Abstract
The performance of six commercially available immunoassay systems for the detection of dengue virus-specific immunoglobulin M (IgM) and IgG antibodies in serum was evaluated. These included two IgM and IgG enzyme immunoassays (EIA) from MRL Laboratories and PanBio, a rapid immunochromatographic test (RIT) from PanBio, immunofluorescence assays (IFA) from Progen, a dot blot assay from Genelabs, and a dipstick EIA from Integrated Diagnostics (INDX). For this study a panel of 132 serum samples, including 90 serum samples from patients with suspected dengue virus infection and 42 serum samples from patients with other viral infections, was used. In addition, serial serum samples from two monkeys experimentally immunized and challenged with dengue virus type 2 were used. Results were considered conclusive when concordant results were obtained with four of the six antibody-specific assays. Based on this definition, the calculated overall agreement for the human serum samples for the respective IgM immunoassays was 97% (128 of 132), with 34% (45 of 132) positive serum samples, 63% (83 of 132) negative samples, and 3% of samples (4 of 132) showing discordant results. The calculated overall agreement for the IgG assays was 94% (124 of 132), with 49% (65 of 132) positive, 45% (59 of 132) negative, and 6% (8 of 132) discordant results, respectively. The sensitivities of the dengue virus-specific assays evaluated varied between 71 and 100% for IgM and between 52 and 100% for IgG, with specificities of 86 to 96% and 81 to 100%, respectively. The relative sensitivities of the respective IgM assays measured with the monkey serum samples were comparable with those obtained with 12 serial serum samples from humans. Overall performance, based on the sum of the agreement, sensitivity, specificity, and Kappa statistics of the IgM and IgG immunoassays, showed that the antibody detection systems from INDX and Genelabs and the MRL and PanBio EIA are useful and reliable assays for dengue virus serodiagnosis.
Dengue virus (DEN) infections are among the most common arthropod-borne infections in tropical and subtropical areas. The four serotypes, DEN 1, DEN 2, DEN 3, and DEN 4, are transmitted by several mosquito species including Aedes aegypti and Aedes albopictus. At least 50 million people are infected with one of the four serotypes annually (3). The majority of DEN infections are asymptomatic or cause mild dengue fever (DF), characterized by flu-like symptoms including fever, chills, headache, and myalgia. Rash, lymphadenopathy, arthralgia, or myalgia usually follows these initial symptoms. In some cases the infection may lead to the more severe dengue hemorrhagic fever (DHF) with plasma leakage. Usually conjunctival suffusion, facial flushing, and truncal erythema are also present. The usually lethal dengue shock syndrome (DSS) may follow DHF after circulatory collapse (6, 8).
Differential diagnosis may be important in order to distinguish DF from influenza, measles, rubella, other arthropod-borne viral infections, malaria, and other hemorrhagic fevers (15). Therefore, a good laboratory diagnosis is important. The classic hemagglutination inhibition (HAI) assay and virus neutralization assay are still widely used, despite their tedious nature (1, 4, 9). Recently other immunosystems for the diagnosis of DEN infection have become commercially available. Among these are enzyme immunoassays (EIA), immunochromatographic assays, and a dot blot assay (2, 10, 11, 12, 13, 16). Differences in assay formats, usage of antigen, and detection systems make it difficult to estimate the value of each individual assay without proper comparison. This prompted us to evaluate six commercially available immunoassay systems for the detection of DEN-specific immunoglobulin M (IgM) and IgG antibodies. Ninety serum samples, both single and serially collected from European and Asian patients with suspected acute DEN infections, and 42 serum samples from Dutch patients with confirmed viral infections other than DEN infection were used to evaluate 11 different assays from five companies. In addition, serial serum samples from experimentally vaccinated monkeys subsequently challenged with DEN 2 were used to study their antibody kinetics in the respective assays.
MATERIALS AND METHODS
Human serum samples.
A panel of 132 human serum samples from patients with suspected DEN infections and patients with other viral infections were included in this study. Serial serum samples were collected from patients with suspected acute DEN infections, living in areas of DEN endemicity. Thirteen patients from Curaçao, with seven paired samples and six single serum samples (n = 20), and 6 patients with paired samples (n = 12) and 12 patients with serial samples (n = 36) from Indonesia were included. Serum samples from patients with suspected primary DEN infections (n = 22), comprising 16 single serum samples and 3 paired samples (n = 6), were collected from Dutch travelers. As controls, serum samples from patients with other viral infections confirmed by the detection of specific IgM antibodies were used. These included sera with specific IgM antibodies to Epstein-Barr virus (EBV) (n = 5), cytomegalovirus (CMV) (n = 8), yellow fever virus (YFV) (n = 4), varicella-zoster virus (VZV) (n = 8), herpes simplex virus (HSV) (n = 6), and tick-borne encephalitis virus (TBEV) (n = 2). Eight samples from chronically infected patients with hepatitis B virus (HBV) (n = 8) were also included.
All samples had been collected between 1993 and 1998 and stored at −20°C until use.
Monkey serum samples.
Serum samples from two cynomolgus monkeys (Macaca fascicularis) experimentally immunized with live attenuated DEN 2 vaccine and subsequently challenged with homologous wild DEN 2, as previously described, were included in this study (14). Serum samples were collected at different times after immunization and challenge and were stored at −20°C until use.
IgG and IgM assays.
The characteristics of the respective immunoassays are depicted in Table 1. Included in this evaluation are two EIA, an immunofluorescence assay (IFA), a rapid immunochromatographic test (RIT), a DipStick EIA, and an immunoblot assay (blot). The MRL EIA (MRL Diagnostics, Cypress, Calif.) and the PanBio EIA (PanBio, Brisbane, Australia) are both based on indirect systems for the detection of IgG serum antibodies using microwell plates coated with the DEN 1 through DEN 4 antigens. The detection of IgM serum antibodies for both these EIA is based on an IgM capture system followed by an incubation with DEN 1 through DEN 4 antigens and virus-specific monoclonal antibodies conjugated with horseradish peroxidase. For the detection of IgG serum antibodies, the assay times are 2 h with the MRL EIA and 1 h with the PanBio EIA; for the detection of IgM serum antibodies, the assay time is 4 h with the MRL EIA and 2 1/2 h for the PanBio EIA. The PanBio RIT is a rapid (7-min) assay based on a capture principle for the detection of IgM and IgG serum antibodies followed by an incubation with a mixture of DEN 1 through DEN 4 antigens and a gold-labeled DEN-specific monoclonal antibody. The IFA from Progen Biotechnik (Heidelberg, Germany) is based on an indirect system for the detection of both IgM and IgG serum antibodies, using IFA slides coated with DEN 2 antigen. To detect DEN-specific IgG antibodies, a goat anti-human IgG-fluorescein isothiocyanate (FITC) conjugate (DAKO, Glostrup, Denmark) was used. To detect DEN-specific IgM antibodies, the IgG-FITC conjugate was replaced by a rabbit anti-human IgM-FITC conjugate (DAKO). Prior to the detection of DEN-specific IgM antibodies by IFA, serum samples were pretreated with Gull-sorb (Gull Laboratories, Salt Lake City, Utah) to remove IgG antibodies. The total assay time is 90 min for detection of IgG serum antibodies and 2 h for IgM detection. The Integrated Diagnostics (INDX; Baltimore, Md.) DipStick EIA is based on an indirect system for the detection of both IgM and IgG serum antibodies. In this assay, a nitrocellulose membrane is coated with DEN 2 antigen and binding antibodies are detected with an anti-human IgM or IgG conjugate labeled with alkaline phosphatase. The assay times for detection of IgM and IgG serum antibodies are 90 and 45 min, respectively. Finally, the Genelabs Diagnostics (Singapore) blot is based on nitrocellulose membranes coated with DEN 1 through DEN 4 antigens; binding IgG antibodies are detected using a peroxidase-labeled protein A conjugate. IgG results were obtained within 2 1/2 h. For the detection of IgM serum antibodies, the nitrocellulose membranes are coated with anti-human IgM. After the binding of IgM antibodies, the membranes are incubated with a mixture of DEN 1 through DEN 4 antigens, followed by incubation with a DEN-specific monoclonal antibody and a rabbit anti-mouse Ig peroxidase-labeled conjugate, and are developed with chloro-naphthol as the substrate. The minimum assay time is 8 h, although the manufacturer recommends an overnight incubation with the antigen.
TABLE 1.
Characteristics of IgG and IgM assays for the detection of DEN antibodies
| Company | Type of assay | Antigen | Principle | Serum dilution | Total assay time (min) |
|---|---|---|---|---|---|
| MRL Diagnostics | IgM EIA | DEN 1, 2, 3, 4 | Capture | 1:101 | 240 |
| IgG EIA | DEN 1, 2, 3, 4 | Indirect | 1:101 | 120 | |
| PanBio | IgM EIA | DEN 1, 2, 3, 4 | Capture | 1:100 | 150 |
| IgG EIA | DEN 1, 2, 3, 4 | Indirect | 1:100 | 60 | |
| IgM-IgG RIT | DEN 1, 2, 3, 4 | Capture | Undiluted | 7 | |
| Progen Biotechnik | IgM IFA | DEN 2 | Indirect | 1:16 | 120 |
| IgG IFA | DEN 2 | Indirect | 1:16 | 90 | |
| INDX | IgM DipStick EIA | DEN 2 | Indirect | 1:200 | 90 |
| IgG DipStick EIA | DEN 2 | Indirect | 1:200 | 45 | |
| Genelabs Diagnostics | IgM blot | DEN 1, 2, 3, 4 | Capture | 1:100 | 480 |
| IgG blot | DEN 1, 2, 3, 4 | Indirect | 1:100 | 210 |
Monkey serum samples were analyzed for both IgM and IgG in the appropriate assays according to the procedures described by the manufacturer, with modifications as described below. In the Progen IFA an FITC-conjugated anti-monkey IgM or anti-monkey IgG was used (DAKO) for detection of IgM and IgG, respectively. In the Genelabs IgG blot, the protein A conjugate was replaced by an anti-monkey IgG-horseradish peroxidase conjugate (Sigma Chemical Co., St. Louis, Mo.). It was not possible to detect IgG antibodies in the monkey sera with the PanBio RIT.
Calculation, statistics, and ranking.
The overall agreement, sensitivities, and specificities of the respective assays were determined in relation to the consensus values as the “gold standard” (17). Results were considered to be “true values” (consensus values) when concordant results were obtained from at least four out of the six assays. When three of the six assays were positive, the result was defined as discordant. Kappa statistics were used to evaluate the level of agreement between concordant results in excess of that expected by chance. If the agreement reached a κ value of 1, this indicated good agreement, while a κ value of 0 indicated no agreement (7). The ranking of each assay group (IgM and IgG) was determined by the sum of the calculated κ value, overall agreement, sensitivity, and specificity. The overall ranking of each diagnostic system was determined by calculating the sum of the IgM and IgG rankings.
RESULTS
The overall agreement between all six immunoassays for the detection of specific IgM and IgG antibodies against DEN in the human sera is summarized in Table 2. Of the 132 samples tested, 45 (34%) gave positive results, 83 (63%) gave negative results, and 4 (3%) gave discordant results for DEN IgM serum antibodies, while 65 (49%) gave positive results, 59 (45%) gave negative results, and 8 (6%) gave discordant results for DEN IgG serum antibodies.
TABLE 2.
Overall agreement of all the respective DEN-specific IgM and IgG immunoassays according to the consensus model
| Result | No. (%) of results for:
|
|
|---|---|---|
| IgM | IgG | |
| Positivea | 45 (34.1) | 65 (49.2) |
| Negativeb | 83 (62.9) | 59 (44.7) |
| Discordantc | 4 (3.0) | 8 (6.1) |
Defined as positive results in at least four out of six assays.
Defined as negative results in at least four out of six assays.
Defined as positive results in three out of six assays and negative results in three out of six assays.
The results of the individual assays with respect to the agreement, sensitivity, specificity and κ value, using the consensus value as a “gold standard,” are summarized in Table 3. In general, the level of agreement between the six IgM assays was good (κ = 0.884 to κ = 0.966). The overall agreements of the respective IgM assays varied from 88 to 98% for the PanBio EIA (κ = 0.912) and PanBio RIT (κ = 0.966), respectively. Sensitivities ranged from 100% for both the PanBio IgM RIT and the Genelabs IgM blot to 71% for the Progen IgM IFA. Specificities varied from 86% for the INDX EIA to 96% for the PanBio EIA.
TABLE 3.
Agreement, specificity, sensitivity, and ranking of DEN antibody assays based on the consensus value
| Assay | IgM detection
|
IgG detection
|
Ranking | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Agreement | Sensitivity | Specificity | κ | Agreement | Sensitivity | Specificity | κ | ||
| MRL EIA | 91 | 96 | 91 | 0.930 | 92 | 100 | 88 | 0.830 | 4 |
| PanBio EIA | 88 | 87 | 96 | 0.912 | 96 | 100 | 98 | 0.917 | 2 |
| PanBio RIT | 98 | 100 | 92 | 0.966 | 75 | 52 | 100 | 0.803 | 5 |
| Progen IFA | 83 | 71 | 89 | 0.884 | 82 | 77 | 86 | 0.785 | 6 |
| INDX EIA | 92 | 96 | 86 | 0.904 | 98 | 97 | 98 | 0.913 | 3 |
| Genelabs blot | 95 | 100 | 92 | 0.950 | 88 | 85 | 95 | 0.847 | 1 |
The overall agreement between the DEN-specific IgG assays varied from 75 to 98% for the INDX EIA (κ = 0.913) and the PanBio RIT (κ = 0.803), respectively. The level of agreement between the respective DEN IgG immunoassays was relatively good (κ = 0.785 to κ = 0.917). High sensitivities of 100, 100, and 97% were obtained with the MRL IgG EIA, PanBio IgG EIA, and INDX IgG EIA, whereas the sensitivities of the Genelabs IgG blot, Progen IgG IFA, and PanBio IgG RIT were, respectively, 85, 77, and 52% compared to the consensus value. Calculations of the specificities of these IgG assays resulted in values between 86 and 100%.
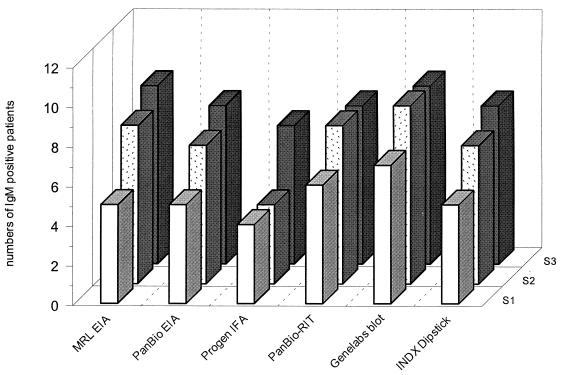
Figure 1 presents the results of the performance of the six different immunoassays for the detection of DEN IgM antibodies measured in serial serum samples from 12 patients with suspected acute DEN infections at different times after the onset of clinical symptoms. During the acute phase, DEN-specific IgM antibodies could be detected in seven patients' samples with the Genelabs blot, whereas five patients tested positive in the MRL, PanBio, and INDX EIA, six tested positive in the PanBio RIT, and four tested positive in the Progen IFA. During the early-convalescent phase, IgM was detected for 9 patients out of 12 in the Genelabs blot, for 8 patients in the MRL EIA and the PanBio RIT, for 7 in the PanBio EIA and the INDX, and for 4 in the Progen IFA. In the convalescent phase, DEN-specific IgM antibodies were detected for nine patients with the MRL EIA and the Genelabs blot. Eight patients' samples were positive for the presence of DEN-specific IgM antibodies in the PanBio EIA, the PanBio RIT, and the INDX. In the Progen IFA, seven patients tested positive.
FIG. 1.
Results of DEN-specific IgM detection of six different assays in sera from 12 patients with suspected acute DEN infections at different time points after the onset of clinical symptoms. S1, serum sample in the acute phase; S2, serum sample in the early-convalescent phase; S3, serum sample in the convalescent phase.
Detection of DEN-specific IgM antibodies in the non-DEN group varied from 0 to 6 positive samples out of 42 tested serum samples. DEN-specific IgM serum antibodies were detected in two samples (VZV) using the PanBio RIT, in three samples (two HBV and one VZV) using the MRL IgM EIA, in four samples (two HBV and two VZV) using the PanBio IgM EIA, and in six samples (one CMV, one HBV, two HSV, one VZV, and one TBEV) using the INDX IgM EIA. DEN-specific IgM serum antibodies were not detected using the Progen IgM IFA or the Genelabs IgM blot. Measurement of serum antibody reactivities of the 42 non-DEN serum samples by the DEN-specific IgG assays showed four positive serum samples with the MRL IgG EIA (one CMV, one TBEV, and two VZV), three positive samples with the PanBio IgG EIA (one TBEV and two VZV), five positive samples with the Progen IgG IFA (two CMV, two TBEV, and one VZV), two positive samples (both VZV) with the INDX IgG EIA, and three positive samples (one CMV, one EBV, and one VZV) with the Genelabs IgG blot. All the non-DEN serum samples were negative with the PanBio IgG RIT.
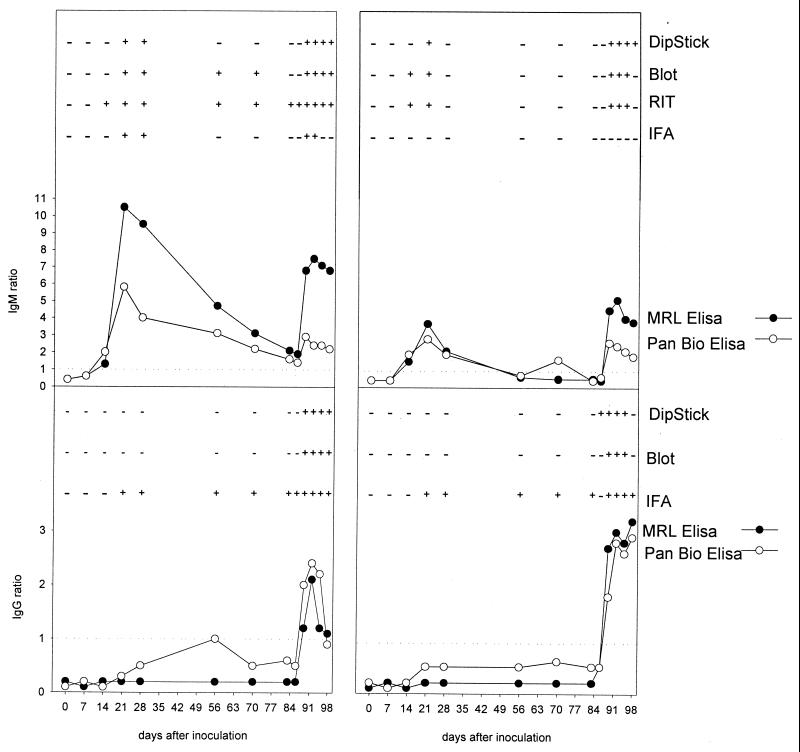
Figure 2 presents the kinetics of IgG and IgM antibodies to DEN 2 in monkeys experimentally vaccinated and subsequently challenged with DEN 2, as measured by different assays. In monkeys E1 and E2, the IgM antibody kinetics measured by the quantitative IgM EIA from MRL and PanBio showed identical patterns after immunization and challenge, although the ratios measured by the IgM PanBio EIA were slightly lower. In sera from monkey E1, the MRL EIA, the PanBio EIA, and the PanBio RIT detected IgM antibodies on day 14, whereas the INDX EIA, blot and Progen IFA results became positive on day 21. The PanBio RIT, PanBio IgM EIA, and MRL IgM EIA remained positive for IgM antibodies during the whole period. The EIA showed a gradual decrease until 3 to 6 days after challenge with homologous DEN 2 virus. In sera from monkey E2, lower IgM antibody responses were detected as shown by the EIA. The Progen IFA remained negative for IgM antibodies during the whole period.
FIG. 2.
Detection by several assays of DEN-specific IgM and IgG antibodies in sera from two monkeys at different time points after experimental immunization (day 0) and subsequent challenge (day 84) with homologous DEN 2. Shown are IgM (top) and IgG (bottom) results for monkey E1 (left) and monkey E2 (right). The cutoff value (ratio of 1) is indicated by a dotted line. The respective assays are indicated on the right.
DEN-specific IgG antibodies were detected only after challenge with homologous DEN 2 in all assays except the Progen IFA, which was already positive on day 21 after immunization and remained positive during the whole period.
DISCUSSION
Recently, a number of DEN-specific immunoassays have become available for the detection of IgM and IgG antibodies in serum, ranging from dipstick-based assays to more-sophisticated enzyme-linked immunoassays. Some of these immunoassays have been evaluated in different studies (2, 10, 16). We have evaluated 11 DEN immunoassays comprising 6 different systems for the detection of IgM and IgG. Based on a consensus model using serum samples from patients with suspected DEN infections and non-DEN patients, the performance of each assay was validated. In addition, the relative sensitivities of the respective assays were studied with serial serum samples from monkeys experimentally infected with DEN 2, followed by homologous challenge with wild-type DEN 2 (14).
In general, all assays were easy to perform, but the simplest and fastest assay to perform is by far the PanBio RIT. The results of the PanBio RIT are available in less than 10 min, and both IgM and IgG antibodies are detected simultaneously. Except the Genelabs blot and the Progen IFA for detection of IgM antibodies and the PanBio RIT for detection of IgG antibodies, all the other DEN immunoassays detected nonspecific DEN IgM and IgG serum antibodies. These reactions were found mainly in patients with a CMV, EBV, or VZV infection, in contrast to a previous study showing no DEN IgM reactivity in serum samples from VZV, CMV, and EBV patients using the INDX DipStick EIA and a homemade DEN-specific IgM capture EIA (16). Although the non-DEN serum panel was carefully selected, using serum samples from patients in The Netherlands, where no flaviviruses are circulating, the presence of flavivirus-specific IgG antibodies cannot be completely ruled out. The TBEV and YFV vaccination statuses of the patients, as well as their histories of possible flavivirus infections, were not available. DEN IgM and IgG reactivities were detected by several assays in serum samples from patients with TBEV infections. These flavivirus cross-reactivities are in agreement with several other studies clearly showing DEN antibody reactivity in patients with YFV and Japanese encephalitis virus infections (2, 5, 16).
Serial serum samples from patients were used to evaluate the respective DEN IgM assays in the acute, early-convalescent, and convalescent phase of disease. The results with these serum samples clearly showed the best performance for the Genelabs IgM blot assay and poor performance for the Progen IgM IFA in the acute and early-convalescent phase. When serial serum samples from humans with DEN infections are used, the estimated time point after infection is variable. Therefore, we used serial serum samples from monkeys experimentally vaccinated and subsequently challenged with wild-type DEN 2 to study the relative sensitivities of the IgM assays. Despite the fact that the monkeys were vaccinated with DEN 2, which is the only virus present in the Vero cells coated on the Progen slides, IgM antibodies could not be detected after vaccination. The Progen IgG IFA, on the other hand, seems to be more sensitive, compared to the other assays (Fig. 2). Therefore, it is not yet clear why the Progen IgM IFA is not performing as well as the other assays. In general, the results obtained with the serial samples from humans, in particular those for the Progen IgM, IFA, are in agreement with the relative sensitivities measured in the serial samples from the monkeys. The monkey samples in this study clearly show the value of well-defined serial serum samples from experimentally infected animals, since the results of these samples are not influenced by previous infections or vaccinations or by geographical background. These samples may also contribute to the composition of quality control panels for flavivirus serology.
The consensus model resulted in an overall agreement of 45 IgM and 65 IgG DEN-positive serum samples, 83 IgM and 59 IgG DEN-negative serum samples, and 4 IgM and 8 IgG DEN-discordant serum samples. On basis of this consensus model, the calculated sensitivities of the DEN immunoassays evaluated varied between 71 and 100% for the respective IgM assays and between 52 and 100% for the respective IgG assays, with specificities of 86 to 92% for the IgM assays and 86 to 100% for the IgG assays.
The variations in sensitivity and specificity are comparable with previously published data (2, 5) and might be caused by the different principles of the assays, different antigens, conjugates (Table 1), or the selection of the respective serum panels. In a multicenter evaluation using a commercial DEN IgM dot assay, it was shown that the sensitivities varied between 80 and 98% depending on the serum samples of the respective collaborating centers (10).
Taken together, we conclude that the best complete DEN IgM and IgG detection systems are the INDX DipStick EIA and the PanBio EIA, followed by the MRL EIA and the Genelabs blot, whereas the PanBio RIT and the Progen IFA perform less well. If separate assays are selected to perform DEN diagnosis in the laboratory, a combination of the PanBio RIT for IgM detection and the PanBio IgG EIA would be the most sensitive and specific combination. The PanBio RIT seems to be in favor for bedside diagnostics and fieldwork because of its high sensitivity and relatively high specificity for IgM, and the simultaneously obtained IgG results are ignored. In our view, for laboratories with a relative high workload of DEN samples, a combination of the MRL IgM EIA and the PanBio IgG EIA could be a good choice, since both assays can easily be automated. The commercially available DEN immunoassay systems offer a good alternative to homemade DEN assays, including HAI- and EIA-based systems. These commercial assays make the serodiagnosis of DEN infection available to general and peripheral laboratories. However, for the isolation, molecular diagnosis, and determination of DEN-specific neutralizing antibodies, reference laboratories will continue to play an important role, which will also be the case for immunopathogenic, epidemiological, and vaccine studies.
REFERENCES
- 1.Clarke D H, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 2.Cuzzubbo A J, Vaughn D W, Nisalak A, Solomon T, Kalayanarooj S, Aaskov J, Dung N, Devine P L. Comparison of PanBio dengue duo enzyme-linked immunosorbent assay (ELISA) and MRL dengue fever virus immunoglobulin M capture ELISA for diagnosis of dengue virus infections in Southeast Asia. Clin Diagn Lab Immunol. 1999;6:705–712. doi: 10.1128/cdli.6.5.705-712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davey S. Key vaccines under development. In: Bellamy C, Nakajima H, editors. State of world's vaccines and immunization. Geneva, Switzerland: World Health Organization-United Nations International Children's Emergency Fund; 1996. pp. 4–143. [Google Scholar]
- 4.Delenda C, Frenkiel M P, Deubel V. Protective efficacy in mice of a secreted form of recombinant dengue-2 virus envelope protein produced in baculovirus infected insect cells. Arch Virol. 1994;139:197–207. doi: 10.1007/BF01309465. [DOI] [PubMed] [Google Scholar]
- 5.Devine P L, Cuzzubbo A, Marlborough D. Dengue fever testing. Today's Life Sci. 1997;9:26–30. [Google Scholar]
- 6.Hayes E B, Gubler D J. Dengue and dengue hemorrhagic fever. Pediatr Infect Dis J. 1992;11:311–317. doi: 10.1097/00006454-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Hesketh L, Charlett A, Farrington P, Miller E, Forsey T, Morgan-Capner P. An evaluation of nine commercial EIA kits for the detection of measles-specific IgG. J Virol Methods. 1997;66:51–59. doi: 10.1016/s0166-0934(97)02210-6. [DOI] [PubMed] [Google Scholar]
- 8.Innis B L. Dengue and dengue hemorrhagic fever. In: Portersfield J S, editor. Exotic viral infections. London, United Kingdom: Chapman & Hall; 1995. pp. 103–146. [Google Scholar]
- 9.Jirakanjanakit N, Sanohsomneing T, Yoksan S, Bhamarapravati N. The micro-focus reduction neutralization test for determining dengue and Japanese encephalitis neutralizing antibodies in volunteers vaccinated against dengue. Trans R Soc Trop Med Hyg. 1997;91:614–617. doi: 10.1016/s0035-9203(97)90050-x. [DOI] [PubMed] [Google Scholar]
- 10.Lam S K, Fong M Y, Chungue E, Doraisingham S, Igarashi A, Khin M A, Kyaw Z T, Nisalak A, Roche C, Vaughn D W, Vorndam V. Multicentre evaluation of dengue IgM dot enzyme immunoassay. Clin Diagn Virol. 1996;7:93–98. doi: 10.1016/s0928-0197(96)00257-7. [DOI] [PubMed] [Google Scholar]
- 11.Lam S K, Devine P L. Evaluation of capture ELISA and rapid immunochromatographic test for the determination of IgM and IgG antibody production during dengue infection. Clin Diagn Virol. 1998;10:75–81. doi: 10.1016/s0928-0197(98)00002-6. [DOI] [PubMed] [Google Scholar]
- 12.Palmer C J, King S D, Cuadrado R R, Perez E, Baum M, Ager A L. Evaluation of the MRL Diagnostics dengue fever virus IgM ELISA and the PanBio rapid immunochromatographic test for diagnosis of dengue fever in Jamaica. J Clin Microbiol. 1999;37:1600–1601. doi: 10.1128/jcm.37.5.1600-1601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaughn D W, Nisalak A, Kalayanarooj S, Solomon T, Dung N M, Cuzzubbo A, Devine P L. Evaluation of a rapid immunochromatographic test for diagnosis of dengue virus infection. J Clin Microbiol. 1998;36:234–238. doi: 10.1128/jcm.36.1.234-238.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velzing J, Groen J, Drouet M T, van Amerongen G, Copra C, Osterhaus A D M E, Deubel V. V. Induction of protective immunity against dengue virus type 2: comparison of candidate live attenuated and recombinant vaccines. Vaccine. 1999;17:1312–1320. doi: 10.1016/s0264-410x(98)00393-4. [DOI] [PubMed] [Google Scholar]
- 15.Wilson M E. Profiles of infections. In: Wilson M E, editor. A world guide to infections. New York, N.Y: Oxford University Press; 1991. pp. 422–702. [Google Scholar]
- 16.Wu S J, Hanson B, Paxton H, Nisalak A, Vaughn D W, Rossi C, Henchal E A, Porter K R, Watts D M, Hayes C G. Evaluation of a dipstick enzyme-linked immunosorbent assay for detection of antibodies to dengue virus. Clin Diagn Lab Immunol. 1997;4:452–457. doi: 10.1128/cdli.4.4.452-457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwieg M H, Robertson E A. Clinical validation of immunoassays: a well-designed approach to a clinical study. In: Can D W, Perstein M T, editors. Immunoassay, a practical guide. Orlando, Fla: Academic Press; 1987. pp. 97–127. [Google Scholar]