Abstract
Conglomerate crystallization is the spontaneous generation of individually enantioenriched crystals from a nonenantioenriched material. This behavior is responsible for spontaneous resolution and the discovery of molecular chirality by Pasteur. The phenomenon of conglomerate crystallization of chiral organic molecules has been left largely undocumented, with no actively curated list available in the literature. While other crystallographic behaviors can be interrogated by automated searching, conglomerate crystallizations are not identified within the Cambridge Structural Database (CSD) and are therefore not accessible by conventional automated searching. By conducting a manual search of the CSD and literature, a list of over 1800 chiral species capable of conglomerate crystallization was curated by inspection of the racemic synthetic routes described in each publication. The majority of chiral conglomerate crystals are produced and published by synthetic chemists who seldom note and rarely exploit the implications this phenomenon can have on the enantiopurity of their crystalline materials. With their structures revealed, we propose that this list of compounds represents a new chiral pool which is not tied to biological sources of chirality.
Keywords: chirality, chiral pool, conglomerate crystallization, CSD, spontaneous resolution, spontaneous deracemization
Introduction
Asymmetric synthesis fundamentally relies on the enantioenriched nature of biological systems. The natural chiral pool is fixed in size, constrained by evolutionary pressures of the organisms that produce its members, and limited in scaffold diversity. Due to the enantiopurity of biological machinery and their chemical precursors, often the resulting compounds are only naturally available in one enantiomeric form. Yet, synthetic chemists have used the chiral information handed to them by the natural world to great effect with increasing levels of stereocontrol (Figure 1).1,2 First, by using the natural chiral pool as a synthetic feedstock, new enriched derivatives are accessible, expanding the library of available enantioenriched materials to include a synthetic chiral pool. Exploiting this expanded library to mediate diastereoselective syntheses allows for the transfer of stereochemical information from the natural chiral pool to new, previously inaccessible stereogenic elements.3−5 However, this reliance on the natural chiral pool to supply chemical scaffolds can limit access to a singular enantiomeric form of a product. The solution to this problem comes with the development of resolution methods using materials derived from the natural and synthetic chiral pools, allowing for the separation of racemic non-natural scaffolds and therefore granting access to both senses of enantioenrichment of compounds not derived from the natural chiral pool. Temporary attachment of these materials and their derivatives, so-called chiral auxiliaries, to molecular frameworks allows for stereoselective transformations on substrates not part of the natural chiral pool. However, this strategy requires derivatization of the substrate molecule, installing stoichiometric amounts of chiral information in a covalent fashion. To overcome this limitation, modern asymmetric catalytic processes take these enantioenriched materials and employ them in transformations which impart stereochemical bias while only requiring substoichiometric amounts of the enriched material to be present, allowing chiral information to be amplified. Ultimately, all materials and asymmetric methods that synthetic chemists currently employ to access enantioenriched synthetic products all rely on the chiral information originally imparted from biology.6
Figure 1.

Euler diagram displaying the relationship between the natural and synthetic chiral pools, and the proposed conglomerate chiral pool.
There is an opportunity to create a chiral pool independent of biological chiral information: one which is based solely on the crystallographic properties of the material itself (Figure 1). In the case of racemic compounds, the most likely outcome from a crystallization is the formation of racemic crystals—crystals in which equal proportions of enantiomers are present. However, in a substantial number of cases, an intriguing crystallographic phenomenon can occur. Here, a racemic material can undergo spontaneous resolution with each crystal containing a single enantiomer in a process referred to as a conglomerate crystallization.200 This phenomenon imparts chiral information on the material and the chemist is no longer in the confines of the natural chiral pool to achieve enantioenrichment. Broadly speaking, there are three methods to exploit conglomerate crystallization for accessing enantioenriched materials. The first is to follow an unbiased spontaneous resolution protocol in which both enantiomers crystallize as discrete crystals from the mixture. This was originally described by Pasteur when he separated individual enantiomorphic crystals of sodium ammonium tartrate by hand.7 While this method was historically fundamental in the discovery of molecular chirality, this method is too arduous to be applicable in modern synthetic processes. The second method in which a conglomerate crystal may be exploited to produce enantioenrichment is to conduct the crystallization in the presence of seed crystals of the desired enantiomer. This seed crystal imparts a kinetic bias to coax the matching enantiomer to crystallize from solution, removing the need to manually sort the resulting crystals.8−11 This method, called preferential crystallization (also known as resolution by entrainment or dédoublement par entraînment), can be regarded as a stereoselective crystallization. The mother liquors will be left enriched in the opposite enantiomer, which can be brought to supersaturation and seeded with the opposite enantiomer. Alternating the enantiomer of the seed crystal and replenishing the mother liquor solution with racemic material allows for indefinite cycles of resolution to occur. As such this method is an attractive resolution strategy and numerous examples of this strategy have been applied in continuous crystallization modes, rendering the process incredibly efficient for large scale industrial processes requiring enantioenriched materials.12−15 A list of conglomerate crystals which have been resolved by preferential crystallization is available in the Supporting Information.
The third and the most recently discovered possibility is to combine a conglomerate crystallization with solution phase racemization and a symmetry breaking event. With careful control of the crystallization/racemization conditions, deracemization of the bulk material can be achieved without external chiral influences, i.e., a spontaneous asymmetric synthesis.16−23 The modern method to achieve a spontaneous asymmetric synthesis is by following an attrition-enhanced deracemization protocol, more commonly known as Viedma ripening.24,25 While the first attrition-enhanced deracemization of conglomerate crystals was performed on sodium chlorate and sodium bromate salts,26−30 this process has since been exploited to produce chiral organic molecules with high enantiopurity, where the chirality of the molecule is maintained upon dissolution of the crystal. The advantage in this strategy is the ability to convert the undesired enantiomer by racemization to the desired enantiomeric form, giving a theoretical maximum yield of 100%. This is in contrast to spontaneous resolution and preferential crystallization protocols which are restricted to 50% yield of a desired enantiomer. Importantly, all three strategies that use conglomerate behavior to achieve enantioenrichment rely solely on the crystallographic behavior of the material without recourse to the natural chiral pool.
Many desirable traits exist for a conglomerate chiral pool based on crystallographic chiral information as opposed to a chiral pool originating from biological sources. There is no limit to which materials could crystallize as a conglomerate, thus providing a vast range of diverse scaffolds which can be spontaneously enantioenriched. A conglomerate crystal is not dependent on a particular organism to produce an abundance of a desired compound to be economically viable. Practically speaking, both enantiomers are equally likely to crystallize (unless a specific enantiomer is deliberately biased from the crystallization), allowing access to both enantiomeric forms of the members of this chiral pool. While the synthetic chiral pool can increase in size through extended chains of resolution or enantioinduction, ultimately the original source of the chiral information remains the natural chiral pool. In contrast, conglomerate crystallization imparts enantioselectivity directly at the crystallization event, providing ever increasing access to sources of chiral information. As chemists continue to synthesize and crystallize new materials, more conglomerate crystals should be discovered every year, thus increasing the structural diversity present in this enantioenriched library. Given these advantages, why is the phenomenon of conglomerate crystallization not currently being widely exploited for augmenting our current sources of chirality and as a means to generate this new chiral pool?
The answer is the lack of documentation. The main hurdle in the adoption of this phenomenon for producing enantioenriched materials is the lack of curated knowledge of which crystals have the capacity to crystallize as conglomerates. The CSD (Cambridge Structural Database) is the largest and most widely adopted crystallographic repository service which is charged with the curation of crystallographic data produced by chemists. At the time of writing, it boasts over 1.1 million structures which can be searched and freely accessed by the community. The development of automated means to search this database with CCDC developed software (ConQuest) and community developed algorithms31 have led to new insights regarding statistical crystal behaviors.32−34 However, the CSD does not require conglomerate crystallization behavior to be identified in their metadata at submission, leading to a loss of this information as a search term in the database. Retrieving conglomerate crystals from this database is further frustrated by the difficulties in their prediction. While efforts to rationalize conglomerate crystallization have been conducted using crystal structure prediction,35 structural modifications,36−38 and supramolecular interactions,39,40 currently only direct measurements of the physical characteristics of a crystal can identify conglomerate behavior conclusively.
The typical work-flow of how X-ray crystallography samples are solved in most academic institutions is not conducive to the communication of conglomerate behavior between the synthetic chemist and the crystallographer, symptomatic of a traditional view of separated scientific disciplines. Often the synthetic chemist will supply a crystalline sample with a proposed structure and the solvent of crystallization to the collaborating crystallographer. Communication of the synthetic origin of the sample is less standardized and whether the starting materials are racemic or enriched, possibly unclear. Without this information it is impossible for the crystallographer to unambiguously identify conglomerate behavior. The sample will then be solved and returned to the synthetic chemist, who is generally interested in the connectivity of the molecule and relative stereochemistry within the crystal (unless they specifically ask for confirmation of absolute configuration). The importance of Sohncke space groups41 or Flack parameters42 in the crystallographic data has the potential to be overlooked, or at least unreported, by the synthetic community leading to the possibility of conglomerate behavior being unidentified. The CIF (crystallographic information file) is deposited in the CSD by the crystallographer and now the synthetic chemist, crystallographer and the wider chemistry community are unaware of the full crystallographic behavior of this sample. Once deposited, the conglomerate crystal can no longer be retrieved selectively without also bringing up thousands of nonconglomerate crystals which have been produced by enantioselective means. Therefore, this foundational phenomenon responsible for the discovery of molecular chirality is currently being undocumented by both the synthetic and crystallographic communities.
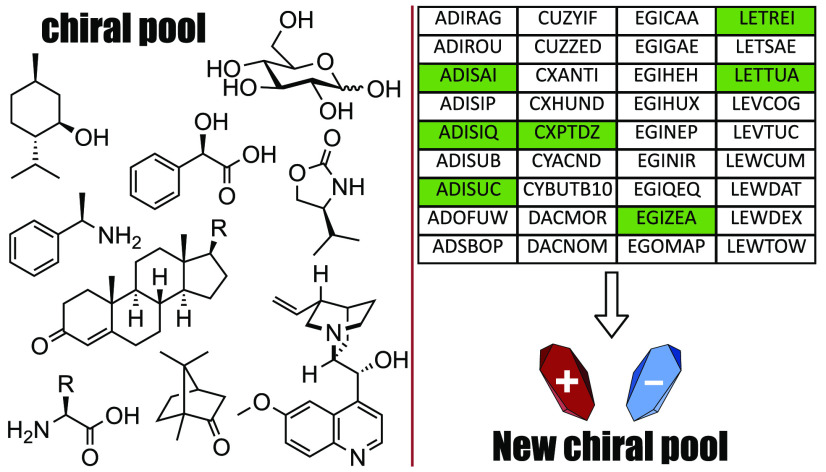
It is only once a phenomenon has been documented that it can be fully exploited for its true potential by members of the chemical community. The most complete list of potential conglomerate crystals was compiled by Jacques, Collet, and Wilen in their influential book published in 1981;43 however, the reports of the crystals contained in this list mostly predate the CSD. There is no actively curated list of chiral conglomerate crystals available in the literature. It is also understood that an automated search of the CSD to identify conglomerate crystallization cannot be achieved without prior recording of metadata; that is to say, conglomerates are hiding in plain sight within the CSD.34 The wealth of crystallographic information present in the CSD represents an untapped resource for confirmed conglomerate behavior. To extract this information, a manual search of crystals in the CSD would have to be conducted, which would interrogate the origin of each chiral crystal to ensure it originated from a racemic synthetic process. This requires manually examining each reported synthetic route. We sought to tap into this wealth of crystallographic and synthetic potential by conducting such a manual search of the CSD for previously unidentified conglomerate crystallizations in order to catalogue this new chiral pool.
Results and Discussion
Methods for Conglomerate Identification
The full list of conglomerate crystals along with their chemical structures and associated references are available in the Supporting Information. While the formation of chiral conglomerate crystals from achiral materials is also possible,44−47 this work focused specifically on documenting the spontaneous resolution phenomenon for chiral organic molecules which will be of interest for the synthetic community. The queries generated to conduct the search are detailed in the Experimental Section. Once a list of candidates (21,098 crystals) was generated from search queries of the CSD mediated by ConQuest, a manual search and interpretation of the reported syntheses for the crystals within the CSD was undertaken to identify conglomerate crystallization.
Caution had to be taken to distinguish between absolute and relative stereochemistry and the use of stereochemical notation to display perspective in compound representations. Crucially, confirming if a crystal had displayed conglomerate behavior relied on the ability to trace the stereochemical enrichment of the starting materials and rule out any use of asymmetric methodology throughout the synthesis. In cases where the synthetic route for the compound was not available, or the described synthetic route was ambiguous in stereochemical information on the precursors, these examples were omitted. As such, all structures which were only available as a CSD Communication were excluded as the origins of these materials was not possible to interrogate. Of course, the following assumptions had to be made while interpreting the reported syntheses and crystallizations within this list. It is assumed that the authors have reported the syntheses and the nature of the enrichment of their reagents/catalysts accurately, that the crystal structure(s) they reported indeed were crystallized from the batch of material as described and that the crystal structures themselves have been solved accurately (i.e., the space groups are correctly assigned).
Trends in Publication and Deposition of Conglomerate Crystals
From this search, 1626 conglomerate crystal structures were identified within the CSD. A further 210 structures were compiled from literature searches from known conglomerate crystallizations and preferential crystallizations, some of which with as-yet undetermined or unreported crystal structures. A recent analysis of the CSD in 2020 by Rekis32 suggests that 9.5% of the chiral compounds which crystallized in Sohncke space groups would be conglomerates, giving an estimated 4281 conglomerates of chiral organic compounds hidden in the CSD. If this estimate is correct, the list curated in this work accounts for 38% of the chiral organic conglomerates currently unaccounted for in the CSD. An intriguing question arises from this search: How many compounds which have been prepared in a nonracemic fashion, and thus were excluded from this list, would show conglomerate behavior? This includes molecules isolated from natural sources, pharmaceuticals, ligands, organocatalysts, and peptide oligomers—most of which have only been prepared in an enantiomerically enriched form and so any conglomerate behaviors would remain obscured.
We sought to determine whether conglomerate crystallization could also be elucidated using the current methods of recording enantioenrichment of deposited crystals in CIF dictionary approved terms. This was achieved by conducting an internal search of the deposited crystallographic data in the CSD for the fields which can record enantioenrichment in a crystalline sample. Only 12 crystals in the CSD contained an entry for the “_chemical_enantioexcess_*” fields, none of which were conglomerate crystals (see the Supporting Information). In comparison, only 17 entries in the CSD have conglomerate behavior unofficially identified using text strings within the deposited CIF, which can be found using ConQuest. These results demonstrate that there is no other means to search the CSD for conglomerate crystals, due to the way that CIFs have been prepared and deposited into the CSD without any attempt to record conglomerate behavior using official CIF dictionary fields or unofficial text strings.
The majority of the conglomerate crystals found from our manual search of the CSD had been originally reported by synthetic groups publishing in non-crystallographic journals, reflecting the vast number of crystallographic samples produced by the synthetic community. A breakdown of the literature sources of conglomerate crystals is shown in Figure 2a. Non-crystallographic journals make up 84% of this conglomerate list. It appears that synthetic chemists publishing in J. Org. Chem., Org. Lett., Tetrahedron, and Tetrahedron Lett. are responsible for 34% of the papers containing conglomerate crystals. In almost all cases where a conglomerate appears in a synthesis focused paper, the phenomenon is not commented on in the CIF or the respective paper. Of the 1,626 conglomerates found from the manual search of the CSD, only 120 mentioned conglomerate behavior in the manuscript text.
Figure 2.
(a) Number of publications by journal which contained a conglomerate crystal identified by manual search of the CSD (n = 1610). (b) Comparison of the distribution of the Sohncke space groups for enantioenriched chiral materials in the CSD (n = 39,894, blue chart) and distribution of the space groups exhibited by chiral conglomerate crystals found by manual searching of the CSD and literature sources (n = 1765, red chart).
Conglomerates have no distinguishing features in their routinely recorded crystallographic metadata which identify them from other enantioenriched compounds. A comparison of the frequency of space groups present in conglomerate crystals (n = 1765; red chart, manual CSD search and literature sources) and the frequency of Sohncke space groups in the CSD for enantioenriched species (n = 39,894; blue chart)32 was conducted (Figure 2b). While there is a slightly greater prevalence of P212121 within the conglomerate data set (65%) than observed in the CSD (52%), the overall trends of space group frequency of conglomerates match those observed in the CSD. The implications of this are clear: once a crystal is deposited in a crystallographic database such as the CSD, only a manual review of the synthetic route to the compound will be able to identify conglomerate behavior.
Structural Observations in Conglomerate Behavior
Conglomerate behavior was observed in all manner of chiral compounds, with no apparent limiting factors on what structures can undergo this process. Carbon, nitrogen, phosphorus, boron, sulfur, silicon, and selenium based stereocenters were among the compounds resolved by conglomerate behavior (Figure 3). Other stereogenic elements are also possible to enrich by crystallization, including axial chirality in the form of atropisomeric (VAWMEM,54 NURHOY56) and twisted structures (KUCGEV57). Larger supramolecular examples also demonstrate the potential to be a conglomerate crystal, including a helical Aib6 foldamer (EYIFOI55) and a helical pyridine-pyrimidine superstructure (KELJAM58). These demonstrate the diversity of structures which are within this list of conglomerate crystals.
Figure 3.
Types of stereocenters resolved by conglomerate crystallization and other chiral elements present in conglomerate crystals. The following crystal structures, labeled by their CSD Refcode, have been identified as conglomerates: TOQCUA,48 QETJII,18,22 REFLIX,49 GOPBOE,50 JAQNEX,51 KIXYOH,52 LONZOG,53 VAWMEM,54 EYIFOI,55 NURHOY,56 KUCGEV,57 KELJAM.58
Conglomerate Crystallization in Natural Product Synthesis
Structural complexity is not a barrier to conglomerate behavior. Since natural product synthesis has been a core area of study for organic chemists for decades, we wished to pay special attention to conglomerate crystals discovered in this area. We have noted a number of natural products and related scaffolds that exhibit conglomerate behavior when prepared in racemic fashion and crystallized (Figure 4). Notably, in these examples, the authors rarely note that spontaneous resolution had occurred during crystallization. There were also notable examples of conglomerate crystals appearing within the synthetic routes of racemic total syntheses. For example, in the synthetic routes to Pallambin C/D59 and Pyrenolide B,60 both routes contained two structures which crystallized as conglomerates within the synthesis. This established that in some synthetic routes there can be multiple instances of conglomerate behavior. The number of observed conglomerate crystals in natural products will be underestimated in this list as it was assumed that any material extracted from a biological source would be enantioenriched and so were discounted. Synthetic chemists have also been incentivised to produce enantioselective routes to natural products, which would also obscure conglomerate crystallization. The use of a conglomerate crystallization resolution or the development of racemization conditions to allow for attrition-enhanced deracemization within these established routes would give access to enantioenriched natural products.
Figure 4.


Natural products and total syntheses which contain a conglomerate crystal. The following crystal structures, labeled by their CSD Refcode, have been identified as conglomerates: LOPJUX,61 UNOJAK,62 HOQSIQ,63 WIWYAD,64 VIQCEF,65 XEXNAP,66 LUJHAB,67 EHATEO,68 TAKBUF,69 CEJWUM,70 YEFMON,71 CEXYOT,72 YOLROI,73 LAGSIW,74 TIGVOX,75 FUGFOC,76 OMUMEP,77 TIJWIS,78,79 VAGBUA,80 UVAXAR,81 TUHLIR,82 EGEZUN,83 DIJBEG,84 GESYOT,85 PELLOJ,86 HIDNEN,60 HIDMUC,60 JIJYOT,87 YEZGAO,88 OKOKEF,89 FOVSEO,90 VEGFAQ,59 VEGDIW,59 TAKFOD.91
Conglomerate Crystallization in Medicinal Chemistry and Crystal Engineering
Conglomerate behavior is not restricted to compounds of academic interest. Materials exhibiting conglomerate behaviors with importance in medicinal chemistry were also compiled (Figure 5), as these compounds have proven industrial interest. The development of a preferential crystallization or spontaneous deracemization protocols of pharmaceuticals will be of interest because of the scalability of crystallization processes, the already present need to find and control crystal polymorphs of the target, and the possibility of removing expensive enantioenriched ligands for transition metal based catalysts from synthetic routes. Similar to the study of natural product conglomerate behavior, the position of the conglomerate can occur at any stage in the synthesis.
Figure 5.

Conglomerate crystals present in medicinally relevant compounds. The following crystal structures, labeled by their CSD Refcode, were identified as conglomerates: RILBET,103 EMOMUQ,104 PANKUL,105 SOWKIZ,105 HONZOC,106 VAQXOC,107 VEFFAP,108 PAXRIR,109,110 QATXER,111 QATXOB,111 DAPHEH,112 BEPPUI,113 VALNII,114 FAYDUF,115,116 SOWKOF,105,117 RAYZAS,118 YISFOW,119 NENXAF,120 PESWAM,121 BUNKOL,19,20 UHUCEH,95 HEGGAD,92,93 NUMZUT.94
Crystal engineering has also been successful in producing conglomerate crystals. Exploring different crystallization methods and conditions can produce conglomerate crystals from structures which previously did not show conglomerate crystallization behaviors. This is a method to remove the probabilistic nature of conglomerate formation and allow for more control over which substrates display this behavior. The use of crystal engineering can be used to formulate cocrystallization conditions which lead to conglomerate crystal structures (HEGGAD,92,93 NUMZUT,94 UHUCEH,95 and others96−99), while retaining favorable biophysical properties. For better or worse, this may also offer a means to evergreen patents on existing pharmaceuticals if a synthetic route is altered to incorporate a conglomerate based asymmetric synthesis or if a final target itself is reformulated to become a conglomerate crystal. The choice of solvent has also been shown to control the formation of a conglomerate crystallization over a racemic crystal.19,20,100 The few cases of analysis of both racemic and conglomerate polymorphs of crystals are invaluable case studies for the development of methods to predict and understand conglomerate formation.101,102 Cases in which a conglomerate crystal formed a racemic twin are also of interest in further understanding this phenomenon and has been collated in the Supporting Information.
Potential Applications of Conglomerate Crystallization Behavior
From surveying the full list of conglomerate crystals, it is possible to identify structures of interest for future applications. Structures with potentially broad utility as chiral ligands and organocatalysts are shown in Figure 6. This highlights the possibility of utilizing conglomerate crystallization as a new chiral pool to provide chemists with sources of chiral information for asymmetric catalysis. Within this list are C2 symmetrical pyrimidine (OBIPAR122), phosphine (LUSZOO123), and imidazole (ROJPOW124) ligands, an atropisomeric quinoline (TUWFAT125), an α-methylpyridine (DOBWUN126), and a chiral salen ligand (TUNMOF127). Potential types of organocatalysts such as the C2 symmetrical diol (NULZEA128), a PTC (phase transfer catalyst) crown ether (NOCNIC129), a chiral phosphoric acid (CUVGAB130), chiral ureas (RIPBUN,131 AZUDAB132), benzotetramisole (YAMBAS133), amino-alcohol (HARFEN134), and imidazole (PURJUJ135) may also find use in asymmetric synthesis. These are only selected examples, and we would encourage the community to view our full list of structures to ascertain which compounds they may deem useful to their research. We acknowledge that the concepts of conglomerate crystallization, preferential crystallization and spontaneous asymmetric synthesis are not original to this paper. Research groups who are aware of the utility of conglomerate crystallization search for chiral structures which crystallize in this manner and which also contain stereocenter(s) suitable for racemization. Once these candidates are identified, spontaneous deracemization protocols have been developed, allowing for the enantioenrichment of materials without conventional forms of asymmetric synthesis. To develop such a spontaneous deracemization protocol, conglomerate crystallization conditions must be unified with racemization conditions such that both may occur simultaneously. Multiple strategies have been employed to allow for solution phase racemization while simultaneously crystallizing the target compound, including base catalysis,136−147 acid catalysis,148 reversible reactions (such as the Mannich,149 aldol,150,151 Diels–Alder,152,153 [2,3]-sigmatropic rearrangements,154 annulation155 reactions), Schiff base formation,156−158 photoracemization,159 and thermal racemization (such as crystallizing from a melt160). With this established, a chiral symmetry breaking event is introduced to allow the system to spontaneously enantioenrich. When conducted in an unbiased fashion, as with attrition/grinding161−163 (Viedma ripening) and ultrasound,164 stochastically enantioenriched material can be produced. Alternatively, these strategies can also be biased using crystal seeding17,165 or CPL,166 allowing for selection of the enantiomer to be formed from crystallization. Researchers working at the interface of crystallography and synthesis have succeeded in achieving impressive examples of spontaneous deracemization and expanding the protocols available to do so, but have been hindered by the lack of documentation of chiral conglomerate species. We present this curated list of conglomerate crystals for the benefit of both the crystallographic and synthetic communities to unlock the potential of this powerful strategy.
Figure 6.

Potential ligands and organocatalysts from conglomerate crystals. The following crystal structures, labeled by their CSD Refcode, were identified as conglomerates: OBIPAR,122 LUZOO,123 PURJUJ,135 NULZEA,128 KAQMEV,167 DOBWUN,126 ROJPOW,124 TUWFAT,125 HARFEN,134 YAMBAS,133 CUVGAB,130 GADTIR,168 NOCNIC,129 TUNMOF,127 QEVSAO,169 VARLIK,170 RIPBUN,131 AZUDAB.132
Future Outlook
Questions on the utility of this curated list of conglomerate crystals may arise:
-
1.
Why should synthetic chemists care about a crystallographic phenomenon? It is a phenomenon that can directly affect the enantiopurity of crystalline materials. If a recrystallization had been performed as a purification step on a racemic material which exhibited conglomerate behavior, selection of a conglomerate crystal from this material not only would give different diffraction properties in SCXRD and PXRD compared to its racemate, but also would affect the recorded melting point, IR spectra, Raman spectra, and interactions with other enantioenriched species, such as those encountered in biological and pharmaceutical studies (IC50, LD50, protein binding, pharmacokinetics, pharmacodynamics).
-
2.
How are conglomerate crystals synthetically useful? The curation of this list of conglomerates should not only aid future research on understanding this fundamental crystallization phenomenon, but also act as a potential source of chiral information for the synthetic community. By tracking materials which undergo this type of crystallization, the possibility of exploiting a powerful mode of chiral amplification can be achieved, whereby a substrate is able to bias its own enantioenrichment.
The exciting synthetic potential of conglomerate crystallization has been demonstrated in the case of the natural product Narwedine.17Figure 7 highlights the process that was developed on a pilot scale, showing this strategy in asymmetric synthesis can reliably produce desired enantioenriched materials in a cost-effective manner for industrial syntheses.165
Figure 7.

Pilot scale spontaneous deracemization of Narwedine.17
The prospective new chiral pool of conglomerate crystals disclosed in the Supporting Information contains a huge variety of structural diversity, with each member being a potential target for spontaneous asymmetric synthesis. A selection of candidates and their hypothesized deracemization conditions are proposed within Figure 8. Armed with a full knowledge of the chemical structures of compounds able to undergo a conglomerate crystallization, we hope that the synthetic and crystallographic communities take advantage of this exciting opportunity to view the structures in this list and use their creativity to develop conditions to exploit this untapped source of chiral information.
Figure 8.
Mechanism of attrition-enhanced deracemization and hypothesized candidates.
Conclusion
A list of over 1800 conglomerate crystals has been compiled from the CSD and literature, representing 38% of the predicted chiral conglomerate compounds contained within the CSD. Incentivizing synthetic chemists to rapidly communicate their crystal structures with a description of the synthetic procedures and reagents which produced the material—even if such crystals are considered unremarkable by the crystallographic community or the synthesis unremarkable to the synthetic community—is the best method to discover new conglomerate crystals. A simple change in the deposition process to the CSD, which could prompt the synthetic chemist/crystallographer to consider if the material originated from a racemic process, would avoid the need to conduct arduous manual searches in the future. We propose that this list of chiral conglomerate crystals could be viewed as a fundamentally new type of chiral pool; one which is not bound to biologically sourced chiral information. We hope that the curation of this list of conglomerate crystals aids the development of preferential crystallization and spontaneous deracemization protocols, while also furthering the understanding of the formation of conglomerate crystal behavior.
Experimental Section
CSD version 5.41 (November 2019) was used for the search. Search queries were generated using Conquest, with the following queries chosen to try and minimize the total number of crystals to be checked while also maximizing the potential number of conglomerate candidates. Crystals must exist in Sohncke space group AND Z′ = 1. Crystals must NOT be in carbohydrate, steroid, peptide, or nucleoside/nucleotide classes. Entries must have a carbon center with C(Nonmetal)4OR H–C(Nonmetal)3. The main focus was put on carbon stereocenters since they make up 98% of all stereocenters within in the CSD. The search was refined such that crystals must be organic, not polymer, not salts, and single crystal only, with R1 < 0.075 and no errors. Disordered structures were allowed. It was also found that specific strings of text could be used to exclude certain natural products, including: “isolated”, “sourced from”, “extracted”, “bark”, “marine”, “sponge”, and “penicillium”. Natural products could be further filtered when sorting the resulting CSD hits by their structure names; generic naming such as “cinchonine”, “strychnine”, and “Striatin A” could be excluded due their natural sources or as targets for asymmetric total syntheses. This generated a list of 30,204 crystals as potential conglomerates. Compounds listed with known stereochemical assignments could be excluded from the list as well. Compound names with the following: (+), (−), d, l, (R), and (S), were removed from the list, as these were either sourced from the natural chiral pool or were produced from enantioselective methodologies and XRD was used for absolute configuration assignment. This left 21,098 crystals to be inspected manually.
Acknowledgments
The authors would like to acknowledge the contributions of Kane A. Bastick during the search of the CSD and discussions on the concept. The authors would also like to thank our colleagues within Durham University for their feedback and fruitful discussions while preparing this manuscript.
Glossary
Abbreviations
- CCDC
Cambridge Crystallographic Data Centre
- CIF
crystallographic information file
- CSD
Cambridge Structural Database
- CPL
circularly polarized light
- SCXRD
single crystal X-ray diffraction
- PXRD
powder X-ray diffraction
The chiral conglomerate crystals identified in this work have been collated as CIF and Refcode list formats (.cif,. txt,. gcd) and are freely available from the Zenodo data repository (10.5281/zenodo.6092203).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.2c00394.
Curated list of conglomerate crystals along with their chemical structures and their associated references (PDF)
Author Contributions
† J.A.B., C.S.B., and J.X. contributed equally to this work. CRediT: James A. Barclay investigation; Callum S. Begg investigation; Jinyi Xuan investigation.
We thank the EPSRC for PhD studentship to C.S.B. (EP/T518001/1, project reference 2456710) and PhD studentship to J.X. through the SOFI2 CDT program (EP/S023631/1, project reference 2531010); the Royal Society for PhD studentship for J.A.B. (RGF\EA\180312) and a research fellowship to M.O.K. (UF150536).
The authors declare the following competing financial interest(s): J.C.C. and N.T.J. currently hold positions within the Cambridge Crystallographic Data Centre.
Notes
The Python script used to extract the CIF fields information from the CIFs within the internal CSD is available from the GitHub repository (https://github.com/walshm78/CSD_conglomerate_search). This manuscript was originally deposited as a preprint on ChemRxiv (10.26434/chemrxiv-2022-3g59b).
This Perspective published ASAP on September 23, 2022. The Supporting Information, Figure 4, Figure 5, and minor text corrections have been updated. The corrected version was reposted on October 14, 2022.
Supplementary Material
References
- Kagan H. B.; Gopalaiah K. Early History of Asymmetric Synthesis: Who Are the Scientists Who Set up the Basic Principles and the First Experiments?. New J. Chem. 2011, 35 (10), 1933–1937. 10.1039/c1nj20216b. [DOI] [Google Scholar]
- Wyatt P.; Warren S. G.. Organic Synthesis: Strategy and Control, 1st ed.; Wiley, 2007. [Google Scholar]
- Fischer E. Notizen Über Einige Säuren Der Zuckergruppe. Berichte der Dtsch. Chem. Gesellschaft 1890, 23 (2), 2625–2628. 10.1002/cber.189002302158. [DOI] [Google Scholar]
- Fischer E. Ueber Die Optischen Isomeren Des Traubenzuckers, Der Gluconsäure Und Der Zuckersäure. Berichte der Dtsch. Chem. Gesellschaft 1890, 23 (2), 2611–2624. 10.1002/cber.189002302157. [DOI] [Google Scholar]
- ″Durch diese Beobachtungen ist meines Wissens zuerst der strenge experimentelle Beweis geliefert worden, dass bei asyrnmetrischen Systemen auch die weitere Synthese im asymmetrischen Sinne vor sich geht.″ ″To my knowledge, these observations furnish the first definitive evidence that further synthesis with asymmetric systems proceeds in an asymmetric manner.″; Fischer E. Synthesen in Der Zuckergruppe II. Berichte der Dtsch. Chem. Gesellschaft 1894, 27 (3), 3189–3232. 10.1002/cber.189402703109. [DOI] [Google Scholar]
- Blaser H. U. The Chiral Pool as a Source of Enantioselective Catalysts and Auxiliaries. Chem. Rev. 1992, 92 (5), 935–952. 10.1021/cr00013a009. [DOI] [Google Scholar]
- Achiral molecules can also crystallize in Sohncke space groups as conglomerate crystals. In this paper, we focus on conglomerate crystals originating from chiral organic materials as these are of greater interest for the synthetic community.
- Flack H. D. Louis Pasteurs Discovery of Molecular Chirality and Spontaneous Resolution in 1848, Together with a Complete Review of His Crystallographic and Chemical Work. Acta Crystallogr. Sect. A Found. Crystallogr. 2009, 65 (5), 371–389. 10.1107/S0108767309024088. [DOI] [PubMed] [Google Scholar]
- Collet A.; Brienne M.-J.; Jacques J. Optical Resolution by Direct Crystallization of Enantiomer Mixtures. Chem. Rev. 1980, 80 (3), 215–230. 10.1021/cr60325a001. [DOI] [Google Scholar]
- Wang Y.; Chen A. M. Enantioenrichment by Crystallization. Org. Process Res. Dev. 2008, 12 (2), 282–290. 10.1021/op700239a. [DOI] [Google Scholar]
- Levilain G.; Coquerel G. Pitfalls and Rewards of Preferential Crystallization. CrystEngComm 2010, 12 (7), 1983–1992. 10.1039/c001895c. [DOI] [Google Scholar]
- Polenske D.; Lorenz H.; Seidel-Morgenstern A. Potential of Different Techniques of Preferential Crystallization for Enantioseparation of Racemic Compound Forming Systems. Chirality 2009, 21 (8), 728–737. 10.1002/chir.20672. [DOI] [PubMed] [Google Scholar]
- Rougeot C.; Hein J. E. Application of Continuous Preferential Crystallization to Efficiently Access Enantiopure Chemicals. Org. Process Res. Dev. 2015, 19 (12), 1809–1819. 10.1021/acs.oprd.5b00141. [DOI] [Google Scholar]
- Vetter T.; Burcham C. L.; Doherty M. F. Separation of Conglomerate Forming Enantiomers Using a Novel Continuous Preferential Crystallization Process. AIChE J. 2015, 61 (9), 2810–2823. 10.1002/aic.14934. [DOI] [Google Scholar]
- Galan K.; Eicke M. J.; Elsner M. P.; Lorenz H.; Seidel-Morgenstern A. Continuous Preferential Crystallization of Chiral Molecules in Single and Coupled Mixed-Suspension Mixed-Product-Removal Crystallizers. Cryst. Growth Des. 2015, 15 (4), 1808–1818. 10.1021/cg501854g. [DOI] [Google Scholar]
- Dunn A. S.; Svoboda V.; Sefcik J.; Ter Horst J. H. Resolution Control in a Continuous Preferential Crystallization Process. Org. Process Res. Dev. 2019, 23 (9), 2031–2041. 10.1021/acs.oprd.9b00275. [DOI] [Google Scholar]
- Hongo C.; Tohyama M.; Yoshioka R.; Yamada S.; Chibata I. Asymmetric Transformation of DL- p -Hydroxyphenylglycine by a Combination of Preferential Crystallization and Simultaneous Racemization of the o -Toluenesulfonate. Bull. Chem. Soc. Jpn. 1985, 58 (2), 433–436. 10.1246/bcsj.58.433. [DOI] [Google Scholar]
- Shieh W. C.; Carlson J. A. Asymmetric Transformation of Either Enantiomer of Narwedine via Total Spontaneous Resolution Process, a Concise Solution to the Synthesis of (−)-Galanthamine. J. Org. Chem. 1994, 59 (18), 5463–5465. 10.1021/jo00097a060. [DOI] [Google Scholar]
- Havinga E. Spotaneous Formation of Optically Acitve Substances. Biochim. Biophys. Acta 1954, 13, 171–174. 10.1016/0006-3002(54)90300-5. [DOI] [PubMed] [Google Scholar]
- Okada Y.; Takebayashi T.; Hashimoto M.; Kasuga S.; Sato S.; Tamura C. Formation of Optically Active Compounds under Achiral Synthetic Conditions. J. Chem. Soc. Chem. Commun. 1983, 14, 784–785. 10.1039/c39830000784. [DOI] [Google Scholar]
- Okada Y.; Takebayashi T.; Sato S. Asymmetric Transformation: III: Crystal Properties and Structures of a 1,4-Benzodiazepinooxa-Zole Derivative. Chem. Pharm. Bull. 1989, 37 (1), 5–8. 10.1248/cpb.37.5. [DOI] [Google Scholar]
- Hongo C.; Yamada S.; Chibata I. Asymmetric Transformation of N -Acetyl-DL-Leucine. Bull. Chem. Soc. Jpn. 1981, 54 (11), 3291–3295. 10.1246/bcsj.54.3291. [DOI] [Google Scholar]
- Kostyanovsky R. G.; Kostyanovsky V. R.; Kadorkina G. K.; Lyssenko K. A. Wedekind–Fock–Havinga Salt Me(Et)N+(All)PhI–·CHCl3 as Historically the First Object for Absolute Asymmetric Synthesis: Spontaneous Resolution, Structure and Absolute Configuration. Mendeleev Commun. 2001, 11 (1), 1–5. 10.1070/MC2001v011n01ABEH001420. [DOI] [Google Scholar]
- Palmans A. R. A. Deracemisations under Kinetic and Thermodynamic Control. Mol. Syst. Des. Eng. 2017, 2 (1), 34–46. 10.1039/C6ME00088F. [DOI] [Google Scholar]
- Buhse T.; Cruz J. M.; Noble-Terán M. E.; Hochberg D.; Ribó J. M.; Crusats J.; Micheau J. C. Spontaneous Deracemizations. Chem. Rev. 2021, 121 (4), 2147–2229. 10.1021/acs.chemrev.0c00819. [DOI] [PubMed] [Google Scholar]
- Weissbuch I.; Lahav M. Crystalline Architectures as Templates of Relevance to the Origins of Homochirality. Chem. Rev. 2011, 111 (5), 3236–3267. 10.1021/cr1002479. [DOI] [PubMed] [Google Scholar]
- Viedma C. Chiral Symmetry Breaking during Crystallization: Complete Chiral Purity Induced by Nonlinear Autocatalysis and Recycling. Phys. Rev. Lett. 2005, 94 (6), 65504. 10.1103/PhysRevLett.94.065504. [DOI] [PubMed] [Google Scholar]
- Szurgot M.; Szurgot J. Chiral Symmetry Breaking in Sodium Chlorate Crystallization from Unstirred Solution. Cryst. Res. Technol. 1995, 30 (7), 949–956. 10.1002/crat.2170300714. [DOI] [Google Scholar]
- Viedma C. Selective Chiral Symmetry Breaking during Crystallization: Parity Violation or Cryptochiral Environment in Control?. Cryst. Growth Des. 2007, 7 (3), 553–556. 10.1021/cg060698d. [DOI] [Google Scholar]
- Shan Monica Cheung P.; Gagnon J.; Surprenant J.; Tao Y.; Xu H.; Cuccia L. A. Complete Asymmetric Amplification of Ethylenediammonium Sulfate Using an Abrasion/Grinding Technique. Chem. Commun. 2008, 8, 987–989. 10.1039/b716977a. [DOI] [PubMed] [Google Scholar]
- Kondepudi D. K.; Kaufman R. J.; Singh N. Chiral Symmetry Breaking in Sodium Chlorate Crystallization. Science 1990, 250 (4983), 975–976. 10.1126/science.250.4983.975. [DOI] [PubMed] [Google Scholar]
- Cole J. C.; Yao J. W.; Shields G. P.; Motherwell W. D. S.; Allen F. H.; Howard J. A. K. Automatic Detection of Molecular Symmetry in the Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. 2001, 57 (1), 88–94. 10.1107/S010876810001380X. [DOI] [PubMed] [Google Scholar]
- Rekis T. Crystallization of Chiral Molecular Compounds: What Can Be Learned from the Cambridge Structural Database?. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2020, 76, 307–315. 10.1107/S2052520620003601. [DOI] [PubMed] [Google Scholar]
- Pidcock E. Achiral Molecules in Non-Centrosymmetric Space Groups. Chem. Commun. 2005, 27, 3457–3459. 10.1039/b505236j. [DOI] [PubMed] [Google Scholar]
- Grothe E.; Meekes H.; De Gelder R. Chirality and Stereoisomerism of Organic Multicomponent Crystals in the CSD. CrystEngComm 2020, 22 (43), 7380–7388. 10.1039/D0CE00403K. [DOI] [Google Scholar]
- Gourlay M. D.; Kendrick J.; Leusen F. J. J. Rationalization of Racemate Resolution: Predicting Spontaneous Resolution through Crystal Structure Prediction. Cryst. Growth Des. 2007, 7 (1), 56–63. 10.1021/cg060364o. [DOI] [Google Scholar]
- Kinbara K.; Hashimoto Y.; Sukegawa M.; Nohira H.; Saigo K. Crystal Structures of the Salts of Chiral Primary Amines with Achiral Carboxylic Acids: Recognition of the Commonly-Occurring Supramolecular Assemblies of Hydrogen-Bond Networks and Their Role in the Formation of Conglomerates. J. Am. Chem. Soc. 1996, 118 (14), 3441–3449. 10.1021/ja9539960. [DOI] [Google Scholar]
- Acosta L. M.; Palma A.; Bahsas A.; Cobo J.; Glidewell C. A Three-Dimensional Hydrogen-Bonded Framework in (2S*,4R*)-7- Fluoro-2-Exo-[(E)-Styr-Yl]-2,3,4,5-Tetra-Hydro-1H-1,4-Ep-Oxy-1-Benzazepine. Acta Cryst. C 2010, 66 (4), o206–o208. 10.1107/S010827011000884X. [DOI] [PubMed] [Google Scholar]
- Guerrero S. A.; Sanabría C. M.; Palma A.; Cobo J.; Glidewell C. Four Related Benzazepine Derivatives in a Reaction Pathway Leading to a Benzazepine Carboxylic Acid: Hydrogen-Bonded Assembly in Zero, One, Two and Three Dimensions. Acta Crystallogr. Sect. C Struct. Chem. 2014, 70 (4), 408–415. 10.1107/S2053229614006007. [DOI] [PubMed] [Google Scholar]
- Pérez-García L.; Amabilino D. B. Spontaneous Resolution, Whence and Whither: From Enantiomorphic Solids to Chiral Liquid Crystals, Monolayers and Macro- and Supra-Molecular Polymers and Assemblies. Chem. Soc. Rev. 2007, 36 (6), 941–967. 10.1039/B610714A. [DOI] [PubMed] [Google Scholar]
- Pérez-García L.; Amabilino D. B. Spontaneous Resolution under Supramolecular Control. Chem. Soc. Rev. 2002, 31 (6), 342–356. 10.1039/B201099M. [DOI] [PubMed] [Google Scholar]
- Flack H. D. Chiral and Achiral Crystal Structures. Helv. Chim. Acta 2003, 86 (4), 905–921. 10.1002/hlca.200390109. [DOI] [Google Scholar]
- Flack H. D. On Enantiomorph-Polarity Estimation. Acta Crystallogr. 1983, A39, 876–881. 10.1107/S0108767383001762. [DOI] [Google Scholar]
- Jacques J.; Collet A.; Wilen S. H.. Enantiomers, Racemates, and Resolutions; Wiley: New York, 1994. [Google Scholar]
- Matsuura T.; Koshima H. Introduction to Chiral Crystallization of Achiral Organic Compounds: Spontaneous Generation of Chirality. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6 (1), 7–24. 10.1016/j.jphotochemrev.2005.02.002. [DOI] [Google Scholar]
- McLaughlin D. T.; Nguyen T. P. T.; Mengnjo L.; Bian C.; Leung Y. H.; Goodfellow E.; Ramrup P.; Woo S.; Cuccia L. A. Viedma Ripening of Conglomerate Crystals of Achiral Molecules Monitored Using Solid-State Circular Dichroism. Cryst. Growth Des. 2014, 14 (3), 1067–1076. 10.1021/cg401577m. [DOI] [Google Scholar]
- Koshima H. Generation of Chirality in Two-Component Molecular Crystals from Achiral Molecules. J. Mol. Struct. 2000, 552 (1–3), 111–116. 10.1016/S0022-2860(00)00478-6. [DOI] [Google Scholar]
- Sivakumar R.; Askari M. S.; Woo S.; Madwar C.; Ottenwaelder X.; Bohle D. S.; Cuccia L. A. Homochiral Crystal Generation: Via Sequential Dehydration and Viedma Ripening. CrystEngComm 2016, 18 (23), 4277–4280. 10.1039/C6CE00119J. [DOI] [Google Scholar]
- Karpiuk J.; Gawryś P.; Karpiuk E.; Suwińska K. Electron Transfer across a Spiro Link: Extreme Solvatofluorochromism of a Compact Spiro-Bridged: N, N -Dimethylaniline-Phthalide Dyad. Chem. Commun. 2019, 55 (58), 8414–8417. 10.1039/C9CC02933H. [DOI] [PubMed] [Google Scholar]
- Kajiyama K.; Kojima S.; Akiba K. Y. Synthesis and Characterization of Intra- and Intermolecular Hydrogen Bonding Isomers of P-H (Apical) Phosphoranes Bearing a Hydroxyl Group and Their Thermal Cyclization. Tetrahedron Lett. 1996, 37 (46), 8409–8412. 10.1016/0040-4039(96)01923-5. [DOI] [Google Scholar]
- Hejda M.; Lyčka A.; Jambor R.; Růžička A.; Dostál L. From C,N- and N,N-Chelated Chloroboranes to Substituted 1H-2,1-Benzazaboroles and 1H-Pyrrolo[1,2-c][1,3,2]Diazaborolidines: A Straightforward Route to Five-Membered Rings Containing the B-N or N-B-N Moiety. Dalt. Trans. 2014, 43 (33), 12678–12688. 10.1039/C4DT01445F. [DOI] [PubMed] [Google Scholar]
- Zhi-Guang X.; Hai-Yang L.; Guo-Bang G.; Xuan X.; Yun-Xiu Z. Benzyl 2-Ethyl-Hexyl Sulfoxide. Acta Cryst. E 2009, 65 (11), o2929. 10.1107/S1600536809044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmes P.; Cowley M. J.; Hartmann M.; Zimmer M.; Huch V.; Scheschkewitz D. From Disilene (Si = Si) to Phosphasilene (Si = P) and Phosphacumulene (P=C=N). Angew. Chem., Int. Ed. 2014, 53 (8), 2216–2220. 10.1002/anie.201308525. [DOI] [PubMed] [Google Scholar]
- Annaka T.; Nakata N.; Ishii A. A Reversible and Turn-on Type Fluorescence Behaviour of Hydrogen Sulfide via a Redox Cycle between Selenoxide and Selenide. New J. Chem. 2019, 43 (29), 11643–11652. 10.1039/C9NJ02813G. [DOI] [Google Scholar]
- Custelcean R.; Ward M. D. Chiral Discrimination in Low-Density Hydrogen-Bonded Frameworks. Cryst. Growth Des. 2005, 5 (6), 2277–2287. 10.1021/cg050118x. [DOI] [Google Scholar]
- Dannecker-Dörig I.; Linden A.; Heimgartner H. Synthesis of Poly-Aib Oligopeptides and Aib-Containing Peptides via the “Azirine/Oxazolone Method”, and Their Crystal Structures. Helv. Chim. Acta 2011, 94 (6), 993–1011. 10.1002/hlca.201100116. [DOI] [Google Scholar]
- Sephton M. A.; Emerson C. R.; Zakharov L. N.; Blakemore P. R. Spontaneous Symmetry Breaking during Interrupted Crystallization of an Axially Chiral Amino Acid Derivative. Chem. Commun. 2010, 46 (12), 2094–2096. 10.1039/b922028c. [DOI] [PubMed] [Google Scholar]
- Schmidt R.; Oh J. H.; Sun Y. S.; Deppisch M.; Krause A. M.; Radacki K.; Braunschweig H.; Könemann M.; Erk P.; Bao Z.; Würthner F. High-Performance Air-Stable n-Channel Organic Thin Film Transistors Based on Halogenated Perylene Bisimide Semiconductors. J. Am. Chem. Soc. 2009, 131 (17), 6215–6228. 10.1021/ja901077a. [DOI] [PubMed] [Google Scholar]
- Ohkita M.; Lehn J. M.; Baum G.; Fenske D. Helicity Coding: Programmed Molecular Self-Organization of Achiral Nonbiological Strands into Multiturn Helical Superstructures: Synthesis and Characterization of Alternating Pyridine-Pyrimidine Oligomers. Chem.—Eur. J. 1999, 5 (12), 3471–3481. . [DOI] [Google Scholar]
- Xu X. S.; Li Z. W.; Zhang Y. J.; Peng X. S.; Wong H. N. C. Total Synthesis of (±)-Pallambins C and D. Chem. Commun. 2012, 48 (68), 8517–8519. 10.1039/c2cc34310j. [DOI] [PubMed] [Google Scholar]
- Moricz A.; Gassmann E.; Bienz S.; Hesse M. Synthesis of (±)-Pyrenolide B. Helv. Chim. Acta 1995, 78 (3), 663–669. 10.1002/hlca.19950780313. [DOI] [Google Scholar]
- Lan P.; Jackson C. J.; Banwell M. G.; Willis A. C. Synthesis of a D-Ring Isomer of Galanthamine via a Radical-Based Smiles Rearrangement Reaction. J. Org. Chem. 2014, 79 (14), 6759–6764. 10.1021/jo501255c. [DOI] [PubMed] [Google Scholar]
- Liu D.; Chen J.; Ai L.; Zhang H.; Liu J. Synthesis of the Putative Structure of (±)-Amarbellisine. Org. Lett. 2013, 15 (2), 410–413. 10.1021/ol3034093. [DOI] [PubMed] [Google Scholar]
- Robertson J.; Naud S. Synthesis of Spiroacetal Enol Ethers by Oxidative Activation of Furan Derivatives. Org. Lett. 2008, 10 (23), 5445–5448. 10.1021/ol802138t. [DOI] [PubMed] [Google Scholar]
- Wakchaure P. B.; Easwar S.; Puranik V. G.; Argade N. P. Facile Air-Oxidation of N-Homopiperonyl-5,6-Dimethoxyhomophthalimide: Simple and Efficient Access to Nuevamine. Tetrahedron 2008, 64 (8), 1786–1791. 10.1016/j.tet.2007.11.104. [DOI] [Google Scholar]
- Peng Y.; Luo Z. B.; Zhang J. J.; Luo L.; Wang Y. W. Collective Synthesis of Several 2,7′-Cyclolignans and Their Correlation by Chemical Transformations. Org. Biomol. Chem. 2013, 11 (43), 7574–7586. 10.1039/c3ob41672k. [DOI] [PubMed] [Google Scholar]
- Brinkworth C.; Rozek T.; Bowie J. H.; Skelton B. W.; White A. H. Angucyclinones Related to Ochromycinone. IV. The Structures and Reactions of Unusual Diels-Alder Adducts Formed from Maleic Anhydride and Racemic 5,5-Dimethyl-3-Vinylcyclohex-2-En-1-Ol. Aust. J. Chem. 2000, 53 (5), 403. 10.1071/CH00034. [DOI] [Google Scholar]
- Ji S.; Qiao X.; Li Z. W.; Wang Y. R.; Yu S. W.; Liang W. F.; Lin X. H.; Ye M. Enantiomeric 3-Arylcoumarins and 2-Arylcoumarones from the Roots of Glycyrrhiza Uralensis as Protein Tyrosine Phosphatase 1B (PTP1B) Inhibitors. RSC Adv. 2015, 5 (56), 45258–45265. 10.1039/C5RA06452J. [DOI] [Google Scholar]
- Liu W.; Li H.; Cai P. J.; Wang Z.; Yu Z. X.; Lei X. Scalable Total Synthesis of Rac-Jungermannenones B and C. Angew. Chem., Int. Ed. 2016, 55 (9), 3112–3116. 10.1002/anie.201511659. [DOI] [PubMed] [Google Scholar]
- Abe H.; Morishita T.; Yoshie T.; Long K.; Kobayashi T.; Ito H. The Total Synthesis of (±)-Naupliolide: A Tetracyclic Sesquiterpene Lactone. Angew. Chem., Int. Ed. 2016, 55 (11), 3795–3798. 10.1002/anie.201600055. [DOI] [PubMed] [Google Scholar]
- Zhai L.; Tian X.; Wang C.; Cui Q.; Li W.; Huang S. H.; Yu Z. X.; Hong R. Construction of Morphan Derivatives by Nitroso–Ene Cyclization: Mechanistic Insight and Total Synthesis of (±)-Kopsone. Angew. Chem., Int. Ed. 2017, 56 (38), 11599–11603. 10.1002/anie.201706018. [DOI] [PubMed] [Google Scholar]
- Roe S. J.; Hughes D. L.; Aggarwal P.; Stockman R. A. Investigation of a Unified Strategy for the Synthesis of Anatoxin Analogues: Scope and Limitations. Synthesis 2009, 22, 3775–3784. 10.1055/s-0029-1217021. [DOI] [Google Scholar]
- Hegedus L. S.; McGuire M. A.; Schultze L. M.; Chen Y.; Anderson O. P. Reaction of Chromium Carbene Complexes with Imines. Synthesis of β-Lactams. J. Am. Chem. Soc. 1984, 106 (9), 2680–2687. 10.1021/ja00321a032. [DOI] [Google Scholar]
- Low Y. Y.; Gan C. Y.; Kam T. S. Andransinine: An Unusual Case of Spontaneous Resolution in an Indole Alkaloid Derivative. J. Nat. Prod. 2014, 77 (6), 1532–1535. 10.1021/np500289t. [DOI] [PubMed] [Google Scholar]
- Quesada E.; Stockley M.; Ragot J. P.; Prime M. E.; Whitwood A. C.; Taylor R. J. K. A Versatile, Non-Biomimetic Route to the Preussomerins: Syntheses of (±)-Preussomerins F, K and L. Org. Biomol. Chem. 2004, 2 (17), 2483–2495. 10.1039/b407895k. [DOI] [PubMed] [Google Scholar]
- Chen J. Q.; Mi Y.; Shi Z. F.; Cao X. P. Construction of the Tetracyclic Core of (±)-Cycloclavine and 4-Amino Uhle’s Ketone. Org. Biomol. Chem. 2018, 16 (20), 3801–3808. 10.1039/C7OB03067C. [DOI] [PubMed] [Google Scholar]
- Paquette L. A.; Schaefer A. G.; Springer J. P. Synthesis of (±)-14-Epiupial by Manganese(III) -γ-Lactone Annulation. Tetrahedron 1987, 43 (23), 5567–5582. 10.1016/S0040-4020(01)87738-3. [DOI] [Google Scholar]
- Sun Y.; Yu B.; Wang X.; Tang S.; She X.; Pan X. Stereoselective Syntheses of Four Diastereomers of 3,9,12- Trihydroxycalamenene via a Benzobicyclo[3.3.1] Intermediate. J. Org. Chem. 2010, 75 (12), 4224–4229. 10.1021/jo1008349. [DOI] [PubMed] [Google Scholar]
- Kerr W. J.; McLaughlin M.; Morrison A. J.; Pauson P. L. Formal Total Synthesis of (±)-α- and β-Cedrene by Preparation of Cedrone. Construction of the Tricyclic Carbon Skeleton by the Use of a Highly Efficient Intramolecular Khand Annulation. Org. Lett. 2001, 3 (19), 2945–2947. 10.1021/ol016054a. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R.; Kerr W. J.; McLaughlin M.; Pauson P. L. Key Tricyclic Synthetic Intermediates for the Preparation of the Sesquiter-Penes α- and β-Cedrene. Acta Cryst. C 2001, 57 (11), 1316–1318. 10.1107/S0108270101013336. [DOI] [PubMed] [Google Scholar]
- Yoshida K.; Nakajima S.; Ban Y.; Shibasaki M.; Ohnuma T.; Aoe K.; Date T. Synthetic Approaches Toward Mitomycins: Construction of p-Quinone Moiety on 1-Benzazocine Derivative. J. Org. Chem. 1988, 53 (22), 5355–5359. 10.1021/jo00257a031. [DOI] [Google Scholar]
- Suzuki H.; Aoyagi S. Total Synthesis of (±)-Chamobtusin A. Chem. Commun. 2011, 47 (27), 7878–7879. 10.1039/c1cc12267c. [DOI] [PubMed] [Google Scholar]
- Bachi M. D.; Bar-Ner N.; Melman A. Stereoselective Synthesis of (±)-α-Kainic Acid Using Free Radical Key Reactions. J. Org. Chem. 1996, 61 (20), 7116–7124. 10.1021/jo9607875. [DOI] [PubMed] [Google Scholar]
- Sudhakar G.; Bayya S.; Kadam V. D.; Nanubolu J. B. Total Synthesis of Gonytolides C and G, Lachnone C, and Formal Synthesis of Blennolide C and Diversonol. Org. Biomol. Chem. 2014, 12 (30), 5601–5610. 10.1039/C4OB00950A. [DOI] [PubMed] [Google Scholar]
- Kalshetti M. G.; Argade N. P. Diastereoselective Synthesis of (±)- Epi-Subincanadine C. ACS Omega 2018, 3 (5), 5308–5316. 10.1021/acsomega.8b00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmark S. E.; Cottell J. J. A Tandem, Nitroalkene Conjugate Addition/[3 + 2] Cycloaddition Approach to the Synthesis of the Pentacyclic Core of (±)-Scandine. Adv. Synth. Catal. 2006, 348 (16–17), 2397–2402. 10.1002/adsc.200600301. [DOI] [Google Scholar]
- Bates R. W.; Sridhar S. A Synthesis of (±)-Stemoamide Using the Intramolecular Propargylic Barbier Reaction. Synlett 2009, 2009 (12), 1979–1981. 10.1055/s-0029-1217540. [DOI] [Google Scholar]
- Wang Z.; Chen L.; Yao Y.; Liu Z.; Gao J. M.; She X.; Zheng H. Dearomatization of Indole via Intramolecular [3 + 2] Cycloaddition: Access to the Pentacyclic Skeleton of Strychons Alkaloids. Org. Lett. 2018, 20 (15), 4439–4443. 10.1021/acs.orglett.8b01720. [DOI] [PubMed] [Google Scholar]
- Hartrampf N.; Winter N.; Pupo G.; Stoltz B. M.; Trauner D. Total Synthesis of the Norhasubanan Alkaloid Stephadiamine. J. Am. Chem. Soc. 2018, 140 (28), 8675–8680. 10.1021/jacs.8b01918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou K. C.; Ortiz A.; Zhang H.; Guella G. Total Synthesis and Structural Revision of Vannusals A and B: Synthesis of the True Structures of Vannusals A and B. J. Am. Chem. Soc. 2010, 132 (20), 7153–7176. 10.1021/ja100742b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. J.; Hong W. P.; Hsu L. Y. Total Synthesis of (±)-Lythrancepine II and (±)-Lythrancepine III. J. Org. Chem. 1987, 52 (21), 4665–4673. 10.1021/jo00230a003. [DOI] [Google Scholar]
- Cheng H.; Zeng F. H.; Yang X.; Meng Y. J.; Xu L.; Wang F. P. Collective Total Syntheses of Atisane-Type Diterpenes and Atisine-Type Diterpenoid Alkaloids: (±)-Spiramilactone B, (±)-Spiraminol, (±)-Dihydroajaconine, and (±)-Spiramines C and D. Angew. Chem., Int. Ed. 2016, 55 (1), 392–396. 10.1002/anie.201508996. [DOI] [PubMed] [Google Scholar]
- Ando S.; Kikuchi J.; Fujimura Y.; Ida Y.; Higashi K.; Moribe K.; Yamamoto K. Physicochemical Characterization and Structural Evaluation of a Specific 2:1 Cocrystal of Naproxen–Nicotinamide. J. Pharm. Sci. 2012, 101 (9), 3214–3221. 10.1002/jps.23158. [DOI] [PubMed] [Google Scholar]
- Neurohr C.; Marchivie M.; Lecomte S.; Cartigny Y.; Couvrat N.; Sanselme M.; Subra-Paternault P. Naproxen-Nicotinamide Cocrystals: Racemic and Conglomerate Structures Generated by CO2 Antisolvent Crystallization. Cryst. Growth Des. 2015, 15 (9), 4616–4626. 10.1021/acs.cgd.5b00876. [DOI] [Google Scholar]
- Shemchuk O.; Song L.; Tumanov N.; Wouters J.; Braga D.; Grepioni F.; Leyssens T. Chiral Resolution of RS-Oxiracetam upon Cocrystallization with Pharmaceutically Acceptable Inorganic Salts. Cryst. Growth Des. 2020, 20 (4), 2602–2607. 10.1021/acs.cgd.9b01725. [DOI] [Google Scholar]
- Surov A. O.; Solanko K. A.; Bond A. D.; Bauer-Brandl A.; Perlovich G. L. Diversity of Felodipine Solvates: Structure and Physicochemical Properties. CrystEngComm 2015, 17 (22), 4089–4097. 10.1039/C5CE00350D. [DOI] [Google Scholar]
- George F.; Norberg B.; Robeyns K.; Wouters J.; Leyssens T. Peculiar Case of Levetiracetam and Etiracetam α-Ketoglutaric Acid Cocrystals: Obtaining a Stable Conglomerate of Etiracetam. Cryst. Growth Des. 2016, 16 (9), 5273–5282. 10.1021/acs.cgd.6b00819. [DOI] [Google Scholar]
- Feng Q.; Wang M.; Dong B.; Xu C.; Zhao J.; Zhang H. Tuning Solid-State Fluorescence of Pyrene Derivatives via a Cocrystal Strategy. CrystEngComm 2013, 15 (18), 3623–3629. 10.1039/c3ce27102a. [DOI] [Google Scholar]
- Kavuru P.; Aboarayes D.; Arora K. K.; Clarke H. D.; Kennedy A.; Marshall L.; Ong T. T.; Perman J.; Pujari T.; Wojtas Ł.; Zaworotko M. J. Hierarchy of Supramolecular Synthons: Persistent Hydrogen Bonds between Carboxylates and Weakly Acidic Hydroxyl Moieties in Cocrystals of Zwitterions. Cryst. Growth Des. 2010, 10 (8), 3568–3584. 10.1021/cg100484a. [DOI] [Google Scholar]
- Davey R. J.; Sadiq G.; Back K.; Wilkinson L.; Seaton C. C. The Isolation of a Metastable Conglomerate Using a Combined Computational and Controlled Crystallization Approach. Chem. Commun. 2012, 48 (14), 1976–1978. 10.1039/C1CC16173C. [DOI] [PubMed] [Google Scholar]
- Kavitha C. V.; Lakshmi S.; Basappa; Mantelingu K.; Sridhar M. A.; Shashidhara Prasad J.; Rangappa K. S. Synthesis and Molecular Structure Analysis of Venlafaxine Intermediate and Its Analog. J. Chem. Crystallogr. 2005, 35 (12), 957–963. 10.1007/s10870-005-5249-y. [DOI] [Google Scholar]
- Ahmed F. R. Three Crystal Structures of 1β-(p -Methoxybenzyl)-9 α,10β-Dihydroxydecahydroisoquinoline and 1β-(p -Methoxybenzyl)-9β,10α-Dihydroxydecahydroisoquinoline, C 17 H 25 NO 3. Acta Cryst. B 1978, 34 (8), 2589–2594. 10.1107/S056774087800864X. [DOI] [Google Scholar]
- Bredikhin A. A.; Zakharychev D. V.; Gubaidullin A. T.; Samigullina A. I.; Bredikhina Z. A. Crystal Landscape of Chiral Drug Chlorphenesin and Its Structural Analogues: Polymorphism of Racemic and Enantiopure Samples, Metastable and Stable Racemic Conglomerates, Diverse in Unity Crystal Motifs. Cryst. Growth Des. 2021, 21 (6), 3211–3224. 10.1021/acs.cgd.0c01570. [DOI] [Google Scholar]
- Kozikowski A. P.; Araldi G. L.; Ball R. G. Dipolar Cycloaddition Route to Diverse Analogues of Cocaine: The 6- and 7-Substituted 3-Phenyltropanes. J. Org. Chem. 1997, 62 (3), 503–509. 10.1021/jo961957g. [DOI] [PubMed] [Google Scholar]
- Takai T.; Koike T.; Nakamura M.; Kajita Y.; Yamashita T.; Taya N.; Tsukamoto T.; Watanabe T.; Murakami K.; Igari T.; Kamata M. Discovery of Novel 5,6,7,8-Tetrahydro[1,2,4]Triazolo[4,3-a]Pyridine Derivatives as γ-Secretase Modulators (Part 2). Bioorg. Med. Chem. 2016, 24 (14), 3192–3206. 10.1016/j.bmc.2016.05.040. [DOI] [PubMed] [Google Scholar]
- Bredikhin A. A.; Gubaidullin A. T.; Bredikhina Z. A.; Krivolapov D. B.; Pashagin A. V.; Litvinov I. A. Absolute Configuration and Crystal Packing for Three Chiral Drugs Prone to Spontaneous Resolution: Guaifenesin, Methocarbamol and Mephenesin. J. Mol. Struct. 2009, 920 (1–3), 377–382. 10.1016/j.molstruc.2008.11.037. [DOI] [Google Scholar]
- Yan X. Q.; Wang Z. C.; Qi P. fei; Li G.; Zhu H. L. Design, Synthesis and Biological Evaluation of 2-H Pyrazole Derivatives Containing Morpholine Moieties as Highly Potent Small Molecule Inhibitors of APC–Asef Interaction. Eur. J. Med. Chem. 2019, 425–447. 10.1016/j.ejmech.2019.05.056. [DOI] [PubMed] [Google Scholar]
- Bonacorso H. G.; Cavinatto S.; Campos P. T.; Porte L. M. F.; Navarini J.; Paim G. R.; Martins M. A. P.; Zanatta N.; Stuker C. Z. New Trifluoromethyl-Containing (E)-N′-Arylidene-[3-Alkyl(Aryl/ Heteroaryl)-4,5-Dihydro-1H-Pyrazol-1-Yl]Carbohydrazides: Synthesis, Crystal Structure and Antimicrobial/Antioxidant Activity. J. Fluor. Chem. 2012, 135, 303–314. 10.1016/j.jfluchem.2011.12.010. [DOI] [Google Scholar]
- Abdel-Wahab B. F.; Abdel-Latif E.; Mohamed H. A.; Awad G. E. A. Design and Synthesis of New 4-Pyrazolin-3-Yl-1,2,3-Triazoles and 1,2,3-Triazol-4-Yl-Pyrazolin-1-Ylthiazoles as Potential Antimicrobial Agents. Eur. J. Med. Chem. 2012, 52, 263–268. 10.1016/j.ejmech.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab B. F.; Abdel-Latif E.; Mohamed H. A.; Awad G. E. A. Design and Synthesis of New 4-Pyrazolin-3-Yl-1,2,3-Triazoles and 1,2,3-Triazol-4-Yl-Pyrazolin-1-Ylthiazoles as Potential Antimicrobial Agents. Eur. J. Med. Chem. 2012, 52, 263–268. 10.1016/j.ejmech.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab B. F.; Mohamed H. A.; Ng S. W.; Tiekink E. R. T. 4-{1-[4-(4-Bromophenyl)-1,3-Thiazol-2-Yl]-5-(4-Fluorophenyl)-4, 5-Dihydro-1H-Pyrazol-3-Yl}-5-Methyl-1-(4-Methylphenyl)-1H-1,2,3-Triazole. Acta Cryst. E 2012, 68 (6), o1956–o1957. 10.1107/S1600536812024257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamski A.; Kruszka D.; Dutkiewicz Z.; Kubicki M.; Gorczyński A.; Patroniak V. Novel Family of Fused Tricyclic [1,4]Diazepines: Design, Synthesis, Crystal Structures and Molecular Docking Studies. Tetrahedron 2017, 73 (24), 3377–3386. 10.1016/j.tet.2017.05.015. [DOI] [Google Scholar]
- Roche E. B.; Nagel D. L.; McPhail A. T. Studies in the Synthesis of C Ring Bridged Morphinans. 2.1 The Synthesis and Structural Verification of a Novel 3, Llc-Ethano-10-Hydroxy-6-Methyl-l, 2, 3, 3a, Llb, Llc-Hexahydroaporphine. J. Org. Chem. 1984, 49 (21), 3881–3887. 10.1021/jo00195a002. [DOI] [Google Scholar]
- Parrish J. P.; Trzupek J. D.; Hughes T. V.; Hwang I.; Boger D. L. Synthesis and Evaluation of N-Aryl and N-Alkenyl CBI Derivatives. Bioorg. Med. Chem. 2004, 12 (22), 5845–5856. 10.1016/j.bmc.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Jardim G. A. M.; Cruz E. H. G.; Valença W. O.; Resende J. M.; Rodrigues B. L.; Ramos D. F.; Oliveira R. N.; Silva P. E. A.; Da Silva Júnior E. N. On the Search for Potential Antimycobacterial Drugs: Synthesis of Naphthoquinoidal, Phenazinic and 1,2,3-Triazolic Compounds and Evaluation against Mycobacterium Tuberculosis. J. Braz. Chem. Soc. 2015, 26 (5), 1013–1027. 10.5935/0103-5053.20150067. [DOI] [Google Scholar]
- Zhang C.; Matzger A. J. A Newly Discovered Racemic Compound of Pioglitazone Hydrochloride Is More Stable than the Commercial Conglomerate. Cryst. Growth Des. 2017, 17 (2), 414–417. 10.1021/acs.cgd.6b01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yathirajan H. S.; Nagaraj B.; Nagaraja P.; Bolte M. Pioglitazone Hydrochloride. Acta Cryst. E 2005, 61 (1), o154–o155. 10.1107/S1600536804033100. [DOI] [Google Scholar]
- Bredikhin A. A.; Zakharychev D. V.; Bredikhina Z. A.; Gubaidullin A. T.; Fayzullin R. R. Crystal Structure and Phase Behavior of the Tolyl Glycerol Ethers. from the Conglomerate Former to the Chirality-Driven Nanogelator. CrystEngComm 2012, 14 (1), 211–222. 10.1039/C1CE05637A. [DOI] [Google Scholar]
- Santini A.; Benedetti E.; Pedone C.; Caliendo G.; Santagada V.; Grieco P.; Perissutti E. Molecular Structures of Quinuclidinic Neurokinin Antagonists: 2-(2-Phenylbenzylidene)-3-(2-X-Benzylamino) Derivatives. Struct. Chem. 1996, 7 (3), 173–181. 10.1007/BF02281228. [DOI] [Google Scholar]
- Van Eupen J. T. H.; Elffrink W. W. J.; Keltjens R.; Bennema P.; De Gelder R.; Smits J. M. M.; Van Eck E. R. H.; Kentgens A. P. M.; Deij M. A.; Meekes H.; Vlieg E. Polymorphism and Migratory Chiral Resolution of the Free Base of Venlafaxine. A Remarkable Topotactical Solid State Transition from a Racemate to a Racemic Conglomerate. Cryst. Growth Des. 2008, 8 (1), 71–79. 10.1021/cg700831z. [DOI] [Google Scholar]
- Kostyanovsky R. G.; Lyssenko K. A.; Kravchenko A. N.; Lebedev O. V.; Kadorkina G. K.; Kostyanovsky V. R. Crystal Properties of N-Alkyl-Substituted Glycolurils as the Precursors of Chiral Drugs. Mendeleev Commun. 2001, 11 (4), 134–136. 10.1070/MC2001v011n04ABEH001469. [DOI] [Google Scholar]
- Xiao T.; Lin Y.; Zhang X. Q.; Chen J.; Wang J. T. (E)-2-[4-(2-Chlorophenyl)-1,3-Dithiolan-2-Ylidene]-2-(Imidazol-1-Yl) Acetonitrile. Acta Cryst. E 2006, 62 (11), o5052–o5053. 10.1107/S1600536806040736. [DOI] [Google Scholar]
- Karmazin L.; Mazzanti M.; Bezombes J. P.; Gateau C.; Pécaut J. Comparative Structural Studies of Iodide Complexes of Uranium(III) and Lanthanide(III) with Hexadentate Tetrapodal Neutral N-Donor Ligands. Inorg. Chem. 2004, 43 (16), 5147–5158. 10.1021/ic049538m. [DOI] [PubMed] [Google Scholar]
- Jones N. D.; Meessen P.; Smith M. B.; Losehand U.; Rettig S. J.; Patrick B. O.; James B. R. Bisphosphine Ligands Containing Two O-N,N-Dimethylanilinyl Substituents at Each Phosphorus Atom. Can. J. Chem. 2002, 80 (11), 1600–1606. 10.1139/v02-127. [DOI] [Google Scholar]
- Lowry R. J.; Veige M. K.; Clément O.; Abboud K. A.; Ghiviriga I.; Veige A. S. New Constrained-Geometry C2-Symmetric Di-N-Heterocyclic Carbene Ligands and Their Mono- and Dinuclear Rhodium(I) Complexes: Design, Synthesis, and Structural Analysis. Organometallics 2008, 27 (20), 5184–5195. 10.1021/om800471m. [DOI] [Google Scholar]
- Howard R. H.; Theobald N.; Bochmann M.; Wright J. A. 1-[2-(2,6-Diisopropyl-Anilino)-1-Naph-Th-Yl]Isoquinoline. Acta Cryst. C 2010, 66 (6), o310–o312. 10.1107/S0108270110018433. [DOI] [PubMed] [Google Scholar]
- Nienkemper K.; Kehr G.; Kehr S.; Fröhlich R.; Erker G. Amidomethyl)Pyridine Zirconium and Hafnium Complexes: Synthesis and Structural Characterization. J. Organomet. Chem. 2008, 693 (8–9), 1572–1589. 10.1016/j.jorganchem.2007.12.004. [DOI] [Google Scholar]
- Black R. S.; Billing D. G.; Bartyzel A.; Cukrowska E. M. N,N′-Bis[(2-Hydroxy-Phen-Yl)(Phen-Yl)Methyl-Idene]Propane-1,2-Diam Ine. Acta Cryst. E 2010, 66 (6), o1256–o1257. 10.1107/S1600536810015291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V. T.; Chan I. Y. H.; Bishop R.; Craig D. C.; Scudder M. L. Crystallisation of C2-Symmetric Endo,Endo-Bicyclo[3.3.1]Nonane- 2–6-Diols: Supramolecular Synthons and Concomitant Degrees of Enantiomer Separation. New J. Chem. 2009, 33 (8), 1736–1741. 10.1039/b900463g. [DOI] [Google Scholar]
- Merz A.; Gromann L.; Karl A.; Parkanyi L.; Schneider O. Conformers and Rotamers of (±)-Trans-2,3-Bis(2-Naphthyl)-15-Crown-5 and −18-Crown-6 and Their Alkali Metal Complexes. Eur. J. Org. Chem. 1998, 1998, 403–408. . [DOI] [Google Scholar]
- Gałdecki Z.; Bartczak T. J.; Wolf W. M.; Krawczyk H.; Majewski P. Structure of 1,2,5-Trihydroxy-2,5-Dimethylphospholane 1-Oxide, C6H13O4P. Acta Cryst. C 1985, 41 (5), 732–734. 10.1107/S0108270185005297. [DOI] [Google Scholar]
- Bosc J. J.; Jarry C.; Léger J. M.; Carpy A. NMR and Crystallographic Evidence for Polymorphism of the N-Phenyl-N′-[1-(3-(Phenyl-4-Piperazinyl)Propan-2-Ol)]Urea. J. Chem. Crystallogr. 1996, 26 (12), 807–814. 10.1007/BF01670313. [DOI] [Google Scholar]
- Choi H.; Shim Y. S.; Lee S. C.; Kang S. K.; Sung C. K. 1-(2-Hydroxy-2-Phenylethyl)-3-(4-Meth-Oxyphenyl)Urea. Acta Cryst. E 2011, 67 (10), o2632–o2632. 10.1107/S1600536811036464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei P.; Amanpour T.; Naderi S.; Soorki A. A. Nef-Isocyanide -Based One-Pot Two-Step Three Component Dihydrobenzo[4,5]Imidazo[2,1-b]Thiazoles Synthesis. J. Heterocycl. Chem. 2016, 53 (6), 1783–1786. 10.1002/jhet.2484. [DOI] [Google Scholar]
- Chmielewski M. K.; Tykarska E.; Markiewicz W. T.; Rypniewski W. Engineering N-(2-Pyridyl)Aminoethyl Alcohols as Potential Precursors of Thermolabile Protecting Groups. New J. Chem. 2012, 36 (3), 603–612. 10.1039/C1NJ20584F. [DOI] [Google Scholar]
- Shih Y. C.; Tsai P. H.; Hsu C. C.; Chang C. W.; Jhong Y.; Chen Y. C.; Chien T. C. Biomimetic Approach toward the Total Synthesis of Rac-2-(Acylmethylene)Pyrrolidine Alkaloids. J. Org. Chem. 2015, 80 (13), 6669–6678. 10.1021/acs.joc.5b00836. [DOI] [PubMed] [Google Scholar]
- Yagishita F.; Ishikawa H.; Onuki T.; Hachiya S.; Mino T.; Sakamoto M. Total Spontaneous Resolution by Deracemization of Isoindolinones. Angew. Chem., Int. Ed. 2012, 51 (52), 13023–13025. 10.1002/anie.201205097. [DOI] [PubMed] [Google Scholar]
- Noorduin W. L.; Izumi T.; Millemaggi A.; Leeman M.; Meekes H.; Van Enckevort W. J. P.; Kellogg R. M.; Kaptein B.; Vlieg E.; Blackmond D. G. Emergence of a Single Solid Chiral State from a Nearly Racemic Amino Acid Derivative. J. Am. Chem. Soc. 2008, 130 (4), 1158–1159. 10.1021/ja7106349. [DOI] [PubMed] [Google Scholar]
- Van Der Meijden M. W.; Leeman M.; Gelens E.; Noorduin W. L.; Meekes H.; Van Enckevort W. J. P.; Kaptein B.; Vlieg E.; Kellogg R. M. Attrition-Enhanced Deracemization in the Synthesis of Clopidogrel - A Practical Application of a New Discovery. Org. Process Res. Dev. 2009, 13 (6), 1195–1198. 10.1021/op900243c. [DOI] [Google Scholar]
- Uemura N.; Hosaka M.; Washio A.; Yoshida Y.; Mino T.; Sakamoto M. Chiral Symmetry Breaking of Thiohydantoins by Attrition-Enhanced Deracemization. Cryst. Growth Des. 2020, 20 (8), 4898–4903. 10.1021/acs.cgd.0c00829. [DOI] [Google Scholar]
- Noorduin W. L.; Kaptein B.; Meekes H.; Van Enckevort W. J. P.; Kellogg R. M.; Vlieg E. Fast Attrition-Enhanced Deracemization of Naproxen by a Gradual in Situ Feed. Angew. Chem., Int. Ed. 2009, 48 (25), 4581–4583. 10.1002/anie.200901386. [DOI] [PubMed] [Google Scholar]
- Baglai I.; Leeman M.; Kellogg R. M.; Noorduin W. L. A Viedma Ripening Route to an Enantiopure Building Block for Levetiracetam and Brivaracetam. Org. Biomol. Chem. 2019, 17 (1), 35–38. 10.1039/C8OB02660B. [DOI] [PubMed] [Google Scholar]
- Engwerda A. H. J.; Mertens J. C. J.; Tinnemans P.; Meekes H.; Rutjes F. P. J. T.; Vlieg E. Solid-Phase Conversion of Four Stereoisomers into a Single Enantiomer. Angew. Chem., Int. Ed. 2018, 57, 15441–15444. 10.1002/anie.201808913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washio A.; Hosaka M.; Uemura N.; Yoshida Y.; Mino T.; Kasashima Y.; Sakamoto M. Asymmetric Anisoin Synthesis Involving Benzoin Condensation Followed by Deracemization. Cryst. Growth Des. 2021, 21 (4), 2423–2428. 10.1021/acs.cgd.1c00036. [DOI] [Google Scholar]
- Baglai I.; Leeman M.; Wurst K.; Kaptein B.; Kellogg R. M.; Noorduin W. L. The Strecker Reaction Coupled to Viedma Ripening: A Simple Route to Highly Hindered Enantiomerically Pure Amino Acids. Chem. Commun. 2018, 54 (77), 10832–10834. 10.1039/C8CC06658B. [DOI] [PubMed] [Google Scholar]
- Sanada K.; Washio A.; Nishihata K.; Yagishita F.; Yoshida Y.; Mino T.; Suzuki S.; Kasashima Y.; Sakamoto M. Chiral Symmetry Breaking of Racemic 3-Phenylsuccinimides via Crystallization-Induced Dynamic Deracemization. Cryst. Growth Des. 2021, 21 (11), 6051–6055. 10.1021/acs.cgd.1c01010. [DOI] [Google Scholar]
- Levilain G.; Rougeot C.; Guillen F.; Plaquevent J. C.; Coquerel G. Attrition-Enhanced Preferential Crystallization Combined with Racemization Leading to Redissolution of the Antipode Nuclei. Tetrahedron Asymmetry 2009, 20 (24), 2769–2771. 10.1016/j.tetasy.2009.11.015. [DOI] [Google Scholar]
- Leeman M.; Noorduin W. L.; Millemaggi A.; Vlieg E.; Meekes H.; Van Enckevort W. J. P.; Kaptein B.; Kellogg R. M. Efficient Havinga-Kondepudi Resolution of Conglomerate Amino Acid Derivatives by Slow Cooling and Abrasive Grinding. CrystEngComm 2010, 12 (7), 2051–2053. 10.1039/c0ce00140f. [DOI] [Google Scholar]
- Engwerda A. H. J.; Maassen R.; Tinnemans P.; Meekes H.; Rutjes F. P. J. T.; Vlieg E. Attrition-Enhanced Deracemization of the Antimalaria Drug Mefloquine. Angew. Chem., Int. Ed. 2019, 58 (6), 1670–1673. 10.1002/anie.201811289. [DOI] [PubMed] [Google Scholar]
- Tsogoeva S. B.; Wei S.; Freund M.; Mauksch M. Generation of Highly Enantioenriched Crystalline Products in Reversible Asymmetric Reactions with Racemic or Achiral Catalysts. Angew. Chem., Int. Ed. 2009, 48 (3), 590–594. 10.1002/anie.200803877. [DOI] [PubMed] [Google Scholar]
- Flock A. M.; Reucher C. M. M.; Bolm C. Enantioenrichment by Iterative Retro-Aldol/Aldol Reaction Catalyzed by an Achiral or Racemic Base. Chem.—Eur. J. 2010, 16 (13), 3918–3921. 10.1002/chem.200903497. [DOI] [PubMed] [Google Scholar]
- Shimizu W.; Uemura N.; Yoshida Y.; Mino T.; Kasashima Y.; Sakamoto M. Attrition-Enhanced Deracemization and Absolute Asymmetric Synthesis of Flavanones from Prochiral Precursors. Cryst. Growth Des. 2020, 20 (9), 5676–5681. 10.1021/acs.cgd.0c00955. [DOI] [Google Scholar]
- Uemura N.; Toyoda S.; Ishikawa H.; Yoshida Y.; Mino T.; Kasashima Y.; Sakamoto M. Asymmetric Diels-Alder Reaction Involving Dynamic Enantioselective Crystallization. J. Org. Chem. 2018, 83 (16), 9300–9304. 10.1021/acs.joc.8b01273. [DOI] [PubMed] [Google Scholar]
- Uemura N.; Toyoda S.; Shimizu W.; Yoshida Y.; Mino T.; Sakamoto M. Absolute Asymmetric Synthesis Involving Chiral Symmetry Breaking in Diels–Alder Reaction. Symmetry 2020, 12 (6), 910. 10.3390/sym12060910. [DOI] [Google Scholar]
- Engwerda A. H. J.; Koning N.; Tinnemans P.; Meekes H.; Bickelhaupt F. M.; Rutjes F. P. J. T.; Vlieg E. Deracemization of a Racemic Allylic Sulfoxide Using Viedma Ripening. Cryst. Growth Des. 2017, 17 (8), 4454–4457. 10.1021/acs.cgd.7b00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engwerda A. H. J.; Van Schayik P.; Jagtenberg H.; Meekes H.; Rutjes F. P. J. T.; Vlieg E. Solid Phase Deracemization of an Atropisomer. Cryst. Growth Des. 2017, 17 (10), 5583–5585. 10.1021/acs.cgd.7b01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spix L.; Meekes H.; Blaauw R. H.; Van Enckevort W. J. P.; Vlieg E. Complete Deracemization of Proteinogenic Glutamic Acid Using Viedma Ripening on a Metastable Conglomerate. Cryst. Growth Des. 2012, 12 (11), 5796–5799. 10.1021/cg301343a. [DOI] [Google Scholar]
- Spix L.; Alfring A.; Meekes H.; Van Enckevort W. J. P.; Vlieg E. Formation of a Salt Enables Complete Deracemization of a Racemic Compound through Viedma Ripening. Cryst. Growth Des. 2014, 14 (4), 1744–1748. 10.1021/cg4018882. [DOI] [Google Scholar]
- Viedma C.; Ortiz J. E.; De Torres T.; Izumi T.; Blackmond D. G. Evolution of Solid Phase Homochirality for a Proteinogenic Amino Acid. J. Am. Chem. Soc. 2008, 130 (46), 15274–15275. 10.1021/ja8074506. [DOI] [PubMed] [Google Scholar]
- Belletti G.; Tortora C.; Mellema I. D.; Tinnemans P.; Meekes H.; Rutjes F. P. J. T.; Tsogoeva S. B.; Vlieg E. Photoracemization-Based Viedma Ripening of a BINOL Derivative. Chem.—Eur. J. 2020, 26 (4), 839–844. 10.1002/chem.201904382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondepudi D. K.; Laudadio J.; Asakura K. Chiral Symmetry Breaking in Stirred Crystallization of 1,1′-Binaphthyl Melt. J. Am. Chem. Soc. 1999, 121 (7), 1448–1451. 10.1021/ja983418u. [DOI] [Google Scholar]
- Steendam R. R. E.; Brouwer M. C. T.; Huijs E. M. E.; Kulka M. W.; Meekes H.; Van Enckevort W. J. P.; Raap J.; Rutjes F. P. J. T.; Vlieg E. Enantiopure Isoindolinones through Viedma Ripening. Chem.—Eur. J. 2014, 20 (42), 13527–13530. 10.1002/chem.201404320. [DOI] [PubMed] [Google Scholar]
- Xiouras C.; Ter Horst J. H.; Van Gerven T.; Stefanidis G. D. Coupling Viedma Ripening with Racemic Crystal Transformations: Mechanism of Deracemization. Cryst. Growth Des. 2017, 17 (9), 4965–4976. 10.1021/acs.cgd.7b00908. [DOI] [Google Scholar]
- Hein J. E.; Huynh Cao B.; Viedma C.; Kellogg R. M.; Blackmond D. G. Pasteur’s Tweezers Revisited: On the Mechanism of Attrition-Enhanced Deracemization and Resolution of Chiral Conglomerate Solids. J. Am. Chem. Soc. 2012, 134 (30), 12629–12636. 10.1021/ja303566g. [DOI] [PubMed] [Google Scholar]
- Rougeot C.; Guillen F.; Plaquevent J. C.; Coquerel G. Ultrasound-Enhanced Deracemization: Toward the Existence of Agonist Effects in the Interpretation of Spontaneous Symmetry Breaking. Cryst. Growth Des. 2015, 15 (5), 2151–2155. 10.1021/cg501765g. [DOI] [Google Scholar]
- Küenburg B.; Czollner L.; Frôhlich J.; Jordis U. Development of a Pilot Scale Process for the Anti-Alzheimer Drug (−)-Galanthamine Using Large-Scale Phenolic Oxidative Coupling and Crystallisation-Induced Chiral Conversion. Org. Process Res. Dev. 1999, 3 (6), 425–431. 10.1021/op990019q. [DOI] [Google Scholar]
- Sakamoto M.; Uemura N.; Saito R.; Shimobayashi H.; Yoshida Y.; Mino T.; Omatsu T. Chirogenesis and Amplification of Molecular Chirality Using Optical Vortices. Angew. Chem., Int. Ed. 2021, 60 (23), 12819–12823. 10.1002/anie.202103382. [DOI] [PubMed] [Google Scholar]
- Weber E.; Meinhold D.; Haase R.; Seichter W.; Rheinwald G. Inclusion Compounds of Bulky Binaphthyl-Type Bis-Fluorenol Hosts. Supramol. Chem. 2005, 17 (4), 303–314. 10.1080/10610270500076245. [DOI] [Google Scholar]
- Kaga A.; Peng X.; Hirao H.; Chiba S. Diastereo-Divergent Synthesis of Saturated Azaheterocycles Enabled by TBuOK-Mediated Hydroamination of Alkenyl Hydrazones. Chem.—Eur. J. 2015, 21 (52), 19112–19118. 10.1002/chem.201504160. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Cai L.; Yang Z.; Houk K. N.; Kwon O. Bridged [2.2.1] Bicyclic Phosphine Oxide Facilitates Catalytic γ-Umpolung Addition-Wittig Olefination. Chem. Sci. 2018, 9 (7), 1867–1872. 10.1039/C7SC04381C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. W.; Tissot O.; Mattison A.; Hooper M. W.; Brown J. M.; Cowley A. R.; Hulmes D. I.; Blacker A. J. Practical Preparation and Resolution of 1-(2′-Diphenylphosphino-1′-Naphthyl)Isoquinoline: A Useful Ligand for Catalytic Asymmetric Synthesis. Org. Process Res. Dev. 2003, 7 (3), 379–384. 10.1021/op034007n. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.