Abstract
Objective:
To evaluate the continued effect of a sequential treatment strategy (fluoxetine followed by continued medication plus relapse prevention cognitive-behavioral therapy [RP-CBT]) on relapse prevention beyond the treatment phase.
Method:
Youth (aged 8–17 years) with major depressive disorder (MDD) were treated with fluoxetine for 6 weeks. Responders (≥50% reduction on the Children’s Depression Rating Scale–Revised [CDRS-R]) were randomized to continued medication management alone (MM) or continued medication management plus RP-CBT (MM+CBT) for an additional 6 months. Long-term follow-up assessments were conducted at weeks 52 and 78.
Results:
Of 144 youth randomized to MM (n = 69) or MM+CBT (n = 75), 67% had at least 1 follow-up assessment, with equal rates in the 2 groups. Remission rates were high, although most had remitted during the 30-week treatment period. Only 6 additional participants remitted during long-term follow-up, and there were no differences on time to remission between MM+CBT and MM. The MM+CBT group had a significantly lower risk of relapse than the MM group throughout the 78-week follow-up period (hazard ratio = 0.467, 95% CI = 0.264 to 0.823; χ2 = 6.852, p = .009). The estimated probability of relapse during the 78-week period was lower with MM+CBT than MM only (36% versus 62%). Mean time to relapse was also significantly longer with MM+CBT compared to MM alone by approximately 3 months (p = .007).
Conclusion:
The addition of RP-CBT after acute response to medication management had a continued effect on reducing risk of relapse even after the end of treatment.
Clinical trial registration information
Sequential Treatment of Pediatric MDD to Increase Remission and Prevent Relapse; http://clinicaltrials.gov/; NCT00612313.
Keywords: depression, relapse, youth, CBT, continuation treatment
Acute-phase treatment interventions for pediatric depression have demonstrated efficacy in achieving response (typically defined as a 50% improvement in symptoms) and remission (no or minimal symptoms) compared to placebo or usual care. Effective acute treatments include antidepressant medications,1,2 psychotherapy,3,4 and a combination of medication and psychotherapy.5–7 Although these interventions have been shown to be effective in acute-phase treatments, high rates of relapse are common in youth.8–10 The goal of continuation-phase treatments is to consolidate response and to prevent relapse (defined as depressive episode after attaining remission). Continuation-phase treatment studies of adults with depression have found that continuation-phase cognitive therapy has been beneficial in reducing rates of relapse compared to placebo.11–20
Few continuation phase trials have been conducted in youth with major depressive disorder (MDD). Emslie et al. demonstrated reduced relapse rates in youth (N = 102) with MDD who remained on fluoxetine, compared to switching to placebo, after response to 3 months of open treatment with fluoxetine.9 In a pilot study comparing youth with MDD treated with acute-phase cognitive-behavioral therapy (CBT) plus 6 months of continuation-phase CBT to historical controls, Kroll et al. found lower rates of relapse in those who were treated with CBT.21 Clarke et al. added booster CBT sessions at the end of acute-phase CBT treatment for adolescents with MDD and found no impact on relapse rates but an acceleration of remission.22 Kennard et al., in a pilot study, found that the risk of relapse was about 8 times lower in youth with MDD (aged 11–17 years) who were treated with a sequential treatment strategy of adding continuation CBT (relapse prevention–CBT [RP-CBT]) to medication compared to those treated with medication alone after response to acute antidepressant treatment.23
The authors conducted a larger continuation trial to further examine RP-CBT in youth with MDD. A total of 200 children and adolescents (ages 8–17 years) with MDD were treated with fluoxetine for 6 weeks. Responders were then randomized to continue medication management alone (MM; n = 69) or continue medication management plus RP-CBT (MM+CBT; n = 75). Remission rates were high (>80%) across the 30-week continuation treatment period for both groups, although the 2 groups did not differ significantly on time to remission during the 30-week continuation-treatment period. Adding CBT led to lower relapse rates compared to fluoxetine alone over a 30-week treatment period (9% versus 26.5%).24
In this report, we report on the continued effects of adding RP-CBT to medication treatment for depression beyond the treatment phase of the study (through week 78).
METHOD
The study results presented are based on the long-term follow-up outcomes from a National Institute of Mental Health (NIMH)–funded, single-site study examining a sequential treatment strategy (initial treatment with fluoxetine followed by continued medication plus CBT compared to continued medication alone) to improve remission and prevent relapse (see Kennard et al.24 for additional details). A detailed description of the full methodology and primary outcomes from the treatment period (30 weeks) has been previously reported (see Kennard et al.24). The study was approved by the University of Texas Southwestern Medical Center Institutional Review Board.
Study Participants
Participants were outpatients (aged 8–17 years) in a general pediatric psychiatry outpatient clinic. Participants had a primary diagnosis of MDD for at least 4 weeks, a Children’s Depression Rating Scale–Revised (CDRS-R)25 score of ≥40, and a Clinical Global Impression– Severity (CGI-S)26 score of ≥4. Although MDD was the primary diagnosis, other concurrent disorders were allowed, with the exception of lifetime bipolar disorder, psychosis, anorexia, and bulimia, or substance abuse or dependence within the past 6 months. Additional inclusion/exclusion criteria have been presented previously.24
Procedures
At the initial visit, participants and their parents provided written consent and assent after the purpose, procedures, risks and benefits, and the rights of study participants were explained, and all questions were answered. They were then assessed by trained masters-level independent evaluators (IEs) using the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL)27 to identify all psychiatric illnesses. Additional measures of depression and suicidality severity, including the CDRS-R, CGI-S, Columbia Suicide History form, Columbia Suicide Severity Rating Scale–Short (C-SSRS),28 and the Family History Screen,29 were used to identify psychiatric illnesses within first-degree relatives. Youth were then evaluated 1 week later by a child psychiatrist to determine eligibility and appropriateness for the study. The child psychiatrist reviewed the K-SADS-PL with the participant and family, and completed the CDRS-R, CGI-S, and C-SSRS.
Following assessment, eligible participants began 6 weeks of fluoxetine (10–40mg). At week 6, responders (based on ≥50% decrease on the CDRS-R total score by the IE) were randomized to continue medication management (MM) alone (n = 69) or MM plus RP-CBT (n = 75) for an additional 6 months (see Kennard et al.24 for additional details). Visits during continuation treatment for MM occurred at weeks 8, 10, 12, 14, 18, 24, and 30. For participants in medication management plus RP-CBT (MM+CBT), visits were weekly for 1 month, then every other week for 1 month, and then every 4 to 6 weeks until the end of the 30-week continuation treatment. Beyond week 30, treatment was uncontrolled.
IEs blinded to treatment assignment assessed participants every 6 weeks throughout continuation treatment, and again at 52 and 78 weeks postbaseline. At the 52- and 78-week follow-up assessments, the IE completed the CDRS-R, C-SSRS, CGI, and course of illness using the Adolescent–Longitudinal Interval Follow-Up Evaluation (A-LIFE).30 Participants continued with IE assessments even if they discontinued study treatments. Treatment beyond week 30 was not controlled but was tracked through the A-LIFE.
Outcomes were time to remission and rate of relapse. During continuation treatment (30 weeks), remission was defined as CDRS-R ≤28, with timing of remission determined through clinical interview and A-LIFE, and relapse was defined as a CDRS-R ≥40 for at least 2 weeks or a CDRS-R <40, but with significant clinical deterioration that would suggest full relapse if the participant’s treatment was not altered.9 During the long-term follow-up period (beyond week 30), remission and relapse were determined through clinical interview and rating of the A-LIFE. The A-LIFE (adapted from the Longitudinal Interval Follow-Up Evaluation30) is a psychiatric diagnostic interview for use in longitudinal studies. A rating of 1 or 2 on the A-LIFE indicates no or minimal symptoms; a rating of 3 indicates possible depression; a rating of 4 indicates probable depression, but below criteria for MDD; and a rating of 5 or 6 indicates definite MDD (moderate or severe). Remission during the long-term follow-up was defined as at least 2 months with a rating of 1 or 2. Relapse during the long-term follow-up period included ratings of ≥4 for at least 2 weeks, with clear evidence of worsening depressive symptoms. Although ratings of >5 signify a full MDD episode, clear worsening of depressive symptoms not yet meeting full criteria (that is, a rating of 4) were included as relapses to remain consistent with the clinical deteriorations noted during the treatment phase of the study. Only youth who had achieved remission (at any point in the study) were assessed for relapse (i.e., cannot relapse if not remitted).
“Time spent well in the study” was measured via the A-LIFE. Time spent well was defined as each week that the youth was rated as a 1 or 2 (no or minimal disorder) on the A-LIFE, and was operationalized as the number of weeks of a rating of 1 or 2 divided by the total number of weeks in the study.
Statistical Analysis
Demographic and clinical characteristics were compared between the MM+CBT and MM treatment groups, as well as the 2 relapse groups (relapse versus no relapse), using an independent-sample t test (continuous outcomes) and a χ2 or Fisher exact test (categorical outcomes).
A Cox proportional hazards regression with adjustment for CDRS-R total score, age (child versus adolescent), and gender was used to compare time to remission and, in a separate model, time to relapse between participants in the 2 treatment groups during the long-term (78-week) follow-up period. Hazard ratios (HRs) were also estimated and interpreted as the effect size estimator for our Cox regression. As part of the survival analysis, right censoring was used and occurred when incomplete information was available about the survival time of a given participant; the information was incomplete because the participant did not have an event during the study. Censoring (or a censored observation) meant a participant who dropped out or completed the study without remitting/relapsing. We also estimated the probability of remitting/relapsing or the rates of remission/relapse at each week during the long-term (78-week) follow-up period.
A negative binomial regression (which is a generalization of the Poisson model for overdispersed count data), with adjustment for CDRS-R total score, age (child versus adolescent), and gender, was used to compare the log “time spent well” rates, or incidence of time spent well in the study (through the 78-week follow-up period), between the 2 groups. Maximum likelihood estimation and robust standard errors (Huber Sandwich Estimator) along with type 3 tests of fixed effects were used with the Wald χ2 statistic.
Finally, to examine the potential “therapist effect” on our basic survival findings, in a sensitivity analysis, we replicated our survival models described above using a frailty model (random effects survival model) with therapist included as a random effect. A separate frailty model was conducted for time to relapse and time to remission. We also ran separate intercept-only frailty models with therapist included as a random effect.
Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC) as well as MedCalc for Windows, version 145.8 (MedCalc Software, Ostend, Belgium). All analyses were intent-to-treat. Medication management alone was the reference group in our analytic models. The level of significance for all tests was set at α = 0.05 (2-tailed) and to address multiple testing (multiplicity), where applicable, p values were adjusted using the false discovery rate procedure. Hedges’ g was also calculated to estimate effect sizes for between-group comparisons. The magnitude of Hedges’ g can be interpreted using Cohen’s (1988) convention as “small effect (0.20),” “medium effect (0.50),” and “large effect (0.80),” with the caveat that this conventional frame of reference (or rules of thumb regarding the size of the effect) is relative not only to each other but also to its substantive context, its operational definition, or even more particularly, to the specific content and research method being used in any given investigation.31
RESULTS
Participant Characteristics
A total of 144 youth were randomized to MM (n = 69) or MM+CBT (n = 75) for 6 months. The sample consisted of 53.5% (n = 77) females, with 54.2% (n = 78) being non-Hispanic white. The mean age was 13.8 ± 2.6 years, and the majority (78.5%, n = 113) were adolescent (aged ≥12 years). The mean CDRS-R total score at baseline (before treatment) was 58.0 ± 7.2, which was reduced at the randomization point (week 6) to 30.9 ± 5.7. The 2 treatment groups did not differ on baseline demographic and clinical characteristics. Additional details about sample characteristics can be found in Kennard et al.24
Attrition and Treatment During the Follow-Up Period
Figure 1 delineates the number of participants continuing with assessments across the continuum of the study. The average length of time in assessments was similar for those in MM+CBT and MM only (58.8 ± 25.5 weeks versus 54.3 ± 27.7 weeks; p = .32). Of the 144 randomized youth, 97 (67.4%; 55 in MM+CBT and 42 in MM) remained in the study assessments through 1 year (week 52), and 82 (56.9%) remained in study assessments through 1.5 years (week 78). Attrition rates were similar for MM+CBT and MM by week 78 (58.7% versus 55.1%; p = .89). As noted in Kennard et al.,24 the overall rate of treatment completion (defined as remaining in the assigned treatment condition up to week 30) was 66% (95 of 144), with a slightly higher (but nonsignificant) treatment completion rate for the MM+CBT group than for the MM group (70.7% versus 60.9%; p = .22). Table 1 lists demographic and clinical characteristics for those who had at least 1 visit in the follow-up period versus those who did not return after week 30.
FIGURE 1.
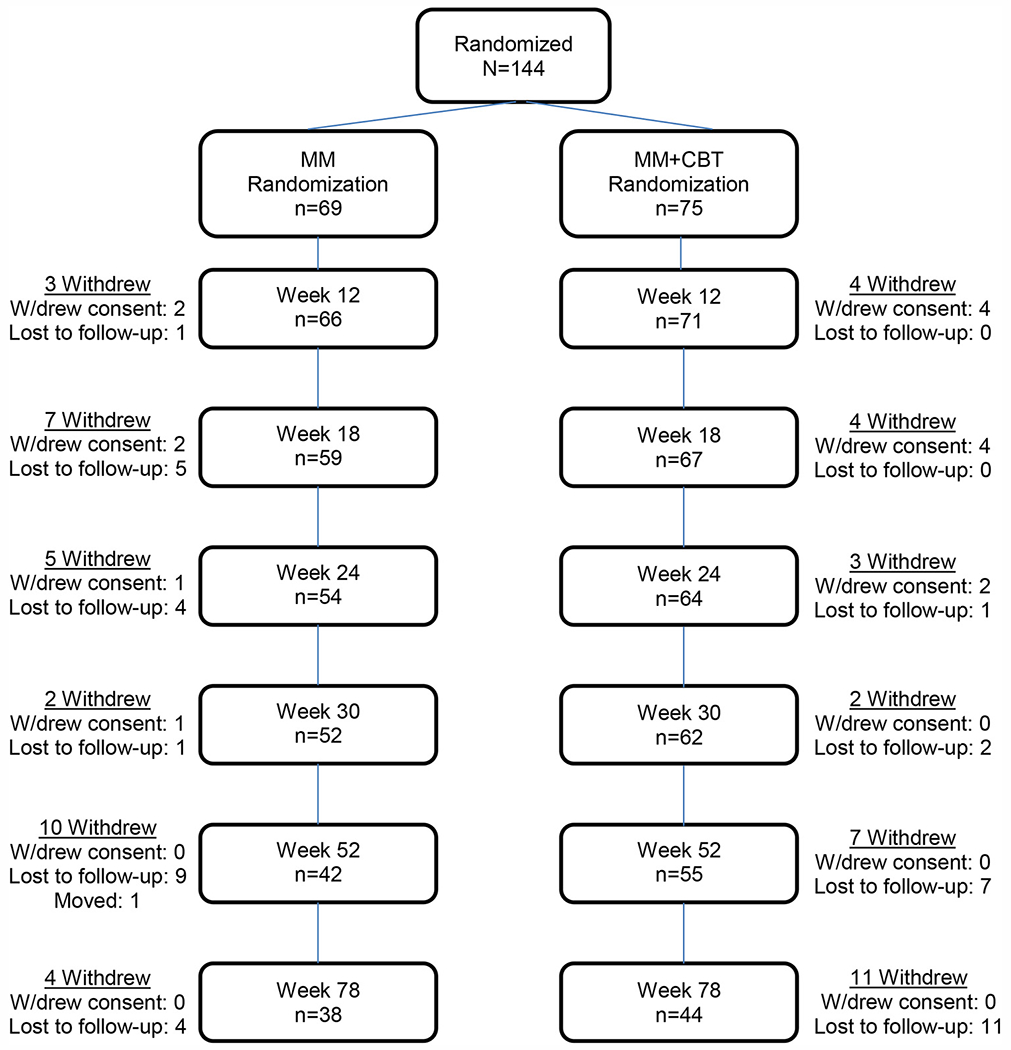
Participants continuing in assessments. Note: MM = medication management; MM + CBT = medication management plus relapse prevention cognitive-behavioral therapy; W/drew = withdrew.
TABLE 1.
Demographic and Clinical Characteristics of Study Sample
| All Randomized (N = 144) |
Had at Least 1 Follow-Up Assessment (n = 97) |
No Follow-Up Assessments (n = 47) |
||||
|---|---|---|---|---|---|---|
| Categorical Variables | n | % | n | % | n | % |
| Female | 77 | 53.5 | 56 | 57.7 | 21 | 44.7 |
| Ethnicity | ||||||
| Hispanic | 43 | 29.9 | 29 | 29.9 | 14 | 29.8 |
| Non-Hispanic | 101 | 70.1 | 68 | 70.1 | 33 | 70.2 |
| Race | ||||||
| White | 118 | 81.9 | 81 | 83.5 | 37 | 78.7 |
| African American | 15 | 10.4 | 12 | 12.4 | 3 | 6.4 |
| Asian | 1 | 0.7 | 0 | 0 | 1 | 2.1 |
| American Indian | 1 | 0.7 | 0 | 0 | 1 | 2.1 |
| Multiracial | 9 | 6.3 | 4 | 4.1 | 5 | 10.6 |
| No. of Episodes | ||||||
| 1 | 127 | 88.2 | 86 | 88.7 | 41 | 87.2 |
| 2 | 15 | 10.4 | 10 | 10.3 | 5 | 10.6 |
| 3+ | 2 | 1.4 | 1 | 1.0 | 1 | 2.1 |
| Baseline CGI Severity | ||||||
| 4 | 25 | 17.4 | 19 | 19.6 | 6 | 12.8 |
| 5 | 69 | 47.9 | 45 | 46.4 | 24 | 51.1 |
| 6 | 50 | 34.7 | 33 | 34.0 | 17 | 36.2 |
| Randomization CGI Severity | ||||||
| 1 | 3 | 2.1 | 2 | 2.1 | 1 | 2.1 |
| 2 | 48 | 33.3 | 36 | 37.1 | 12 | 25.5 |
| 3 | 76 | 52.8 | 48 | 49.5 | 28 | 59.6 |
| 4 | 16 | 11.1 | 11 | 11.3 | 5 | 10.6 |
| 5 | 1 | 0.7 | 0 | 0 | 1 | 2.1 |
| Comorbid anxiety disorder | 32 | 22.2 | 25 | 25.8 | 7 | 14.9 |
| Comorbid behavior disorder | 48 | 33.3 | 29 | 29.9 | 19 | 40.4 |
| Comorbid dysthymia | 24 | 16.7 | 14 | 14.4 | 10 | 21.3 |
| Positive family psychiatric history | 108 | 75.0 | 75 | 77.3 | 33 | 70.2 |
| Baseline suicidal ideation | ||||||
| None | 42 | 29.2 | 25 | 25.8 | 17 | 36.2 |
| Morbid thoughts/death wishes | 23 | 16.0 | 15 | 15.5 | 8 | 17.0 |
| Suicidal thoughts | 53 | 36.8 | 40 | 41.2 | 13 | 27.7 |
| Suicidal plans | 5 | 3.5 | 4 | 4.1 | 1 | 2.1 |
| Suicide attempts | 21 | 14.6 | 13 | 13.4 | 8 | 17.0 |
| Assigned to MM+CBT | 75 | 52.1 | 55 | 56.7 | 20 | 42.6 |
| Attained remission by week 6 | 52 | 43.0 | 39 | 41.9 | 13 | 46.4 |
| Attained remission at any time | 121 | 84.0 | 93 | 95.9 | 28 | 59.6*** |
| Relapsed at any time | 49/121 | 40.5 | 41/93 | 44.1 | 8/28 | 28.6** |
| Continuous Variables | Mean | SD | Mean | SD | Mean | SD |
|
| ||||||
| Age | 13.8 | 2.6 | 13.8 | 2.6 | 13.9 | 2.7 |
| Duration of episode (weeks) | 43.7 | 38.6 | 43.9 | 37.3 | 43.4 | 41.6 |
| Baseline CDRS-R | 58.0 | 7.2 | 58.2 | 7.6 | 57.5 | 6.1 |
| Randomization CDRS-R | 30.9 | 5.7 | 30.4 | 5.6 | 32.1 | 5.8 |
| Week of treatment exit | 25.7 | 6.9 | 28.4 | 4.4 | 20.4 | 7.9*** |
| Week remitted | 11.0 | 10.5 | 11.5 | 11.5 | 9.5 | 6.1 |
| % Time well | 54.7 | 33.4 | 66.4 | 27.5 | 30.5 | 31.5*** |
Note: CBT = cognitive-behavioral therapy; CDRS-R = Childhood Depression Rating Scale–Revised; CGI = Clinical Global Impression; MM = medication management.
p < .05
p < .01
p < .001.
The mean number of weeks on antidepressant treatment (with any antidepressant) for the full sample during follow-up was 50.7 ± 24.0 for youth assigned to MM+CBT compared to 46.2 ± 24.7 for those assigned to MM (p = .40). Of the 97 youth who remained in the study assessments for the follow-up period, most (92.8%; 90 of 97) remained on medication beyond the 30-week treatment study (no difference between the 2 groups).
Among youth who had at least 1 follow-up assessment, 46 youth reported attending at least 1 individual therapy session beyond the 30-week continuation treatment study: 32 of 55 (58.2%) in MM+CBT and 14 of 42 (33.3%) in MM. The mean number of individual therapy sessions during the follow-up period was much lower for MM+CBT compared to MM (4.5 ± 5.1 versus 16.6 ± 11.1; p < .001).
Hazard of Remission Through 78-Week Follow-Up
As noted in Kennard et al.,24 115 youth experienced remission by week 30, with no difference between groups. An additional 6 remitted during the follow-up assessment period. After adjustment for CDRS-R total score at baseline, age, and gender, Cox regression revealed that participants in the MM+CBT group did not differ significantly on time to remission from those in the MM group during the 78-week follow-up period (HR = 1.255, 95% CI = 0.874–1.801; χ2 = 1.50, p = .220).
Throughout the entire 78-week follow-up period, the mean time to remission for the MM+CBT group was 14.26 weeks (SE = 2.13) compared with 18.37 weeks (SE = 2.94) for the MM group (Figure 2). A log-rank test showed that the mean time to remission between the 2 treatment groups was not significantly different (p = .122), g = 0.258. At the follow-up weeks (weeks 52 and 78), the estimated probability of remitting with MM+CBT was about 94% and 96%, respectively, compared with about 89% and 92%, respectively, with MM alone.
FIGURE 2.
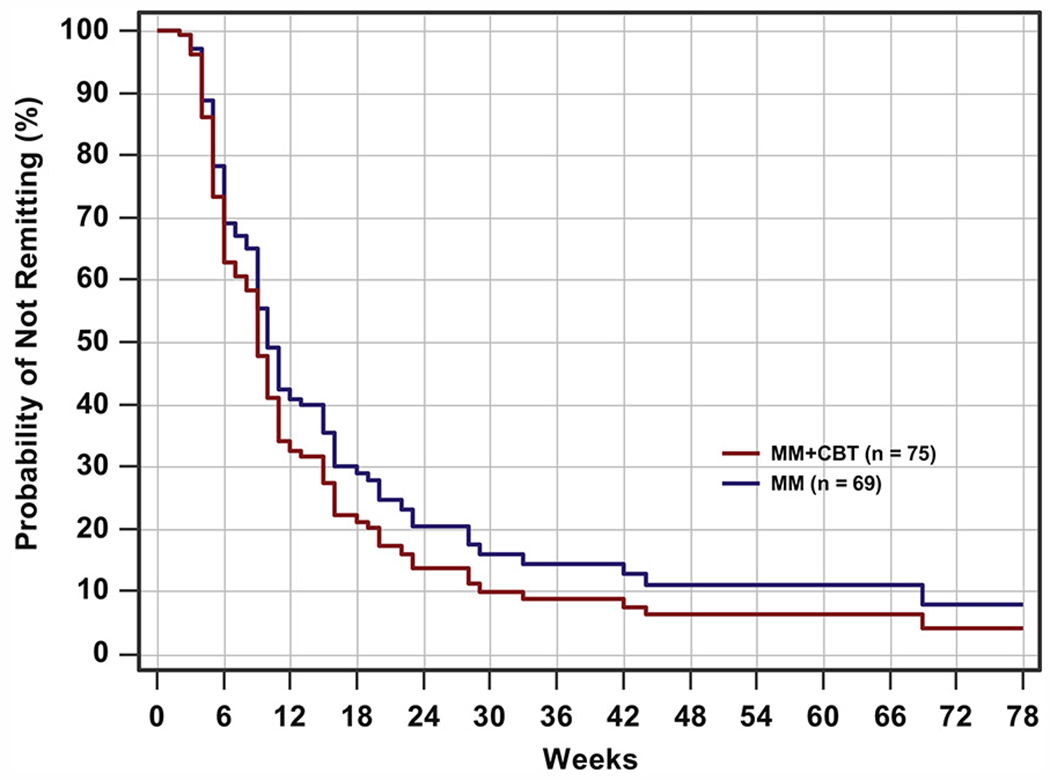
Remission survival curves through week 78. Note: For medication management plus relapse prevention cognitive-behavioral therapy (MM+CBT), n = 75; for medication management (MM), n = 69.
Hazard of Relapse Through 78-Week Follow-Up
Relapse was assessed in participants who achieved remission at any time during the study (n = 121). After adjustment for CDRS-R total score at randomization, age, and gender, Cox regression revealed that participants in the MM+CBT group had a significantly lower risk of relapse than those in the MM group throughout the 78-week follow-up period (HR = 0.467, 95% CI = 0.264–0.823; χ2 = 6.852, p = .009). Thus, throughout the full 78-week period, the hazard of relapse for those who received MM+CBT was 0.467 times that of those who received MM alone; an estimated HR < 1 here (0.467) indicated a lower risk of relapse for the MM+CBT group compared to the MM-only group.
During the full 78-week follow-up period, the mean time to relapse for MM+CBT was 64.40 weeks (SE = 2.68) compared with 50.93 weeks (SE = 3.61) for MM only (log-rank test: p = .007, g = 0.499). Figure 3 displays survival curves, plotted at the mean of all covariates in the model, and indicates that the survival probability of relapsing was lower at all time periods for MM+CBT compared to MM alone. At weeks 52 and 78, the estimated probability of relapse for the MM+CBT group was about 27% and 36%, respectively, compared with about 49% and 62%, respectively, for the MM-only group. Moreover, the 2 treatment groups differed significantly on relapse rates at follow-up week 52 (raw p = .021, false discovery rate–adjusted p = .021) and week 78 (raw p = .008, false discovery rate–adjusted p = .015).
FIGURE 3.
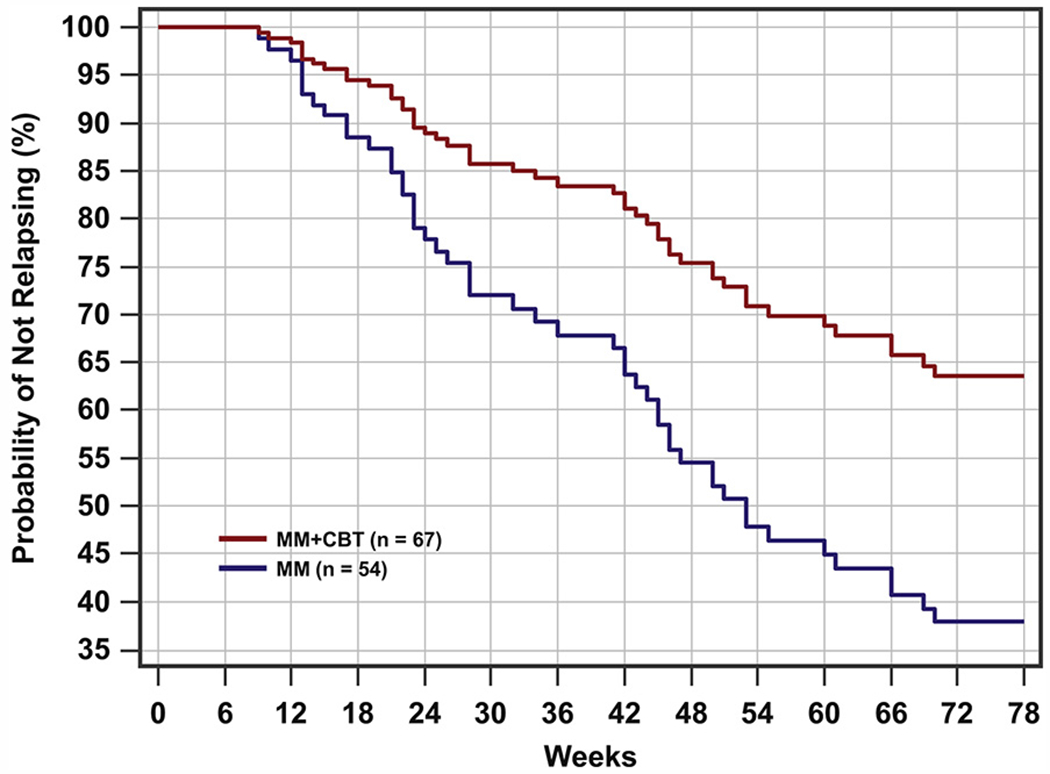
Relapse survival curves through week 78. Note: For medication management plus relapse prevention cognitive-behavioral therapy (MM+CBT), n = 67; for medication management (MM), n =54.
Table 2 provides the demographic and clinical characteristics of youth who experienced a relapse of depression versus those who did not, independent of treatment assignment during the continuation phase. There were few differences between those who relapsed and those who did not. However, those who relapsed had more severe depression at baseline (based on both the CDRS-R Total score and the CGI-Severity score). There was also a higher proportion of Hispanic participants among those who relapsed.
TABLE 2.
Demographic and Clinical Characteristics of Relapsers and Nonrelapsers
| Relapse (n = 49) |
No Relapse (n =72) |
|||
|---|---|---|---|---|
| Categorical Variables | n | % | n | % |
| Female | 28 | 57.1 | 35 | 48.6 |
| Ethnicity | ||||
| Hispanic | 19 | 38.8 | 15 | 20.8* |
| Non-Hispanic | 30 | 61.2 | 57 | 79.2* |
| Race | ||||
| Caucasian | 40 | 81.6 | 60 | 83.3 |
| African American | 6 | 12.2 | 6 | 8.3 |
| Asian | 0 | 0 | 0 | 0 |
| American Indian | 0 | 0 | 1 | 1.4 |
| Multiracial | 3 | 6.1 | 5 | 6.9 |
| No. of Episodes | ||||
| 1 | 45 | 91.8 | 61 | 84.7 |
| 2 | 2 | 4.1 | 11 | 15.3 |
| 3+ | 2 | 4.0 | 0 | 0 |
| Baseline CGI Severity | ||||
| 4 | 5 | 10.2 | 19 | 26.4 |
| 5 | 20 | 40.8 | 39 | 54.2 |
| 6 | 24 | 49.0 | 14 | 19.4*** |
| Comorbid anxiety disorder | 13 | 26.5 | 15 | 20.8 |
| Comorbid behavior disorder | 15 | 30.6 | 26 | 36.1 |
| Comorbid dysthymia | 9 | 18.4 | 9 | 12.5 |
| Positive family psychiatric history | 35 | 71.4 | 56 | 77.8 |
| Baseline suicidal ideation | ||||
| None | 10 | 20.4 | 27 | 37.5 |
| Morbid thoughts/death wishes | 7 | 14.3 | 13 | 18.1 |
| Suicidal thoughts | 21 | 42.9 | 25 | 34.7 |
| Suicidal plans | 2 | 4.1 | 2 | 2.8 |
| Suicide attempts | 9 | 18.4 | 5 | 6.9 |
| Attained remission by week 6 | 21 | 42.9 | 31 | 43.1 |
| Continuous Variables | Mean | SD | Mean | SD |
|
| ||||
| Age | 13.7 | 2.4 | 13.9 | 2.7 |
| Duration of episode (wk) | 42.9 | 32.1 | 41.3 | 40.5 |
| Baseline CDRS-R | 60.4 | 7.5 | 55.8 | 6.8*** |
| Randomization CDRS-R | 30.5 | 5.8 | 29.7 | 5.5 |
| Week of study exit | 67.1 | 19.3 | 60.0 | 23.8 |
| Week of treatment exit | 28.1 | 4.4 | 27.3 | 5.4 |
| Week remitted | 10.5 | 7.7 | 11.4 | 12.1 |
| % Time well | 52.0 | 22.6 | 73.3 | 25.2*** |
Note: CBT = cognitive-behavioral therapy; CDRS-R = Childhood Depression Rating Scale—Revised; CGI = Clinical Global Impression.
p < .05;
p < .01;
p < .001.
Time Spent Well
The incidence of “time spent well” was evaluated using a negative binomial regression model with adjustment for CDRS-R Total score at randomization, age, and gender. The results revealed that participants in the MM+CBT group had a significantly higher estimated rate of time spent well than those in the MM group (59.3% versus 48.8%, χ2 = 4.09, p = .043, g = 0.341). After adjustment for the above-mentioned covariates, a negative binomial regression also revealed that the MM+CBT group spent 38.3 (SE = 2.82) weeks well versus 30.4 (SE = 2.95) weeks well with MM only (χ2 = 3.37, p = .066, g = 0.308).
DISCUSSION
This article reports on the long-term follow-up period for children and adolescents with MDD who completed a 30-week continuation treatment study examining the effect of medication alone versus a sequential strategy of acute treatment with medication followed by continuation treatment with medication plus CBT. The outcomes during the continuation treatment period of the study (30 weeks) demonstrated reduced relapse rates for youth who were in the MM+CBT arm compared to those who received medication alone (9% versus 26.5%; HR = 0.31).24 In the long-term assessments, these differences were maintained for up to 78 weeks, with the MM+CBT group continuing to have substantially lower relapse rates compared to MM only (36% versus 62%). In addition, the mean time to relapse was about 3 months longer for those who received MM+CBT compared to those who received MM alone. Despite efficacy of adding RP-CBT to prevent relapse, the relapse rates over 1.5 years were still considerable at 36%. Identifying which patients are at greater risk for relapse will be an important next step. In this report, youth who relapsed had greater depression severity at baseline. Additional examination of predictors and moderators of relapse of this sample may provide key factors related to relapse, which in turn could lead to important targets for future intervention.
Both the MM+CBT and MM groups had similarly high rates of remission by 78 weeks (>90%). In fact, remission rates for the 2 groups during the long-term follow-up period were similar to those during the 30-week continuation treatment portion of the study. Like the 30-week continuation treatment, time to remission was also not statistically different between the 2 groups during the 78-week follow-up, although the MM+CBT group remitted approximately 1 month earlier than the MM group. The MM+CBT group was also well (i.e., had minimal or no depressive symptoms based on the A-LIFE) for approximately 2 months longer than the MM group; although not statistically significant (p = .06), 2 months in the life of a teen is arguably clinically meaningful.
One interesting finding in this study was the continued use of medication beyond the 30-week treatment portion of the study. More than 90% of the sample continued medication beyond the treatment phase of the study (30 weeks), with the mean length of medication treatment from 46 to 50 weeks. Thus, most patients remained on medication for 6-9 months, as recommended by treatment guidelines. Of note, although the RP-CBT manual did not have a specific module for medication adherence, the entire study team (therapists, psychiatrists, and study coordinators) frequently reminded patients and families about the importance of medication adherence, and pill counts were conducted at each medication management visit.
We acknowledge that this study has some limitations. As noted in Kennard et al.,24 1 limitation was that the participants were not blinded to treatment assignment, which could have also affected participant and parent perception of outcomes. In addition, treatment was not controlled after 30 weeks. One-third of youth who were assigned to MM received psychotherapy after the 30-week continuation treatment phase, with a mean number of 16 sessions, which would be considered an adequate dose of psychotherapy treatment. Although more youth assigned to MM+CBT did continue to have psychotherapy sessions beyond the treatment phase (58%), the number of sessions was minimal (mean = 4) and more indicative of booster sessions than full-dose psychotherapy. Finally, attrition affects results; however, attrition during the long-term assessment period was similar to the attrition rates in the TADS study beyond the treatment phase.32
We did examine a potential “therapist effect” through a sensitivity analysis by replicating our survival models using a frailty model (random effects survival model) with therapist included as a random effect. A separate frailty model was conducted for time to relapse and time to remission. In each frailty model, with therapist included as a random effect, our basic survival results and conclusions did not change (results not reported). We also ran separate intercept-only frailty models with therapist included as a random effect. Results (not reported) from separate intercept-only frailty models, with therapist included as a random effect, suggested that therapists did not introduce any significant concomitant variability concerning the interpretation of our basic survival findings. We also conducted a separate sensitivity analysis by replicating our Cox and negative binomial regression models, excluding the 14 youth in the MM-only group who received at least 1 individual therapy session beyond the 30 week continuation treatment, and the results revealed that our basic findings persisted with no meaningful change (results not reported).
Despite these limitations, this study suggests that adding CBT after treatment response to medication improves outcomes not only during the actual continuation treatment phase, but also has enduring effects for up to a full year after completion of therapy. Yet, current guidelines for acute treatment continue to suggest beginning treatment for moderate to severe depression with psychotherapy alone, medication alone, or psychotherapy plus medication simultaneously (AACAP Practice Parameters).33 Current guidelines regarding continuation treatment state to continue the treatment for 6 to 9 months after remission. The findings from this study, however, are consistent with prior adult studies, which suggest that adding CBT to antidepressant treatment after initial response as a continuation phase treatment for depression reduces rates of relapse.20 Thus, although most clinicians begin reducing the frequency of treatment sessions once a patient has shown improvement, the findings in this pediatric trial support that increasing visit frequency by adding therapy after response will reduce relapse rates. Furthermore, providing treatment to participants who are in remission, who are therefore “less ill,” could also reduce the frequency of therapy sessions (10 sessions versus the 16–20 typical in clinical trials of CBT), which may in turn increase the cost-effectiveness of CBT treatment in the continuation phase.34
Unfortunately, we currently do not have long-term strategies for treating youth with depression, as the focus to date has been on acute strategies. To date, there has been only 1 published maintenance study in youth with depression.35 Maintenance treatment with antidepressants looks promising for preventing recurrence; however, more long-term studies are needed. Strategies that are designed to target the prevention of relapse and recurrence are critical in such a chronic, episodic illness as pediatric depression. Although the field has made great gains in treating the symptoms of this illness, more work is needed in maintaining wellness once symptoms subside. &
Acknowledgments
Funding for this study was provided by National Institute of Mental Health (NIMH) grant R01MH39188 (Principle Investigators Graham Emslie and Betsy Kennard).
The authors are grateful to all of the children and families who participated in this study, and to all of the faculty and staff who worked diligently on this research project. The authors especially thank the study coordinators and research assistants, without whom they could not have successfully completed this project: Shauna Barnes, BA, Krystle Joyner, MS, and Tabatha Melton, BA, all of whom were employed by UT Southwestern Medical Center during their work on the study. The authors also recognize the contributions of their consultants: David Brent, MD (University of Pittsburgh), Greg Clarke, PhD (Kaiser Permanente Research), Michael Frisch, PhD (Baylor University), Robin Jarrett, PhD (UT Southwestern), John Rush, MD (UT Southwestern), and Kevin Stark, PhD (UT Austin).
Disclosure:
Dr. Emslie has received research support from Duke University, Forest Laboratories, and Mylan, and has served as a consultant for Alkermes, Allergan, Bristol-Myers Squibb, Eli Lilly and Co., INC Research Inc., Lundbeck, Merck, NCS Pearson, Neuronetics, Otsuka, and Pfizer Inc. Dr. Kennard has received research support from the National Institute of Mental Health. Drs. Nakonezny and Foxwell and Mss. Mayes, Moore, Jones, and King report no biomedical financial interests or potential conflicts of interest.
Footnotes
Dr. Nakonezny served as the statistical expert for this research.
Previous Presentation: Data from this study have been previously presented at the annual meetings of the American Academy of Child and Adolescent Psychiatry (AACAP 2013), the New Clinical Drug Evaluation Unit (NCDEU, 2013), European Society for Child and Adolescent Psychiatry (ESCAP, 2013), and the International Conference on Child and Adolescent Psychopathology (2013).
Contributor Information
Graham J. Emslie, University of Texas Southwestern Medical Center and Children’s Health Children’s Medical Center, Dallas.
Betsy D. Kennard, University of Texas Southwestern Medical Center and Children’s Health Children’s Medical Center, Dallas.
Taryn L. Mayes, University of Texas Southwestern Medical Center and Children’s Health Children’s Medical Center, Dallas.
Paul A. Nakonezny, Division of Biostatistics, University of Texas Southwestern Medical Center, Dallas.
Jarrette Moore, University of Texas Southwestern Medical Center and Children’s Health Children’s Medical Center, Dallas.
Jessica M. Jones, University of Texas Southwestern Medical Center and Children’s Health Children’s Medical Center, Dallas.
Aleksandra A. Foxwell, University of Texas Southwestern Medical Center and Children’s Health Children’s Medical Center, Dallas.
Jessica King, University of Texas Southwestern Medical Center and Children’s Health Children’s Medical Center, Dallas.
REFERENCES
- 1.Bridge J, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. J Am Med Assoc. 2007;297:1683–1696. [DOI] [PubMed] [Google Scholar]
- 2.Cheung A, Emslie GJ, Mayes TL. Review of the efficacy and safety of antidepressants in youth depression. J Child Psychol Psychiatry. 2005;46:735–754. [DOI] [PubMed] [Google Scholar]
- 3.Compton SN, March JS, Brent DA, Albano AM, Weersing VR, Curry JF. Cognitive-behavioral psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry. 2004;43:930–959. [DOI] [PubMed] [Google Scholar]
- 4.Weisz JR, McCarty CA, Valeri SM. Effects of psychotherappy for depression in children and adolescents: a meta-analysis. Psychol Bull. 2006;132:132–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–820. [DOI] [PubMed] [Google Scholar]
- 6.Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299:901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodyer IM, Dubicka B, Wilkinson P, et al. A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial. Health Technol Assess. 2008;12:iii–iiv, ix-60. [DOI] [PubMed] [Google Scholar]
- 8.Emslie GJ, Heiligenstein JH, Hoog SL, et al. Fluoxetine treatment for prevention of relapse of depression in children and adolescents: a double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 2004;43:1397–1405. [DOI] [PubMed] [Google Scholar]
- 9.Emslie GJ, Kennard BD, Mayes TL, et al. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry. 2008;165:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennard BD, Emslie GJ, Mayes TL, Hughes JL. Relapse and recurrence in pediatric depression. Child Adolesc Psychiatr Clin North Am. 2006;15:1057–1079. [DOI] [PubMed] [Google Scholar]
- 11.Jarrett RB, Minhajuddin A, Gershenfeld H, Friedman ES, Thase ME. Preventing depressive relapse and recurrence in higher-risk cognitive therapy responders: a randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo. JAMA Psychiatry. 2013;70:1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fava G, Grandi S, Zielezny M, Canestrari R, Morphy MA. Cognitive behavioral treatment of residual symptoms in primary major depressive disorder. Am J Psychiatry. 1994;151:1295–1299. [DOI] [PubMed] [Google Scholar]
- 13.Fava G, Grandi S, Zielezny M, Rafanelli C, Canestrari R. Four-year outcome for cognitive behavioral treatment of residual symptoms in major depression. Am J Psychiatry. 1996;153:945–947. [DOI] [PubMed] [Google Scholar]
- 14.Fava G, Rafanelli C, Grandi S, Canestrari R, Morphy MA. Six-year outcome for cognitive behavioral treatment of residual symptoms in major depression. Am J Psychiatry. 1998;155:1443–1445. [DOI] [PubMed] [Google Scholar]
- 15.Fava G, Rafanelli C, Grandi S, Conti S, Belluardo P. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry. 1998;55:816–820. [DOI] [PubMed] [Google Scholar]
- 16.Fava GA, Fabbri S, Sonino N. Residual symptoms in depression: an emerging therapeutic target. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1019–1027. [DOI] [PubMed] [Google Scholar]
- 17.Nierenberg AA, Petersen TJ, Alpert JE. Prevention of relapse and recurrence in depression: the role of long-term pharmacotherapy and psychotherapy. J Clin Psychiatry. 2003;64(Suppl 15):13–17. [PubMed] [Google Scholar]
- 18.Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56:829–835. [DOI] [PubMed] [Google Scholar]
- 19.Teasdale JD, Williams JMG, Soulsby JM, Segal ZV, Ridgeway VA, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68: 615–625. [DOI] [PubMed] [Google Scholar]
- 20.Guidi J, Fava GA, Fava M, Papakostas GI. Efficacy of the sequential integration of psychotherapy and pharmacotherapy in major depressive disorder: a preliminary meta-analysis. Psychol Med. 2011;41:321–331. [DOI] [PubMed] [Google Scholar]
- 21.Kroll L, Harrington R, Jayson D, Fraser J, Gowers S. Pilot study of continuation cognitive-behavioral therapy for major depression in adolescent psychiatric patients. J Am Acad Child Adolesc Psychiatry. 1996;35:1156–1161. [DOI] [PubMed] [Google Scholar]
- 22.Clarke GN, Rohde P, Lewinsohn PM, Hops H, Seeley JR. Cognitive-behavioral treatment of adolescent depression: efficacy of acute group treatment and booster sessions. J Am Acad Child Adolesc Psychiatry. 1999;38:272–279. [DOI] [PubMed] [Google Scholar]
- 23.Kennard BD, Emslie GJ, Mayes TL, et al. Cognitive-behavioral therapy to prevent relapse in pediatric responders to pharmacotherapy for major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2008;47: 1395–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennard BD, Emslie GJ, Mayes TL, et al. Sequential treatment with fluoxetine and relapse—prevention CBT to improve outcomes in pediatric depression. Am J Psychiatry. 2014;171:1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poznanski EO, Mokros H. Children’s Depression Rating Scale–Revised (CDRS-R). Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- 26.Guy W ECDEU Assessment Manual for Psychopharmacology. Washington DC: US Government Printing Office; 1976. [Google Scholar]
- 27.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 28.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168: 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57:675–682. [DOI] [PubMed] [Google Scholar]
- 30.Keller MB, Lavori PW, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 32.March J, Silva S, Petrycki S, et al. The Treatment for Adolescents with Depression Study (TADS): long-term effectiveness and safety outcomes. Arch General Psychiatry. 2007;64:1132–1144. [DOI] [PubMed] [Google Scholar]
- 33.Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503–1526. [DOI] [PubMed] [Google Scholar]
- 34.Scott J, Palmer S, Paykel ES, Teasdale JD, Hayhurst H. Use of cognitive therapy for relapse prevention in chronic depression: cost effectiveness study. Br J Psychiatry. 2003;182:221–227. [DOI] [PubMed] [Google Scholar]
- 35.Cheung A, Kusumakar V, Kutcher S, et al. Maintenance study for adolescent depression. J Child Adolesc Psychopharmacol. 2008;18:389–394. [DOI] [PubMed] [Google Scholar]


