Abstract
The fishery industry generates large amounts of waste (20–75% (w/w) of the total caught fish weight). The recovery of bioactive compounds from residues and their incorporation in cosmetics represents a promising market opportunity and may contribute to a sustainable valorisation of the sector. In this work, protein-rich extracts obtained by high-pressure technologies (supercritical CO2 and subcritical water) from sardine (Sardina pilchardus) waste and codfish (Gadus morhua) frames were characterized regarding their cosmeceutical potential. Antioxidant, anti-inflammatory and antibacterial activities were evaluated through chemical (ORAC assay), enzymatic (inhibition of elastase and tyrosinase), antimicrobial susceptibility (Klebsiella pneumoniae, Staphylococcus aureus and Cutibacterium acnes) and cell-based (in keratinocytes-HaCaT) assays. Sardine extracts presented the highest antibacterial activity, and the extract obtained using higher extraction temperatures (250 °C) and without the defatting step demonstrated the lowest minimum inhibitory concentration (MIC) values (1.17; 4.6; 0.59 mg/mL for K. pneumoniae, S. aureus and C. acnes, respectively). Codfish samples extracted at lower temperatures (90 °C) were the most effective anti-inflammatory agents (a concentration of 0.75 mg/mL reduced IL-8 and IL-6 levels by 58% and 47%, respectively, relative to the positive control). Threonine, valine, leucine, arginine and total protein content in the extracts were highlighted to present a high correlation with the reported bioactivities (R2 ≥ 0.7). These results support the potential application of extracts obtained from fishery industry wastes in cosmeceutical products with bioactive activities.
Keywords: fish waste streams valorisation, antioxidant activity, anti-inflammatory activity, antimicrobial activity, anti-ageing, anti-hyperpigmentation, cosmeceuticals
1. Introduction
With the constant search for innovation, especially for active ingredients, the cosmetic industry is growing and has demonstrated the intention to replace petroleum-derived components moving forward toward natural compounds [1]. The antioxidant properties of natural active ingredients can help in the prevention of several skin issues caused by oxidative stress and ageing [2,3]. Skin ageing can be induced by both intrinsic (such as inflammation or telomere shortening) and extrinsic (environmental) factors [4]. Skin ageing leads to the loss of mature collagen and alterations at the extracellular matrix (ECM) which compromises the barrier function, resulting in a dry appearance and susceptibility to external aggressors, increasing the risk for skin disorders [5]. This process can be accelerated by several enzymes, such as elastases, matrix metalloproteinases (MMPs) and hyaluronidases that can induce ECM degradation [6], or even by the accumulation of excessive reactive oxygen species (ROS) that can compromise the normal cell function [7]. Environmental factors, such as exposure to UV radiation, leads to the generation of high quantities of ROS that induces the same molecular and cellular responses as intrinsic ageing, but with amplified effects. Importantly, ROS can intensify the activity of enzymes related to skin ageing or skin pigmentation processes [8,9], and thus the presence of antioxidants can play an important role in the cosmetic field.
In 2018, world fish consumption was estimated by FAO to stand at 20.5 kg per capita [10], which leads to large quantities of by-products, mostly skin and bones. The generated residues correspond to 20–75% (w/w) of the total caught fish weight, potentially leading to environmental problems [11,12]. However, these residues still contain a significant amount of lipids, proteins, and minerals and should be adequately valorised. In recent years, extracts derived from waste generated by the fish industry have shown bioactive properties such as antihypertensive, antioxidative, antimicrobial, neuroprotective, antihyperglycemic, anti-ageing, and anti-inflammatory [13,14,15,16,17,18,19]. Atlantic codfish (Gadus morhua) and sardine (Sardina pilchardus) are among the most consumed fish in Portugal and extracts derived from its residues have shown promising nutraceutical potential, such as antioxidant, antiproliferative or anti-inflammatory activities [11,20,21,22]. However, since the exploitation and valorisation of fish industry wastes is still in an early stage, there is plenty of room to explore opportunities for the industry to convert this waste into high-value market bioproducts, including cosmetic ingredients.
In a previous work, we explored the use of high-pressure technologies (supercritical CO2 and subcritical water), to isolate bioactive fractions from sardine waste and codfish frames with promising health benefits [11,22]. For sardine wastes, we demonstrated that by applying a first step with supercritical carbon (ScCO2) (to remove lipid fraction) followed by an extraction process with subcritical water (SW) it was possible to obtain protein hydrolysates with high antioxidant potential and antiproliferative effect in colorectal cancer cells [22]. Subcritical water extraction/hydrolysis were also applied to obtain proteins-, peptides- and amino acid-enriched extracts from codfish frames and we showed that lower processing temperatures (90 °C) favour the extraction of compounds with anti-inflammatory potential in a human intestinal epithelial cell model [11]. Most of the proteins present in codfish frames extracts were collagen and collagen fragments. Other compounds include minor quantities of lipids, ash and some sugars. Sardine extracts were rich in peptides and amino acids, and lipids, ash and sugars were also present. Since fish-derived proteins and peptides may become an important resource for cosmetic industries, the present study aims to further evaluate the bioactive potential of these extracts derived from fish-processing wastes and by-products focused on the assessment of their cosmeceutical potential [23]. For this purpose, a range of chemical, enzymatic, and cell-based assays were applied to explore the antioxidant, anti-ageing, anti-hyperpigmentation, anti-inflammatory, and antimicrobial effects of the extracted samples. Correlation studies were also performed to identify the main bioactive constituents with cosmeceutical potential.
2. Materials and Methods
2.1. Reagents
3,4-dihydroxy-l-phenylalanine (L-DOPA), mushroom tyrosinase, porcine pancreatic elastase (PPE) type III, N-succinyl-Ala-Ala-Ala-p-nitroanilide (AAAPVN), Tris (2-amino-2-hydroxymethyl-propane-1,3-diol), 2,2′-azobis (2-methylpropionamidine)dihydrochlo-ride (AAPH), and 2′,7′-dichlorofluorescein diacetate (DCFH-DA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Calcium-adjusted Mueller Hinton broth (CAMHB) was purchased from BD (Sparks, MD, USA). Brain-heart infusion (BHI) was purchased from Avantor (Radnor, PA, USA). AnaeroGen™ Compact sachets were purchased from Oxoid (Hampshire, UK). PrestoBlue™, Dulbecco’s Modified Eagle Medium (DMEM), heat-inactivated Fetal Bovine Serum (FBS) and Penicillin-Streptomycin were obtained from Invitrogen (San Diego, CA, USA). Human immortalized non-tumorigenic keratinocyte cell line HaCaT was obtained from Cell Line Service (Eppelheim, Germany). Human IL-8 and IL-6 Mini TMB ELISA Development Kits were obtained from Peprotech (London, UK). All other reagents and solvents used in the present study were of analytical grade and purchased from available suppliers.
2.2. Samples
The extracts used in this work were the ones developed in our previous studies focused on process optimization [11,22]. Briefly, codfish frames were supplied by Pascoal and Filhos S.A. (Gafanha da Nazaré, Portugal) and consisted of fish backbone and adhered muscle. Sardine waste, made of heads, spines and viscera, was supplied by Conservas A Poveira S.A. (Póvoa de Varzim, Portugal). The proximate composition of the raw materials used have been presented in our earlier works [11,22]. Protein (47 wt %) and ash (39 wt %) were the major components of codfish frames, with small quantity of lipids and carbohydrates (2 wt % and 0.3 wt %, respectively). Collagen is found to be the major protein in codfish frames, and in this case, it accounts for ca. 65% of the total protein content of original waste. In contrast, sardine is an oily fish, thus its waste is much richer in lipids than codfish frames. Sardine wastes showed a lipid content of 26 wt % and a protein content of 52 wt %, the rest being ash (17 wt %) and carbohydrates (3 wt %).
The extracts from codfish frames (Cf1, Cf2, Cf3 and Cf4) and sardine wastes (S1, S2 and S3) selected for this work were obtained by high pressure technology in a lab-scale apparatus as previously described [11,22,24] using the conditions summarized in Table 1. Briefly, 60 g of ground codfish frames or sardine waste (defatted or non-defatted) were loaded into a high-pressure reactor that was put inside an oven. The water pump was switch on at desired flowrate (ca. 10 mL/min) and pressure was set to 100 bar. As soon as pressure reached that value, the electrical oven was switch on, and the experiment started. The different extracts were collected during 30 min at different temperatures (90–250 °C). S1 and S3 extracts were obtained after a defatting process of the sardine waste by ScCO2 before SW extraction. Subcritical water extraction experiments were duplicated. For each extract sample, 25 mL were taken in triplicate, lyophilized, and weighed to calculate the corresponding extraction yield. Analytical data—protein content—are expressed as mean ± standard deviation (SD) of triplicates. The information regarding the characterization of these extracts in terms of protein content, amino acid profile, major mineral compounds or toxic and heavy metals is described in our previous works [11,22].
Table 1.
Extraction process techniques and parameters used for each sample. Extraction yield and protein content are expressed as mean ± standard deviation (SD).
| Sample | Defatting Conditions | SW Extraction Conditions | Extraction Yield (g/100 g Feed) [11,22] |
Protein Content (wt %) [11,22] |
|---|---|---|---|---|
| Cf1 | - | 90 °C, 100 bar | 13.2 ± 0.5 | 81.6 ± 0.3 |
| Cf2 | - | 140 °C, 100 bar | 27.7 ± 0.5 | 93.6 ± 0.3 |
| Cf3 | - | 190 °C, 100 bar | 41.4 ± 0.5 | 95 ± 0.3 |
| Cf4 | - | 250 °C, 100 bar | 53.9 ± 0.5 | 84.4 ± 0.3 |
| S1 | ScCO2 (40 °C, 250 bar) | 190 °C, 100 bar | 45.7 ± 2.8 | 87.5 ± 2.7 |
| S2 | - | 250 °C, 100 bar | 58.5 ± 0.4 | 57.5 ± 1.8 |
| S3 | ScCO2 (40 °C, 250 bar) | 250 °C, 100 bar | 61.7 ± 2.0 | 85.2 ± 0.6 |
Cf1, Cf2, Cf3, Cf4—extracts from codfish frames; S1, S2, S3—extracts from sardine wastes.
Stock solutions of Cf1, Cf2, Cf3, Cf4, and S2 were prepared in Milli-Q H2O at a concentration of 100 mg/mL. The other samples, namely S1 and S3, were dissolved in DMSO (300 and 550 mg/mL, respectively) due to their lower solubility in water. Samples were frozen and kept at −20 °C until further use. For cellular assays, the samples were previously sterilized by heat (121 °C, 15 min) in an autoclave (Tuttnauer 3870 el, Breda, Netherlands).
2.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
ORAC assay was performed to evaluate the antioxidant capacity of the samples towards peroxyl radicals (ROO•), following the method developed by Huang et al. [25], with some adjustments as reported previously [26]. Briefly, in a black 96-well microplate, 150 µL disodium fluorescein (0.3 μM) was added to 25 µL of sample dilutions and incubated for 10 min at 37 °C. Afterwards, the reaction was initiated by the addition of 25 μL of 2,2′-Azobis (2-amidinopropane) dihydrochloride (AAPH, 153 mM) and fluorescence (Ex/Em 485 ± 20/528 ± 20 nm) was measured for 40 min at 37 °C in a FLx800 fluorescence microplate reader (FL800 Bio-Tek Instruments, Winooski, VT, USA). A standard curve was prepared using 5, 10, 20, 30 and 40 μM of (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox)). All solutions were prepared in phosphate-buffered saline (PBS), 75 mM, pH 7.4. The results are expressed as micromoles of Trolox equivalent antioxidant capacity per gram of extract (μ mol TEAC/g extract).
2.4. Enzymatic Assays
2.4.1. Elastase Inhibition Assay
This assay was based on the work of Wittenauer et al. [27] with some modifications as described previously [6]. Elastase inhibitory activity is determined by a spectrophotometric method using porcine pancreatic elastase (PPE) and N-succinyl-Ala-Ala-Ala-p-nitroanilide (AAAPVN) as the enzyme-substrate, by monitoring the release of p-nitroaniline at 410 nm. PPE was dissolved in 100 mM Tris (2-amino-2-hydroxymethyl-propane-1,3-diol)-HCl buffer (pH = 8.0) to a concentration of 1 mg/mL and stored at 20 °C in aliquots. On the day of the assay, an aliquot was taken and diluted in buffer to a concentration of 0.03 U/mL, 10 µL was loaded in the wells of the microtiter plates together with 100 μL of the Tris-HCl buffer and 30 μL of each sample. After 20 min of pre-incubation at 25 °C, the reaction was initiated by the addition of 40 µL of the substrate AAAPVN (0.55 mM). Absorbance was measured for 20 min after the addition of AAAPVN at a BioTek Instruments EPOCH 2 spectrophotometer microplate reader. The calculations were made as described in Equation (1), where Acontrol and Asample represent the absorbance at 410 nm in the absence or presence of the sample, respectively. Since DMSO was used to dissolve samples S1 and S3, this solvent was also tested and used as control for these samples. The potential of the extracts to inhibit elastase was evaluated with increasing concentrations, to determine dose-dependent relations and establish the half maximal inhibitory concentrations (IC50) values, indicating the capacity of each sample in enzymatic activity inhibition to an extent of 50%.
| (1) |
All results are expressed as IC50 mean value with the lower and upper limits of a 95% confidence interval, obtained from at least three independent experiments.
2.4.2. Tyrosinase Inhibition Assay
The tyrosinase inhibitory potential of the extracts was evaluated spectrophotometrically using mushroom tyrosinase and L-DOPA as the substrate [28]. Tyrosinase converts L-DOPA to dopaquinone, which will sequentially cyclize to form dopachrome. The dopachrome formation can be observed by measurement of the absorbance at 475 nm. The substrate was added to the enzyme in the presence of the sample dilutions, to a final concentration of 30 U/mL tyrosinase and 2.5 mM L-DOPA. Since DMSO was used to dissolve samples S1 and S3, this solvent was also tested and used as control for these samples. After incubation at 37 °C for 30 min, absorbance was measured at 475 nm on a BioTek Instruments EPOCH 2 microplate spectrophotometer. All the reagents were prepared in sodium phosphate buffer (SPB; 0.1 M, pH 6.8), prepared by mixing sodium phosphate dibasic dihydrate and sodium phosphate monobasic monohydrate, and the calculations were made as described in Equation (1). All results are expressed as IC50 mean value with the lower and upper limits of a 95% confidence interval, obtained from at least three independent experiments.
2.5. Antimicrobial Susceptibility Testing
The target microorganisms selected for the antibacterial activity assays were the gram-negative bacteria Klebsiella pneumoniae CECT 8453 and the gram-positive bacteria Staphylococcus aureus ATCC 6538 and Cutibacterium acnes ATCC 6919T. For K. pneumoniae CECT 8453 and S. aureus ATCC 6538, assays were performed according to the broth microdilution method of CLSI M07-A10 guidelines as previously described by Rodrigues et al. [29]. In short, extract stock solutions were distributed in a round bottom microtiter 96-well plate and 2-fold serially diluted in calcium-adjusted Mueller Hinton broth (CAMHB; BD, Sparks, MD, USA) to obtain a concentration range of solutions. The inoculum was prepared using the growth method to achieve a homogenous suspension in saline solution. The adjusted inoculum was additionally diluted in CAMHB to guarantee that, following inoculation, each well contained around 5 × 104 CFU. The plates were incubated under aerobic conditions at 37 °C for 16 to 20 h. For C. acnes, the assays were performed as previously described with the use of brain-heart infusion (BHI) (Avantor, Radnor, PA, USA) broth instead of CAMHB and by incubating the microtiter plates for 70–74 h at 37 °C in anaerobic jars containing the atmosphere generation system AnaeroGen™ Compact (Oxoid, Hampshire, UK).
For each stock solution analysed, a positive control (CAMHB or BHI and diluted inoculum), a medium sterility control (uninoculated CAMHB or BHI), and an extract sterility control (uninoculated 2-fold extract stock solution in CAMHB or BHI) were performed accordingly. Minimum inhibitory concentration (MIC) values were the lowest concentration of a sample that visibly inhibited microbial growth after incubation. When needed, MIC values were confirmed using the cell viability reagent PrestoBlue™ (Invitrogen, San Diego, CA USA) following the manufacturer’s guidelines. An additional MIC value, designated MIC*, was also established and defined as the lowest concentration of a sample at which bacterial growth was visually and differentially affected in comparison to the positive control. Minimum bactericidal concentration (MBC) values were reported as the lowest concentration of a sample leading to at least 99.9% reduction in viable bacterial counts in comparison to the initial inoculum and for equal incubation time. Results were expressed as a median of the values obtained after three biological replicates. Since DMSO was used to dissolve samples S1 and S3, this solvent was also tested to ensure that the final concentration used did not interfere with the target microorganism, hence the assay.
2.6. Cell-Based Assays
2.6.1. Cell Culture
Human keratinocyte cell line HaCaT (CLS, Germany) was cultured in a standard Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin. The cells were routinely maintained as monolayers in 75 cm2 culture flasks and incubated at 37 °C with 5% CO2 in a humidified atmosphere.
2.6.2. In Vitro Cytotoxicity
Cytotoxicity assays were performed according to previous works [6]. Briefly, HaCaT cells were seeded at a density of 1.4 × 105 cells/cm2 in 96 well plates. After 3 days, cells were incubated with different concentrations of each sample (Cf1; Cf2; Cf3; Cf4; S2—50, 25, 12.5, 6.25, 3.13, 1.56, 0.78, 0.39; S1—3, 1.5, 0.75, 0.38, 0.19, 0.09, 0.05, 0.02; S3—5.5, 2.75, 1.38, 0.69, 0.34, 0.17, 0.09, 0.04 mg/mL) diluted in culture medium (DMEM medium containing 0.5% FBS). Wells containing cells incubated only with culture medium supplemented with 0.5% (v/v) of FBS were used as control. Solvent controls with 50% water or 1% DMSO in culture medium were also performed to exclude solvent toxicity. After 24 h of incubation, the cell viability was evaluated using PrestoBlue® (5% v/v in culture medium) for 2 h at 37 °C, 5% CO2, according to the manufacturer’s instructions. After this, the fluorescence of each well was measured (Ex./Em. 560 ± 20/590 ± 20 nm) in an FLx800 fluorescence microplate reader (BioTek Instruments, Winooski, VT, USA). Cell viability was expressed as the percentage of viable cells relative to the control. Three independent experiments were performed in triplicate.
2.6.3. Cellular Antioxidant Activity
Cellular antioxidant activity was evaluated following previously described methods [6,30], with some modifications. Briefly, HaCaT cells were seeded at a density of 1.4 × 105 cells/cm2 in 96 well plates and the formation of intracellular ROS was monitored using 2′,7′-dichlorofluorescein diacetate (DCFH-DA) as a fluorescent probe. 72 h after seeding, cells were washed with PBS and incubated with non-toxic concentrations of the samples (0.1875 mg/mL; 0.375 mg/mL; 0.75 mg/mL) plus 25 µM DCFH-DA in PBS for 1 h. Subsequently, cells were washed again with PBS and incubated with the stress inducer (600 µM AAPH in PBS) for 1 h. After that, fluorescence was measured in an FL800 microplate fluorescence reader (Bio-Tek Instruments, Winooski, VT, USA) (Ex/Em 485 ± 20/528 ± 20 nm). The results are expressed as ROS percentage relative to the untreated control (cells treated with DCFH-DA and AAPH). Three independent experiments were performed in triplicate.
2.6.4. Evaluation of IL-6 and IL-8 Secretion
Experiments were performed as previously described [31], with several modifications. Briefly, HaCaT cells were seeded at a density of 1 × 105 cells/cm2 in 12 well plates. After 3 days, cells were stimulated with 15 μg/mL of lipopolysaccharides (LPS) from Escherichia coli and co-incubated with three different concentrations of each extract (0.1875 mg/mL; 0.375 mg/mL; 0.75 mg/mL) diluted in culture medium (DMEM medium containing 0.5% FBS). Cells incubated only with LPS and cells incubated with only culture media were used as positive and negative controls, respectively. After 24 h, supernatants were collected, centrifuged for 10 min at 2000 g and stored at −80 °C until further analysis. IL-6 and IL-8 levels were assessed by enzyme-linked immunosorbent assay (ELISA), using commercially available kits (PeproTech; London, UK), according to the manufacturer’s instructions, with absorbance measured at 450 nm with wavelength correction set at 620 nm in a microplate spectrophotometer (EPOCH 2, BioTek Instruments, Winooski, VT, USA). The results are expressed as IL-6 or IL-8 percentage relative to the positive control (cells stimulated with LPS). Three independent experiments were performed in triplicate.
2.7. Statistical Analysis
ORAC and cell-based assays results are expressed as the mean value ± SD, obtained from at least three independent experiments. For the enzymatic and cytotoxicity assays, the IC50 values were determined from dose-response curves through log10 plots using GraphPad Prism 8.4.3. software (GraphPad Software, Inc., La Jolla, CA, USA). Results are presented as IC50 with a 95% confidence interval. Statistical analysis of the results was performed using the former software. When homogeneous variance and a normal distribution of the data were verified, the results were analysed by one-way analysis of variance (ANOVA), followed by the Tukey test for multiple comparisons. In the case of heterogeneous variances or if the data were not normally distributed, an appropriate unpaired Student’s t-test was performed to determine whether the means were significantly different. A p-value ≤ 0.05 was accepted as statistically significant in all cases. Antimicrobial susceptibility testing results are expressed as the median value, obtained from at least three independent experiments.
3. Results and Discussion
This study aims to investigate the cosmeceutical potential of protein-rich extracts that were produced by high-pressure technologies from fishery industry wastes, namely sardine wastes and codfish frames [11,22]. The selection of extracts was based on previous results regarding their characterization and process conditions. For codfish, all extracts were selected aiming at evaluating the impact of the extraction temperature on the recovery of compounds with promising bioactive effects on the skin. For sardine extracts, only three extracts derived by both defatted and non-defatted raw materials, processed at higher temperatures (190 and 250 °C) and with the highest extraction yield (>45.7 g/100 g) were chosen. The results regarding the total protein content of each extract are presented in Table 1.
3.1. Antioxidant, Anti-Ageing and Anti-Hyperpigmentation Activities
The potential cosmeceutical effect of the extracts was initially screened using chemical and enzymatic assays to evaluate their antioxidant, anti-ageing, and anti-hyperpigmentation effect. For the antioxidant capacity, the ORAC assay was selected as it measures the ability of samples to scavenge biologically relevant ROS, namely peroxyl radicals, which are considered one of the main inducers of skin ageing [2,32]. The anti-ageing effect was also evaluated through elastase inhibition since this enzyme is reported to be responsible for the degradation of elastin and other ECM proteins [6]. For the anti-hyperpigmentation effect, the tyrosinase assay was used, to evaluate the capacity of samples to inhibit melanin production. Table 2 summarizes the ORAC and IC50 values of all extracts.
Table 2.
Antioxidant, anti-ageing, and anti-hyperpigmentation activities of codfish and sardine residues extracts. ORAC values are expressed as the mean value ± SD. Enzymatic assays IC50 values are expressed as mean with a 95% confidence interval.
| Sample | Antioxidant Activity | Anti-Ageing Activity | Anti-Hyperpigmentation Activity |
|---|---|---|---|
| ORAC | Elastase Inhibition | Tyrosinase Inhibition | |
| (µmol TEAC/mg extract) | (IC50, mg extract/mL) | (IC50, mg extract/mL) | |
| Cf1 | 0.64 ± 0.18 | 42.58 (37.38, 49.88) | >100 ** |
| Cf2 | 0.54 ± 0.22 | 60.35 (50.71, 73.43) | 78.59 (61.41, 100.70) |
| Cf3 | 0.59 ± 0.26 | 34.74 (24.57, 49.16) | >100 ** |
| Cf4 | 1.29 ± 0.26 | 38.11 (30.42, 49.52) | 40.89 (30.26, 54.43) |
| S1 | 1.94 ± 0.08 | 44.29 (39.10, 53.44) | 82.51 (57.12, 108.39) |
| S2 | 1.19 ± 0.10 | >300 * | 3.70 (3.26, 4.42) |
| S3 | 1.24 ± 0.06 | 17.96 (12.36, 25.83) | 10.40 (5.02, 18.15) |
* The maximum concentration tested was 300 mg/mL; ** The maximum concentration tested was 100 mg/mL.
Our results show that sardine and codfish extracts presented antioxidant activity and inhibition effects on elastase and tyrosinase enzymes’ activity. Among sardine samples, S1 showed the highest ORAC value (1.94 ± 0.08 µmol TEAC/mg extract) followed by S3 and S2. These results are in accordance with a previous antioxidant evaluation through an alternative method (2,2-diphenyl-1-picrylhydrazyl-DPPH assay) where the extract S1 presented the lowest IC50 values [22]. The higher scavenging capacity towards peroxyl radicals of the S1 sample could be derived from peptides with different amino acid sequences present in the extracts since interactions among them can influence the radical scavenging ability [33]. Additionally, compounds generated in Maillard or other thermo-oxidation reactions might influence the antioxidant activity of the samples [34]. Despite the lowest ORAC value, samples S2 and S3 were the ones with the highest capacity in inhibiting tyrosinase and elastase activities, respectively.
Among codfish extracts, Cf4 was shown to have the highest antioxidant and anti-hyperpigmentation activities. SEC-GPC analysis of the extracts has shown that peptides of decreasing molecular weight were obtained when increasing the extraction temperature up to 250 °C [11]. The elastase inhibition capacity of this sample is within the range of values found for other Cf extracts, namely Cf1 and Cf3. However, for the concentrations tested, these two extracts showed no inhibition activity towards tyrosinase.
In the literature there are some reports showing that extracts from marine by-products presents relevant antioxidant activity and inhibition of tyrosinse. For instance, extracts derived from marine (Scophthalmus maximus) by-products by alkaline hydrolysis, have demonstrated antioxidant activity accessed by different chemical assays: 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging ability (36.12% in relation to control), ABTS (2,2′-azinobis-(3-ethyl-benzothiazoline-6-sulphonic acid) method (12.81 μg BHT/mL) and crocin bleaching assay (8.03 μg Trolox/mL) [35]. In another study, enzymatic extraction of alum-salted jellyfish (Lobonema smithii) showed to produce hydrolysates with high antioxidant activity (IC50 = 0.9 mg/mL for ABTS e DPPH assays) and tyrosinase inhibitory potential, with IC50 values ranging between 14.1 and 24.5 mg/mL [36], which are in the same order of magnitude as the ones obtained in this work. Additionally, extracts with similar antioxidant values (ORAC values – 0.4 to 3.5 µmol TEAC/mg extract) were obtained from another type of food industry residues, namely winemaking waste streams [6]. However, these winery residues extracts presented higher tyrosinase (IC50 from 4.0 to 0.14 mg extract/mL) and elastase (IC50 from 3.4 to 0.1 mg extract/mL) inhibitory capacities than those obtained in this work, probably due to the presence of phenolic compounds that are recognized to have several bioactivities. It is important to mention that in our study we used mushroom tyrosinase to screen the anti-hyperpigmentation effect of extracts, as this enzyme has been widely used in high throughput assays [37]. Nevertheless, since there are some controversies regarding the similarity and homology of this enzyme with mammalian/human tyrosinase [38,39,40], future studies involving mammalian cell lines should be considered to evaluate the potential anti-hyperpigmentation effect of sardine and codfish extracts.
To identify which compounds could be responsible for the bioactive response of extracts, correlation studies between bioactivity data and the extracts’ amino acid composition reported previously [11,22] were performed (Table S1). For antioxidant activity, the highest correlations (R2 ≥ 0.7) were obtained between ORAC value and total threonine, free valine, as well as free and total leucine for all extracts. In the case of sardine samples, a high correlation (R2 ≥ 0.94) was also obtained for free and total tryptophan content. Accordingly, all these amino acids have been previously reported to have antioxidant properties in several model systems [41,42,43]. For elastase and tyrosinase inhibition activities, the highest correlation coefficients (R2 ≥ 0.8) were obtained for total protein content and free arginine, respectively, suggesting that these compounds could have an important role in the potential anti-ageing and anti-hyperpigmentation effect of fish industry waste streams extracts.
3.2. Antibacterial Activity
S. aureus and K. pneumoniae were chosen as representative gram-positive and -negative bacteria to evaluate the antimicrobial capacity of the extracts. The antibacterial activity assays performed with codfish frame extracts revealed that all of them were able to affect both bacterial strains’ growth behaviour (MIC* results in Table 3 and Table 4). However, true growth inhibition (MIC values) did not occur in the presence of any of the extracts at the concentrations tested.
Table 3.
Antibacterial activity of codfish and sardine extracts against S. aureus.
| Sample | MIC* Median | MIC Median | MBC Median |
|---|---|---|---|
| (mg/mL) | (mg/mL) | (mg/mL) | |
| (n = 1/n = 2/n = 3) | (n = 1/n = 2/n = 3) | (n = 1/n = 2/n = 3) | |
| Cf1 | 0.39 | >50.00 | >50.00 |
| (0.39/0.39/0.20) | (>50.00/>50.00/>50.00) | (>50.00/>50.00/>50.00) | |
| Cf2 | 0.78 | >50.00 | >50.00 |
| (0.78/0.78/0.39) | (>50.00/>50.00/>50.00) | (>50.00/>50.00/>50.00) | |
| Cf3 | 0.39 | >50.00 | >50.00 |
| (0.39/0.39/0.20) | (>50.00/>50.00/>50.00) | (>50.00/>50.00/>50.00) | |
| Cf4 | 0.10 | >50.00 | >50.00 |
| (0.10/0.10/0.10) | (>50.00/>50.00/>50.00) | (>50.00/>50.00/>50.00) | |
| S1 | 1.56 | 25.00 | 50.00 |
| (1.56/1.56/0.78) | (25.00/25.00/25.00) | (50.00/50.00/50.00) | |
| S2 | 0.07 | 1.17 | 9.38 |
| (0.07/0.07/0.07) | (1.17/2.34/1.17) | (9.38/9.38/9.38) | |
| S3 | 0.27 | 68.75 | 68.75 |
| (0.27/0.54/0.27) | (68.75/68.75/34.38) | (68.75/68.75/68.75) |
MIC*—Lowest concentration of a sample at which bacterial growth was visually and differentially affected; MIC—Minimum inhibitory concentration; MBC—Minimum bactericidal concentration.
Table 4.
Antibacterial activity of codfish and sardine extracts against K. pneumoniae.
| Sample | MIC* Median | MIC Median | MBC Median |
|---|---|---|---|
| (mg/mL) | (mg/mL) | (mg/mL) | |
| (n = 1/n = 2/n = 3) | (n = 1/n = 2/n = 3) | (n = 1/n = 2/n = 3) | |
| Cf1 | 12.50 | >50.00 | > 50.00 |
| (12.50/12.50/12.50) | (>50.00/>50.00/>50.00) | (>50.00/>50.00/>50.00) | |
| Cf2 | 25.00 | >50.00 | >50.00 |
| (25.00/25.00/50.00) | (>50.00/>50.00/>50.00) | (>50.00/>50.00/>50.00) | |
| Cf3 | 0.78 | >50.00 | >50.00 |
| (0.39/0.78/0.78) | (>50.00/>50.00/>50.00) | (>50.00/>50.00/>50.00) | |
| Cf4 | 0.39 | >50.00 | >50.00 |
| (0.39/0.39/0.20) | (>50.00/>50.00/>50.00) | (>50.00/>50.00/>50.00) | |
| S1 | 3.13 | 50.00 | 50.00 |
| (3.13/3.13/6.25) | (50.00/50.00/50.00) | (50.00/50.00/50.00) | |
| S2 | 0.07 | 4.69 | 18.75 |
| (0.07/0.07/0.07) | (4.69/4.69/9.38) | (18.75/18.75/18.75) | |
| S3 | 0.54 | 68.75 | 68.75 |
| (0.54/1.07/0.54) | (68.75/68.75/68.75) | (68.75/>68.75/68.75) |
MIC*—Lowest concentration of a sample at which bacterial growth was visually and differentially affected; MIC—Minimum inhibitory concentration; MBC—Minimum bactericidal concentration.
As S1 and S3 were solubilized in DMSO, assays were performed with this solvent to evaluate its influence on the results obtained for the sardine extracts. The results reveal that the maximum DMSO concentration used in the antibacterial activity testing of the different extracts (up to 12.5%) did not affect the growth of both bacterial strains.
In general, all sardine extracts inhibited the bacterial growth of both gram-positive and gram-negative selected strains, but S. aureus was shown to be more susceptible than K. pneumoniae, which is expected since the outer membrane of gram-negative bacteria poses an additional barrier to prevent the interference of different molecules with the cell [44]. S2 was shown to be the extract with the most promising antibacterial potential, indicating that higher extraction temperatures favoured the extraction of anti-bacterial compounds. Taking these results into account, S2 was selected to be tested against another gram-positive bacteria, namely C. acnes, an aerotolerant anaerobe linked to the acne skin condition [45]. To evaluate the impact of the defatting process on the inhibition capacity of C. acnes growth, sample S3 was also selected to be tested in this assay. The results are summarized in Table 5 showing that C. acnes behaves similarly to S. aureus with its growth being affected by both sardine extracts. Between both samples, S2 presented the highest inhibitory effect (lower MIC and MBC values) suggesting that the defatting process could modify or eliminate some anti-microbial compounds from the sardine samples before the extraction process, especially free fatty acids and lipid oxidation products [22].
Table 5.
Antibacterial activity of sardine extracts against C. acnes.
| Sample | MIC* Median | MIC Median | MBC Median |
|---|---|---|---|
| (mg/mL) | (mg/mL) | (mg/mL) | |
| (n = 1/n = 2/n = 3) | (n = 1/n = 2/n = 3) | (n = 1/n = 2/n = 3) | |
| S2 | 0.29 | 0.59 | 2.34 |
| (0.29/0.29/0.29) | (0.59/0.29/0.59) | (4.69/1.17/2.34) | |
| S3 | 2.15 | 17.19 | > 68.75 |
| (2.15/2.15/2.15) | (34.38/17.19/17.19) | (>68.75/68.75/>68.75) |
MIC*—Lowest concentration of a sample at which bacterial growth was visually and differentially affected; MIC—Minimum inhibitory concentration; MBC—Minimum bactericidal concentration.
Previous studies showed that some amino acid residues present in peptides can lead to different antibacterial activities [46]. Rodrigues et al. showed that protein derivative-rich extracts obtained by extraction with deep eutectic solvents (DES) from sardine processing waste streams have antibacterial activity toward S. aureus and Escherichia coli [47]. However, the MIC*, MIC and MBC values of these extracts were lower than those obtained in this work for the same raw material, which could be explained by the synergic and/or additive effect between the extracts’ components and DES, as described previously [29]. Nevertheless, the antimicrobial effect of S2 extract is similar to other extracts obtained by subcritical water extraction, namely using kānuka leaves (S. aureus—MIC: 0.9 mg/mL, MBC: 3.8–5 mg/mL; E. coli—MIC: 3.8–7.5 mg/mL, MBC: 4.4–7.5 mg/mL) [48], that are rich in phenolic compounds already recognized as presenting relevant antimicrobial effects [49].
In this study, the highest correlation coefficient obtained was for MIC values of S. aureus and the total glutamic acid content (R2 = 0.6, Table S1).
3.3. Cellular Antioxidant and Anti-Inflammatory Effect
In this work, a human keratinocyte cell line (HaCaT) was used to better understand the bioactivity, namely antioxidant and anti-inflammatory effects, of extracts as some of the processes related to the uptake, distribution, and metabolism of bioactive compounds are better addressed [50]. HaCaT cells are one of the predominant cell types encountered in the skin, being responsible for skin integrity and, when affected by senescence or oxidative stress, can accelerate the skin ageing process [51]. Additionally, keratinocytes play an important role in the regulation of skin inflammation, responding to external stimuli, such as bacterial LPS, actively contributing to inflammation pathways especially by releasing proinflammatory cytokines or chemokines [31,52].
In a first approach, cytotoxicity assays were performed to evaluate the safety of samples and to select non-toxic concentrations for further bioactivity studies. Table 6 presents the IC50 values of each sample showing that S1 presented the highest cytotoxic effect. In previous studies, these samples showed higher IC50 values in Caco-2, a model for crypt enterocytes, than in HaCaT, which indicates that the samples are more toxic for keratinocytes than intestinal cells [11,22].
Table 6.
IC50 values obtained for all the extracts in HaCaT cells, with 24 h incubation. IC50 values are expressed as mean with a 95% confidence interval.
| Sample | IC50 (mg Extract/mL) |
|---|---|
| Cf1 | 9.7 (9.5, 9.9) |
| Cf2 | 3.6 (3.5, 3.7) |
| Cf3 | 17.3 (15.2, 19.6) |
| Cf4 | 2.0 (1.9, 2.2) |
| S1 | 0.6 (0.5, 0.7) |
| S2 | 3.4 (3.3, 3.6) |
| S3 | >5.5 |
IC50—concentration of the sample that leads to a decrease of 50 % of the cell population after 24 h incubation.
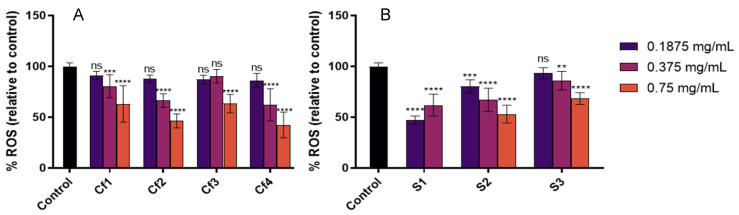
The cellular antioxidant activity was then assessed by evaluating the capacity of samples in scavenging intracellular ROS generated by the chemical stressor AAPH. In parallel, anti-inflammatory assays were also performed to investigate the effect of samples in reducing the secretion of IL-8, which has been consistently reported as an important skin inflammation biomarker [53,54,55], upon pro-inflammatory stimulus with LPS. In these assays, non-cytotoxic concentrations of the extracts were used (0.1875, 0.375 and 0.75 mg/mL for all samples except S1 where 0.75 mg/mL was not tested since this concentration presented a cytotoxic effect, Table 6). In general, all samples inhibited ROS formation in HaCaT, and a dose-dependent effect was observed (Figure 1). Among codfish samples, Cf2 and Cf4 showed the highest cellular antioxidant activities, and amongst sardine samples, S1 showed the highest ROS percentage reduction relative to control.
Figure 1.
Cellular antioxidant capacity, expressed as % of ROS inhibition relative to the control, of each extract. (A) Codfish frame extracts; (B) Sardine wastes extracts. The results are expressed as mean ROS percentage relative to the control ± SD. The symbol * indicates significance relative to the control ** p-value ≤ 0.01, *** p-value ≤ 0.001, **** p-value ≤ 0.0001); ns—not significant.
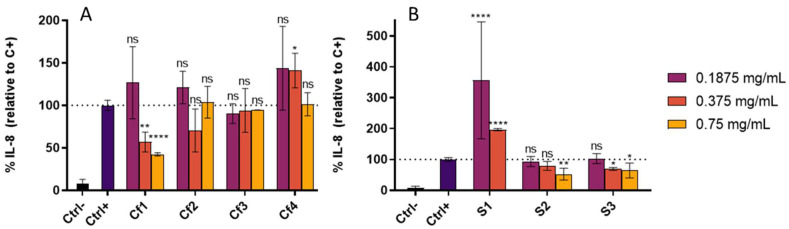
Concerning the anti-inflammatory effect, only Cf1 (0.375 and 0.75 mg/mL), S2 (0.75 mg/mL) and S3 (0.375 and 0.75 mg/mL) revealed capacity to inhibit IL-8 secretion by HaCaT cells after LPS-induced inflammation (Figure 2). In contrast, the other samples did not reduce IL-8 and, for some concentrations, Cf4 and S1 showed a pro-inflammatory effect. These two extracts were the ones that demonstrated the highest cytotoxic effect in HaCaT cells and thus the concentrations tested, although not leading to cell death, could induce the activation of inflammatory pathways since injured cells can release danger signals that alert other cells to cell death [56].
Figure 2.
IL-8 secreted by HaCaT cells treated for 24 h with different extracts concentrations and 15 µg/mL LPS. (A) Codfish residues extracts; (B) Sardine residues extracts. Ctrl-—cells incubated with only culture media; Ctrl+—Cells incubated with culture media + inflammation inductor (LPS); Cf1, Cf2, Cf3, Cf4—cells incubated with the different extracts from codfish frames + inflammation inductor (LPS); S1, S2, S3—cells incubated with the different extracts from sardine wastes + inflammation inductor (LPS). The results are expressed as mean IL-8 percentage relative to the positive control ± SD. The symbol * indicates significance relative to the positive control (* p-value ≤ 0.05, ** p-value ≤ 0.01, **** p-value ≤ 0.0001); ns—not significant.
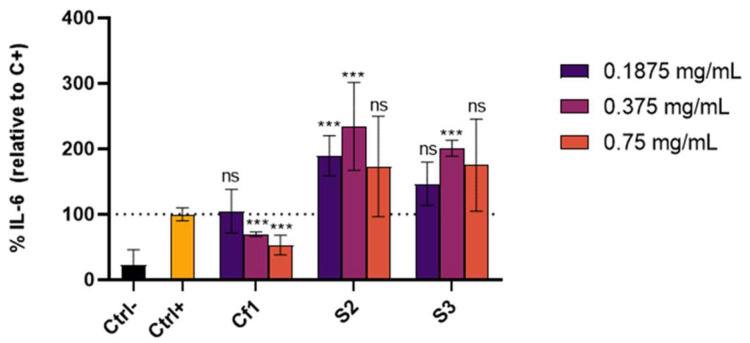
The extracts with the highest anti-inflammatory effect, namely Cf1, S2 and S3, were further selected to evaluate their capacity in inhibiting IL-6 secretion, a cytokine also recognized as an important biomarker in skin disorders [53]. Results show that Cf1 was the only sample able to significantly reduce IL-6 secretion by 69.4 ± 3.5 and 53.0 ± 14.7% IL-6 (relative to the positive control, p-value < 0.001) at 0.375 and 0.75 mg/mL, respectively, whereas sardine extracts increased in IL-6 levels of the supernatants (Figure 3). Overall, only Cf1 revealed the capacity to reduce both IL-8 and IL-6 secretion by HaCaT cells, suggesting that this codfish extract could be further explored for anti-inflammatory applications in skin conditions.
Figure 3.
IL-6 secreted by HaCaT cells treated for 24 h with different extracts concentrations and 15 µg/mL LPS. Ctrl-—cells incubated with only culture media; Ctrl+—Cells incubated with culture media + inflammation inductor (LPS); Cf1– cells incubated with Cf1 extract from codfish frames + inflammation inductor (LPS); S2, S3—cells incubated S2 and S3 extracts from sardine wastes + inflammation inductor (LPS). The results are expressed as mean IL-6 percentage relatively to the positive control ± SD. The symbol * indicates significance relative to the positive control (*** p-value ≤ 0.001); ns—not significant.
The anti-inflammatory activity of codfish extracts was previously evaluated in an intestinal cell line (Caco-2 cells) [11] and the results were in line with the data presented in this study for HaCaT cells. In both cell lines, Cf1 presented the highest anti-inflammatory effect, reinforcing the use of lower temperatures to extract bioactive compounds from codfish frames.
Our results are in accordance with previous studies supporting the idea that fish-derived extracts/hydrolysates display a broad spectrum of bioactivities, including antioxidant, antimicrobial, anti-ageing, anti-hypertensive, anti-human immunodeficiency virus, anti-proliferative, or anticoagulant activities [23,57]. Song et al. showed that enzymatic hydrolysates of the marine fish half-fin anchovy contained antibacterial peptide fractions, with activity against E. coli [58]. Fish skin and collagen hydrolysed by subcritical water hydrolysis also showed high antioxidant and antimicrobial activity against Bacillus cereus, S. aureus and Pseudomonas putida [59]. Additionally, Wang and co-workers produced extracts derived from fish side streams of two fish species (rainbow trout and sole) that could inhibit the growth of pathogenic bacteria (S. aureus or Salmonella) and with anti-inflammatory properties [60].
4. Conclusions
In this work, we applied a platform of in vitro bioassays to evaluate for the first time the cosmeceutical potential of protein extracts derived from fishery wastes, namely sardine residues and codfish frames, obtained by high-pressure technology. We demonstrated that both types of extracts showed antioxidant, anti-ageing, and anti-hyperpigmentation potential. Among all, sardine extracts presented the highest anti-bacterial activity, and this effect was more pronounced for samples obtained using higher extraction temperatures (250 °C) and without the defatting step. Codfish samples were the most effective anti-inflammatory agents, and in this case, lower temperatures (90 °C) favoured the extraction of these bioactive compounds. Although further studies are needed to identify which compounds could be responsible for the bioactive effects, total threonine, free valine as well as free and total leucine were identified to highly correlate with the antioxidant activities of samples. Total protein content and free arginine correlated with elastase and tyrosinase inhibition activities, respectively.
This work is a step forward in the development of potential cosmeceutical ingredients with antioxidant, skin whitening, antimicrobial, and anti-inflammatory effects from sardine residues and codfish frames, adding potential high value to these fishery industry wastes.
Acknowledgments
We acknowledge Pascoal and Filhos S.A. (Gafanha da Nazaré, Portugal) and Conservas A Poveira S.A. (Portugal) for providing us, respectively, the Atlantic codfish (Gadus morhua) frames and Sardine (Sardina pilchardus) waste used in this work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11101925/s1, Table S1: Correlation coefficients between protein and amino acids content and different bioactivities of sardine waste and codfish frames extracts.
Author Contributions
Conceptualization, I.C.L., F.B.G., P.S., A.P., R.M., N.F. and A.T.S.; methodology, M.C., A.B., I.C.L., F.B.G., N.F. and A.T.S.; investigation, M.C., I.C.L., F.B.G., M.M., R.M., N.F. and A.T.S.; resources, F.B.G., N.F. and A.T.S.; writing—original draft preparation, M.C.; writing—review and editing, I.C.L., F.B.G., R.M., P.S., N.F. and A.T.S.; supervision, F.B.G., P.S., N.F. and A.T.S.; funding acquisition, F.B.G., P.S., N.F. and A.T.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available within the article and its supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior (FCT/MCTES, Portugal) through project PTDC/ASPPES/28399/2017 and national funds iNOVA4Health (UIDB/04462/2020 and UIDP/04462/2020) and the Associate Laboratories LS4FUTURE (LA/P/0087/2020) and LAQV (UIDB/QUI/50006/2020). Funding from INTERFACE Programme, through the Innovation, Technology and Circular Economy Fund (FITEC), is gratefully acknowledged. ATS also acknowledges FCT/MCTES for the Individual Grant CEECIND/04801/2017.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guillerme J.B., Couteau C., Coiffard L. Applications for marine resources in cosmetics. Cosmetics. 2017;4:35. doi: 10.3390/cosmetics4030035. [DOI] [Google Scholar]
- 2.e Silva S.A.M., Leonardi G.R., Michniak-Kohn B. An overview about oxidation in clinical practice of skin aging. An. Bras. Dermatol. 2017;92:367–374. doi: 10.1590/abd1806-4841.20175481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoang H.T., Moon J.Y., Lee Y.C. Natural antioxidants from plant extracts in skincare cosmetics: Recent applications, challenges and perspectives. Cosmetics. 2021;8:106. doi: 10.3390/cosmetics8040106. [DOI] [Google Scholar]
- 4.Tobin D.J. Introduction to skin aging. J. Tissue Viability. 2017;26:37–46. doi: 10.1016/j.jtv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Hashizume H. Skin aging and dry skin. J. Dermatol. 2004;31:603–609. doi: 10.1111/j.1346-8138.2004.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 6.Matos M.S., Romero-Díez R., Álvarez A., Bronze M.R., Rodríguez-Rojo S., Mato R.B., Cocero M.J., Matias A.A. Polyphenol-rich extracts obtained from winemaking waste streams as natural ingredients with cosmeceutical potential. Antioxidants. 2019;8:355. doi: 10.3390/antiox8090355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordberg J., Arnér E.S.J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31:1287–1312. doi: 10.1016/S0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 8.Rinnerthaler M., Bischof J., Streubel M.K., Trost A., Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5:545. doi: 10.3390/biom5020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H., Zheng Y.-W., Liu Q., Liu L.-P., Luo F.-L., Zhou H.C., Isoda H., Ohkohchi N., Li Y.-M. Reactive oxygen species in skin repair, regeneration, aging, and inflammation. React. Oxyg. Species ROS Living Cells. 2017;8:69–88. doi: 10.5772/INTECHOPEN.72747. [DOI] [Google Scholar]
- 10.The State of World Fisheries and Aquaculture 2020. [(accessed on 11 January 2022)]. Available online: https://www.fao.org/state-of-fisheries-aquaculture.
- 11.Melgosa R., Marques M., Paiva A., Bernardo A., Fernández N., Sá-Nogueira I., Simões P. Subcritical water extraction and hydrolysis of cod (Gadus morhua) frames to produce bioactive protein extracts. Foods. 2021;10:1222. doi: 10.3390/foods10061222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferraro V., Carvalho A.P., Piccirillo C., Santos M.M., Paula P.M., Pintado M.E. Extraction of high added value biological compounds from sardine, sardine-type fish and mackerel canning residues—A review. Mater. Sci. Eng. C. 2013;33:3111–3120. doi: 10.1016/j.msec.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Thuanthong M., De Gobba C., Sirinupong N., Youravong W., Otte J. Purification and characterization of angiotensin-converting enzyme-inhibitory peptides from nile tilapia (Oreochromis niloticus) skin gelatine produced by an enzymatic membrane reactor. J. Funct. Foods. 2017;36:243–254. doi: 10.1016/j.jff.2017.07.011. [DOI] [Google Scholar]
- 14.Chi C.F., Wang B., Hu F.Y., Wang Y.M., Zhang B., Deng S.G., Wu C.W. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res. Int. 2015;73:124–129. doi: 10.1016/j.foodres.2014.08.038. [DOI] [Google Scholar]
- 15.Seo J.K., Lee M.J., Go H.J., Kim Y.J., Park N.G. Antimicrobial function of the GAPDH-related antimicrobial peptide in the skin of skipjack tuna, katsuwonus pelamis. Fish Shellfish. Immunol. 2014;36:571–581. doi: 10.1016/j.fsi.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Wang T.Y., Hsieh C.H., Hung C.C., Jao C.L., Chen M.C., Hsu K.C. Fish skin gelatin hydrolysates as dipeptidyl peptidase IV inhibitors and glucagon-like peptide-1 stimulators improve glycaemic control in diabetic rats: A comparison between warm- and cold-water fish. J. Funct. Foods. 2015;19:330–340. doi: 10.1016/j.jff.2015.09.037. [DOI] [Google Scholar]
- 17.Cai L., Wu X., Zhang Y., Li X., Ma S., Li J. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods. 2015;16:234–242. doi: 10.1016/j.jff.2015.04.042. [DOI] [Google Scholar]
- 18.Lu J., Hou H., Fan Y., Yang T., Li B. Identification of MMP-1 inhibitory peptides from cod skin gelatin hydrolysates and the inhibition mechanism by MAPK signaling pathway. J. Funct. Foods. 2017;33:251–260. doi: 10.1016/j.jff.2017.03.049. [DOI] [Google Scholar]
- 19.Abdallah M.M., Leonardo I.C., Krstić L., Enríquez-De-salamanca A., Diebold Y., González-García M.J., Gaspar F.B., Matias A.A., Bronze M.R., Fernández N. Potential ophthalmological application of extracts obtained from tuna vitreous humor using lactic acid-based deep eutectic systems. Foods. 2022;11:342. doi: 10.3390/foods11030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues L.A., Pereira C.V., Carvalho Partidario A.M., Gouveia L.F., Simoes P., Paiva A., Matias A.A. Supercritical CO2 extraction of bioactive lipids from canned sardine waste streams. J. CO2 Util. 2021;43:101359. doi: 10.1016/j.jcou.2020.101359. [DOI] [Google Scholar]
- 21.Šližyte R., Mozuraityte R., Martínez-Alvarez O., Falch E., Fouchereau-Peron M., Rustad T. Functional, bioactive and antioxidative properties of hydrolysates obtained from cod (Gadus morhua) backbones. Process Biochem. 2009;44:668–677. doi: 10.1016/j.procbio.2009.02.010. [DOI] [Google Scholar]
- 22.Melgosa R., Trigueros E., Sanz M.T., Cardeira M., Rodrigues L., Fernández N., Matias A.A., Bronze M.R., Marques M., Paiva A., et al. Supercritical CO2 and subcritical water technologies for the production of bioactive extracts from sardine (Sardina pilchardus) waste. J. Supercrit. Fluids. 2020;164:104943. doi: 10.1016/j.supflu.2020.104943. [DOI] [Google Scholar]
- 23.Venkatesan J., Anil S., Kim S.K., Shim M.S. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs. 2017;15:143. doi: 10.3390/md15050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedras B., Salema-Oom M., Sá-Nogueira I., Simões P., Paiva A., Barreiros S. Valorization of white wine grape pomace through application of subcritical water: Analysis of extraction, hydrolysis, and biological activity of the extracts obtained. J. Supercrit. Fluids. 2017;128:138–144. doi: 10.1016/j.supflu.2017.05.020. [DOI] [Google Scholar]
- 25.Huang D., Ou B., Hampsch-Woodill M., Flanagan J.A., Prior R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002;50:4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira-Alves S.C., Andrade F., Prazeres I., Silva A.B., Capelo J., Duarte B., Caçador I., Coelho J., Serra A.T., Bronze M.R. Impact of Drying Processes on the Nutritional Composition, Volatile Profile, Phytochemical Content and Bioactivity of Salicornia ramosissima J. Woods. Antioxidants. 2021;10:1312. doi: 10.3390/antiox10081312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittenauer J., MäcKle S., Sußmann D., Schweiggert-Weisz U., Carle R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia. 2015;101:179–187. doi: 10.1016/j.fitote.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Chan E.W.C., Lim Y.Y., Wong L.F., Lianto F.S., Wong S.K., Lim K.K., Joe C.E., Lim T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008;109:477–483. doi: 10.1016/j.foodchem.2008.02.016. [DOI] [Google Scholar]
- 29.Rodrigues L.A., Pereira C.V., Leonardo I.C., Fernández N., Gaspar F.B., Silva J.M., Reis R.L., Duarte A.R.C., Paiva A., Matias A.A. Terpene-based natural deep eutectic systems as efficient solvents to recover astaxanthin from brown crab shell residues. ACS Sustain. Chem. Eng. 2020;8:2246–2259. doi: 10.1021/acssuschemeng.9b06283. [DOI] [Google Scholar]
- 30.Serra A.T., Matias A.A., Frade R.F.M., Duarte R.O., Feliciano R.P., Bronze M.R., Figueira M.E., de Carvalho A., Duarte C.M.M. Characterization of traditional and exotic apple varieties from portugal. Part 2—Antioxidant and antiproliferative activities. J. Funct. Foods. 2010;2:46–53. doi: 10.1016/j.jff.2009.12.005. [DOI] [Google Scholar]
- 31.Di Caprio R., Lembo S., Di Costanzo L., Balato A., Monfrecola G. Anti-inflammatory properties of low and high doxycycline doses: An in vitro study. Mediat. Inflamm. 2015;2015:329418. doi: 10.1155/2015/329418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee P.K., Maity N., Nema N.K., Sarkar B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine. 2011;19:64–73. doi: 10.1016/j.phymed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Chalamaiah M., Dinesh Kumar B., Hemalatha R., Jyothirmayi T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012;135:3020–3038. doi: 10.1016/j.foodchem.2012.06.100. [DOI] [PubMed] [Google Scholar]
- 34.Asaduzzaman A.K.M., Chun B.S. Hydrolyzates produced from mackerel Scomber japonicus skin by the pressurized hydrothermal process contain amino acids with antioxidant activities and functionalities. Fish. Sci. 2014;80:369–380. doi: 10.1007/s12562-014-0705-2. [DOI] [Google Scholar]
- 35.Vázquez J.A., Rodríguez-Amado I., Sotelo C.G., Sanz N., Pérez-Martín R.I., Valcárcel J. Production, characterization, and bioactivity of fish protein hydrolysates from aquaculture turbot (Scophthalmus maximus) wastes. Biomolecules. 2020;10:310. doi: 10.3390/biom10020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upata M., Siriwoharn T., Makkhun S., Yarnpakdee S., Regenstein J.M., Wangtueai S. Tyrosinase inhibitory and antioxidant activity of enzymatic protein hydrolysate from jellyfish (Lobonema smithii) Foods. 2022;11:615. doi: 10.3390/foods11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan Y.F., Zhu S.X., Hou F.B., Zhao D.F., Pan Q.S., Xiang Y.W., Qian X.K., Ge G.B., Wang P. Spectrophotometric assays for sensing tyrosinase activity and their applications. Biosensors. 2021;11:290. doi: 10.3390/bios11080290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Promden W., Viriyabancha W., Monthakantirat O., Umehara K., Noguchi H., De-Eknamkul W. Correlation between the potency of flavonoids on mushroom tyrosinase inhibitory activity and melanin synthesis in melanocytes. Molecules. 2018;23:1403. doi: 10.3390/molecules23061403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zolghadri S., Bahrami A., Hassan Khan M.T., Munoz-Munoz J., Garcia-Molina F., Garcia-Canovas F., Saboury A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019;34:279–309. doi: 10.1080/14756366.2018.1545767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strzępek-Gomółka M., Gaweł-Bęben K., Angelis A., Antosiewicz B., Sakipova Z., Kozhanova K., Głowniak K., Kukula-Koch W. Identification of mushroom and murine tyrosinase inhibitors from Achillea biebersteinii afan. Extract. Molecules. 2021;26:964. doi: 10.3390/molecules26040964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji S., Qi X., Ma S., Liu X., Min Y. Effects of dietary threonine levels on intestinal immunity and antioxidant capacity based on cecal metabolites and transcription sequencing of broiler. Animals. 2019;9:739. doi: 10.3390/ani9100739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cojocaru E., Filip N., Ungureanu C., Filip C., Danciu M. Effects of valine and leucine on some antioxidant enzymes in hypercholesterolemic rats. Health. 2014;6:2313–2321. doi: 10.4236/health.2014.617266. [DOI] [Google Scholar]
- 43.Nayak B.N., Buttar H.S. Evaluation of the antioxidant properties of tryptophan and its metabolites in in vitro assay. J. Complement. Integr. Med. 2016;13:129–136. doi: 10.1515/jcim-2015-0051. [DOI] [PubMed] [Google Scholar]
- 44.Jones S. Permeability rules for antibiotic design. Nat. Biotechnol. 2017;35:639. doi: 10.1038/nbt.3919. [DOI] [PubMed] [Google Scholar]
- 45.Mayslich C., Grange P.A., Dupin N. Cutibacterium acnes as an opportunistic pathogen: An update of its virulence-associated factors. Microorganisms. 2021;9:303. doi: 10.3390/microorganisms9020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huan Y., Kong Q., Mou H., Yi H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020;11:2559. doi: 10.3389/fmicb.2020.582779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodrigues L.A., Leonardo I.C., Gaspar F.B., Roseiro L.C., Duarte A.R.C., Matias A.A., Paiva A. Unveiling the potential of betaine/polyol-based deep eutectic systems for the recovery of bioactive protein derivative-rich extracts from sardine processing residues. Sep. Purif. Technol. 2021;276:119267. doi: 10.1016/j.seppur.2021.119267. [DOI] [Google Scholar]
- 48.Essien S.O., Young B., Baroutian S. The antibacterial and antiproliferative ability of kānuka, Kunzea ericoides, leaf extracts obtained by subcritical water extraction. J. Chem. Technol. Biotechnol. 2021;96:1308–1315. doi: 10.1002/jctb.6647. [DOI] [Google Scholar]
- 49.Lu C., Li C., Chen B., Shen Y. Composition and antioxidant, antibacterial, and anti-HepG2 cell activities of polyphenols from seed coat of amygdalus pedunculata pall. Food Chem. 2018;265:111–119. doi: 10.1016/j.foodchem.2018.05.091. [DOI] [PubMed] [Google Scholar]
- 50.Wolfe K.L., Rui H.L. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007;55:8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- 51.Csekes E., Račková L. Skin Aging, Cellular senescence and natural polyphenols. Int. J. Mol. Sci. 2021;22:12641. doi: 10.3390/ijms222312641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li S., Xie R., Jiang C., Liu M. Schizandrin A Alleviates LPS-Induced Injury in human keratinocyte cell hacat through a microRNA-127-dependent regulation. Cell. Physiol. Biochem. 2018;49:2229–2239. doi: 10.1159/000493826. [DOI] [PubMed] [Google Scholar]
- 53.Zampetti A., Mastrofrancesco A., Flori E., Maresca V., Picardo M., Amerio P., Feliciani C. Proinflammatory cytokine production in HaCaT cells treated by eosin: Implications for the topical treatment of psoriasis. Int. J. Immunopathol. Pharmacol. 2009;22:1067–1075. doi: 10.1177/039463200902200423. [DOI] [PubMed] [Google Scholar]
- 54.Colombo I., Sangiovanni E., Maggio R., Mattozzi C., Zava S., Corbett Y., Fumagalli M., Carlino C., Corsetto P.A., Scaccabarozzi D., et al. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat. Inflamm. 2017;2017:7435621. doi: 10.1155/2017/7435621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeong S.J., Lim H.S., Seo C.S., Jin S.E., Yoo S.R., Lee N., Shin H.K. Anti-inflammatory actions of herbal formula Gyejibokryeong-hwan regulated by inhibiting chemokine production and STAT1 activation in HaCaT cells. Biol. Pharm. Bull. 2015;38:425–434. doi: 10.1248/bpb.b14-00660. [DOI] [PubMed] [Google Scholar]
- 56.Rock K.L., Kono H. The inflammatory response to cell death. Annu. Rev. Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ngo D.H., Vo T.S., Ngo D.N., Wijesekara I., Kim S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012;51:378–383. doi: 10.1016/j.ijbiomac.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Song R., Wei R.B., Luo H.Y., Wang D.F. Isolation and characterization of an antibacterial peptide fraction from the pepsin hydrolysate of half-fin anchovy (Setipinna taty) Molecules. 2012;17:2980. doi: 10.3390/molecules17032980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmed R., Chun B.S. Subcritical water hydrolysis for the production of bioactive peptides from tuna skin collagen. J. Supercrit. Fluids. 2018;141:88–96. doi: 10.1016/j.supflu.2018.03.006. [DOI] [Google Scholar]
- 60.Wang M., Zhou J., Pallarés N., Bäuerl C., Collado M.C., Dar B.N., Barba F.J. Role of extracts obtained from rainbow trout and sole side streams by accelerated solvent extraction and pulsed electric fields on modulating bacterial and anti-inflammatory activities. Separations. 2021;8:187. doi: 10.3390/separations8100187. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its supplementary material.