Abstract
Background
An increased prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in children was observed in various diabetes centres worldwide during the COVID-19 pandemic. We aimed to evaluate trends in the prevalence of diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes before and during the COVID-19 pandemic, and to identify potential predictors of changes in diabetic ketoacidosis prevalence during the pandemic.
Methods
For this international multicentre study, we used data from 13 national diabetes registries (Australia, Austria, Czechia, Denmark, Germany, Italy, Luxembourg, New Zealand, Norway, Slovenia, Sweden, USA [Colorado], and Wales). The study population comprised 104 290 children and adolescents aged 6 months to younger than 18 years, who were diagnosed with type 1 diabetes between Jan 1, 2006, and Dec 31, 2021. The observed diabetic ketoacidosis prevalence in 2020 and 2021 was compared to predictions based on trends over the pre-pandemic years 2006–19. Associations between changes in diabetic ketoacidosis prevalence and the severity of the COVID-19 pandemic and containment measures were examined with excess all-cause mortality in the whole population and the Stringency Index from the Oxford COVID-19 Government Response Tracker.
Findings
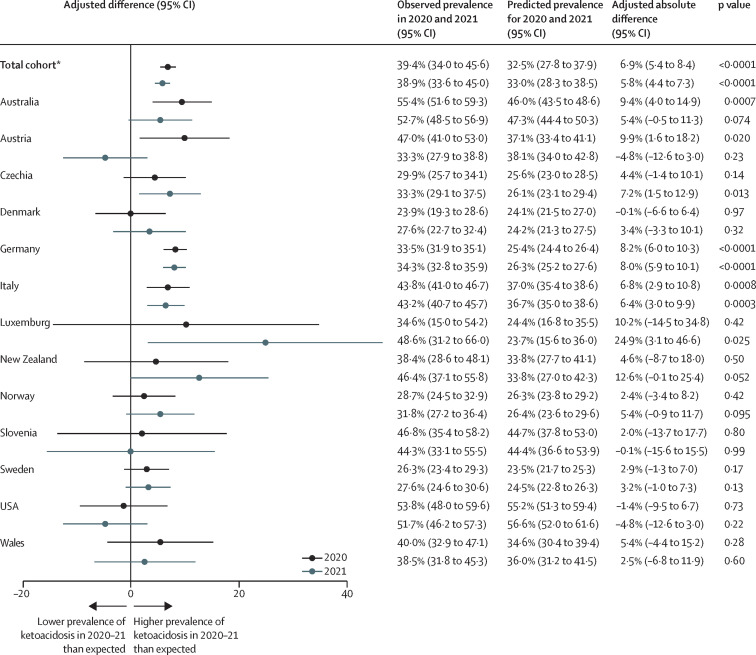
87 228 children and adolescents were diagnosed with type 1 diabetes between 2006 and 2019, 8209 were diagnosed in 2020, and 8853 were diagnosed in 2021. From 2006 to 2019, diabetic ketoacidosis at diagnosis of type 1 diabetes was present in 23 775 (27·3%) of 87 228 individuals and the mean annual increase in the prevalence of diabetic ketoacidosis in the total cohort from 2006 to 2019 was 1·6% (95% CI 1·3 to 1·9). The adjusted observed prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes was 39·4% (95% CI 34·0 to 45·6) in 2020 and 38·9% (33·6 to 45·0) in 2021, significantly higher than the predicted prevalence of 32·5% (27·8 to 37·9) for 2020 and 33·0% (28·3 to 38·5) for 2021 (p<0·0001 for both years). The prevalence of diabetic ketoacidosis was associated with the pandemic containment measures, with an estimated risk ratio of 1·037 (95% CI 1·024 to 1·051; p<0·0001) per ten-unit increase in the Stringency Index for 2020 and 1·028 (1·009 to 1·047; p=0·0033) for 2021, but was not significantly associated with excess all-cause mortality.
Interpretation
During the COVID-19 pandemic, there was a marked exacerbation of the pre-existing increase in diabetic ketoacidosis prevalence at diagnosis of type 1 diabetes in children. This finding highlights the need for early and timely diagnosis of type 1 diabetes in children and adolescents.
Funding
German Federal Ministry for Education and Research, German Robert Koch Institute, German Diabetes Association, German Diabetes Foundation, Slovenian Research Agency, Welsh Government, Central Denmark Region, and Swedish Association of Local Authorities and Regions.
Introduction
Diabetic ketoacidosis at the time of diagnosis of type 1 diabetes is an emergency condition and is associated with complications including cerebral oedema, higher rates of mortality, longer hospital stay, and poorer long-term glycaemic control.1, 2, 3 In children, even a single episode of moderate or severe diabetic ketoacidosis at diagnosis is associated with lower cognitive scores and impaired brain growth, with potentially long-term effects on neurocognitive function.4, 5 From the onset of hyperglycaemia, progression to diabetic ketoacidosis usually occurs over a period of several days to weeks.2 Therefore, diabetic ketoacidosis at the time of diagnosis of type 1 diabetes suggests delayed diagnosis of diabetes and commencement of insulin treatment.6, 7 Delayed referral for new-onset type 1 diabetes might be due to parents' and caregivers' inadequate knowledge of the symptoms of diabetes, or due to non-recognition of the signs of diabetes early in children presenting to primary health care.6, 7, 8
Research in context.
Evidence before the study
We searched PubMed on July 22, 2022, for articles using the following search criteria: “(diabetic ketoacidosis OR DKA) AND (children OR pediatrics) AND type 1 diabetes AND (newly diagnosed OR manifestation OR new onset) AND (Covid OR SARS-CoV-2 OR pandemic)”. No language restrictions were applied. We retrieved 78 results, of which 53 articles matched the searched topic, including ten case reports, 15 single-centre studies, 25 multicentre studies, one review, and two meta-analyses. All multicentre studies were conducted within single countries and were mostly limited to the first lockdown period in 2020, the first few months of the pandemic, or, rarely, the first year of the pandemic (ie, up to Feb 28, 2021). No multicentre study has, to the best of our knowledge, compared rates of diabetic ketoacidosis at diagnosis between countries before and during the COVID-19 pandemic or examined the prevalence of diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes beyond February, 2021.
Added value of this study
This international multicentre study found that the increase in the prevalence of diabetic ketoacidosis during the COVID-19 pandemic was mainly related to an existing increasing trend in diabetic ketoacidosis prevalence before the pandemic. These findings indicate that the COVID-19 pandemic and the subsequently enforced containment measures created ideal conditions for uncovering pre-existing problems with the timely diagnosis and care of children with new-onset type 1 diabetes.
Implication of all the available evidence
Both the long-term pre-pandemic increase in the prevalence of diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes and the additional pronounced increase during the COVID-19 pandemic were general phenomena observed in most registries from different countries and continents. The results of our study show that there is insufficient early diagnosis of type 1 diabetes in childhood worldwide. Therefore, universal efforts are needed to reverse the increasing trend of diabetic ketoacidosis.
A previous international analysis of data from 2006 to 2016 showed large differences in the prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes between countries, being generally high and slightly increasing over the years in most countries.8
The COVID-19 pandemic caused by SARS-CoV-2 has resulted in considerable morbidity and mortality worldwide. In response to the pandemic, nationwide health policy measures to contain the spread of SARS-CoV-2 were implemented in most countries during 2020.9 As a result, various diagnoses were delayed, leading to diseases being identified at a more advanced stage and increased rates of out-of-hospital mortality.10, 11 An increased prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes was observed during the initial COVID-19 wave in diabetes centres in different parts of the world.12, 13, 14, 15, 16, 17
We aimed to explore the pre-pandemic time-trend of diabetic ketoacidosis prevalence at diagnosis of paediatric type 1 diabetes in international registries from 2006 to 2019, and to compare the prevalence of diabetic ketoacidosis observed during the pandemic in 2020 and 2021 to a prediction derived from the years 2006–19. We also aimed to identify potential predictors of changes in diabetic ketoacidosis prevalence during the pandemic, such as pandemic severity or containment measures.
Methods
Study design and population
This international, multicentre study used data from 13 diabetes registries: a register encompassing two diabetes centres in New Zealand, a register encompassing ten diabetes centres in Australia, a register of the state of Colorado in the USA, and ten national registries from Europe (one each from Austria, Czechia, Denmark, Germany, Italy, Luxembourg, Norway, Slovenia, Sweden, and Wales; for the list of registries see the appendix p 3). The study population comprised children and adolescents aged 6 months to younger than 18 years, with a diagnosis of type 1 diabetes between Jan 1, 2006, and Dec 31, 2021. To exclude cases of neonatal diabetes, children younger than 6 months were not included in this analysis. The final study population comprised 104 290 individuals. The coverage of the above data sources was above 90% (national European registries and Colorado, USA), with the exception of Austria (80%) and the sub-national registries of Australia and New Zealand (60% of children diagnosed in the country).
Variables
We analysed demographic data (sex, age, month, and year of clinical diagnosis of type 1 diabetes) and diabetic ketoacidosis status: serum bicarbonate, venous pH, or clinical diagnosis of diabetic ketoacidosis, or a combination of the above. Diabetic ketoacidosis was defined according to the International Society for Pediatric and Adolescent Diabetes (ISPAD) criteria as venous pH less than 7·3 or serum bicarbonate less than 15 mmol/L1 or the documented clinical diagnosis of diabetic ketoacidosis (yes vs no) by the physician providing medical care at diagnosis. The leads of the individual diabetes registries provided clarification on how data on diabetic ketoacidosis was coded and how potential missing values could be interpreted. The absence of laboratory or clinical values was then handled accordingly, either as missing values (in 2752 of 11 589 individuals in Australia and in 1771 of 13 476 individuals in Sweden) or as a type 1 diabetes diagnosis without diabetic ketoacidosis (in the other 11 countries).
Measures of COVID-19 severity and containment
The severity of the COVID-19 pandemic was indirectly measured as excess all-cause mortality in the whole population from the World Mortality Dataset.18 To quantify the perception of pandemic severity by individual governments, we used the Stringency Index from the open-access global database of pandemic policies, the Oxford COVID-19 Government Response Tracker (OxCGRT).9 The OxCGRT offers several composite indices that provide snapshots of the intensity of government policies applied in different countries. The indices are based on eight indicators of containment and closure, four indicators of economic response, and seven indicators of health systems. We chose the composite Stringency Index as the variable to examine, since this is a simple additive composite index containing the indicators of school closure, workplace closure, cancellation of public events, restrictions on gathering size, closure of public transport, stay-at-home requirements, restrictions on internal movement, restrictions on international travel, and public information campaigns. Data on excess all-cause mortality and the Stringency Index were averaged per country and month, centred to the 15th day to bring them in accord with the monthly aggregation of diabetic ketoacidosis prevalence data.
Ethics
In some countries, by law, people with diabetes are entered into national databases without their consent, whereas in other countries verbal or written informed consent is obtained from individuals or their guardians. The present study and audit received ethical approvals in each country, either from the authors' institutional review boards or from relevant national bodies. Anonymised person-level data were analysed at the Institute of Epidemiology and Medical Biometry, University of Ulm, Ulm, Germany. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies.19
Statistical analysis
Summary statistics were calculated and presented as medians (IQRs) for continuous variables and as proportions for dichotomous variables. The prevalence of diabetic ketoacidosis was compared between sexes and age groups with χ2 tests. To analyse a time trend of the prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes from 2006 to 2019, a log-binomial regression analysis adjusted for sex and age groups, including country as a random intercept, was implemented to estimate the annual percentage change. Here, we used year of diagnosis (integer number) as the term for time. The analysis was done on individual individuals as observations. Age was categorised as 6 months to younger than 6 years, 6 years to younger than 12 years, and 12 years to younger than 18 years. The resulting regression coefficients were used to predict the prevalence of diabetic ketoacidosis for 2020 and 2021. We compared the observed prevalence in 2020 and 2021 with the predicted prevalence for 2020 and 2021, by calculating the difference with corresponding 95% CIs and applying a Z test based on standard normal distribution. In an additional exploratory non-linear model, we used year and year squared (ie, linear plus quadratic) as the terms for time.
An exploratory joinpoint regression analysis was also done, as described in the appendix (p 2).
To analyse predictors for diabetic ketoacidosis at diagnosis of type 1 diabetes in 2020 and 2021, we used a log-binomial model based on data from 2006 to 2019 with age groups, sex, calendar month, country, and year as predictors. That is, each individual with a type 1 diabetes diagnosis in 2020 or 2021 could be assigned a specific likelihood of developing diabetic ketoacidosis, based on their age, sex, the country they live in, calendar month, and the time trend observed in 2006–19. To explore associations of excess diabetic ketoacidosis prevalence with the overall excess mortality and the Stringency Index, we implemented a model with observed diabetic ketoacidosis as the dependent variable and the likelihood of diabetic ketoacidosis predicted from the model above, with overall excess mortality or Stringency Index as predictors. The estimates were presented as risk ratios (with 95% CIs) of developing diabetic ketoacidosis.
A two-sided p value less than 0·05 was considered statistically significant. All analyses were done with SAS (version 9.4), build TS1M7 on a Windows Server 2019 mainframe.
Role of the funding source
The funders were not involved in any part of the conduct of this study or in the decision to submit this work.
Results
We obtained and analysed data from 104 290 children and adolescents who were diagnosed with type 1 diabetes in 13 countries (figure 1 ; table 1 ). 87 228 were diagnosed with type 1 diabetes from Jan 1, 2006, to Dec 31, 2019 (table 2 ), 8209 were diagnosed in 2020, and 8853 were diagnosed in 2021 (table 3 ). The study population consisted of 47 762 (45·8%) female and 56 528 (54·2%) male individuals. The median age of chidlren and adolescents at diagnosis was 9·5 years (IQR 5·9–12·6), 26 417 (25·3%) children were younger than 6 years at diagnosis, 46 434 (44·5%) were between 6 years and younger than 12 years, and 31 439 (30·1%) were between 12 years and younger than 18 years. The underlying distribution of age at diagnosis remained fairly stable over the study period, with an increase of 0·042 years of age (95% CI 0·036–0·048) per year of study, and the proportion of male individuals increased by 0·18% (95% CI 0·06–0·30) per year of study. The number of documented cases of children with newly diagnosed type 1 diabetes with and without diabetic ketoacidosis for each participating registry for the individual years is shown in the appendix (pp 7–8).
Figure 1.
Participating countries
Number of individuals over the study period from 2006 to 2021 are shown in table 1.
Table 1.
Number of individuals from the participating countries over the study period from 2006 to 2021
| 2006–21 | 2020 | 2021 | |
|---|---|---|---|
| Australia | 8837 | 644 | 545 |
| Austria | 3024 | 268 | 288 |
| Czechia | 5148 | 461 | 490 |
| Denmark | 5085 | 330 | 330 |
| Germany | 40 272 | 3379 | 3672 |
| Italy | 16 127 | 1162 | 1521 |
| Luxembourg | 387 | 26 | 35 |
| New Zealand | 1127 | 99 | 112 |
| Norway | 5160 | 453 | 396 |
| Slovenia | 952 | 77 | 79 |
| Sweden | 11 696 | 835 | 865 |
| USA* | 3716 | 290 | 315 |
| Wales | 2759 | 185 | 205 |
The state of Colorado, USA
Table 2.
Children and adolescents with newly diagnosed type 1 diabetes from 2006 to 2019, with prevalence of diabetic ketoacidosis and corresponding relative annual percentage changes
| All individuals, 2006–19 | Individuals with no diabetic ketoacidosis, 2006–19 | Individuals with diabetic ketoacidosis, 2006–19 | Adjusted mean annual percentage change in diabetic ketoacidosis prevalence, 2006–19 (95% CI) | p value for annual trend | ||
|---|---|---|---|---|---|---|
| Overall dataset | 87 228 (100%) | 63 453 (72·7%) | 23 775 (27·3%) | 1·6% (1·3 to 1·9) | <0·0001 | |
| Age, years | 9·5 (5·9–12·5) | 9·5 (6·0–12·6) | 9·4 (5·1–12·3) | .. | .. | |
| Male | 47 066 (54·0%) | 34 486 (73·3%) | 12 580 (26·7%)* | 1·7% (1·3 to 2·1) | <0·0001 | |
| Female | 40 162 (46·0%) | 28 967 (72·1%) | 11 195 (27·9%)* | 1·4% (1·0 to 1·8) | <0·0001 | |
| By age | ||||||
| ≥6 months to <6 years | 22 192 (25·4%) | 15 515 (69·9%) | 6677 (30·1%)† | 1·1% (0·6 to 1·6) | <0·0001 | |
| ≥6 years to <12 years | 38 934 (44·6%) | 28 600 (73·5%) | 10 334 (26·5%)† | 1·7% (1·2 to 2·1) | <0·0001 | |
| ≥12 years to <18 years | 26 102 (29·9%) | 19 338 (74·1%) | 6764 (25·9%)† | 2·2% (1·7 to 2·8) | <0·0001 | |
| By country | ||||||
| Australia | 7648 | 4823 (63·1%) | 2825 (36·9%) | 4·0% (3·1 to 4·8) | <0·0001 | |
| Austria | 2468 | 1598 (64·7%) | 870 (35·3%) | 1·1% (−0·4 to 2·5) | 0·14 | |
| Czechia | 4197 | 3224 (76·8%) | 973 (23·2%) | 1·8% (0·3 to 3·3) | 0·020 | |
| Denmark | 4425 | 3498 (79·1%) | 927 (20·9%) | 1·9% (0·4 to 3·3) | 0·013 | |
| Germany | 33 221 | 26 486 (79·7%) | 6735 (20·3%) | 3·5% (2·9 to 4·0) | <0·0001 | |
| Italy | 13 444 | 7986 (59·4%) | 5458 (40·6%) | −1·1% (−1·6 to −0·6) | <0·0001 | |
| Luxemburg | 326 | 231 (70·9%) | 95 (29·1%) | −2·5% (−6·8 to 2·0) | 0·27 | |
| New Zealand | 916 | 639 (69·8%) | 277 (30·2%) | 2·0% (−0·9 to 5·1) | 0·18 | |
| Norway | 4311 | 3225 (74·8%) | 1086 (25·2%) | 0·6% (−0·7 to 1·9) | 0·36 | |
| Slovenia | 796 | 492 (61·8%) | 304 (38·2%) | 2·6% (0·4 to 4·9) | 0·022 | |
| Sweden | 9996 | 7925 (79·3%) | 2071 (20·7%) | 1·9% (0·9 to 2·8) | 0·0001 | |
| USA‡ | 3111 | 1604 (51·6%) | 1507 (48·4%) | 2·5% (1·2 to 3·9) | 0·0002 | |
| Wales | 2369 | 1722 (72·7%) | 647 (27·3%) | 3·5% (1·8 to 5·2) | <0·0001 | |
Data are n (%) or median (IQR), unless otherwise stated. The analysis of the temporal trend for diabetic ketoacidosis was adjusted for sex and age group, except for the age-stratified and sex-stratified subanalysis. Trend analysis for the international cohort included country as random intercept.
Significantly higher diabetic ketoacidosis prevalence in female individuals (p=0·0002).
Significantly different diabetic ketoacidosis prevalence between age groups (p<0·0001).
The state of Colorado, USA.
Table 3.
Characteristics of children and adolescents with newly diagnosed type 1 diabetes in 2020 and 2021 by diabetic ketoacidosis status
|
All individuals |
No diabetic ketoacidosis |
Diabetic ketoacidosis |
|||||
|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Overall dataset | 8209 | 8853 | 5204 (63·4%) | 5587 (63·1%) | 3005 (36·6%) | 3266 (36·9%) | |
| Age, years | 9·9 (6·1–12·8) | 9·5 (5·9–12·6) | 9·9 (6·2–12·9) | 9·3 (5·8–12·7) | 9·9 (6·0–12·7) | 9·7 (6·0–12·6) | |
| Male | 4521 | 4941 | 2879 (63·7%) | 3139 (63·5%) | 1642 (36·3%) | 1802 (36·5%) | |
| Female | 3688 | 3912 | 2325 (63·0%) | 2448 (62·6%) | 1363 (37·0%) | 1464 (37·4%) | |
| By age | |||||||
| ≥6 months to <6 years | 1965 | 2260 | 1217 (61·9%) | 1455 (64·4%) | 748 (38·1%) | 805 (35·6%) | |
| ≥6 years to <12 years | 3588 | 3912 | 2280 (63·6%) | 2454 (62·7%) | 1308 (36·4%) | 1458 (37·3%) | |
| ≥12 years to <18 years | 2656 | 2681 | 1707 (64·3%) | 1678 (62·6%) | 949 (35·7%) | 1003 (37·4%) | |
| By country | |||||||
| Australia | 644 | 545 | 287 (44·6%) | 258 (47·3%) | 357 (55·4%) | 287 (52·7%) | |
| Austria | 268 | 288 | 142 (53·0%) | 192 (66·7%) | 126 (47·0%) | 96 (33·3%) | |
| Czechia | 461 | 490 | 323 (70·1%) | 327 (66·7%) | 138 (29·9%) | 163 (33·3%) | |
| Denmark | 330 | 330 | 251 (76·1%) | 239 (72·4%) | 79 (23·9%) | 91 (27·6%) | |
| Germany | 3379 | 3672 | 2246 (66·5%) | 2411 (65·7%) | 1133 (33·5%) | 1261 (34·3%) | |
| Italy | 1162 | 1521 | 653 (56·2%) | 864 (56·8%) | 509 (43·8%) | 657 (43·2%) | |
| Luxemburg | 26 | 35 | 17 (65·4%) | 18 (51·4%) | 9 (34·6%) | 17 (48·6%) | |
| New Zealand | 99 | 112 | 61 (61·6%) | 60 (53·6%) | 38 (38·4%) | 52 (46·4%) | |
| Norway | 453 | 396 | 323 (71·3%) | 270 (68·2%) | 130 (28·7%) | 126 (31·8%) | |
| Slovenia | 77 | 79 | 41 (53·3%) | 44 (55·7%) | 36 (46·7%) | 35 (44·3%) | |
| Sweden | 835 | 865 | 615 (73·7%) | 626 (72·4%) | 220 (26·3%) | 239 (27·6%) | |
| USA* | 290 | 315 | 134 (46·2%) | 152 (48·3%) | 156 (53·8%) | 163 (51·8%) | |
| Wales | 185 | 205 | 111 (60·0%) | 126 (61·5%) | 74 (40·0%) | 79 (38·5%) | |
Data are n (%) or median (IQR), unless otherwise stated.
The state of Colorado, USA.
Diabetic ketoacidosis at diagnosis of type 1 diabetes was present in 23 775 (27·3%) of 87 228 people diagnosed between 2006 and 2019. The prevalence of diabetic ketoacidosis was significantly higher in female (27·9%) than in male individuals (26·7%; p=0·0002), and higher in the youngest age group (30·1% in children <6 years, 26·5% in children aged 6 years to <12 years, and 25·9% in children and adolescents aged 12 years to <18 years; p<0·0001 for age groups). The prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes differed markedly between countries and ranged from 20·3% in Germany to 48·4% in Colorado, USA (table 2).
From 2006 to 2019, the estimated mean annual increase relative to the preceding year in diabetic ketoacidosis prevalence at diagnosis of type 1 diabetes was 1·6% (95% CI 1·3 to 1·9; table 2, figure 2 ). An increasing prevalence of diabetic ketoacidosis was evident in both sexes, although slightly higher in male individuals. The mean annual rise in the prevalence of diabetic ketoacidosis increased with age at diagnosis of type 1 diabetes, from 1·1% (95% CI 0·6 to 1·6) in children younger than 6 years to 2·2% (95% CI 1·7 to 2·8) in adolescents aged 12 years to younger than 18 years (table 2). Stratified by country, the mean annual increase in diabetic ketoacidosis prevalence at diagnosis of type 1 diabetes between 2006 and 2019 was most pronounced in Australia (4·0% [95% CI 3·1 to 4·8]), Germany (3·5% [2·9 to 4·0]), Wales (3·5% [1·8 to 5·2]), Slovenia (2·6% [0·4 to 4·9]), and Colorado, USA (2·5 [1·2 to 3·9]). Only Italy showed a significant mean annual decrease in diabetic ketoacidosis at diagnosis of type 1 diabetes between 2006 and 2019 (−1·1% [–1·6 to –0·6]; table 2).
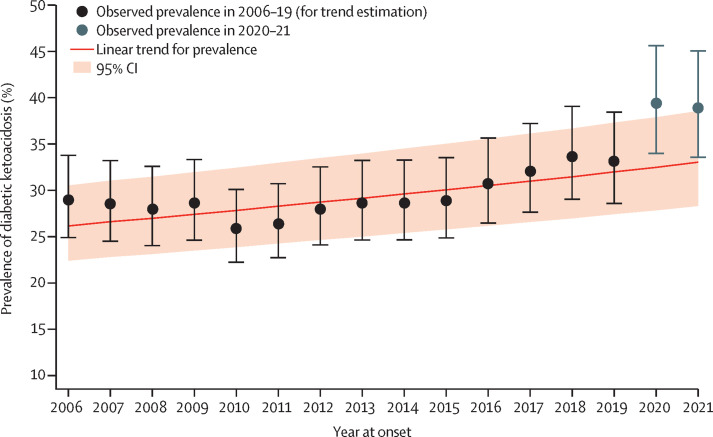
Figure 2.
Prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in children in 2020 and 2021 compared with the trend from 2006 to 2019
To analyse predictors for diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes in 2020 and 2021, we used a log-binomial model based on data from 2006 to 2019 with age groups, sex, and year as predictors, including countries as random intercepts. The error bars indicate the corresponding 95% CI of the observed diabetic ketoacidosis prevalence.
The rising trend of diabetic ketoacidosis was not steady throughout the study period, as suggested by visual inspection of the trends in individual registries, as well as by joinpoint regression applied to pooled data: the overall prevalence of diabetic ketoacidosis first decreased relative to the preceding year at –1·6% (95% CI –4·2 to 1·1) per year between 2006 and 2010, and then increased from 2010 (95% CI 2009 to 2013) to 2019 relative to the preceding year by 3·0% (95% CI 2·4 to 3·6) per year (appendix p 4). Because of the heterogeneity in trends among countries as suggested by joinpoint regression (data not shown), for the predictive models below we utilised the log-binomial trend model.
The highest number of yearly documented cases of diabetic ketoacidosis in the 13 registries before the emergence of COVID-19 was 2338, in 2019 (appendix p 7); this increased to 3005 cases (an increase of 28·5%) in 2020 and 3266 cases (an increase of 39·7%) in 2021. The adjusted observed prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes was 39·4% (95% CI 34·0–45·6) in 2020 and 38·9% (33·6–45·0) in 2021 (figure 2). According to the multivariable log-binomial trend model, the adjusted observed prevalence of diabetic ketoacidosis in 2020 and 2021 was significantly higher than the predicted prevalence of 32·5% (95% CI 27·8–37·9) for 2020 and 33·0% (28·3–38·5) for 2021 (figure 2). The adjusted absolute percentage difference was 6·9% (95% CI 5·4–8·4; p<0·0001) for 2020 and 5·8% (4·4–7·3; p<0·0001) for 2021 (figure 3 ). In contrast to the previous years, there were no differences in the prevalence of diabetic ketoacidosis between female and male individuals or between age groups. Stratified by country, the diabetic ketoacidosis prevalence in 2020 ranged from 23·9% in Denmark to 55·4% in Australia, and in 2021 the prevalence ranged from 27·6% in Denmark and Sweden to 52·7% in Australia (table 3). Figure 3 shows the absolute percentage differences for each country between the observed and predicted prevalence of diabetic ketoacidosis for 2020 and 2021.
Figure 3.
Difference between the observed prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in children in 2020 and 2021, and the predicted prevalence based on data from 2006 to 2019
Error bars indicate 95% CIs. *For the total cohort, the analysis was adjusted for age and sex and included county as random intercept.
An exploratory joinpoint regression analysis showed that the increase in the prevalence of diabetic ketoacidosis during the pandemic followed the existing pre-pandemic trend, showing a sharp increase in the prevalence of diabetic ketoacidosis since 2015. According to the results of the joinpoint regression analysis, the rate of diabetic ketoacidosis relative to the preceding year increased by 5·6% (95% CI 3·9 to 7·3) per year after 2015 (95% CI 2009 to 2019), compared to 0·9% (−0·6 to 2·4) per year before 2015 (appendix p 4). Furthermore, a non-linear log-binomial model (with the year term both linear and as year squared) indicated that the prevalence of diabetic ketoacidosis was significantly increased only in 2020, during the first year of the pandemic, and not in 2021 (appendix p 5).
The measures of the severity of the pandemic are presented along with the excess diabetic ketoacidosis prevalence in the appendix (p 6) for the ten registries with more than 100 cases each year in 2020 and 2021. Our multivariable log-binomial regression model using data from individuals diagnosed during the pandemic years (2020–21) indicated that the likelihood of diabetic ketoacidosis increased with a higher Stringency Index, with an estimated risk ratio of having diabetic ketoacidosis per ten-unit increase in the Stringency Index of 1·037 (95% CI 1·024–1·051; p<0·0001) for 2020 and 1·028 (1·009–1·047; p=0·0033) for 2021. By contrast, the likelihood of diabetic ketoacidosis at diagnosis of type 1 diabetes in 2020 and 2021 was not significantly associated with excess all-cause mortality in the whole population (risk ratio per ten-unit increase in mortality of 0·986 [95% CI 0·969–1·004], p=0·121, in 2020 and 0·986 [0·961–1·011], p=0·275, in 2021).
Discussion
This international multicentre study found a significant increase in the prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in children and adolescents from 2006 to 2019, with a marked additional increase observed during the COVID-19 pandemic in 2020 and 2021. Therefore, the increase in diabetic ketoacidosis during the COVID-19 pandemic was not a short-term phenomenon restricted to the early months of the pandemic. Notably, our segmented joinpoint regression analysis suggests that a significant increase in the prevalence of diabetic ketoacidosis at type 1 diabetes diagnosis occurred well before the pandemic. Even though this analysis did not detect a separate joinpoint for the first 2 years of the pandemic, a comparison of the two analyses, one without and one with the two pandemic years, showed a clear increase in the prevalence of diabetic ketoacidosis in 2020–21, indicating an aggravation of the pre-existing increasing trend during the pandemic.
Our data also show a crisis of diabetic ketoacidosis in children with newly diagnosed type 1 diabetes during the pandemic. The massive increase in diabetic ketoacidosis cases is the multiplied impact of two factors: the increase in the number of children with newly diagnosed type 1 diabetes during the pandemic20, 21 and the higher proportion of children presenting with diabetic ketoacidosis at diagnosis compared with previous years.
The development of diabetic ketoacidosis is thought to be due to delayed diagnosis and initiation of insulin replacement therapy.6, 7 A study done in Germany by Kamrath and colleagues22 suggested that elevated HbA1c along with an increased prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in children during the COVID-19 pandemic might be due to a delay in diagnosis and initiation of type 1 diabetes therapy as a result of the pandemic. By contrast, insulin deficiency due to direct destruction of beta cells by SARS-CoV-2 does not seem to play an important role in children with newly diagnosed type 1 diabetes.23
Both the long-term trend of an increasing prevalence of diabetic ketoacidosis and the additional increase in diabetic ketoacidosis during the COVID-19 pandemic were observed in most registries. Therefore, we assume this trend is due to underlying mechanisms that are independent of the specific developments in children's health care in the individual countries. Consequently, universal efforts are needed to reverse the increasing trend of diabetic ketoacidosis worldwide. For instance, educational campaigns have been effective in raising awareness of the symptoms of diabetes, and screening programmes for islet autoantibodies aimed at reducing the incidence of diabetic ketoacidosis at diagnosis of diabetes have been reported.24, 25
We found a significant association between the likelihood of diabetic ketoacidosis at type 1 diabetes diagnosis and the pandemic containment measures, as expressed by the Stringency Index. However, the magnitude of this association was small, which means that the contribution of the pandemic containment measures to the excess prevalence of diabetic ketoacidosis must be assessed as being low. Notably, the Stringency Index reflects the perception of the pandemic by governments and authorities, and their intents, so its level runs in parallel with many other factors, including those that cannot be measured directly, such as individual concerns, fears, or misperceptions of individual risk. All components might have contributed to delayed presentation and diagnosis of type 1 diabetes in children during the pandemic.12, 17 In contrast to the pandemic containment measures, there was no association between the prevalence of diabetic ketoacidosis and excess all-cause mortality in the whole population as a measure of pandemic severity. Therefore, COVID-19 did not appear to be the sole cause of the increased prevalence of diabetic ketoacidosis. Rather, it seems that the pandemic provided the ideal conditions to uncover pre-existing problems associated with timely diagnosis of children with new-onset type 1 diabetes.
In our analysis, the USA cohort from the state of Colorado showed no increase in the prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes during the pandemic. This observation contrasts with other reports from the USA, where an increased prevalence of diabetic ketoacidosis during the COVID-19 pandemic has been reported.21, 26 Besides the fact that different regions were studied, this discrepancy might also be due to methodological reasons. For example, we did not simply compare the prevalence of diabetic ketoacidosis during the pandemic years with the average prevalence in previous years, as other studies have done. Instead, we took into account the increasing trend in recent years. Additionally, we compared the prevalence of diabetic ketoacidosis over a whole year, whereas other studies only examined the prevalence of diabetic ketoacidosis over a shorter period, such as the first lockdown or the first few months of the pandemic.
The strengths of the study are the large number of cases from 13 countries, with most national registries having high coverage over the 15-year study period, and the fact that our analysis covered the first 2 years of the pandemic. Additionally, by comparing the changes in the prevalence of diabetic ketoacidosis between these countries, each with different pandemic severities and different pandemic control measures, we were able to analyse the impact of these factors on the increase in diabetic ketoacidosis prevalence during the COVID-19 pandemic.
Limitations of the study include the heterogeneity in the way data on diabetic ketoacidosis were coded in the registries, with Australia and Sweden handling “no information on diabetic ketoacidosis” as missing values, while other countries registered no information on diabetic ketoacidosis as no diabetic ketoacidosis. Additionally, the impact of race and ethnicity on the prevalence of diabetic ketoacidosis could not be analysed because of the varying definitions for racial and ethnic background across the 13 registries.
In summary, we found an increasing trend in the prevalence of diabetic ketoacidosis cases at diagnosis of type 1 diabetes in children, which was increasing well before the emergence of COVID-19 and was then further increased during the pandemic. Comprehensive education about the classic symptoms of type 1 diabetes in childhood needs to be provided within the general public, within those working in the childcare or daycare sector, and among general practitioners in the primary care setting to raise awareness of the symptoms of type 1 diabetes, and public health interventions may also be considered, such as implementation of general screening programmes for islet autoantibodies in preschool children. The rising prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in children is a global concern that should be addressed.
Data sharing
Aggregated data might be made available upon reasonable request via email to the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank Andreas Hungele and Ramona Ranz for their support and the development of the DPV documentation software (clinical data managers at the Institute of Epidemiology and Medical Biometry, Ulm University, Ulm, Germany). We also express our sincere thanks to Stefanie Lanzinger (Institute of Epidemiology and Medical Biometry, Ulm University, Ulm, Germany) for her statistical support in preparing the revision, and Joachim Rosenbauer (German Diabetes Center, Institute for Biometrics and Epidemiology, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany) for his support in the segmented regression analysis. We thank all centres participating in the registries. We would like to commemorate the late Ann-Marie Svensson and thank for her long-term commitment to epidemiology of childhood type 1 diabetes and acknowledge her contribution to the planning phase of this Article and to the generation of hypotheses. The German, Austrian, and Luxemburg DPV registry is funded by the German Federal Ministry for Education and Research (BMBF) within the German Center for Diabetes Research (grant number 82DZD14E03), the German Robert Koch Institute (diabetes surveillance), the German Diabetes Association, and the German Diabetes Foundation (grant number FP-0446-2022). The Slovenian Childhood Diabetes Registry is supported by Slovenian Research Agency grants J3-6798, V3-1505, and P3-0343. The Children and Young People's Wales Diabetes Network (and Brecon Group) is funded by the Welsh Government. NHB is partially funded by The Health Research Foundation of the Central Denmark Region. SWEDIABKIDS is financially supported by the Swedish Association of Local Authorities and Regions.
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps.
Contributors
RWH had the idea for and conceptualised the study, coordinated and supervised data collection, acquired funding for the analysis, and critically reviewed the manuscript for important intellectual content. JMG and AJE analysed the data and designed the statistical analyses. NHB, CK, and OC interpreted the analyses, visualised the results, and wrote the initial draft of the manuscript. All other authors collected data, contributed intellectually to this study, and critically reviewed the scientific content of the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work. JMG, AJE, and RWH are the guarantors of this work and, as such, had full access to all the data in the study, verified the data, and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(suppl 27):155–177. doi: 10.1111/pedi.12701. [DOI] [PubMed] [Google Scholar]
- 2.White NH. Diabetic ketoacidosis in children. Endocrinol Metab Clin North Am. 2000;29:657–682. doi: 10.1016/s0889-8529(05)70158-4. [DOI] [PubMed] [Google Scholar]
- 3.Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6:40. doi: 10.1038/s41572-020-0165-1. [DOI] [PubMed] [Google Scholar]
- 4.Aye T, Mazaika PK, Mauras N, et al. Impact of early diabetic ketoacidosis on the developing brain. Diabetes Care. 2019;42:443–449. doi: 10.2337/dc18-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghetti S, Kuppermann N, Rewers A, et al. Cognitive function following diabetic ketoacidosis in children with new-onset or previously diagnosed type 1 diabetes. Diabetes Care. 2020;43:2768–2775. doi: 10.2337/dc20-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bui H, To T, Stein R, Fung K, Daneman D. Is diabetic ketoacidosis at disease onset a result of missed diagnosis? J Pediatr. 2010;156:472–477. doi: 10.1016/j.jpeds.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Wersäll JH, Adolfsson P, Forsander G, Ricksten SE, Hanas R. Delayed referral is common even when new-onset diabetes is suspected in children. A Swedish prospective observational study of diabetic ketoacidosis at onset of type 1 diabetes. Pediatr Diabetes. 2021;22:900–908. doi: 10.1111/pedi.13229. [DOI] [PubMed] [Google Scholar]
- 8.Cherubini V, Grimsmann JM, Åkesson K, et al. Temporal trends in diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes between 2006 and 2016: results from 13 countries in three continents. Diabetologia. 2020;63:1530–1541. doi: 10.1007/s00125-020-05152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hale T, Angrist N, Goldszmidt R, et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker) Nat Hum Behav. 2021;5:529–538. doi: 10.1038/s41562-021-01079-8. [DOI] [PubMed] [Google Scholar]
- 10.Lai PH, Lancet EA, Weiden MD, et al. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiol. 2020;5:1154–1163. doi: 10.1001/jamacardio.2020.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Primessnig U, Pieske BM, Sherif M. Increased mortality and worse cardiac outcome of acute myocardial infarction during the early COVID-19 pandemic. ESC Heart Fail. 2021;8:333–343. doi: 10.1002/ehf2.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamrath C, Mönkemöller K, Biester T, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA. 2020;324:801–804. doi: 10.1001/jama.2020.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho J, Rosolowsky E, Pacaud D, et al. Diabetic ketoacidosis at type 1 diabetes diagnosis in children during the COVID-19 pandemic. Pediatr Diabetes. 2021;22:552–557. doi: 10.1111/pedi.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence C, Seckold R, Smart C, et al. Increased paediatric presentations of severe diabetic ketoacidosis in an Australian tertiary centre during the COVID-19 pandemic. Diabet Med. 2021;38 doi: 10.1111/dme.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman S, Pinhas-Hamiel O, Weinberg A, et al. Alarming increase in ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the first wave of the COVID-19 pandemic in Israel. Pediatr Diabetes. 2022;23:10–18. doi: 10.1111/pedi.13296. [DOI] [PubMed] [Google Scholar]
- 16.Nagl K, Waldhör T, Hofer SE, et al. Alarming increase of ketoacidosis prevalence at type 1 diabetes-onset in Austria—results from a nationwide registry. Front Pediatr. 2022;10 doi: 10.3389/fped.2022.820156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alfayez OM, Aldmasi KS, Alruwais NH, et al. Incidence of diabetic ketoacidosis among pediatrics with type 1 diabetes prior to and during COVID-19 pandemic: a meta-analysis of observational studies. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.856958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlinsky A, Kobak D. Tracking excess mortality across countries during the COVID-19 pandemic with the World Mortality Dataset. eLife. 2021;10:10. doi: 10.7554/eLife.69336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 20.Kamrath C, Rosenbauer J, Eckert AJ, et al. Incidence of type 1 diabetes in children and adolescents during the COVID-19 pandemic in Germany: results from the DPV Registry. Diabetes Care. 2022;45:1762–1771. doi: 10.2337/dc21-0969. [DOI] [PubMed] [Google Scholar]
- 21.Gottesman BL, Yu J, Tanaka C, Longhurst CA, Kim JJ. Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr. 2022;176:414–415. doi: 10.1001/jamapediatrics.2021.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamrath C, Rosenbauer J, Eckert AJ, et al. Glycated hemoglobin at diagnosis of type 1 diabetes and at follow-up in children and adolescents during the COVID-19 pandemic in Germany. Pediatr Diabetes. 2022;23:749–753. doi: 10.1111/pedi.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamrath C, Rosenbauer J, Tittel SR, et al. Frequency of autoantibody-negative type 1 diabetes in children, adolescents, and young adults during the first wave of the COVID-19 pandemic in Germany. Diabetes Care. 2021;44:1540–1546. doi: 10.2337/dc20-2791. [DOI] [PubMed] [Google Scholar]
- 24.Chiarelli F, Rewers M, Phillip M. Screening of islet autoantibodies for children in the general population: a Position Statement endorsed by the European Society for Pediatric Endocrinology. Horm Res Paediatr. 2022 doi: 10.1159/000525824. published online July 1. [DOI] [PubMed] [Google Scholar]
- 25.Ghalwash M, Dunne JL, Lundgren M, et al. Two-age islet-autoantibody screening for childhood type 1 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol. 2022;10:589–596. doi: 10.1016/S2213-8587(22)00141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf RM, Noor N, Izquierdo R, et al. Increase in newly diagnosed type 1 diabetes in youth during the COVID-19 pandemic in the United States: a multi-center analysis. Pediatr Diabetes. 2022;23:433–438. doi: 10.1111/pedi.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Aggregated data might be made available upon reasonable request via email to the corresponding author.