Abstract
Introduction
Fascioloides magna is a parasite of high veterinary importance due to its pathogenicity for wild and domestic ruminants. The aim of our study was to describe the presence of trematode infection in the red deer population in the Lower Silesian Wilderness, one of the established fascioloidosis foci in Central Europe, and to assess the overall prevalence of F. magna in the studied area. In order to achieve this, a coprological study of different cervid species was performed.
Material and Methods
The livers of 99 red deer were collected over three years and examined for the presence of trematodes. Prevalence and infection intensity was estimated and a histopathological analysis was performed. In addition, 172 faecal samples from red deer, fallow deer and roe deer were examined.
Results
By year, Fascioloides magna was isolated from the livers of 2/30 (6.7%), 9/34 (26.5%) and 10/35 (28.6%) red deer. Severe hepatic lesions, including massive tissue damage, extensive fibrosis, and cirrhotic changes in the liver parenchyma were observed. Faecal examination revealed the presence of F. magna eggs, with a prevalence of approximately 40%, 50% and 53% in roe deer, fallow deer and red deer, respectively.
Conclusion
The eggs of F. magna may be commonly excreted in the faeces of roe deer, as well as those of red deer and fallow deer. The presence of F. magna throughout the cervid population in the Lower Silesian Wilderness favours the risk of the trematode’s transmission to livestock or farmed deer.
Keywords: cervids, faecal examination, giant liver fluke, histopathology, liver
Introduction
Fascioloides magna is a giant liver fluke that primarily infects wild and domestic ruminants in North America (15, 24). The life cycle is typical for the Fasciolidae family and includes a wild ruminant as the definitive host, and the pond snail (Lymnaeidae) as the intermediate (6). It was introduced to Europe together with the North American cervids, from where it spread to new species of definitive hosts: red deer, fallow deer and roe deer (10, 15). Some natural fascioloidosis foci have become established in Europe, specifically in the Czech Republic, Italy, Croatia, Austria, Slovakia, Hungary and southwestern Poland (8, 9, 11, 15, 19, 27, 32).
Fascioloides magna demonstrates high pathogenicity for infected wildlife, and hence is of high veterinary importance (16). The migration of juvenile flukes and the fibrous encapsulation of adult trematodes inside the liver result in mechanical damage of the parenchyma, organ enlargement and possibly liver failure (8). Therefore, the presence of flukes is associated with significant losses in body girth and weight, as well as impaired reproduction in infected animals (10).
The introduction of F. magna into Europe and its establishment as endemic, together with the recently increasing population of wild cervids (2) and number of deer farms (4), might favour the expansion of fascioloidosis foci and increase the risk of infection for domestic ruminants.
The aim of our study was to describe F. magna infection in the red deer population in a natural fascioloidosis focus in Poland over three subsequent seasons, paying special attention to the pathomorphological changes taking place in the liver in the course of the disease. The study also investigates the occurrence of trematode eggs in the faeces of red deer, roe deer and fallow deer to assess the prevalence and epidemiological risk of F. magna infection.
Material and Methods
Study area. The Lower Silesian Wilderness is one of the largest continuous forests of central Europe, with a total area of 1,650 square kilometres. It is located in southwestern Poland, in the Lower Silesian and the Lubusz Voivodeships, near the border with Germany. The mean annual air temperature is 8.3°C. Annual precipitation is around 850 mm, with most of the rain falling in the summer. The area is predominantly flat, covered mostly by pine trees and crossed by a number of rivers, including the Kwisa, the Bóbr and the Szprotawa. The characteristic features of the region are the abundant mid-forest ponds, peat bogs, swamps and inland dunes (12). The Lower Silesian Wilderness has long been appreciated by hunters, both in Poland and abroad, because of the large number of red deer, roe deer, fallow deer and wild boar (33).
Post-mortem examination. A total of 99 red deer were culled in the Ruszów Forest District, Lower Silesian Wilderness during the following hunting seasons: 2014 (n = 30), 2015 (n = 34) and 2017 (n = 35). The age and sex were determined for 60 animals. The animals were dissected by the hunters; the liver was collected and then immediately transported to the laboratory for examination. The size, shape, surface and cross-sections of the organs were assessed.
Organs were cut into pieces 5 cm in diameter, then compressed and rinsed in tap water so that the flukes and their eggs could leave the bile ducts. The decanted liver sediment was examined under a stereoscopic microscope (PZO, Warsaw, Poland) at 10× magnification. The isolated flukes were identified on the basis of morphometrical features (6, 31) and counted. The number of identified flukes was assumed to reflect the intensity of infection.
Histopathological analysis. Liver samples were collected from the areas of visible gross lesions, fixed in 10% buffered formalin and taken for histopathology examination.
Under laboratory conditions, the organ samples were macroscopically examined. Subsequently, tissue samples were taken from the borderline areas of macroscopic lesions and normal tissue. Liver sections were placed in histological cassettes, washed out with formalin and then dehydrated in graded ethanol and xylene baths. The tissue samples were then embedded in paraffin blocks, cut into 4 μm-thick histological sections, and then stained with haematoxylin and eosin. Microscopic evaluation was performed at 10× and 40× magnification, and the stained tissue section was photographed. The liver tissue and abomasum wall tissue were examined using an Axiolab A5 light microscope with an Axiocam 208 Color microscope camera and ZEN 3.0 software (Zeiss, Jena, Germany). The liver analysis was performed by two independent blinded observers: each had a PhD in veterinary medicine and was a specialist in veterinary laboratory diagnostics and the pathology of wild animals.
Faecal examination. A total of 170 faecal samples were collected in February and June, 2015 and 2017, in the Krzystkowice Forest District, Lower Silesian Wilderness: 73 from red deer, 52 from roe deer and 45 from fallow deer. To avoid pseudoreplication, the sampling strategy included both temporal and spatial stratification, i.e. no more than two faecal samples were collected from a given area at the same time. The samples were transported at 4°C and examined in the laboratory within five days of collection. In the laboratory, the samples were examined for trematode presence: briefly, 3 g amounts of each sample were decanted in tap water according to Żarnowski and Josztowa (34). The samples were observed under a stereoscopic microscope (PZO) at 40× magnification. The eggs were identified to the species or genus level on the basis of morphometrical features (6, 18). The degree of parasitic infection was characterised by prevalence, defined as the percentage of deer faecal samples in which at least one F. magna egg was detected, and by intensity, defined as the number of F. magna eggs in 1 g of faeces (EPG).
Statistical analysis. Categorical variables were presented as counts and percentages. These values were compared between groups using the maximum-likelihood G test; however, if the expected count in any cell of the contingency table was < 5, Fisher’s exact test was used. The 95% confidence intervals (CI 95%) for the proportions were calculated using the Wilson score method (1). Numerical variables were summarised based on median and range; interquartile range was included if the number of measurements was > 5. Forward error correction was compared using the Mann–Whitney U-test (two groups) in the case of two groups and the Kruskal–Wallis H-test for more than two groups. If the result was significant, it was followed by Dunn’s post-hoc test. All tests were two-sided. The significance level (α) was set at 0.05. Statistical analysis was performed in TIBCO Statistica 13.3 (TIBCO Software Inc., Palo Alto, CA, USA).
Results
Parasitological findings. Fascioloides magna (Fig. 1) was isolated from the livers of two (6.7%) red deer examined in 2014, nine (26.5%) in 2016 and ten (28.6%) in 2017. A significantly higher prevalence of infection was observed in the red deer examined in 2016 and 2017 compared to 2014 (P = 0.040). Infection intensity varied from 2 to 24 trematodes (Table 1). All infected animals except one were adults.
Fig. 1.
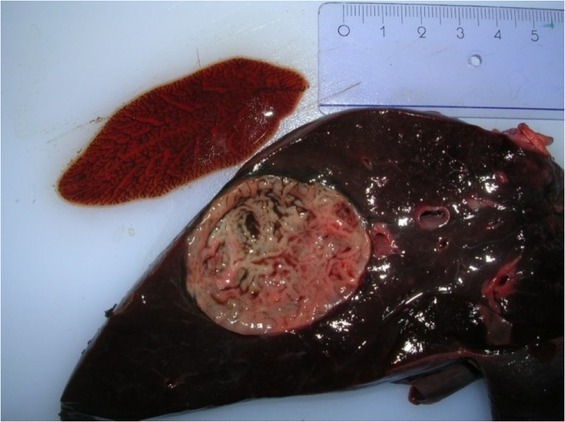
Fascioloides magna isolated from a pseudocyst in the liver parenchyma of an infected red deer
Table 1.
Prevalence and intensity of infection of Fascioloides magna in the livers of red deer from the Lower Silesian Wilderness during the three hunting seasons of the study
| Year | Number of examined red deer | Number of infected red deer | Prevalence (CI 95%) | Intensity |
|---|---|---|---|---|
| 2014 | 30 | 2 | 6.7 (1.8–21.3) | 2, 9 |
|
| ||||
| 2016 | 34 | 9 | 26.5 (14.6–43.1) | 2–19 a |
|
| ||||
| 2017 | 35 | 10 | 28.6 (16.3–45.1) | 9, 3–24 b |
range
median and range
Macroscopic examination. Gross examination revealed the presence of severe hepatic lesions in the infected animals. The organs were enlarged, with rounded edges and a tender consistency. The lymph nodes were also enlarged and darkly pigmented. Large areas of the liver surface on the diaphragm side were covered with fibrin, and dark irregular linear streaks were sometimes observed (Fig. 2A). One to five superficial cystic yellowish structures were visible through the liver capsule (Fig. 2B). In cross section, they resembled thick-walled pseudocysts, 2 to 7 cm in diameter, and were connected with the bile ducts; these were filled with flukes and dark brown liquid, together with hundreds of trematode eggs (Fig. 1). One to six trematodes 5.5 to 8.5 cm long were isolated from each capsule; however, smaller parasites 4.0 to 4.5 cm long were also found migrating through the liver parenchyma. Some cysts contained partially decomposed dead flukes. Fibrous connective tissue with haematin deposits of various sizes was visible in the liver parenchyma.
Fig. 2.
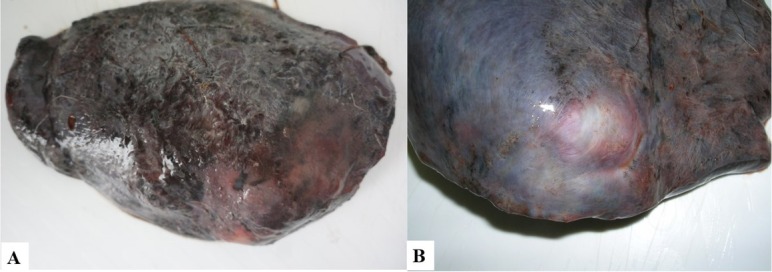
A – Surface of infected red deer liver covered with fibrin; B – A pseudocyst visible through the liver capsule
Histopathological findings. In samples derived from different animals (n = 5), the lesions all had a similar character and severity, although in one animal, the lesions were less severe. All individuals showed moderate to severe disturbances in liver architecture, with the lobular structure of the parenchyma being obliterated. A higher degree of fibrosis was visible, especially around and between the portal fields, but also around the central veins, accompanied by stimulation and hyperplasia of the liver stroma (Fig. 3D). Around the hypertrophic connective tissue, bile ducts with mucosal epithelial hyperplasia and arterial blood vessels with medial (muscular) membrane hypertrophy could be seen. Numerous small and medium-sized hepatocyte necrosis foci were also visible in the parenchyma (Fig. 3B). The described changes were accompanied by a significant lymphocytic-plasmacytic infiltration, with a smaller component of histiocytes (Fig. 3A). Numerous iridescent oval structures (suggesting parasite eggs) were observed in the liver tissues (Fig. 3C). The pathological changes were suggestive of cirrhosis of the liver, resulting mainly from chronic damage to the gates and bile spaces with the accompanying lymphocytic-histiocytic inflammation.
Fig. 3.
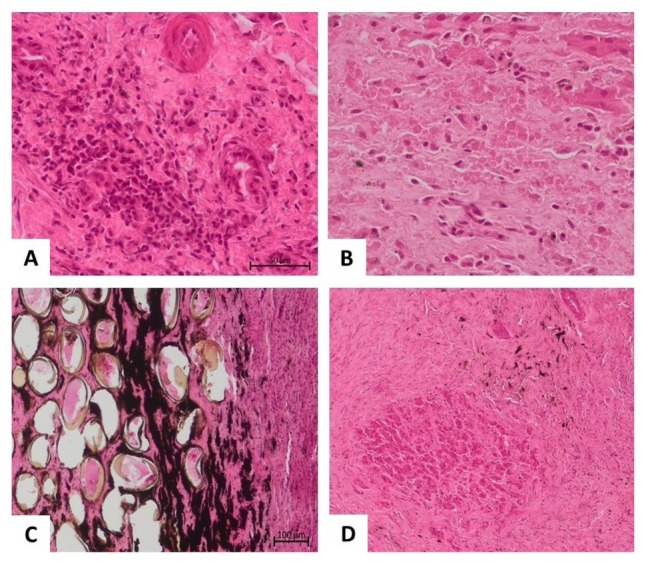
Histopathological picture of the liver parenchyma in the course of trematode infection. A – Focus of lymphoplasmacytic inflammatory infiltrates in the liver parenchyma; B – Coagulative necrosis of individual hepatocytes mixed with inflammatory cells; C – Oval structures with an opaque sheath embedded in the liver parenchyma suggestive of parasite eggs; D – Extensive areas of fibrosis of the liver parenchyma with a central focus of the necrotic area
Coprological examination. Fascioloides magna eggs (Fig. 4) were found in 53.4% (CI 95%: 42.1%–64.4%) of the studied red deer faeces, as well as in 51.1% (CI 95%: 37.0%–65.0%) of those of fallow deer, and 40.4% (CI 95%: 28.2%–53.9%) of roe deer material. No significant differences in the prevalence or the intensity were found between the studied cervid species or years of sample collection (Table 2). Fascioloides magna was found to be the most prevalent parasite in fallow deer and the third most prevalent in roe and red deer.
Fig. 4.
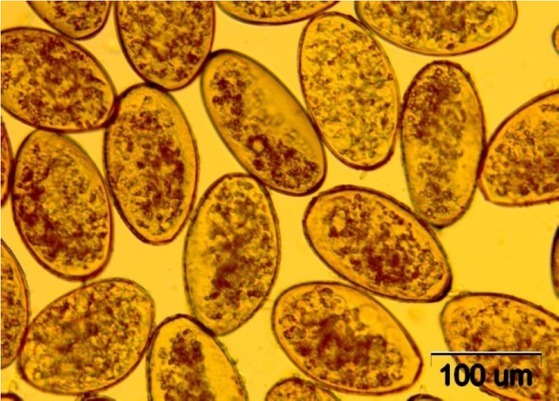
Eggs of Fascioloides magna isolated from the liver of an infected red deer
Table 2.
Prevalence and number of Fascioloides magna eggs per gram of faeces (EPG) in roe deer, red deer and fallow deer in 2015 and 2017
| Year of sampling |
|||
|---|---|---|---|
| 2015 | 2017 | P value | |
| Roe deer | |||
|
| |||
| n = 35 | n = 17 | ||
| Prevalence a | 16 (45.7; 30.5–61.8) | 5 (29.4; 13.3–53.1) | 0.256 |
| FEC (EPG) b | 18, 4–35 (1–84) | 2, 1–6 (1–83) | 0.160 |
|
| |||
| Red deer | |||
|
| |||
| n = 61 | n = 12 | ||
| Prevalence a | 32 (52.5; 40.2–64.5) | 7 (58.3; 32.0–80.7) | 0.709 |
| FEC (EPG) b | 25, 11–43 (1–97) | 31, 11–219 (3–395) | 0.419 |
|
| |||
| Fallow deer | |||
|
| |||
| n = 33 | n = 12 | ||
| Prevalence a | 16 (48.5; 32.5–64.8) | 7 (58.3; 32.0–80.7) | 0.558 |
| FEC (EPG) b | 12, 5–26 (1–76) | 33, 14–57 (11–63) | 0.181 |
FEC – faecal egg count; a – presented as the number of infected (%; 95% confidence interval) and compared using the maximum-likelihood G-test; b – presented as the median, interquartile range (range) and compared using the Mann–Whitney U test
Discussion
Fascioloides magna was introduced to Europe in the 19th century with imported game animals (3, 30). Since then, it has become a parasite of veterinary importance, having spread to a variety of wild and domestic ruminant species in a number of European countries on both sides of the Danube: over 90% of red deer in some regions of the Czech Republic were found to be infected with F. magna (11), as well as 20%–100% of animals in Austria (29), over 50% in Croatia (9) and 33% to over 90% in Hungary (22).
The fascioloidosis foci known to exist in Europe continue to expand (11, 23), and this was reflected in our study. The prevalence of infection in the studied area was found to significantly increase from 2014 to 2016 and 2017, and previous studies of cervids in the Lower Silesian Wilderness confirmed a similar trend of parasite expansion to new hosts and territories (5, 8, 26). Two key environmental risk factors for F. magna infection are soil type and the possibility of flooding and water accumulation, both of which favour the presence of the intermediate host (13); furthermore, parasite spread may be exacerbated by the growing population of cervids in the Lower Silesian Wilderness, integral to which are the recent introduction of fallow deer and the increasing popularity of deer farming (8).
In the present study, only one infected calf was observed among all the examined red deer. This is to be expected, as the prevalence of F. magna in the definitive host has been found to increase with age (17, 21). This is probably due to the different diet and immunological status of younger animals (8).
The pathomorphological changes observed in the livers of infected red deer indicate considerable and permanent damage to the parenchyma, one such change being scarring, leading to cirrhosis and possible permanent loss of organ function. The histopathological examination confirmed the presence of parasites and eggs in the liver parenchyma. The pathogenic effect of fascioloidosis depends on the host type (7). Red deer, the typical definitive host in Europe, often do not show any clinical signs of the disease or deterioration of the condition, even when a considerable burden of infection and changes in the liver tissue are present (8, 19, 20); this was also confirmed in our study. However, the observed damage to the liver parenchyma was extensive and permanent, which would have affected the haemodynamics of the hepatic circulation and thus the function of the organ.
Coprological examination revealed the presence of F. magna eggs in all examined cervid species. While red deer and fallow deer are considered the definitive hosts of F. magna in Europe, participating in its spread in the environment (8, 16), roe deer are thought to be an aberrant host, i.e. one less adapted to infection and not passing trematode eggs in faeces. In aberrant hosts, pseudocysts fail to develop in the liver parenchyma, leading to constant migration of the juvenile fluke in the liver. This movement causes excessive tissue damage and severe haemorrhage, which often bring about the death of the animal (16). However, recent reports of fascioloidosis in roe deer have indicated the presence of juvenile trematodes in the liver parenchyma and of adults in the pseudocysts producing eggs (5, 13); this could reflect a potential adaptation of roe deer to survive F. magna infection. As such, roe deer should also be considered to play a potential role in F. magna transmission. The excretion by over 40% of the examined roe deer of F. magna eggs into the environment is also suggestive of their involvement in transmission. It is therefore possible that this co-evolutionary host–parasite relationship, together with the co-occurrence of numerous different cervid species, may be responsible for the relatively high prevalence of the F. magna eggs observed in the Lower Silesian Wilderness.
Limiting the proliferation of trematode infection among the different wild cervid species in the Lower Silesian Wilderness might be challenging. Attempts to counteract the spread of fascioloidosis have already been made in the Krzystkowice Forest District, where a drug containing albendazole was administered to red deer in the form of licks (Demiaszkiewicz, personal communication), albeit unsuccessfully. Deworming of wildlife species is often inefficient because of numerous difficulties in the selection of the appropriate anthelminthic dose, the method of drug administration, reliable measurement of the deworming effectiveness and the risk of encouraging anthelminthic resistance (14, 25).
Although the hosts appear to have adapted to some degree to accommodate fascioloidosis, it should still be considered one of the most dangerous parasitic diseases of wild and domestic ruminants (10, 13, 28). The presence of F. magna in all cervid species in the Lower Silesian Wilderness increases the risk of transmission to livestock and farmed deer. Therefore, special attention must be paid to regular deworming and parasitological examination of farmed animals at risk of fascioloidosis and to limiting their contact with both definitive and intermediate hosts of the trematode. Further studies are needed to monitor the spread of the parasite, to determine the potential expansion of natural fascioloidosis foci in the Lower Silesian Wilderness, and to follow the changing relationships of F. magna to its European hosts.
Footnotes
Conflict of Interest
Conflict of Interest Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Animal Rights Statement:
The experimental procedures in the study did not require any consent, as they were carried out on animals killed in accordance with the provisions in law on hunting.
Financial Disclosure Statement:
The source of the funding of research was the Witold Stefanski Institute of Parasitology of the Polish Academy of Sciences, and publication was funded by Warsaw University of Life Sciences.
References
- 1.Altman D.G., Machin D., Bryant T.N., Gardner M.J. Statistics with Confidence. BMJ Books; London: 2000. pp. 46–47. [Google Scholar]
- 2.Apollonio M., Andersen R., Putman R. European Ungulates and Their Management in the 21st Century. Cambridge University Press; New York: 2010. [Google Scholar]
- 3.Bassi R.. Sulla cachessia ittero-verminosa, o marciaia, causata dal Distomum magnum (On helmintic cachexia and jaundice caused by Distomum magnum – in Italian) Med Vet Torino. 1875;44:497–515. [Google Scholar]
- 4.Borys B., Bogdaszewska Z., Bogdaszewski M.. A rapid increase in fallow deer and red deer farming in Poland (in Polish) Wiad Zootech. 2012;50:33–44. [Google Scholar]
- 5.Demiaszkiewicz A.W., Kowalczyk R., Filip K.J., Pyziel A.M.. Fascioloides magna: a parasite of roe deer in Bory Zielonogórskie (in Polish) Med Weter. 2018;74:257–260. doi: 10.21521/mw.6037. [DOI] [Google Scholar]
- 6.Demiaszkiewicz A.W., Pyziel A.M., Kuligowska I., Lachowicz J.. Fascioloides magna, a parasite of red deer in Lower Silesian Wilderness (in Polish) Med Weter. 2016;72:110–112. [Google Scholar]
- 7.Foreyt W.J., Todd A.C., Foreyt K.M.. Fascioloides magna (Bassi 1875) in feral swine from southern Texas. J Wildl Dis 1975. 11:554–559. doi: 10.7589/0090-3558-11.4.554. [DOI] [PubMed] [Google Scholar]
- 8.Houszka M., Piekarska J., Podkowik M., Gorczykowski M., Bania J.. Morphology and molecular study of Fascioloides magna – a growing threat to cervids (Cervidae) in Poland. J Vet Res. 2016;60:435–439. doi: 10.1515/jvetres-2016-0065. [DOI] [Google Scholar]
- 9.Janicki Z., Konjević D., Severin K.. Monitoring and treatment of Fascioloides magna in semi-farm red deer husbandry in Croatia. Vet Res Commun. 2005;29:83–88. doi: 10.1007/s11259-005-0027-z. [DOI] [PubMed] [Google Scholar]
- 10.Karamon J., Larska M., Jasik A., Sell B.. First report of the giant liver fluke (Fascioloides magna) infection in farmed fallow deer (Dama dama) in Poland - pathomorphological changes and molecular identification. Bull Vet Inst Pulawy. 2015;59:339–344. doi: 10.1515/bvip-2015-0050. [DOI] [Google Scholar]
- 11.Kašný M., Beran L., Siegelová V., Siegel T., Leontovyč R., Berankova K., Pankrác J., Košťáková M., Horák P.. Geographical distribution of the giant liver fluke (Fascioloides magna) in the Czech Republic and potential risk of its further spread. Vet Med Czech. 2012;57:101–109. doi: 10.17221/5256-VETMED. [DOI] [Google Scholar]
- 12.Kondracki J. Geografia regionalna Polski (Regional geography of Poland – in Polish) Polskie Wydawnictwo Naukowe; Warsaw: 2002. [Google Scholar]
- 13.Konjević D., Bujanić M., Beck A., Beck R., Martinković F., Janicki Z.. First record of chronic Fascioloides magna infection in roe deer (Capreolus capreolus) Int J Parasitol: Paras Wild. 2021;15:173–176. doi: 10.1016/j.ijppaw.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozakiewicz B., Kowalski J., Maszewska I., Przygrodzki H.. Extensiveness of invasion and attempts to control Capreocaulus capreoli (Stroh and Schmid 1938) in roe-deer in the Wielkopolska region (in Polish) Med Weter 1986. 42:478–480. [Google Scholar]
- 15.Králová-Hromadová I., Bazsalovicsová E., Štefka J., Špakulová M., Vávrová S., Szemes T., Tkach V., Trudgett A., Pybus M.. Multiple origins of European population of the giant liver fluke Fascioloides magna (Trematoda: Fascioloidae), a liver parasite of ruminants. Int J Parasitol. 2011;41:373. doi: 10.1016/j.ijpara.2010.10.010. –. [DOI] [PubMed] [Google Scholar]
- 16.Králová-Hromadová I., Juhásová L., Bazsalovicsová E. Springer Briefs in Animal Sciences. The Giant Liver Fluke, Fascioloides Magna: Past, Present and Future Research. Springer International Publishing AG; Cham, Switzerland: 2016. [Google Scholar]
- 17.Lankester M.W., Luttich S.. Fascioloides magna (Trematoda) in woodland caribou (Rangifer tarandus caribou) of the George River herd, Labrador. Can J Zool. 66:475–479. doi: 10.1139/z88-067. [DOI] [Google Scholar]
- 18.Leontovyč R., Košťáková M., Siegelová V., Melounová K., Pankrác J., Vrbová K., Horák P., Kašný M.. Highland cattle and Radix labiata, the hosts of Fascioloides magna. BMC Vet Res. 2014;10:41. doi: 10.1186/1746-6148-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malcicka M.. Life history and biology of Fascioloides magna (Trematoda) and its native and exotic hosts. Ecol Evol. 2015;5:1381–1397. doi: 10.1002/ece3.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marinković D., Nešić V. Changes on the liver of fallow deer (Dama dama) caused by American giant liver fluke (Fascioloides magna) infection. Proceedings of the 20th Conference of Serbian Veterinarians, 9–12 September. Zlatibor, Serbia: 2008. [Google Scholar]
- 21.Mulvey M., Aho J.M.. Parasitism and mate competition – liver flukes in white-tailed deer. Oikos. 1993;66:187–192. doi: 10.2307/3544804. [DOI] [Google Scholar]
- 22.Nagy E., Jócsák I., Csivincsik Á., Zsolnai A., Halász T., Nyúl A., Plucsinszki Z., Simon T., Szabó S., Turbók J., Nemes C., Sugár L., Nagy G.. Establishment of Fascioloides magna in a new region of Hungary: case report. Parasitol Res. 2018;117:3683–3687. doi: 10.1007/s00436-018-6099-9. [DOI] [PubMed] [Google Scholar]
- 23.Novobilský A., Horáčková E., Hirtová L., Modrý D., Koudela B.. The giant liver fluke Fascioloides magna (Bassi 1875) in cervids in the Czech Republic and potential of its spreading to Germany. Parasitol Res 2007. 100:549–553. doi: 10.1007/s00436-006-0299-4. [DOI] [PubMed] [Google Scholar]
- 24.Pybus M.J.. Survey of hepatic and pulmonary helminths of wild cervids in Alberta, Canada. J Wild Dis. 1990;26:453–459. doi: 10.7589/0090-3558-26.4.453. [DOI] [PubMed] [Google Scholar]
- 25.Pyziel A.M., Björck S., Wiklund R., Skarin M., Demiaszkiewicz A.W., Höglund J.. Gastrointestinal parasites of captive European bison Bison bonasus (L.) with a sign of reduced efficacy of Haemonchus contortus to fenbendazole. Parasitol Res. 2018;117:295–302. doi: 10.1007/s00436-017-5663-z. [DOI] [PubMed] [Google Scholar]
- 26.Pyziel A.M., Demiaszkiewicz A.W., Kuligowska I.. Molecular identification of Fascioloides magna (Bassi 1875) from red deer from South-Western Poland (Lower Silesian Wilderness) on the basis of internal transcribed spacer 2 (ITS-2) Pol J Vet Sci 2014. 17:523–525. doi: 10.2478/pjvs-2014-0077. [DOI] [PubMed] [Google Scholar]
- 27.Rajković-Janje R., Bosnić S., Rimac D., Gojmerac T.. The prevalence of American liver fluke Fascioloides magna (Bassi 1875) in red deer from Croatian hunting grounds. Eur J Wildl Res 2008. 54:525–528. doi: 10.1007/s10344-007-0163-6. [DOI] [Google Scholar]
- 28.Rajský D., Čorba J., Várady M., Špakulová M., Cabadaj R.. Control of fascioloidosis (Fascioloides magna Bassi, 1875) in red deer and roe deer. Helminthol 2002. 39:67–70. [Google Scholar]
- 29.Sattmann H., Hörweg C., Gaub L., Feix A.S., Haider M., Walochnik J., Rabitsch W., Prosl H.. Wherefrom and whereabouts of an alien: the American liver fluke Fascioloides magna in Austria: an overview. Wien Klin Wochenschr. 2014;126:23–31. doi: 10.1007/s00508-014-0499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swales W.E.. The life cycle of Fascioloides magna (Bassi 1875), the large liver fluke of ruminants in Canada with observations on the bionomics of the larval stages and the intermediate hosts, pathology of fascioloidiasis magna, and control measures. Can J Res 1935. 12:177–215. doi: 10.1139/cjr35-015. [DOI] [Google Scholar]
- 31.Taylor M.A., Coop R.L., Wall R.L. Veterinary Parasitology, Third Edition. Blackwell Publishing; Oxford: 2007. p. 89. p. [Google Scholar]
- 32.Ursprung J., Joachim A., Prosl H.. Incidence and control of the American giant liver fluke, Fascioloides magna, in a population of wild ungulates in the Danubian wetlands east of Vienna (in German) Berl Munch Tierarztl Wochenschr. 2006;119:316–323. [PubMed] [Google Scholar]
- 33.Zawadzka D., Kwiecień E. Puszcze i lasy Polski. Encyklopedia ilustrowana (Primeval forests and woods in Poland. Illustrated encyclopedia – in Polish) Multico Oficyna Wydawnicza; Warsaw: 2011. p. 182. p. [Google Scholar]
- 34.Ziomko I., Cencek T. Outline of Laboratory Diagnostic of Farm Animal Parasites. Wydawnictwo PIWet-PIB; Puławy: 1995. [Google Scholar]


