Abstract
Procalcitonin (PCT) is an early marker of bacterial infection but little is known about its value in neutropenic allogeneic bone marrow transplant (BMT) recipients. We collected plasma from 12 recipients of T-cell-depleted HLA-matched related BMT recipients who had been treated preemptively with meropenem from the day after BMT for at least 15 days. PCT and C-reactive protein (CRP) concentrations were determined on BMT days 1, 5, 8, 12, and 15, and their relationship to inflammatory events (IE), including mucositis, microbiologically and clinically defined infections, acute graft-versus-host disease (GVDH), and unexplained fever, was then determined. The PCT concentrations were all low and never exceeded 4 μg/liter, unlike CRP concentrations, which spanned the full range up to 350 mg/liter. All patients had mucositis, and there was no significant difference between PCT concentrations associated with mucositis alone and those associated with an additional IE on BMT days 1 to 12. However, on BMT day 15, the mean concentrations of PCT were 0.37 ± 0.05 μg/liter for the 10 patients that had an additional IE, compared with 0.11 ± 0.03 μg/liter for the 2 patients with mucositis only (P = 0.012), and GVHD rather than infection was involved in six cases. PCT was also not a sensitive marker of gram-positive bacteremia or pulmonary aspergillosis. Thus, PCT is of little value in discriminating infections from other inflammatory complications that occur following allogeneic BMT.
Procalcitonin (PCT) has been proposed as a new discriminative marker of bacterial infections (1). PCT is the propeptide of calcitonin devoid of hormonal activity and is normally produced in C cells of the thyroid gland. The substance consists of 116 amino acids and has a long serum half-life of 25 to 30 h. Levels of PCT are undetectable (<0.1 μg/liter) in healthy individuals, and small to modest increases (<1.5 μg/liter) are seen in severe viral infections (e.g., human immunodeficiency virus infection) and noninfectious inflammatory responses such as autoimmune diseases and pancreatitis (8). In contrast, severe bacterial, parasitic, and fungal infections are associated with increased PCT levels exceeding 1.5 μg/liter, including those found in neutropenic patients (4). Moreover, the diagnostic value of PCT has been found to be more discriminative than C-reactive protein (CRP) for differentiating infections from other inflammatory responses accompanied by fever in neutropenic patients (8, 10). Therefore, we sought to determine whether PCT could be useful for discriminating inflammatory responses from infectious complications in allogeneic bone marrow transplant (BMT) recipients receiving meropenem preemptively after BMT.
MATERIALS AND METHODS
We selected 12 adult patients (5 female and 7 male) out of a homogeneous cohort of 30 consecutive patients who had received an HLA-matched, mixed lymphocyte culture negative T-cell-depleted sibling BMT and had participated in a pharmacokinetic study of meropenem in which the drug had been given preemptively from day 1 posttransplantation onward for at least 15 days after transplantation until neutrophil recovery (>0.5 × 109/liter).
All patients had received the transplants for a hematological malignancy (CML4, AML3, ALL2, MM2, and MDS1), and each had a double-lumen intravascular venous catheter (IVC) inserted in the subclavian vein on BMT day −13, after which idarubicin at 42 mg/m2 was given by continuous infusion for 48 h as the first part of the conditioning regimen. Cyclophosphamide at 60 mg/kg was given intravenously (i.v.) a week later on BMT days −6 and −5 and was followed by total body irradiation with 4.5 Gy on BMT days −2 and −1. Anti-infective prophylaxis consisted of ciprofloxacin at 500 mg every 12 h from BMT day −13 to BMT day 0, acyclovir at 400 mg every 6 h from BMT day −13 to BMT day 60, and cotrimoxazole (sulfamethoxazole-trimethoprim) at 1,960 mg twice weekly from BMT day −13 onwards. No systemic antifungal agents were given. Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporin at 3 mg/kg/day given i.v. from BMT day −1 until BMT day 15, followed by 5 mg/kg/day given orally. Meropenem at 1 g every 8 h (given i.v) was administered preemptively from BMT day 1 for at least 15 days. Other antimicrobial agents were only given when there were objective grounds for doing so, such as a microbiologically defined infection (MDI) with persistent bacteremia due to coagulase-negative staphylococci (CoNS) or a nonbacterial MDI or clinically defined infection (CDI) such as folliculitis.
Vital signs and the presence of mucositis were recorded daily. Ten milliliters of blood was obtained on BMT days 1, 5, 8, 12, and 15 for culture from each lumen of the IVC before the 9:00 a.m. dose of meropenem was given. At the onset of fever (≥38.5°C once or >38°C for >8 h), two blood cultures were obtained from each lumen of the IVC, together with two 20-ml samples drawn through two separate peripheral veins within 30 min of each other. The plasma samples for PCT and CRP were also taken before the 9:00 a.m. dose of meropenem on BMT days 1, 5, 8, 12, and 15 and then 1, 2, 4, 6, and 8 h later.
PCT was measured using an immunoluminometric assay (LUMItest; Brahms-Diagnostica Berlin, Berlin, Germany) with a lower detection limit of <0.3 μg/liter. All measurements were done in duplicate. CRP was measured using a nephelometric assay (Turbidtimer; Dade-Behring). The clinical manifestations of acute GVHD were classified as grades I to IV according to standard criteria (6).
The occurrence of fever, a CDI, an MDI, GVHD, or mucositis was initially considered evidence of an inflammatory process but, since all patients had mucositis throughout the 15-day study period, those without an additional inflammatory event (IE) were used as a control group. Furthermore, a patient might be febrile, have bacteremia, and have GVHD at the same time, so the presence of any one of these complications was considered to represent an IE.
For the purposes of analysis, any additional IE that occurred on any one of the five study days was assumed to represent a separate event. Hence, there were 12 possible additional IEs on each study day, resulting in a total of 60 IEs altogether. All six PCT and CRP measurements obtained for each patient during any one of the 5 study days were assumed to be replicates of each other, since there was little evidence of fluctuation during the sampling period (data not shown). The patients were divided into a control group, i.e., those with mucositis but no other IE, and those with mucositis and an additional IE, and PCT and CRP data were analyzed using the Student t test. A P value of <0.05 was taken to indicate significance after applying Bonferroni's correction to compensate for multiple tests.
RESULTS
All 12 patients had at least one additional IE at some point during the study period. As shown in Table 1, four patients had IEs on BMT days 1 and 5, six patients had IEs on BMT days 8 and 12, and ten patients had IEs on BMT day 15. Three patients had repeated episodes of infection: patient 6 had folliculitis accompanied by fever and bacteremia due to Staphylococcus epidermidis on BMT days 1 and 5 and then folliculitis alone on BMT day 8; patient 22 had bacteremia due to S. epidermidis on each of the study days, and patient 23 developed pulmonary aspergillosis on BMT day 5, which persisted until 31 days after transplant, when she died. Patients 11 and 17 developed GVHD on day 12, which was still present on BMT day 15. All other IEs occurred separately at different times during the study period. Infection affected 7 patients and was present on 17 study days, 12 of which involved bacteremia (S. epidermidis alone, 8; S. epidermidis plus Bacillus species, 2; S. epidermidis plus a gram-negative nonfermentative bacillus, 1; and 1 Acinetobacter species). Eight patients had fever on 15 study days, and eight patients had GVHD on 10 study days.
TABLE 1.
IE results for BMT days 1, 5, 8, 12, and 15
| BMT day | Additional IE(s) | No. of patients |
|---|---|---|
| 1 | Folliculitis and bacteremia due to S. epidermidis and Bacillus species accompanied by fevera | 1 |
| Bacteremia due to S. epidermidisb | 1 | |
| Bacteremia due to S. epidermidis and a nonfermentative gram-negative bacillus | 1 | |
| Unexplained fever | 1 | |
| 4 (subtotal) | ||
| 5 | Folliculitis and bacteremia due to S. epidermidis and Bacillus species accompanied by fevera | 1 |
| Bacteremia due to S. epidermidisb | 1 | |
| Pulmonary aspergillosisc | 1 | |
| Unexplained fever | 1 | |
| 4 (subtotal) | ||
| 8 | Bacteremia due to Acinetobacter species | 1 |
| Bacteremia due to S. epidermidisb | 1 | |
| Folliculitisa | 1 | |
| Pulmonary aspergillosisc | 1 | |
| Unexplained fever | 2 | |
| 6 (subtotal) | ||
| 12 | Bacteremia due to S. epidermidis | 2 |
| GVHD accompanied by feverd | 2 | |
| Pulmonary aspergillosisc | 1 | |
| Unexplained fever | 1 | |
| 6 (subtotal) | ||
| 15 | Bacteremia due to S. epidermidis | 1 |
| Bacteremia due to S. epidermidis accompanied by fever | 1 | |
| GVHD accompanied by feverd | 5 | |
| GVHD and bacteremia due to S. epidermidisb | 1 | |
| GVHD and pulmonary aspergillosisc | 1 | |
| GVHD | 1 | |
| 10 (subtotal) |
Patient 6.
Patient 22.
Patient 23.
Patients 11 and 17.
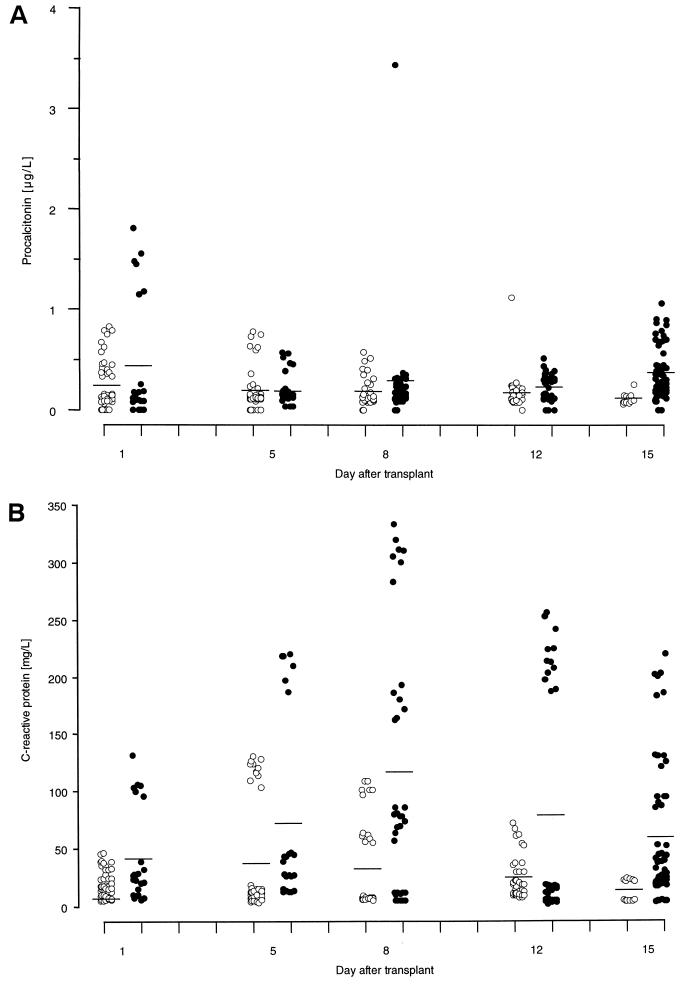
The PCT concentrations were all low and never exceeded 4 μg/liter; in contrast, CRP concentrations spanned the full range up to 350 mg/liter (Fig. 1). There was no significant difference in PCT concentrations between those associated with an additional IE and the control group except on BMT day 15, when the mean concentrations were 0.37 ± 0.05 and 0.11 ± 0.03 μg/liter, respectively (P = 0.012). GVHD rather than infection was involved in 6 of the 10 IEs. In contrast, the CRP concentrations differed significantly between those associated with an additional IE and the control group on BMT day 1 (41.9 ± 15.9 and 20.4 ± 3.3 mg/liter, respectively; P = 0.009), on BMT day 8 (117.0 ± 36.3 and 31.9 ± 12.4 mg/liter, respectively; P ≤ 0.001), and on BMT day 12 (79.1 ± 32.6 and 24.8 ± 6.2 mg/liter, respectively; P = 0.020). Infection was involved in 10 of the 16 IEs. The highest PCT and CRP levels (3.4 and 333 mg/liter, respectively) occurred only 1 h after Acinetobacter species had been detected in the blood cultures which had been taken just before meropenem was administered to patient 27. PCT and CRP concentrations had both declined 1 h later. In contrast, the highest PCT concentration associated with other infections was 1.81 μg/liter, and this was associated with the folliculitis and bacteremia due to S. epidermidis that patient 6 had developed. The highest PCT concentration associated with pulmonary aspergillosis (patient 23) was only 0.89 μg/liter, and that associated with GVHD was 1.06 μg/liter.
FIG. 1.
Relationship of PCT (A) and CRP (B) to mucositis and additional inflammatory events. (A) There is little fluctuation in the PCT concentrations and no difference between patients with mucositis only (○) and those with additional inflammatory events (●) from the first 12 days posttransplantation. However, on BMT day 15 the concentrations of PCT are significantly higher in 10 patients with an additional inflammatory event than in the 2 patients who had mucositis alone. GVHD rather than infection accounted for the increase in six patients. (B) In contrast to PCT, there are marked fluctuations in the CRP concentrations and significant differences between patients with mucositis only (○) and those with additional inflammatory events (●) on BMT days 1, 8, and 12 posttransplantation, and infection accounted for 10 of the 16 events.
DISCUSSION
We were able to observe some remarkable fluctuations in PCT and CRP levels after BMT, which illustrate clearly how difficult it is to relate laboratory findings to clinical events. It was also interesting that, except for the PCT concentration associated with transient bacteremia due to the gram-negative Acinetobacter species, all other concentrations were <2 μg/liter. Thus, the concentrations of PCT fell within a very narrow band at the lower limits of detection of the assay, so it would be difficult to discriminate between the various inflammatory processes, even were such a difference to exist. In contrast, CRP concentrations spanned the full range of the assay, making it at least feasible to discriminate reliably the inflammatory events associated with high concentrations from those associated with lower concentrations. However, in contrast to CRP, the results of serial sampling during 8 h show that a single determination of PCT appears to be sufficient to monitor any changes that might be expected.
Although only a small number of patients were studied, the population was very homogeneous since all were treated uniformly, all were monitored in five separate study periods, and no sampling data were missing. Surprisingly, instead of a relationship between infection and PCT, there appeared to be a possible relationship between acute GVHD and moderately elevated PCT and CRP levels, since this was observed in five of the eight cases. Unfortunately, we had no more plasma with which to follow the progress of PCT and CRP, so we cannot exclude the possibility that this might only have been a transient increase in these substances. In the absence of infection, fever, and GVHD, PCT and CRP levels were elevated to some extent, suggesting that there is an inflammatory process associated with mucositis. Admittedly, the levels were relatively low and did not extend much beyond 1.5 μg/liter, but these would normally be considered indicative of infection until proven otherwise. Endotoxin can induce PCT expression (5), and its translocation from the gut might also explain the low levels of PCT, since this has been shown to occur in similar patients (7).
What attracted our attention initially was the expectation that PCT should have a high negative predictive value for infection. Our use of meropenem preemptively was clearly successful in achieving its goal, namely, to prevent the occurrence of infection due to gram-negative bacilli except for the single, brief episode of bacteremia due to an Acinetobacter species. This occurred just before meropenem was given at 9:00 a.m. and coincided with a very high but apparently short-lived rise in both PCT and CRP levels. It is conceivable that bacteremia had already been present for 8 h at most (the dosing interval for meropenem); thus, the PCT and CRP levels may have already risen in response to the bacteremia, and the subsequent dose of meropenem may have ensured rapid eradication of the organism and, hence, a return of the PCT and CRP levels to normal. We had expected to find some use for PCT in detecting bacteremia due to gram-positive bacteria, but we found, like other investigators, that PCT was not a sensitive indicator of bacteremia due to CoNS (10). Nor does this appear to be the case for fungal infections, since PCT failed to indicate pulmonary aspergillosis in patient 23, as has been noted before (3). Moreover, PCT levels, and more markedly those of CRP, were elevated during mucositis and acute GVHD. Both clinical conditions are associated with or are the result of an inflammatory response elicited by the conditioning regimen used in allogeneic transplantation. These results cast considerable doubt on the utility of PCT in BMT recipients, especially since the gram-positive opportunistic cocci (particularly CoNS and, to a lesser extent, the viridans group streptococci) account for almost all episodes of bacteremia encountered within the first 2 to 3 weeks posttransplantation when patients are neutropenic, whereas the gram-negative bacilli and the more “professional” pathogens are rarely, if ever, involved.
The exact function or even the site of PCT production during sepsis is still uncertain, although PCT activity has been identified in human leukocytes (9), while others have suggested that lungs or neuroendocrine cells are possible production sites (11). The absence of leukocytes may explain in part the lower levels measured in patients with neutropenic infections (2). In BMT recipients, the reappearance of donor leukocytes that usually coincides with the occurrence of acute GVHD could result in higher PCT levels. The precise role of PCT as only a marker or even as a mediator of this inflammatory process remains unclear, however. Therefore, although PCT does satisfy one of the criteria for a surrogate marker of inflammation, it appears, like CRP, to be of little value in discriminating infection from other inflammatory complications that occur during the neutropenia that follows allogeneic BMT.
REFERENCES
- 1.Al-Nawas B, Krammer I, Shah P M. Procalcitonin in diagnosis of severe infections. Eur J Med Res. 1996;1:331–333. [PubMed] [Google Scholar]
- 2.Al-Nawas B, Shah P M. Procalcitonin in patients with and without immunosuppression and sepsis. Infection. 1996;24:434–436. doi: 10.1007/BF01713044. [DOI] [PubMed] [Google Scholar]
- 3.Beaune G, Bienvenu F, Ondarre C, Monneret G, Bienvenu J, Souillet G. Serum procalcitonin rise is only slight in two cases of disseminated aspergillosis. Infection. 1998;26:168–169. doi: 10.1007/BF02771844. [DOI] [PubMed] [Google Scholar]
- 4.Bernard L, Ferriere F, Casassus P, Malas F, Leveque S, Guillevin L, Lortholary O. Procalcitonin as an early marker of bacterial infection in severely neutropenic febrile adults. Clin Infect Dis. 1998;27:914–915. doi: 10.1086/517175. [DOI] [PubMed] [Google Scholar]
- 5.Dandona P, Nix D, Wilson M F, Aljada A, Love J, Assicot M, Bohuon C. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605–1608. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]
- 6.Glucksberg H, Storb R, Fefer A, Buckner C D, Nieman P E, Clift R A, Lerner K G, Thomas E D. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Jackson S K, Parton J, Barnes R A, Poynton C H, Fegan C. Effect of IgM-enriched intravenous immunoglobulin (Pentaglobin) on endotoxaemia and anti-endotoxin antibodies in bone marrow transplantation. Eur J Clin Investig. 1993;23:540–545. doi: 10.1111/j.1365-2362.1993.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 8.Karzai W, Oberhoffer M, Meier H A, Reinhart K. Procalcitonin—a new indicator of the systemic response to severe infections. Infection. 1997;25:329–334. doi: 10.1007/BF01740811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oberhoffer M, Stonans I, Russwurm S, Stonane E, Vogelsang H, Junker U, Jager L, Reinhart K. Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis-related cytokines in vitro. J Lab Clin Med. 1999;134:49–55. doi: 10.1016/s0022-2143(99)90053-7. [DOI] [PubMed] [Google Scholar]
- 10.Ruokonen E, Nousiainen T, Pulkki K, Takala J. Procalcitonin concentrations in patients with neutropenic fever. Eur J Clin Microb Infect Dis. 1999;18:283–285. doi: 10.1007/s100960050277. [DOI] [PubMed] [Google Scholar]
- 11.Snider R H J, Nylen E S, Becker K L. Procalcitonin and its component peptides in systemic inflammation: immunochemical characterization. J Investig Med. 1997;45:552–560. [PubMed] [Google Scholar]