Abstract
Iron deficiency anemia is a common public health problem in the Alaska Native population. Yet, a clear etiology has eluded researchers for decades. Previous studies suggested a link between Helicobacter pylori infection, gastrointestinal blood loss due to hemorrhagic gastritis, and generalized iron deficiency anemia in adult Alaska Natives. Therefore, we examined the association between the prevalence of H. pylori-specific immunoglobulin G (IgG) and serum ferritin levels, a marker of iron deficiency. A random sample of 2,080 serum samples from Alaska Native residents drawn between 1980 and 1986 from residents in 13 regions was selected, and the samples were stratified by age, sex, and region. Overall, 75% were positive for H. pylori-specific IgG. The rate of H. pylori seropositivity increased with age; by age 14 years, 78% of the residents were positive. There were no gender differences in H. pylori seropositivity. However, marked regional differences were observed. Serum ferritin levels of <12 ng/ml were found most commonly among persons <20 years of age and among women of childbearing age. A significant association between low serum ferritin levels and prevalence of H. pylori-specific IgG was found, particularly for people aged less than 20 years. H. pylori may be a factor contributing to the iron deficiency anemia in the Alaska Native population.
Iron deficiency anemia is a persistent public health problem among Alaska Natives. Early studies documented rates of anemia ranging from 10 to 35% in school-aged children (3, 17, 19, 20). Subsequent studies that have used serum ferritin levels, the level of transferrin saturation, and the need for iron supplementation as markers of anemia indicated that the observed anemia is caused by iron deficiency (2, 13). More recently, a meta analysis of multiple cross-sectional surveys focusing on dietary iron intake, hemoglobin concentrations, and iron storage found that 30 to 50% of Alaska Native children had depleted iron stores, and both male and female adults were twice as likely to have low iron stores than the general U.S. population (18). These findings are surprising since the dietary iron and vitamin C intakes of Alaska Natives between 21 and 60 years of age are 25 to 35% higher than the U.S. average (15).
Gastrointestinal blood loss in the absence of other etiologies (e.g., chronic disease) is by far the most prevalent cause of iron deficiency anemia in children and adults (11). Occult gastrointestinal bleeding has been reported in the Alaska Native population and has been explored as a cause of the observed widely pervasive iron deficiency (22). Using a semiquantitative measure of the amount of stool heme per gram weight of stool (i.e., Hemaquant), investigators found stool heme concentrations of more than 2 mg/g of stool in 90% of stool samples collected from adults living in one region of Alaska (22). In comparison, only 3% of individuals in an asymptomatic U.S. reference population had >2 mg of heme/g of stool. Upper and lower endoscopy of the Alaska Native adults in this cohort with elevated stool heme concentrations revealed no abnormal lower gastrointestinal findings but did reveal significant macroscopic and microscopic upper gastrointestinal tract disease. Ninety-seven percent had an abnormal gastric mucosal appearance including diffuse mucosal hemorrhage and ulceration, and 99% had histopathologic evidence of chronic active pangastritis associated with Helicobacter pylori.
The hypothesis that H. pylori infection and the associated host gastric disease (i.e., hemorrhagic gastritis) resulted in the observed iron deficiency anemia required a population follow-up study to evaluate the overall prevalence of H. pylori-specific immunoglobulin G (IgG) in a large cross-sectional cohort of Alaska Natives. In addition, we aimed to determine the prevalence H. pylori infection and the correlation or lack thereof of H. pylori infection with serum ferritin levels to confirm whether H. pylori was a factor contributing to iron deficiency anemia.
MATERIALS AND METHODS
Study population.
The study included a sample of serum specimens obtained from approximately 85,000 Alaska Natives residing in Alaska. The specific study cohort comprises a sample of an ethnically and linguistically diverse group including Eskimos, Aleuts, and Indians that reside in approximately 150 villages of 100 to 800 individuals per village throughout the state. About 25% of Alaska Natives reside in the urban areas. A random sample of stored sera from this population was available from studies conducted from 1980 to 1986. These sample sera were stratified by the subject's region of residence, gender, and age. Sera were drawn from 10 males and 10 females in each of the following age groups of residents of 13 regions across the state: <5, 5 to 9, 10 to 14, 15 to 19, 20 to 29, 30 to 39, 40 to 49, and 50+ years. Thus, sera from 260 persons per age group were included in the final cohort of serum samples to be tested.
H. pylori serology and serum ferritin level testing.
Serum samples were tested for antibody to H. pylori by using an in-house enzyme-linked immunosorbent assay by the Foodborne and Diarrheal Diseases Laboratory, National Center for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Ga. (7, 10). The assay uses whole-cell antigens and has a sensitivity of 92% and a specificity of 98% compared with the results of upper endoscopy performed with Alaska Natives and had positive and negative predictive values superior to those of widely used commercial serologic assays (10). Serum ferritin levels in all subject sera were measured at the Arctic Investigations Program in Anchorage by using a commercial assay (Quant-immune RIA; Bio-Rad, Hercules, Calif.).
In this blinded study the names of all individuals and any other patient identifiers were removed from samples prior to testing in order to maintain patient confidentiality. Therefore, individual subjects or their physicians could not be notified of test results. The Alaska Area Native Health Service Research and Publication Committee approved the study.
Statistical analysis.
Rates of H. pylori infection were calculated for Alaska Natives, including age-, sex-, and region-specific rates. For the purposes of analysis sera with H. pylori-specific IgG values within the indeterminate range were considered positive. For comparisons of the presence of H. pylori-specific IgG and low serum ferritin level prevalence and for correlation of these two factors, chi-square, Fisher's exact, and Mantel-Haenszel (MH) tests were used for trend as appropriate.
RESULTS
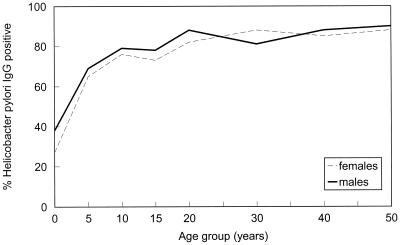
A total of 2,080 serum samples were tested for IgG antibody to H. pylori; 1,386 of the 2,080 samples (66.6%) were positive and an additional 170 serum samples were indeterminate, for an overall seropositivity rate of 74.8% (1,556 of 2,080). The rate of seropositivity increased with increasing age (z = 15.05; P < 0.001) (Fig. 1). Thirty-two percent (84 of 260) of individuals age 0 to 4 years were positive for H. pylori. By age 5 to 9 years, the rate of H. pylori seropositivity increased to 66.9% (174 of 260), and by age 10 to 14 years it reached 77.7% (202 of 260). There was a slightly higher rate of seropositivity among males, 76.5% (796 of 1,040), than among females, 73.1% (760 of 1,040). However, this difference was not significant, even after adjusting for age (χ2 = 3.71 [MH test]; P = 0.054).
FIG. 1.
Rates of H. pylori infection among Alaska Natives by age and sex, 1980 to 1986.
H. pylori seroprevalence rates varied significantly by region (χ2 = 66.9; degrees of freedom = 12; P < 0.001), from a low of 61.3% (98 of 160) in the south-central region (Anchorage and vicinity) to a high of 84.4% (135 of 160) in the interior regions (Fairbanks and vicinity). This difference was particularly pronounced in the youngest subjects (ages 0 to 4 years), among whom the seropositivity rates ranged from 5% (1 of 20) in the south-central region to 65% (13 of 20) in the interior (χ2 = 40.1; degrees of freedom = 12; P < 0.001).
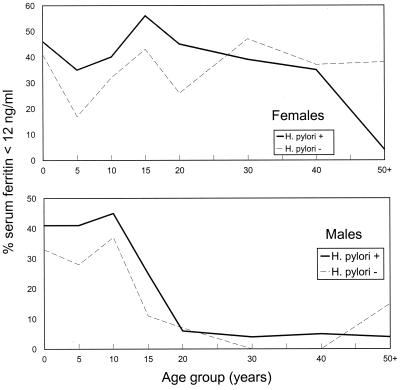
Low serum ferritin levels (<12 ng/ml) were found in 19.6% (204 of 1,040) of males and 35.8% (372 of 1,040) of females. Low levels were most common among persons <20 years of age and among women of childbearing age (Fig. 2). Of persons with serum ferritin levels <12 ng/ml, 73.5% (150 of 204) of males and 73.6% (274 of 372) of females were also seropositive for H. pylori. Overall, an association was observed between low serum ferritin levels and H. pylori seropositivity. When adjusted for age and sex, the relative risk for low serum ferritin levels among persons seropositive for H. pylori compared to that among persons seronegative for H. pylori was 1.13 (χ2 = 6.24 [MH test]; P = 0.013); for persons less than 20 years of age the relative risk (RR) was 1.15 (χ2 = 10.0 [MH test]; P < 0.002). Among males, the overall risk for low serum ferritin levels was statistically significant (RR = 1.16; χ2 = 4.22 [MH test]; P = 0.04), with an increased RR for low serum ferritin levels among those <20 years of age (RR = 1.14; χ2 = 4.61 [MH test]; P = 0.032). Among females, the overall association was not significant when adjusted for age but demonstrated significance when the analysis was restricted to those <20 years of age (RR = 1.16; χ2 = 4.96 [MH test]; P = 0.026).
FIG. 2.
Percentage of Alaska Natives with low serum ferritin levels (<12 ng/ml), by age, gender, and presence of H. pylori-specific IgG.
The association between low serum ferritin levels and H. pylori seropositivity was stronger when the 170 serum samples with indeterminate results were excluded from the analysis. When adjusting for age and sex, the RR among persons who were H. pylori positive compared to that among those who were H. pylori negative was 1.39 (P = 0.055), and for persons less than 20 year of age the RR was 1.71 (P < 0.001).
DISCUSSION
This cross-sectional population-based serosurvey demonstrates a high rate of past or current infection with H. pylori in the Alaska Native population during the 1980s. The age when infection is acquired appears to be quite young, in that up to 40% of children were seropositive by the age of 4 years and almost 70% were seropositive by the age of 10 years. The cross-sectional prevalence of H. pylori infection in Alaska Natives is similar to that observed in populations in developing countries. Previous seroepidemiological studies in developing countries have shown infection rates among children to be quite high, approaching 70 to 90% among children under age 10 years in some countries (6, 8). However, marked differences in the seroprevalence of H. pylori have been observed between various ethnic groups living in developed countries (8, 12, 21). Graham et al. (8) found a significantly higher seroprevalence of H. pylori among Hispanics and African Americans than among non-Hispanic whites. Conversely, the rate of acquisition in persons over 20 years of age was similar in all three groups. This suggests that the risk of acquisition is greater in childhood or early adolescence for Hispanics and African Americans and may be related not to ethnic background but to the lower socioeconomic conditions experienced by these groups during childhood. This concept may explain the lower rates of infection found in Alaska Native children living in Anchorage than in Alaska Native children living in rural regions of the state. However, this observation did not hold true for Alaska Native children living in urban Fairbanks, where rates of infection were similar to those for Alaska Native children living in rural regions of Alaska.
The finding of persistently high rates of iron deficiency in persons <20 years of age and women of childbearing age in our study cohort is compatible with the rates found in earlier studies performed in Alaska (2, 3, 5, 13, 17–19, 20). These high rates of iron deficiency are in contrast to declining rates of iron deficiency among all age groups of the general U.S. population (4). While menstrual blood loss can be considered a contributing factor for iron deficiency in women of childbearing age, factors for iron deficiency among those Alaska Natives <20 years of age may include inadequate intake of dietary iron or foods that enhance iron absorption (5). In general, however, the typical Alaska Native subsistence diet (e.g., fish and marine and land mammals) is high in dietary bioavailable iron (15).
The association of low serum ferritin levels, a marker of iron deficiency, with the presence of H. pylori-specific IgG supports the earlier endoscopic findings of H. pylori-associated gastritis as a cause of elevated stool heme levels (22). This association appeared to be particularly strongest for those Alaska Natives <20 years of age, regardless of whether the analysis either included or excluded sera with indeterminate results. We have since found a similar association between both anemia and iron deficiency and H. pylori seropositivity among young children residing in one rural Alaska village (5). Our findings are also supported by another large serosurvey for H. pylori in adults in Denmark in which serum ferritin levels were found to be significantly lower in adult men and postmenopausal women who were H. pylori IgG positive than in noninfected persons (14). A smaller study in Australia also showed that serum ferritin levels were significantly lower in women who were H. pylori IgG positive than in women who were H. pylori negative (16). While blood loss associated with gastritis or bleeding duodenal or gastric ulcers may play an important role in the development of iron deficiency in Alaska Natives, other factors related to H. pylori infection may also contribute. These include the sequestering of iron by the host in response to H. pylori-related chronic gastric inflammation (1) and the binding of iron by H. pylori outer membrane iron binding proteins (9, 23). Both of these mechanisms would act in the host to reduce the iron normally available for storage and utilization. Approximately one-quarter of iron-deficient persons had no serologic evidence of infection by H. pylori, suggesting that other factors may be responsible for the observed iron deficiency in this population. These factors may include low levels of intake of iron or substances that enhance iron adsorption (i.e., vitamin C), high levels of intake of iron-inhibiting substances (i.e., tannins), or chronic infection by infectious agents other than H. pylori.
In summary, the statewide serosurvey described here found a high prevalence of H. pylori-specific IgG among Alaska Natives. The rate of seropositivity increased with increasing age, in that 78% of the study cohort was positive by age 14 years. There were marked regional differences, with high rates of seropositivity occurring in the northern, western, and central regions of Alaska. However, an explanation for this regional variation remains to be determined. Overall, low serum ferritin levels were found in 20% of males and 36% of females, underscoring the persistence of iron deficiency anemia within this population. An association between low serum ferritin levels and the presence of H. pylori-specific IgG was found, particularly among persons less than 20 years of age, providing further support for a possible role of H. pylori in the etiology of the iron deficiency anemia observed among Alaska Natives.
ACKNOWLEDGMENT
B. D. Gold is supported, in part, by a grant (DK-53708-01) from the National Institutes of Health.
REFERENCES
- 1.Blaser M. Hypothesis on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 2.Burks J M, Siimes M A, Mentzer W C, Dallman P R. Iron deficiency in an Eskimo village. J Pediatr. 1976;88:224–228. doi: 10.1016/s0022-3476(76)80986-9. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. High prevalence of iron deficiency anemia among Alaska Native children. Morb Mortal Wkly Rep. 1988;37:200–202. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. Morb Mortal Wkly Rep. 1988;47(RR-3):1–36. [Google Scholar]
- 5.Centers for Disease Control and Prevention. Iron deficiency anemia in Alaska Native children—Hooper Bay, Alaska 1999. Morb Mortal Wkly Rep. 1999;48:714–716. [PubMed] [Google Scholar]
- 6.The EUROGAST Study Group. Epidemiology of and risk factors for Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. Gut. 1993;34:1672–1676. doi: 10.1136/gut.34.12.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold B D, Khanna B, Huang L M, Lu C, Banatvala N. Helicobacter pylori acquisition in infancy after decline of maternal passive immunity. Pediatr Res. 1997;41:641–646. doi: 10.1203/00006450-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Graham D Y, Malaty H M, Evans D G, Doyle J E, Klein P D, Adams E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States: effect of age, race, sex, and socioeconomic status. Gastroenterology. 1991;100:1495–1501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 9.Husson M A, Legrand D, Spik G, Leclerc H. Iron acquisition by Helicobacter pylori: importance of human lactoferrin. Infect Immun. 1993;61:2694–2697. doi: 10.1128/iai.61.6.2694-2697.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna B, Cutler A, Israel N R, Perry M, Lastovica A, Fields P, Gold B D. Use caution with serologic testing for Helicobacter pylori in children. J Infect Dis. 1998;178:460–465. doi: 10.1086/515634. [DOI] [PubMed] [Google Scholar]
- 11.Koerper M A, Mintger W L, Brecher G, Dallman P R. Developmental changes in red blood cell volume. Implications in screening infants and children for iron deficiency and thalassemia trait. J Pediatr. 1976;89:580–583. doi: 10.1016/s0022-3476(76)80390-3. [DOI] [PubMed] [Google Scholar]
- 12.Louw J A, Jaskiewicz K, Girdwood A H, Zak J, Trey G, Lucke W, Truter H, Kotze T J. Helicobacter pylori prevalence in nonulcer dyspepsia—ethnic and socioeconomic differences. S Afr Med J. 1993;83:169–171. [PubMed] [Google Scholar]
- 13.Margolis H S, Hardison H H, Bender T R, Dallman P R. Iron deficiency anemia in children: the relationship between laboratory tests and subsequent hemoglobin response to therapy. Am J Clin Nutr. 1981;34:2158–2168. doi: 10.1093/ajcn/34.10.2158. [DOI] [PubMed] [Google Scholar]
- 14.Milman N, Rosenstock S J, Andersen L P, Jorgensen T, Bonnevie O. Serum ferritin, hemoglobin and Helicobacter pylori infection: a seroprevalence survey comprising 2794 Danish adults. Gastroenterology. 1998;115:268–274. doi: 10.1016/s0016-5085(98)70192-1. [DOI] [PubMed] [Google Scholar]
- 15.Nobmann E D, Byers T, Lanier A L, Hankin J H, Jackson M Y. The diet of Alaska Natives: 1987–1988. Am J Clin Nutr. 1992;55:1024–1032. doi: 10.1093/ajcn/55.5.1024. [DOI] [PubMed] [Google Scholar]
- 16.Peach H G, Bath N E, Farish S J. Helicobacter pylori infection: added stressor on iron status of women in the community. Med J Aust. 1998;169:188–190. doi: 10.5694/j.1326-5377.1998.tb140218.x. [DOI] [PubMed] [Google Scholar]
- 17.Petersen K M, Grant L J. Growth and hematological changes in the Eskimo population of Wainwright, Alaska: 1968 to 1977. Am J Clin Nutr. 1984;39:460–465. doi: 10.1093/ajcn/39.3.460. [DOI] [PubMed] [Google Scholar]
- 18.Petersen K M, Parkinson A J, Nobmann E D, Bulkow L, Yip R, Mokdad A. Iron deficiency anemia among Alaska Natives may be due to fecal loss rather than inadequate intake. J Nutr. 1996;126:2774–2783. doi: 10.1093/jn/126.11.2774. [DOI] [PubMed] [Google Scholar]
- 19.Sauberlich H E, Goad W, Herman Y F, Milan F, Jamison P. Biochemical assessment of the nutritional status of the Eskimos of Wainwright Alaska. Am J Clin Nutr. 1972;25:437–447. doi: 10.1093/ajcn/25.4.437. [DOI] [PubMed] [Google Scholar]
- 20.Scott E M, Wright R C, Hanan B Y. Anemia in Alaskan Eskimos. J Nutr. 1955;55:137–149. doi: 10.1093/jn/55.1.137. [DOI] [PubMed] [Google Scholar]
- 21.Smoak B L, Kelly P W, Taylor D N. Seroprevalence of Helicobacter pylori infection in a cohort of US Army recruits. Am J Epidemiol. 1994;139:513–519. doi: 10.1093/oxfordjournals.aje.a117034. [DOI] [PubMed] [Google Scholar]
- 22.Yip R, Limberg P J, Ahlquist D A, Carpenter H A, O'Neill A, Kruse D, Sitham S, Gold B D, Gunter E W, Looker A C, Parkinson A J, Nobmann E, Petersen K M, Ellefsen M, Schwartz S. Pervasive occult gastrointestinal bleeding in an Alaska Native population with prevalent iron deficiency: role of Helicobacter pylori gastritis. JAMA. 1997;277:1135–1139. doi: 10.1001/jama.1997.03540380049030. [DOI] [PubMed] [Google Scholar]
- 23.Worst D J, Otto B R, DeGraaff J. Iron repressible outer membrane proteins of Helicobacter pylori involved in heme uptake. Infect Immun. 1996;63:4161–4165. doi: 10.1128/iai.63.10.4161-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]