Abstract
Background
Childhood asthma has substantial effects on children's health. It is important to identify factors in early life that influence childhood asthma. Accumulating evidence indicates that Helicobacter pylori may protect against allergic diseases. This study aimed to evaluate the relationship between H. pylori infection and pediatric asthma in Chongqing, China.
Materials and methods
This cross-sectional study included healthy children aged 4–18 years who underwent a 13C urea breath test during medical checkups in 2021. All medical information was extracted from electronic medical records and a big data system. Logistic regression was used to evaluate the association between H. pylori infection and pediatric asthma, and multivariate regression models were adjusted for covariates.
Results
In our study, 2241 participants, including 1240 boys (55.33%) and 1001 girls (44.67%), underwent urea breath testing (average age: 8.67 ± 2.70 years). Among them, 292 (13.03%) were positive for H. pylori and 152 (6.78%) had asthma. The rates of asthma diagnosis in H. pylori-negative and -positive children were 7.23% and 3.77%, respectively (odds ratio = 1.995; 95% confidence interval: 1.003–3.968; P < .05). Furthermore, family history of asthma and the percentage of eosinophils in routine blood examination were associated with childhood asthma; however, the body mass index, platelet count, and serum vitamin D level were not.
Conclusions
We demonstrated a significant inverse association between H. pylori infection and pediatric asthma in Chongqing, China. Further studies are required to determine the causal association and underlying mechanisms to prevent and control childhood asthma.
Keywords: Asthma1, Children2, Helicobacter pylori3, Infecition4, Association5
Introduction
Asthma is one of the most common chronic respiratory diseases worldwide and particularly affects children [1]. The latest data from the World Health Organization show that there are approximately 334 million patients with asthma worldwide [2], and approximately 250,000 deaths are attributable to asthma annually [3]. Several risk factors have been linked with asthma susceptibility, including host genetics, specific environmental exposures, and respiratory tract infections during early life. The classic risk factors include air pollution, smoking, infections, and personal allergy history, but the list is incomplete. Attention is turning to the role of the human microbiome in asthma pathogenesis and protection [4]. Identification of potential risk factors in cases of childhood asthma that are not explained by traditional risk factors might improve preventative strategies.
Helicobacter pylori is a spiral-shaped, Gram-negative, microaerophilic bacterium belonging to the genus Helicobacteraceae. It infects approximately 50% of the world population [5]. The prevalence of H. pylori infection varies widely between different parts of the world, with higher rates in developing countries. An up-to-date meta-analysis reported that the prevalence of H. pylori infection in China ranges from 35.4% to 66.4%, and varies according to socioeconomic status and the hygiene level [6]. In most instances, H. pylori colonizes the gastric stomach from early childhood [7]. H. pylori infection may lead to gastritis, peptic ulcer disease, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma; however, most infected subjects remain asymptomatic [8]. In addition, an increasing body of evidence indicates that there is a link between H. pylori infection and extra-gastric diseases [9].Recently, Sahin y et al. evaluated the association between neutrophil/lymphocyte ratio and mean platelet volume values with H. pylori infection, severity classification, and pre-and post-treatment status, however, no relationship was found [10].
As the incidence of asthma has increased, the prevalence of H. pylori infections has decreased. The hygiene hypothesis proposes that increased exposure to microorganisms in early life may confer a protective effect against asthma and allergic diseases [11]. Several epidemiological studies have reported an inverse association between H. pylori infection and allergic asthma, especially in children and adolescents [12, 13]. Nevertheless, There are still some conflicting views on whether there is an association between H. pylori infection and childhood asthma [14].
Our previous meta-analysis, which included 18 observational studies with 17,196 enrolled children, reported a significant negative association between H. pylori infection and the risk of childhood asthma, but lacked data about children from China [15]. Chongqing, a city in southwestern China, usually suffers from wet weather, and also most homes here have poor indoor ventilation, are usually plagued with dampness and mold, and prefer to use hygienic incense and mosquito coils, so that children in Chongqing have a significantly increased risk of childhood asthma [16]. Thus, we conducted a cross-sectional study to screen patients with childhood asthma and H. pylori-infected populations, to investigate the prevalence of childhood asthma and H. pylori infection in southwest China, and to explore the association between H. pylori infection and childhood asthma. Our findings can help to understand the different genetic and environmental contributors of asthma in order to better manage this disease.
Methods
Study population
This cross-sectional study included healthy children who underwent medical checkups at the Children’s Hospital of Chongqing Medical University from January 1, 2021 to December 31, 2021. Participants fulfilled all of the following inclusion criteria: 1) underwent a 13C urea breath test for H. pylori infection; 2) lived in urban areas of Chongqing; 3) had records of their sex, age, height, weight, family history of asthma, routine blood examination, and serum vitamin D test (not all children underwent a routine blood test and serum vitamin D test during physical examination); and 4) aged 4–18 years. The reliability of the 13C urea breath test results is lower for patients younger than 4 years due to technical difficulties performing the test in this age group; therefore, the analysis was limited to children aged 4–18 years. Participants who had a medical history of peptic ulcers or H. pylori infection were excluded. Participants who had received antibiotics, bismuth compounds, or proton pump inhibitors in the previous 2 weeks were also excluded. This study was exempted from informed consent and was approved by the Ethics Committee of the Children’s Hospital of Chongqing Medical University (approval number: K2022198). All procedures were performed in accordance with the guidelines of our institutional ethics committee and adhered to the tenets of the Declaration of Helsinki. All participants’ information was anonymous.
Asthma diagnosis
Medical information of the children was retrieved from the electronic medical records and big data system of the hospital, including all diagnosis information and family history of various diseases. Asthma was defined as a diagnosis of “asthma” by a respiratory physician with lung function results in the big data system. This limitation was used to minimize the inadvertent inclusion of children who actually had alternative diagnoses such as bronchiolitis and viral-induced wheezing as infants that were misclassified by other pediatricians.
Diagnosis of H. pylori infection
The 13C urea breath test is based on the ability of H. pylori-produced urease to hydrolyze swallowed urea labeled with 13C into carbon dioxide and ammonia. A baseline breath sample was collected by asking participants to exhale into a bag after overnight fasting. Then, a capsule containing 13C-urea was administered to the participants with 80–100 mL water. The second breath sample was obtained from participants after 30 min of sitting. H. pylori infection was determined by comparing the 13CO2 content of the baseline and 30 min samples, and a ratio ≥ 4.0 was considered positive (Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd, Guangdong, China). This was based on the Fourth Chinese National Consensus Report on the management of H. pylori infection [17].
Other factors
The following factors were selected a priori and included in the analyses: sex, age, family history of asthma, body mass index (BMI), percentage of eosinophils in routine blood examination, and serum vitamin D level. Routine physical examinations, including measurement of height and weight, were performed by trained medical personnel. BMI was calculated by dividing weight in kilograms by height in meters squared to estimate general overweight and obesity. The BMI values corresponding to overweight and obesity differ according to age. The standards of overweight and obesity were based on the “Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years” [18, 19]. Family history of asthma was reported based on first degree relatives (parents and siblings).
Statistical analysis
Data for categorical variables(sex, family history of asthma, and H. pylori infection) are expressed as the number and percentage. Non-normally continuous distributed data(age, BMI, percentage of eosinophils in routine blood examination, and serum vitamin D level) are expressed as the median and interquartile range. Prior to investigating the association between H. pylori infection and asthma, univariate analyses were performed to identify possible confounders. The χ2 test and Mann–Whitney U test were used to compare the difference between the two groups. Logistic regression was used to test the association between H. pylori infection and asthma by calculating odds ratios (ORs) and 95% confidence intervals (CIs). Multivariate regression models were adjusted for covariates. Data were analyzed using Statistical Package for Social Sciences software (version 26.0). P < 0.05 was considered statistically significant.
Results
Characteristics of participants
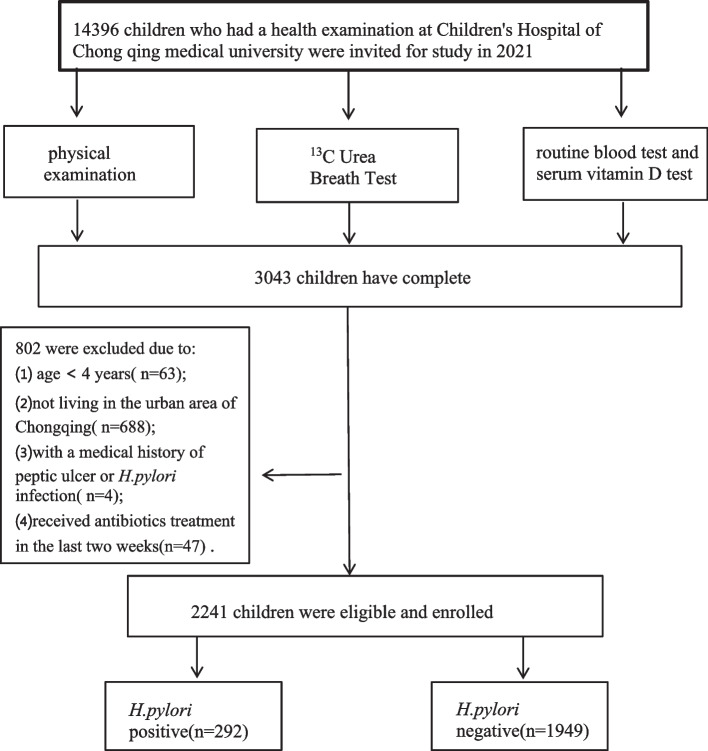
According to the inclusion and exclusion criteria, we selected 2241 participants aged 4–18 years (Fig. 1). The sample included 1240 boys (55.33%) and 1001 girls (44.67%) with a mean age of 8.67 ± 2.70 years. Among them, 292 (13.03%) participants were H. pylori-positive and 1949 (86.97%) participants were H. pylori-negative. The average number of outpatient visits for all participants was 21.45 ± 3.70. The number of outpatient visits did not significantly differ between the H. pylori-positive (22.50 ± 3.33) and H. pylori-negative (21.35 ± 2.92) groups (P > 0.05). A total of 152 (6.78%) participants were diagnosed with asthma. The number of outpatient visits was significantly higher for patients diagnosed with asthma (31.43 ± 5.58) than for patients not diagnosed with asthma (20.97 ± 3.74) (P < 0.05). Table 1 shows the basic characteristics of the subjects and univariate analysis of each factor related to asthma.
Fig. 1.
Flow chart of the study population enrollment
Table 1.
Basic characteristics of the study participants and univariate analysis for each factor related to asthma
| Characteristic | Total No. (%) |
Asthma No. (%) |
No asthma No. (%) |
P value | χ2/Z |
|---|---|---|---|---|---|
| Total | 2241 | 152 | 2089 | ||
| Sex | .302 | 1.064 | |||
| Male | 1240 (55.33) | 78(51.32) | 1162(55.62) | ||
| Female | 1001(44.67) | 74(48.68) | 927(44.38) | ||
| Age,years | .174 | 3.501 | |||
| Median(P25,P75) | 8.50(6.58,10.41) | 8.21(6.25,9.73) | 8.50(6.63,10.46) | .099 | -1.648 |
| 4–5 | 395(17.63) | 31(20.40) | 364(17.43) | ||
| 6–11 | 1567(69.92) | 109(71.71) | 1458(69.79) | ||
| 12–18 | 279(12.45) | 12(7.89) | 267(12.78) | ||
| BMI | .481 | 1.464 | |||
| Median(P25,P75) | 15.94(14.78,17.82) | 15.95(14.81,17.75) | 15.94(14.77,17.82) | .875 | -0.158 |
| No overweight | 1802(80.41) | 125(82.24) | 1677(80.28) | ||
| Overweight | 317(14.15) | 17(11.18) | 300(14.36) | ||
| Obesity | 122(5.44) | 10(6.58) | 112(5.36) | ||
| Family history | < .001 | 489.433 | |||
| Positive | 169(7.54) | 81(53.29) | 88(4.21) | ||
| Negative | 2072(92.46) | 71(46.71) | 2001(95.79) | ||
| Platelet,1012 | |||||
| Median(P25,P75) | 285(244,327) | 285(250,320) | 285(243,327) | .869 | -0.165 |
| Vitamin D | |||||
| Median(P25,P75) | 19.29(14.87,23.40) | 19.52(14.84,23.69) | 19.29(14.87,23.39) | .483 | -0.702 |
| Eosinophils(%) | |||||
| Median(P25,P75) | 2.94(1.95,4.05) | 4.01(2.05,5.96) | 2.92(1.94,4.02) | < .001 | -5.714 |
| H.pylori | .028 | 4.829 | |||
| Positive | 292(13.03) | 11(7.24) | 281(13.45) | ||
| Negative | 1949(86.97) | 141(92.76) | 1808(86.55) | ||
H.pylori Helicobacter pylori, BMI Body mass index
Association between H. pylori infection and asthma
There were 11 (3.77%) and 141 (7.23%) patients diagnosed with asthma in the H. pylori-positive and -negative groups, respectively, and this difference was significant (P < 0.05). The percentage of patients with a family history of asthma and the percentage of eosinophils in routine blood examination also significantly differed between the two groups (P < 0.05), but other characteristics (sex, age, BMI, platelet count, and serum vitamin D level) did not (P > 0.05). Univariate analysis revealed an association between H. pylori infection and asthma (P = 0.028), but the effects of other factors could not be ignored. A multinomial logistic regression model was further used to explore the factors that influenced asthma. We included all the factors in the basic multivariate logistic regression model, and this revealed an association between H. pylori infection and asthma (OR = 1.995, 95% CI: 1.003–3.968). Three adjusted multivariate logistic regression models were used to identify the potential association between H. pylori infection and asthma. There was an inverse relationship between H. pylori infection and asthma in all covariate-adjusted multivariate models (Table 2), suggesting that H. pylori infection is an independent protective factor for asthma (OR range: 1.887–2.008, P < 0.05).
Table 2.
The risk of pediatric asthma according to the infection of H.pylori
| Model | variant | OR(95%CI) | P value |
|---|---|---|---|
| Basic model | H.pylori | 1.995(1.003 ~ 3.968) | .049 |
| Family history | 0.039(0.026 ~ 0.058) | < 0.001 | |
| Eosinophils | 1.079(1.022 ~ 1.139) | 0.006 | |
| Platelet | 0.999(0.996 ~ 1.002) | 0.511 | |
| Vitamin D | 0.997(0.967 ~ 1.027) | 0.837 | |
| BMI | 0.422 | ||
| BMI(1) | 1.005(0.452 ~ 2.234) | 0.99 | |
| BMI(2) | 0.672(0.261 ~ 1.728) | 0.409 | |
| Age | 1.006(0.931 ~ 1.086) | 0.885 | |
| Sex | 0.758(0.517 ~ 1.112) | 0.157 | |
| Model 1 | H.pylori | 1.887(1.007 ~ 3.538) | 0.048 |
| Platelet | 0.999(0.997 ~ 1.002) | 0.582 | |
| Vitamin D | 1.008(0.982 ~ 1.035) | 0.542 | |
| BMI | 0.577 | ||
| BMI(1) | 0.783(0.397 ~ 1.545) | 0.48 | |
| BMI(2) | 0.649(0.287 ~ 1.467) | 0.299 | |
| Age | 0.948(0.887 ~ 1.013) | 0.116 | |
| Sex | 0.854(0.611 ~ 1.193) | 0.355 | |
| Model 2 | H.pylori | 1.960(1.040 ~ 3.692) | 0.037 |
| Eosinophils | 1.112(1.063 ~ 1.163) | < 0.001 | |
| Platelet | 0.999(0.997 ~ 1.002) | 0.579 | |
| Vitamin D | 1.008(0.981 ~ 1.035) | 0.579 | |
| BMI | 0.564 | ||
| BMI(1) | 0.740(0.374 ~ 1.465) | 0.388 | |
| BMI(2) | 0.641(0.283 ~ 1.451) | 0.286 | |
| Age | 0.947(0.885 ~ 1.013) | 0.111 | |
| Sex | 0.812(0.579 ~ 1.138) | 0.227 | |
| Model 3 | H.pylori | 2.008(1.008 ~ 4.000) | 0.047 |
| Family history history | 0.036(0.024 ~ 0.054) | < 0.001 | |
| Platelet | 0.999(0.996 ~ 1.002) | 0.551 | |
| Vitamin D | 0.997(0.967 ~ 1.027) | 0.822 | |
| BMI | 0.393 | ||
| BMI(1) | 1.049(0.473 ~ 2.327) | 0.906 | |
| BMI(2) | 0.691(0.269 ~ 1.772) | 0.441 | |
| Age | 1.009(0.935 ~ 1.089) | 0.822 | |
| Sex | 0.787(0.538 ~ 1.152) | 0.217 |
Model 1 is adjusted for family history and eosinophils. Model 2 is adjusted for family history. Model 3 is adjusted for eosinophils
OR Odd ratio, CI Confidence intervals
Discussion
This study of 2241 children who underwent urea breath testing in 2021 shows that H. pylori infection is an independent predictor for a positive diagnosis of pediatric asthma. To the best of our knowledge, this is the largest study documenting such an association for children in China and the first to use pediatric urea breath test results rather than serologies, which are considered less reliable in children from a healthy childhood population.
The Children’s Hospital of Chongqing Medical University is a medical center for children in southwest China and the only children’s hospital in Chongqing. All the participants lived in urban areas of Chongqing, meaning that the effect of living in an urban area, which is an independent risk factor for pediatric asthma [20], was eliminated. The average number of outpatient visits was higher than 20, and almost all disease information was recorded by electronic medical records and a big data system of the hospital. It was considered more reliable to use asthma diagnoses recorded by a respiratory physician with lung function results in the system rather than questionnaires completed by parents. In addition, we included two asthma-related laboratory tests, the percentage of eosinophils in routine blood examination and the serum vitamin D level, which were not included in previous studies. Moreover, there was no heterogeneity in the study population, including differences in sex, age, BMI, and other confounders. To eliminate the possible influence of other variables, propensity score matching (PSM) analysis was performed using a logistic regression model based on age, sex, and other confounders to assess the relationship between H. pylori infection and childhood asthma. PSM refers to screening of the experimental and control groups using certain statistical methods so that the screened study subjects are comparable in terms of clinical characteristics (adjusting for potential confounders). However, there was little difference between matched and unmatched data; therefore, we did not describe PSM in Section Methods. This homogeneity may improve the comparability of our study to a certain extent.
The prevalence of childhood asthma in our study was 6.78%, which is higher than reported by epidemiological surveys in 2013 in Chongqing (4.43%) [21] and a meta-analysis in 2020 in China (2.6%) [22]. This may be because the prevalence of childhood asthma has increased in recent years and the prevalence of asthma is high in Chongqing [21], and all participants of our study lived in urban areas. The H. pylori infection rate was 13.03% in the present study. This is lower than that reported by Ren et al. in a meta-analysis, which demonstrated that the pooled prevalence of H. pylori infection was 44.2% in mainland China, 46.6% in Southwest China, and 28.0% in children and adolescents [6]. This difference may be because the prevalence of H. pylori infection increases with age and varies across geographic areas [5, 6]. In addition, all our participants lived in urban areas and had better sanitary conditions, higher family incomes, and healthier lifestyles.
Our study showed an inverse association between H. pylori infection and asthma in children, consistent with our previous meta-analysis [15]. Furthermore, several other studies have suggested that H. pylori infection significantly protects children younger than 10 years against allergic asthma [23, 24]. Zevit et al. demonstrated that the prevalence of asthma was 7.3% in children with H. pylori infection and 9.1% in healthy children, meaning that the prevalence of asthma was reduced in patients with H. pylori infection [12]. Another case–control study performed in Greece, which included 27 pediatric patients with asthma and 54 controls, also reported an inverse association between H. pylori infection and asthma [25]. Moreover, a cohort study performed in 2020 found that 16.4% of children who were H. pylori-negative at 2 and 10 years of age had asthma at 16 years of age, whereas children who were H. pylori-positive at 12 years of age did not have asthma at 16 years of age, suggesting that early exposure to H. pylori can prevent asthma [26]. The results of these studies are consistent with ours.
The possible protective mechanisms of H.pylori against allergic asthma, including (1) according to the “hygiene hypothesis”, infectious agents can inhibit allergic T helper (Th) 2 cell response, thus eliciting a Th1-type immune response, (2) H.pylori neutrophil-activating protein(HP-NAP) is one of the major virulence factors of H. pylori, which can increase interferon-γ production and decrease the level of interleukin-4, thereby stimulating Th1 activation and attenuating Th2 response in allergy-related asthma, (3)Another possibility involves the inverse correlation between H. pylori infection and gastroesophageal reflux disease (GERD), because gastroesophageal reflux can induce or aggravate asthma, but H.pylori can reduce gastroesophageal reflux, (4) H. pylori can also inhibit dendritic cells to promote immune tolerance and enhance the protective effect against asthma, and this inhibition is highly dependent on the suppressed regulatory T cells, (5) The gut-lung axis theory suggests remote regulation of lung immune function by H. pylori, (6)H. pylori infection may also prevent allergic asthma by altering stomach hormones levels, affecting the autonomic nervous system, and reducing the expression of heat shock protein 70 [27, 28].
It is well known that family history of allergies and blood eosinophil counts are strongly associated with childhood asthma [29, 30]. Recently, the association between BMI and childhood asthma has received increasing attention. Several studies have shown that BMI is significantly associated with the prevalence and incidence of allergic asthma [31, 32]. However, our study did not show such relationship. A recent systematic review and meta-analysis of case–control studies of children by Azizpour et al. [31] reported that the risk of asthma was 1.64 times and 1.92 times higher in individuals who were overweight and obese than in individuals who were underweight/normal weight, respectively. However, by removing the confounding factors, the effect size was reduced from 1.64 and 1.92 to 1.30. This suggests that although obesity increases the risk of asthma, but the confounding factors might influence the relationship between BMI and asthma. Therefore, in future epidemiological studies, it is important to investigate the confounding factors that affect the relationship between BMI and asthma. In addition, we found that children with asthma had similar vitamin D leves to healthy children, consistent with the previous studies by Yang et al. and Reinehr et al. [33, 34]. However, in contrast to our study, a meta-analysis by Wang et al., which included 35 studies with 27,271 participants, reported that children with asthma had significantly lower vitamin D levels than children without asthma. Furthermore, children with asthma who received vitamin D supplements had significantly lower recurrence rates than those who received a placebo [35].
Our study has several limitations. First, it was a single-center study and the results may not reflect the general population. Second, the participants only underwent urea breath testing, not endoscopy with biopsy, and there was a lack of data about the cytotoxin-associated gene A status, which is an important factor in H. pylori infection and is negatively associated with asthma and allergies [36]. Third, other potential confounding factors that might interfere with asthma, such as passive smoking, birth mode, and early antibiotic use, were not collected. Finally, our study was cross-sectional and lacked a comparison intervention, and its results only reflected epidemiological links, but failed to draw a cause-effect inference. Larger studies with an appropriate epidemiological design are required to investigate the potential causal relationship between H. pylori infection and asthma in the future.
Conclusion
Our study demonstrated an inverse relationship between H. pylori infection and asthma in children. Further studies are required to determine the causal association and specific mechanisms, which will help to provide a scientific basis for the establishment of preventative and therapeutic strategies for childhood asthma.
Acknowledgements
The authors acknowledge the big date center and Ethics Committee of the Children’s Hospital of Chongqing Medical University.
Data statement
The data used to support the findings of this study are included within the supplementary files. They included the original data and the collated data.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- GERD
Gastroesophageal reflux disease
- OR
Odds ratio
- PSM
Propensity score matching
- Th
T helper
- HP-NAP
H.pylori Neutrophil-activating protein
Authors’ contributions
CYX, WDH, and DY contributed to conception and design of the study. CYX, WDH, and TJW organized the database. CYX and WDH performed the statistical analysis. CYX and WDH wrote the first draft of the manuscript. CYX, WDH, DY, and TJW wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This research was funded by Children Health Management and Disease Prevention (NCRCCHD-2019-HP-10).
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Children's Hospital of Chongqing Medical University (approval number: K2022198). Informed consent waiver statement, because this is a study of medical records obtained form previous clinical treatment.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuxia Chen and Yuan Ding contributed equally to this work and share corresponding authorship.
Contributor Information
Yuxia Chen, Email: 1009957044@qq.com.
Yuan Ding, Email: dingyuan1981444@aliyun.com.
References
- 1.Ferrante G, La Grutta S. The Burden of Pediatric Asthma. Front Pediatr. 2018;6:186. doi: 10.3389/fped.2018.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246. doi: 10.3389/fped.2019.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Amato G, et al. Asthma-related deaths. Multidiscip Respir Med. 2016;11:37. doi: 10.1186/s40248-016-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borbet TC, Zhang X, Müller A, Blaser MJ. The role of the changing human microbiome in the asthma pandemic. J Allergy Clin Immunol. 2019;144(6):1457–1466. doi: 10.1016/j.jaci.2019.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooi J, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Ren S, et al. Prevalence of Helicobacter pylori infection in China: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37(3):464–470. doi: 10.1111/jgh.15751. [DOI] [PubMed] [Google Scholar]
- 7.McColl KE. Clinical practice Helicobacter pylori infection. N Engl J Med. 2010;362(17):1597–604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 8.Reshetnyak VI, Burmistrov AI, Maev IV. Helicobacter pylori: commensal, symbiont or pathogen. World J Gastroenterol. 2021;27(7):545–560. doi: 10.3748/wjg.v27.i7.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gravina AG, et al. Helicobacter pylori and extragastric diseases: a review. World J Gastroenterol. 2018;24(29):3204–3221. doi: 10.3748/wjg.v24.i29.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahin Y, Gubur O, Tekingunduz E. Relationship between the severity of Helicobacter pylori infection and neutrophil and lymphocyte ratio and mean platelet volume in children. Arch Argent Pediatr. 2020;118(3):e241–e245. doi: 10.5546/aap.2020.eng.e241. [DOI] [PubMed] [Google Scholar]
- 11.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noam Z, et al. Inverse association between Helicobacter pylori and pediatric asthma in a high-prevalence population. Helicobacter. 2012;17(1):30–5. doi: 10.1111/j.1523-5378.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 13.Nael E, et al. Associations of Helicobacter pylori seropositivity and gastric inflammation with pediatric asthma. Pediatr Pulmonol. 2020;55(9):2236–2245. doi: 10.1002/ppul.24905. [DOI] [PubMed] [Google Scholar]
- 14.Rasoul N, et al. Evaluation of the Relationship Between Childhood Asthma and Helicobacter pylori Sero-Prevalence. Acta Med Iran. 2019;57(5):299-302.
- 15.Yuxia C, Xue Z, Donghai W. Association between Helicobacter pylori and risk of childhood asthma: a meta-analysis of 18 observational studies. J Asthma. 2021;59(5):890–900. doi: 10.1080/02770903.2021.1892752. [DOI] [PubMed] [Google Scholar]
- 16.Li W, et al. Effects of indoor environment and lifestyle on respiratory health of children in Chongqing. China. J Thorac Dis. 2020;12(10):6327–6341. doi: 10.21037/jtd.2020.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong LW, et al. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013;14(5):211–21. doi: 10.1111/1751-2980.12034. [DOI] [PubMed] [Google Scholar]
- 18.Dang JJ, et al. [Methods for evaluating overweight and obesity among children and adolescents and application in SPSS and SAS] Zhonghua Yu Fang Yi Xue Za Zhi. 2022;56(1):75–81. doi: 10.3760/cma.j.cn112150-20210319-00271. [DOI] [PubMed] [Google Scholar]
- 19.Hui L, Cheng-Ye J, Xin-Nan Z, Ya-Qin Z. [Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years] Zhonghua Er Ke Za Zhi. 2009;47(7):493–8. [PubMed] [Google Scholar]
- 20.Gern JE. The urban environment and childhood asthma study. J Allergy Clin Immunol. 2010;125(3):545–549. doi: 10.1016/j.jaci.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu CH, et al. Comparison of asthma prevalence in children from 16 cities of China in 20 years. Chin J Pract Pediatr. 2015;30(8):596–600. [Google Scholar]
- 22.Shu W, Li ML, Li ZA, Yi-fei H. Meta-analysis of asthma prevalence of children aged 0–14 in surveillance cities of China. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54(8):875–883. doi: 10.3760/cma.j.cn112150-20191015-00788. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198(4):553–560. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raj SM, Choo KE, Noorizan AM, Lee YY, Graham DY. Evidence against Helicobacter pylori being related to childhood asthma. J Infect Dis. 2009;199(6):914–5. doi: 10.1086/597066. [DOI] [PubMed] [Google Scholar]
- 25.Tsigalou C, et al. Inverse association between Helicobacter pylori infection and childhood asthma in Greece: a case-control study. Germs. 2019;9(4):182–7. doi: 10.18683/germs.2019.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melby KK, Carlsen KL, Håland G, Samdal HH, Carlsen KH. Helicobacter pylori in early childhood and asthma in adolescence. BMC Res Notes. 2020;13(1):79. doi: 10.1186/s13104-020-04941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durazzo M, Adriani A, Fagoonee S, Saracco GM, Pellicano R. Helicobacter pylori and respiratory diseases: 2021 UPDATE. Microorganisms. 2021;9(10):2033. doi: 10.3390/microorganisms9102033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo ZT, et al. The protective effects of Helicobacter pylori infection on allergic asthma. Int Arch Allergy Immunol. 2021;182(1):53–64. doi: 10.1159/000508330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29."Global strategy for asthma management and prevention: GINA executive summary." E.D. Bateman, S.S. Hurd, P.J. Barnes, J. Bousquet, J.M. Drazen, J.M. FitzGerald, P. Gibson, K. Ohta, P. O'Byrne, S.E. Pedersen, E. Pizzichini, S.D. Sullivan, S.E. Wenzel and H.J. Zar. Eur Respir J 2008; 31: 143–178. Eur Respir J. 2018;51(2). 10.1183/13993003.51387-2007. [DOI] [PubMed]
- 30.Benson VS, et al. Blood eosinophil counts in the general population and airways disease: a comprehensive review and meta-analysis. Eur Respir J. 2022;59(1). 10.1183/13993003.04590-2020. [DOI] [PMC free article] [PubMed]
- 31.Yosra A, Ali D, Zahra M, Kourosh S, Behzad D. Effect of childhood BMI on asthma: a systematic review and meta-analysis of case-control studies. BMC Pediatri. 2018;18(1):143. doi: 10.1186/s12887-018-1093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egan Kathryn B, Ettinger Adrienne S, Bracken Michael B. Childhood body mass index and subsequent physician-diagnosed asthma: a systematic review and meta-analysis of prospective cohort studies. BMC Pediatr. 2013;13(1):121. doi: 10.1186/1471-2431-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang HK, et al. The association between hypovitaminosis D and pediatric allergic diseases: a Korean nationwide population-based study. Allergy Asthma Proc. 2016;37(4):64–69. doi: 10.2500/aap.2016.37.3957. [DOI] [PubMed] [Google Scholar]
- 34.Reinehr T, et al. 25-Hydroxvitamin D concentrations are not lower in children with bronchial asthma, atopic dermatitis, obesity, or attention-deficient/hyperactivity disorder than in healthy children. Nutr Res. 2018;52:39–47. doi: 10.1016/j.nutres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, Ying Q, Zhu W, Chen J. Vitamin D and asthma occurrence in children: a systematic review and meta-analysis. J Pediatr Nurs. 2022;62:e60–e68. doi: 10.1016/j.pedn.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Chen C, Xun P, Tsinovoi C, He K. Accumulated evidence on Helicobacter pylori infection and the risk of asthma: a meta-analysis. Ann Allergy Asthma Immunol. 2017;119(2):137–45.e2. doi: 10.1016/j.anai.2017.05.021. [DOI] [PubMed] [Google Scholar]