Abstract
This literature review investigates the significant overlap between myelin-repair signaling pathways and pathways known to contribute to hallmark pathologies of Alzheimer’s disease (AD). We discuss previously investigated therapeutic targets of amyloid, tau, and ApoE, as well as other potential therapeutic targets that have been empirically shown to contribute to both remyelination and progression of AD. Current evidence shows that there are multiple AD-relevant pathways which overlap significantly with remyelination and myelin repair through the encouragement of oligodendrocyte proliferation, maturation, and myelin production. There is a present need for a single, cohesive model of myelin homeostasis in AD. While determining a causative pathway is beyond the scope of this review, it may be possible to investigate the pathological overlap of myelin repair and AD through therapeutic approaches.
Keywords: Myelin, Alzheimer’s disease, Myelin repair, Oligodendrocyte, Remyelination
Background
Alzheimer’s disease (AD) is the leading cause of dementia and currently has few avenues of effective treatment. AD has been well-characterized as a disease involving primary pathologies of intracellular neurofibrillary tau tangles and extracellular amyloid beta (Aβ) plaques. Pharmaceuticals have been developed to target these hallmark pathologies, but even therapeutics successful at reducing Aβ plaque load have been unable to sufficiently halt disease progression. Recently, it was proposed that the only FDA-approved amyloid-targeting drug, Aducanumab, may be more efficacious when combined with drugs that target oligodendrocytes and encourage remyelination [1].
Myelin is the lipid-based sheathing which surrounds axons to protect and promote nerve conduction. Demyelination has been observed in vivo in patients with mild cognitive impairment and dementia using myelin water fraction (MWF), a sophisticated neuroimaging method that is preferentially sensitive to myelin, indicating its potential utility as a clinical biomarker for dementia [2, 3]. Additionally, myelination in the elderly without dementia, measured through MWF, has been associated with episodic and semantic memory capacity [4] and the AD risk allele apolipoprotein E (APOE) ε4 [5]. In cognitively normal older adults at risk for AD, evidence supports the association of MWF with cerebrospinal fluid biomarkers of AD such as phosphorylated tau 181, total tau, and Aβ [6]. Thus, it is possible that promyelinating strategies may potentially ameliorate hallmark AD pathology and cognitive decline.
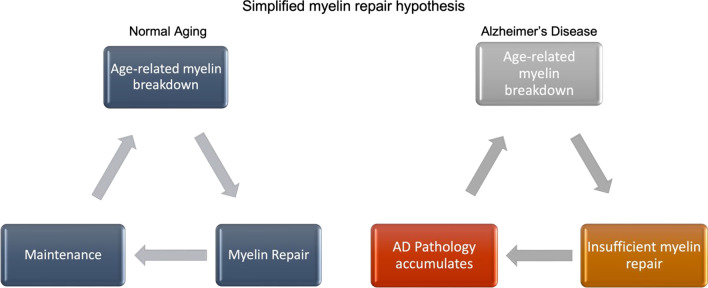
In considering potential therapeutic targets, early research by George Bartzokis and colleagues on myelin in AD and the association of Aβ with late-myelination regions is especially relevant [7–9]. Bartzokis proposed the theory that Aβ and tau are secondary, rather than primary, pathologies and may result from the brain attempting to maintain myelin homeostasis through a cycle of damage, repair, and maintenance [9] (Fig. 1).
Fig. 1.
Dysfunctional myelin repair mechanisms in individuals with AD, which may interrupt the normal myelin repair and maintenance cycle and lead to downstream amyloid-beta deposition and tau pathology as previously proposed by Bartzokis et al. [4]
In his hypothesis, Bartzokis theorizes that the complexity of human evolution and the related development of sophisticated myelination put humans at a unique vulnerability to neurological diseases stemming from the breakdown of and subsequent effort to maintain myelination [9]. Many pathways involved in myelin damage, maintenance, and repair overlap with genetic correlates of AD. However, demyelination and injury alone are not sufficient to cause AD pathology [9–12]. Typical myelin maintenance patterns follow a well-characterized, normal, age-related, quadratic trajectory [7, 13–15], which suggests that age-related pathology may not be due to mechanisms of myelin damage or maintenance but rather occur in the attempts to repair myelin. Although an increase in oligodendrocyte progenitor cell (OPC) proliferative rate suggestive of endogenous repair has been observed in an AD mouse model, this finding was not observed in post-mortem AD brain tissues, further suggesting insufficient repair mechanisms unique to clinical AD [16].
In this comprehensive review, we focus on the overlaps of myelin repair pathways empirically demonstrated to induce remyelination and AD-relevant pathways, to identify targetable mechanisms for potentially preventing AD progression. Other therapeutics that act on the intersection of myelin repair and AD pathology that should be investigated further are also reviewed.
Search criteria
The following keywords and terms were used to search for literature within the scope of this review: (myelin repair) AND (tau); (myelin repair) AND (amyloid); (myelin repair) AND (apoe4); (myelin repair) AND (apoe); ((myelin repair) AND (alzheimer's)) AND (gene); (myelin repair) AND (alzheimer's); (remyelination) AND (alzheimer's); ((remyelination) AND (alzheimer's)) AND (gene); (remyelination) AND (apoe); (remyelination) AND (apoe4); (remyelination) AND (amyloid); (remyelination) AND (tau); (myelin repair) AND (iron); (remyelination) AND (iron); ((myelin repair) AND (alzheimer's)) AND (iron); ((remyelination) AND (alzheimer's)) AND (iron).
The initial search yielded 318 articles after removal of duplicate retrievals. Exclusion criteria included: publication before 1992, review article or meta-analysis, organisms other than humans or murine, studies that did not have empirical evidence of affecting remyelination or myelin repair, studies that only investigated protection, injury, or early development of myelin, and studies not related to AD. After removal based on these criteria, 68 studies remained and are covered in this review. If studies covered myelin repair with indirect relationships to AD pathology, citations and PubMed were further searched for relevance to AD before exclusion was determined. Reviews were cited if the gene or pathway target has been thoroughly researched beyond the scope of this review. These parameters yielded another 50 results, bringing the total references cited to 118.
Examining overlaps between myelin repair and AD signaling pathways
Amyloid precursor protein (APP) and Aβ
Myelin pathology has been demonstrated to co-localize to Aβ plaque deposition in a commonly used AD mouse model with 5 familial AD mutations (5× FAD). The model, which expresses AD-associated mutations in APP and presenilin 1 (PSEN1) genes, is reportedly absent of confounding tau pathology [17], supporting the association of Aβ and myelin pathology. Additionally, soluble Aβ oligomers have been shown to: (a) specifically inhibit the survival of mature oligodendrocytes (OLs), cells that mainly function to assemble myelin sheathing, and (b) prevent myelin sheath formation [18]. In contrast, soluble Aβ oligomers can also induce myelin basic protein (MBP) expression, a vital protein in myelination, as well as promoting OL differentiation and maturation [19]. When Aβ toxicity is reduced, myelin integrity is rescued, but regeneration is not affected [20, 21]. Low-sulfated modified heparin mimetics have been shown to bind to Aβ and specifically prevent it from inhibiting OPC differentiation into mature OLs, leading to rescued remyelination [22].
Upstream of Aβ plaque deposition is APP, which is necessary for myelin repair, as knocking out APP leads to very delayed or no remyelination [23]. In a transgenic mouse model that overexpresses amyloid pathology, early disease stages of Aβ plaque deposition are associated with increased OPCs and their subsequent differentiation into mature OLs [16]. On the other hand, too much APP may also impair remyelination, as the same study also found decreased OLs in analogous human AD post-mortem tissues. A possible mechanism of myelin repair may be tied to Arginase 1 (Arg1) expression, as bulk RNA transcriptome analysis and cell type-profiling of APP mice demonstrated a significant association between insufficient Arg1 expression in myeloid cells, including OLs and other glial and phagocytic cells, and subsequent neurodegeneration and Aβ deposition [24]. Counterintuitively, Arg1 deficiency promotes OLs; more expectedly, it upregulates pro-inflammatory markers. Arg1 is also known to be significantly reduced in demyelination [25], further implicating Arg1 deficiency in overall myelin pathology.
APP is processed through several downstream pathways that may mechanistically explain the protein’s impact on myelination. First, APP can be processed by a family of α-secretases, known as a disintegrin and metalloproteinase (ADAM, such as ADAM10 and ADAM17), to form the non-neurotoxic soluble APP alpha (sAPPα) [26]. Upregulated sAPPα appears to be not just repair-oriented, but also protective in a demyelinating context. The administration of a sAPPα promoter, etazolate, in a mouse model of demyelination subsequently restored damaged myelin, upregulated MBP and mature OLs, and protected from further demyelination [27]. sAPPα may be targeted with the FDA-approved acetylcholinesterase inhibitor (AChEI) rivastigmine, which has been shown to encourage α-secretase processing activity in both 3× TG mice and human post-mortem tissues [28]. However, there is evidence that rivastigmine does not directly affect oligodendrogenesis [29].
APP can also be cleaved by β-secretase, known as BACE-1, which cleaves APP into Aβ [30]. The β-secretase also cleaves neuregulin 1 (NRG1) proteins, which are necessary for initiating remyelination [31, 32]. Aged APP/PSEN1 transgenic mice with vascular pathology, as well as their non-APP aged, stroked counterparts, demonstrated chronic upregulation of BACE1/NRG1 expression, as well as increased amyloid pathology [33]. Selective deletion of BACE1 leads to subsequent NRG1 loss in peripheral injury [34, 35], suggesting the effects of BACE1 signaling on remyelination may be mediated through the neuregulin family. While NRG1 can also be cleaved by ADAMs, specific cleavage by BACE1 seems both necessary and sufficient for NRG1 to signal myelin production [36]. However, bypassing this pathway is possible, as promoting downstream protein kinase B (Akt) expression in OLs rescues the NRG1-associated production of myelin in a BACE1-deficient model [37]. On the other hand, remyelination does not occur even in the presence of BACE1 in an APP-knock out (KO) model [23].
γ-Secretase also cleaves Aβ [38], and inhibition of γ-secretase can likewise decrease Aβ levels [39]. γ-Secretase inhibition also promotes remyelination, encourages stem cell maturation [40], and is associated with significantly quicker disease recovery and milder pathology in mice with experimental autoimmune encephalomyelitis (EAE), a demyelinating animal model similar to multiple sclerosis (MS) [41]. Inhibition of γ-secretase specifically inhibits the Notch1 signaling pathway, which in turn creates a pro-myelinating environment [41].
Taken together, Aβ and upstream APP may have both pro- and anti-remyelinating properties that are pathway-dependent. sAPPα, BACE1, and γ-secretase are known to affect the deposition of toxic Aβ oligomers in AD, and evidence suggests that these pathways may play a larger role than previously thought in myelin regulation in AD. Additionally, vascular pathology has been shown to contribute to general myelin pathology outside of AD [33, 42], though amyloid pathology appears to exacerbate the injurious effects of vascular injury on myelin [33]. Finally, the myelin repair pathways may be more effective initially in younger APP models [33] regardless of vascular contribution [16]. Further studies are needed to determine to what degree the amyloid and myelin changes are linked both spatially and temporally, as current literature suggests that AD-related demyelination occurs in a heterogenous manner in relation to amyloid pathology [16, 43–45].
In summary, APP and remyelination have significant overlaps in pathway biology. Solely targeting Aβ has been unsuccessful to date in stopping disease progression. Upregulating pro-myelinating pathways and downregulating Aβ concurrently may result in amelioration of pathology beyond what current Aβ clearance drug trials have demonstrated. Future studies testing this hypothesis are warranted.
ApoE and lipid metabolism
ApoE is a potential key player overlapping several neurodegenerative conditions in which myelin is affected (for review see [46]). APOE genotype, especially the ε4 allele, has been associated with multiple sclerosis (MS, a myelin-centric disorder) [47, 48], as well as lower apparent diffusion coefficient and fractional anisotropy values by diffusion tensor imaging in normal populations, indicating impaired white matter [49]. APOE ε4 carriers have shown developmental differences in white matter and cognition compared to non-carriers [15, 50]. The APOE ε2 allele is also associated with impaired remyelination in MS [51], though this finding has not been replicated. In fact, the ε2 allele has been shown to be protective against AD [52, 53] and is associated with higher myelin content compared to noncarriers, as observed via MWF [15]. A worse disease trajectory in APOE-deficient EAE mice compared with EAE controls, along with inhibited remyelination with concomitant immune activity, has been observed [54], potentially because the microglia and macrophages become overloaded by the large amount of cholesterol resulting from injured myelin and subsequent breakdown, which leads to their inability to keep up with phagocytic activities to drive downstream remyelination [55]. ATP-binding cassette transporter A1 (ABCA1) is a key player in cholesterol transport and metabolism (for reviews see [56–58]). ABCA1 is essential for astrocytic and glial synthesis of ApoE [59], and is also involved in transporting cholesterol from cells to high-density lipoproteins (HDLs), of which ApoE is a component [59, 60]. ABCA1-deficient mice exhibit significant and sustained reductions of OLs and myelin density, and reduced oligodendrogenesis post-stroke; ApoE2 and HDL3 expression directly rescues neurological deficits, promotes OPC differentiation, and significantly attenuates reductions in myelin, OLs, and oligodendrogenesis [61]. ApoE also binds to microglia-expressed triggering receptor expressed on myeloid cells 2 (TREM2) (for review see [62]). TREM2, a rare variant strongly associated with AD [63], is present in early cell proliferation and has been shown to co-localize with OPCs and OLs in APP/PSEN1 mice [64]. TREM2 is related to myelin repair as it is necessary for the formation of lipid droplets through cholesterol esterification [65]. Additionally, TREM2 plays an essential role in mediating the phagocytosis of myelin and other cellular debris [66, 67]. Single-cell RNA sequencing in mice has identified a TREM2-dependent white matter-associated microglial phenotype (WAM), which has been observed to clump with myelin debris and is involved in debris degradation, hypoxia-inducible factor signaling, and lysosomal and cholesterol pathways [68]. WAM also overlap in genetic signature with disease-associated microglia (DAM), an abnormal microglial type previously observed in transgenic AD mice [69]. While WAM are TREM2-dependent, they are typically APOE-independent in wild-type mouse models of aging. However, in mouse models of AD, ApoE is necessary for WAM development in addition to TREM2 [68]. ApoE is also related to the regulation of the enzyme lipoprotein lipase (LPL). LPL, which mediates the reparative phenotype of microglia, is specifically involved in the uptake and phagocytosis of myelin-related lipids, and it is associated with the initiation of improved demyelination-related clinical symptoms in EAE-induced mice at the temporal junction where demyelination ends and remyelination begins [25]. LPL deficiency has been thoroughly investigated as a possible contributing factor in the development of AD [25, 70–72]. LPL administration results in elevated cellular Arg1 levels [25], which has been previously implicated in myelin repair [24]. Lipid uptake may also be mediated by colony-stimulating factor 1 receptor, which, when inhibited, reduces microglia but potentially enhances the phagocytic capacity of remaining microglia, thus enabling remyelination [73]. Evidence also shows that remyelination may be encouraged through the upregulation of specific lipid receptors such as liver X receptors (LXR), oxysterol-activated nuclear receptors that maintain cholesterol homeostasis. These receptors are present in oligodendrocytes and have been demonstrated to enhance lipid transfer from other cells to OLs when LXR is activated [55, 74]. LXR agonists have been shown to improve remyelination, reduce inflammation, and reduce overall cholesterol overload that occurs in demyelination, through inducing target genes including ABCA1, APOE, and others [55, 74]. The nuclear receptor retinoid X receptor (RXR) signaling can similarly upregulate the expression of ABCA1 and APOE to directly increase OPC and OL maturation and improve AD-related cognitive functioning [75].
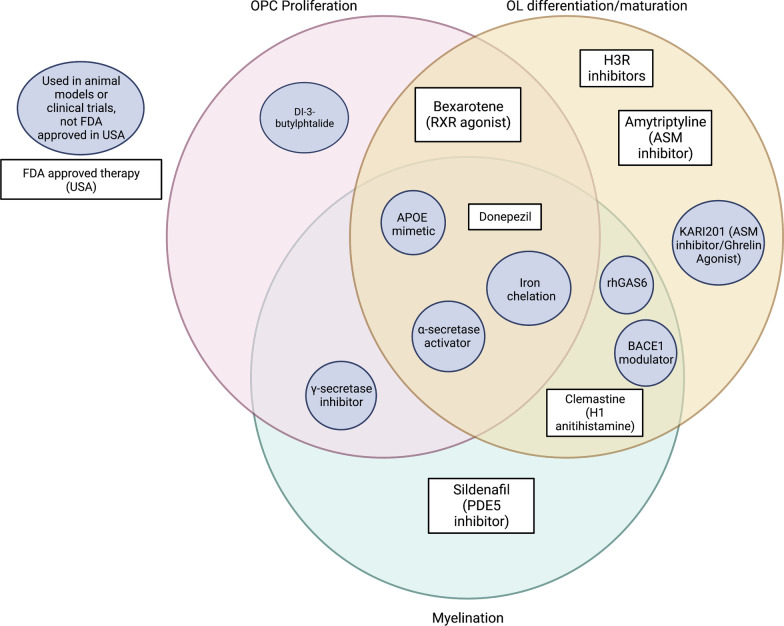
ApoE mimetics have been able to rescue myelin repair while also suppressing macrophage activity in the peripheral nervous system [76]. Inhibition of low-density lipoprotein receptor 1, an essential receptor for myelin phagocytosis [77], blocks mimetic effects, indicating a potential role for this receptor. LXR and RXR pathways can also be upregulated by synthetic agonists. RXR is a clinically applicable target, and the FDA-approved RXR agonist, bexarotene, is associated with remyelination in triple transgenic (3× TG) mouse models of AD [75] and remyelination-associated cognitive recovery in stroked mice [75, 78]. Additionally, TREM2 has the potential to enter the brain from peripheral sites and directly modulate OPC and OL activity [64].
Taken together, modulation of ApoE-related signaling pathways seems to both improve cognition and encourage remyelination in the context of AD as well as other models of demyelination and vascular injury.
Tau and neurofilament proteins
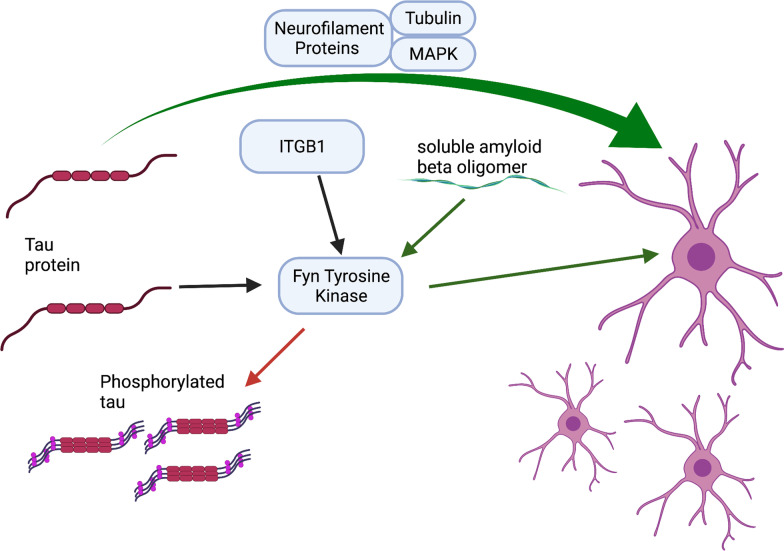
Selective myelin injury occurs early in tauopathy models followed closely by cognitive deficits, and in turn, remyelination has been shown to ameliorate cognitive decline [79]. Tau, which becomes hyperphosphorylated and aggregates as neurofibrillary tangles in AD, has been shown to modulate OPC differentiation along with other axonal cytoskeleton proteins like tubulin and microtubule-associated proteins [80, 81]. In addition, tau has been shown to bind to the cytoskeleton of OLs via the truncating tyrosine kinase Fyn [82] (Fig. 2), which is also involved in the phosphorylation of tau [83]. When phosphorylated tau (p-tau) is specifically reduced without affecting the total tau levels, myelin repair is increased and functional outcomes are improved [84]. Tau is also associated with axonal neurofilament proteins (NFPs). Specific fractions of NFP, such as NFP2 and NFP5, have been associated with OL lineage and development in vitro [81]. The ratio of NFP to tubulin may specifically affect OL lineage. NFP2, which is associated specifically with OPC proliferation, contains a higher tubulin concentration. NFP5, which affects OL maturation and promotes differentiation, contains less tubulin [81]. Interestingly, soluble Aβ oligomers can promote OL differentiation/maturation and induce MBP expression through the oligodendrocytic Fyn/Ca2/CAMKII signaling cascade and its upstream activator ITGB1, suggesting that Fyn may serve as a target for simultaneously modulating oligodendrocytic machinery and tau hyperphosphorylation [19] (Fig. 2).
Fig. 2.
Simplified rendering of tau-associated pathways that may influence OL differentiation, including the binding of tau and Fyn to the OL cytoskeleton, which may simultaneously encourage hyperphosphorylation of tau, an upstream activator of Fyn, ITGB1, and other axonal and neurofilament proteins that may interact with tau to also promote OL differentiation beyond Fyn
Exploring other targets implicated in both myelin repair and AD
Phosphoinositide 3-kinase (PI3k)/Akt/mammalian target of rapamycin (mTOR) signaling
The PI3k signaling cascade and its upstream and downstream components can promote remyelination and overlap with pathways that contribute to AD pathology. Akt expression, downstream of PI3k, was previously discussed in the context of BACE1 cleavage, where Akt expression may serve as a “bypass” to allow BACE1 inhibitors to suppress amyloid without affecting remyelination [37]. Activating the PI3k pathway directly or indirectly has been shown to promote remyelination [85–87]. The traditional Chinese herbal compound Shen-zhi-ling (SZL) oral liquid has been shown to increase PI3k and downstream Akt mRNA expression, as well as significantly increasing mTOR-positive cells and myelin-related proteins in APP mice [88]. Similar effects have also been seen with donepezil, an AChEI that has been approved by the FDA for treatment of AD and has also been investigated for myelin-promoting properties. Donepezil promotes differentiation of OPCs to OLs, encourages the formation of myelin sheathing, and upregulates myelin-specific proteins [29, 89]. Notably, rivastigmine did not have a significant effect on OPCs and myelination when compared to donepezil. While the exact remyelinating mechanism of donepezil is presently unclear, the PI3k/Akt/mTOR pathway modulation is thought to be a contributor [89]. In contrast, low doses of a PI3k antagonist can upregulate OPCs and encourage OL maturation, though it has yet to be determined whether this is a PI3k-specific effect or a result of off-target Wnt and RAF-MAPK signaling [90]. In summary, PI3k/Akt modulation may be useful for promoting remyelination specifically in the context of AD. Further investigations of PI3k and especially Akt in the dual contexts of myelination and AD may offer insight into relevant biological pathways that contribute to AD.
Histamine receptors
Clemastine, an FDA-approved H1 antihistamine with anti-inflammatory and anti-muscarinic effects, has been shown to reduce Aβ deposition and ameliorate cognitive deficits while simultaneously enhancing OPC differentiation and maturation, as well as myelin integrity, in mouse models of AD [91, 92]. Clemastine can additionally upregulate mTOR to inhibit OPC senescence. OPC senescence has been associated with Aβ in mouse models of AD and in post-mortem brain tissues of AD patients, implicating a possible therapeutic role for senolytic and senescence-inhibiting therapeutics in inducing myelin repair in the context of AD [93].
Histamine 3 receptor (H3R) antagonists and inverse agonists may also be a potential target, as H3R antagonism has been shown to reduce Aβ load, possibly through cAMP response element-binding protein (CREB)-mediated autophagy [94, 95], and improve memory deficits [96]. H3R inhibition acts on the cAMP/CREB/HDAC-1/HES-5 signaling cascade and has been shown to improve remyelination by promoting OL differentiation and maturation by reducing cAMP. Hes Family BHLH Transcription Factor 5 (HES-5) inhibition alone may ameliorate OPC differentiation [97]. HES-5 is also a downstream product of the Notch signaling pathway, which is cleaved by both the ADAM metalloprotease family and γ-secretase (for review see [98]). Abnormal Notch signaling and related NOTCH gene mutations are associated with tau and Aβ as well as vascular components of AD (for review see [99]). Notch is also involved in myelination, as inhibition of the Notch signaling pathway in OLs results in quicker recovery and milder clinical manifestations in a demyelinating context, as well as upregulated remyelination [41].
Clemastine has been assessed in a trial in MS patients for its remyelinating properties [100], and it potentially proves useful for AD as well [1]. Additionally, the H3R selective antagonist/inverse agonist, Pitolisant, is FDA-approved to treat narcolepsy with cataplexy, demonstrating feasibility of targeting H3R. In conclusion, selectively targeting histamine, and in turn mediating Notch signaling, may offer a clinically relevant pathway for both myelination and AD.
Acid sphingomyelinase (ASM)
KARI201, an ASM inhibitor, has been recently developed and may have direct effects on both AD pathology and OL-lineage effects. KARI201 normalizes ASM activity without affecting protein level of ASM or mRNA transcript level of SMPD1, the gene coding for ASM. However, this drug is associated with significantly reduced Aβ levels and improved autophagic and phagocytic microglial activity [101]. ASM inhibition also encourages OL maturation and/or survival [102]. KARI201 is also found to have a dual action as a ghrelin receptor agonist; agonism of this receptor is known to promote hippocampal synaptic density, plasticity, and neurogenesis in the context of AD [101].
Other approved or experimental drugs also target the ASM pathway. Amitriptyline is an FDA-approved tricyclic antidepressant that is a potent ASM inhibitor [103], which could be explored further for effects on OL pathways. Though still experimental, mesenchymal stem cell exosomes have been found to directly stimulate OPC proliferation, maturation, and remyelination while also encouraging reparative microglial phenotypes [104]. Moreover, sphingomyelin-driven neuronal exosomes can specifically bind and sequester Aβ [105], which can then be phagocytosed [106], indicating a larger role for sphingomyelin metabolism as a therapeutic target.
Growth arrest-specific protein 6 (GAS6)
GAS6 has been associated with clinical AD [107] and clearance of Aβ [108]. Additionally, direct delivery of recombinant human GAS6 (rhGAS6) protein to the corpus callosum in demyelinated mice demonstrated a beneficial effect on the clearance and reduction of myelin and lipid debris and encourages accelerated maturation of OPCs [109]. Microglia are not directly affected by rhGAS6, but based on the amelioration of debris clearance in previous studies, it appears that GAS6 may promote microglia to target Aβ, as well as encouraging existing microglia to clear Aβ instead of myelin and/or to restore reparative phagocytic properties in disease contexts [110].
Klotho
Enhanced expression of the anti-aging gene KLOTHO [111] reduces AD-related cognitive deficits [112]. The KLOTHO-VS allele heterozygosity has been shown to reduce amyloid and tau pathology [113] in APOE ε4 carriers [114]. In addition to its AD-specific effects, Klotho enhances remyelination [115] and promotes OL maturation [116, 117]. Taken together, Klotho appears to specifically encourage myelin repair pathways while also ameliorating amyloid and tau pathology. The recent advance of a CRISPR-Cas9 model for studying KLOTHO activation will allow for further research into this gene and its effects [118].
Phosphodiesterase-5 (PDE5) inhibition
PDE5 inhibition has also been investigated in AD animal models as a potential treatment. Specifically, a rat model of AD treated with a PDE5 inhibitor showed increases in vascular endothelial growth factor A and cyclic GMP, decreases in vascular cell adhesion molecule 1 and tumor necrosis factor alpha, and increased memory performance compared to the non-treated group [119]. Additionally, PDE5 inhibition has been shown to encourage remyelination in demyelinating mouse models while also exhibiting immune-modulating effects [120–122]. These studies indicate that sildenafil, an FDA-approved PDE5 inhibitor, commonly known as Viagra, may have an ameliorating effect on AD pathology along with positive effects on myelination. However, a study of sildenafil treatment of OPCs showed evidence that PDE5 inhibition may also negatively impact myelin gene transcription and impair oligodendrocyte proliferation [123]. Further clinical studies are warranted due to the differing conclusions between in vitro and in vivo studies, which suggest that PDE5 inhibition alone may not be sufficient to induce remyelination, and immune modulation may be necessary with this treatment.
Nogo-A signaling pathway
Dl-3-butylphtalide (dl-NBP) can ameliorate neuropathology related to AD [124] and may ameliorate myelin injury in vascular models. Specifically, dl-NBP has been shown to promote OPC proliferation through the neurite outgrowth inhibitor (Nogo-A) and brain-derived neurotrophic factor signaling pathways [125]. Nogo-A-deficient mice demonstrate delayed OL maturation and myelination [126]. Leucine-rich repeat and Ig domain containing 1 (LINGO-1), downstream of Nogo-A, directly interacts with and encourages degradation of APP [127], while also negatively impacting myelination and OL differentiation [128]. In contrast, anti-LINGO-1 therapy has been shown to improve spatial learning and, at least partially, to restore MBP levels [12], and genetic deletion of LINGO-1 in animal models also results in enhanced remyelination [129–131]. Relevant to AD, anti-LINGO-1 therapy in a 5 × FAD mouse model ameliorated memory impairment before abnormal Aβ deposition was apparent [132]. In an APP/PS1 mouse model, anti-Lingo-1 therapy decreased the existing Aβ deposition and restored OL function [133]. Single-cell transcriptome analysis of post-mortem human AD brain tissue has further implicated LINGO-1 as a regulator of myelin integrity across not just OPCs and OLs, but also most neuronal and glial cell types, which may reflect a central response attempting to restore myelin homeostasis [134]. Additionally, single-nucleus transcriptome analysis has indicated upregulated LINGO-1 in astrocytes of AD prefrontal cortical samples [128]. Taken together, it appears that modulating the Nogo-A signaling pathway and the downstream LINGO-1 activity may prevent AD pathology and simultaneously encourage OPC proliferation, possibly enhancing remyelination.
Protein-tyrosine phosphatase receptor type Z (PTPRZ) signaling
PTPRZ acts on the same target in OLs as Fyn; however, it functions conversely. Inhibition of PTPRZ has been shown to upregulate OPC differentiation, as PTPRZ potentially prevents OPC maturation [135]. In addition to competing with Fyn, as discussed previously for its effects on tau, PTPRZ expression is also mediated through metalloproteinase and γ-secretase pathways [136]. PTPRZ, which overlaps with both tau and amyloid processing pathways, along with its direct effects on OPC differentiation, merits further investigations of its effects in relation to AD.
Iron homeostasis
Iron homeostasis is essential for glial function and myelination, and multiple recent literature reviews implicate dysfunctional iron homeostasis in the pathogenesis of AD [137–139]. Additionally, clinical neuroimaging studies have demonstrated the association between iron accumulation and myelination in normal aging [140, 141] as well as the association between tau accumulation and iron in AD patients [142]. Cerebrospinal fluid levels of iron transport proteins are also associated with cognitive decline in AD [137].
The literature is divided on the benefits of inhibiting or activating iron storage and transport proteins. For example, the iron storage protein ferritin is known to encourage remyelination and OL function through microglial H-ferritin release [143, 144], as OLs themselves express an H-ferritin receptor known as T cell immunoglobulin and mucin domain-containing protein-2 (TIM-2) [145]. H-ferritin iron storage impairment in OPCs and deletion of astrocytic H-ferritin are also associated with a delay in myelin repair [146, 147]. However, despite observations of OL proliferation in inflammatory environments, toxicity of excess ferritin to OL lineage has also been observed [143]. Inflammatory milieu such as the presence of activated microglia and associated cytokine production may also play a role in inducing ferritin toxicity to OLs [144]. Additionally, excess iron in animal models can increase APP, inhibit α-secretase cleavage, and increase β-secretase cleavage [137]; however, APP, in turn, can stabilize the iron export protein ferroportin (Fpn) [137, 148], which is involved in OL maturation and myelination [149]. Another iron import protein, Divalent metal transporter 1, is involved in APP processing and co-localizes with Aβ plaques [150], and its deletion is associated with reductions in OPC maturation and myelination [147]. Transferrin, another iron importer, binds with tau [151] and has been associated specifically with p-tau in AD patients [152]. It also enhances microglial phagocytic capacity, improves lipid and myelin debris uptake, and encourages OL maturation [153, 154].
While the overall upregulation of proteins related to iron homeostasis is evidently associated with AD pathology, the overall downregulation may negatively impact myelination. Modulation of iron and associated transport proteins could be achieved through iron chelators modified to cross the blood–brain barrier and has previously been clinically trialed in several neurodegenerative diseases (for review, see [155]), including AD [156, 157]. Studies examining combined therapeutics that act through iron accumulation and transport to encourage microglial and oligodendrocyte function while also mediating APP cleavage and tau phosphorylation are still needed.
Summary
This comprehensive literature review highlights the overlaps of signaling pathways involved in myelin repair and AD. Evidence from this review also suggests that dysfunctional myelin repair may occur early in the disease spectrum, and thus, it may be more beneficial to target myelin repair in early disease stages. Further investigation is needed to confirm the spatial and temporal relationship of insufficient myelin repair with AD pathology. The accumulation of amyloid and tau pathology that occur after myelin pathology [79, 132] suggests that early targeting of remyelination could be a potential therapeutic choice.
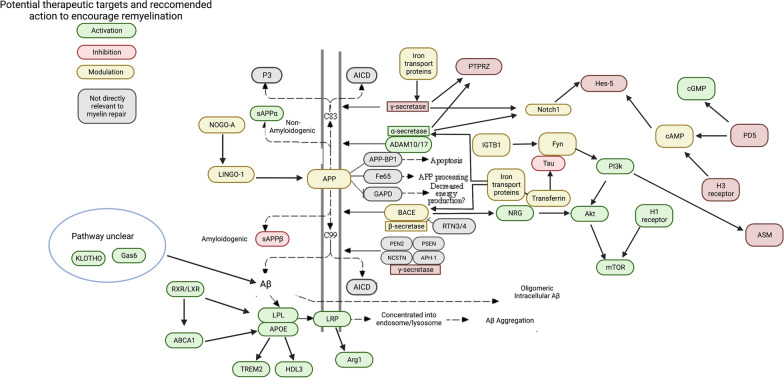
Pathways directly related to AD, including α-, β-, and γ-secretase pathways, may have dual targets that ameliorate both Aβ and myelin repair mechanisms (Figs. 3, 4 and 5). These pathways may also be synergistically acting with dysfunctional myelin repair mechanisms. Inability of pro-remyelinating α-secretase processing may lead to overwhelming cleavage by β- and γ-secretases, where the excess Aβ may either impair or overpower concomitant β-secretase cleavage of pro-myelinating NRG1. NRG1 inhibition leading to limited remyelination may offer a possible explanation for the failure of BACE1 inhibitors in clinical trials. Moreover, considering the role of NRG1 during BACE1 inhibition may also offer a potential solution, as BACE1 inhibitors may still be used as an anti-amyloid treatment if they are used in tandem with a protein kinase B (Akt) activator.
Fig. 3.
A schematic expanding the canonical KEGG pathway for AD, superimposed with proposed specific modulations that may upregulate myelination based on literature search. Targets are color-coded to indicate what action, based on empirical studies covered in this review, may simultaneously (1) encourage at least part of the remyelination cascade and (2) discourage development of AD pathology simultaneously. Green indicates target activation, red indicates inhibition, and yellow indicates conflicting literature on whether activation or inhibition is beneficial. Gray targets are not relevant in the scope of this review
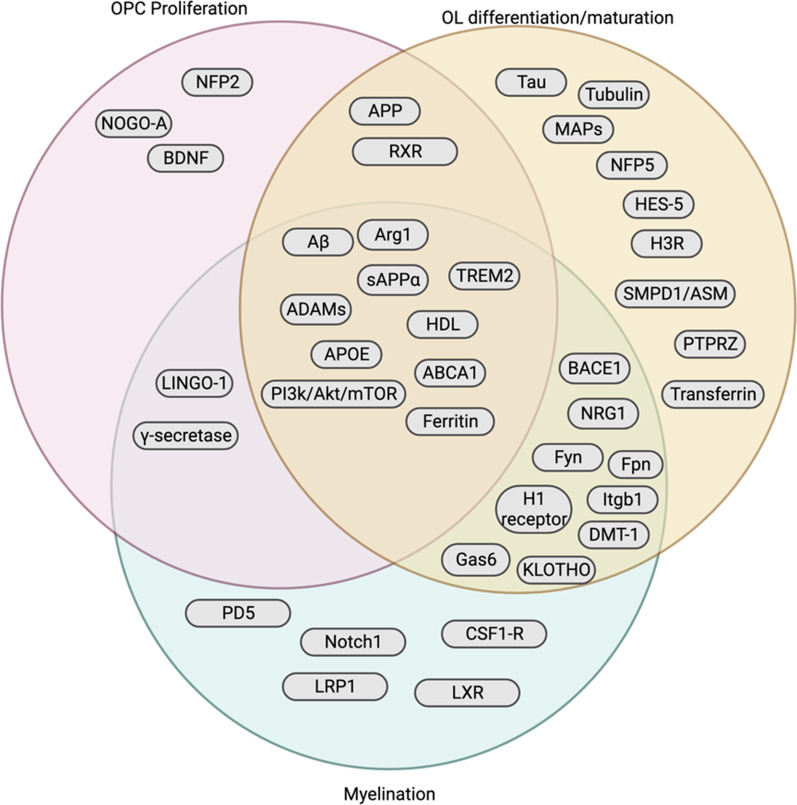
Fig. 4.
Organization of gene and pathway targets as described within the scope of this review based on their impact on OPC proliferation, OL differentiation/maturation, and/or myelination
Fig. 5.
Existing compounds that have been investigated thus far in animal studies and clinical trials (circle) or have FDA-approved formulations (rectangle) by mechanism of impact on OPC proliferation, OL differentiation/maturation, and/or myelination
Additionally, the ABCA1/ApoE/HDL pathway may be critical for remyelination. The effects of ApoE mimetics, which rescue myelin repair while also suppressing macrophagic activity, suggest that macrophages seen in disease-related inflammation may be over-clearing debris. ApoE mimetics can also act to upregulate TREM2, which may increase OPC proliferation and encourage the remyelination cascade. The relationship of ApoE, TREM2, and phagocytic activity to myelin in AD is further emphasized by the presence of a TREM2-dependent WAM. WAM may precede DAM gene signatures, indicating the possibility of upstream myelin pathology in early AD, though more research is needed to confirm this and to elucidate the overlap between the two types [68]. Further research is needed to understand the biological premise for observed ApoE dependence of WAM and if it indeed is related to AD pathophysiology. ApoE mimetics and LXR/RXR agonists may be therapeutically beneficial for targeting these pathways. Further lipidomic analysis is warranted to understand the broader impact of lipid metabolism, such as the implication of glycolipids like sulfatide and ganglioside in demyelination and AD-like cognitive impairment [158–160].
Hyperphosphorylation of tau may be directly related to the inhibition of myelin repair. When p-tau is specifically reduced without affecting the total tau levels, increased myelin repair and improved functional outcomes were observed [84]. Targeting tau, NFP, or the IGTB1/Fyn/Ca2/CAMKII signaling pathway may allow for precise control of the OL lineage and remyelinating properties. The involvement of Fyn with Aβ, tau, and oligodendrocytes implies considerable overlap of AD pathology, myelin repair, and Fyn activity that should be further investigated.
The present review also identifies studies supporting the roles of other pathways and targets in both AD and myelin repair. All targets discussed have been demonstrated to play a role in both AD and remyelination. Further research is warranted into these pathways in the context of AD, as the literature does not unanimously support activation or inhibition of many of the signaling pathways, and studies examining the overlap of myelination and AD pathology are scarce. These targets may offer therapeutic potential alone or in combination with other targeted AD-specific therapeutics, warranting further studies with animal models, single-cell and single-nucleus analysis, and clinical trials when applicable.
This review emphasizes a body of work which demonstrates an overlap between myelin repair mechanisms and AD pathogenesis. However, many studies using preclinical models have fallen short of thoroughly addressing the development of hallmark AD pathology in this shared context. Thus, despite evidence of involvement of myelin repair in different signaling pathways of the amyloid/tau/neurodegeneration cascade, proposing a single, cohesive model is beyond the scope of this review. Limitations regarding scope also include the focus on myelin repair, as it does not comprehensively address mechanisms of injury or maintenance, though other such reviews do cover this topic [9–12]. Much work remains to be done to fully reveal the implications of myelin repair in AD. Due to the urgent need for disease-modifying therapies, it may be possible to reverse-engineer the relationship of myelination with AD progression through therapeutic approaches discussed in this review.
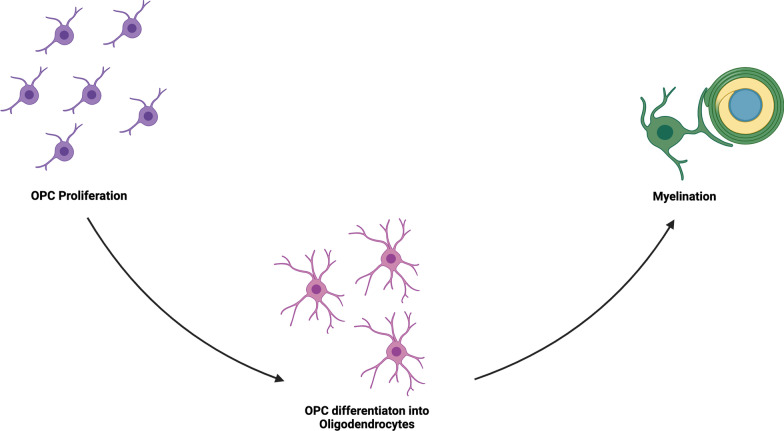
In conclusion, we have shown that there are numerous target pathways that directly overlap with both myelin repair and AD pathophysiology, including APP processing, ApoE signaling, and tau-Fyn processing. Additionally, other pathways and druggable targets have been shown to both ameliorate AD and restore remyelination. From this review, three targets for myelin repair have become apparent: (1) OPC proliferation, (2) OL maturation, and (3) myelin sheath production (Fig. 6). These targets may also be employed to prevent initial myelin damage, although the injurious mechanisms are beyond the scope of this review. More research needs to be done to elucidate which part(s) of these processes are most impaired in AD, if myelin repair dysfunction indeed occurs earlier in the disease course than previously suggested, and which, if any, of the pathways above can ameliorate myelin pathology and potentially restore myelin function.
Fig. 6.
Schematic of the main constituents of myelin repair that can be targeted by pathway modulation: OPC proliferation, OPC differentiation and oligodendrocyte maturation/survival, and the actual formation of myelin sheath and associated proteins
Acknowledgements
The authors would like to thank Dr. Michelle Block, Dr. Yu-Chien Wu, and Dr. Sha Cao for valuable discussions, as well as Dr. Paula Bice for editorial assistance.
Abbreviations
- ADAM
A disintegrin and metalloproteinase
- AChEI
Acetylcholinesterase inhibitor
- ASM
Acid sphingomyelinase
- AD
Alzheimer’s disease
- Aβ
Amyliod beta
- APP
Amyloid precursor protein
- ApoE
Apolipoprotein E
- Arg1
Arginase 1
- ABCA1
ATP-binding cassette transporter A1
- BACE1
Beta-secretase 1
- CREB
CAMP response element-binding protein
- DAM
Disease-associated microglia
- dl-NBP
Dl-3-butylphtalide
- EAE
Experimental autoimmune encephalomyelitis
- GAS6
Growth-arrest specific protein 6
- HES-5
Hes family BHLH transcription factor 5
- HDL
High density lipoprotein
- H3R
Histamine 3 receptor
- LINGO-1
Leucine rich repeat and Ig domain containing 1
- LPL
Lipoprotein lipase
- LXR
Liver X receptors
- MS
Multiple sclerosis
- MBP
Myelin basic protein
- NRG1
Neuregulin 1
- NFP
Neurofilament protein
- OPC
Oligodendrocyte progenitor cells
- OL
Oligodendrocytes (s)
- PDE5
Phosphodiesterase-5
- PI3K
Phosphoinositide 3-kinase
- PTPRZ
Protein-tyrosine phosphatase receptor type Z
- RXR
Retinoid X receptor
- TREM2
Triggering receptor expressed on myeloid cells 2
- WAM
White matter associated microglia
Author contributions
LH contributed to the conception, design, literature search, and drafting of the manuscript. SR, KN, and AS contributed to critical revision of the manuscript. All authors read and approved the final version of the manuscript.
Funding
Ms. Hirschfeld received support from multiple grants during the preparation of this manuscript: T32AG071444 and F31AG074700. Dr. Saykin receives support from multiple NIH grants (P30 AG010133, P30 AG072976, R01 AG019771, R01 AG057739, U19 AG024904, R01 LM013463, R01 AG068193, T32 AG071444, and U01 AG068057 and U01 AG072177). Dr. Risacher receives support from NIH grants AG061788 and K01AG049050. Dr. Nho receives support from NIH grants R01 LM012535 and R03 AG054936.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Saykin has received support from Avid Radiopharmaceuticals, a subsidiary of Eli Lilly (in kind contribution of PET tracer precursor); Bayer Oncology (Scientific Advisory Board); Eisai (Scientific Advisory Board); Siemens Medical Solutions USA, Inc. (Dementia Advisory Board); NIH NHLBI (MESA Observational Study Monitoring Board); Springer-Nature Publishing (Editorial Office Support as Editor-in-Chief, Brain Imaging and Behavior).
Contributor Information
Lauren Rose Hirschfeld, Email: pricelr@iu.edu.
Andrew J. Saykin, Email: asaykin@iu.edu
References
- 1.Fessel J. Reversing Alzheimer's disease dementia with clemastine, fingolimod, or rolipram, plus anti-amyloid therapy. Alzheimers Dement. 2022;8(1):e12242. doi: 10.1002/trc2.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouhrara M, Reiter DA, Bergeron CM, Zukley LM, Ferrucci L, Resnick SM, et al. Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimers Dement. 2018;14(8):998–1004. doi: 10.1016/j.jalz.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacKay AL, Laule C. Magnetic resonance of myelin water: an in vivo marker for myelin. Brain Plast. 2016;2(1):71–91. doi: 10.3233/BPL-160033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kavroulakis E, Simos PG, Kalaitzakis G, Maris TG, Karageorgou D, Zaganas I, et al. Myelin content changes in probable Alzheimer's disease and mild cognitive impairment: associations with age and severity of neuropsychiatric impairment. J Magn Reson Imaging. 2018;47(5):1359–1372. doi: 10.1002/jmri.25849. [DOI] [PubMed] [Google Scholar]
- 5.Park M, Lee HP, Kim J, Kim DH, Moon Y, Moon WJ. Brain myelin water fraction is associated with APOE4 allele status in patients with cognitive impairment. J Neuroimaging. 2022;32(3):521–529. doi: 10.1111/jon.12960. [DOI] [PubMed] [Google Scholar]
- 6.Dean DC, III, Hurley SA, Kecskemeti SR, O’Grady JP, Canda C, Davenport-Sis NJ, et al. Association of amyloid pathology with myelin alteration in preclinical Alzheimer disease. JAMA Neurol. 2017;74(1):41–49. doi: 10.1001/jamaneurol.2016.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol Aging. 2004;25(7):843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Bartzokis G, Lu PH, Mintz J. Human brain myelination and amyloid beta deposition in Alzheimer’s disease. Alzheimers Dement. 2007;3(2):122–125. doi: 10.1016/j.jalz.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartzokis G. Alzheimer's disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging. 2011;32(8):1341–1371. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasrabady SE, Rizvi B, Goldman JE, Brickman AM. White matter changes in Alzheimer's disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun. 2018;6(1):22. doi: 10.1186/s40478-018-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papuć E, Rejdak K. The role of myelin damage in Alzheimer's disease pathology. Arch Med Sci. 2018;16(2):345–351. doi: 10.5114/aoms.2018.76863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J, Zhou H, Bai F, Ren Q, Zhang Z. Myelin injury induces axonal transport impairment but not AD-like pathology in the hippocampus of cuprizone-fed mice. Oncotarget. 2016;7(21):30003. doi: 10.18632/oncotarget.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouhrara M, Rejimon AC, Cortina LE, Khattar N, Bergeron CM, Ferrucci L, et al. Adult brain aging investigated using BMC-mcDESPOT–based myelin water fraction imaging. Neurobiol Aging. 2020;85:131–139. doi: 10.1016/j.neurobiolaging.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartzokis G. Quadratic trajectories of brain myelin content: unifying construct for neuropsychiatric disorders. Neurobiol Aging. 2004;25(1):49–62. [Google Scholar]
- 15.Triebswetter C, Kiely M, Khattar N, Ferrucci L, Resnick SM, Spencer RG, et al. Differential associations between apolipoprotein E alleles and cerebral myelin content in normative aging. Neuroimage. 2022;251:118988. doi: 10.1016/j.neuroimage.2022.118988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrendt G, Baer K, Buffo A, Curtis MA, Faull RL, Rees MI, et al. Dynamic changes in myelin aberrations and oligodendrocyte generation in chronic amyloidosis in mice and men. Glia. 2013;61(2):273–286. doi: 10.1002/glia.22432. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Wu N, Wang S, Yao Z, Xiao F, Lu J, et al. Neuronal loss and microgliosis are restricted to the core of Aβ deposits in mouse models of Alzheimer's disease. Aging Cell. 2021;20(6):e13380. doi: 10.1111/acel.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horiuchi M, Maezawa I, Itoh A, Wakayama K, Jin L-W, Itoh T, et al. Amyloid β1–42 oligomer inhibits myelin sheet formation in vitro. Neurobiol Aging. 2012;33(3):499–509. doi: 10.1016/j.neurobiolaging.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quintela-López T, Ortiz-Sanz C, Serrano-Regal MP, Gaminde-Blasco A, Valero J, Baleriola J, et al. Aβ oligomers promote oligodendrocyte differentiation and maturation via integrin β1 and Fyn kinase signaling. Cell Death Dis. 2019;10(6):1–16. doi: 10.1038/s41419-019-1636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezra A, Rabinovich-Nikitin I, Rabinovich-Toidman P, Solomon B. Multifunctional effect of human serum albumin reduces Alzheimer’s disease related pathologies in the 3xTg mouse model. J Alzheimers Dis. 2016;50(1):175–188. doi: 10.3233/JAD-150694. [DOI] [PubMed] [Google Scholar]
- 21.Fleming CE, Saraiva MJ, Sousa MM. Transthyretin enhances nerve regeneration. J Neurochem. 2007;103(2):831–839. doi: 10.1111/j.1471-4159.2007.04828.x. [DOI] [PubMed] [Google Scholar]
- 22.McCanney GA, McGrath MA, Otto TD, Burchmore R, Yates EA, Bavington CD, et al. Low sulfated heparins target multiple proteins for central nervous system repair. Glia. 2019;67(4):668–687. doi: 10.1002/glia.23562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truong PH, Ciccotosto GD, Merson TD, Spoerri L, Chuei MJ, Ayers M, et al. Amyloid precursor protein and amyloid precursor-like protein 2 have distinct roles in modulating myelination, demyelination, and remyelination of axons. Glia. 2019;67(3):525–538. doi: 10.1002/glia.23561. [DOI] [PubMed] [Google Scholar]
- 24.Ma C, Hunt JB, Kovalenko A, Liang H, Selenica M-LB, Orr MB, et al. Myeloid arginase 1 insufficiency exacerbates amyloid-β associated neurodegenerative pathways and glial signatures in a mouse model of Alzheimer’s disease: a targeted transcriptome analysis. Front Immunol. 2021;12:628156. doi: 10.3389/fimmu.2021.628156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruce KD, Gorkhali S, Given K, Coates AM, Boyle KE, Macklin WB, et al. Lipoprotein lipase is a feature of alternatively-activated microglia and may facilitate lipid uptake in the CNS during demyelination. Front Mol Neurosci. 2018;11:57. doi: 10.3389/fnmol.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17(1):7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 27.Llufriu-Dabén G, Carrete A, Chierto E, Mailleux J, Camand E, Simon A, et al. Targeting demyelination via α-secretases promoting sAPPα release to enhance remyelination in central nervous system. Neurobiol Dis. 2018;109:11–24. doi: 10.1016/j.nbd.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Ray B, Maloney B, Sambamurti K, Karnati HK, Nelson PT, Greig NH, et al. Rivastigmine modifies the α-secretase pathway and potentially early Alzheimer’s disease. Transl Psychiatry. 2020;10(1):1–17. doi: 10.1038/s41398-020-0709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui X, Guo Y-E, Fang J-H, Shi C-J, Suo N, Zhang R, et al. Donepezil, a drug for Alzheimer’s disease, promotes oligodendrocyte generation and remyelination. Acta Pharmacol Sin. 2019;40(11):1386–1393. doi: 10.1038/s41401-018-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. β-Secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 31.Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9(12):1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 32.Kataria H, Alizadeh A, Karimi-Abdolrezaee S. Neuregulin-1/ErbB network: an emerging modulator of nervous system injury and repair. Prog Neurobiol. 2019;180:101643. doi: 10.1016/j.pneurobio.2019.101643. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen T-VV, Hayes M, Zbesko JC, Frye JB, Congrove NR, Belichenko NP, et al. Alzheimer’s associated amyloid and tau deposition co-localizes with a homeostatic myelin repair pathway in two mouse models of post-stroke mixed dementia. Acta Neuropathol Commun. 2018;6(1):1–25. doi: 10.1186/s40478-018-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, et al. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22(8):2970–2980. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu X, Hu J, Dai L, Trapp B, Yan R. Axonal and Schwann cell BACE1 is equally required for remyelination of peripheral nerves. J Neurosci. 2015;35(9):3806–3814. doi: 10.1523/JNEUROSCI.5207-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo X, Prior M, He W, Hu X, Tang X, Shen W, et al. Cleavage of neuregulin-1 by BACE1 or ADAM10 protein produces differential effects on myelination. J Biol Chem. 2011;286(27):23967–23974. doi: 10.1074/jbc.M111.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu X, Schlanger R, He W, Macklin WB, Yan R. Reversing hypomyelination in BACE1-null mice with Akt-DD overexpression. FASEB J. 2013;27(5):1868–1873. doi: 10.1096/fj.12-224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, et al. Modulation of γ-secretase reduces β-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Neuron. 2010;67(5):769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dovey H, John V, Anderson J, Chen L, de Saint AP, Fang L, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76(1):173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 40.Okubo T, Nagoshi N, Kohyama J, Tsuji O, Shinozaki M, Shibata S, et al. Treatment with a gamma-secretase inhibitor promotes functional recovery in human iPSC-derived transplants for chronic spinal cord injury. Stem Cell Rep. 2018;11(6):1416–1432. doi: 10.1016/j.stemcr.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jurynczyk M, Jurewicz A, Bielecki B, Raine CS, Selmaj K. Overcoming failure to repair demyelination in EAE: γ-secretase inhibition of Notch signaling. J Neurol Sci. 2008;265(1–2):5–11. doi: 10.1016/j.jns.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 42.McQueen J, Reimer MM, Holland PR, Manso Y, McLaughlin M, Fowler JH, et al. Restoration of oligodendrocyte pools in a mouse model of chronic cerebral hypoperfusion. PLoS ONE. 2014;9(2):e87227. doi: 10.1371/journal.pone.0087227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai MK, Sudol KL, Janelsins MC, Mastrangelo MA, Frazer ME, Bowers WJ. Triple-transgenic Alzheimer's disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia. 2009;57(1):54–65. doi: 10.1002/glia.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian J, Shi J, Bailey K, Mann D. Relationships between arteriosclerosis, cerebral amyloid angiopathy and myelin loss from cerebral cortical white matter in Alzheimer's disease. Neuropathol Appl Neurobiol. 2004;30(1):46–56. doi: 10.1046/j.0305-1846.2003.00510.x. [DOI] [PubMed] [Google Scholar]
- 45.Kaya I, Jennische E, Lange S, Tarik Baykal A, Malmberg P, Fletcher JS. Brain region-specific amyloid plaque-associated myelin lipid loss, APOE deposition and disruption of the myelin sheath in familial Alzheimer’s disease mice. J Neurochem. 2020;154(1):84–98. doi: 10.1111/jnc.14999. [DOI] [PubMed] [Google Scholar]
- 46.Troutwine BR, Hamid L, Lysaker CR, Strope TA, Wilkins HM. Apolipoprotein E and Alzheimer's disease. Acta Pharm Sin B. 2021;12(2):496–510. doi: 10.1016/j.apsb.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaillard O, Gervais A, Meillet D, Plassart E, Fontaine B, Lyon-Caen O, et al. Apolipoprotein E and multiple sclerosis: a biochemical and genetic investigation. J Neurol Sci. 1998;158(2):180–186. doi: 10.1016/s0022-510x(98)00118-x. [DOI] [PubMed] [Google Scholar]
- 48.Rafiei M, Yeganeh MZ, Sheikholeslami S, Gozalpour E, Ghaffarpour M, Hedayati M. Apolipoprotein E polymorphisms status in Iranian patients with multiple sclerosis. J Neurol Sci. 2012;320(1–2):22–25. doi: 10.1016/j.jns.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 49.Ryan L, Walther K, Bendlin BB, Lue L-F, Walker DG, Glisky EL. Age-related differences in white matter integrity and cognitive function are related to APOE status. Neuroimage. 2011;54(2):1565–1577. doi: 10.1016/j.neuroimage.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Remer J, Dean DC, III, Chen K, Reiman RA, Huentelman MJ, Reiman EM, et al. Longitudinal white matter and cognitive development in pediatric carriers of the apolipoprotein ε4 allele. Neuroimage. 2020;222:117243. doi: 10.1016/j.neuroimage.2020.117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carlin C, Murray L, Graham D, Doyle D, Nicoll J. Involvement of apolipoprotein E in multiple sclerosis: absence of remyelination associated with possession of the APOE ε2 allele. J Neuropathol Exp Neurol. 2000;59(5):361–367. doi: 10.1093/jnen/59.5.361. [DOI] [PubMed] [Google Scholar]
- 52.Corder E, Saunders AM, Risch N, Strittmatter W, Schmechel D, Gaskell P, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7(2):180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 53.Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE ɛ2. Neurosci Biobehav Rev. 2013;37(10):2878–2886. doi: 10.1016/j.neubiorev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Karussis D, Michaelson D, Grigoriadis N, Korezyn A, Mizrachi-Koll R, Chapman S, et al. Lack of apolipoprotein-E exacerbates experimental allergic encephalomyelitis. Mult Scler J. 2003;9(5):476–480. doi: 10.1191/1352458503ms950oa. [DOI] [PubMed] [Google Scholar]
- 55.Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil M-T, Su M, Sen P, et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science. 2018;359(6376):684–688. doi: 10.1126/science.aan4183. [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Smith JD. ABCA1 and nascent HDL biogenesis. BioFactors. 2014;40(6):547–554. doi: 10.1002/biof.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao G-J, Yin K, Fu Y-C, Tang C-K. The interaction of ApoA-I and ABCA1 triggers signal transduction pathways to mediate efflux of cellular lipids. Mol Med. 2012;18(2):149–158. doi: 10.2119/molmed.2011.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santamarina-Fojo S, Remaley AT, Neufeld EB, Brewer HB. Regulation and intracellular trafficking of the ABCA1 transporter. J Lipid Res. 2001;42(9):1339–1345. [PubMed] [Google Scholar]
- 59.Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, et al. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem. 2004;279(39):41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- 60.Schmitz G, Langmann T. Structure, function and regulation of the ABC1 gene product. Curr Opin Lipidol. 2001;12(2):129–140. doi: 10.1097/00041433-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Li L, Li R, Zacharek A, Wang F, Landschoot-Ward J, Chopp M, et al. ABCA1/APOE/HDL signaling pathway facilitates myelination and oligodendrogenesis after stroke. Int J Mol Sci. 2020;21(12):4369. doi: 10.3390/ijms21124369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolfe CM, Fitz NF, Nam KN, Lefterov I, Koldamova R. The role of APOE and TREM2 in Alzheimer′ s disease—current understanding and perspectives. Int J Mol Sci. 2018;20(1):81. doi: 10.3390/ijms20010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raha AA, Henderson JW, Stott SR, Vuono R, Foscarin S, Friedland RP, et al. Neuroprotective effect of TREM-2 in aging and Alzheimer’s disease model. J Alzheimers Dis. 2017;55(1):199–217. doi: 10.3233/JAD-160663. [DOI] [PubMed] [Google Scholar]
- 65.Gouna G, Klose C, Bosch-Queralt M, Liu L, Gokce O, Schifferer M, et al. TREM2-dependent lipid droplet biogenesis in phagocytes is required for remyelination. J Exp Med. 2021;218(10):e20210227. doi: 10.1084/jem.20210227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4(4):e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petković F, Campbell IL, Gonzalez B, Castellano B. Astrocyte-targeted production of interleukin-6 reduces astroglial and microglial activation in the cuprizone demyelination model: Implications for myelin clearance and oligodendrocyte maturation. Glia. 2016;64(12):2104–2119. doi: 10.1002/glia.23043. [DOI] [PubMed] [Google Scholar]
- 68.Safaiyan S, Besson-Girard S, Kaya T, Cantuti-Castelvetri L, Liu L, Ji H, et al. White matter aging drives microglial diversity. Neuron. 2021;109(7):1100–1117. doi: 10.1016/j.neuron.2021.01.027. [DOI] [PubMed] [Google Scholar]
- 69.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169(7):1276–90.e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 70.Baum L, Chen L, Masliah E, Chan YS, Ng HK, Pang CP. Lipoprotein lipase mutations and Alzheimer's disease. Am J Med Genet. 1999;88(2):136–139. doi: 10.1002/(sici)1096-8628(19990416)88:2<136::aid-ajmg8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 71.Pedrini S, Chatterjee P, Hone E, Martins RN. High-density lipoprotein-related cholesterol metabolism in Alzheimer’s disease. J Neurochem. 2021;159(2):343–377. doi: 10.1111/jnc.15170. [DOI] [PubMed] [Google Scholar]
- 72.Blain JF, Aumont N, Théroux L, Dea D, Poirier J. A polymorphism in lipoprotein lipase affects the severity of Alzheimer's disease pathophysiology. Eur J Neurosci. 2006;24(5):1245–1251. doi: 10.1111/j.1460-9568.2006.05007.x. [DOI] [PubMed] [Google Scholar]
- 73.Wies Mancini VSB, Pasquini JM, Correale JD, Pasquini LA. Microglial modulation through colony-stimulating factor-1 receptor inhibition attenuates demyelination. Glia. 2019;67(2):291–308. doi: 10.1002/glia.23540. [DOI] [PubMed] [Google Scholar]
- 74.Nelissen K, Mulder M, Smets I, Timmermans S, Smeets K, Ameloot M, et al. Liver X receptors regulate cholesterol homeostasis in oligodendrocytes. J Neurosci Res. 2012;90(1):60–71. doi: 10.1002/jnr.22743. [DOI] [PubMed] [Google Scholar]
- 75.Santos-Gil DF, Arboleda G, Sandoval-Hernández AG. Retinoid X receptor activation promotes re-myelination in a very old triple transgenic mouse model of Alzheimer’s disease. Neurosci Lett. 2021;750:135764. doi: 10.1016/j.neulet.2021.135764. [DOI] [PubMed] [Google Scholar]
- 76.Li F-Q, Fowler KA, Neil JE, Colton CA, Vitek MP. An apolipoprotein E-mimetic stimulates axonal regeneration and remyelination after peripheral nerve injury. J Pharmacol Exp Ther. 2010;334(1):106–115. doi: 10.1124/jpet.110.167882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaultier A, Wu X, Le Moan N, Takimoto S, Mukandala G, Akassoglou K, et al. Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis. J Cell Sci. 2009;122(8):1155–1162. doi: 10.1242/jcs.040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song S, Yu L, Hasan MN, Paruchuri SS, Mullett SJ, Sullivan ML, et al. Elevated microglial oxidative phosphorylation and phagocytosis stimulate post-stroke brain remodeling and cognitive function recovery in mice. Commun Biol. 2022;5(1):1–15. doi: 10.1038/s42003-021-02984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jackson J, Bianco G, Rosa AO, Cowan K, Bond P, Anichtchik O, et al. White matter tauopathy: transient functional loss and novel myelin remodeling. Glia. 2018;66(4):813–827. doi: 10.1002/glia.23286. [DOI] [PubMed] [Google Scholar]
- 80.Ossola B, Zhao C, Compston A, Pluchino S, Franklin RJ, Spillantini MG. Neuronal expression of pathological tau accelerates oligodendrocyte progenitor cell differentiation. Glia. 2016;64(3):457–471. doi: 10.1002/glia.22940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fressinaud C, Berges R, Eyer J. Axon cytoskeleton proteins specifically modulate oligodendrocyte growth and differentiation in vitro. Neurochem Int. 2012;60(1):78–90. doi: 10.1016/j.neuint.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 82.Belkadi A, LoPresti P. Truncated Tau with the Fyn-binding domain and without the microtubule-binding domain hinders the myelinating capacity of an oligodendrocyte cell line. J Neurochem. 2008;107(2):351–360. doi: 10.1111/j.1471-4159.2008.05600.x. [DOI] [PubMed] [Google Scholar]
- 83.Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, et al. Phosphorylation of tau by fyn: implications for Alzheimer's disease. J Neurosci. 2004;24(9):2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu Y, Yang Y, Shi J, Bishayee K, Lin L, Lin Y, et al. Acori tatarinowii rhizoma extract ameliorates Alzheimer's pathological syndromes by repairing myelin injury and lowering Tau phosphorylation in mice. Pharmazie. 2020;75(8):395–400. doi: 10.1691/ph.2020.0492. [DOI] [PubMed] [Google Scholar]
- 85.Hochmeister S, Romauch M, Bauer J, Seifert-Held T, Weissert R, Linington C, et al. Re-expression of N-cadherin in remyelinating lesions of experimental inflammatory demyelination. Exp Neurol. 2012;237(1):70–77. doi: 10.1016/j.expneurol.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 86.Liu SQ, Zhang ML, Zhang HJ, Liu FZ, Chu RJ, Zhang GX, et al. Matrine promotes oligodendrocyte development in CNS autoimmunity through the PI3K/Akt signaling pathway. Life Sci. 2017;180:36–41. doi: 10.1016/j.lfs.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 87.Kopec BM, Kiptoo P, Zhao L, Rosa-Molinar E, Siahaan TJ. Noninvasive brain delivery and efficacy of BDNF to stimulate neuroregeneration and suppression of disease relapse in EAE mice. Mol Pharm. 2019;17(2):404–416. doi: 10.1021/acs.molpharmaceut.9b00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qin G, Wang Y, Liu Z, Mana L, Huang S, Wang P. Shenzhiling oral solution promotes myelin repair through PI3K/Akt-mTOR pathway in STZ-induced SAD mice. 3 Biotech. 2021;11(7):1–15. doi: 10.1007/s13205-021-02900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Imamura O, Arai M, Dateki M, Takishima K. Donepezil promotes differentiation of neural stem cells into mature oligodendrocytes at the expense of astrogenesis. J Neurochem. 2017;140(2):231–244. doi: 10.1111/jnc.13856. [DOI] [PubMed] [Google Scholar]
- 90.Rivera A, Pieropan F, Williams G, Calzolari F, Butt A, Azim K. Drug connectivity mapping and functional analysis reveal therapeutic small molecules that differentially modulate myelination. Biomed Pharmacother. 2022;145:112436. doi: 10.1016/j.biopha.2021.112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie YY, Pan TT, Xu DE, Huang X, Tang Y, Huang W, et al. Clemastine ameliorates myelin deficits via preventing senescence of oligodendrocytes precursor cells in Alzheimer’s disease model mouse. Front Cell Dev Biol. 2021;9:733945. doi: 10.3389/fcell.2021.733945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen J-F, Liu K, Hu B, Li R-R, Xin W, Chen H, et al. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron. 2021;109(14):2292–2307. doi: 10.1016/j.neuron.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci. 2019;22(5):719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Liu B, Xu Y, Yang M, Wang C, Song M, et al. Activation of CREB-mediated autophagy by thioperamide ameliorates β-amyloid pathology and cognition in Alzheimer’s disease. Aging Cell. 2021;20(3):e13333. doi: 10.1111/acel.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Falkenstein M, Reiner-Link D, Zivkovic A, Gering I, Willbold D, Stark H. Histamine H3 receptor antagonists with peptidomimetic (keto) piperazine structures to inhibit Aβ oligomerisation. Bioorg Med Chem. 2021;50:116462. doi: 10.1016/j.bmc.2021.116462. [DOI] [PubMed] [Google Scholar]
- 96.Alachkar A, Łażewska D, Kieć-Kononowicz K, Sadek B. The histamine H3 receptor antagonist E159 reverses memory deficits induced by dizocilpine in passive avoidance and novel object recognition paradigm in rats. Front Pharmacol. 2017;8:709. doi: 10.3389/fphar.2017.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y, Zhen W, Guo T, Zhao Y, Liu A, Rubio JP, et al. Histamine receptor 3 negatively regulates oligodendrocyte differentiation and remyelination. PLoS ONE. 2017;12(12):e0189380. doi: 10.1371/journal.pone.0189380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baron M. An overview of the notch signalling pathway. Semin Cell Dev Biol. 2003;14(2):113–119. doi: 10.1016/s1084-9521(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 99.Kapoor A, Nation DA. Role of notch signaling in neurovascular aging and Alzheimer's disease. Semin Cell Dev Biol. 2021;116:90–97. doi: 10.1016/j.semcdb.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Green AJ, Gelfand JM, Cree BA, Bevan C, Boscardin WJ, Mei F, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481–2489. doi: 10.1016/S0140-6736(17)32346-2. [DOI] [PubMed] [Google Scholar]
- 101.Park MH, Park KH, Choi BJ, Han WH, Yoon HJ, Jung HY, et al. Discovery of a dual-action small molecule that improves neuropathological features of Alzheimer’s disease mice. Proc Natl Acad Sci U S A. 2022;119(3):e2115082119. doi: 10.1073/pnas.2115082119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chami M, Halmer R, Schnoeder L, Anne Becker K, Meier C, Fassbender K, et al. Acid sphingomyelinase deficiency enhances myelin repair after acute and chronic demyelination. PLoS ONE. 2017;12(6):e0178622. doi: 10.1371/journal.pone.0178622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beckmann N, Sharma D, Gulbins E, Becker KA, Edelmann B. Inhibition of acid sphingomyelinase by tricyclic antidepressants and analogons. Front Physiol. 2014;5:331. doi: 10.3389/fphys.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J, Buller BA, Zhang ZG, Zhang Y, Lu M, Rosene DL, et al. Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp Neurol. 2022;347:113895. doi: 10.1016/j.expneurol.2021.113895. [DOI] [PubMed] [Google Scholar]
- 105.Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T, et al. A potential function for neuronal exosomes: sequestering intracerebral amyloid-β peptide. FEBS Lett. 2015;589(1):84–88. doi: 10.1016/j.febslet.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 106.Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J Biol Chem. 2012;287(14):10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sainaghi PP, Bellan M, Lombino F, Alciato F, Carecchio M, Galimberti D, et al. Growth arrest specific 6 concentration is increased in the cerebrospinal fluid of patients with Alzheimer’s disease. J Alzheimers Dis. 2017;55(1):59–65. doi: 10.3233/JAD-160599. [DOI] [PubMed] [Google Scholar]
- 108.Owlett LD, Karaahmet B, Le L, Belcher EK, Dionisio-Santos D, Olschowka JA, et al. Gas6 induces inflammation and reduces plaque burden but worsens behavior in a sex-dependent manner in the APP/PS1 model of Alzheimer’s disease. J Neuroinflamm. 2022;19(1):1–17. doi: 10.1186/s12974-022-02397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsiperson V, Li X, Schwartz GJ, Raine CS, Shafit-Zagardo B. GAS6 enhances repair following cuprizone-induced demyelination. PLoS ONE. 2010;5(12):e15748. doi: 10.1371/journal.pone.0015748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang Y, Happonen KE, Burrola PG, O’Connor C, Hah N, Huang L, et al. Microglia use TAM receptors to detect and engulf amyloid β plaques. Nat Immunol. 2021;22(5):586–594. doi: 10.1038/s41590-021-00913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 112.Dubal DB, Zhu L, Sanchez PE, Worden K, Broestl L, Johnson E, et al. Life extension factor klotho prevents mortality and enhances cognition in hAPP transgenic mice. J Neurosci. 2015;35(6):2358–2371. doi: 10.1523/JNEUROSCI.5791-12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Neitzel J, Franzmeier N, Rubinski A, Dichgans M, Brendel M, Malik R, et al. KL-VS heterozygosity is associated with lower amyloid-dependent tau accumulation and memory impairment in Alzheimer’s disease. Nat Commun. 2021;12(1):1–12. doi: 10.1038/s41467-021-23755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Belloy ME, Eger SJ, Le Guen Y, Napolioni V, Deters KD, Yang H-S, et al. KL∗ VS heterozygosity reduces brain amyloid in asymptomatic at-risk APOE∗ 4 carriers. Neurobiol Aging. 2021;101:123–129. doi: 10.1016/j.neurobiolaging.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zeldich E, Chen C-D, Avila R, Medicetty S, Abraham CR. The anti-aging protein Klotho enhances remyelination following cuprizone-induced demyelination. J Mol Neurosci. 2015;57(2):185–196. doi: 10.1007/s12031-015-0598-2. [DOI] [PubMed] [Google Scholar]
- 116.Chen C-D, Li H, Liang J, Hixson K, Zeldich E, Abraham CR. The anti-aging and tumor suppressor protein Klotho enhances differentiation of a human oligodendrocytic hybrid cell line. J Mol Neurosci. 2015;55(1):76–90. doi: 10.1007/s12031-014-0336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen C-D, Sloane JA, Li H, Aytan N, Giannaris EL, Zeldich E, et al. The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J Neurosci. 2013;33(5):1927–1939. doi: 10.1523/JNEUROSCI.2080-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen C-D, Zeldich E, Li Y, Yuste A, Abraham CR. Activation of the anti-aging and cognition-enhancing gene klotho by CRISPR-dCas9 transcriptional effector complex. J Mol Neurosci. 2018;64(2):175–184. doi: 10.1007/s12031-017-1011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ibrahim M, Haleem M, AbdelWahab S, Abdel-Aziz A. Sildenafil ameliorates Alzheimer disease via the modulation of vascular endothelial growth factor and vascular cell adhesion molecule-1 in rats. Hum Exp Toxicol. 2021;40(4):596–607. doi: 10.1177/0960327120960775. [DOI] [PubMed] [Google Scholar]
- 120.Pifarre P, Prado J, Baltrons MA, Giralt M, Gabarro P, Feinstein DL, et al. Sildenafil (Viagra) ameliorates clinical symptoms and neuropathology in a mouse model of multiple sclerosis. Acta Neuropathol. 2011;121(4):499–508. doi: 10.1007/s00401-010-0795-6. [DOI] [PubMed] [Google Scholar]
- 121.Pifarré P, Gutierrez-Mecinas M, Prado J, Usero L, Roura-Mir C, Giralt M, et al. Phosphodiesterase 5 inhibition at disease onset prevents experimental autoimmune encephalomyelitis progression through immunoregulatory and neuroprotective actions. Exp Neurol. 2014;251:58–71. doi: 10.1016/j.expneurol.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 122.de Santana Nunes AK, Rapôso C, de Almeida Luna RL, da Cruz-Höfling MA, Peixoto CA. Sildenafil (Viagra®) down regulates cytokines and prevents demyelination in a cuprizone-induced MS mouse model. Cytokine. 2012;60(2):540–551. doi: 10.1016/j.cyto.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 123.Muñoz-Esquivel J, Göttle P, Aguirre-Cruz L, Flores-Rivera J, Corona T, Reyes-Terán G, et al. Sildenafil inhibits myelin expression and myelination of oligodendroglial precursor cells. ASN Neuro. 2019;11:1759091419832444. doi: 10.1177/1759091419832444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chang Y, Yao Y, Ma R, Wang Z, Hu J, Wu Y, et al. Dl-3-n-butylphthalide reduces cognitive deficits and alleviates neuropathology in P301S tau transgenic mice. Front Neurosci. 2021;15:17. doi: 10.3389/fnins.2021.620176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheng X, Wang H, Liu C, Zhong S, Niu X, Zhang X, et al. Dl-3-n-butylphthalide promotes remyelination process in cerebral white matter in rats subjected to ischemic stroke. Brain Res. 2019;1717:167–175. doi: 10.1016/j.brainres.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 126.Pernet V, Joly S, Christ F, Dimou L, Schwab ME. Nogo-A and myelin-associated glycoprotein differently regulate oligodendrocyte maturation and myelin formation. J Neurosci. 2008;28(29):7435–7444. doi: 10.1523/JNEUROSCI.0727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Laat R, Meabon JS, Wiley JC, Hudson MP, Montine TJ, Bothwell M. LINGO-1 promotes lysosomal degradation of amyloid-β protein precursor. Pathobiol Aging Age Relat Dis. 2015;5(1):25796. doi: 10.3402/pba.v5.25796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lau S-F, Cao H, Fu AK, Ip NY. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2020;117(41):25800–25809. doi: 10.1073/pnas.2008762117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Y, Zhang YP, Pepinsky B, Huang G, Shields LB, Shields CB, et al. Inhibition of LINGO-1 promotes functional recovery after experimental spinal cord demyelination. Exp Neurol. 2015;266:68–73. doi: 10.1016/j.expneurol.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 130.Mi S, Sandrock A, Miller RH. LINGO-1 and its role in CNS repair. Int J Biochem Cell Biol. 2008;40(10):1971–1978. doi: 10.1016/j.biocel.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 131.Andrews JL, Fernandez-Enright F. A decade from discovery to therapy: Lingo-1, the dark horse in neurological and psychiatric disorders. Neurosci Biobehav Rev. 2015;56:97–114. doi: 10.1016/j.neubiorev.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 132.Wu D, Tang X, Gu LH, Li XL, Qi XY, Bai F, et al. LINGO-1 antibody ameliorates myelin impairment and spatial memory deficits in the early stage of 5 XFAD mice. CNS Neurosci Ther. 2018;24(5):381–393. doi: 10.1111/cns.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang H, Jiang L, Zhang Y, Liang X, Tang J, He Q, et al. Anti-LINGO-1 antibody treatment alleviates cognitive deficits and promotes maturation of oligodendrocytes in the hippocampus of APP/PS1 mice. J Comp Neurol. 2022;530(10):1606–1621. doi: 10.1002/cne.25299. [DOI] [PubMed] [Google Scholar]
- 134.Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019;570(7761):332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuboyama K, Fujikawa A, Suzuki R, Noda M. Inactivation of protein tyrosine phosphatase receptor type Z by pleiotrophin promotes remyelination through activation of differentiation of oligodendrocyte precursor cells. J Neurosci. 2015;35(35):12162–12171. doi: 10.1523/JNEUROSCI.2127-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chow JPH, Fujikawa A, Shimizu H, Suzuki R, Noda M. Metalloproteinase-and γ-secretase-mediated cleavage of protein-tyrosine phosphatase receptor type Z. J Biol Chem. 2008;283(45):30879–30889. doi: 10.1074/jbc.M802976200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tran D, DiGiacomo P, Georgiadis M, Zeineh M. Iron and Alzheimer’s disease: from pathology to imaging. Front Hum Neurosci. 2022;16:838692. doi: 10.3389/fnhum.2022.838692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gleason A, Bush AI. Iron and ferroptosis as therapeutic targets in Alzheimer’s disease. Neurotherapeutics. 2021;18(1):252–264. doi: 10.1007/s13311-020-00954-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Peng Y, Chang X, Lang M. Iron homeostasis disorder and Alzheimer’s disease. Int J Mol Sci. 2021;22(22):12442. doi: 10.3390/ijms222212442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khattar N, Triebswetter C, Kiely M, Ferrucci L, Resnick SM, Spencer RG, et al. Investigation of the association between cerebral iron content and myelin content in normative aging using quantitative magnetic resonance neuroimaging. Neuroimage. 2021;239:118267. doi: 10.1016/j.neuroimage.2021.118267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Möller HE, Bossoni L, Connor JR, Crichton RR, Does MD, Ward RJ, et al. Iron, myelin, and the brain: neuroimaging meets neurobiology. Trends Neurosci. 2019;42(6):384–401. doi: 10.1016/j.tins.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 142.Spotorno N, Acosta-Cabronero J, Stomrud E, Lampinen B, Strandberg OT, van Westen D, et al. Relationship between cortical iron and tau aggregation in Alzheimer’s disease. Brain. 2020;143(5):1341–1349. doi: 10.1093/brain/awaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schonberg DL, Goldstein EZ, Sahinkaya FR, Wei P, Popovich PG, McTigue DM. Ferritin stimulates oligodendrocyte genesis in the adult spinal cord and can be transferred from macrophages to NG2 cells in vivo. J Neurosci. 2012;32(16):5374–5384. doi: 10.1523/JNEUROSCI.3517-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang X, Surguladze N, Slagle-Webb B, Cozzi A, Connor JR. Cellular iron status influences the functional relationship between microglia and oligodendrocytes. Glia. 2006;54(8):795–804. doi: 10.1002/glia.20416. [DOI] [PubMed] [Google Scholar]
- 145.Todorich B, Zhang X, Slagle-Webb B, Seaman WE, Connor JR. Tim-2 is the receptor for H-ferritin on oligodendrocytes. J Neurochem. 2008;107(6):1495–1505. doi: 10.1111/j.1471-4159.2008.05678.x. [DOI] [PubMed] [Google Scholar]
- 146.Wan R, Cheli VT, Santiago-González DA, Rosenblum SL, Wan Q, Paez PM. Impaired postnatal myelination in a conditional knockout mouse for the ferritin heavy chain in oligodendroglial cells. J Neurosci. 2020;40(40):7609–7624. doi: 10.1523/JNEUROSCI.1281-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheli VT, González DAS, Marziali LN, Zamora NN, Guitart ME, Spreuer V, et al. The divalent metal transporter 1 (DMT1) is required for iron uptake and normal development of oligodendrocyte progenitor cells. J Neurosci. 2018;38(43):9142–9159. doi: 10.1523/JNEUROSCI.1447-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wong BX, Tsatsanis A, Lim LQ, Adlard PA, Bush AI, Duce JA. β-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS ONE. 2014;9(12):e114174. doi: 10.1371/journal.pone.0114174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schulz K, Kroner A, David S. Iron efflux from astrocytes plays a role in remyelination. J Neurosci. 2012;32(14):4841–4847. doi: 10.1523/JNEUROSCI.5328-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zheng W, Xin N, Chi ZH, Zhao BL, Zhang J, Li JY, et al. Divalent metal transporter 1 is involved in amyloid precursor protein processing and Aβ generation. FASEB J. 2009;23(12):4207–4217. doi: 10.1096/fj.09-135749. [DOI] [PubMed] [Google Scholar]
- 151.Jahshan A, Esteves-Villanueva JO, Martic-Milne S. Evaluation of ferritin and transferrin binding to tau protein. J Inorg Biochem. 2016;162:127–134. doi: 10.1016/j.jinorgbio.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 152.Hoshi K, Ito H, Abe E, Fuwa TJ, Kanno M, Murakami Y, et al. Transferrin biosynthesized in the brain is a novel biomarker for Alzheimer’s disease. Metabolites. 2021;11(9):616. doi: 10.3390/metabo11090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Carden TR, Correale J, Pasquini JM, Pérez MJ. Transferrin enhances microglial phagocytic capacity. Mol Neurobiol. 2019;56(9):6324–6340. doi: 10.1007/s12035-019-1519-0. [DOI] [PubMed] [Google Scholar]
- 154.Adamo A, Paez P, Cabrera OE, Wolfson M, Franco P, Pasquini J, et al. Remyelination after cuprizone-induced demyelination in the rat is stimulated by apotransferrin. Exp Neurol. 2006;198(2):519–529. doi: 10.1016/j.expneurol.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 155.Nuñez MT, Chana-Cuevas P. New perspectives in iron chelation therapy for the treatment of neurodegenerative diseases. Pharmaceuticals. 2018;11(4):109. doi: 10.3390/ph11040109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7(9):779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 157.Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Aβ amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol. 2003;60(12):1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 158.Pernber Z, Blennow K, Bogdanovic N, Månsson J-E, Blomqvist M. Altered distribution of the gangliosides GM1 and GM2 in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;33(2–3):174–188. doi: 10.1159/000338181. [DOI] [PubMed] [Google Scholar]
- 159.Qiu S, Palavicini JP, Wang J, Gonzalez NS, He S, Dustin E, et al. Adult-onset CNS myelin sulfatide deficiency is sufficient to cause Alzheimer’s disease-like neuroinflammation and cognitive impairment. Mol Neurodegen. 2021;16(1):1–24. doi: 10.1186/s13024-021-00488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yanagisawa K. GM1 ganglioside and Alzheimer’s disease. Glycoconj J. 2015;32(3):87–91. doi: 10.1007/s10719-015-9579-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.