Abstract
Objective
Placebo-induced adverse events, or nocebo effects, occur when doctor-patient communication anticipates the onset of negative symptoms. They have been found to correlate with the anxiety-related activity of the hypothalamic-pituitary-adrenal system. Here we try to determine if prenatal hyperactivity of this system, as assessed through plasma cortisol, may influence nocebo effects in adulthood.
Method
We investigated the rate and magnitude of nocebo effects in 378 adults whose prenatal maternal plasma cortisol was measured during the first, second and third trimester of pregnancy. The healthy subjects underwent a nocebo oxygen challenge. This consisted of the inhalation of fake (placebo) oxygen and assessment of the following adverse events: headache, chest pain, abdominal pain, and cough. Plasma cortisol responses during the nocebo adverse events were also measured.
Results
41 out of 46 (89.1%) subjects who reported 3 adverse events, and 37 out of 37 (100%) subjects who reported 4 adverse events had prenatal maternal cortisol above normal levels. By contrast, only 10 out of 143 (7%) subjects who reported 0 adverse events showed prenatal maternal cortisol above the normal range. Moreover, whereas subjects who reported 3 and 4 adverse events showed a significant increase in plasma cortisol following the nocebo challenge, subjects who reported 0 adverse events showed no changes.
Conclusions
These findings emphasize the importance of the doctor-patient communication in perceiving symptoms like pain, and suggest that those subjects with high prenatal maternal cortisol may be more sensitive to the effects of a negative communication in adulthood.
Keywords: placebo, nocebo, hyperalgesia, cortisol, prenatal, adverse events, oxygen
Introduction
Today there is compelling evidence that a number of functions, behaviors and disorders during life are influenced by the exposure to stress and high maternal cortisol levels during pregnancy (Davis et al., 2007; de Weerth et al., 2003; Entringer et al., 2009; Nathanielsz et al., 2003; Teicher et al., 2003; Zijlmans et al., 2015a,b). For example, maternal stress hormone levels in pregnancy have been found to be related to the development of different brain regions, such as the amygdala, along with the occurrence of affective disorders, thus suggesting that the origins of neuropsychiatric disorders may have their foundations as early as in the intrauterine life (Zijlmans et al., 2015a,b). Likewise, a change in neuronal and endocrine functioning following high levels of prenatal cortisol has been found both in humans, where abnormal maternal cortisol may lead to affective problems of the child (Bussa et al., 2012), and in animals, where prenatal stress affects hippocampal cell proliferation (Koehl et al., 2009) and the expression of corticotrophin-releasing hormone (CRH) and its receptors in the hypothalamus and amygdala (Zohar & Weinstock, 2011). Likewise, pain in animals during the lifespan has been found to be affected by early life experience, for example prenatal and perinatal stress (Butkevich et al., 2007; Butkevich & Vershinina, 2001, 2003; Butkevich, 2002; Knaepen et al., 2014; Knaepen et al., 2013; Mikhailenko et al., 2010; Said et al., 2015; Sandercock et al., 2011; Wang et al., 2018; Winston et al., 2014). For example, prenatal stress in rodents alters formalin-induced nociceptive behavior in rats (Butkevich et al., 2007; Butkevich & Vershinina, 2001, 2003; Butkevich, 2002) and visceral hypersensitivity (Wang et al., 2018; Winston et al., 2014), thus indicating that pain perception and behavior is tightly linked to early life experience, both prenatal and perinatal.
In a recent study, we found that negative communication that anticipates the onset of negative symptoms increases both anxiety and the hypothalamic-pituitary-adrenal system following placebo administration (Benedetti et al., 2021). These negative effects, or nocebo effects, show how verbally-induced anticipation of pain generates negative expectations which, in turn, amplify the intensity of the pain experience (Colloca & Benedetti, 2007; Colloca & Finniss, 2012; Keltner et al., 2006).
Therefore, whereas the placebo effect is due to positive communication and expectations of improvement, whose biological underpinnings have been partially unraveled (Benedetti, 2014; Benedetti et al., 2011), the nocebo effect is attributable to negative communication and expectations of worsening. The nocebo effect thus represents an excellent model to understand how negative communication may lead to negative expectations which, in turn, may generate symptoms from nothing, such as pain (Colloca & Benedetti, 2007; Benedetti et al., 2007, 2016), as occurs when adverse events are reported following placebo administration (Benedetti et al., 2021).
On the basis of all these considerations, we hypothesized that stress-related factors in the intrauterine milieu could also affect nocebo hyperalgesic effects in adulthood. Accordingly, we analyzed the nocebo hyperalgesic effects in adulthood to understand whether they are linked to maternal cortisol levels during pregnancy. To do this, we assessed the adverse events to a nocebo oxygen challenge, as already done in our previous study (Benedetti et al., 2021), whereby expectations of headache, chest pain, abdominal pain, and cough were induced through verbal suggestions. In addition, we also measured the plasma cortisol responses to the nocebo challenge.
Materials and methods
Subjects
We studied 1357 healthy subjects (figure 1) who underwent a nocebo oxygen challenge and who were recruited from the University of Turin Medical School, the Psychology School, and other institutions, like hospitals, medical centers and the high-altitude school, after approval of the Institutional Review Board and after signing a written informed consent form, including the consent for collecting data on prenatal maternal cortisol. In addition, the principles outlined in the Declaration of Helsinki were followed. All subjects are original and not reused from previous studies. In order to study a population as homogeneous as possible, we discarded those subjects with a body mass index (BMI) lower than 17.5 and higher than 30, as we usually do when studying oxygen challenges. We were able to find the prenatal clinical records of 485 subjects but for 107 of them maternal cortisol was not assessed throughout pregnancy. Therefore, the results of this study come from the remaining 378 subjects, the nocebo group (NOC), whose prenatal maternal plasma cortisol was measured during the first, second and third trimester of pregnancy (figure 1).
Figure 1.

Workflow and selection of the study participants. BMI=body mass index
Nocebo challenge
As part of our ongoing project on hypoxia (Benedetti et al., 2015, 2021), we used fake oxygen as a nocebo challenge. This consisted of the administration of placebo (fake) oxygen through an oxygen mask while comfortably seated on an armchair. This procedure was always performed in the morning. To do this, the oxygen tank was empty but the subjects were told it was full of 100% oxygen. The participants were told that inhalation of 100% oxygen induces frequent adverse events, such as headache, burning chest pain, abdominal pain, and cough. They were asked to report these side effects at the end of 10 min breathing according to a numerical rating scale (NRS) ranging from 0= no pain to 10=unbearable pain. In addition, the number of coughs were recorded during the inhalation of placebo oxygen. The placebo oxygen challenge was performed according to a double-blind paradigm, whereby neither the participant nor the experimenter knew whether real or placebo oxygen was delivered. Actually, the oxygen tank was always empty and was prepared by a different experimenter who was not present during the challenge.
Cortisol responses to the nocebo challenge
Blood samples were taken 1 h before placebo oxygen and at the end of the 10 min breathing for the assessment of plasma cortisol concentration according to standard procedures (Benedetti et al., 2021). Samples were collected in sterile tubes and immediately centrifuged at 4°C, and the plasma was stored at -80°C until assayed. Plasma cortisol concentrations were measured using commercially available kit [CORTCTK-125 (Sorin, Saluggia, Italy)]. The sensitivity of cortisol was 5 μg/L, and the intra-assay and inter-assay coefficients of variation were 3.8 and 5.7%.
Prenatal maternal cortisol assessment
Prenatal clinical records with assessment of maternal plasma cortisol during the first, second and third trimester of pregnancy were found for 378 subjects (figure 1). The reason why prenatal maternal was available for only 378 subjects was due to the fact that cortisol assessment is not routine clinical practice: it was only measured in some hospitals to assess possible stress and this was repeated over the three trimesters of pregnancy to find out whether the high cortisol was caused by stress or a disease. All measurements were performed in the morning in order to avoid circadian variability. Normal range of plasma cortisol was recorded according to standard procedures (Abbassi-Ghanavati et al., 2009). The following ranges were considered.
Nonpregnant Adult: 0 to 25 μg/dL.
Pregnancy Trimester One: 7 to 19 μg/dL.
Pregnancy Trimester Two: 10 to 42 μg/dL.
Pregnancy Trimester Three: 12 to 50 μg/dL
Natural history
To study the natural history of the adverse events during the 10 minutes breathing through the oxygen mask, 70 additional subjects underwent the same procedure as described above, but no verbal instructions about possible adverse events were given (NH group). Therefore, this group provides information about the occurrence of possible adverse events by merely wearing an oxygen mask and can be compared to the subjects who received the negative verbal instructions (i.e., the nocebo challenge).
Statistical analysis
The results are presented as the differences of the means and their 95% confidence intervals. Relative risk (RR) was also calculated. The Kolmogorov-Smirnov test was used to verify the normality of the distribution; in no case the normality was violated. ANOVA was used to assess statistical significance, where level of significance is P<0.05. In all cases, Bonferroni correction for multiple comparisons was applied. In addition, Chi-square test and linear regression analysis were performed.
Results
No differences in age, gender and BMI were found across the groups (table 1). Likewise, the baseline assessment of both pain and cough, that was performed before the nocebo challenge, showed that neither pain nor cough were present in all tested subjects.
Table 1.
Age, gender and body mass index (BMI) for the natural history group (NH) and for those subjects who reported 0, 1, 2, 3, 4 adverse events (AE) during the nocebo challenge
| age | gender | BMI | |
|---|---|---|---|
| NH | 22.5+2.6 SD | 66F, 58M | 22.2+2.1 |
| 0AE | 22.3±2 | 76F, 67M | 22.4±2.5 |
| 1AE | 22.3±2.2 | 27F, 34M | 22.3±2.4 |
| 2AE | 22.3±2.1 | 51F, 40M | 22.4±2.3 |
| 3AE | 22.3±2.4 | 24F, 22M | 22.5±2.4 |
| 4AE | 22.6±2.3 | 14F, 23M | 22.3±2.4 |
None of the 70 subjects of the natural history group reported 2, 3 or 4 adverse events, whereas 7 subjects (10%) reported 1 adverse event during the 10 minutes breathing through the oxygen mask. By contrast, 61 subjects who received the nocebo challenge (16.1%) reported 1 adverse event (1AE), 91 subjects (24.1%) 2 adverse events (2AE), 46 subjects (12.2%) 3 adverse events (3AE), and 37 subjects (9.8%) 4 adverse events (4AE). The difference between the two groups is highly significant (χ2 = 60.31, P<0.00001). Therefore, the adverse event induced by the nocebo challenge can be attributed to the nocebo negative instructions about the occurrence of headache, chest pain, abdominal pain, and cough, and not to the wearing of a mask.
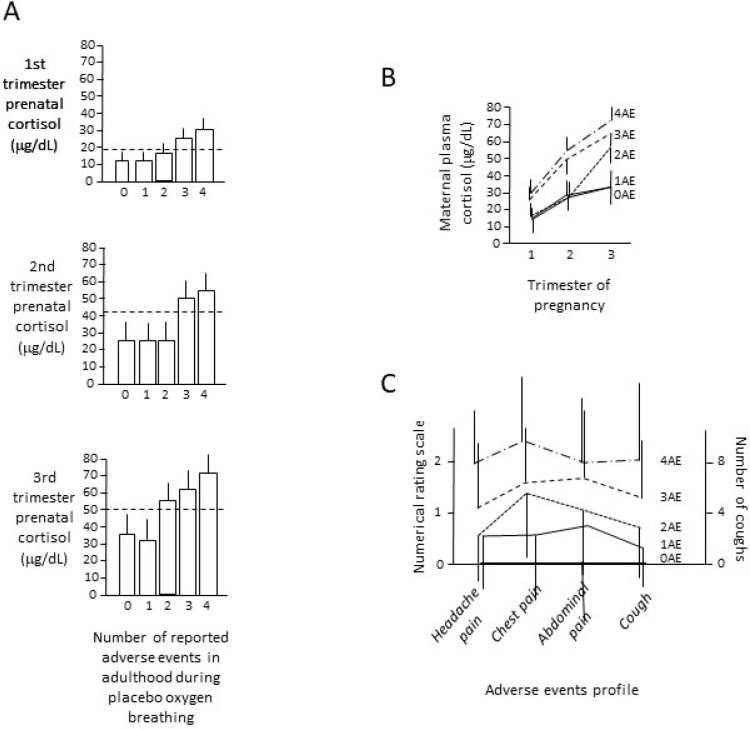
Whereas in the prenatal clinical records of subjects 0AE and 1AE, the average levels of maternal plasma cortisol were normal throughout pregnancy, in subjects 3AE and 4AE the average prenatal maternal cortisol was above the upper limit of the normal range (see Methods and Abbassi-Ghanavati et al. (2009)) across all three trimesters (P<0.01), and in subjects 2AE only in the third trimester (P<0.05) (figure 2A). In fact, in group 0AE, only 10 out of 143 subjects (7%) showed prenatal maternal cortisol above the normal range, whereas in group 3AE 41 out of 46 (89.1%) had abnormal maternal cortisol (RR=12.74, CI=6.95 to 23.36P<0.01and in 4AE 37 out of 37 (100%) had maternal cortisol above the upper limit of events in relation to the increase of prenatal maternal normal range (RR=14.3, CI=7.86 to 25.99, P<0.01). Similarly, group 1AE, with 3 out of 62 subjects (4.8%) with abnormal prenatal maternal cortisol, differed from 3AE (RR=18.12, CI=5.98 to 54.88, P<0.01) and 4AE (RR=20.33, CI=6.74 to 61.29, P<0.01).
Figure 2.
A) The number of adverse events is shown in relation to prenatal maternal cortisol in the 1st, 2nd, 3rd trimester of pregnancy. Horizontal broken lines show the upper limit of the normal range of maternal plasma cortisol. Error bars are standard deviations. B) Increase in maternal plasma cortisol over the 3 trimesters of pregnancy in relation to no adverse events reported in adulthood (0AE), 1 adverse event (1AE), 2 adverse events (2AE), 3 adverse events (3AE), 4 adverse events (4AE). C) Adverse events profile for 0AE, 1AE, 2AE, 3AE, 4AE, whereby the magnitude of adverse events is measured by means of a numerical rating scale from 0 to 10 for headache pain, chest pain, abdominal pain, and by considering the number of coughs for cough. Error bars are standard deviations.
Prenatal maternal cortisol increased differently during pregnancy in the five groups, with a lower increase in subjects 0AE-1AE and a larger increase in subjects 2AE-3AE-4AE (figure 2B and table 2). In fact, subjects 3AE-4AE differed significantly from 0AE-1AE for the three trimesters of pregnancy, whereas 2AE differed from 0AE-1AE only for the third trimester and from 3AE-4AE for all trimesters (table 2). Therefore, there was a progressive increase of adverse cortisol. Likewise, the adverse events profiles showed a progressive increase in the magnitude of adverse events across the different groups (figure 2C).
Table 2.
Comparisons among groups 0AE, 1AE, 2AE, 3AE, 4AE. The diferences of the means and their 95% confidence intervals are shown. Statistical differences are in bold with asterisk
| Comparison of nocebo adverse events | Prenatal plasma cortisol in Trimester 1 | Prenatal plasma cortisol in Trimester 2 | Prenatal plasma cortisol in Trimester 3 |
|---|---|---|---|
| 0AE vs 1AE | 0.68 (0.18 to 2.59) | 1.17 (0.21 to 6.6) | 0.76 (0.23 to 2.47) |
| 0AE vs 2AE | 1.82 (0.74 to 4.49) | 2.89 (0.82 to 10.18 | *32.27 (15.14 to 68.8) |
| 0AE vs 3AE | *109.06 (35.25 to 337.33) | *764.5 (135.41 to >1000) | *505.16 (63.52 to >1000) |
| 0AE vs 4AE | *495.1 (61 to >1000) | *608.12 (107 to >1000) | *405.9 (50.84 to >1000) |
| 1AE vs 2AE | 2.65 (0.7 to 9.95) | 2.45 (0.49 to 12.25 | *42.13 (13.76 to 128.94) |
| 1AE vs 3AE | *158.53 (35.86 to 700.73) | *649 (87.96 to >1000) | *655.5 (70.8 to >1000) |
| 1AE vs 4AE | *718.33 (71.68 to >1000) | *516.25 (69.58 to >1000) | *527.25 (56.69 to >1000) |
| 2AE vs 3AE | *59.63 (19.41 to 183.17) | *264 (52.59 to >1000) | *15.55 (2.02 to 119.27) |
| 2AE vs 4AE | *270.1 (33.48 to >1000) | *210 (41.55 to >1000) | *12.51 (1.62 to 96.41) |
| 3AE vs 4AE | 4.51 (0.5 to 40.2) | 0.79 (0.1 to 5.93) | 0.8 (0.04 to 13.29) |
We also assessed the cortisol response to the nocebo oxygen challenge. No effect was found in the NH group (figure 3A). Conversely, whereas subjects 0AE and 1AE of the NOC group did not show any increase in cortisol plasma concentration at the end of the nocebo challenge, subjects 2AE, 3AE, 4AE showed a significant increase (figure 3B). In addition, 2AE differed from both 3AE and 4AE, suggesting a progressive increase of the cortisol response to nocebo across the 0,1,2,3,4 AE groups.
Figure 3.
A) Cortisol response in the NH group shows no efect by merely wearing the oxygen mask. B) In the NOC group, whereas cortisol response to the nocebo oxygen challenge was absent for groups 0AE and 1AE, an increase in cortisol occurred after nocebo administration in groups 2AE, 3AE and 4AE. In addition, cortisol response in 2AE difered significantly from both 3AE and 4AE, suggesting a gradual increase in cortisol secretion as the number of reported adverse events increased. The diferences of the means and their 95% confidence intervals are also shown (significant differences are in bold). Error bars are standard deviations
By considering all subjects together, we performed a linear regression analysis of prenatal maternal cortisol versus pre- and post-nocebo challenge (figure 4). It can be seen that, whereas no correlation was present between the three trimesters of pregnancy and baseline cortisol (pre-nocebo), a significant correlation was found for the first trimester (F(1,375)=63.539, P<0.001), second (F(1,375)=42.793, P<0.001), and third trimester (F(1,375)=103.518, P<0.001) versus post-nocebo cortisol. This indicates that only post-nocebo cortisol depended on prenatal maternal cortisol, whereas baseline values were normal.
Figure 4.
Regression lines and 95% CI of prenatal maternal cortisol (trimesters 1, 2, 3) versus baseline cortisol before (left) and after (right) the nocebo challenge in all the 378 subjects of the NOC group analyzed
Discussion
A variety of studies show that changes in the intrauterine milieu are important determinants of behavior and illness in adulthood. In particular, maternal stress has been found to be associated with the subsequent neural development of the child. For example, the anatomical organization of the amygdala and hippocampus, as assessed by means of volume measurements of these regions, can be affected by maternal cortisol during pregnancy, and these neuroanatomical changes are associated with affective disorders (Bussa et al., 2012). Likewise, a number of functions, behaviors and disorders during life are influenced by the exposure to stress and high maternal cortisol levels during pregnancy. These include cardiovascular and metabolic functions (Nathanielsz et al., 2003), infant behavior and temperament (Davis et al., 2007; de Weerth et al., 2003). In addition, prenatal stress may be related to memory impairment, as shown in humans by prefrontal cortex-dependent working memory performance following prenatal psychosocial stress exposure (Entringer et al., 2009). Likewise, in animals, brain regions tightly related to memory have been found to be affected by prenatal stress; for example, a change in cell proliferation in the hippocampus has been observed (Koehl et al., 2009) as well as a change in the expression of corticotrophin-releasing hormone (CRH) receptors in the hypothalamus and amygdala (Zohar & Weinstock, 2011).
Similarly, stressful early life experience, both prenatal and perinatal, can lead to altered nociceptive behavior in animals during life (Butkevich et al., 2007; Butkevich & Vershinina, 2001, 2003; Butkevich, 2002; Knaepen et al., 2014; Knaepen et al., 2013; Mikhailenko et al., 2010; Said et al., 2015; Sandercock et al., 2011; Wang et al., 2018; Winston et al., 2014). This has been investigated by using different pain models, such as the formalin test (Butkevich et al., 2007; Butkevich & Vershinina, 2001, 2003; Butkevich, 2002) and by studying visceral hypersensitivity as a model of irritable bowel syndrome (Wang et al., 2018; Winston et al., 2014).
It is important to highlight that nocebo hyperalgesic effects represent a complex phenomenon whereby nociceptive mechanisms and psychological factors cooperate together to generate increased pain perception (Colloca & Benedetti, 2007; Colloca & Finniss 2012; Benedetti et al., 2007). In fact, nocebo hyperalgesia is related to increased activity of different areas that are known to be involved in pain processing (Keltner et al., 2006), and they are known to modulate different biochemical pathways, such as cholecystokinin and cyclooxygenase (Benedetti et al., 1997; Benedetti et al., 2006; Benedetti et al., 2014), and hormones, like CRH, ACTH and cortisol (Benedetti et al., 2006, 2021). One of the main determinants of these neural and endocrine effects is represented by negative expectations, thus the nocebo effect represents an excellent model to understand the psychogenic generation of pain, and more in general of illness.
On the basis of these considerations, the present study suggests that the intrauterine milieu may be an important determinant of nocebo hyperalgesia in adulthood, thereby highlighting how psychogenic symptoms in adult life can be determined at very early stages of antenatal life. Therefore, these findings underscore a close relationship between adverse events reporting after placebo administration in adulthood and prenatal maternal cortisol levels. As nocebo effects are related to negative communication, anticipatory anxiety and expectations about negative outcomes, these results are in agreement with all the other studies showing that maternal stress during pregnancy is related to behavioral changes and affective disorders during life (Davis et al., 2007; de Weerth et al., 2003; Entringer et al., 2009; Nathanielsz et al., 2003; Teicher et al., 2003; Zijlmans et al., 2015a,b). It should also be emphasized that in the present study we found a larger cortisol response to the nocebo oxygen challenge in those subjects who reported 3-4 adverse events, thus suggesting that the pattern of cortisol secretion in adulthood following a nocebo procedure may depend on prenatal maternal cortisol levels. This endocrine finding is in keeping with previous studies, in which a change in neuronal and endocrine functioning following high levels of prenatal cortisol has been found both in humans and in animals (Bussa et al., 2012; Koehl et al., 2009; Zohar & Weinstock, 2011).
The method we used to induce these nocebo hyperalgesic effects comes from previous work of our group with placebo oxygen, a procedure which powerfully affect different physiological functions, such as ventilation, heart rate, blood pH, cyclooxygenase and prostaglandin activation (Benedetti et al., 2015). In particular, in our previous study we found that the whole hypothalamic-pituitary-adrenal activity, as assed through the measurement of CRH, ACTH and cortisol, is increased in those subjects who show a high rate of adverse events following the administration of a placebo (Benedetti et al., 2021). Accordingly, in the present study we used placebo oxygen, along with negative verbal suggestions of adverse events, to induce the following nocebo hyperalgesic responses: headache, chest pain, abdominal pain. Whereas these are subjective outcomes, we also considered objective measurements, like cough and plasma cortisol responses to the nocebo challenge. Therefore, we were able to investigate both subjective and objective outcome measures, which certainly strengthened our findings. Indeed, we found a very good correlation across all these subjective and objective measurements, which indicates that nocebo hyperalgesia is associated with a variety of behavioral and physiological changes.
Whereas headache, chest pain and cough are more commonly associated to oxygen side effects, abdominal pain is usually not related to the possible adverse events induced by breathing oxygen. Nonetheless, abdominal pain was often reported as adverse event. In this regard, it is interesting to note that prenatal stress has been found to be associated to visceral hypersensitivity, an effect that seems to be mediated in rodents by factors such as the brain-derived neurotrophic factor (BDNF) (Winston et al., 2014) and cystathionine-β-synthase (Wang et al., 2018). It is also interesting to note that maternal prenatal stress is also associated to the human intestinal microbiota (Zijlmans et al., 2015a,b), a finding that could be importantly correlated to visceral hypersensitivity. In light of the complexity of the nocebo phenomenon, involving both nociceptive mechanisms and psychological factors, it should also be noted that animal models found a double effect of prenatal stress on both anxiety, as assessed through the elevated plus maze test, and nociceptive behavior, as measured by means of the tail flick test in rodents (Said et al., 2015). Therefore, the combined effect of prenatal stress on anxiety and pain in rodents seems to be highly relevant to human studies of nocebo hyperalgesia, whereby both anxiety and pain are involved.
It should also be pointed out that the adverse events generated by the nocebo challenge were closely related to the negative verbal instructions about the possible occurrence of adverse events, as wearing a mask for 10 minutes did not induce per se any negative effect. Indeed, 90% of the subjects of the NH group did not report any adverse events, which represents a highly significant difference compared to the NOC group. Likewise, no cortisol response to the nocebo challenge occurred in the NH group, which strongly indicate that the hormonal effects we observed in the NOC group were attributable to the negative instructions. This is in agreement with our previous study, in which 96 subjects of the natural history group were tested (Benedetti et al., 2021).
At least three limitations of our study should be acknowledged. First, we did not measure expectations, thus we could not perform a correlation between the degree of negative expectations and the magnitude of the nocebo effects. Second, we did not measure anxiety, for example by means of the State-Trait Anxiety Inventory, thus no correlation could be performed between plasma cortisol levels and anxiety. A future challenge will be to assess psychosocial stress, anxiety, depression by means of the appropriate questionnaires. Third, in the clinical records we could not find any indication of psychosocial maternal stress from a clinical and behavioral point of view, such as anxiety or depression: the only indication we found in the clinical records was the level of plasma maternal cortisol. Thus, we do not know the causes that led to increased maternal cortisol during the three trimesters of pregnancy.
In spite of these limitations, we believe that our study is the first attempt to correlate the intrauterine milieu to a complex phenomenon such as the nocebo effect. Although the observational nature of these data does not allow for causal inferences, the present results suggest that changes in physiological stress levels during pregnancy may influence the adverse events reporting after placebo administration in adulthood.
These findings may have important implications in clinical practice (Benedetti et al., 2016). First, the psychogenic generation of pain, as occurs in the nocebo hyperalgesic effect, should be considered as an effect that might be associated to negative doctor-patient communication and relationship. Second, these findings emphasize the importance of the longitudinal assessment of a patient with pain, specifically the antenatal period, which may account for variation in the generation and responsiveness of pain in later life. In order to reduce abnormal response to pain in adulthood, efforts should be made both to avoid negative doctor-patient communication and to lower stress during pregnancy.
Acknowledgements
This work was supported by grants from the Innovative Clinical Training, Trials & Healthcare Worldwide Initiative to F.B., and from the Nerve and Muscle Center of Texas to A.S.
References
- Abbassi-Ghanavati, M., Greer, L. G., & Cunningham, F.G. (2009). Pregnancy and laboratory studies: a reference table for clinicians. Obstetrics & Gynecology, 114, 1326-1331. [DOI] [PubMed] [Google Scholar]
- Benedetti, F., Amanzio, M., Casadio, C., Oliaro, A., & Maggi, G. (1997). Blockade of nocebo hyperalgesia by the cholecystokinin antagonist proglumide. Pain, 71, 135-140. [DOI] [PubMed] [Google Scholar]
- Benedetti, F., Amanzio, M., Giovanelli, F., Craigs-Brackhahn, K., & Shaibani, A. (2021). Hypothalamic-pituitary-adrenal activity in adverse events reporting after placebo administration. Clinical Pharmacology & Therapeutics, 110, 1349-1357. [DOI] [PubMed] [Google Scholar]
- Benedetti, F., Amanzio, M., Rosato, R., & Blanchard, C. (2011). Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nature Medicine, 17, 1228-1230. [DOI] [PubMed] [Google Scholar]
- Benedetti, F., Amanzio, M., Vighetti, S., & Asteggiano, G. (2006). The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. Journal of Neuroscience, 26, 12014-12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti, F., Carlino, E., & Piedimonte, A. (2016). Increasing uncertainty in CNS clinical trials: the role of placebo, nocebo, and Hawthorne effects. Lancet Neurology, 15, 736-747. [DOI] [PubMed] [Google Scholar]
- Benedetti, F., Durando, J., Giudetti, L., Pampallona, A., & Vighetti, S. (2015). High altitude headache: the effects of real versus sham oxygen administration. Pain, 156, 2326– 2336. [DOI] [PubMed] [Google Scholar]
- Benedetti, F., Durando, J., & Vighetti, S. (2014). Nocebo and placebo modulation of hypobaric hypoxia headache involves the cyclooxygenase-prostaglandins pathway. Pain, 155, 921-928. [DOI] [PubMed] [Google Scholar]
- Benedetti, F., Lanotte, M., Lopiano, L., & Colloca, L. (2007). When words are painful: unraveling the mechanisms of the nocebo effect. Neuroscience, 147, 260-271. [DOI] [PubMed] [Google Scholar]
- Benedetti, F. (2014). Placebo effects: from the neurobiological paradigm to translational implications. Neuron, 84, 623637. [DOI] [PubMed] [Google Scholar]
- Bussa, C., Davis, E. P., Shahbaba, B., Pruessner, J.C., Head, K., & Sandman, C.A. (2012). Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Science USA, 109, E1312-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkevich, I. P. (2002). Effects of prenatal stress on formalin-induced acute and persistent pain in adult male rats. Bulletin of Experimental Biology and Medicine, 133, 130-132. [DOI] [PubMed] [Google Scholar]
- Butkevich, I. P., Barr, G. A., & Vershinina, E.A. (2007). Sex differences in formalin-induced pain in prenatally stressed infant rats. European Journal of Pain, 11, 888-894. [DOI] [PubMed] [Google Scholar]
- Butkevich, I. P., & Vershinina, E. A. (2001). Effect of prenatal stress on tonic pain in rats. Bulletin of Experimental Biology and Medicine, 131, 515-517. [DOI] [PubMed] [Google Scholar]
- Butkevich, I. P., & Vershinina, E. A. (2003). Maternal stress differently alters nociceptive behaviors in the formalin test in adult female and male rats. Brain Research, 961, 159165. [DOI] [PubMed] [Google Scholar]
- Colloca, L., & Benedetti, F. (2007). Nocebo hyperalgesia: how anxiety is turned into pain. Current Opinion in Anaesthesiology, 20, 435-439. [DOI] [PubMed] [Google Scholar]
- Colloca, L., & Finniss, D. (2012). Nocebo effects, patient-clinician communication, and therapeutic outcomes. Journal of American Medical Association, 307, 567-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, E. P., Glynn, L. M., Schetter, C. D., Hobel, C., Chicz-Demet, A., & Sandman, C.A. (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of American Academy of Child Adolescent Psychiatry, 46, 737–746. [DOI] [PubMed] [Google Scholar]
- de Weerth, C., van Hees, Y., & Buitelaar, J. K. (2003). Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Human Development, 74, 139–151. [DOI] [PubMed] [Google Scholar]
- Entringer, S., Buss, C., Kumsta, R., Hellhammer, D. H., Wadhwa, P.D., & Wuest, S. (2009). Prenatal psychosocial stress exposure is associated with subsequent working memory performance in young women. Behavioral Neuroscience, 123, 886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner, J. R., Furst, A., Fan, C., Redfern, R., Inglis, B., & Fields, H. L. (2006). Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. Journal of Neuroscience, 26, 4437-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaepen, L., Pawluski, J. L., Patijn, J., van Kleef, M., Tibboel, D., & Joosten, E. A. (2014). Perinatal maternal stress and serotonin signaling: effects on pain sensitivity in offspring. Developmental Psychobiology, 56, 885-896. [DOI] [PubMed] [Google Scholar]
- Knaepen, L., Rayen, I., Charlier, T. D., Fillet, M., Houbart, V., van Kleef, M., Steinbusch, H. W., Patijn, J., Tibboel, D., Joosten, E. A., & Pawluski, J. L. (2013). Developmental fluoxetine exposure normalizes the long-term effects of maternal stress on post-operative pain in Sprague-Dawley rat offspring. PLoS ONE, 8, e57608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl, M., Lemaire, V., Le Moal, M. & Abrous, D. N. (2009). Age-dependent effect of prenatal stress on hippocampal cell proliferation in female rats. European Journal of Neuroscience, 29, 635–640. [DOI] [PubMed] [Google Scholar]
- Mikhailenko, V. A., Butkevich, I. P., Vershinina, E. A., & Semenov, P.O. (2010). Interrelationship between measures of pain reactions in inflammation and levels of depression in prenatally stressed rat pups. Neuroscience and Behavioral Physiology, 40, 179-184. [DOI] [PubMed] [Google Scholar]
- Nathanielsz, P. W., Berghorn, K. A., Derks, J. B., Giussani, D. A., Docherty, C., Unno, N., Davenport, A., Kutzlers, M., Koenen, S., Visser, G. H., & Nijland, M. J. (2003). Life before birth: effects of cortisol on future cardiovascular and metabolic function. Acta Paediatrica, 92, 766–772. [PubMed] [Google Scholar]
- Said, N., Lakehayli, S., Battas, O., Hakkou, F., & Tazi, A. (2015). Effects of prenatal stress on anxiety-like behavior and nociceptive response in rats. Journal of Integrative Neuroscience,14, 223-234. [DOI] [PubMed]
- Sandercock, D. A., Gibson, I. F., Rutherford, K. M., Donald, R. D., Lawrence, A. B., Brash, H. M., Scott, E. M., & Nolan, A. M. (2011). The impact of prenatal stress on basal nociception and evoked responses to tail-docking and inflammatory challenge in juvenile pigs. Physiology & Behavior, 104, 728-737. [DOI] [PubMed] [Google Scholar]
- Teicher, M. H., Andersen, S. L., Polcari, A., Anderson, C. M., Navalta, C. P., & Kim, D. M. (2003). The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews, 27, 33–44. [DOI] [PubMed] [Google Scholar]
- Wang, H. J., Xu, X., Xie, R. H., Rui, Y. Y., Zhang, P. A., Zhu, X. J., & Xu, G. Y. (2018). Prenatal maternal stress induces visceral hypersensitivity of adult rat offspring through activation of cystathionine-β-synthase signaling in primary sensory neurons. Molecular Pain, 14, 1744806918777406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston, J. H., Li, Q., & Sarna, S. K. (2014). Chronic prenatal stress epigenetically modifies spinal cord BDNF expression to induce sex-specific visceral hypersensitivity in offspring. Neurogastroenterology & Motility, 26, 715730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans, M. A., Korpela, K., Riksen-Walraven, J. M., de Vos, W. M., & de Weerth, C. (2015a). Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology, 53, 233-245. [DOI] [PubMed] [Google Scholar]
- Zijlmans, M. A., Riksen-Walraven, J. M., & de Weerth, C. (2015b). Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neuroscience and Biobehavioral Reviews, 53, 1-24. [DOI] [PubMed] [Google Scholar]
- Zohar, I., & Weinstock, M. (2011). Differential effect of prenatal stress on the expression of corticotrophin-releasing hormone and its receptors in the hypothalamus and amygdala in male and female rats. Journal of Neuroendocrinology, 23, 320–328. [DOI] [PubMed] [Google Scholar]