e.g., SO42–, ClO4–, and NO3–) were used in the electrolytes, the deposited Co-OEC catalysts degraded rapidly in the OER region because of severe Co dissolution. However, the degraded catalysts were recovered after Pi anions were added to the electrolyte, thereby corroborating the requirement for buffering anions in the self-healing catalyst mode. During the OER, water oxidation discharges protons and decreases the local pH at the catalyst–electrolyte interface, which can degrade metal hydr(oxy)oxide active sites through chemical dissolution. Therefore, pH-buffering species such as Pi can provide stable local pH conditions on the catalyst surface by reacting as a base in place of the Co-OEC catalyst and allowing stable OER electrocatalysis.
Figure 2.

Self-healing Co-based oxygen-evolving catalyst (Co-OEC). (a) Dissolution rate of isotope-labeled 57Co from Co-OEC catalyst layer into electrolyte under open-circuit potential (red) or applied potential (with on/off switching) of 1.3 V (vs NHE). Reproduced from ref (5). Copyright 2009 American Chemical Society. (b) Electrodeposition mechanism of self-healing Co-OEC with Pi. Reproduced with permission from ref (21). Copyright 2017 National Academy of Sciences. (c) Ranges of electrolyte pH for self-healing mechanism, determined on the basis of Pourbaix diagrams of Co-OEC catalyst deposition (black) and OER electrocatalysis (red). Reproduced from ref (20). Copyright 2012 American Chemical Society.
The detailed mechanism of the self-healing mode in Co-OEC was later investigated by Surendranath et al.20 and Costentin et al.21 They found that the electrodeposition rate of Co-OEC had first-order, inverse third-order, and inverse first/second-order dependences on the ionic activities of Co2+, H+, and Pi, respectively.20 From these results, the self-healing mechanism can be derived as shown in Figure 2b.21 First, solvated [Co(H2O)6]2+ is deprotonated to [Co(OH)(H2O)5]+. Simultaneously, the Co3+ site in Co-OEC is oxidized to Co4+ through the proton-coupled electron transfer (PCET) step, and the Pi anion dissociates from the oxidized Co4+ site via deprotonation. Then, [Co(OH)(H2O)5]+ in the electrolyte is oxidatively deposited onto the Pi-free Co4+ site, which results in the addition of Co3+ to the edge where Pi dissociated. This inverse third-order dependence of protons on the catalyst deposition rate uniquely imparts strong pH dependency on the self-healing capability of the system. As discussed above, the self-healing mode is viable only if the catalyst self-assembly potential is lower than the OER potential. Thus, considering that the OER rate is inversely first-order dependent on the H+ activity,22 the available pH ranges for the self-healing mechanism can be plotted in the Pourbaix diagram between Co-OEC deposition and OER catalysis (Figure 2c). The Pourbaix diagram shows that the self-healing mechanism is operative at pH > 6, where catalyst corrosion is counterbalanced by much more favorable catalyst deposition during the OER.
Beyond Co-OECs from Pi-based electrolytes, many efforts have been made to expand the chemical inventory of self-healing catalysts (Table 1). Inspired by the critical role of Pi in the self-healing mechanism, other proton-accepting buffer anions were also explored, including methylphosphate (MePi) and borate (Bi).23 Similar to Pi anion, both MPi and Bi anions enable the oxidative electrodeposition of amorphous Co-OEC catalysts. Notably, both Co-OEC catalysts feature negligible Co dissolution into the electrolyte within the OER potential window, which corroborates the need for proton-accepting anions as functional building blocks for self-healing OER catalysts. The self-healing OER catalyst was extended to Ni- and Mn-based active sites. Dincâ et al. reported that Bi anions could effectively assemble Ni into amorphous Ni-OEC catalysts under oxidative polarization.24 Similar to Co-OEC, Ni-OEC was only formed in the presence of proton-accepting Bi anions in the electrolyte and showed relatively stable OER electrocatalysis. In addition, Huynh et al. demonstrated that MnOx catalyst deposited from Mn2+-containing MePi electrolyte also showed self-healing characteristics.6 From different levels of proton dependence between MnOx electrodeposition and OER kinetics, the MnOx catalyst was functionally stabilized through self-healing within the pH region where its deposition potential was lower than the OER-operative potential, similar to self-healing Co-OEC.
Table 1. Summary of Self-Healing Oxygen Evolution Catalysts.
| catalyst
deposition |
||||||
|---|---|---|---|---|---|---|
| catalyst | metal | anion type | pH | potential (V vs NHE) | self-healing condition | ref |
| CoPi | Co2+ (0.5 mM) | phosphate | 7.0 | +1.3 V | pH > 5.2 | (11, 20) |
| CoMePi | Co2+ (>0.5 mM) | methylphosphonate | 8.0 | +1.3 V | (23) | |
| CoBi | Co2+ (0.5 mM) | borate | 9.2 | +1.3 V | (23) | |
| MnMePi | Mn2+ (0.5 mM) | methylphosphonate/phospate | 8.0 | +0.54 V | pH > −0.5 | (6) |
| NiBi | Ni2+ (1 mM) | borate | 9.2 | +1.3 V | pH > 9.2 | (24) |
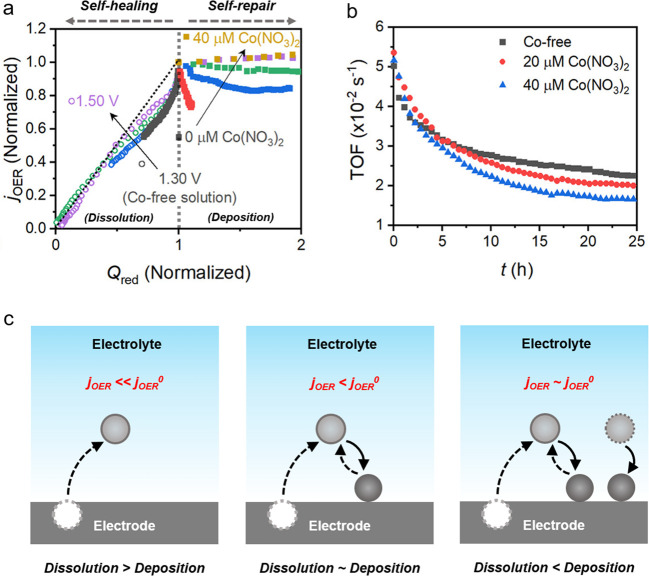
Although self-healing catalysts with continuous regeneration appear to be ideal systems for sustainable OER electrocatalysis, more detailed investigations are required to ensure the catalytic efficacy of the regenerated catalyst sites. For this purpose, Mohammadi et al. recently revisited the Co-OEC catalyst in a Pi-based electrolyte to investigate the limitations of its self-healing (in Co-free electrolyte) and self-repair (in Co-containing electrolyte) mechanisms during long-term operation.25 Electrodeposited Co-OEC catalysts were systematically exposed to chronoamperometry, and both Co-loss and OER-deactivation were monitored (Figure 3a). For the self-healing mode in Co2+-free electrolytes, Co dissolution was confirmed to be mitigated between 1.1 and 1.3 V, which corresponds to the self-healing regime in the previous study.5 Normalized OER current density also followed the same trend as Co dissolution, which indicated both atomic dissolution (as major OER degradation) and improved OER stability around 1.3 V by the self-healing mechanism. Nevertheless, the Co-OEC still suffered significant Co dissolution and critical OER deactivation at 1.3 V after 24 h (Figure 3a). Therefore, the self-healing mode cannot completely secure the initial catalyst activity during long-term OER. The self-repair mode was tested in Co2+-containing electrolytes to overcome the limitations of self-healing. The presence of Co2+ in the electrolyte increased the Co-OEC deposition rate to counterbalance Co2+ dissolution during OER electrocatalysis. Therefore, the Co loss within the catalyst film was significantly decreased and was no longer observed beyond the 3 μM Co2+ mark. Nonetheless, the catalyst still suffered OER deactivation despite the preserved Co amounts, which implies that the self-repair mode played an incomplete role in OER activity preservation. Stable OER activity was achieved only at high Co2+ concentrations of 20 μM and above, where additional Co-OEC films were continuously deposited (Figure 3a). However, this led to a decrease in the OER turnover frequency (TOFOER) at each Co active site because the number of Co sites increased, while the overall OER activity remained the same. Indeed, when the time-dependent TOFOER of various Co sites were compared, both Co2+-free and Co2+-containing conditions showed relatively similar features of rapidly decreasing TOFOER (Figure 3b). Thus, it can be implied that there is the same deactivation mechanism within the Co-OEC, which cannot be resolved by either self-healing or self-repair modes. On the basis of X-ray absorption spectroscopy analysis, OER deactivation was attributed to structural ordering within the amorphous Co-OEC, regardless of the presence of Co2+ in the electrolyte. Consequently, both the self-healing and self-repair mechanisms are highly beneficial for mitigating Co dissolution in Co-OEC, but they cannot prevent the intrinsic OER deactivation mechanism of structural ordering within the amorphous Co-OEC film. This result demonstrates that the suppression of Co dissolution is not sufficient for stable OER activity because of catalyst self-deactivation and that additional deposition of new Co-OEC layers is required for truly stable long-term OER electrocatalysis (Figure 3c).
Figure 3.
Limitations of self-healing and self-repair modes on Co-OEC. (a) Summary of both Co content and OER activity changes in Co-OEC under self-healing (Co-free electrolyte) and self-repair (Co-containing electrolyte) conditions. (b) Time-dependent OER turnover frequency (TOF) of Co sites in Co-free (black, self-healing) and Co-containing (red and blue, self-repair) electrolytes. Reproduced from ref (25). Copyright 2020 American Chemical Society. (c) A broadened scope for understanding the behavior and stability of Co-OEC.
2.2. Active Site–Stable Host Interaction
Previously, the active sites were considered to be fixed if the loading mass or surface area of the catalyst was determined; this was known as the so-called “frozen” surface. However, as clearly validated, the number of active sites changes during the reaction via vigorous dissolution and redeposition, which indicates that the “frozen” surface concept can no longer be applied to dynamic interfaces.19 As discussed in the previous chapter, self-healing provides a unique opportunity to regenerate active sites during electrochemical reactions. While it opens a new direction to overcome thermodynamic limitations on stability from a frozen surface to a dynamic surface, it can be limited to certain conditions (or elements), such as Co and Mn systems. If the active element cannot be regenerated by itself, the self-healing concept is no longer valid; therefore, an alternative strategy is required to regenerate the active site. In this part, we focus on a recent strategy to utilize active species by introducing stable host materials, especially focusing on active species–host interactions.3,18
When OER catalysis on a metal hydroxide (M hydroxide) surface was studied with a controlled (Fe-purified) electrolyte,3 the inverse correlation was identified between activity and stability as in the acid media.26−28 Ni hydroxide is both the most stable (Ni hydroxide > Co hydroxide ≫ Fe hydroxide) and the least OER-active (Ni hydroxide < Co hydroxide < Fe hydroxide) catalyst. In the case of Fe-incorporated metal hydroxides, the activity and stability were highly affected by the small amount of Fe(aq) in the electrolytes. When a 0.1 ppm Fe(aq)-containing solution existed in the electrolyte, there was no activity drop. However, a significant performance drop was observed in the purified electrolytes. The puzzling behavior of stability difference depending on the existence of Fe(aq) in the electrolyte was demonstrated through isotope experiments.3 Starting with 56Fe in the electrode as Fe–Ni hydroxide and 57Fe in the electrolyte, the loss of 56Fe (initially from the electrode) and the gain of 57Fe (initially from the electrolyte) at the electrode can be traced back to the dissolution and deposition events occurring during the OER, respectively. The overall Fe content in the electrode remained constant, thereby preserving the total number of active sites made available through Fe dissolution and redeposition at the interface during the OER (Figure 4a,b): a concept which is similar to that of self-healing. However, it differs from previously reported self-healing in that Fe cannot regenerate itself into Fe hydroxide. That is, to regenerate Fe active sites, a support on which Fe can be electrochemically or chemically deposited is required, and the degree of iron redeposition varies depending on the support. With the introduction of a second element, Ni hydroxide (NiM alloy hydroxide; M: Cu, Co, Mn), the OER activity is significantly affected, and the trend is followed by the stabilization of Fe in the host materials during the OER.3 This observation strongly supports the idea that the interaction between the active site (Fe) and the host materials can be regarded as an important design criterion for active electrochemical interfaces under dynamic conditions.
Figure 4.
Active site–stable host interactions at the dynamic electrochemical interface. (a) Total amount of Fe (active sites) in electrode during OER. (b) OER activity during chronoamperometry in Fe(aq) in electrolyte. (c) Scheme of active site and host interactions. Reproduced with permission from ref (3). Copyright 2020 Nature Publishing Group.
On the basis of the activity–stability trend and operando analysis, a new concept considering dynamic electrochemical interfaces, which is supported by the active site–support interaction, is proposed (Figure 4c).3 This dynamic electrochemical interface is compromised by two factors. First, it indicates the extent to which an active site can be maximally stabilized and utilized by the interaction with host materials as a thermodynamic factor. Second, it shows the equilibrium condition at the electrochemical interface between the electrode and electrolyte via dissolution from the electrode to the electrolyte and redeposition from the electrolyte to the electrode. On the basis of this concept, two general strategies are effective for increasing the overall electrochemical performance. First, it is crucial to design active sites and host pairs with strong interactions. For example, Fe is the active site in alkaline oxygen evolution; however, it cannot be regenerated (self-healed) because Fe cannot be self-regenerated on the Fe hydroxide surface because of the weak interaction between the Fe hydroxide (host) and Fe atom (active site).3 It is critical to find a suitable host surface that can stabilize the active sites, which implies that the number of active sites and the overall activity can be controlled by host engineering. The second important factor is the equilibrium between the electrode and the electrolyte. In particular, the concentration of active sites in the electrolyte is the key to optimizing equilibrium. If the concentration is too low or negligible, the number of dynamic active sites keeps decreasing during the reaction, followed by activity decay. If the concentration is sufficiently high at a certain level to lead equilibrium, the active sites can be continuously regenerated. Because both thermodynamic and chemical equilibrium factors are equally important for determining the number of dynamic active sites, the design principles should be revisited to utilize dynamic electrochemical interfaces.
The concept of the active site–host interaction can be generally adopted in various phases and structures of transition metals, such as oxides, nitrides, and phosphides because hydroxides and oxyhydroxides are thermodynamically stable phases from the Pourbaix diagram under OER conditions.10,29−35 Recently, this type of reconstruction issue was discussed using a precatalyst/catalyst scenario whereby the starting materials were “precatalysts” while the reconstructed surfaces were “catalysts”.36−38 Not surprisingly, surface reconstruction issues were also raised in perovskite samples during the OER.10,39−41 The Schmidt group clearly observed the reconstruction of perovskites using nanosized Ba0.5Sr0.5Co0.8Fe0.2O3-δ (BSCF).10 The surface of BSCF was transformed to (Co/Fe)O(OH), a self-assembled active layer via a lattice oxygen evolution reaction (LOER). The LOER process leads to Ba2+ and Sr2+ dissolution and triggers the dissolution of transition metals (Co and Fe). However, being rather insoluble species, Co and Fe can be easily redeposited on the catalyst surface, especially near the electrode surface. The cation concentration (because of the initial dissolution) becomes significant, which presents that a dynamic self-assembled surface layer appears to be the key to a mechanistic understanding of the OER (Figure 5a). A recent study also validated that the activity trend of perovskites is highly affected by the Fe in electrolytes and suggested that the number of dynamic active sites could also be the reason for these systems using Sr-doped LaCoO3 (La1–xSrxCoO3) model study.42 When Sr is introduced to La sites, dissolution of Sr activates the surface reconstruction of La1–xSrxCoO3 from crystalline perovskite to amorphous (oxy)hydroxide (note: the dissolution of La is negligible in the same potential window). The (oxy)hydroxide surface can potentially stabilize Fe and form dynamic active (Fe)–stable host interactions, whereas the oxide surface without surface reconstruction cannot, thereby indicating that the activity of the Sr-doped samples was highly affected only when the electrolyte contained Fe. The higher the amount of Sr, the more easily the surface was reconstructed, and the surface area that could interact with Fe increased, thereby showing high OER activity (Figure 5b). When there is no Fe in the electrolyte, the activities of all samples are identical, which indicates that the origin of the difference in the activities depending on Sr doping can be attributed to the dynamic interaction between the Fe active site (solution) with the host surface (hydroxide/oxyhydroxide). Recent reports also highlight similar phenomena using B-site-controlled perovskites (SrCo1–xFexO3).43 Both SrCoO3 and SrCo0.8Fe0.2O3 perovskites underwent surface amorphization during electrochemical cycling in 0.1 M KOH. After spiking in the electrolyte, Fe was incorporated into the amorphized surface of SrCoO3, which led to remarkable activity compared with the conditions in the absence of an Fe spike (Figure 5c). Because several perovskite samples can be subjected to surface reconstruction, the activity trend and mechanistic study should be carefully conducted after considering dynamic reconstruction and interaction with electrolytes.44
Figure 5.
Reconstruction of perovskite during oxygen evolution reaction. (a) Scheme of dissolution and redeposition mechanisms to form the active surface layer. Reproduced with permission from ref (10). Copyright 2017 Nature Publishing Group. (b) Activity trend of Sr doped La1–xSrxCoO3 with and without Fe(aq) in the electrolyte. Reproduced from ref (42). Copyright 2021 American Chemical Society. (c) Schematic illustration of two ways to incorporate Fe into perovskites and their OER performance. Reproduced from ref (43). Copyright 2021 American Chemical Society.
2.3. Redox Mediator as Charge Agents
Charge transfer at the electrode–electrolyte interface requires considerable activation energy, which makes electrochemical reactions less efficient, particularly at high rates. This drawback can be overcome by introducing a redox mediator (or soluble catalyst) with fast kinetics into electrolytes to enhance charge transfer properties. This diffusible additive can easily move around in the electrolyte and have frequent contact with active materials, thereby maximizing the areas of interaction for catalysis.45,46 Unlike the abovementioned two cases, redox mediators are not deposited on the solid surface of the electrocatalysts. They actively participate in these reactions as charge carriers. The redox-mediated interface can be functionally active through the (electro)chemical transition of mediators. This so-called soluble catalyst can be electrochemically activated on the electrode surface, diffused into the electrolyte for chemical reactions with reactants, and reactivated on the electrode surface. This electrochemical–chemical-coupled system can be operated when the molecular orbital energy level of the redox mediator is properly aligned to drive the chemical reactions of interest while minimizing parasitic side reactions.
Redox mediators are usually a series of molecules and ions that can be reversibly oxidized or reduced in a target electrochemical reaction. They act as intermediate carriers and electron reservoirs without influencing the final products. To act as good mediators, these species require some key properties such as high reversibility, fast kinetics, negligible side reactions, good solubility as a component of electrolytes, and good stability under operating conditions.47 The careful choice of species with proper redox potential is important, but there are no comprehensive descriptions and/or guidelines for redox mediators for various electrochemical applications. In this section, we provide a brief review of the progress, challenges, and prospects of redox mediators in electrochemical energy-storage systems.
Li–O2 batteries have attracted significant interest as next-generation batteries because of their exceptionally high theoretical energy densities.48,49 Li–O2 batteries consist of a lithium anode, an electrolyte, and a carbon material as the air electrode. During the discharge process, O2 is reduced, and solid Li2O2 is produced on the air electrode. This process is referred to as the oxygen reduction reaction from the perspective of the oxygen species. During the charging process, solid Li2O2 decomposes into Li+ and O2 (another type of OER). Electronic insulating discharge products, such as Li2O/Li2O2 crystals, contribute to poor rate capability, low active material utilization, and high polarization.50,51 These issues can be circumvented during the charging process by introducing into the electrolytes redox-active species that can act as electron–hole transport mediators.52 The use of these mediators is considered an efficient solution to facilitate the electrochemical reaction between lithium and oxygen, thus improving the cycling performance. These redox mediators enable charge transfer at the solid–liquid interface, thereby accelerating the kinetics of the discharge intermediates and products (Figure 6a). In addition, they are usually soluble molecules and can migrate to longer distances from the electrode surface; thus, a large number of them can directly affect (maximize) energy density.
Figure 6.
(a) Mechanism of a redox mediator on a Li–O2 battery and (b) the voltage profiles of Li–O2 cells with and without a redox mediator. Schematic of the unified mechanisms in a lithium–sulfur battery. Reproduced with permission from ref (54). Copyright 2016 Nature Publishing Group. (c) Li2S nucleation and growth via conductive surface pathway and solution pathway in the presence of a redox mediator and (d) discharge curves of cells with redox mediators at different concentrations. Reproduced with permission from ref (58). Copyright 2016 Nature Publishing Group.
In 2013, Bruce et al. reported a redox mediator that could act as an electron–hole transfer agent between the electrode and Li2O2 interfaces, thereby activating the insulating discharge products.45 Unlike conventional solid electrodes, this soluble redox mediator (molecule) could easily move around with the discharge product (Li2O2) and maximize the interaction for catalysis. In a follow-up report, Bruce et al. suggested that the redox potential of redox mediators should be close to that for Li2O2 formation.53 They further proposed that the equilibrium potential of Li2O2 should be slightly lower than the oxidation potential of the redox mediators and that the redox mediators should be inert to the electrolyte or counter Li metal anode. Recently, Kang et al. proposed an innovative methodology to screen a series of redox mediators and formulate design rules for selecting a suitable redox mediator for the OER process in Li–O2 batteries (Figure 6b).54 Their work established a link between the ionization energy of the redox mediator and its ability to serve as a soluble catalyst. They also suggested that the highest occupied molecular orbital (HOMO) energy level of the catalyst should be considered in comparison with the formation energy of Li2O2 and the HOMO energy level of the electrolyte. Thus far, redox mediators are susceptible to degradation during battery operation and, in some cases, they can induce the degradation of other cell components. Moreover, they can act as electron shuttles between the cathode and anode rather than as Li2O2-oxidation mediators. Therefore, careful design of the redox mediator and evaluation of its efficiency as an OER catalyst are exceptionally important for the development of advanced Li–O2 batteries with high performance and long cycle lifetimes.
As another system, lithium–sulfur batteries have attracted recent attention because of their high theoretical specific capacity and the low cost of sulfur.55 However, the sluggish electrochemical kinetics of sulfur species have impeded the widespread adoption of lithium–sulfur batteries. One of the main challenges associated with Li2S intermediates is the initial activation of Li2S on the cathode side to obtain full theoretical capacity. Li2S intermediates are insoluble and have a high charge-transfer resistance. Therefore, redox mediators can play vital roles in increasing the conductivity of the cathode and provide better utilization through the continuous activation of Li2S.56 The key point in choosing an appropriate redox mediator is that the reduction potential of the mediator should be less than the voltage for the reduction of Li2S4 to Li2S. Another problem that deteriorates the performance of lithium–sulfur batteries is the poor conductivity of sulfur cathode electrodes.57 To circumvent this problem, large amounts of conductive agents and binders are employed in the cathode, but this reduces the loading of sulfur and therefore decreases the energy density. In this case, the redox mediator can be a solution with a higher sulfur mass load because it can be introduced into the electrolyte. A recent study suggested that soluble cobaltocene molecules were introduced as the redox mediator for sulfur activation (Figures 6c,d).58 The cobaltocene could act as a redox mediator to facilitate electron transfer between the polysulfide and the conductive surface because its redox potential lies within the polysulfide reduction region. With the charge-shuttling effect, the discharge capacitance increased with an increase in the mediator concentration.
Generally, the energy density of supercapacitors is determined by both the electrode material and the electrolyte. Most studies have focused on electrode-modifying methods to design materials with tailored structures and morphologies to improve the electrochemical capacitive performance of supercapacitors. However, the capacitance of electrode materials is limited by slow diffusion and penetration of electrolyte ions. Thus, the addition of redox-active molecules into the electrolytes has recently proven to be an alternative method to enhance system performance (Figure 7a).59 A fast reversible redox reaction of redox mediators can efficiently increase the ionic conductivity and store extra charges via valence change during the redox process, thereby providing more charge capacity compared with electrostatic interfacial capacitors. As iodine has stable redox properties with various oxidation states, KI salt has been widely used as an aqueous redox mediator for supercapacitors with a carbon electrode.60 When KI is dissolved in an electrolyte solution, an exceptionally high specific capacitance is achieved, one which is several times higher than that without KI. Na2MoO4 is another redox-active additive under acidic and neutral conditions.61 The introduction of Na2MoO4 into the Li2SO4 electrolyte increases the specific capacitance. Moreover, when this salt was introduced into an acidic electrolyte, a high specific capacitance was observed, one that was approximately 3.75 times higher than that of the H2SO4 electrolyte system alone. Complex ions are another example of compounds that can also act as redox mediators. Liu et al. reported that [Fe(CN)6]3-/4- improved the capacitance of the Co–Al hydroxide electrode.62 Organic redox mediators are equally attractive candidates because of their structural diversity. Santamaría et al. showed that on two occasions, a higher specific capacitance was achieved when hydroquinone was added as a mediator.63 Despite the merits of redox mediators in improving the electrochemical capacitive performance of supercapacitors, several issues remain. Generally, the energy density of supercapacitors involving redox mediators depends on the interaction between the mediators and the electrode surface and the solubility of the mediators (Figure 7b).64 The former is related to the rates of adsorption/desorption of redox mediators on electrodes, while the latter is related to the solvation energy of redox mediators. The shuttling problem on the opposite electrode can be suppressed in the presence of strong interactions with the target electrode. However, if this interaction is too intense, it could hinder the desorption of redox mediators from the electrode. Likewise, the enormous solvation energy can slow down the solvation process if the mediator and solvent have strong interactions. Therefore, the optimal balance for such interactions between redox mediators and other components requires further investigation.
Figure 7.
(a) Charge-storage mechanism in electrical double-layer (EDLC) capacitor, pseudocapacitor, and redox mediator in a supercapacitor. Reproduced with permission from ref (59). Copyright 2019 Wiley-VCH. (b) Working mechanism of redox mediators for enhancing supercapacitors. Reproduced with permission from ref (46). Copyright 2020 Royal Society of Chemistry.
3. Conclusions and Perspectives
Understanding the interfaces between the electrode and electrolyte during the electrochemical process is crucial for achieving high-performance energy storage and conversion systems. To date, most studies have focused on electrode-modifying approaches that aim to change the adsorption energetics of reactants on static active sites to achieve enhanced electrochemical performance. However, the electrochemical system can be further improved in terms of both functionality and stability when its dynamics are fundamentally understood, as evidenced by the three classes of dynamic electrochemical interfaces. Moreover, in recent times, a growing number of investigations have proposed a new viewpoint that the active sites can undergo structure-evolving processes during electrochemical reactions. That is, the as-synthesized starting electrode is not the catalyst itself; instead, it serves as a “precatalyst” that can be reconstructed electrochemically into the real active “catalyst” or “precatalyst–catalyst interface.” The dissolved catalysts or ions in the electrolyte can also critically influence catalytic performance and stability. Thus, an extensive study of the exact dynamics of the electrified interface is required to understand the role of chemistry in the performance of the electrochemical system on the basis of the following issues.
3.1. Dynamic Behaviors at Electrochemical Interfaces in Many Aspects
The structural dynamics of the electrochemical interfaces determine the chemistry in all electrochemical reactions, but the dynamic nature is still poorly understood. In the time frame aspect, how electrocatalytic active sites and local structures dynamically evolve is mainly considered. However, from the interaction-related dynamic viewpoint, how the electrochemical reaction-related species (e.g., intermediates or electrolytes) participate in the catalytic process is a primary concern. Much remains as challenging issues to elucidate how ions, protons, and even insertion/deinsertion of electrolytes can trigger changes in structural and electronic states in order to clearly understanding the dynamic electrochemical processes. For example, the hydroxide materials usually have a layered structure, so their electrocatalytic activity could be easily influenced by the inserted anions, hydroxide, and water molecules.65,66 Furthermore, the unpredictable dynamic nature of electroanalytical measurements is complicated under operating conditions, so they are not adequately understood in many areas so far. It might be difficult to figure out dynamic behavior in electrochemical interface because of the insufficient techniques for direct observation of reactive intermediates generated. Likewise, recent studies show that intentionally modified operating protocols for electrochemical measurements could show different phenomena. As an example, the square wave at each electrochemical cycling demonstrates a more vigorous effect compared with that of the conventional triangle wave protocol in terms of degradation of electrocatalyst materials at a given period.67 Thus, we should carefully consider dynamic and intricate behaviors at the interfaces when treating the electrochemical environment that surrounds electrodes and also during electroanalytical methods.
3.2. Atomic and Molecular Understanding On the Basis of Theoretical and Operando Analytical Method
Of related importance, careful design of the experimental work is exceptionally important when it comes to an atomistic understanding of interface dynamics. Utilization of only certain spectroscopy tools and simple electrochemical measurements within a certain time interval could easily provide misleading data about improved performance and electrochemical findings. It is essential to provide direct insights into the molecular dynamics of the true time scales of the reaction at electrified interfaces with more delicate operando analyses. For example, despite recent advancements in the understanding of catalysts with operando techniques, some previous studies might have been oversimplifications based on conventional observations. Significant progress has been achieved with newly developed characterization techniques, but observation of the exact reaction active sites and dynamics at electrochemical interfaces remains challenging.68−74 Therefore, a fundamental understanding of atomic and molecular level dynamics using operando techniques and its continuous tracking in various time frames can shed light on enigmatic features at electrochemical interfaces. Furthermore, a theoretical understanding of density functional theory and molecular dynamics is invaluable in deciphering the dynamic behavior and guiding the effective design of electrochemical interfaces.75−80 Since it is almost impossible to control all variables experimentally in dynamic conditions, and there is a limit to experimentally capturing all changes, theory and computational approaches and comparison of experimental results will be very important factors for future research.81,82
3.3. Different Varieties of Surface Reconstruction and Chemical Evolution Dynamics in Electrochemical Interfaces
As noted above, most electrode surfaces usually suffer from unavoidable surface reconstruction in electrochemical operating conditions, which can have either harmful or beneficial effects on electrocatalysis.83 In particular, the instability of electrode materials, such as metal corrosion, dendrite growth, and volume expansion at the electrolyte/electrode interface, is among the most critical issues hindering the development of advanced energy storage techniques. In general, a passivation layer called the solid electrolyte interphase (SEI) is usually formed on the battery electrode surface as a decomposition product of electrolytes. After formation at the electrolyte/electrode interface, it would suppress the further continuous electrolyte consumption and dendrite growth inhibition. However, the structurally dense SEIs can act as resistive interfacial layers that hinder the fast electrochemical performance. As the formation of SEI is common dynamic behavior in battery systems, a new strategy should be proposed to yield high ion-conductive and stable interlayers, thereby enabling long-term stability and fast charge transfer kinetics.84,85 Furthermore, electrochemical interfaces even undergo severe reconstructions involving compositional change and crystallographic domain transformation (e.g., grain boundary formation, and amorphization) during electrocatalysis.10,86,87 To resolve and understand the aforementioned interfacial behaviors, recent studies propose some strategies guiding the reconstruction process in a controllable direction to break from the conventional catalyst design path. In these cases, surface reconstruction sometimes generates unique active sites with enhanced catalytic activity and selectivity for specific products.
3.4. Understanding and Designing the Microenvironment around Dynamic Interfaces
In addition, it is critical to implement high functionality of dynamic electrochemical interfaces into practical device systems. Considering most research has focused on the fundamental aspects of reaction mechanisms, engineering strategies to utilize them at the device level are rather limited. It is critical to understand the local environment of interfaces on electrocatalysts, which can mediate the transport and local concentration of reactants and other related species (e.g., electrolyte salts, proton, or water molecules) influencing reaction pathways. For instance, water or CO2 electrolyzer often uses gas diffusion electrodes with a flow-type cell configuration to increase surface area and improve mass transport on the electrode surface, respectively.88,89 Compared with the conventional microelectrode-based catalyst film, a different mode of dynamic electrochemical interface can be formulated in practical device environments. However, there is still a lot to understand about how the catalyst microenvironment and reaction interfaces could affect the mass transport and kinetics of electrocatalytic behaviors. For example, during the electrochemical gas evolution reaction, the continuously produced gas bubbles can block the electrode surface.90 They might impede the transport of electrolytes and reactants to and away from the catalytic sites, thereby influencing the reaction kinetics. In addition, during the self-healing mechanism in the OER process, Ostwald ripening and consequent surface area loss can be induced within nanoporous catalyst film because of nonuniform dissolution kinetics on each nanoparticle catalyst. Therefore, device-level studies should focus on investigating any limitation of dynamic interface effect imposed on catalyst layers and rational strategies to address them, including electrolyte engineering and system design.
In conclusion, to understand the correlations between structural activity and structural stability in electrochemical conversion and storage systems, it is necessary to understand the interface structure and its dynamics at the atomic scale. From this perspective, we covered three unique cases of dynamic electrochemical behavior at the interfaces and acquired insight into the true picture of electrode–electrolyte interfaces in electrochemical systems. Ultimately, further research efforts should be directed toward balancing the surface dissolution and reconstruction processes at electrochemical interfaces for utilization in industrially relevant electrochemical applications. In addition, it is essential to maximize the solid–liquid(−gas) interface where reactants, electrons, and ions meet with the introduction of some soluble redox-mediated species or by enhancing the interactions of the active site–host pairs.
Acknowledgments
Y.-E. Sung gratefully acknowledges financial support from the Institution for Basic Science (IBS-R006-A2). D.Y. Chung acknowledges financial support from the National Research Foundation of Korea (NRF) through a grant funded by the Korean Government (2022R1C1C1011269, 2021R1F1A105988).
Author Contributions
⊥ H. Shin and J. M. Yoo contributed equally. All the authors contributed to the writing of the manuscript. All authors approved the final version of the manuscript.
The authors declare no competing financial interest.
References
- Stamenkovic V. R.; Strmcnik D.; Lopes P. P.; Markovic N. M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2017, 16 (1), 57–69. 10.1038/nmat4738. [DOI] [PubMed] [Google Scholar]
- Strmcnik D.; Kodama K.; van der Vliet D.; Greeley J.; Stamenkovic V. R.; Markovic N. M. The role of non-covalent interactions in electrocatalytic fuel-cell reactions on platinum. Nat. Chem. 2009, 1 (6), 466–472. 10.1038/nchem.330. [DOI] [PubMed] [Google Scholar]
- Chung D. Y.; Lopes P. P.; Farinazzo Bergamo Dias Martins P.; He H.; Kawaguchi T.; Zapol P.; You H.; Tripkovic D.; Strmcnik D.; Zhu Y.; Seifert S.; Lee S.; Stamenkovic V. R.; Markovic N. M. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction. Nature Energy 2020, 5 (3), 222–230. 10.1038/s41560-020-0576-y. [DOI] [Google Scholar]
- Chen F.-Y.; Wu Z.-Y.; Adler Z.; Wang H. Stability challenges of electrocatalytic oxygen evolution reaction: From mechanistic understanding to reactor design. Joule 2021, 5 (7), 1704–1731. 10.1016/j.joule.2021.05.005. [DOI] [Google Scholar]
- Lutterman D. A.; Surendranath Y.; Nocera D. G. A Self-Healing Oxygen-Evolving Catalyst. J. Am. Chem. Soc. 2009, 131 (11), 3838–3839. 10.1021/ja900023k. [DOI] [PubMed] [Google Scholar]
- Huynh M.; Bediako D. K.; Nocera D. G. A Functionally Stable Manganese Oxide Oxygen Evolution Catalyst in Acid. J. Am. Chem. Soc. 2014, 136 (16), 6002–6010. 10.1021/ja413147e. [DOI] [PubMed] [Google Scholar]
- Grimaud A.; Diaz-Morales O.; Han B.; Hong W. T.; Lee Y.-L.; Giordano L.; Stoerzinger K. A.; Koper M. T. M.; Shao-Horn Y. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017, 9 (5), 457–465. 10.1038/nchem.2695. [DOI] [PubMed] [Google Scholar]
- Mefford J. T.; Rong X.; Abakumov A. M.; Hardin W. G.; Dai S.; Kolpak A. M.; Johnston K. P.; Stevenson K. J. Water electrolysis on La1-xSrxCoO3-δ perovskite electrocatalysts. Nat. Commun. 2016, 7 (1), 11053. 10.1038/ncomms11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz L. C.; Dickens C. F.; Nishio K.; Hikita Y.; Montoya J.; Doyle A.; Kirk C.; Vojvodic A.; Hwang H. Y.; Norskov J. K.; Jaramillo T. F. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 2016, 353 (6303), 1011–1014. 10.1126/science.aaf5050. [DOI] [PubMed] [Google Scholar]
- Fabbri E.; Nachtegaal M.; Binninger T.; Cheng X.; Kim B.-J.; Durst J.; Bozza F.; Graule T.; Schäublin R.; Wiles L.; Pertoso M.; Danilovic N.; Ayers K. E.; Schmidt T. J. Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat. Mater. 2017, 16 (9), 925–931. 10.1038/nmat4938. [DOI] [PubMed] [Google Scholar]
- Kanan M. W.; Nocera D. G. In Situ Formation of an Oxygen-Evolving Catalyst in Neutral Water Containing Phosphate and Co2+. Science 2008, 321 (5892), 1072–1075. 10.1126/science.1162018. [DOI] [PubMed] [Google Scholar]
- Bergmann A.; Martinez-Moreno E.; Teschner D.; Chernev P.; Gliech M.; de Araújo J. F.; Reier T.; Dau H.; Strasser P. Reversible amorphization and the catalytically active state of crystalline Co3O4 during oxygen evolution. Nat. Commun. 2015, 6 (1), 8625. 10.1038/ncomms9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilovic N.; Subbaraman R.; Chang K. C.; Chang S. H.; Kang Y. J.; Snyder J.; Paulikas A. P.; Strmcnik D.; Kim Y. T.; Myers D.; Stamenkovic V. R.; Markovic N. M. Activity-Stability Trends for the Oxygen Evolution Reaction on Monometallic Oxides in Acidic Environments. J. Phys. Chem. Lett. 2014, 5 (14), 2474–2478. 10.1021/jz501061n. [DOI] [PubMed] [Google Scholar]
- Nixon P. J.; Michoux F.; Yu J.; Boehm M.; Komenda J. Recent advances in understanding the assembly and repair of photosystem II. Ann. Bot 2010, 106 (1), 1–16. 10.1093/aob/mcq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesendruck C. E.; Sottos N. R.; Moore J. S.; White S. R. Biomimetic Self-Healing. Angew. Chem., Int. Ed. 2015, 54 (36), 10428–10447. 10.1002/anie.201500484. [DOI] [PubMed] [Google Scholar]
- Wang S.; Urban M. W. Self-healing polymers. Nature Reviews Materials 2020, 5, 562–583. 10.1038/s41578-020-0202-4. [DOI] [Google Scholar]
- Cao Y.; Tan Y. J.; Li S.; Lee W. W.; Guo H.; Cai Y.; Wang C.; Tee B. C.-K. Self-healing electronic skins for aquatic environments. Nature Electronics 2019, 2, 75–82. 10.1038/s41928-019-0206-5. [DOI] [Google Scholar]
- Feng C.; Wang F.; Liu Z.; Nakabayashi M.; Xiao Y.; Zeng Q.; Fu J.; Wu Q.; Cui C.; Han Y.; et al. A self-healing catalyst for electrocatalytic and photoelectrochemical oxygen evolution in highly alkaline conditions. Nat. Commun. 2021, 12 (1), 5980. 10.1038/s41467-021-26281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorarinsdottir A. E.; Veroneau S. S.; Nocera D. G. Self-healing oxygen evolution catalysts. Nat. Commun. 2022, 13 (1), 1243. 10.1038/s41467-022-28723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendranath Y.; Lutterman D. A.; Liu Y.; Nocera D. G. Nucleation, Growth, and Repair of a Cobalt-Based Oxygen Evolving Catalyst. J. Am. Chem. Soc. 2012, 134 (14), 6326–6336. 10.1021/ja3000084. [DOI] [PubMed] [Google Scholar]
- Costentin C.; Nocera D. G. Self-healing catalysis in water. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (51), 13380. 10.1073/pnas.1711836114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendranath Y.; Kanan M. W.; Nocera D. G. Mechanistic Studies of the Oxygen Evolution Reaction by a Cobalt-Phosphate Catalyst at Neutral pH. J. Am. Chem. Soc. 2010, 132 (46), 16501–16509. 10.1021/ja106102b. [DOI] [PubMed] [Google Scholar]
- Surendranath Y.; Dincǎ M.; Nocera D. G. Electrolyte-Dependent Electrosynthesis and Activity of Cobalt-Based Water Oxidation Catalysts. J. Am. Chem. Soc. 2009, 131 (7), 2615–2620. 10.1021/ja807769r. [DOI] [PubMed] [Google Scholar]
- Dincă M.; Surendranath Y.; Nocera D. G. Nickel-borate oxygen-evolving catayst that functions under benign conditins. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (23), 10337–10341. 10.1073/pnas.1001859107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M. R.; Loos S.; Chernev P.; Pasquini C.; Zaharieva I.; González-Flores D.; Kubella P.; Klingan K.; Smith R. D. L.; Dau H. Exploring the Limits of Self-Repair in Cobalt Oxide Films for Electrocatalytic Water Oxidation. ACS Catal. 2020, 10 (14), 7990–7999. 10.1021/acscatal.0c01944. [DOI] [Google Scholar]
- Danilovic N.; Subbaraman R.; Chang K.-C.; Chang S. H.; Kang Y. J.; Snyder J.; Paulikas A. P.; Strmcnik D.; Kim Y.-T.; Myers D.; Stamenkovic V. R.; Markovic N. M. Activity-Stability Trends for the Oxygen Evolution Reaction on Monometallic Oxides in Acidic Environments. J. Phys. Chem. Lett. 2014, 5 (14), 2474–2478. 10.1021/jz501061n. [DOI] [PubMed] [Google Scholar]
- Chang S. H.; Danilovic N.; Chang K.-C.; Subbaraman R.; Paulikas A. P.; Fong D. D.; Highland M. J.; Baldo P. M.; Stamenkovic V. R.; Freeland J. W.; Eastman J. A.; Markovic N. M. Functional links between stability and reactivity of strontium ruthenate single crystals during oxygen evolution. Nat. Commun. 2014, 5 (1), 4191. 10.1038/ncomms5191. [DOI] [PubMed] [Google Scholar]
- Danilovic N.; Subbaraman R.; Chang K. C.; Chang S. H.; Kang Y.; Snyder J.; Paulikas A. P.; Strmcnik D.; Kim Y. T.; Myers D.; Stamenkovic V. R.; Markovic N. M. Using Surface Segregation To Design Stable Ru-Ir Oxides for the Oxygen Evolution Reaction in Acidic Environments. Angew. Chem., Int. Ed. 2014, 53 (51), 14016–14021. 10.1002/anie.201406455. [DOI] [PubMed] [Google Scholar]
- Li H.; Chen Y.; Seow J. Z. Y.; Liu C.; Fisher A. C.; Ager J. W.; Xu Z. J. Surface Reconstruction of Perovskites for Water Oxidation: The Role of Initial Oxides’ Bulk Chemistry. Small Science 2022, 2, 2100048. 10.1002/smsc.202100048. [DOI] [Google Scholar]
- Kou Z.; Li X.; Zhang L.; Zang W.; Gao X.; Wang J. Dynamic Surface Chemistry of Catalysts in Oxygen Evolution Reaction. Small Science 2021, 1 (7), 2100011. 10.1002/smsc.202100011. [DOI] [Google Scholar]
- Ding H.; Liu H.; Chu W.; Wu C.; Xie Y. Structural Transformation of Heterogeneous Materials for Electrocatalytic Oxygen Evolution Reaction. Chem. Rev. 2021, 121 (21), 13174–13212. 10.1021/acs.chemrev.1c00234. [DOI] [PubMed] [Google Scholar]
- Liu X.; Ni K.; Wen B.; Guo R.; Niu C.; Meng J.; Li Q.; Wu P.; Zhu Y.; Wu X.; Mai L. Deep Reconstruction of Nickel-Based Precatalysts for Water Oxidation Catalysis. ACS Energy Letters 2019, 4 (11), 2585–2592. 10.1021/acsenergylett.9b01922. [DOI] [Google Scholar]
- Gao L.; Cui X.; Sewell C. D.; Li J.; Lin Z. Recent advances in activating surface reconstruction for the high-efficiency oxygen evolution reaction. Chem. Soc. Rev. 2021, 50 (15), 8428–8469. 10.1039/D0CS00962H. [DOI] [PubMed] [Google Scholar]
- Selvam N. C. S.; Du L.; Xia B. Y.; Yoo P. J.; You B. Reconstructed Water Oxidation Electrocatalysts: The Impact of Surface Dynamics on Intrinsic Activities. Adv. Funct. Mater. 2021, 31 (12), 2008190. 10.1002/adfm.202008190. [DOI] [Google Scholar]
- Zhao Y.; Wang Y.; Dong Y.; Carlos C.; Li J.; Zhang Z.; Li T.; Shao Y.; Yan S.; Gu L.; Wang J.; Wang X. Quasi-Two-Dimensional Earth-Abundant Bimetallic Electrocatalysts for Oxygen Evolution Reactions. ACS Energy Letters 2021, 6 (9), 3367–3375. 10.1021/acsenergylett.1c01302. [DOI] [Google Scholar]
- Wygant B. R.; Kawashima K.; Mullins C. B. Catalyst or Precatalyst? The Effect of Oxidation on Transition Metal Carbide, Pnictide, and Chalcogenide Oxygen Evolution Catalysts. ACS Energy Letters 2018, 3 (12), 2956–2966. 10.1021/acsenergylett.8b01774. [DOI] [Google Scholar]
- Zheng W.; Lee L. Y. S. Metal–Organic Frameworks for Electrocatalysis: Catalyst or Precatalyst?. ACS Energy Letters 2021, 6 (8), 2838–2843. 10.1021/acsenergylett.1c01350. [DOI] [Google Scholar]
- Liu X.; Meng J.; Zhu J.; Huang M.; Wen B.; Guo R.; Mai L. Comprehensive Understandings into Complete Reconstruction of Precatalysts: Synthesis, Applications, and Characterizations. Adv. Mater. 2021, 33 (32), 2007344. 10.1002/adma.202007344. [DOI] [PubMed] [Google Scholar]
- May K. J.; Carlton C. E.; Stoerzinger K. A.; Risch M.; Suntivich J.; Lee Y.-L.; Grimaud A.; Shao-Horn Y. Influence of Oxygen Evolution during Water Oxidation on the Surface of Perovskite Oxide Catalysts. J. Phys. Chem. Lett. 2012, 3 (22), 3264–3270. 10.1021/jz301414z. [DOI] [Google Scholar]
- Risch M.; Grimaud A.; May K. J.; Stoerzinger K. A.; Chen T. J.; Mansour A. N.; Shao-Horn Y. Structural Changes of Cobalt-Based Perovskites upon Water Oxidation Investigated by EXAFS. J. Phys. Chem. C 2013, 117 (17), 8628–8635. 10.1021/jp3126768. [DOI] [Google Scholar]
- Samira S.; Hong J.; Camayang J. C. A.; Sun K.; Hoffman A. S.; Bare S. R.; Nikolla E. Dynamic Surface Reconstruction Unifies the Electrocatalytic Oxygen Evolution Performance of Nonstoichiometric Mixed Metal Oxides. JACS Au 2021, 1 (12), 2224–2241. 10.1021/jacsau.1c00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes P. P.; Chung D. Y.; Rui X.; Zheng H.; He H.; Farinazzo Bergamo Dias Martins P.; Strmcnik D.; Stamenkovic V. R.; Zapol P.; Mitchell J. F.; Klie R. F.; Markovic N. M. Dynamically Stable Active Sites from Surface Evolution of Perovskite Materials during the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2021, 143 (7), 2741–2750. 10.1021/jacs.0c08959. [DOI] [PubMed] [Google Scholar]
- Li H.; Chen Y.; Ge J.; Liu X.; Fisher A. C.; Sherburne M. P.; Ager J. W.; Xu Z. J. Active Phase on SrCo1-xFexO3-δ (0 ≤ x ≤ 0.5) Perovskite for Water Oxidation: Reconstructed Surface versus Remaining Bulk. JACS Au 2021, 1 (1), 108–115. 10.1021/jacsau.0c00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almutlaq J.; Mir W. J.; Gutiérrez-Arzaluz L.; Yin J.; Vasylevskyi S.; Maity P.; Liu J.; Naphade R.; Mohammed O. F.; Bakr O. M. CsMnBr3: Lead-Free Nanocrystals with High Photoluminescence Quantum Yield and Picosecond Radiative Lifetime. ACS Materials Letters 2021, 3 (3), 290–297. 10.1021/acsmaterialslett.0c00603. [DOI] [Google Scholar]
- Chen Y.; Freunberger S. A.; Peng Z.; Fontaine O.; Bruce P. G. Charging a Li–O2 battery using a redox mediator. Nat. Chem. 2013, 5 (6), 489–494. 10.1038/nchem.1646. [DOI] [PubMed] [Google Scholar]
- Tamirat A. G.; Guan X.; Liu J.; Luo J.; Xia Y. Redox mediators as charge agents for changing electrochemical reactions. Chem. Soc. Rev. 2020, 49 (20), 7454–7478. 10.1039/D0CS00489H. [DOI] [PubMed] [Google Scholar]
- Pande V.; Viswanathan V. Criteria and Considerations for the Selection of Redox Mediators in Nonaqueous Li–O2 Batteries. ACS Energy Letters 2017, 2 (1), 60–63. 10.1021/acsenergylett.6b00619. [DOI] [Google Scholar]
- Bruce P. G.; Freunberger S. A.; Hardwick L. J.; Tarascon J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11 (1), 19–29. 10.1038/nmat3191. [DOI] [PubMed] [Google Scholar]
- Girishkumar G.; McCloskey B.; Luntz A. C.; Swanson S.; Wilcke W. Lithium–Air Battery: Promise and Challenges. J. Phys. Chem. Lett. 2010, 1 (14), 2193–2203. 10.1021/jz1005384. [DOI] [Google Scholar]
- Radin M. D.; Siegel D. J. Charge transport in lithium peroxide: relevance for rechargeable metal–air batteries. Energy Environ. Sci. 2013, 6 (8), 2370–2379. 10.1039/c3ee41632a. [DOI] [Google Scholar]
- Aurbach D.; McCloskey B. D.; Nazar L. F.; Bruce P. G. Advances in understanding mechanisms underpinning lithium–air batteries. Nature Energy 2016, 1 (9), 16128. 10.1038/nenergy.2016.128. [DOI] [Google Scholar]
- Lim H.-D.; Song H.; Kim J.; Gwon H.; Bae Y.; Park K.-Y.; Hong J.; Kim H.; Kim T.; Kim Y. H.; Lepró X.; Ovalle-Robles R.; Baughman R. H.; Kang K. Superior Rechargeability and Efficiency of Lithium–Oxygen Batteries: Hierarchical Air Electrode Architecture Combined with a Soluble Catalyst. Angew. Chem., Int. Ed. 2014, 53 (15), 3926–3931. 10.1002/anie.201400711. [DOI] [PubMed] [Google Scholar]
- Gao X.; Chen Y.; Johnson L.; Bruce P. G. Promoting solution phase discharge in Li–O2 batteries containing weakly solvating electrolyte solutions. Nat. Mater. 2016, 15 (8), 882–888. 10.1038/nmat4629. [DOI] [PubMed] [Google Scholar]
- Lim H.-D.; Lee B.; Zheng Y.; Hong J.; Kim J.; Gwon H.; Ko Y.; Lee M.; Cho K.; Kang K. Rational design of redox mediators for advanced Li–O2 batteries. Nature Energy 2016, 1 (6), 16066. 10.1038/nenergy.2016.66. [DOI] [Google Scholar]
- Yin Y.-X.; Xin S.; Guo Y.-G.; Wan L.-J. Lithium–Sulfur Batteries: Electrochemistry, Materials, and Prospects. Angew. Chem., Int. Ed. 2013, 52 (50), 13186–13200. 10.1002/anie.201304762. [DOI] [PubMed] [Google Scholar]
- Liang X.; Hart C.; Pang Q.; Garsuch A.; Weiss T.; Nazar L. F. A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 2015, 6 (1), 5682. 10.1038/ncomms6682. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Dong Y.; Li H.; Zhao Z.; Bin Wu H.; Hao C.; Liu S.; Qiu J.; Lou X. W. Enhancing lithium–sulphur battery performance by strongly binding the discharge products on amino-functionalized reduced graphene oxide. Nat. Commun. 2014, 5 (1), 5002. 10.1038/ncomms6002. [DOI] [PubMed] [Google Scholar]
- Kim K. R.; Lee K.-S.; Ahn C.-Y.; Yu S.-H.; Sung Y.-E. Discharging a Li-S battery with ultra-high sulphur content cathode using a redox mediator. Sci. Rep. 2016, 6 (1), 32433. 10.1038/srep32433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L.; Zhai T.; Li H.; Wang Y. Redox-Mediator-Enhanced Electrochemical Capacitors: Recent Advances and Future Perspectives. ChemSusChem 2019, 12 (6), 1118–1132. 10.1002/cssc.201802450. [DOI] [PubMed] [Google Scholar]
- Sun K.; Feng E.; Peng H.; Ma G.; Wu Y.; Wang H.; Lei Z. A simple and high-performance supercapacitor based on nitrogen-doped porous carbon in redox-mediated sodium molybdate electrolyte. Electrochim. Acta 2015, 158, 361–367. 10.1016/j.electacta.2015.01.185. [DOI] [Google Scholar]
- Xu D.; Hu W.; Sun X. N.; Cui P.; Chen X. Y. Redox additives of Na2MoO4 and KI: Synergistic effect and the improved capacitive performances for carbon-based supercapacitors. J. Power Sources 2017, 341, 448–456. 10.1016/j.jpowsour.2016.12.031. [DOI] [Google Scholar]
- Su L.-H.; Zhang X.-G.; Mi C.-H.; Gao B.; Liu Y. Improvement of the capacitive performances for Co–Al layered double hydroxide by adding hexacyanoferrate into the electrolyte. Phys. Chem. Chem. Phys. 2009, 11 (13), 2195–2202. 10.1039/b814844a. [DOI] [PubMed] [Google Scholar]
- Roldán S.; Blanco C.; Granda M.; Menéndez R.; Santamaría R. Towards a Further Generation of High-Energy Carbon-Based Capacitors by Using Redox-Active Electrolytes. Angew. Chem., Int. Ed. 2011, 50 (7), 1699–1701. 10.1002/anie.201006811. [DOI] [PubMed] [Google Scholar]
- Qin W.; Zhou N.; Wu C.; Xie M.; Sun H.; Guo Y.; Pan L. Mini-Review on the Redox Additives in Aqueous Electrolyte for High Performance Supercapacitors. ACS Omega 2020, 5 (8), 3801–3808. 10.1021/acsomega.9b04063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionigi F.; Zeng Z.; Sinev I.; Merzdorf T.; Deshpande S.; Lopez M. B.; Kunze S.; Zegkinoglou I.; Sarodnik H.; Fan D.; Bergmann A.; Drnec J.; Araujo J. F.; Gliech M.; Teschner D.; Zhu J.; Li W.-X.; Greeley J.; Cuenya B. R.; Strasser P. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 2020, 11 (1), 2522. 10.1038/s41467-020-16237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S.; Peng J.; Cai B.; Huang Z.; Garcia-Esparza A. T.; Sokaras D.; Zhang Y.; Giordano L.; Akkiraju K.; Zhu Y. G.; Hübner R.; Zou X.; Román-Leshkov Y.; Shao-Horn Y. Tunable metal hydroxide–organic frameworks for catalysing oxygen evolution. Nat. Mater. 2022, 21 (1), 673–680. 10.1038/s41563-022-01199-0. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Liu Z.; Zhang A.; Yan X.; Xue W.; Peng B.; Xin H. L.; Pan X.; Duan X.; Huang Y. Graphene-nanopocket-encaged PtCo nanocatalysts for highly durable fuel cell operation under demanding ultralow-Pt-loading conditions. Nat. Nanotechnol. 2022, 17, 968. 10.1038/s41565-022-01170-9. [DOI] [PubMed] [Google Scholar]
- Mefford J. T.; Akbashev A. R.; Kang M.; Bentley C. L.; Gent W. E.; Deng H. D.; Alsem D. H.; Yu Y.-S.; Salmon N. J.; Shapiro D. A.; Unwin P. R.; Chueh W. C. Correlative operando microscopy of oxygen evolution electrocatalysts. Nature 2021, 593 (1), 67–73. 10.1038/s41586-021-03454-x. [DOI] [PubMed] [Google Scholar]
- Budiyanto E.; Salamon S.; Wang Y.; Wende H.; Tüysüz H. Phase Segregation in Cobalt Iron Oxide Nanowires toward Enhanced Oxygen Evolution Reaction Activity. JACS Au 2022, 2 (3), 697–710. 10.1021/jacsau.1c00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S.; Yang Y.; Tang Z. Insight into Structural Evolution, Active Sites, and Stability of Heterogeneous Electrocatalysts. Angew. Chem., Int. Ed. 2021, 61 (11), e202110186 10.1002/anie.202110186. [DOI] [PubMed] [Google Scholar]
- Chen S.; Ma L.; Huang Z.; Liang G.; Zhi C. In situ/operando analysis of surface reconstruction of transition metal-based oxygen evolution electrocatalysts. Cell Reports Physical Science 2022, 3 (1), 100729. 10.1016/j.xcrp.2021.100729. [DOI] [Google Scholar]
- Liu L.; Li W.; He X.; Yang J.; Liu N. In Situ/Operando Insights into the Stability and Degradation Mechanisms of Heterogeneous Electrocatalysts. Small 2022, 18 (7), 2104205. 10.1002/smll.202104205. [DOI] [PubMed] [Google Scholar]
- Zuo S.; Wu Z.-P.; Zhang H.; Lou X. W. Operando Monitoring and Deciphering the Structural Evolution in Oxygen Evolution Electrocatalysis. Adv. Energy Mater. 2022, 12 (8), 2103383. 10.1002/aenm.202103383. [DOI] [Google Scholar]
- Zhang Z.; Carlos C.; Wang Y.; Dong Y.; Yin X.; German L.; Berg K. J.; Bu W.; Wang X. Nucleation Kinetics and Structure Evolution of Quasi-Two-Dimensional ZnO at the Air–Water Interface: An In Situ Time-Resolved Grazing Incidence X-ray Scattering Study. Nano Lett. 2022, 22 (7), 3040–3046. 10.1021/acs.nanolett.2c00300. [DOI] [PubMed] [Google Scholar]
- Man I. C.; Su H.-Y.; Calle-Vallejo F.; Hansen H. A.; Martínez J. I.; Inoglu N. G.; Kitchin J.; Jaramillo T. F.; Nørskov J. K.; Rossmeisl J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem. 2011, 3 (7), 1159–1165. 10.1002/cctc.201000397. [DOI] [Google Scholar]
- Shi X.; Lin X.; Luo R.; Wu S.; Li L.; Zhao Z.-J.; Gong J. Dynamics of Heterogeneous Catalytic Processes at Operando Conditions. JACS Au 2021, 1 (12), 2100–2120. 10.1021/jacsau.1c00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J. S.; Rong X.; Liu Y.; Kolpak A. M. Role of Lattice Oxygen Participation in Understanding Trends in the Oxygen Evolution Reaction on Perovskites. ACS Catal. 2018, 8 (5), 4628–4636. 10.1021/acscatal.8b00612. [DOI] [Google Scholar]
- Martirez J. M. P.; Carter E. A. Noninnocent Influence of Host β-NiOOH Redox Activity on Transition-Metal Dopants’ Efficacy as Active Sites in Electrocatalytic Water Oxidation. ACS Catal. 2020, 10 (4), 2720–2734. 10.1021/acscatal.9b05092. [DOI] [Google Scholar]
- Martirez J. M. P.; Carter E. A. Unraveling Oxygen Evolution on Iron-Doped β-Nickel Oxyhydroxide: The Key Role of Highly Active Molecular-like Sites. J. Am. Chem. Soc. 2019, 141 (1), 693–705. 10.1021/jacs.8b12386. [DOI] [PubMed] [Google Scholar]
- Shin S.-J.; Kim D. H.; Bae G.; Ringe S.; Choi H.; Lim H.-K.; Choi C. H.; Kim H. On the importance of the electric double layer structure in aqueous electrocatalysis. Nat. Commun. 2022, 13 (1), 174. 10.1038/s41467-021-27909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resasco J.; Abild-Pedersen F.; Hahn C.; Bao Z.; Koper M. T. M.; Jaramillo T. F. Enhancing the connection between computation and experiments in electrocatalysis. Nature Catalysis 2022, 5 (1), 374–381. 10.1038/s41929-022-00789-0. [DOI] [Google Scholar]
- Seh Z. W.; Kibsgaard J.; Dickens C. F.; Chorkendorff I.; Nørskov J. K.; Jaramillo T. F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355 (6321), 146. 10.1126/science.aad4998. [DOI] [PubMed] [Google Scholar]
- Han Z.; Han D.; Chen Z.; Gao J.; Jiang G.; Wang X.; Lyu S.; Guo Y.; Geng C.; Yin L.; Weng Z.; Yang Q.-H. Steering surface reconstruction of copper with electrolyte additives for CO2 electroreduction. Nat. Commun. 2022, 13 (1), 3158. 10.1038/s41467-022-30819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J.; Kim D. O.; Bekaert L.; Song M.; Chung J.; Lee D.; Hubin A.; Lim J. Non-fluorinated non-solvating cosolvent enabling superior performance of lithium metal negative electrode battery. Nat. Commun. 2022, 13 (1), 4538. 10.1038/s41467-022-32192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Huang W.; Pei A.; Li Y.; Shi F.; Yu X.; Cui Y. Improving cyclability of Li metal batteries at elevated temperatures and its origin revealed by cryo-electron microscopy. Nature Energy 2019, 4 (1), 664–670. 10.1038/s41560-019-0413-3. [DOI] [Google Scholar]
- Li C. W.; Kanan M. W. CO2 Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu2O Films. J. Am. Chem. Soc. 2012, 134 (17), 7231–7234. 10.1021/ja3010978. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Wang T.; Liu B.; Cheng D.; Hu C.; Zhang G.; Zhu W.; Wang H.; Zhao Z.-J.; Gong J. Grain-Boundary-Rich Copper for Efficient Solar-Driven Electrochemical CO2 Reduction to Ethylene and Ethanol. J. Am. Chem. Soc. 2020, 142 (15), 6878–6883. 10.1021/jacs.0c00971. [DOI] [PubMed] [Google Scholar]
- Xing Z.; Hu L.; Ripatti D. S.; Hu X.; Feng X. Enhancing carbon dioxide gas-diffusion electrolysis by creating a hydrophobic catalyst microenvironment. Nat. Commun. 2021, 12 (1), 136. 10.1038/s41467-020-20397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A.; Jansonius R. P.; Mowbray B. A.W.; Cao Y.; Wheeler D. G.; Chau J.; Dvorak D. J.; Berlinguette C. P. Managing Hydration at the Cathode Enables Efficient CO2 Electrolysis at Commercially Relevant Current Densities. ACS Energy Letters 2020, 5 (5), 1612–1618. 10.1021/acsenergylett.0c00637. [DOI] [Google Scholar]
- Xia C.; Back S.; Ringe S.; Jiang K.; Chen F.; Sun X.; Siahrostami S.; Chan K.; Wang H. Confined local oxygen gas promotes electrochemical water oxidation to hydrogen peroxide. Nature Catalysis 2020, 3 (1), 125–134. 10.1038/s41929-019-0402-8. [DOI] [Google Scholar]