Abstract
BALB/c mice were immunized subcutaneously with soluble Neospora caninum tachyzoite antigen (NSO) entrapped in nonionic surfactant vesicles (NISVs) or administered with Freund's complete adjuvant (FCA). Following virulent parasite challenge, groups of mice immunized with NSO and either NISVs or FCA had clinical neurological disease and increased numbers of brain lesions compared to groups of mice inoculated with FCA, NISVs, or phosphate-buffered saline (PBS) alone. Increased numbers of brain lesions were statistically significant only between mice immunized with NISV-NSO and NISV- or PBS-treated mice. Following parasite challenge, brain inflammatory infiltrates in all experimental and control groups of mice were relatively similar and consisted of compact infiltrates of macrophages admixed with various numbers of lymphoid cells. Increased brain lesions in NSO-immunized mice were associated with increased antigen-specific interleukin 4 (IL-4) secretion and increased IL-4:gamma interferon secretion ratios from splenocytes in vitro and increased antigen-specific immunoglobulin G1 (IgG1):IgG2a ratios in vivo. Thus, immunization with whole killed N. caninum antigen and either liposoidal or Freund's adjuvant induced a type 2 immune response that was associated with worsened disease. The present studies emphasize the need to identify specific N. caninum antigens or other delivery systems that will elicit protective immune responses to neosporosis.
Neospora caninum is an apicomplexan protozoan parasite that causes neurological disease and abortion in multiple animal species (9). Abortion as a result of N. caninum infection and congenital N. caninum infection in cattle cause significant economic loss to the cattle industry. Unlike other major abortofacients in cattle, there is no effective vaccine to control abortion that results from neosporosis. Congenital infection is the primary mode of parasite transmission identified (6, 9, 27, 32), and infected hosts can transmit the parasite repeatedly during successive pregnancies, suggesting a lack of long-term immunity (6, 27). There is a major need in neosporosis research to identify the immune responses effective at reducing parasite load and associated pathologic effects such as transplacental transmission and host tissue damage.
The type of immune response generated following infection determines protective immunity. Immunity to intracellular protozoa in general is cell mediated and is dominated by type 1 T-lymphocyte responses (25, 34). Resistance to neosporosis is associated with the type 1 cytokines gamma interferon (IFN-γ) and interleukin 12 (IL-12) (4, 16), while susceptibility to both N. caninum encephalitis and congenital transmission is associated with the type 2 cytokine IL-4 (21, 22). To investigate whether a protective type 1 immune response could be induced by immunization, soluble antigen from whole tachyzoites coupled with liposoidal adjuvant nonionic surfactant vesicles (NISVs) to selectively target activation of type 1 immune responses was used as an immunogen in a BALB/c mouse model of N. caninum encephalitis (7, 20).
MATERIALS AND METHODS
Experimental design.
Groups of 10 female BALB/c mice were inoculated with either (i) 50 μg of killed tachyzoite N. caninum antigen (NSO) with NISV adjuvant, (ii) 50 μg of NSO with Freund's complete adjuvant (FCA), (iii) NISVs with phosphate-buffered saline (PBS) (adjuvant control), (iv) FCA with PBS (adjuvant control), or (v) PBS alone (no-adjuvant, no-antigen control). Mice were immunized subcutaneously twice at 3-week intervals, and 3 weeks later they were challenged with 2 × 105 low-passage, virulent N. caninum tachyzoites inoculated subcutaneously. All experiments were repeated at least once. Three mice from the NSO-immunized groups (groups 1 and 2) were killed on the day of N. caninum challenge infection to measure parasite-specific immune parameters prior to challenge infection. The remaining mice in each group were killed at 6 weeks after the challenge infection to measure the level of parasite-induced disease and parasite-specific immune parameters. Disease severity was measured postmortem by quantitative microscopic enumeration of brain lesions and qualitative assessment of inflammatory infiltrates in the brain. Immune status was measured by antigen-specific splenocyte production of IFN-γ and IL-4 in vitro by enzyme-linked immunosorbent assay (ELISA), and antigen-specific serum immunoglobulin G (IgG) isotype production was measured in vivo by ELISA.
Mice.
Inbred 6-week-old female BALB/c (H-2d) mice were used for all experiments. The mice were obtained from a disease-free colony maintained at the Washington State University College of Veterinary Medicine. All mice underwent quarterly serologic surveillance testing for common murine pathogens (Charles River Laboratories, Inc., Wilmington, Mass.) and were determined to be seronegative for antibody to N. caninum by competitive ELISA (3) before inclusion in the study.
Parasites and parasite antigen.
The N. caninum isolate used in the study (isolate NC-1) was generously provided by J. P. Dubey and was passaged at least once in vivo in mice prior to initiation of the present studies. After isolation from mice, tachyzoites were passaged in vitro in Vero cell cultures in Dulbecco modified Eagle medium and 5% fetal calf serum at 37°C in a 95% air–5% CO2 atmosphere (18). Viability was determined by fluorescence staining with fluorescein diacetate (10). Fluorescing tachyzoites were counted in a hemocytometer. The organisms were resuspended in PBS for inoculation to a final volume of 200 μl/mouse. All challenge infections were done with tachyzoites passed less than 10 times in Vero cell culture to ensure parasite virulence (unpublished observations).
NSO was obtained by a sonication and centrifugation procedure described previously (3, 35). Infected Vero cells were scraped and passed through a 27-gauge needle to rupture the host cells, and tachyzoites were separated from the host cell debris by centrifugation in a 40% Percoll density gradient. Cell-free tachyzoites were pelleted (800 × g for 20 min), washed three times in PBS, resuspended in sterile distilled water, and sonicated for six 30-s pulses (Branson 450 sonifier). Cell debris and unlysed cells were removed by centrifugation (1,000 × g for 20 min at 4°C). The protein in the supernatant was quantified by a commercial protein assay (bicinchoninic acid assay [BCA]; Pierce, Rockford, Ill.) with bovine serum albumin as a standard and stored at −80°C until use.
SDS-PAGE and immunoblotting.
Aliquots of NSO in PBS were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and probed by Western blotting as described previously (3). Nitrocellulose filters containing transferred antigens were probed with bovine serum obtained from a cow with naturally occurring N. caninum-induced abortion (Washington Animal Disease Diagnostic Laboratory) or with goat serum from a hyperimmune goat experimentally inoculated with N. caninum (VMRD Inc., Pullman, Wash.). Bovine and goat serum were diluted 1:100, and antibody binding to nitrocellulose membranes was detected with peroxidase-conjugated protein G (Zymed Laboratories Inc., South San Francisco, Calif.) visualized by enhanced chemiluminescence (ECL; Amersham Life Science, Arlington Heights, Ill.). Western blot assay controls consisted of samples in which the primary antibody was replaced by with nonimmune sera or buffer alone.
NISV adjuvant.
Multilamellar NISVs were formed in PBS (pH 7.4) from a mixture of 1-monopalmitoyl-glycerol (nonionic surfactant), cholesterol, and dicetyl phosphate in a molar ratio of 5:4:1 as described previously (7). Soluble NSO protein was entrapped in the vesicles by a direct hydration and freeze-thaw method described previously (24). The entrapped protein concentration was determined by a BCA protein assay (Pierce) following vesicle lysis with propanol.
Brain histopathology and lesion enumeration.
Evaluation of brain lesions as a disease parameter was based upon previous temporal N. caninum infection studies (19, 20; unpublished observations). Two investigators (T.V.B. and T.F.M.) did all evaluations in a double-blind design. Brain lesions, consisting of random, distinct foci of inflammation and gliosis with or without necrosis in the neuropil, were enumerated from coronal brain sections as described previously (4, 22). The mean number of lesions was calculated within each group, and differences between groups were compared by a Mann-Whitney test (P ≤ 0.05). The character of the inflammatory infiltrates comprising the brain lesions was assessed qualitatively by histopathology.
Splenocyte IL-4 and IFN-γ analysis.
The levels of N. caninum antigen-specific IFN-γ and IL-4 cytokine secretion from cultured splenocytes were measured by ELISA as described previously (4, 22). Dispersed spleen cells were plated at a density of 3 × 106 cells/well. Six wells were incubated for each lymphocyte preparation and consisted of three wells stimulated with 10 μg of NSO antigen per well and three unstimulated wells that served as negative controls. After 72 h of incubation, supernatants from the cells were collected for measurement of IL-4 and IFN-γ levels. Standards consisted of serial dilutions of known concentrations of recombinant IL-4 and recombinant IFN-γ, with the highest concentration of IL-4 being 2 ng/ml and the highest concentration of IFN-γ being 10 ng/ml. The optical density (OD) at 495 nm (OD495) for each test sample was converted to nanograms per milliliter by regression analysis. The mean ± standard deviation cytokine level for the NSO-immunized and adjuvant control-treated groups were calculated and analyzed by a Student t test after conditions of population normality were satisfied (P ≤ 0.05).
Serum IgG1 and IgG2a analysis.
Serum was taken from mice via tail vein bleeding prior to the experiment and immediately before challenge infection (9 weeks after the initial immunization) and via cardiac puncture in euthanatized mice evaluated at 6 weeks after the challenge infection. Antibody isotypes were measured by ELISA as described previously (4, 22). Sera from all mice in each experimental group were pooled. A 1:500 dilution of pooled sera from each experimental group was placed in triplicate into 96-well plates coated with NSO. An electronic plate reader at a wavelength of 450 nm well determined the OD of each sample. The background cutoff in OD units was determined from wells containing pooled pretreatment sera from the entire group of mice. OD450s and IgG1:IgG2a ratios were compared between treatment groups by two-way analysis of variance (P ≤ 0.05).
RESULTS
Western blot characterization of NSO antigen.
Western blot analysis was done to affirm that the NSO antigen preparation used as immunogen had a wide range of N. caninum antigen sizes. NSO was separated under reducing conditions by SDS-PAGE and was analyzed by Western blot analysis with polyclonal antibodies. Individually, antiserum from a hyperimmunized goat (VMRD Inc.) and from a naturally infected cow bound multiple antigens between 12 and 190 kDa (Fig. 1). In addition, multiple shared antigens ranging from 15 to 190 kDa were recognized by both antisera.
FIG. 1.
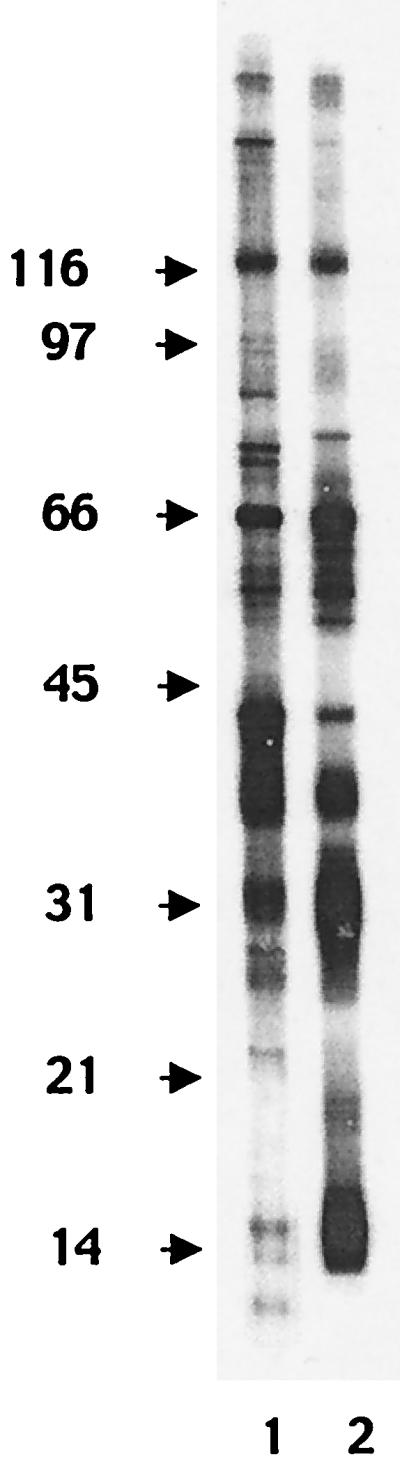
Western blot analysis of NSO antigen. Lane 1, probed with antiserum (1:100) from a goat experimentally infected with N. caninum; lane 2, probed with antiserum (1:100) from a naturally infected cow with confirmed N. caninum-induced abortion. The positions of the molecular mass standards (in kilodaltons) are indicated on the left.
Parasite-induced brain lesions.
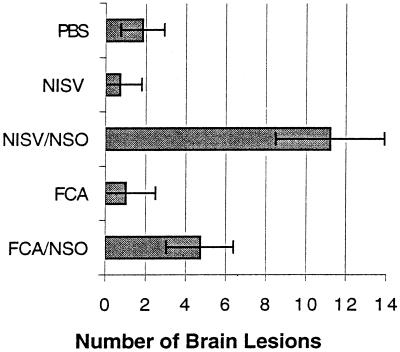
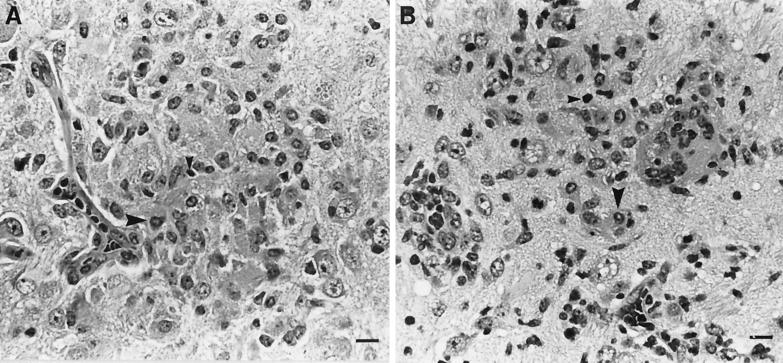
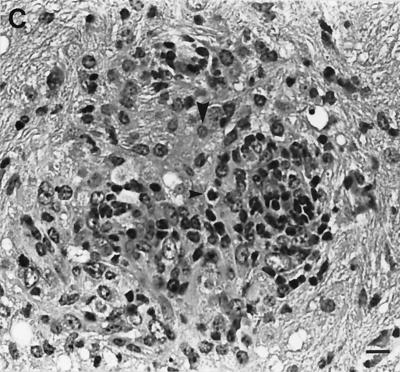
Following challenge infection with a virulent dose of N. caninum, foci of brain inflammation and necrosis were enumerated in groups of mice immunized with NSO in FCA adjuvant, NSO incorporated into NISVs, FCA alone, NISVs alone, or PBS alone (Fig. 2). At least 75% of mice immunized with NISVs-NSO and FCA-NSO developed clinical neurological disease (kyphosis, ataxia, and head tilt), while all mice injected with NISVs, FCA, or PBS alone prior to challenge infection remained clinically normal. The number of brain lesions was significantly higher (P ≤ 0.05) in NISV-NSO-immunized mice (11.2 ± 2.7) than in NISV-immunized control mice (0.7 ± 1.2). FCA-NSO-immunized mice also had a larger number of brain lesions (4.71 ± 1.68) than FCA-treated control mice (1.0 ± 1.5), but the difference was not significant (P = 0.09). Both groups of mice immunized with NISVs-NSO and FCA-NSO had significantly larger (P ≤ 0.05) numbers of brain lesions than PBS-treated control mice (1.8 ± 1.3). There were also increased numbers of brain lesions in NISV-NSO-immunized mice than FCA-NSO-immunized mice, but the difference was not statistically significant (P = 0.07). The composition of individual brain lesions, as determined by histopathology, was relatively similar in all groups of mice and consisted of compact infiltrates of macrophages admixed with lymphocytes and occasional plasma cells (Fig. 3). Brain lesions in NISV-NSO-immunized mice tended to contain fewer lymphoid cells (Fig. 3A), but the composition of inflammatory infiltrates was variable between individual mice within each group, making any generalized statements about the overall character of brain inflammation between groups capricious.
FIG. 2.
Quantitative analysis of N. caninum-induced brain lesions. The total foci of necrosis were enumerated in four coronal brain sections.
FIG. 3.
Representative histopathology of brain lesions induced by N. caninum. All experimental groups had compact collections of macrophages (large arrowheads) and lymphoid cells (small arrowheads) in the neuropil often associated with gliosis and central necrosis. (A) NISV-NSO-immunized mice. (B) Mice immunized with NISVs alone. (C) PBS-treated control mice. Hematoxylin and eosin stain was used. Bar, 16 μm.
Parasite-specific IL-4 and IFN-γ from splenocytes in vitro.
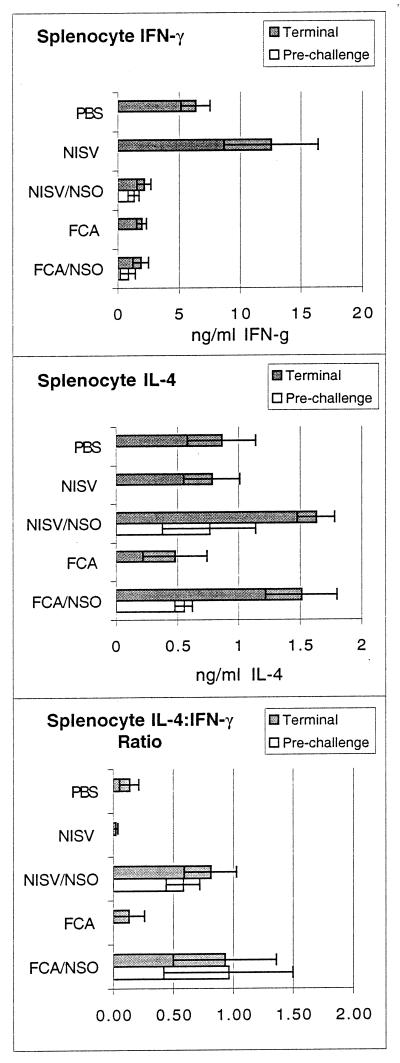
To determine whether the severity of brain pathology between treatment groups was associated with a systemic immune cytokine bias, the level of parasite-specific IL-4 and IFN-γ secretion was measured in supernatants of cultured splenocytes stimulated in vitro with NSO (Fig. 4). Prior to challenge infection, mice immunized with either NISVs-NSO or FCA-NSO had a mixed IL-4 and IFN-γ response and a relatively high IL-4:IFN-γ ratio. Mice from the NISV- and FCA-treated control groups produced no parasite-specific IL-4 or IFN-γ before challenge infection. Six weeks following challenge infection, at the termination of the experiment, mice immunized with NISVs-NSO had significantly lower levels of IFN-γ, significantly higher levels of IL-4, and a significantly higher IL-4:IFN-γ ratio than control mice inoculated with NISVs or PBS alone prior to challenge infection (P < 0.05). Comparison of the FCA-NSO-immunized group and the group treated with FCA alone revealed relatively equal levels of production of IFN-γ but significantly higher levels of IL-4 secretion and a significantly higher IL-4:IFN-γ ratio (P < 0.05) in the FCA-NSO-immunized mice after challenge.
FIG. 4.
Secretion of antigen-specific IFN-γ and IL-4 from splenocytes in vitro. Prechallenge values are for mice killed immediately prior to challenge infection. Terminal values are for mice killed 6 weeks after challenge infection. IFN-g, IFN-γ.
Parasite-specific IgG1 and IgG2a production in vivo.
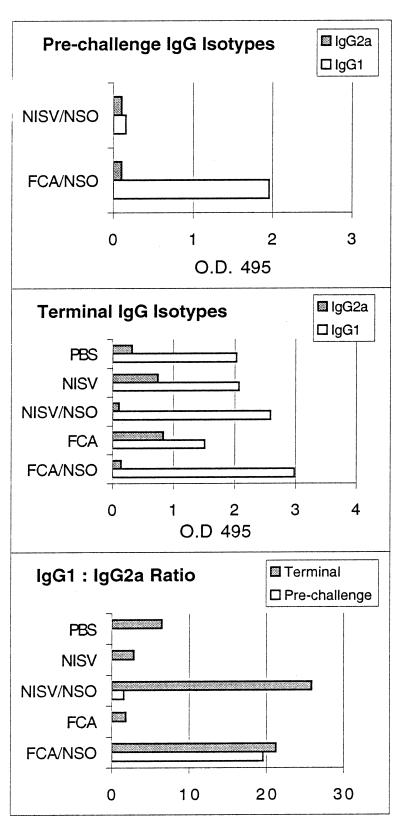
Parasite-specific serum antibody isotype was measured as an in vivo indicator of antigen-specific type 1 or type 2 immune response (36) (Fig. 5). Prior to challenge infection, NISV-NSO-inoculated mice produced low levels of both IgG1 and IgG2a, while FCA-NSO-inoculated mice produced abundant IgG1 and had a high IgG1:IgG2a ratio. Mice from the NISV- and FCA-treated control groups produced no parasite-specific IgG1 or IgG2a prior to challenge infection. At 6 weeks after the challenge infection, sera from mice immunized with either NISVs-NSO or FCA-NSO, both groups of which displayed higher levels of parasite-specific IL-4 production in vitro than the controls, had significantly lower levels of parasite-specific IgG2a and a significantly higher IgG1:IgG2a ratio than control mice inoculated with NISVs, FCA, or PBS alone prior to challenge infection (P ≤ 0.05).
FIG. 5.
Production of antigen-specific serum antibody isotypes IgG1 and IgG2a in vivo. Prechallenge values are for mice killed immediately prior to challenge infection. Terminal values are for mice killed 6 weeks after challenge infection.
DISCUSSION
Immunization of BALB/c mice with a soluble antigen derived from whole N. caninum tachyzoites resulted in exacerbated encephalitis and clinical neurological disease following challenge with virulent parasites. While strictly polarized type 1 or type 2 immune responses were not demonstrated, increased disease and severity of brain lesions following immunization with NSO and NISV adjuvant or FCA was associated with a bias toward a type 2 response. There was a significantly increased level of parasite-specific IL-4 secretion from splenocytes, a significantly decreased ratio of IFN-γ:IL-4 in vitro, and a significantly increased ratio of parasite-specific IgG1:IgG2a production in vivo that correlated with more severe disease parameters.
In previous studies of murine neosporosis, disease was also associated with IL-4 production (22). There was an increased severity of encephalitis and an increased parasite load, as measured by quantification of brain lesions and counting of the intralesional tachyzoites by immunohistochemistry, in mouse strains that responded to N. caninum infection with a high level of IL-4 secretion and a high level of IgG1 production compared to those in mouse strains that lacked significant levels of IL-4 secretion and that produced primarily IgG2a in response to infection. The interpretation that increased parasite load is due to IL-4 production is supported by recent studies showing that maternal in vivo neutralization of IL-4 concurrent with live tachyzoite priming significantly reduced the level of N. caninum transplacental transmission following challenge infection in a BALB/c mouse model (21). In addition, the present data show better control of disease and brain lesions in mice immunized with FCA alone than in FCA-NSO-immunized mice, despite a relative low level of production of IFN-γ, a cytokine generally associated with protection against intracellular protozoa. This suggests that protection in the mice immunized with FCA alone was due to low levels of production of IL-4 and IgG1. Thus, multiple data from the murine neosporosis model are consistent with the hypothesis that parasite-specific type 2 immune responses enhance the pathologic effects of N. caninum infection.
The association of enhanced disease with increased IL-4 production also occurs with other intracellular protozoan, fungal, and bacterial infections. In vivo neutralization of IL-4 results in increased survival in mice infected with Toxoplasma gondii, Leishmania major, Listeria monocytogenes, and Candida albicans (11, 30, 31, 39). Although toxoplasmosis-susceptible strains of IL-4 −/− knockout mice have increased rates of mortality during acute infection compared to the rates for wild-type IL-4 +/+ mice (28, 37), during chronic infection IL-4 −/− knockout mice have decreased numbers of brain cysts and brain pathology (28). In addition, pregnant IL-4 −/− knockout mice are less susceptible to both maternal infection and congenital transmission than control IL-4 +/+ mice following infection with T. gondii (1, 38).
The specific subfraction(s) of the NSO antigen that induced a disease-promoting type 2 response was not determined in this study. Western blot analysis of NSO revealed multiple large and small N. caninum-specific antigens and multiple previously published immunodominant antigens (3, 5, 12, 14). Disease-exacerbating antigens have also been identified during immunization studies in mice challenged with other protozoan parasites such as L. major and T. gondii (2, 15, 33). These previous studies and results from the current study emphasize the need to identify specific parasite antigens that either induce or impede development of protective immune responses to protozoan infections so that effective vaccines can be designed.
NISVs are multilamellar synthetic phospholipid vesicles that efficiently entrap soluble protein antigens and have been shown to induce type 1 immune responses and protective immunity to toxoplasmosis (7, 29). Even antigens shown to be nonprotective can be directed toward offering protective immunity with liposoidal adjuvants (8, 23). Why nonionic surfactant vesicles were unsuccessful as an adjuvant for NSO to induce a type 1 immune response in the current study was not determined. Previous studies have shown that the use of high doses of antigen and the subcutaneous inoculation route, as carried out in the current study, can result in a bias toward disease-enhancing type 2 responses (13, 17, 26), regardless of the adjuvant used. A similar phenomenon could be occurring in N. caninum immunizations. If so, the use of more potent type 1 cytokine adjuvants, such as IL-12 or IL-15, or other routes and doses of antigen delivery may be necessary to induce immune protection.
ACKNOWLEDGMENTS
We are indebted to the histology laboratory of the Washington Animal Disease Diagnostic Laboratory and the Laboratory Animal Resource Center at Washington State University for maintenance of the animals used in this study. We thank D. P. Knowles for critical review of the manuscript.
This work received support from Animal Health Formula Funds from CSRS, U.S. Department of Agriculture, to Washington State University (grant 0168435).
REFERENCES
- 1.Alexander J, Jebbari H, Bleuthmann H, Brombacher F, Roberts C. The role of IL-4 in adult acquired and congenital toxoplasmosis. Int J Parasitol. 1998;28:113–120. doi: 10.1016/s0020-7519(97)00168-9. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J, Roberts C, Brewer J. Progress towards the development of a vaccine against congenital toxoplasmosis: identification of protective antigens and the selection of appropriate adjuvants. In: Smith J E, editor. NATO-ISI on toxoplasmosis. Vol. 78. Cambridge, Mass: Elsevier Publications; 1993. pp. 217–229. [Google Scholar]
- 3.Baszler T V, Knowles D P, Dubey J P, Gay J M, Mathison B A, McElwain T F. Serological diagnosis of bovine neosporosis by Neospora caninum monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J Clin Microbiol. 1996;34:1423–1428. doi: 10.1128/jcm.34.6.1423-1428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baszler T, Long M, McElwain T, Mathison B. Interferon-γ and IL-12 mediate protection to acute Neospora caninum infection in BALB/c mice. Int J Parasitol. 1999;29:1635–1646. doi: 10.1016/s0020-7519(99)00141-1. [DOI] [PubMed] [Google Scholar]
- 5.Bjerkas I, Jenkins M C, Dubey J P. Identification and characterization of Neospora caninum tachyzoite antigens useful for diagnosis of neosporosis. Clin Diagn Lab Immunol. 1994;1:214–221. doi: 10.1128/cdli.1.2.214-221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorkman C, Johansson O, Stenlund S, Holmdahl O J, Uggla A. Neospora species infection in a herd of dairy cattle. J Am Vet Med Assoc. 1996;208:1441–1444. [PubMed] [Google Scholar]
- 7.Brewer J, Alexander J. The adjuvant activity of non-ionic surfactant vesicles (niosomes) on the BALB/c humoral response to bovine serum albumin. Immunology. 1992;75:570–575. [PMC free article] [PubMed] [Google Scholar]
- 8.Bulow R, Boothroyd J C. Protection of mice from fatal Toxoplasma gondii infection by immunization with p30 antigen in liposomes. J Immunol. 1991;147:3496–3500. [PubMed] [Google Scholar]
- 9.Dubey J P, Lindsay D S. A review of Neospora caninum and neosporosis. Vet Parasitol. 1996;67:1–59. doi: 10.1016/s0304-4017(96)01035-7. [DOI] [PubMed] [Google Scholar]
- 10.Erp E, Ristic M, Carson C. Fluorescein diacetate staining of Anaplasma marginale as a possible measure of viability. Am J Vet Res. 1978;39:344–346. [PubMed] [Google Scholar]
- 11.Haak-Frendscho M, Brown J, Iizawa Y, Wagner R, Czuprynski C. Administration of anti-IL-4 monoclonal antibody 11B11 increases the resistance of mice to Listeria monocytogenes infection. J Immunol. 1992;148:3978–3985. [PubMed] [Google Scholar]
- 12.Harkins D, Clements D N, Maley S, Marks J, Wright S, Esteban I, Innes E A, Buxton D. Western blot analysis of the IgG responses of ruminants infected with Neospora caninum and with Toxoplasma gondii. J Comp Pathol. 1998;119:45–55. doi: 10.1016/s0021-9975(98)80070-4. [DOI] [PubMed] [Google Scholar]
- 13.Hosken N A, Shibuya K, Heath A W, Murphy K M, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha/beta transgenic model. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howe D K, Crawford A C, Lindsay D, Sibley L D. The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii. Infect Immun. 1998;66:5322–5328. doi: 10.1128/iai.66.11.5322-5328.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasper L H, Currie K M, Bradley M S. An unexpected response to vaccination with a purified major membrane tachyzoite antigen (p30) of Toxoplasma gondii. J Immunol. 1985;134:3426–3431. [PubMed] [Google Scholar]
- 16.Khan I A, Schwartzman J D, Fonseka S, Kasper L H. Neospora caninum: role for immune cytokines in host immunity. Exp Parasitol. 1997;85:24–34. doi: 10.1006/expr.1996.4110. [DOI] [PubMed] [Google Scholar]
- 17.Liew F Y, Hodson K, Lelchuk R. Prophylactic immunization against experimental leishmaniasis. VI. Comparison of protective and disease promoting T cells. J Immunol. 1987;139:3112–3117. [PubMed] [Google Scholar]
- 18.Lindsay D S, Dubey J P. In vitro development of Neospora caninum (Protozoa: Apicomplexa) from dogs. J Parasitol. 1989;75:163–165. [PubMed] [Google Scholar]
- 19.Lindsay D S, Dubey J P. Infections in mice with tachyzoites and bradyzoites of Neospora caninum (Protozoa: Apicomplexa) J Parasitol. 1990;76:410–413. [PubMed] [Google Scholar]
- 20.Lindsay D S, Lenz S D, Cole R A, Dubey J P, Blagburn B L. Mouse model for central nervous system Neospora caninum infections. J Parasitol. 1995;81:313–315. [PubMed] [Google Scholar]
- 21.Long M, Baszler T. Neutralization of maternal IL-4 modulates congenital protozoal transmission: comparison of innate and acquired immune responses. J Immunol. 2000;164:4768–4774. doi: 10.4049/jimmunol.164.9.4768. [DOI] [PubMed] [Google Scholar]
- 22.Long M T, Baszler T V, Mathison B A. Comparison of intracerebral parasite load, lesion development, and systemic cytokines in mouse strains infected with Neospora caninum. J Parasitol. 1998;84:316–320. [PubMed] [Google Scholar]
- 23.Lunden A, Lovgren K, Uggla A, Araujo F G. Immune responses and resistance to Toxoplasma gondii in mice immunized with antigens of the parasite incorporated into immunostimulating complexes. Infect Immun. 1993;61:2639–2643. doi: 10.1128/iai.61.6.2639-2643.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer L D, Hope M J, Cullis P R, Janoff A S. Solution distributions and trapping efficiencies observed in freeze-thawed multilamellar vesicles. Biochem Biophys Acta. 1985;817:193–196. doi: 10.1016/0005-2736(85)90084-7. [DOI] [PubMed] [Google Scholar]
- 25.Mossmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. An Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 26.Nabors G S, Nolan T, Croop W, Li J, Farrell P. The influence of the site of parasite inoculation on the development of Th1 and Th2 type immune responses in (BALB/c X C57BL/6) F1 mice infected with Leishmania major. Parasite Immunol. 1995;17:569–579. doi: 10.1111/j.1365-3024.1995.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 27.Pare J, Thurmond M C, Hietala S K. Congenital Neospora caninum infection in dairy cattle and associated calfhood mortality. Can J Vet Res. 1996;60:133–139. [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts C, Ferguson D, Jebbari H, Satoskar A, Bleuthmann H, Alexander J. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect Immun. 1996;64:897–904. doi: 10.1128/iai.64.3.897-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts C W, Brewer J M, Alexander J. Congenital toxoplasmosis in the BALB/c mouse: prevention of vertical transmission and fetal death by vaccination. Vaccine. 1994;12:1389–1396. doi: 10.1016/0264-410x(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 30.Romani L, Mencacci A, Grohmann U, Mocci S, Mosci P, Puccetti P, Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1 associated immunity in murine candidiasis. J Exp Med. 1992;176:19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadick M D, Heinzel F P, Holaday B J, Pu R T, Dawkins R S, Locksley R M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-dependent mechanism. J Exp Med. 1990;171:115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schares G, Peters M, Wurm R, Barwald A, Conraths F J. The efficiency of vertical transmission of Neospora caninum in dairy cattle analysed by serological techniques. Vet Parasitol. 1998;80:87–98. doi: 10.1016/s0304-4017(98)00195-2. [DOI] [PubMed] [Google Scholar]
- 33.Scott P, Pearce E, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in a murine model. II. Immunologic properties of protective and nonprotective subfraction of a soluble promastigote extract. J Immunol. 1987;139:3118–3125. [PubMed] [Google Scholar]
- 34.Seder R, Paul W. Acquisition of lymphokine-producing phentype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 35.Sharma S D, Mullenax J, Araujo F G, Erlich H A, Remington J S. Western blot analysis of the antigens of Toxoplasm gondii recognized by human IgM and IgG antibodies. J Immunol. 1983;131:977–983. [PubMed] [Google Scholar]
- 36.Snapper C M, Finkelman C R. Immunoglobulin class switching. In: Paul W E, editor. Fundamental immunology. 3rd ed. New York, N.Y: Raven Press; 1994. pp. 837–864. [Google Scholar]
- 37.Suzuki Y, Yang Q, Yang S, Nguyen N, Lim S, Liesenfeld O, Kojima T, Remington J. IL-4 is protective against development of toxoplasmic encephalitis. J Immunol. 1996;157:2564–2569. [PubMed] [Google Scholar]
- 38.Thouvenin, M., E. Candolfi, O. Villard, J. Klein, and T. Kien. Immune response in a murine model of congenital toxoplasmosis: increased susceptibility of pregnant mice and transplacental passage of Toxoplasma gondii are type 2-dependent. Parasitologia 39:279–283. [PubMed]
- 39.Villard O, Candolfi E, Despringre J, Derouin F, Marcellin L, Viville S, Kien T. Protective effect of low doses of an anti-IL-4 monoclonal antibody in a murine model of acute toxoplasmosis. Parasite Immunol. 1995;17:233–236. doi: 10.1111/j.1365-3024.1995.tb01020.x. [DOI] [PubMed] [Google Scholar]