Abstract
Objective
To quantify the comparative risk of thrombosis with thrombocytopenia syndrome or thromboembolic events associated with use of adenovirus based covid-19 vaccines versus mRNA based covid-19 vaccines.
Design
International network cohort study.
Setting
Routinely collected health data from contributing datasets in France, Germany, the Netherlands, Spain, the UK, and the US.
Participants
Adults (age ≥18 years) registered at any contributing database and who received at least one dose of a covid-19 vaccine (ChAdOx1-S (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), or Ad26.COV2.S (Janssen/Johnson & Johnson)), from December 2020 to mid-2021.
Main outcome measures
Thrombosis with thrombocytopenia syndrome or venous or arterial thromboembolic events within the 28 days after covid-19 vaccination. Incidence rate ratios were estimated after propensity scores matching and were calibrated using negative control outcomes. Estimates specific to the database were pooled by use of random effects meta-analyses.
Results
Overall, 1 332 719 of 3 829 822 first dose ChAdOx1-S recipients were matched to 2 124 339 of 2 149 679 BNT162b2 recipients from Germany and the UK. Additionally, 762 517 of 772 678 people receiving Ad26.COV2.S were matched to 2 851 976 of 7 606 693 receiving BNT162b2 in Germany, Spain, and the US. All 628 164 Ad26.COV2.S recipients from the US were matched to 2 230 157 of 3 923 371 mRNA-1273 recipients. A total of 862 thrombocytopenia events were observed in the matched first dose ChAdOx1-S recipients from Germany and the UK, and 520 events after a first dose of BNT162b2. Comparing ChAdOx1-S with a first dose of BNT162b2 revealed an increased risk of thrombocytopenia (pooled calibrated incidence rate ratio 1.33 (95% confidence interval 1.18 to 1.50) and calibrated incidence rate difference of 1.18 (0.57 to 1.8) per 1000 person years). Additionally, a pooled calibrated incidence rate ratio of 2.26 (0.93 to 5.52) for venous thrombosis with thrombocytopenia syndrome was seen with Ad26.COV2.S compared with BNT162b2.
Conclusions
In this multinational study, a pooled 30% increased risk of thrombocytopenia after a first dose of the ChAdOx1-S vaccine was observed, as was a trend towards an increased risk of venous thrombosis with thrombocytopenia syndrome after Ad26.COV2.S compared with BNT162b2. Although rare, the observed risks after adenovirus based vaccines should be considered when planning further immunisation campaigns and future vaccine development.
Introduction
By May 2021, four covid-19 vaccines had been granted conditional marketing authorisation by the European Medicines Agency after showing high efficacy and safety in phase 3 clinical trials.1 2 3 ChAdOx1-S (Oxford-AstraZeneca) and Ad26.COV2.S (Janssen/Johnson & Johnson) are both adenovirus based vaccines. BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) are both mRNA based vaccines. After millions of vaccine doses were given in large scale immunisation campaigns, rare cases of thrombosis with thrombocytopenia syndrome were reported, often after the first dose of adenovirus vaccines.4 5 6 Although fewer concerns have been raised about the safety of mRNA vaccines, instances of immune thrombocytopenia have also been observed in recipients of BNT162b2.7
A causal relation between these vaccines and thrombosis with thrombocytopenia syndrome was considered by the EMA’s pharmacovigilance risk assessment committee, leading to an update of the product information for ChAdOx1-S to include thrombosis with thrombocytopenia syndrome as a very rare side effect.8 Because these unusual blood clots in combination with thrombocytopenia were reported predominantly in women aged under 60 years, several European countries restricted the use of adenovirus vaccines in younger age groups as a precautionary measure. While the pathogenesis is not yet fully understood, an immune response leading to the development of pathological platelet activating antibodies has been suggested and named as vaccine induced immune thrombotic thrombocytopenia.6 9 Although these events are very rare, absolute numbers of affected patients could become substantial owing to the large numbers of vaccine doses administered worldwide.
Although some observational studies have examined the risk of thrombosis with thrombocytopenia syndrome after covid-19 vaccination in some European countries,10 11 12 13 no clear evidence exists on the comparative safety profile of different vaccines. Given the high number of SARS-CoV-2 infections and reinfections seen worldwide, and the known effectiveness of covid-19 vaccines in minimising severe infection and complications, understanding the risks of the available vaccines compared with each other is essential, rather than comparing them with no vaccination. We therefore aimed to quantify the comparative risk of thrombosis with thrombocytopenia syndrome or thromboembolic events associated with the use of adenovirus based covid-19 vaccines versus mRNA based covid-19 vaccines.
Methods
Study design
We conducted an international network cohort study using routinely collected healthcare data mapped to the OMOP CDM (observational medical outcomes partnership common data model). The OMOP CDM allowed the study to be run by each site with common analytical code. Results were aggregated without sharing patient level data.14 15 16
Data sources
Datasets from five European countries (France, Germany, the Netherlands, Spain, and the UK) and two datasets from the US informed the analyses. IQVIA Longitudinal Patient Data (LPD) France is a centralised anonymised patient electronic medical records database contributed by general practices.17 IQVIA Disease Analyser (DA) Germany is collected from extracts of patient management software used by general medicine and specialists practising in ambulatory care settings. The Integrated Primary Care Information (IPCI) database contains electronic healthcare records collected from patients registered with general practices in the Netherlands.18 The Information System for Research in Primary Care (SIDIAP) is a primary care records database that covers about 80% of the population of Catalonia, Spain. SIDIAP was linked to the regional vaccination registry and to hospital discharge data (CMBD-HA) for this study.19 The Clinical Practice Research Datalink (CPRD) Aurum database collects anonymised primary care electronic health records from general practices across the UK, which are linked at origin to national vaccination records.20 The IQVIA hospital charge data master (US Hospital CDM) dataset comprises records from hospital charge data master files from the US and records both inpatient and outpatient encounters.21 The US Open Claims dataset includes medical claims covering about 191 million people across the US, with patient level office visit, outpatient, and inpatient information (table 1).
Table 1.
Descriptions of medical records databases used in study
| Database full (short) names | Country | Active size of database (by mid-2021; No of people) | Latest data available time | Key data available | ||||
|---|---|---|---|---|---|---|---|---|
| Covid-19 vaccines | Hospital treatments | Hospital outcomes | Outpatient treatments | Platelet counts | ||||
| Clinical Practice Research Datalink Aurum (UK CPRD) | UK | 13m | May 2021 | Complete | No | Incomplete | Yes | Yes |
| Information System for Research in Primary Care with minimum basic set of hospital discharge data (CMBD-HA; Spain SIDIAP) | Spain | 6m | June 2021 | Complete | No | Linked | Yes | Yes |
| Integrated Primary Care Information (Netherlands IPCI) | The Netherlands | 2m | June 2021 | Incomplete | No | Incomplete | Yes | Yes |
| IQVIA Longitudinal Patient Data France (France LPD) | France | 2.3m | September 2021 | Incomplete | No | Incomplete | Yes | Yes |
| IQVIA Disease Analyser Germany (Germany DA) | Germany | 8.5m | August 2021 | Incomplete | No | Incomplete | Yes | Yes |
| Medical and Institutional Claims (US Open Claims) | US | 187m | September 2021 | Incomplete | Incomplete | Incomplete | Yes | Yes |
| Charge Data Master (US Hospital CDM) | US | 30m | July 2021 | Incomplete | Yes | Yes | Incomplete | Incomplete |
The study period to identify vaccinations and outcomes started from December 2020 (first vaccines administered) to the latest data release available in each of the contributing databases (ie, mid-2021).
Study participants
The study population were adults (aged 18 years or over at date of the first dose vaccination)registered in any of the contributing databases and exposed to at least one dose of a covid-19 vaccine during the study period. We required a minimum of one year of history available in the database before the index vaccination date. We excluded individuals who did not have a vaccine brand specified (unspecific vaccine codes) during the study period. We also excluded people who received their second dose within 14 days of the first dose, as these were likely errors in vaccination records. We included only people with complete records for age and sex.
Four covid-19 vaccines were included: ChAdOx1-S, BNT162b2, mRNA-1273, and Ad26.COV2.S. Vaccines were identified by procedure, drug, or observation codes in each database (supplementary B). We built first and second dose cohorts for each brand. In the second dose cohorts, we did not include individuals whose second dose vaccine brand was different from their first dose. A single dose cohort was built for Ad26.COV2.S as it was approved for a single dose schedule at the time of protocol approval. Comparisons were made between the adenovirus based vaccines (ChAdOx1-S or Ad26.COV2.S; ie, the target) and mRNA vaccines (BNT162b2 or mRNA-1273; ie, the comparator).
The index dates for the first and second dose vaccination cohorts were defined as the dates of the first and second covid-19 vaccinations for a specific brand, respectively. We followed individuals from their index date to 28 days after vaccination, death, or loss of visibility in the database (eg, person leaving the practice in electronic health records data, or end of continuous enrolment in claims data), whichever came first. The risk window of 28 days is based on the World Health Organization’s definition and the UK Medicines and Healthcare Products Regulatory Agency (MHRA) guidelines.22 23 Owing to the expense of computing, we used a random sample of 20% of each cohort when using US Open Claims data.
Primary outcomes
The primary outcomes were thromboembolic events and thrombosis with thrombocytopenia syndrome. Thromboembolic events of interest included deep vein thrombosis, pulmonary embolism, venous thromboembolism as a composite of deep vein thrombosis or pulmonary embolism, cerebral venous sinus thrombosis, splanchnic and visceral vein thrombosis ischaemic stroke, myocardial infarction, arterial thromboembolism as a composite of ischaemic stroke, and other rare arterial thromboembolisms such as intestinal infarction (supplementary B).
The definition of thrombosis with thrombocytopenia syndrome (supplementary B) was based on that proposed by the Brighton Collaboration and encompassed the occurrence of any thromboembolic event of interest with concurrent thrombocytopenia within 10 days before or after a thromboembolic event occurring within 28 days after vaccination. Thrombocytopenia was identified by a diagnostic code or measurement of <150 000 platelets per μL of blood, as proposed by the Brighton Collaboration.24 This definition has been used in previous OMOP CDM based studies.25
We used two alternative definitions for thrombosis with thrombocytopenia syndrome in sensitivity analyses. The first analysis required concurrent thrombocytopenia to have happened within five days before or after the thromboembolic event after vaccination. The second analysis reduced the threshold to <100 000 platelets/µL for the definition of thrombocytopenia, based on laboratory data.
Negative control outcomes
Negative control outcomes are outcome events that are not expected to be causally associated with the vaccination. We used 92 negative control outcomes previously used for vaccine safety.26 They were prespecified on the basis of clinical knowledge and previous literature, validated by two clinicians, and tested in previous work on other vaccine safety projects.27 Supplementary A table 1 shows the codes for these negative control outcomes.
Covariates
We defined baseline patient characteristics as potential confounders based on information recorded before index date, including personal data (age, sex, index year, and index month), clinical condition at any time before cohort index, composite comorbidity (Romano’s adapted Charlson Comorbidity Index28), and thrombosis score (CHA2DS2-VASc, congestive heart failure, hypertension, vascular disease29), and total number of medicines, procedures, and measurement records in the six months before the cohort index date.
Statistical analysis
Descriptive statistics were used to report the baseline characteristics for each cohort. We reported the database specific incidence rate at 28 days and corresponding 95% confidence intervals for each event.
We matched on propensity scores to minimise observed confounding. We calculated propensity scores for each pair of vaccines being compared (target and comparator) using large scale L1 regularised logistic regression,30 which included all available baseline patient characteristics in the databases. The derived propensity score was used to match patients using greedy matching with a caliper width of 0.2 standard deviations of the logit at a ratio of up to 1:4. If the target cohort was larger than the comparator cohort, reverse matching was allowed, and a ratio of 4:1 was used.
We used three diagnostic tools to evaluate measured confounding, statistical power, and unmeasured confounding. We did not complete any database specific analysis that failed the measured confounding or statistical power diagnostics to avoid bias. Firstly, regarding measured confounding, only vaccine pairs of target and comparator with all covariates showing a standardised mean difference below 0.1 after the matching of propensity scores were considered satisfactory. Secondly, for statistical power, we calculated the minimum detectable rate ratio using α of 0.05 and power of 80% for each outcome of interest in both the crude cohorts and those matched with propensity scores.31
No estimates of incidence rate ratios specific to outcome were reported where the minimum detectable rate ratio was >5 for an outcome combination of database, target, and comparator, because a minimum detectable rate ratio >5 was deemed too underpowered making any such comparison unreliable. Thirdly, regarding unmeasured confounding, we studied associations with negative control outcomes to assess residual bias after matching propensity scores. We prespecified that <20% of negative control outcomes should be associated with vaccination to deem an analysis reliable in terms of residual confounding. Results for those that failed the unmeasured confounding diagnostic are reported, but only empirically calibrated estimates should be relied on (see below).
We used Poisson regression to calculate the incidence rate ratio and 95% confidence intervals of outcomes according to the target and comparator vaccinations. Following reviewers’ suggestions, we also estimated incidence rate difference and 28 day absolute risk differences for associations with a significant calibrated incidence rate ratio.
We used empirical calibration to account for residual systematic error due to potential unobserved confounding.32 33 To perform calibration, we first derived an empirical null distribution from the actual effect estimates for the negative control outcomes. We then used the null distribution to compute the calibrated P value and confidence intervals. This approach has been used in many previous studies in different clinical areas, including covid-19 repurposed treatments,34 35 36 and was acknowledged in the latest version of the ENCePP guide on methodological standards in pharmacoepidemiology.37 We only presented estimates specific to databases where empirical calibrations were conducted.
Finally, we conducted random effect meta-analysis to pool results across databases. Estimates from combinations of database, target, and comparator that passed the covariate balance diagnostic were included, regardless of the diagnostics on power or systematic error. Empirical calibration was conducted for meta-analysis as well.
We stratified all analyses by age (10 year bands) and sex as prespecified in the study protocol. Only groups with sufficient power (minimum detectable rate ratio <5) were reported. All analyses were prespecified in a registered study protocol (https://www.encepp.eu/standards_and_guidances/methodologicalGuide.shtml), and conducted in R 3.6.0 using the open source OHDSI (observational health data science and informatics) tool stack. The Cyclops and EvidenceSynthesis packages are available via CRAN. All our analytical code is available for review in a dedicated Github repository (https://github.com/oxford-pharmacoepi/ROC22_CovVaxComparativeSafety/tree/main/CovVaxComparativeSafety).
Patient and public involvement
Owing to the nature of this study and data privacy constraints, no patients or members of the public were involved in the study design, analysis, interpretation of data, or revision of the manuscript.
Results
We identified 4.6 million people vaccinated with a first dose of ChAdOx1-S (3 789 631 UK CPRD, 606 399 Spain SIDIAP, 98 562 Germany DA, 27 698 France LPD, and 71 083 the Netherlands IPCI) and 1.6 million people vaccinated with a second dose of ChAdOx1-S (1 195 626 UK CPRD, 307 344 Spain SIDIAP, 31 200 Germany DA, 15 067 France LPD, and 38 884 the Netherlands IPCI) from all participating databases. We identified 1.1 million people vaccinated with single dose Ad26.COV2.S in three databases (37 723 Germany DA, 138 351 Spain SIDIAP, and 939 748 US Open Claims). We identified 10.6 million people vaccinated with a first dose of BNT162b2 (1 840 240 UK CPRD, 391 063 Germany DA, 6 055 754 US Open Claims, and 2 027 950 Spain SIDIAP), and 7.7 million people vaccinated with a second dose (1 369 238 UK CPRD, 321 099 Germany DA, 4 450 735 US Open Claims, and 1 357 509 Spain SIDIAP). We identified 4 261 016 people vaccinated with a first dose of mRNA-1273 in US Open Claims, and 2 938 023 people vaccinated with a second dose in US Open Claims. Cohort characteristics are summarised in supplementary A tables 2-7.
Noticeable differences existed in baseline patient characteristics before matching when comparing first dose ChAdOx1-S with first dose BNT162b2 recipients in UK CPRD data (supplementary A table 2). BNT162b2 recipients were more likely to be female (1 050 372 (58.2%) v 1 926 800 (51.5%)) and older and had a higher prevalence of comorbidities of interest. They were also more likely to use common medications such as treatments for hypertension and diabetes.
To reduce confounding, we estimated propensity scores for each vaccine pair and database. Supplementary A table 15 summarises the top 10 variables with stronger association with vaccine type in each of the databases. Propensity score matching led to a final cohort of 1.2 million ChAdOx1-S and 1.8 million BNT162b2 recipients (fig 1, supplementary A table 2). Patient characteristics after matching were comparable for most vaccination cohort pairs and databases, and are described in detail in supplementary A tables 2-7. The cohort selection process of all included cohorts is detailed in supplementary A table 14.
Fig 1.
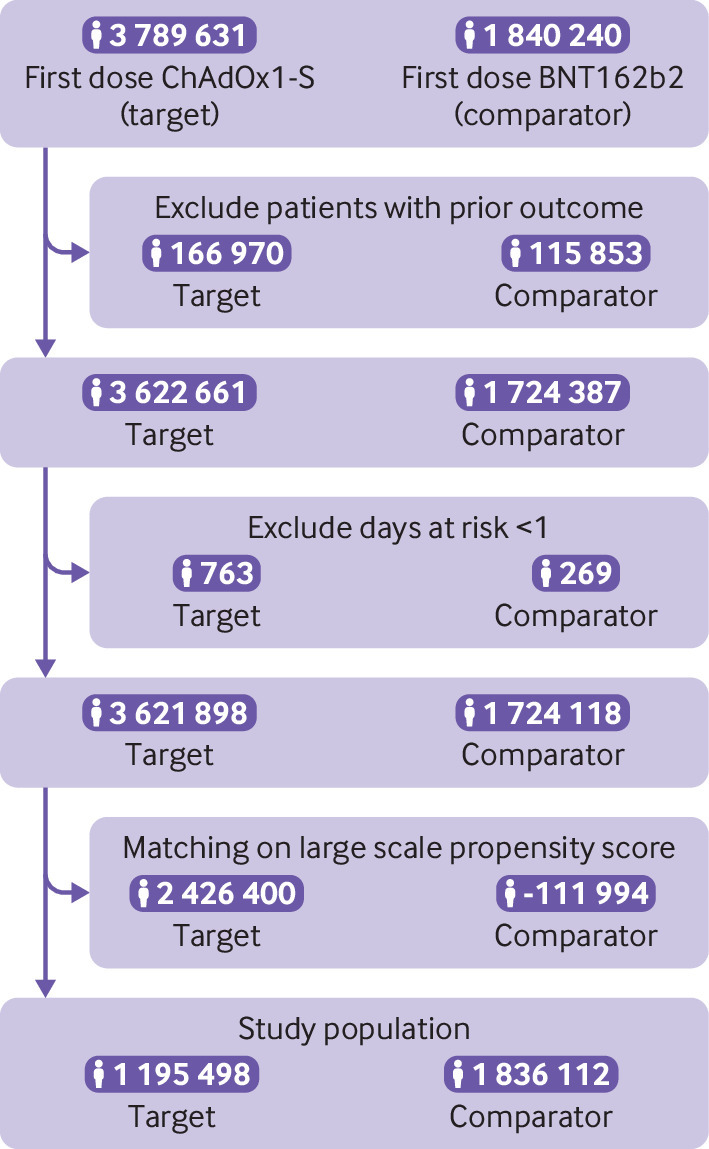
Study cohort selection. Example shows data from the UK database Clinical Practice Research Datalink Aurum used to compare the risk of thrombocytopenia after a first dose of ChAdOx1-S vaccine (target) compared with a first dose of BNT162b2 vaccine (comparator)
Study diagnostics: confounding and statistical power
We applied three diagnostic tests to evaluate the robustness of our analyses, based on measured confounding, statistical power, and unmeasured confounding. Supplementary A table 8 summarises the diagnostics. Firstly, to avoid bias due to confounding, we did not analyse cohorts with substantial differences after matching: 14 analyses passed this diagnostic, where no patient characteristic had a standardised mean difference of ≥0.1 after propensity score matching. All available comparisons in UK CPRD, the Netherlands IPCI, and US Open Claims met the measured confounding requirements. In Spain SIDIAP, only Ad26.COV2.S compared with BNT162b2 showed covariate balance after matching. Other combinations of database, target, and comparator that passed the covariate balance test included: first and second dose Germany DA ChAdOx1-S compared with BNT162b2, Germany DA Ad26.COV2.S compared with BNT162b2, France LPD ChAdOx1-S compared with first dose BNT162b2, and France LPD ChAdOx1-S compared with first dose mRNA-1273. Conversely, no analysis was conducted in the US Hospital CDM, because residual confounding was noted (standardised mean difference >0.1 for ≥1 variables).
Secondly, eight analyses had sufficient statistical power for at least one outcome, as noted by a minimum detectable rate ratio <5. However, France LPD failed the power diagnostics for all study outcomes. Therefore, no database specific estimates were reported for France LPD, although this database contributed to meta-analyses (see below).
Thirdly, negative control outcomes were used to identify residual confounding. Of seven combinations of database, target, and comparator with sufficient negative control outcomes, three had >20% associated with vaccine use (Germany DA Ad26.COV2.S v BNT162b2, US Open Claims Ad26.COV2.S v BNT162b2, and US Open Claims Ad26.COV2.S v mRNA-1273), suggesting the presence of substantial systematic error (supplementary A figs 1 and 2). Most of the estimates for these negative control outcomes had an incidence rate ratio >1, suggesting that our uncalibrated results overestimated risks, and that only calibrated results should be considered adequate.
For Germany DA ChAdOx1-S compared with second dose BNT162b2, France DA ChAdOx1-S compared with first dose mRNA-1273, and all comparisons within the Netherlands IPCI, too few negative control outcomes were observed, which precluded the use of empirical calibration.
Comparative safety
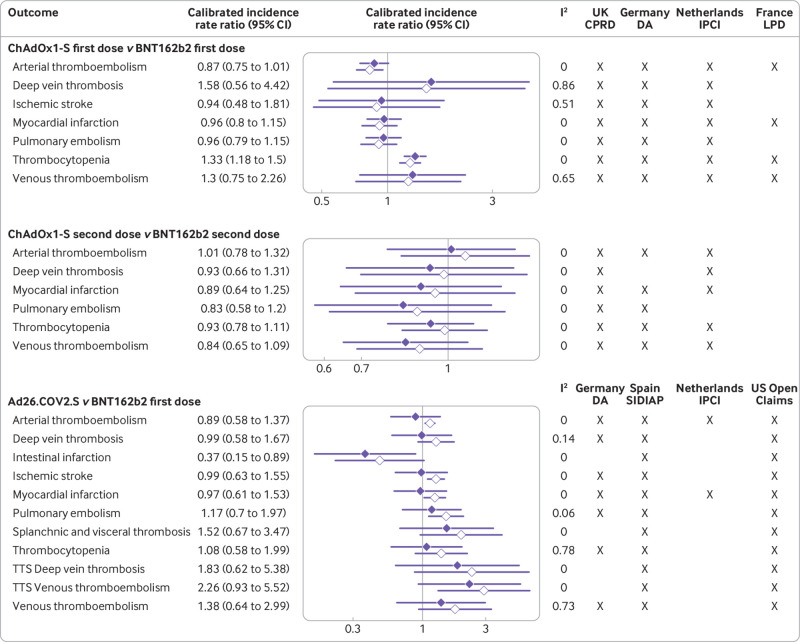
Crude incidence rates before matching are available in supplementary A tables 9 and 10. Database specific results from the seven combinations that passed all three diagnostics after matching are reported in table 2 and table 3. Figure 2 depicts meta-analytical incidence rate ratios for all analyses where two or more databases contributed after diagnostics for three comparisons: first and second dose ChAdOx1-S compared with BNT162b2, and Ad26.COV2.S compared with BNT162b2.
Table 2.
Incidence rates per 1000 person years and incidence rate ratios of developing thrombosis with thrombocytopenia syndrome or venous or arterial thromboembolic events in the 28 days after use of ChAdOx1-S versus mRNA based covid-19 vaccines in analyses passing diagnostic tests among matched cohorts
| Vaccination and outcome | Database | No of participants after propensity score matching* | No of person years | No of events | Incidence rates (95% CI)/1000 person years | Calibrated incidence rate ratio (95% CI) |
|---|---|---|---|---|---|---|
| First dose ChAdOx1-S v BNT162b2 | ||||||
| Arterial thromboembolism | UK CPRD |
1 227 495 | 92 807 | 331 | 3.57 (3.19 to 3.97) | Reference |
| 1 886 308 | 140 256 | 416 | 2.97 (2.69 to 3.27) | 0.85 (0.73 to 0.99) | ||
| Germany DA |
204 702 | 15 530 | 44 | 2.83 (2.06 to 3.8) | Reference | |
| 82 643 | 6261 | 19 | 3.03 (1.83 to 4.74) | 0.76 (0.41 to 1.39) | ||
| Deep vein thrombosis | UK CPRD |
1 247 556 | 94 341 | 150 | 1.59 (1.35 to 1.87) | Reference |
| 1 912 752 | 142 268 | 193 | 1.36 (1.17 to 1.56) | 0.89 (0.71 to 1.11) | ||
| Germany DA |
211 587 | 16 056 | 21 | 1.31 (0.81 to 2) | Reference | |
| 85 163 | 6,454 | 21 | 3.25 (2.01 to 4.97) | 2.62 (1.34 to 5.13) | ||
| Intestinal infarction | UK CPRD | 1 270 917 | 96 126 | 14 | 0.15 (0.08 to 0.24) | Reference |
| 1 945 248 | 144 743 | 22 | 0.15 (0.1 to 0.23) | 1.06 (0.53 to 2.13) | ||
| Ischaemic stroke | UK CPRD | 1 264 894 | 95 666 | 76 | 0.79 (0.63 to 0.99) | Reference |
| 1 936 816 | 144 104 | 75 | 0.52 (0.41 to 0.65) | 0.66 (0.48 to 0.92) | ||
| Germany DA | 210 616 | 15 982 | 15 | 0.94 (0.53 to 1.55) | Reference | |
| 84 835 | 6429 | 11 | 1.71 (0.85 to 3.06) | 1.34 (0.58 to 3.09) | ||
| Myocardial infarction | UK CPRD | 1 233 874 | 93 294 | 201 | 2.15 (1.87 to 2.47) | Reference |
| 1 895 358 | 140 942 | 283 | 2.01 (1.78 to 2.26) | 0.94 (0.78 to 1.14) | ||
| Germany DA | 208 975 | 15 856 | 26 | 1.64 (1.07 to 2.4) | Reference | |
| 84 048 | 6368 | 10 | 1.57 (0.75 to 2.89) | 0.70 (0.31 to 1.57) | ||
| Pulmonary embolism | UK CPRD | 1 254 781 | 94 894 | 197 | 2.08 (1.8 to 2.39) | Reference |
| 1 922 818 | 143 038 | 269 | 1.88 (1.66 to 2.12) | 0.93 (0.77 to 1.12) | ||
| Germany DA | 212 362 | 16 115 | 20 | 1.24 (0.76 to 1.92) | Reference | |
| 85 493 | 6479 | 6 | 0.93 (0.34 to 2.02) | 0.69 (0.26 to 1.83) | ||
| Thrombocytopenia | UK CPRD | 1 195 498 | 90 381 | 442 | 4.89 (4.45 to 5.37) | Reference |
| 1 836 112 | 136 523 | 827 | 6.06 (5.65 to 6.48) | 1.31 (1.16 to 1.49) | ||
| Germany DA | 204 508 | 15 516 | 78 | 5.03 (3.97 to 6.27) | Reference | |
| 82 281 | 6234 | 35 | 5.61 (3.91 to 7.81) | 1.01 (0.63 to 1.62) | ||
| Any thrombosis (venous thromboembolism or arterial thromboembolism) with thrombocytopenia syndrome | UK CPRD | 1 263 613 | 95 571 | 64 | 0.67 (0.52 to 0.86) | Reference |
| 1 934 651 | 143 950 | 121 | 0.84 (0.7 to 1) | 1.29 (0.94 to 1.77) | ||
| Venous thromboembolism | UK CPRD | 1 233 788 | 93 290 | 314 | 3.37 (3 to 3.76) | Reference |
| 1 893 469 | 140 803 | 420 | 2.98 (2.7 to 3.28) | 0.91 (0.78 to 1.06) | ||
| Germany DA | 209 244 | 15 878 | 40 | 2.52 (1.8 to 3.43) | Reference | |
| 84 436 | 6398 | 25 | 3.91 (2.53 to 5.77) | 1.61 (0.92 to 2.83) | ||
| Second dose ChAdOx1-S v BNT162b2 | ||||||
| Thrombocytopenia | UK CPRD | 1 012 563 | 60 302 | 347 | 5.75 (5.16 to 6.39) | Reference |
| 747 810 | 38 474 | 230 | 5.98 (5.23 to 6.8) | 0.94 (0.76 to 1.16) | ||
| Any thrombosis (venous thromboembolism or arterial thromboembolism) with thrombocytopenia syndrome | UK CPRD | 1 076 722 | 64 277 | 42 | 0.65 (0.47 to 0.88) | Reference |
| 795 629 | 41 080 | 38 | 0.93 (0.65 to 1.27) | 1.16 (0.71 to 1.89) | ||
| Deep vein thrombosis | UK CPRD | 1 063 064 | 63 456 | 96 | 1.51 (1.23 to 1.85) | Reference |
| 784 878 | 40 506 | 61 | 1.51 (1.15 to 1.93) | 0.93 (0.65 to 1.34) | ||
| Pulmonary embolism | UK CPRD | 1 069 375 | 63 835 | 92 | 1.44 (1.16 to 1.77) | Reference |
| 789 797 | 40 767 | 53 | 1.3 (0.97 to 1.7) | 0.86 (0.58 to 1.26) | ||
| Venous thromboembolism | UK CPRD | 1 050 916 | 62 715 | 179 | 2.85 (2.45 to 3.3) | Reference |
| 775 486 | 39 998 | 105 | 2.63 (2.15 to 3.18) | 0.87 (0.66 to 1.16) | ||
| Ischaemic stroke | UK CPRD | 1 078 360 | 64 368 | 28 | 0.43 (0.29 to 0.63) | Reference |
| 796 695 | 41 129 | 23 | 0.56 (0.35 to 0.84) | 1.20 (0.66 to 2.18) | ||
| Myocardial infarction | UK CPRD | 1 050 018 | 62 656 | 109 | 1.74 (1.43 to 2.1) | Reference |
| 774 713 | 39 952 | 61 | 1.53 (1.17 to 1.96) | 0.91 (0.64 to 1.3) | ||
| Arterial thromboembolism | UK CPRD | 1 044 491 | 62 307 | 153 | 2.46 (2.08 to 2.88) | Reference |
| 770 339 | 39 705 | 101 | 2.54 (2.07 to 3.09) | 1.05 (0.78 to 1.4) | ||
UK CPRD=Clinical Practice Research Datalink Aurum; Germany DA=IQVIA Disease Analyser Germany.
Numbers of participants differ for each outcome, because patients with a previous history of a given outcome of interest were excluded before the propensity score matching for that analysis.
Table 3.
Incidence rates per 1000 person years and incidence rate ratios of developing thrombosis with thrombocytopenia syndrome or venous or arterial thromboembolic events in the 28 days after use of Ad26.COV2.S versus mRNA based covid-19 vaccines in analyses passing diagnostic tests among matched cohorts
| Vaccination and outcome | Database | No of participants after propensity score matching† | No of person years | No of events | Incidence rates (95% CI)/1000 person years | Calibrated incidence rate ratio (95% CI) |
|---|---|---|---|---|---|---|
| Ad26.COV2.S v BNT162b2 | ||||||
| Thrombocytopenia | Germany DA |
65 217 | 4894 | 14 | 2.86 (1.56 to 4.8) | Reference |
| 17 933 | 1213 | 12 | 9.89 (5.11 to 17.28) | 1.30 (0.57 to 2.93)* | ||
| Spain SIDIAP |
386 334 | 19 944 | 197 | 9.88 (8.55 to 11.36) | Reference | |
| 106 217 | 5037 | 49 | 9.73 (7.2 to 12.86) | 0.77 (0.55 to 1.08) | ||
| US Open Claims | 2 364 195 | 172 698 | 470 | 2.72 (2.48 to 2.98) | Reference | |
| 628 293 | 46 997 | 170 | 3.62 (3.09 to 4.2) | 1.03 (0.63 to 1.7)* | ||
| US Open Claims | 2 231 498 | 169 780 | 484 | 2.85 (2.6 to 3.12) | Reference | |
| 628 459 | 47 007 | 170 | 3.62 (3.09 to 4.2) | 0.88 (0.56 to 1.4)* | ||
| Venous thromboembolism with thrombocytopenia syndrome | US Open Claims | 2 404 904 | 175 752 | 13 | 0.07 (0.04 to 0.13) | Reference |
| 639 269 | 47 828 | 11 | 0.23 (0.11 to 0.41) | 2.45 (0.95 to 6.29)* | ||
| Any thrombosis (venous thromboembolism or arterial thromboembolism) with thrombocytopenia syndrome | US Open Claims | 2 365 254 | 172 778 | 378 | 2.19 (1.97 to 2.42) | Reference |
| 628 571 | 47 019 | 146 | 3.11 (2.62 to 3.65) | 1.11 (0.67 to 1.84)* | ||
| Deep vein thrombosis | Spain SIDIAP | 421 532 | 22 028 | 33 | 1.5 (1.03 to 2.1) | Reference |
| 116 087 | 5582 | 10 | 1.79 (0.86 to 3.29) | 0.94 (0.45 to 1.96) | ||
| US Open Claims | 2 363 428 | 172 627 | 347 | 2.01 (1.8 to 2.23) | Reference | |
| 628 002 | 46 974 | 121 | 2.58 (2.14 to 3.08) | 0.98 (0.59 to 1.63)* | ||
| Pulmonary embolism | Spain SIDIAP | 422 330 | 22 072 | 14 | 0.63 (0.35 to 1.06) | Reference |
| 116 315 | 5593 | 5 | 0.89 (0.29 to 2.09) | 1.06 (0.37 to 3.07) | ||
| US Open Claims | 2 380 869 | 173 941 | 250 | 1.44 (1.26 to 1.63) | Reference | |
| 632 834 | 47 339 | 105 | 2.22 (1.81 to 2.69) | 1.18 (0.7 to 1.98)* | ||
| Venous thromboembolism | Spain SIDIAP | 420 502 | 21 960 | 42 | 1.91 (1.38 to 2.59) | Reference |
| 115 760 | 5562 | 14 | 2.52 (1.38 to 4.22) | 1.03 (0.55 to 1.93) | ||
| US Open Claims | 2 348 419 | 171 499 | 506 | 2.95 (2.7 to 3.22) | Reference | |
| 624 001 | 46 670 | 190 | 4.07 (3.51 to 4.69) | 1.06 (0.64 to 1.74)* | ||
| Ischaemic stroke | Spain SIDIAP | 417 793 | 21 749 | 61 | 2.8 (2.15 to 3.6) | Reference |
| 114 999 | 5,509 | 18 | 3.27 (1.94 to 5.16) | 1.04 (0.59 to 1.81) | ||
| US Open Claims | 2 348 140 | 171 471 | 540 | 3.15 (2.89 to 3.43) | Reference | |
| 623 396 | 46 622 | 193 | 4.14 (3.58 to 4.77) | 1.02 (0.62 to 1.67)* | ||
| Myocardial infarction | Spain SIDIAP | 418 734 | 21 822 | 38 | 1.74 (1.23 to 2.39) | Reference |
| 115 276 | 5528 | 10 | 1.81 (0.87 to 3.33) | 0.81 (0.38 to 1.71) | ||
| US Open Claims | 2 356 142 | 172 074 | 472 | 2.74 (2.5 to 3) | Reference | |
| 625 168 | 46 757 | 168 | 3.59 (3.07 to 4.18) | 1.02 (0.62 to 1.68)* | ||
| Intestinal infarction | US Open Claims | 2 401 293 | 175 480 | 53 | 0.3 (0.23 to 0.4) | Reference |
| 638 257 | 47 752 | 7 | 0.15 (0.06 to 0.3) | 0.35 (0.14 to 0.87) | ||
| Arterial thromboembolism | Spain SIDIAP | 413 039 | 21 426 | 119 | 5.55 (4.6 to 6.65) | Reference |
| 113 588 | 5421 | 34 | 6.27 (4.34 to 8.76) | 0.93 (0.62 to 1.39) | ||
| US Open Claims | 2 304 844 | 168 208 | 2231 | 13.26 (12.72 to 13.83) | Reference | |
| 610 895 | 45 673 | 720 | 15.76 (14.63 to 16.96) | 0.92 (0.57 to 1.48)* | ||
| Splanchnic and visceral thrombosis | US Open Claims | 2 404 366 | 175 711 | 19 | 0.11 (0.07 to 0.17) | Reference |
| 639 111 | 47 816 | 10 | 0.21 (0.1 to 0.38) | 1.46 (0.59 to 3.61)* | ||
| Ad26.COV2.S v mRNA-1273 | ||||||
| Deep vein thrombosis with thrombocytopenia syndrome | US Open Claims | 2 271 774 | 172 851 | 12 | 0.07 (0.04 to 0.12) | Reference |
| 639 496 | 47 843 | 6 | 0.13 (0.05 to 0.27) | 1.35 (0.45 to 4.05)* | ||
| Venous thromboembolism with thrombocytopenia syndrome | US Open Claims | 2 271 552 | 172 835 | 14 | 0.08 (0.04 to 0.14) | Reference |
| 639 432 | 47 838 | 11 | 0.23 (0.11 to 0.41) | 1.92 (0.77 to 4.8)* | ||
| Any thrombosis (venous thromboembolism or arterial thromboembolism) with thrombocytopenia syndrome | US Open Claims | 2 232 550 | 169 861 | 380 | 2.24 (2.02 to 2.47) | Reference |
| 628 737 | 47 028 | 146 | 3.1 (2.62 to 3.65) | 0.97 (0.61 to 1.55)* | ||
| Deep vein thrombosis | US Open Claims | 2 230 157 | 169 676 | 336 | 1.98 (1.77 to 2.2) | Reference |
| 628 164 | 46 983 | 121 | 2.58 (2.14 to 3.08) | 0.92 (0.57 to 1.48)* | ||
| Pulmonary embolism | US Open Claims | 2 247 746 | 171 017 | 227 | 1.33 (1.16 to 1.51) | Reference |
| 632 997 | 47 349 | 105 | 2.22 (1.81 to 2.68) | 1.15 (0.71 to 1.87)* | ||
| Venous thromboembolism | US Open Claims | 2 215 499 | 168 558 | 488 | 2.9 (2.64 to 3.16) | Reference |
| 624 163 | 46 679 | 190 | 4.07 (3.51 to 4.69) | 0.99 (0.62 to 1.56)* | ||
| Ischaemic stroke | US Open Claims | 2 214 613 | 168 485 | 533 | 3.16 (2.9 to 3.44) | Reference |
| 623 557 | 46 632 | 193 | 4.14 (3.58 to 4.77) | 0.93 (0.59 to 1.47)* | ||
| Myocardial infarction | US Open Claims | 2 222 711 | 169 104 | 513 | 3.03 (2.78 to 3.31) | Reference |
| 625 329 | 46 766 | 168 | 3.59 (3.07 to 4.18) | 0.86 (0.54 to 1.36)* | ||
| Intestinal infarction | US Open Claims | 2 267 972 | 172 560 | 54 | 0.31 (0.24 to 0.41) | Reference |
| 638 418 | 47 761 | 7 | 0.15 (0.06 to 0.3) | 0.29 (0.12 to 0.73)* | ||
| Arterial thromboembolism | US Open Claims | 2 171 445 | 165 188 | 2246 | 13.6 (13.04 to 14.17) | Reference |
| 611 054 | 45 682 | 720 | 15.76 (14.63 to 16.96) | 0.83 (0.54 to 1.28)* | ||
| Splanchnic and visceral thrombosis | US Open Claims | 2 271 071 | 172 798 | 17 | 0.1 (0.06 to 0.16) | Reference |
| 639 274 | 47 826 | 10 | 0.21 (0.1 to 0.38) | 1.48 (0.60 to 3.65)* | ||
Germany DA=IQVIA Disease Analyser Germany; Spain SIDIAP=Information System for Research in Primary Care.
Did not pass the systematic error diagnostic test of >80% uncalibrated confidence intervals covering 1.
Numbers of participants differ for each outcome, because patients with a previous history of that outcome were excluded before the propensity score matching for that analysis.
Fig 2.
Meta-analytical estimates of incidence rate ratios of developing thrombosis with thrombocytopenia syndrome or venous or arterial thromboembolic events in the 28 days after covid-19 vaccination, according to information from routinely collected health databases. Lines with solid diamonds=calibrated estimates; lines with clear diamonds=uncalibrated estimates; TTS=thrombosis with thrombocytopenia syndrome; UK CPRD=Clinical Practice Research Datalink Aurum; Germany DA=IQVIA Disease Analyser Germany; Netherlands IPCI=Integrated Primary Care Information; France LPD=IQVIA Longitudinal Patient Data
We observed a total of 862 thrombocytopenia events were in the matched first dose ChAdOx1-S recipients from Germany and the UK, and 520 events after a first dose of BNT162b2. Meta-analyses showed an increased risk of thrombocytopenia after first dose ChAdOx1-S compared with BNT162b2, with a pooled calibrated incidence rate ratio of 1.33 (95% confidence interval 1.18 to 1.50; fig 2), a calibrated incidence rate difference of 1.18 (0.57 to 1.8) per 1000 person years, and an absolute risk difference of 8.21 (3.59 to 12.82) per 100 000 recipients. In UK CPRD data, 827 and 442 thrombocytopenia events occurred after first dose ChAdOx1-S and BNT162b2, respectively. Incidence rates were 6.06 per 1000 person years (95% confidence interval 5.65 to 6.48) and 4.89 (4.45 to 5.37), respectively, with a calibrated incidence rate ratio of 1.31 (1.16 to 1.49). This finding was not replicated in the Germany DA data, where the calibrated incidence rate ratio was 1.01 (0.63 to 1.62). No differential risk of thrombocytopenia was seen after the second dose of ChAdOx1-S versus second dose BNT162b2 (meta-analytical calibrated incidence rate ratio 0.93 (0.78 to 1.11); fig 2). Similarly, no increased risk of thrombocytopenia was noted after Ad26.COV2.S compared with first dose BNT162b2 (meta-analytical calibrated incidence rate ratio 1.08 (0.58 to 1.99); fig 2).
For venous thromboembolism and deep vein thrombosis, the meta-analysis was unreliable because of heterogeneity (I2 values of 65% and 86%, respectively). No increased risk of venous thromboembolism was seen after the first dose of ChAdOx1-S versus BNT162b2 either in Germany DA (calibrated incidence rate ratio 1.61 (95% confidence interval 0.92 to 2.83)) or UK CPRD (0.91 (0.78 to 1.06); table 2). An increased risk of deep vein thrombosis was seen after first dose ChAdOx1-S compared with BNT162b2 in Germany DA (2.62, 1.34 to 5.13), but not replicated in UK CPRD data (0.89, 0.71 to 1.11; table 2). No increased risk of pulmonary embolism was seen in either database, with calibrated incidence rate ratio 0.93 (0.77 to 1.12) and 0.69 (0.26 to 1.83) in UK and German data respectively. No differential risks of venous thromboembolism, deep vein thrombosis, or pulmonary embolism were noted when comparing second dose ChAdOx1-S with BNT162b2 in pooled meta-analysis or database specific analyses (table 2, fig 2). In line with this, no association was seen between vaccination with Ad26.COV2.S and any venous thromboembolic event in database specific (table 3) or pooled meta-analysis (fig 2). Regarding rare thrombosis, the meta-analysis showed a lower risk of intestinal infarction for the single dose Ad26.COV2.S users compared with first dose BNT162b2, with a pooled calibrated incidence rate ratio of 0.37 (0.15 to 0.89), an incidence rate difference of −0.41 (−1.17 to 0.35) per 1000 person years, and an absolute risk difference of −3.34 (−9.77 to 3.09) per 100 000 vaccinations (fig 2). No other rare thrombotic events had differential risks between cohorts.
For composite arterial thromboembolism, the pooled calibrated incidence rate ratio for first dose ChAdOx1-S compared with first dose BNT162b2 was 0.87 (95% confidence interval 0.75 to 1.01; fig 2). The two reliable database specific analyses in table 2 showed consistent findings—the calibrated incidence rate ratio was 0.85 (0.73 to 0.99) in CPRD UK and 0.76 (0.41 to 1.39) in Germany DA. In line with this, no differences in risk of arterial thromboembolism, ischaemic stroke, or myocardial infarction were seen after second dose ChAdOx1-S versus two dose BNT162b2 or after Ad26.COV2.S versus first dose BNT162b2. Similar results were seen also for ischaemic stroke and myocardial infarction when analysed separately (table 3, fig 2).
Thrombosis with thrombocytopenia syndrome was very rare, and could only be analysed in UK data for ChAdOx1-S and in US and Spanish data for Ad26.COV2.S. A trend towards an increase in risk of thrombosis with thrombocytopenia syndrome was observed in UK CPRD after first dose ChAdOx1-S compared with first dose BNT162b2 (calibrated incidence rate ratio 1.29 (95% confidence interval 0.94 to 1.77)). The calibrated incidence rate ratio after second dose was 1.16 (0.71 to 1.89). For comparing Ad26.COV2.S with BNT162b2, meta-analyses were possible for venous thromboembolism with thrombocytopenia syndrome and deep vein thrombosis with thrombocytopenia syndrome. A similar association was seen for venous thromboembolism with thrombocytopenia syndrome in the meta-analysis of US and Spanish data (pooled calibrated incidence rate ratio 2.26 (0.93 to 5.52)) and, with much more uncertainty, for deep vein thrombosis with thrombocytopenia syndrome (1.83 (0.62 to 5.38); fig 2). Database specific estimates from US Open Claims were in line with the pooled results (table 3).
Sensitivity and subgroup analyses
Sensitivity analyses restricting the time window for thrombosis with thrombocytopenia syndrome to five days or reducing the threshold of platelet count (to lower than 100 000 platelets per μL) found results consistent with the main analysis (table 4).
Table 4.
Sensitivity analysis of incidence rates per 1000 person years and incidence rate ratios of developing thrombosis with thrombocytopenia syndrome or venous or arterial thromboembolic events in the 28 days after use of adenovirus versus mRNA based covid-19 vaccination in analyses passing diagnostics
| Sensitivity analysis and medical records database | Target comparator combination | Event | No of individuals after propensity score matching | No of person years | No of events | Incidence rates (95% CI)/1000 person years | Calibrated incidence rate ratio (95% CI) |
|---|---|---|---|---|---|---|---|
| Thrombocytopenia window to five days before/after thrombosis after vaccination | |||||||
| UK CPRD |
First dose BNT162b2 (comparator) | Any thrombosis (venous thromboembolism or arterial thromboembolism) with thrombocytopenia syndrome | 1 934 829 | 95 580 | 63 | 0.66 (0.51 to 0.84) | Reference |
| First dose ChAdOx1-S (target) | 1 934 829 | 143 963 | 120 | 0.83 (0.69 to 1) | 1.3 (0.95 to 1.79) | ||
| Second dose BNT162b2 (comparator) | 1 076 870 | 64 286 | 38 | 0.59 (0.42 to 0.81) | Reference | ||
| Second dose ChAdOx1-S (target) | 795 723 | 41 085 | 37 | 0.9 (0.63 to 1.24) | 1.23 (0.74 to 2.04) | ||
| US Open Claims |
First dose BNT162b2 (comparator) | 2 365 342 | 172 785 | 376 | 2.18 (1.96 to 2.41) | Reference | |
| First dose Ad26.COV2.S (target) | 628 592 | 47 020 | 143 | 3.04 (2.56 to 3.58) | 1.09 (0.66 to 1.81)* | ||
| First dose mRNA-1273 (comparator) | 2 232 627 | 169 867 | 378 | 2.23 (2.01 to 2.46) | Reference | ||
| Ad26.COV2.S (target) | 628 758 | 47 030 | 143 | 3.04 (2.56 to 3.58) | 0.96 (0.6 to 1.53)* | ||
| Thrombocytopenia threshold of <100 000 platelets per microlitre | |||||||
| US Open Claims |
First dose mRNA-1273 (comparator) | Deep vein thrombosis with thrombocytopenia syndrome | 2 271 774 | 172 851 | 12 | 0.07 (0.04 to 0.12) | Reference |
| Ad26.COV2.S (target) | 639 496 | 47 843 | 6 | 0.13 (0.05 to 0.27) | 1.35 (0.45 to 4.05)* | ||
| US Open Claims |
First dose BNT162b2 (comparator) | Venous thromboembolism with thrombocytopenia syndrome | 2 404 904 | 175 752 | 13 | 0.07 (0.04 to 0.13) | Reference |
| Ad26.COV2.S (target) | 639 269 | 47 828 | 11 | 0.23 (0.11 to 0.41) | 2.45 (0.95 to 6.29)* | ||
| First dose mRNA-1273 (comparator) | 2 271 552 | 172 835 | 14 | 0.08 (0.04 to 0.14) | Reference | ||
| Ad26.COV2.S (target) | 639 432 | 47 838 | 11 | 0.23 (0.11 to 0.41) | 1.92 (0.77 to 4.8)* | ||
| UK CPRD |
First dose BNT162b2 (comparator) | Any thrombosis (venous thromboembolism or arterial thromboembolism) with thrombocytopenia syndrome | 1 263 960 | 95 597 | 63 | 0.66 (0.51 to 0.84) | Reference |
| First dose ChAdOx1-S (target) | 1 935 138 | 143 986 | 119 | 0.83 (0.68 to 0.99) | 1.29 (0.94 to 1.78) | ||
| Second dose BNT162b2 (comparator) | 1 077 077 | 64 299 | 39 | 0.61 (0.43 to 0.83) | Reference | ||
| Second dose ChAdOx1-S (target) | 795 893 | 41 094 | 37 | 0.9 (0.63 to 1.24) | 1.25 (0.76 to 2.06) | ||
| US Open Claims |
First dose BNT162b2 (comparator) | 2 365 254 | 172 778 | 378 | 2.19 (1.97 to 2.42) | Reference | |
| Ad26.COV2.S (target) | 628 571 | 47 019 | 146 | 3.11 (2.62 to 3.65) | 1.11 (0.67 to 1.84)* | ||
| First dose mRNA-1273 (comparator) | 2 232 550 | 169 861 | 380 | 2.24 (2.02 to 2.47) | Reference | ||
| Ad26.COV2.S (target) | 628 737 | 47 028 | 146 | 3.1 (2.62 to 3.65) | 0.97 (0.61 to 1.55)* | ||
UK CPRD=Clinical Practice Research Datalink Aurum.
Did not pass the systematic error diagnostics of >80% uncalibrated confidence intervals covering 1.
Stratified analyses are reported in supplementary A tables 11 and 12, and include findings from the UK CPRD and US Open Claims databases, as these were the only ones with sufficient power (minimum detectable rate ratio <5) for at least one outcome. An increased risk of thrombocytopenia was observed in those aged 40-49 years, 70-79 years, and among women in the UK data receiving first dose ChAdOx1-S compared with first dose BNT162b2. Additionally, the calibrated incidence rate ratio for composite arterial thromboembolism after ChAdOx1-S compared with BNT162b2 vaccination was lower in men, with a calibrated incidence rate ratio of 0.75 (95% confidence interval 0.61 to 0.92) (supplementary A table 11). Conversely, a subgroup analysis in US Open Claims data found an increased risk of arterial thromboembolism after Ad26.COV2.S compared with BNT162b2 and mRNA-1273 vaccination in people aged 20-29 years (calibrated incidence rate ratio 4.64 (2.16 to 9.97) and 5.10 (1.71 to 15.19), respectively). This finding was not replicated in any other subgroups.
Discussion
Principal findings
To our knowledge, this is the first multinational analysis of the comparative safety of adenovirus based compared with mRNA based covid-19 vaccines. In this matched cohort study, we compared the rates of thrombosis and of thrombosis with thrombocytopenia within 28 days after vaccination. We used routinely collected health data from five European countries and the US, and produced risk estimates after applying methods to minimise confounding and systematic error. After excluding many analyses because of identified confounding or limited statistical power, we observed a 30% increased risk of thrombocytopenia following first dose ChAdOx1-S compared with first dose BNT162b2.
Findings in context
Thrombosis with concomitant thrombocytopenia was very rare, and we did not find any statistically significant increase in risk with either adenovirus based vaccine compared with any mRNA based vaccine. However, this finding should be put in context with previous research, because some of our estimates were close to significance, suggesting a potential increased risk of venous thromboembolism with thrombocytopenia syndrome after vaccination with Ad26.COV2.S. While thrombosis events and thrombocytopenia have been studied as separate outcomes, thrombosis with thrombocytopenia syndrome has rarely been studied as an individual outcome in previous real world studies owing to the complexity of the case definition and rare nature of the outcome in case definition.38 A US case series using the Vaccine Adverse Event Reporting System estimated rates of thrombosis with thrombocytopenia syndrome to be 3.83 per 1 million vaccine doses of Ad26.COV2.S and 0.00855 per 1 million vaccine doses of mRNA based covid-19 vaccines.39 Yet the authors stated that cases of thrombosis with thrombocytopenia syndrome reported after mRNA vaccines were associated with different demographic characteristics and medical history compared with cases after Ad26.COV2.S. By comparison, we used routinely collected health data and were able to estimate the comparative risks between vaccines, therefore minimising surveillance bias.
Subgroup analyses showed a 25% lower risk of arterial thromboembolism after first dose ChAdOx1-S versus BNT162b2 in men based in the UK, and a fourfold to fivefold increased risk of arterial thromboembolism in younger people (aged 20-29 years) vaccinated with Ad26.COV2.S compared with either mRNA vaccine in the US. However, these findings were not replicated in other contributing data sources or in other age groups, and deserve further research.
Thrombosis with thrombocytopenia syndrome or vaccine induced immune thrombotic thrombocytopenia was first reported after use of the ChAdOx1-S vaccine in early 2021.4 5 A disproportionality analysis using WHO’s VigiBase database reported a safety signal for cerebral venous sinus thrombosis and ischaemic stroke for ChAdOx1-S, BNT162b2, and mRNA-1273.40 The authors called for well designed comparative safety studies on adverse events of all three vaccines. A study based on Danish and Norwegian data also found higher than expected rates of venous thromboembolism, pulmonary embolism, and cerebral venous sinus thrombosis after vaccination compared with background rates.10 While these studies provided important insights into the incidence of adverse outcomes reported after vaccination, they failed to adjust for potential confounders including comorbidity, frailty, nursing home residence, or history of other risk factors for thrombosis or coagulopathy.
The risk of thrombocytopenia after covid-19 vaccination has been studied by comparing vaccinated with unvaccinated groups, and using self-controlled designs. Hippisley-Cox et al conducted a self-controlled case series analysis of English data including about 30 million vaccinated people.12 They provided epidemiological evidence of a 30% increased risk of thrombocytopenia and venous thromboembolism after ChAdOx1-S vaccination, and an elevated risk of cerebral venous sinus thrombosis after ChAdOx1-S and BNT162b2. In a population based cohort study in England, Whiteley et al reported increased rates of thrombocytopenia during the 28 days after ChAdOx1-S compared with unvaccinated people among those aged under 70 years, but no association with BNT162b2.41 Our study compares both vaccines, and we found a 30% excess risk of thrombocytopenia after ChAdOx1-S compared with BNT162b2, consistent with previous studies.
Regarding arterial thromboembolism, a study from Scotland found an increased risk of arterial thromboembolic events in nested case-control analyses, which was attenuated in self-controlled case series analyses.13 An English self-controlled case series study found an increased risk of arterial thromboembolism after BNT162b2 but not ChAdOx1-S vaccination.12 Whiteley et al reported lower rates of major arterial thromboembolism and venous thromboembolism after vaccination with both ChAdOx1-S and BNT162b2 compared with unvaccinated people, after adjusting for potential confounding factors.41 Partially consistent with these results, we observed a lower rate of arterial thromboembolism after ChAdOx1-S compared with BNT162b2 in UK CPRD data, not replicated elsewhere or with other adenovirus based vaccines (Ad26.COV2.S v BNT162b2). The observed increase in risk of arterial thromboembolism in young people after Ad26.COV2.S versus mRNA based vaccines in US data was not replicated elsewhere or with ChAdOx1-S, and needs further research.
Study outcomes of cerebral venous sinus thrombosis and splanchnic and visceral thrombosis were also very rare. Kerr et al reported that cerebral venous sinus thrombosis was observed in about 16.34 per million doses of ChAdOx1-S, and 12.60 per million doses of BNT162b2. In a self-controlled case series analysis using data from England, Scotland, and Wales, ChAdOx1-S was associated with an elevated risk of cerebral venous sinus thrombosis in the 28 days after ChAdOx1-S vaccination (incidence rate ratio 1.93 (95% confidence interval 1.20 to 3.11)) but not after BNT162b2 vaccination.11 Similarly, a large record linkage study of hospital admissions in England showed an increased risk of cerebral venous sinus thrombosis after first dose ChAdOx1-S, seen only in adults aged under 65 years, and not after BNT162b2.42 In a previous study, we reported that background incidence rates varied across data sources, and suggested the use of analyses within databases for historical rate comparisons.43 In the present study, while we did not see large heterogeneity of incidence rates after vaccination between databases, relative rates varied. In our meta-analysis, the pooled estimates were largely driven by databases with larger sample sizes such as UK CPRD and US Open Claims data.
Strengths and limitations
The results of our study should be interpreted in the context of its known limitations. Owing to heterogeneity across data sources, misclassification of vaccine use and outcomes might be problematic. Regarding vaccination, the UK and Spanish data sources captured vaccine information more reliably than previous studies through linkage to official vaccination registries. By contrast, the German and French records and US datasets are expected to include incomplete vaccine records. The use of comparative safety analyses minimises the impact of this problem, because only vaccinated cohorts are included for analysis.
Information bias due to outcome ascertainment was likely to be present in our study. We used robust methods for the creation and transportation of algorithms for the identification of all of the study events.25 However, some study events typically treated in hospital could be incompletely captured in some of our databases, including the German and French data sources. But inpatient data were available for the Spanish database through linkage and for US claims based on reimbursement. Our choice of matched cohort design should additionally minimise the impact of misclassification, because we do not expect incompleteness to be conditional on the vaccine received.
As in any observational study, analyses are susceptible to unmeasured confounders. Although the routinely collected health data and the use of large scale propensity scores allowed us to control for many potential confounders, we observed evidence of systematic errors in some analyses, especially in the US Open Claims database. Factors such as health seeking behaviour or family history of study outcomes were unmeasured or partially measured. In our study, we used empirical calibration to account for the unmeasured confounding.
Each country has its own immunisation schedule, and the studied vaccines were not all approved at the same time. For example, the vaccination campaign began on 8 December 2020 in England, and BNT162b2 was first given to care home residents, people aged ≥80 years, and frontline health workers, followed by vulnerable people and those aged ≥70 years. Individuals vaccinated earlier therefore have higher background rates, especially for thromboembolic events. Age and calendar time were therefore essential confounders, accounted for in our propensity score models. Propensity score matching created comparable cohorts, at the cost of excluding those with extreme propensity score values, who could not find a match. For example, in the UK CPRD, while 11% of the original BNT162b2 cohort was indexed in December 2020, almost none was included after matching. This factor should be taken into account when interpreting our findings.
We analysed data up to mid-2021, so only the first and second waves of the pandemic were represented. However, the proportion of included people with a history of covid-19 infection before vaccination was balanced in all eligible comparisons, both before and after matching.
In our study, we reported the database specific incidence rates of outcomes for both the original full cohorts and the propensity score matched cohorts. The incidence rates from the full cohorts were crude without any adjustment. While reflecting the real world incidence, they were highly subjected to the population characteristics and thus were not directly comparable between cohorts. The incidence rates from matched cohorts, on the other hand, can be compared since the propensity score matching accounted for the measured confounding. Caution is needed when interpreting these incidence rates as the generalisability of the rates is limited.
Finally, and despite the use of large international data sources, we had limited power for the analysis of thrombosis with thrombocytopenia syndrome, a rare event, resulting in only three databases (UK, Spain, and the US) contributing to our findings. In addition, meta-analysis was only meaningful for the analysis of Ad26.CoV.S, and resulted in wide confidence intervals and borderline (not significant) estimates. These analyses therefore warrant replication elsewhere.
Our study also has important strengths. While other epidemiological methods have been used in vaccine safety studies, a cohort study with active comparators enabled us to directly estimate the relative risk of developing thromboembolic events or thrombosis with thrombocytopenia syndrome after different covid-19 vaccines, which is not feasible in self-controlled designs or in observed to expected analyses. Our study therefore answers a more reliable question at this stage of the pandemic (ie, “which vaccine is safer” rather than “are vaccines safer than no vaccination”). The OMOP CDM allowed us to replicate the exact same analysis across different databases, therefore improving robustness, transparency, and reproducibility.
To reduce bias and confounding and ensure the reported results are reliable, we used robust diagnostics in our study design and statistical analysis plan. We used large scale propensity score modelling based on an L1 regularised logistic regression to minimise observed confounding. This approach has been shown to preferable to traditional propensity score estimation.44 We examined residual confounding after matching, and did not perform analyses where relevant confounding was observed. Further, we leveraged previously validated negative control outcomes 27 45 to assess risk of residual (unobserved) bias. Empirical calibration was then used to minimise any remaining systematic error.
What is already known on this topic
Thrombosis with thrombocytopenia syndrome is being investigated as an adverse reaction of adenovirus based covid-19 vaccines
The comparative risk of thrombosis with thrombocytopenia syndrome or thromboembolic events after vaccination with different covid-19 vaccines remains unclear
What this study adds
This multinational analysis of comparative safety of covid-19 vaccines used routinely collected data from Europe and the US
A 30% increased risk of thrombocytopenia was seen after first dose ChAdOx1-S compared with first dose BNT162b2 vaccination
A trend towards an increased risk of venous thrombosis with thrombocytopenia was observed after a first vaccine dose of Ad26.COV2.S, which needs replication elsewhere
Although rare, the observed risks after adenovirus based vaccines should be considered when planning further immunisation campaigns and future vaccine development
Acknowledgments
We thank Jennifer A de Beyer (Centre for Statistics in Medicine, University of Oxford) for English language editing; and the Hospital del Mar for data availability (especially J P Horcajada, Robert Güerri, Jordi Martínez Roldán, and Ana Aldea).
Web extra.
Extra material supplied by authors
Web appendix 1: Supplementary materials A
Web appendix 2: Supplementary materials B
Contributors: XL, VS, and DP-A designed the study, and KV and CC reviewed and approved the study protocol. XL, VS, and DP-A wrote the related analysis plan, and KV and CC reviewed and approved it. TD-S, CY, CR, AD, PR, MM, LHJ, M-AM, J-MR-A, EB, and XL curated or analysed the data. MAS and KL contributed to analytical coding and related software. DP-A, KV, and PR were responsible for funding applications and project management. XL and DP-A wrote the first draft of the current manuscript, and all coauthors provided feedback and approved the final version for submission. XL and DP-A are guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by the European Medicines Agency. The views expressed in this article are the personal views of the authors, and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its committees or working parties. The funder had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the EMA for the submitted work; DP-A receives funding from the UK National Institute for Health Research (NIHR) in the form of a senior research fellowship and from the Oxford NIHR Biomedical Research Centre. XL receives the Clarendon Fund and Brasenose College scholarship (University of Oxford) to support her DPhil study. DP-A’s research group has received research grants from the EMA; Innovative Medicines Initiative; and Amgen, Chiesi, and UCB Biopharma; and consultancy or speaker fees from Astellas, Amgen, AstraZeneca, and UCB Biopharma. LHJ works for a research group who receives unconditional research grants from Yamanouchi, Pfizer-Boehringer Ingelheim, Novartis, GSK, Chiesi, Astra-Zeneca, Amgen, Janssen Research and Development. PR works for a research group who receives unconditional research grants from Yamanouchi, Pfizer-Boehringer Ingelheim, Novartis, GSK, Chiesi, Astra-Zeneca, Amgen, Janssen Research and Development. CC has no conflicts of interest. None of these declarations relate to the content of this paper.
The lead author (XL) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We will disseminate a lay summary of our findings through our Twitter and other social media accounts. Members of the general public could also help disseminate the paper, especially on social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The protocol for this research was approved by the independent scientific advisory committee for Medicine and Healthcare Products Regulatory Agency database research (protocol No 21_000641). Informed consent of individual patients was not required as anonymised information was obtained from medical records.
Data availability statement
Patient level data cannot be shared without approval from data custodians owing to local information governance and data protection regulations. The analytical code is available at: https://github.com/oxford-pharmacoepi/ROC22_CovVaxComparativeSafety/tree/main/CovVaxComparativeSafety. Additional correspondence and requests for materials should be addressed to the corresponding author (EB).
References
- 1. Baden LR, El Sahly HM, Essink B, et al. COVE Study Group . Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021;384:403-16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group . Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603-15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voysey M, Clemens SAC, Madhi SA, et al. Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124-30. 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092-101. 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med 2021;384:2254-6. 10.1056/NEJMe2106315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee E-J, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol 2021;96:534-7. 10.1002/ajh.26132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaxzevria EMA. further advice on blood clots and low blood platelets. European Medicines Agency. 2021.https://www.ema.europa.eu/en/news/vaxzevria-further-advice-blood-clots-low-blood-platelets.
- 9. Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2202-11. 10.1056/NEJMoa2105385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ 2021;373:n1114. 10.1136/bmj.n1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kerr S, Joy M, Torabi F, et al. First dose ChAdOx1 and BNT162b2 COVID-19 vaccinations and cerebral venous sinus thrombosis: A pooled self-controlled case series study of 11.6 million individuals in England, Scotland, and Wales. PLoS Med 2022;19:e1003927. 10.1371/journal.pmed.1003927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ 2021;374:n1931. 10.1136/bmj.n1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simpson CR, Shi T, Vasileiou E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med 2021;27:1290-7. 10.1038/s41591-021-01408-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voss EA, Makadia R, Matcho A, et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc 2015;22:553-64. 10.1093/jamia/ocu023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hripcsak G, Duke JD, Shah NH, et al. Observational Health Data Sciences and Informatics (OHDSI): Opportunities for Observational Researchers. Stud Health Technol Inform 2015;216:574-8. [PMC free article] [PubMed] [Google Scholar]
- 16. Overhage JM, Ryan PB, Reich CG, Hartzema AG, Stang PE. Validation of a common data model for active safety surveillance research. J Am Med Inform Assoc 2012;19:54-60. 10.1136/amiajnl-2011-000376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jouaville SL, Miotti H, Coffin G, et al. Validity and limitations of the longitudinal patient database France for use in pharmacoepidemiological and pharmacoeconomics studies. Value Health 2015;18:A18 10.1016/j.jval.2015.03.115. [DOI] [Google Scholar]
- 18. de Ridder MAJ, de Wilde M, de Ben C, et al. Data resource profile: The integrated primary care information (IPCI) database, the Netherlands. Int J Epidemiol 2022;19:dyac026. 10.1093/ije/dyac026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. García-Gil MdelM, Hermosilla E, Prieto-Alhambra D, et al. Construction and validation of a scoring system for the selection of high-quality data in a Spanish population primary care database (SIDIAP). Inform Prim Care 2011;19:135-45. [DOI] [PubMed] [Google Scholar]
- 20. Wolf A, Dedman D, Campbell J, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. Int J Epidemiol 2019;48:1740-1740g. 10.1093/ije/dyz034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Available IQVIA data . https://www.iqvia.com/insights/the-iqvia-institute/available-iqvia-data.
- 22.Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19). 2021.https://apps.who.int/iris/bitstream/handle/10665/342999/WHO-2019-nCoV-TTS-2021.1-eng.pdf?sequence=1&isAllowed=y. [PubMed]
- 23.Information for healthcare professionals on blood clotting following COVID-19 vaccination. GOV.UK. https://www.gov.uk/government/publications/covid-19-vaccination-blood-clotting-information-for-healthcare-professionals/information-for-healthcare-professionals-on-blood-clotting-following-covid-19-vaccination
- 24.Chen RT, Monash JB. November 11 v 2b DRAFT updated Brighton collaboration case definition for thrombosis with thrombocytopenia syndrome (TTS). https://brightoncollaboration.us/wp-content/uploads/2021/11/TTS-Updated-Brighton-Collaboration-Case-Defintion-Draft-Nov-11-2021.pdf.
- 25. Burn E, Li X, Kostka K, et al. Background rates of five thrombosis with thrombocytopenia syndromes of special interest for COVID-19 vaccine safety surveillance: Incidence between 2017 and 2019 and patient profiles from 38.6 million people in six European countries. Pharmacoepidemiol Drug Saf 2022;31:495-510. 10.1002/pds.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schuemie MJ, Arshad F, Pratt N, et al. Vaccine safety surveillance using routinely collected healthcare data – An empirical evaluation of epidemiological designs. bioRxiv. 2021;2021.08.09.21261780. 10.1101/2021.08.09.21261780 [DOI] [PMC free article] [PubMed]
- 27. Li X, Lai LY, Ostropolets A, et al. Bias, precision and timeliness of historical (background) rate comparison methods for vaccine safety monitoring: An empirical multi-database analysis. Front Pharmacol 2021;12:773875. 10.3389/fphar.2021.773875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46:1075-9, discussion 1081-90. 10.1016/0895-4356(93)90103-8 [DOI] [PubMed] [Google Scholar]
- 29. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 30. Suchard MA, Simpson SE, Zorych I, Ryan P, Madigan D. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans Model Comput Simul 2013;23:1-17. 10.1145/2414416.2414791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Armstrong B. A simple estimator of minimum detectable relative risk, sample size, or power in cohort studies. Am J Epidemiol 1987;126:356-8. 10.1093/aje/126.2.356 [DOI] [PubMed] [Google Scholar]
- 32. Schuemie MJ, Ryan PB, DuMouchel W, Suchard MA, Madigan D. Interpreting observational studies: why empirical calibration is needed to correct p-values. Stat Med 2014;33:209-18. 10.1002/sim.5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. Proc Natl Acad Sci U S A 2018;115:2571-7. 10.1073/pnas.1708282114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGrath LJ, Spangler L, Curtis JR, et al. Using negative control outcomes to assess the comparability of treatment groups among women with osteoporosis in the United States. Pharmacoepidemiol Drug Saf 2020;29:854-63. 10.1002/pds.5037 [DOI] [PubMed] [Google Scholar]
- 35. Lane JCE, Weaver J, Kostka K, et al. OHDSI-COVID-19 consortium . Risk of hydroxychloroquine alone and in combination with azithromycin in the treatment of rheumatoid arthritis: a multinational, retrospective study. Lancet Rheumatol 2020;2:e698-711. 10.1016/S2665-9913(20)30276-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morales DR, Conover MM, You SC, et al. Renin-angiotensin system blockers and susceptibility to COVID-19: an international, open science, cohort analysis. Lancet Digit Health 2021;3:e98-114. 10.1016/S2589-7500(20)30289-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.European Medicines Agency. Amsterdam, the Neherlands. ENCePP Guide on Methodological Standards in Pharmacoepidemiology (Version 9). https://www.encepp.eu/standards_and_guidances/methodologicalGuide.shtml.
- 38. Shoaibi A, Rao GA, Voss EA, et al. Phenotype algorithms for the identification and characterization of vaccine-induced thrombotic thrombocytopenia in real world data: A multinational network cohort study. Drug Saf 2022;45:685-98. 10.1007/s40264-022-01187-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. See I, Lale A, Marquez P, et al. Case series of thrombosis with thrombocytopenia syndrome after COVID-19 vaccination-United States, December 2020 to August 2021. Ann Intern Med 2022;175:513-22. 10.7326/M21-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sodhi M, Samii A, Etminan M. A comparative safety study of reported neurological adverse events with three COVID-19 vaccines. J Neurol 2022;269:2301-3. 10.1007/s00415-021-10919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whiteley WN, Ip S, Cooper JA, et al. CVD-COVID-UK consortium . Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLoS Med 2022;19:e1003926. 10.1371/journal.pmed.1003926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andrews NJ, Stowe J, Ramsay ME, Miller E. Risk of venous thrombotic events and thrombocytopenia in sequential time periods after ChAdOx1 and BNT162b2 COVID-19 vaccines: A national cohort study in England. Lancet Reg Health Eur 2022;13:100260. 10.1016/j.lanepe.2021.100260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li X, Ostropolets A, Makadia R, et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study. BMJ 2021;373:n1435. 10.1136/bmj.n1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol 2018;47:2005-14. 10.1093/ije/dyy120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schuemie MJ. Vaccine safety surveillance using routinely collected healthcare data - An empirical evaluation of epidemiological designs. medRxiv . [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix 1: Supplementary materials A
Web appendix 2: Supplementary materials B
Data Availability Statement
Patient level data cannot be shared without approval from data custodians owing to local information governance and data protection regulations. The analytical code is available at: https://github.com/oxford-pharmacoepi/ROC22_CovVaxComparativeSafety/tree/main/CovVaxComparativeSafety. Additional correspondence and requests for materials should be addressed to the corresponding author (EB).