Abstract
Population-based studies of Staphylococcus aureus contribute to understanding the epidemiology of S. aureus infection. We enrolled surgical inpatients admitted to an African tertiary-care hospital in order to prospectively analyze the nosocomial impact of S. aureus. Data collection included an active sampling of the anterior nares and infectious foci within 48 h after admission and subsequently when clinically indicated. All S. aureus isolates were spa and agr genotyped. Possession of Panton-Valentine leukocidin (PVL) and other toxin genes was determined. We analyzed antibiotic susceptibility profiles by VITEK 2 systems and verified methicillin-resistant S. aureus (MRSA) by mecA/C PCR. Among 325 patients, 15.4% carried methicillin-susceptible S. aureus (MSSA) at admission, while 3.7% carried MRSA. The incidence densities of nosocomial infections due to MSSA and MRSA were 35.4 and 6.2 infections per 10,000 patient-days, respectively. Among all 47 nosocomial infections, skin and soft-tissue (40.4%) and bones or joints’ (25.5%) infections predominated. Six (12.7%) infection-related S. aureus isolates harbored PVL genes including two (4.2%) MRSA: overall, seventeen (36.2%) isolates carried pyrogenic toxin superantigens or other toxin genes. This study illustrates the considerable nosocomial impact of S. aureus in a Nigerian University hospital. Furthermore, they indicate a need for effective approaches to curtail nosocomial acquisition of multidrug-resistant S. aureus.
Keywords: Staphylococcus aureus, MRSA, nosocomial infection, surgical patients, skin and soft-tissue infections, Panton-Valentine leukocidin, pyrogenic toxin superantigens, exfoliative toxins, epidermal differentiation inhibitors, agr
1. Introduction
Staphylococcus aureus infections are one of the leading causes of morbidity and mortality worldwide, and their management is challenged by the emergence of MRSA in the past decades [1]. This opportunistic pathogen causes a plethora of infections, ranging from mild skin and soft-tissue abscesses to potentially life-threatening conditions including, e.g., bacteremia, pneumonia, septic arthritis, osteomyelitis, and toxin-mediated illnesses, resulting in increased antibiotic consumption and healthcare costs [2,3]. Presumably, the risk for S. aureus infection depends on both patient-related factors and strain-specific differences [1]. Numerous reports described the changing epidemiology and taxonomy of the S. aureus complex and addressed the evolution of hospital-adapted, community-associated, and livestock-originated S. aureus clonal lineages, as well as changes in antibiotic resistance [4,5,6,7,8,9,10]. In contrast to developed countries, only a limited number of studies elucidated the nosocomial impact of S. aureus acquisition in risk patients in resource-limited settings, particularly in sub-Saharan Africa excluding South Africa [11,12,13]. Therefore, analyzing the association with S. aureus infection, of patient-specific risks and comorbidities as well as the ecological reservoirs of locally and regionally circulating S. aureus genetic lineages can enhance a more targeted, secondary prevention of S. aureus infection. Hence, studying the specific characteristics and molecular epidemiology of S. aureus in African countries is needed, because, compared with other continents, Africa has different demographics, such as a lower average life expectancy, higher population growth rates and higher total fertility rates, resulting in a generally younger population [14]. In addition, often less stringent antibiotic use policies both in the community and in hospitals and high rates of anti-staphylococcal drug resistance are reported [13,15]. Moreover, toxin gene-harboring S. aureus isolates, in particular, those carrying the PVL-encoding genes, were more prevalent in those strains recovered in studies performed in sub-Saharan African countries [16,17,18,19,20]. Therefore, we aimed (i) to prospectively determine the prevalence of nosocomial S. aureus infection in a cohort of S. aureus carriers and non-carriers; (ii) to determine risk factors for nosocomial S. aureus infection; and (iii) to analyze the contribution of PVL toxin gene-positive S. aureus, to nosocomial S. aureus infection.
2. Results
2.1. Baseline Characteristics of the Enrolled Surgical Inpatient Cohort and S. aureus Carriage
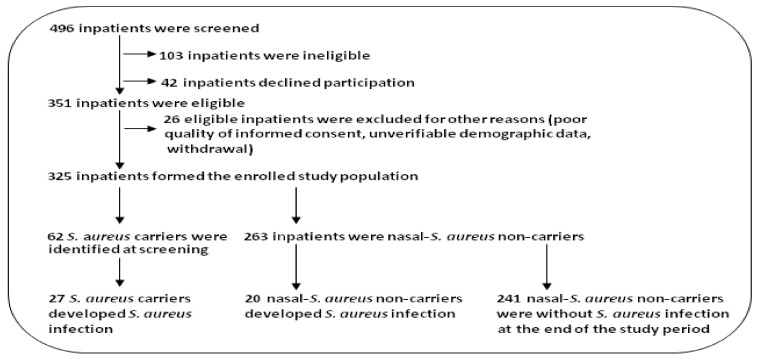
A total of 325 of 496 surgical inpatients were enrolled (Figure 1). These comprised 182 males and 143 females (mean age ± SD in years: 36.0 ± 18.6 and 37.4 ± 20.3, respectively).
Figure 1.
Flow chart of patient screening and passage through the present study.
Among the 325 patients, 62 (19.0%) nasal S. aureus carriers were identified comprising twelve (3.7%) MRSA and 50 (15.4%) MSSA carriers (the latter including three penicillin-susceptible isolates). Among these 62 carriers, 8 (i.e., 2.5% of all 325 patients) were PVL gene-positive MSSA nasal carriers (16.0% of all MSSA carriers); 3 (i.e., 0.9% of all 325 patients) were PVL gene-positive MRSA nasal carriers (25% of all MRSA carriers). Two-hundred-seventy-nine inpatients (85.8%) had various co-morbidities on admission.
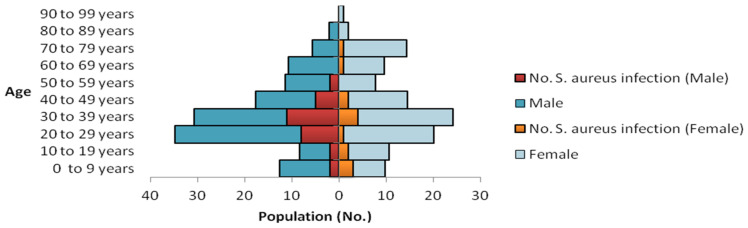
The characteristics of all enrolled inpatients are shown in Table 1. Overall, 47 patients developed S. aureus infections. Hence, the incidence density of S. aureus infections per 10,000 patient-days was 41.6 (i.e., 35.4 for MSSA and 6.2 for MRSA). The population age–sex structure of the cohort is shown in Figure 2.
Table 1.
Baseline characteristics of the surgical inpatient cohort (n = 325).
| Characteristics * | Male | Female | Total |
|---|---|---|---|
| Total number of patients enrolled | 182 | 143 | 325 |
| Age, mean ± SD | 36.0 ± 18.6 | 37.4 ± 20.3 | 36.6 ± 19.4 |
| Median (range) | 35.6 (1–83) | 30.4 (1–90) | 35.8 (1–90) |
| LOS, mean days ± SD | 33.9 ± 32.5 | 36.0 ± 40.3 | 34.8 ± 36.1 |
| Specialty affiliation of patients: | |||
| General Surgery | 12 (6.6) | 13 (9.1) | 25 (7.7) |
| Cardiothoracic surgery | 1 (0.5) | 2 (1.4) | 3 (1.0) |
| Abdominal surgery | 9 (4.9) | 18 (12.6) | 27 (8.3) |
| Plastic surgery | 25 (13.7) | 5 (3.5) | 30 (9.2) |
| Orthopedic surgery | 119 (65.4) | 77 (53.8) | 196 (60.3) |
| Urology | 7 (2.1) | 2 (1.4) | 9 (2.7) |
| Gynecology | - | 13 (9.0) | 13 (4.0) |
| Pediatric surgery | 8 (4.4) | 11 (7.7) | 19 (5.8) |
| Unspecified type of surgery | 1 (0.5) | 2 (1.4) | 3 (0.9) |
| No. of patients with S. aureus carriage within 48 h after admission | 45 (24.7) | 17 (11.9) | 62 (19.0) |
| No. of patients with MRSA carriage | 8 (4.4) | 4 (2.8) | 12 (3.7) |
| No. of patients with MSSA carriage | 37 (20.3) | 13 (9.0) | 50 (15.4) |
| No. of patients with PVL-positive S. aureus carriage: | 7 (3.8) | 4 (2.8) | 11 (3.4) |
| Of which PVL-positive MSSA carriage | 5 (2.7) | 3 (2.1) | 8 (2.5) |
| Of which PVL-positive MRSA carriage | 2 (1.1) | 1 (0.7) | 3 (0.9) |
| No. of patients without S. aureus carriage at screening | 136 (74.7) | 127 (88.8) | 263 (80.9) |
| Total no. of patients with co-morbid conditions | 153 (84.1) | 129 (90.2) | 279 (85.8) |
| McCabe score (at screening): Mean (Range) | 1.0 (1.0–2.0) | 1.1 (1.0–2.0) | 1.1 (1.0–2.0) |
| No. patients receiving antibiotics without activity against MRSA | 156 (85.7) | 106 (74.1) | 262 (80.6) |
| No. patients receiving antibiotic treatment active against MRSA | 1 (0.5) | 2 (1.4) | 3 (1.0) |
| No. of patients who underwent surgical procedures | 138 (75.8) | 98 (68.5) | 236 (72.6) |
| No. of patients with two or more surgical procedures | 56 (30.7) | 34 (23.7) | 90 (27.7) |
| Surgical procedures (CDC classification) | |||
| Clean | 26 (12.7) | 31 (22.8) | 57 (16.7) |
| Clean-Contaminated | 178 (87.3) | 106 (77.9) | 284 (83.3) |
| Emergency | 18 (8.8) | 15 (11.0) | 33 (9.7) |
| Elective | 186 (91.2) | 122 (88.9) | 308 (90.3) |
| Total number of surgical procedures | 204 (59.8) | 137 (40.2) | 341 (100) |
| Incomplete medical records | 9 (4.9) | 5 (3.5) | 14 (4.3) |
| Intravenous devices > 24 h | 99 (54.4) | 71 (49.6) | 170 (52.3) |
| Urinary catheters > 24 h | 52 (28.5) | 41 (28.6) | 93 (28.6) |
| Hemodialysis | 1 (0.5) | 0 (0.0) | 1 (0.31) |
| Total no. of inpatient-days | 6,137 | 5,155 | 11,292 |
* Data are no. (%) of patients, except indicated differently. (mean McCabe score (range): 1.1 (1.0–2.0). For all inpatients enrolled, an aggregate of 11,292 inpatient-days was accumulated during the period of the study.
Figure 2.
Population age–sex structure of the surgical inpatient cohort (n = 325). Superposed within each age group is the age-group specific occurrence of S. aureus infection observed within the cohort.
2.2. Characteristics of Inpatients with Nosocomial S. aureus Infection
In addition to well-known characteristics (recent hospital stay, surgery or antibiotic use), inpatients with nosocomial S. aureus infection (n = 47) differed from those without S. aureus infection with respect to sex and age distribution, the highest odds for S. aureus infection was found among males within the age subgroup 30–39 (15/47; 31.9% vs. 56/278; 20.1%, p = 0.071, Odds Ratio (OR) = 1.86, 95% confidence interval (CI): 0.87–3.82, Figure 2). While MSSA carriage was clearly associated with nosocomial S. aureus infection (24/47; 51.06% vs. 25/278; 8.9%, p = 0.0001, OR = 10.56, 95% CI: 4.90–22.6), there was no significant difference in MRSA carriage between the subgroup with S. aureus infection and those without S. aureus infection (3/47; 6.4% vs. 8/278; 2.8%, p = 0.219, OR 2.30, 95% CI: 0.38–10.03). The proportion of patients who received antibiotics was different between the subgroup with and those without S. aureus infection (46/47; 97.8% vs. 217/278; 78.1%, p = 0.001)). In addition, emergency procedures or two or more surgical procedures were more frequent among patients with nosocomial S. aureus infection (11/47; 23.4% vs. 22/278; 7.9% p = 0.007, OR 3.06, 95% CI: 1.13-7.63, or 26/47; 55.3% vs. 60/278; 21.6%, p = 0.0001, OR 4.50, 95% CI: 2.25–9.01 respectively). Moreover, an association was found with nosocomial S. aureus infection among pediatric surgery patients (9/47; 19.1% vs. 10/278; 3.6%, p = 0.0002, OR 6.35, 95% CI: 2.12–18.49)). Furthermore, bone and skin and soft-tissue diseases were more frequently observed in patients with S. aureus infection (22/47; 46.8% vs. 35/278; 12.6%, p = 0.0001, OR 6.11, 95% CI: 2.93–12.59). There was a difference in mean lengths of stay (LOS) ± SD and variance of LOS between the subgroup with (51.36 ± 45.96), and without (31.85 ± 32.67) S. aureus infection (p = 0.007; for variance: p = 0.0006) (Supplementary Table S2).
2.3. Antibiotic Resistance Geno- and Phenotypes of Infection-Related S. aureus
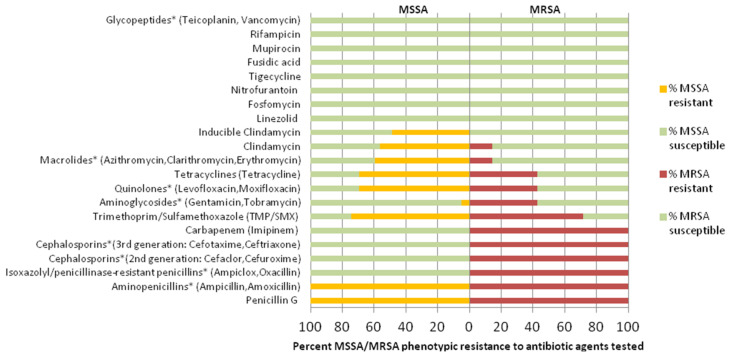
Of all infection-related isolates, 40 S. aureus isolates (85.1%) were susceptible to methicillin (including one penicillin-susceptible S. aureus isolate) while 7 were MRSA isolates (14.9%, all harboring mecA). The antibiotic resistance pattern of MSSA and MRSA isolates is shown (Figure 3).
Figure 3.
Antibiotic class/agent-specific resistance profiles of nosocomial infection-related MSSA (n = 40) and MRSA (n = 7) in the form of a gapped population pyramid (MSSA left; MRSA right).
Among all 47 isolates associated with infections, 61.7% were multidrug-resistant (MDR) according to a consensus definition (either MRSA or resistance to at least 1 antibiotic of ≥3 antibiotic classes) [21]. Table 2 shows resistance profiles for 62 carriage-associated S. aureus isolates derived from patients within 48 h after admission, of which 74.2% were MDR.
2.4. Distribution of Toxin Genes, Agr Types and Spa/MLST Types
All 47 infection-related S. aureus isolates harbored the nuc gene. The distribution of toxin-encoding genes and genotypes in these isolates is shown in Table 2. Briefly, all isolates carried the gamma-hemolysin (hlg) gene, 15 carried at least one PTSAg or other toxin gene (32.0%), while 13 carried two or more toxin gene combinations (27.6%). Overall, 63.8% (30/47) isolates did not harbor any of the toxin genes tested. The staphylococcal enterotoxin A (sea), B (seb), C (sec), D (sed), D–J (sed-sej) combination, and E (see) genes occurred in 17.0%, 4.2%, 0%, 2.5%, 0% and 0% of the isolates, respectively. Of note, toxic shock syndrome toxin (tst) and exfoliative toxin A-, B-, and C-encoding genes were not found in infection-related S. aureus isolates. Among the non-classical pyrogenic toxin superantigen genes, seg occurred in three (3/47; 6.4%) MSSA isolates (in combination with sei). One isolate harboring PVL-encoding genes and sei was recovered from an episode of necrotizing fasciitis of the left lower extremity in a 48-year-old female, diabetic patient. seh and sej were found in 3/47 (6.4%) and 0% of the isolates, respectively. The epidermal differentiation inhibitor gene (edinA) was detected in 2/47 (4.2%) isolates, and were isolated from a pediatric patient with pyomyositis and a young adult patient hospitalized due to trauma arising from superficial partial-thickness burn. lukF and lukS PV genes encoding PVL were detected in six (12.7%) infection-related S. aureus comprising two MRSA (Table 2).
Spa typing identified thirteen distinct spa types among 47 infection-related S. aureus isolates (Table 2) with t091 (n = 28; 59.6%), t084 (n = 4), t355 (n = 3), t127 (n = 2), and t786 (n = 2) occurring more than once. The seven MRSA isolates belonged to t091, t355, t786, t037, and t008. ST7 (n = 27; 57.4%), ST15 (n = 7; 14.9%), and ST1 (n = 3) were predominant among MSSA, while ST88 and ST152 (both n = 2) were most frequent among MRSA isolates.
Table 2.
Pattern analyses of all S. aureus isolates according to antibiotic resistance phenotype, toxin possession, and genotype.
| Pattern | No. of Isolates | *MDR | MSSA/MRSA | P E N |
G E N |
M O X |
E R Y |
C L I |
T E T |
S X T |
V A N |
PTSAgs, lukS-PV and lukF-PV, and EDIN Genes | spa Type | MLST | agr Group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infection-related S. aureus isolates (n = 47) | |||||||||||||||
| A | 2 | Y | MRSA | R | S | S | S | S | S | R | S | ND | t786 | ST88 | IV |
| B | 1 | N | MSSA | R | S | S | S | S | R | R | S | sea | t084 | ST15 | II |
| C | 1 | N | MSSA | R | S | S | S | S | R | R | S | sea, lukS-PV and lukF-PV | t084 | ST15 | IV |
| D | 2 | N | MSSA | R | S | S | S | S | S | R | S | sea, lukS-PV and lukF-PV | t084 | ST15 | II |
| E | 1 | Y | MSSA | R | S | S | R | R | R | R | S | sea, lukS-PV and lukF-PV | t091 | ST7 | III |
| F | 1 | Y | MRSA | R | R | R | S | S | R | R | S | ND | t091 | ST7 | III |
| G | 26 | Y # | MSSA | R | S | R | R | R | R | R | S | ND | t091 | ST7 | III |
| I2 | S6 | S6 | S3 | S8 | |||||||||||
| H | 1 | N | MSSA | R | S | S | S | S | S | S | S | sea, seh | t127 | ST1 | III |
| I | 1 | N | MSSA | R | R | S | S | S | R | S | S | seh | t127 | ST1 | IV |
| J | 1 | N | MSSA | R | S | S | R | S | S | R | S | seg-sei, edinA | t2724 | ST15 | II |
| K | 1 | N | MSSA | S | S | S | S | S | S | R | S | ND | t355 | ST152 | III |
| L | 1 | Y | MRSA | R | S | S | S | S | S | S | S | sei, lukS-PV and lukF-PV | t355 | ST152 | III |
| M | 1 | Y | MRSA | R | S | S | S | S | S | S | S | lukS-PV and lukF-PV | t355 | ST152 | III |
| N | 1 | N | MSSA | R | S | S | S | S | S | R | S | seg-sei, edinA | t311 | ST15 | II |
| O | 1 | N | MSSA | R | S | S | S | S | S | S | S | seg-sei | t2731 | ST5 | III |
| P | 1 | N | MSSA | R | S | S | S | S | S | R | S | sea, seb | t085 | ST1 | II |
| Q | 1 | Y | MSSA | R | R | S | R | S | R | R | S | sed | t064 | ST8 | II |
| R | 1 | Y | MRSA | R | R | I | R | R | R | R | S | ND | t037 | ST241 | III |
| S | 1 | N | MSSA | R | S | S | S | S | S | R | S | seh | t7762 | ST1 | IV |
| T | 1 | Y | MRSA | R | S | I | S | S | R | R | S | sea, seb | t008 | ST8 | III |
| Carriage-associated S. aureus isolates (n = 62) | |||||||||||||||
| A | 29 | Y ## | MSSA | R | S | R | R | R | R | R | S | ND | t091 | ST7 | III |
| I2 | S3 | S5 | S8 | ||||||||||||
| B | 4 | Y | MRSA | R | S | S | S | S | S | R | S | ND | t786 | ST88 | IV |
| C | 1 | Y | MRSA | R | S | S | S | S | S | R | S | sea, luk S-PV and luk F-PV | t786 | ST88 | II |
| D | 2 | Y | MSSA | R | S | S | S | S | R1 | R1 | S | ND | t084 | ST15 | II |
| E | 1 | N | MSSA | S | S | R | S | S | S | R | S | ND | t091 | ST7 | III |
| F | 1 | Y | MSSA | R | S | R | R | R | R | R | S | sea, luk S-PV and luk F-PV | t091 | ST7 | III |
| G | 2 | Y | MSSA | R | R | S | S | S | R | R1 | S | sea, seh, luk S-PV and luk F-PV | t127 | ST1 | IV |
| H | 2 | Y | MRSA | R | S | R1 | R | S | R | R | S | seg-sei, edin-A | t311 | ST15 | II |
| I | 4 | Y | MSSA | R | S | S | R | S | S | R1 | S | seg-sei, edin-A | t2724 | ST15 | II |
| J | 1 | N | MSSA | S | S | S | S | S | S | S | S | seg-sei | t2724 | ST15 | II |
| K | 2 | Y | MRSA | R | S | S | S | S | S | S | S | luk S-PV and luk F-PV | t355 | ST152 | III |
| L | 2 | Y | MRSA | R | S | S | S | S | R | S1 | S | ND | t355 | ST152 | III |
| M | 2 | N | MSSA | R | S | S | S | S | R1 | S | S | ND | t355 | ST152 | III |
| N | 1 | N | MSSA | R | S | S | S | S | R | S | S | sea, seb, sec, luk S-PV and luk F-PV | t064 | ST8 | III |
| O | 1 | Y | MRSA | R | S | S | R | S | R | R | S | sea, seb | t064 | ST8 | III |
| P | 1 | N | MSSA | R | S | S | S | S | R | S | S | luk S-PV and luk F-PV | t4690 | ST153 | III |
| Q | 2 | N | MSSA | R | S | S | S | S | S | R | S | luk S-PV and luk F-PV | t355 | ST152 | III |
| R | 1 | N | MSSA | R | S | S | S | S | S | R | S | sea, luk S-PV and luk F-PV | t084 | S15 | II |
| S | 1 | N | MSSA | S | S | S | S | S | R | S | S | seg-sei | t091 | ST7 | II |
| T | 1 | Y | MSSA | R | S | R | R | R | R | R | S | tst | t091 | ST7 | III |
| U | 1 | Y | MSSA | R | S | R | R | R | R | S | S | ND | t1685 | ST7 | III |
*Multidrug resistant [21]. # Twenty infection-related S. aureus isolates were multidrug resistant. ## Twenty-three carriage-related S. aureus isolates were multidrug resistant. Y- Yes; N- No; spa, staphylococcal protein A; MSSA, methicillin-susceptible S. aureus; MRSA, methicillin resistant S. aureus; PTSAg, pyrogenic toxin superantigen; agr, accessory gene regulator; MLST, multilocus sequence typing; hlg, gamma-hemolysin; sea-sei, staphylococcal enterotoxin genes A-I; edinA, epidermal differentiation inhibitor A gene; lukS-PV and lukF-PV, Panton-Valentine leucocidin genes; ND, none of the tested virulence genes detected. R-resistant, S-susceptible, I-intermediate PEN penicillin, GEN gentamicin, MOX. moxifloxacin, ERY erythromycin, CLI clindamycin, TET tetracycline, SXT trimethoprim/sulfamethoxazole, VAN vancomycin. Detection of susceptible or resistant variant(s) is (are) indicated. (Numbers indicate variants ≥ 1). Grey row(s) identify genotypes common to infection-related and carriage-related S. aureus isolates. Black row indicates putative USA300 ST 8-related, isolate. Mean (median) number of antibiotics to which infection-related S. aureus isolates (n = 47) were resistant: 10.5 (10.4). Mean (median) number of antibiotics to which carriage-related S. aureus isolates (n = 62) bore resistance: 10.5 (9.67). Total number of antibiotics tested, n = 30. Note: pattern nomenclatures (e.g., A, B, C, etc) are for identification purposes. Pattern nomenclatures do not generally suggest pattern correlation across both isolate collections.
All isolates were agr-typeable (agr II: n = 7, 14.9%; agr III: n = 35, 74.4%; and agr IV: n = 5; 10.6%); agr I was not detected. While PTSAg gene-carrying S. aureus more frequently belonged in agr group II (n = 5; 41.6%) and agr group III (n = 4; 28.5%), edinA gene-carrying S. aureus detected in the present study exclusively belonged to agr group II (n = 2; 100%) and strictly carried the seg-sei gene combination.
PVL-encoding genes frequently belonged to agr group III (n = 3; 6.4%) and agr group II (n = 2; 4.2%). MSSA were mostly associated with agr group III (77.5%; 31/40) and agr group II (15.0%; 6/40), while MRSA predominantly belonged to agr group III (57.1%; 4/7) and agr group IV (28.5%; 2/7). Table 2 shows a pattern analysis of the characteristics of infection-related and carriage-associated S. aureus isolated from surgical inpatients. The pattern analysis revealed 15 and 16 distinct genotypes of infection-related and carriage-associated S. aureus strains, respectively (Table 2). Five genotypes (t786/ST88, t084/ST15, t091/ST7, t2724/ST15, and t355/ST152) represented 25.0% and 23.8% of both infection-related and carriage-associated S. aureus genotypes, respectively. In general, MLSTs frequently linked to toxin gene possession included ST1, ST5, ST8, ST15, and ST152 whereas ST7 and ST88 rarely possessed toxin genes. In contrast, ST7, ST8, ST15, ST88, and ST241 displayed a high frequency of antibiotic resistance traits compared to ST1 and ST5, which comprised mostly susceptible strains. A notable combination of toxin gene possession and multi-resistance traits was observed in ST8 and ST15. On the level of individual patients, we found that, of 47 S. aureus isolates identified from sites of nosocomial infection, 27 (57.4%) shared the same spa type as those colonizing the respective patient at (or within 48h after) admission. Among the 27 spa types, heterogeneity within spa types was apparent in two cases.
2.5. Stratification of Patients with Nosocomial S. aureus Infection According to Nasal S. aureus Carriage
Supplementary Table S1 shows the characteristics of patients with S. aureus infection stratified by nasal S. aureus carriage status at admission. Table 3 summarizes the risk factors observed, and those associated with nosocomial S. aureus infection in carriers vs. non-carriers. Overall, the nosocomial S. aureus infection rate was 27/62 (43.5%) among patients carrying S. aureus within the 48 h after admission vs. 20/263 (7.6%) among S. aureus non-carriers (p = 0.0001; RP 5.73 99% CI (2.93–11.13)). We observed that S. aureus carriers and non-S. aureus carriers differed regarding their risk to develop a nosocomial infection. In particular, S. aureus carriers with underlying bone disease (p = 0.008; RP 4.04 (0.54–19.5)), cardiovascular disease (RP 8.50; 1.54–47.04), or diabetes mellitus (RP: 8.00; 0.72–89.03) had a higher risk for nosocomial S. aureus infection compared with non-carriers affected by these comorbidities. In addition, the total number of patient-days was significantly higher among S. aureus carriers with S. aureus infection than among S. aureus non-carriers (p = 0.0005; RP: 5.01 (4.58–5.48)), suggesting added healthcare costs for S. aureus carriers with nosocomial S. aureus infection. Among inpatients who received invasive devices, an intravenous device received intermittently for more than 24 h was associated with a significantly increased risk for S. aureus infection in S. aureus carriers than non-carriers (p = 0.0001; RP 6.00; 1.45–24.82). Similarly, emergency surgery (RP 7.88; 1.32–46.93), or two or more surgeries (RP 3.37; 1.42–7.95) was associated with an increased risk for nosocomial S. aureus infection. Overall, the stratification analyses not only confirmed a higher incidence density of nosocomial S. aureus infection in S. aureus carriers vs. non-carriers (106.7 vs. 23.1 S. aureus infections per 10,000 patient days, respectively), it also proportionately revealed risks associated with nosocomial S. aureus infection in S. aureus carriers versus (vs.) S. aureus non-carriers in the setting studied (Table 3).
Table 3.
Risk factors for nosocomial S. aureus infection in nasal S. aureus carriers and nasal S. aureus non-carriers among surgical inpatients (n = 325).
| Variable |
S. aureus Carriers (n = 62) |
S. aureus Non-Carriers (n = 263) |
p-Value a | Relative Prevalence (99% CI) | ||
|---|---|---|---|---|---|---|
| n | n with Infection (% Carriers) | n | n with Infection (% Non-Carriers) | |||
| Nosocomial S. aureus infection | 62 | 27 (43.5) | 263 | 20 (7.6) | 0.0001 | 5.73 (2.93-11.13) |
| MRSA | 12 | 3 (25.0) | 313 | 4 (1.3) | 0.0001MH | 19.56 (3.18-120.2) |
| Hospitalized ≤ 12 months | 15 | 7 (46.6) | 53 | 7 (13.2) | 0.002MH | 3.53 (1.12-11.2) |
| Antibiotic therapy ≤ 12 months | 6 | 4 (66.6) | 11 | 4 (36.36) | 0.122MH | 1.83 (0.51-6.51) |
| Intravenous device ≤ 12 months | 12 | 5 (41.6) | 27 | 3 (11.1) | 0.015MH | 3.75 (0.71-19.63) |
| Surgery ≤ 12 months | 9 | 3 (33.3) | 24 | 5 (20.8) | 0.231MH | 1.60 (0.33-7.84) |
| LOS (This study) ≤ 3days | 3 | 1 (33.3) | 28 | 0 (0.0) | 0.096F | Undefined |
| LOS ≤ 2 weeks | 14 | 3 (21.4) | 83 | 5 (6.0) | 0.026MH | 3.55 (0.63-20.02) |
| LOS ≤ 4 weeks | 12 | 7 (58.3) | 60 | 6 (10.0) | 0.0001MH | 5.83 (1.79-18.97) |
| LOS ≤ 8 weeks | 19 | 8 (42.1) | 50 | 3 (6.0) | 0.0001MH | 7.02 (1.42-34.75) |
| LOS ≤ 12 weeks | 8 | 5 (62.5) | 25 | 4 (16.0) | 0.005MH | 3.91 (0.98-15.45) |
| LOS > 12 weeks | 6 | 3 (50.0) | 17 | 2 (11.7) | 0.088F | 4.25 (0.57-31.66) |
| Antibiotic use (this study) | 58 | 26 (44.8) | 207 | 20 (9.6) | 0.0001 | 4.64 (2.38-9.01) |
| Intravenous device (˃24 h) | 24 | 9 (37.5) | 64 | 4 (6.3) | 0.0001MH | 6.00 (1.45-24.82) |
| Urinary catheter (˃24 h) | 2 | 1 (50.0) | 8 | 0 (0.0) | 0.200F | Undefined |
| Hemodialysis | 1 | 0 (0.0) | 0 | 0 | - | Undefined |
| Surgery (this study) | 53 | 22 (41.5) | 184 | 15 (8.2) | 0.0001 | 5.09 (2.37-10.92) |
| Emergency | 12 | 9 (75.0) | 21 | 2 (9.5) | 0.0001MH | 7.87 (1.32-46.93) |
| Elective | 41 | 13 (31.7) | 163 | 13 (7.9) | 0.0001 | 3.98 (1.61-9.82) |
| ≥ 2 surgeries (This study) | 29 | 16 (55.2) | 61 | 10 (16.4) | 0.0001 | 3.37 (1.42-7.95) |
| Comorbidities | 49 | 24 (48.9) | 176 | 20 (11.4) | 0.0001 | 4.31 (2.23-8.34) |
| Bone disease. | 17 | 5 (29.4) | 55 | 4 (7.3) | 0.008MH | 4.04 (0.84-19.5) |
| Skin and soft-tissue disease | 7 | 3 (42.8) | 28 | 4 (14.3) | 0.047MH | 3.00 (0.58-15.45) |
| Bone, skin and soft-tissue disease | 19 | 13 (68.4) | 38 | 9 (23.7) | 0.001MH | 2.89 (1.23-6.76) |
| Cardiovascular disease | 2 | 2 (100) | 17 | 2 (11.7) | 0.035F | 8.50 (1.54-47.04) |
| Pulmonary disease | 1 | 0 (0.0) | 4 | 2 (50.0) | 0.600F | 2.0 (0.55-7.25) |
| Genitourinary disease | 1 | 0 (0.0) | 15 | 1 (6.6) | 0.125F | 15.0 (1.25-180.6) |
| Neurological disease | 0 | 0 (0.0) | 8 | 2 (25.0) | - | Undefined |
| Diabetes mellitus | 1 | 1 (100) | 8 | 1 (12.5) | 0.222F | 8.00 (0.72-89.03) |
| Total number of patient-days | 2,529 | 1,441 (56.9) | 8,763 | 996 (11.4) | 0.0001 | 5.01 (4.58-5.48) |
a Uncorrected chi-square p-values were derived (p ≤ 0.01). For uniform reporting of p-values, and for continuity corrections (cell frequencies ≤ 10), Mantel–Haenszel-corrected chi-square p-values superseded crude uncorrected chi-square p-values, Fisher exact p-values were derived for cell frequencies ≤ 2. All p-values are one-tailed. Degree of freedom = 1. Dashed lines indicate any row or column total = 0, for which no statistical- or p-values are derivable. Undefined risk ratios are indicated. MRSA—methicillin-resistant S. aureus, F—Fisher exact p-value, MH—Mantel–Haenszel, LOS—length of stay.
3. Discussion
In this prospective study, we describe the local epidemiology of and risks for nosocomial S. aureus infection in a cohort comprising surgical inpatients. Regarding antibiotic resistance profiles, antistaphylococcal benzylpenicillin or aminopenicillin resistance reached 98% and 100% among infection-related isolates in the present study, consistent with findings from neighboring African countries [13]. All MRSA strains displayed, as expected, complete resistance to isoxazolyl penicillins, second and third generation cephalosporins, and carbapenem, in sharp contrast to MSSA strains which tested completely susceptible to these anti-infective agents. Interestingly, we found apparently lower MRSA vs. MSSA resistance (71.4% vs. 74.3%, respectively) to trimethoprim/sulfamethoxazole, a widely used antibiotic in community settings in this region while a similar pattern of MRSA vs. MSSA resistance was displayed to quinolones (42.8% vs. 69.2%), tetracycline (42.8% vs. 69.2%), macrolides (14.3% vs. 59.6%), and clindamycin (14.3% vs. 56.4%) making us think that appropriate combinations of these antibiotics may preserve efficacy against certain MRSA infections. Moreover, high rates of inducible clindamycin resistance seen among MSSA strains (48.7%) suggest that to checkmate the growing threat of clindamycin resistance among MSSA strains (56.4%), clindamycin use, where possible, should be discouraged for treatment of certain MSSA infections. Overall, all MSSA and MRSA strains were completely susceptible to linezolid, fosfomycin, and vancomycin, in line with previous findings [16]. The same was true for nitrofurantoin, fusidic acid, mupirocin, and rifampicin (Figure 3). However, resistances to fusidic acid [22], mupirocin [23], nitrofurantoin [24], rifampicin [25] and vancomycin [24] have been reported in other studies performed in Africa. During the study period, we were able to include 92.3% (325/351) of the eligible patients screened after admission. We found that in this setting, the crude rate of nosocomial S. aureus infection was 14.5% (47/325). This was equivalent to an overall incidence density of 41.6 S. aureus (MSSA: 35.4 and MRSA: 6.2) infections per 10,000 patient days. This rate is comparable to the findings of a WHO study [26] performed in 14 countries worldwide where, on average, 8.7% of the patients treated in hospitals developed nosocomial infections (due to all causative agents). This rate ranged from 5.0% in North America to 40% in Asia, Latin America, and the Sub-Saharan regions of Africa. Allegranzi et al. found that the prevalence of nosocomial infections was 15.5% in developing countries when pooling data from more than 100 studies performed until 2008 [27]. In our study, surgical-site infection was the most important infection occurring in 5.6% of 341 surgical interventions. Hence, our data are comparable, as S. aureus substantially contributes to the burden of healthcare-associated infection, especially in surgical patients, but exceed infection rates found in surgical patients in industrialized settings. For example, in the Netherlands, 40 of 1,980 patients (2%) developed sternal S. aureus wound infections after cardiac surgery [28], in Switzerland, 1.06% developed MRSA surgical site infections [29], and in the UK, 4.5% developed S. aureus wound infections in a historical cohort in the 1950s [30]. Compared to industrialized settings, one explanation for the high rate of S. aureus infection in the present study could be the very long length of hospitalization of the patients included in this study (>30 days). This exceeds by far the averages for patients in industrialized countries (5–6 days) and increases the risk of nosocomial infection irrespective of the causative agent. The LOS observed might be explained by the fact that most surgical patients enrolled in this study (60%; 196/325, Table 1) were hospitalized for trauma surgery; correspondingly, rates of clean-contaminated procedures were high, > 80% in this study (Table 1, Supplementary Figure S1) in contrast to rates seen in industrialized settings (<10%), altogether suggesting increased antibiotic use or resistance and nosocomial burden of infection, as shown. Importantly, we found that the overall risk of nosocomial S. aureus infection was not equally distributed among the included patients. We observed a clearly significant difference of the infection risk between S. aureus carriers (27/62 (43.5%) or 106.7 infections/10,000 patients-days) vs. S. aureus non-carriers (20/263 (7.6%) or 23.1 infections/10,000 patients-days; Table 3). However, it should be borne in mind that it was not the intent of the present study to prove that carriage of S. aureus in the nasal cavity, which is known as the principle Habitat of S. aureus [31,32,33], caused subsequent S. aureus infection. This subject has been addressed in earlier reports [34,35,36]. Rather, owing to the role of nosocomial reservoirs in the epidemiology of infection, we collected prior screening samples from the anterior nares in order to better understand the extent of involvement of this ecological niche in S. aureus infection in the hospital setting in view, further aiding risk classification, and separation of endogenous S. aureus infection for infection control purposes. Whereas 20 of 263 previously uncolonized patients had at least an episode of S. aureus nosocomial infection, 2 of 27 patients colonized before had an infection caused by a strain associated with a spa type and phenotypic antibiogram other than the colonizing strain. Regarding the types of nosocomial infections detected, S. aureus skin and soft tissue infections’ (SSTIs) isolates predominated (40.4%; 19/47) in this surgical inpatient cohort, mostly associated with multi-resistant MSSA genotypes such as t091 ST7 agr III, t786 ST88 agr IV, t355 ST152 agr III, t127 ST1 agr IV. Intriguingly, ST1-, ST7-, and ST88-related S. aureus SSTIs have been detected in an Asian healthcare setting [37], signaling inter-regional dissemination. S. aureus bone and joint infections (25.5%; 12/47) were also recorded frequently among a subset of patients with a recent history of road traffic-related trauma of the extremities, implying direct implantation or contiguous-focus acquisition. Since S. aureus binding to host tissue is an important precedent to infection, we speculate that the observed predominance in the anatomical distribution of S. aureus infections may reflect, in part, a gradient of S. aureus selectivity for host tissue, i.e., binding avidly to stratified squamous epithelial, or periosteal tissue including bone matrix and collagen, than to columnar or transitional epithelium [38]. An explanation for this discrepancy also could be that most of the surgical-site infections (63.1%, 12/19) occurred among patients admitted with open, traumatic wounds. These are patients with an increased infection risk, because compared to elective procedures, aseptic techniques are more likely to fail, and preoperative decolonization therapies are likely not to be applicable.
Recovery of S. aureus from the nares in patients with a previous history of hospitalization suggests that previous hospitalization may predispose to host adaptation, prolonged carrier state, or subsequent infection. In the present study, prior healthcare contact (p = 0.0002, RR (95% CI): 3.64 (1.60-7.96)) was associated with patients with S. aureus infection (Supplementary Table S2), in line with similar findings elsewhere [39]. In general, we observed a significant risk for S. aureus infection in carriers, where the duration of hospitalization was ≤8 weeks (p = 0.0001, RR (95% CI): 7.02 (2.08-23.70)), invasive devices were present (p = 0.0001, RR 4.16 (2.30–7.50)), emergency surgery (p = 0.0001, RR 7.88 (2.02–30.63)) or two or more surgeries (p = 0.0001, RR 3.37 (1.75–6.48)) were received, or where comorbid conditions such as bone disease (p = 0.008, RR 4.04 (1.22-13.39)) or bone, skin, and soft tissue diseases (p = 0.0005, 2.89 (1.51-5.52)) were present (Table 3).
PVL-possessing S. aureus strains are mainly associated with deep, often recurrent SSTIs [40], with a plausible but controversial role in virulence. In the present study, PVL toxin genes-positive S. aureus was detected more frequently in acute SSTIs (66.7%; 4/6) than in other clinical specimens (33.3%; 2/6). This is consistent with the findings of a previous report [40]. Whilst two of eleven PVL toxin genes-positive S. aureus carriers (18.2%; 2/11) developed S. aureus skin infections, other PVL toxin genes-positive S. aureus infections were observed in non-S. aureus carriers. Besides putative transmission of the clones, our observation may also reflect the mobility of PVL phages between S. aureus lineages. Of note, when screening for nasal carriage, we detected a PVL-positive MSSA ST 8 isolate in one patient, which had a spa repeat pattern (t064: 11-19-12-05-17-34-24-34-22-25) similar to the USA300/ST8 (t008: 11-19-12-21-17-34-24-34-22-25) clone, indicating that a rarely detected S. aureus clone with similar characteristics to USA300 is present in sub-Saharan Africa, in the form of aberrant/mutant spa types, mostly MSSA [41] whereas most PVL-positive S. aureus isolates in this study were associated with agr group III (50%; 3/6 for infection-related S. aureus, 81.2%; 9/11 for carriage S. aureus), which confirms previous findings [42,43] and might suggest that agr independently, or together with specific virulent factors, may explain certain clinical features of S. aureus infection [43]. Concerning the low occurrence of PVL-positive S. aureus infection in PVL toxin genes-positive S. aureus carriers, we think that this might either be due to the small number of carriers detected or reflect immunologically modulated protection, already shown for toxic shock toxin (TSST)-producing S. aureus carriers [44]. It could also be that altered C5a or CD45 PVL domains on neutrophils [45] are equally important determinants of PVL-positive S. aureus infection.
Apart from PVL toxin genes, we found 32.0% PTSAg gene-encoding, infection-related S. aureus predominantly in patients with a recent history of trauma. In addition to classical sympathoadrenal and metabolic responses to trauma, the proinflammatory cytokine component may be aggravated by PTSAg-producing S. aureus strains [46], with consequent adverse, localized, or systemic sequelae. Further on, we detected EDIN A toxin gene in exclusive association with seg-sei genes, in two infection-related S. aureus isolates (spa type t2724, t311). EDIN toxins are Ras-homolog-A GTPase-targeting and may interrupt intracellular signaling in S. aureus-infected host cells, disrupting molecular switches governing cytoskeletal architecture and other vital cell processes, with important consequences for host cell regulation and survival [47]. Although we observed a low prevalence of EDIN A toxin gene-positive S. aureus infection (2/47; 4.2%) and apparent lineage specificity (being limited to ST15), a higher prevalence of edin A positive S. aureus has been reported elsewhere [48]. In contrast to edin A (4.2%), PVL toxin genes (12.7%), and other variably detected PTSAg genes (32.0%), the invariable detection of leukotoxic hlg genes in all infection-related (100%; 47/47) and carriage-related (98.3%; 61/62, not shown) S. aureus isolates in the present study, might reflect a strong role for hlg in molecular patho-mechanistic events preceding S. aureus disease. A recent experimental study suggests a direct role for hlgAB in the competition for and successful hijacking of atypical chemokine receptor 1 (ACKR1, a G protein-coupled receptor) in the early stages of S. aureus disease [49], whereas the cytotoxic effect of the PVL toxin, but not HLG, may be neutralized [50].
Although we aimed for 95% statistical power to detect 50% S. aureus infections in S. aureus carriers, with one-half difference in prevalence between non-S. aureus carriers, since we enrolled less than the required number of patients, a post-hoc power evaluation undertaken to find the actual power of the present study (given n = 325, α = 0.01, other design parameters unchanged) showed that the present study had 82.8% power to detect 43.5% S. aureus infection among S. aureus carriers, with approximately one-half distance in prevalence from non-S. aureus carriers. Although we observed a lower (0.435) than assumed (0.50) proportion of S. aureus infection among S. aureus carriers, we found no significant difference between both proportions using the z test, the z score (0.80) being less than 0.01 and 0.05 values of z (2.58 and 1.96, respectively), thus confirming initial study assumption(s) among S. aureus carriers. Given adequate attention to statistical power evaluation, the probable error associated with infection control decisions is known. Since we quantified the effect of classical and perceived risk factors including co-morbidities (Table 3), our findings demonstrate opportunities for a priori determination of risk for S. aureus infection, but also for evaluation of costs for infection control approaches. Limitations included that many patients received prophylactic broad-spectrum antibiotic therapy. Therefore, the clinical impact of S. aureus infection might not have been fully captured. In addition, due to limitations of spa typing in resolving genetic differences, an allele-based typing method could further clarify genetic links between spatiotemporally related S. aureus strains. Overall, we observed a discrete, non-random occurrence of nosocomial S. aureus infection, with a tendency for clustering, especially among those surgical specialties comprising inpatients admitted with comorbidities involving the integumentary or musculoskeletal system.
4. Materials and Methods
4.1. Study Design and Setting
At the time of the study described here (2012/2013), the study site, the Obafemi Awolowo University Teaching Hospitals Complex in Ile-Ife, was an approximately 500-bed hospital serving an estimated 4,140,228 inhabitants of Osun State, Nigeria. The hospital is a major referral healthcare facility in southwestern Nigeria and provides specialized medical services across various specialties including in internal medicine, obstetrics and gynecology, pediatrics, and surgery. Specialty or subspecialty services from where patients were screened and enrolled are listed in the body of the report (Table 1).
We planned to screen eligible patients for MSSA and MRSA carriage within 48 h after admission in order to be able to delineate the proportion of subsequent S. aureus infections attributable to endogenous sources. We simulated effective inpatient sample sizes at different α and β levels, by optimizing for two effect sizes: the effect size of S. aureus carriage [16], and secondly, the effect size of S. aureus infection. The average ratio of non-S. aureus carriers to S. aureus carriers (3.1–4.0: 1.0) in this setting was also determined [16,17,18]. The theoretical probability of the prevalence of S. aureus infection was deduced from the S. aureus carriage rate. PVL toxin gene proportions from selected studies in this region [16,17,18] were pooled to estimate the occurrence of PVL toxin gene-positive S. aureus (≥ 0.113 PVL ± 0.063 (95% SE)). Criteria for selection of pooled studies included, performance in a healthcare setting in sub-Saharan Africa, a clearly defined population focus, analysis of carried S. aureus strains, and a prospective approach. Assumptions included: (i) a discrete asymptotic distribution of S. aureus infection cases, (ii) α = 0.01; β = 0.05, (iii) a one-tailed test (degree of freedom (df) = 1)). We hypothesized that at least 50% of enrolled inpatients who carried S. aureus would develop an S. aureus infection episode during the same (or later, related) hospital stay, while this risk was diminished arbitrarily by half (i.e., 25%) for non-S. aureus carriers, in order to justify the possibility of inadvertent S. aureus acquisition. Our assumptions implied an optimal sample comprising 376 inpatients (301 non-S. aureus carriers vs. 75 S. aureus carriers) for an unmatched cohort design. Critical value of χ2: 5.41.
4.2. Patients
All inpatients who met the inclusion criteria and who gave written (occasionally, if not literate, oral) informed consent were considered eligible. For each patient, anonymized data were collected at study inception by a review of the medical record including age, gender, occupation, specialty/subspecialty affiliation, date and indication of admission, ward, co-morbid conditions, prior hospitalization, prior antibiotic use, prior surgery, presence of indwelling device(s), presence of any open wounds or potentially infectious foci. Records with missing data were clarified by patient interviews or excluded. McCabe scores were derived as previously described [51]. Subsequent data collection comprised clinical specimens, surgical treatment classification (emergency or elective), length of stay, number of patient-days and antibiotic use (all antibiotics belonging to class J01 of the World Health Organization/Anatomical Therapeutic Chemical Classification (WHO/ATCC) database).
The following definitions were used:
Nosocomial (or healthcare-associated) MSSA and MRSA acquisition; was defined as isolation of MSSA and MRSA from any specimen including surveillance probes obtained from a surgical inpatient more than 48 h after admission who was previously identified as non-carrier (as determined by non-recovery of S. aureus from nasal swabs and/or open wound swabs of the same patient);
Nosocomial (or healthcare-associated) infection by MSSA and MRSA, respectively; was defined as the detection of MSSA and MRSA in a purulent specimen, superficial or deep soft-tissue lesion, skin abscesses, blood, sputum, or urine samples, obtained 48 h or more after admission. In addition, infection was only assumed, if at least two clinical symptoms of infection (e.g., fever, localized pain) associated with the respective site were present;
Surgical-site S. aureus infection; was defined as recovery of S. aureus from a superficial or deep surgical incision site, with or without drainage within 30 days after surgery.
Hospitalized surgical patients of the Obafemi Awolowo University Teaching Hospitals Complex in Ile-Ife and aged 01–90 years were included. Inclusion criteria were (i) hospitalization for a minimum period of 48 h, (ii) clinical evaluation for possible elective or emergency surgical intervention (irrespective of the ward to which the patient was initially admitted), (iii) antibiotic use after admission, (iv) and written (occasionally, if not literate, oral) informed consent to participate in the study. For pediatric patients, consent was sought from caregivers. Exclusion from the present study occurred if (i) hospitalization was less than 48 h, or medical records not accessible at the time of screening, (ii) clinical and laboratory evidence of S. aureus infection was documented at the time of admission, (iii) intermittent nasal discharge (e.g., catarrh, epistaxis), compromised nasal mucosal epithelium (e.g., cuts, abrasions), or a nasal device (e.g., endotracheal or nasogastric intubation, other devices e.g., oxygenation mask) was present, and (iv) convalescent patient(s) on discharge list. Surgical inpatients were recruited by a letter of invitation (in English).
4.3. Samples
Within the first 48 h after admission, swabs from the anterior nares were taken using a dry, sterile, cotton-tipped applicator (MicroPoint Diagnostics). If patients had open wounds or fractures at admission (i.e., open wounds from traffic accidents, gun shots, machetes, burns, other skin and soft tissue lesions such as superficial pressure ulcers or diabetic ulcers), these sites were also sampled. Subsequently, follow-up (peri-operative) specimens were obtained from various anatomical sites suspected of infection including skin abscesses and other symptomatic skin lesions, subcutaneous soft-tissue, deep soft-tissue, musculoskeletal tissue, peripheral blood, sputum, urine, or surgical incision sites. All wards were visited at least twice weekly for subsequent data collection and validation of nursing charts, drug prescription sheets, microbiology laboratory notes, etc. for all patients enrolled. All enrolled surgical inpatients considered eligible for surgical intervention also received standard peri-operative antibiotic prophylaxis, in accordance with prevailing anti-infective procedures within Obafemi Awolowo University Teaching Hospitals in Ile-Ife, at the time of the present study.
4.4. Microbiological Techniques
Clinical specimens and nasal swabs were cultivated according to standard procedures on Mueller–Hinton broth at 37 °C for 24 h, mannitol salt agar at 37 °C for 48 h, and on Columbia blood agar at 37 °C for 24 h, before molecular studies. S. aureus was presumably identified by catalase slide test, coagulase tube test, and Pastorex Staph-Plus test, performed according to the manufacturer’s protocol (Bio-rad, Marnes-la-Coquette, France) [2,52]. Antimicrobial susceptibility testing was achieved by the VITEK-2 Antimicrobial Susceptibility Testing automated systems, using the AST-P580 card according to the manufacturer’s specifications (bioMérieux, Marcy l’Etoile, France) and EUCAST clinical breakpoints (version 1.3) to define susceptibility. Species confirmation was achieved by Matrix-Assisted Laser Desorption Ionization Time-of-Flight mass spectrometry (MALDI Biotyper system, Bruker Daltonics, Bremen, Germany) and, additionally, by PCR targeting the S. aureus nuc gene as described elsewhere [53,54,55]. DNA was extracted from S. aureus cells using the QIA amp tissue kit (Qiagen, Hilden, Germany) by following the manufacturer’s recommendations. Methicillin resistance was confirmed by PCR targeting mecA and mecC, respectively [56,57].
4.5. Toxin Genes Detection
In accordance with standard S. aureus genotyping protocols, multiplex PCRs for detection of staphylococcal exotoxin genes including exfoliative toxin genes (eta, etb, and etd), epidermal differentiation inhibitor genes (edinA, edinB, and edinC), the staphylococcal pyrogenic toxin superantigen (PTSAg) genes including the toxic shock toxin 1 gene (tst) and enterotoxin/enterotoxin-like genes (sea, seb, sec, sed, see, seg, seh, sei, and sej), and lukF-PV and lukS-PV genes encoding PVL, were conducted for every S. aureus isolate as previously described [58,59,60].
4.6. Typing of S. aureus Isolates
For every S. aureus isolate, the polymorphic X-region of the S. aureus protein A gene (spa) was sequence-typed as previously described [61]. The “based upon repeat patterns” (BURP) algorithm of the StaphType software (Ridom GmbH, version 1.5, Münster, Ger-many) was applied, to cluster related spa types, using the default parameters described by Mellmann et al. [62]. Subtypes of the accessory gene regulator (agr I, II, III, and IV) were detected by multiplex PCR as previously described [63]. We performed multilocus sequence typing (MLST) for representative spa types in the present study [64]. MLST sequence types were clustered into groups as described by Mellmann et al. [62].
4.7. Statistical Approaches
The Student’s t-test, or F-test, was applied to test the significance of the difference between mean values, or variances, respectively. Comparison between groups and stratification analyses, were accomplished by chi-square test. The difference between expected and observed proportions of S. aureus infection among S. aureus carriers was tested using z-test. To obviate erroneous conclusions of significance, a continuity correction for cell frequencies less than 10 was implemented in (Mantel–Haenszel) chi-square. Where cell frequencies were appreciably low (≤2), Fisher’s exact results superseded chi-square-derived p-values. The utility function “StatCalc” in Epi InfoTM version 3.5.4 (Centers for Disease Control and Prevention, (CDC) Atlanta, USA) was utilized for sample size derivation and calculations. For exploratory comparison of patient characteristics, p-values were assessed for statistical significance on a case-by-case basis (at p < 0.05). For the hypothesis based on the chi-square test (implemented in the study design), a more stringent p-value (p≤ 0.01) determined significance of the outcome(s).
5. Conclusions
In contrast to widely varied epidemiology of S. aureus infection in industrialized settings, S. aureus more commonly was implicated in opportunistic SSTIs and bone and joint-related infections than in bacteremia, respiratory tract infection, or urinary tract infection, in the present study. Essentially, acute S. aureus infection developed during hospital stay (involving mostly MLST (lineages) ST1, ST7, ST15, ST88, or ST152) was 4.6-fold higher among S. aureus carriers; in particular, those admitted with recent bone or skin and soft tissue trauma, whereas, compared to the high detection rate of hlg (100%; 47/47) in infection-related S. aureus isolates, PVL-encoding genes were less frequent (12.7%; 6/47). This might reflect the mobility of PVL genes or lineage-specific PVL restrictions and might indirectly suggest a more pertinent contribution of HLG-encoding genes (which share bicomponent leukotoxicity with PVL [46]), to S. aureus infection. Together, these findings reflect the nosocomial burden of toxigenic, MDR S. aureus infection among surgical inpatients admitted with co-morbidities involving the integumentary or musculoskeletal system, who may benefit from strategies to block potentially virulent MDR S. aureus in an African country.
Acknowledgments
This work was supported by the open access fund of the University of Münster, Germany. The erstwhile Genomics and Environmental Determinants of Health Initiative of the Institute of Public Health in Obafemi Awolowo University, Ile-Ife, Nigeria provided administrative support for this work. The Hematology Unit of the Obafemi Awolowo University Teaching Hospitals Complex in Ile-Ife is appreciated for its kind provision of human anticoagulated plasma for staphylococcal coagulase tests. We are grateful to consulting surgeons, physicians in residency, nurses, and patients cared for in various specialties of Obafemi Awolowo University Teaching Hospitals Complex in Ile-Ife, Nigeria, for their voluntary cooperation through the period of the study. Kemi Awotipe, Foyeke Olafimihan and Sylvester Udoh are thanked for technical advice. Toyin Elemiele, Damayanti Kaiser, Sayo Olutola, Richard Omole, Femi Oyetoke, and Martina Schulte are appreciated for technical assistance. We especially thank the governing authorities of the respective institutions, for their kind cooperation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11101372/s1, Table S1: Occurrence of S. aureus infection among S. aureus nasal-carriers and non-carriers, stratified according to indication for admission. Table S2: Characteristics of inpatients with, or without any type of nosocomial S. aureus infection. Figure S1; Surgical procedures related to, or not related to S. aureus infection.
Author Contributions
Conceptualization, A.A. (Adeniran Adeyanju), N.T., A.O., A.A. (Akinyele Akinyoola), Y.A., O.L. and K.B.; Methodology, A.A. (Adeniran Adeyanju), A.O., A.A. (Akinyele Akinyoola), Y.A., O.L., F.S., R.K. and K.B.; Validation, A.A. (Adeniran Adeyanju), A.O., A.A. (Akinyele Akinyoola), T.A. (Taofeeq Adeyemi), T.A. (Temilade Adeyanju), N.T., Y.A., O.L., D.A., D.K., F.S., R.K., C.K. and K.B.; Formal Analysis, A.A. (Adeniran Adeyanju), F.S. and R.K.; Investigation A.A. (Adeniran Adeyanju), A.A. (Akinyele Akinyoola), T.A. (Taofeeq Adeyemi), T.A. (Temilade Adeyanju), O.U., F.S. and R.K.; Resources, A.A. (Adeniran Adeyanju), A.A. (Akinyele Akinyoola), Y.A., O.L., T.A. (Taofeeq Adeyemi), T.A. (Temilade Adeyanju), O.U., D.A., N.T., F.S. and K.B.; Data Curation, A.A. (Adeniran Adeyanju) and F.S.; Original Draft Preparation, A.A. (Adeniran Adeyanju), R.K. and K.B; Review and Editing, A.A. (Adeniran Adeyanju), O.U., N.T., D.A., D.K., F.S., R.K., C.K. and K.B.; Visualization, A.A. (Adeniran Adeyanju), T.A. (Taofeeq Adeyemi), T.A. (Temilade Adeyanju), O.U., F.S., R.K., C.K. and K.B; Supervision, D.K., D.A., F.S. and K.B.; Project Administration, A.O., D.K. and K.B.; Funding Acquisition, A.A. (Adeniran Adeyanju), D.K., F.S. and K.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria (protocol code ERC2012/12/12 on 30 November 2012).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data analyzed in the present study are available within the article and Supplementary Materials section.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was funded by grants of the Deutscher Akademischer Austauschdienst e.V. (DAAD) (A/12/90661) to AA and of the Deutsche Forschungsgemeinschaft (DFG) (SCHA 1994/5-1) to FS.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tong S.Y., Davis J.S., Eichenberger E., Holland T.L., Fowler V.J., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker K., David M.Z., Skov R.L., von Eiff C. Staphylococcus, Micrococcus, and Other Catalase-Positive Cocci. In: Jorgensen J.H., Carroll K.C., Funke G., Pfaller M.A., Landry M.L., Richter S.S., Warnock D.W., editors. Manual of Clinical Microbiology. ASM Press; Washington, DC, USA: 2019. pp. 367–398. [Google Scholar]
- 3.Noskin G.A., Rubin R.J., Schentag J.J., Kluytmans J., Hedblom E.C., Smulders M., Lapetina E., Gemmen E. The burden of Staphylococcus aureus infections on hospitals in the United States: An analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch. Intern. Med. 2005;165:1756–1761. doi: 10.1001/archinte.165.15.1756. [DOI] [PubMed] [Google Scholar]
- 4.Rasigade J.P., Dumitrescu O., Lina G. New Epidemiology of Staphylococcus aureus infections. Clin. Microbiol. Infect. 2014;20:587–588. doi: 10.1111/1469-0691.12718. [DOI] [PubMed] [Google Scholar]
- 5.Tong S.Y., Schaumburg F., Ellington M.J., Corander J., Pichon B., Leendertz F., Bentley S.D., Parkhill J., Holt D.C., Peters G., et al. Novel staphylococcal species that form part of a Staphylococcus aureus-related complex: The non-pigmented Staphylococcus argenteus sp. nov. and the non-human primate-associated Staphylococcus schweitzeri sp. nov. Int. J. Syst. Evol. Microbiol. 2015;65:15–22. doi: 10.1099/ijs.0.062752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker K., Schaumburg F., Kearns A., Larson A.R., Lindsay J.A., Skov R.L., Westh H. Implications of identifying the recently defined members of the Staphylococcus aureus complex S. argenteus and S. schweitzeri: A position paper of the ESCMID Study Group for Staphylococci and Staphylococcal Diseases (ESGS) Clin. Microbiol. Infect. 2019;25:1064–1070. doi: 10.1016/j.cmi.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 7.Hiramatsu K., Okuma K., Ma X.X., Yamamoto M., Hori S., Kapi M. New trends in Staphylococcus aureus infections: Glycopeptides resistance in hospital and methicillin resistance in the community. Curr. Opin. Infect. Dis. 2002;15:407–413. doi: 10.1097/00001432-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Vandenesch F., Naimi T., Enright M.C., Lina G., Nimmo G.R., Heffernan H., Liassine N., Bes M., Greenland T., Reverdy M.E., et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: Worldwide emergence. Emerg. Infect. Dis. 2003;9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Köck R., Schaumburg F., Mellmann A., Köksal M., Jurke A., Becker K., Friedrich A.W. Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) as causes of human infection and colonization in Germany. PLoS ONE. 2013;8:e55040. doi: 10.1371/journal.pone.0055040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinross P., Petersen A., Skov R., van Hauwermeiren E., Pantosti A., Laurent F., Voss A., Kluytmans J., Struelens M.J., Heuer O., et al. Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) among human MRSA isolates, European Union/European Economic Area Countries, 2013. Eur. Surveill. 2017;22:16–00696. doi: 10.2807/1560-7917.ES.2017.22.44.16-00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaumburg F., Alabi A.S., Mombo-Ngoma G., Kaba H., Zoleko R.M., Diop D.A., Mackanga J.R., Basra A., Gonzalez R., Menendez C., et al. Transmission of Staphylococcus aureus between mothers and infants in an African setting. Clin. Microbiol. Infect. 2014;20:O390–O396. doi: 10.1111/1469-0691.12417. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez J.M., Dobrick J.B., Jadavji A., Adam R.D. Staphylococcus aureus bacteremia at a referral medical center in Kenya: A retrospective review of cases from 2010 to 2018. PLoS ONE. 2020;15:e0234914. doi: 10.1371/journal.pone.0234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaumburg F., Alabi A.S., Peters G., Becker K. New epidemiology of Staphylococcus aureus infection in Africa. Clin. Microbiol. Infect. 2014;20:589–596. doi: 10.1111/1469-0691.12690. [DOI] [PubMed] [Google Scholar]
- 14.United Nations, World Population Prospects. 2017. [(accessed on 6 May 2022)]. Available online: https://population.un.org/wpp/Maps/
- 15.Van Boeckel T.P., Gandra S., Ashok A., Caudron Q., Grenfell B.T., Levin S.A., Laxminarayan R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014;14:742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 16.Kolawole D., Adeyanju A., Schaumburg F., Akinyoola A., Lawal O., Amusa Y., Köck R., Becker K. Characterization of colonizing Staphylococcus aureus isolated from surgical wards’ patients in a Nigerian university hospital. PLoS ONE. 2013;8:e68721. doi: 10.1371/journal.pone.0068721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaumburg F., Ngoa U.A., Kösters K., Köck R., Adegnika A.A., Kremsner P.G., Lell B., Peters G., Mellmann A., Becker K. Virulence factors and genotypes of Staphylococcus aureus from infection and carriage in Gabon. Clin. Microbiol. Infect. 2011;17:1507–1513. doi: 10.1111/j.1469-0691.2011.03534.x. [DOI] [PubMed] [Google Scholar]
- 18.Ruimy R., Maiga A., Armand-Lefevre L., Maiga I., Diallo A., Koumare K.A., Ouattara K., Soumare S., Gaillard K., Lucet J.C., et al. The carriage population of Staphylococcus aureus from Mali is composed of a combination of pandemic clones and the divergent Panton-Valentine leukocidin-positive genotype ST152. J. Bacteriol. 2008;190:3962–3968. doi: 10.1128/JB.01947-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okon K.O., Shittu A.O., Kudi A.A., Umar H., Becker K., Schaumburg F. Population dynamics of Staphylococcus aureus from Northeastern Nigeria in 2007 and 2012. Epidemiol. Infect. 2014;142:1737–1740. doi: 10.1017/S0950268813003117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraef C., Alabi A.S., Peters G., Becker K., Kremsner P.G., Rossatanga E.G., Mellmann A., Grobusch M.P., Zanger P., Schaumburg F. Co-detection of Panton-Valentine leukocidin encoding genes and cotrimoxazole resistance in Staphylococcus aureus in Gabon: Implications for HIV-patients’ care. Front. Microbiol. 2015;6:60. doi: 10.3389/fmicb.2015.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magiorakos A.-P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 22.Egyir B., Guardabassi L., Sørum M., Nielsen S.S., Kolekang A., Frimpong E., Addo K.K., Newman M.J., Larson A.R. Molecular Epidemiology and Antimicrobial Susceptibility of Clinical Staphylococcus aureus from Healthcare Institutions in Ghana. PLoS ONE. 2014;9:e89716. doi: 10.1371/journal.pone.0089716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shittu A.O., Kaba M., Abdulgader S.M., Ajao Y.O., Abiola M.O., Olatimehin A.O. Mupirocin-resistant Staphylococcus aureus in Africa: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2018;7:101. doi: 10.1186/s13756-018-0382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tadesse B.T., Ashley E.A., Ongarello S., Havumaki J., Wijegoonewardena M., Gonzalez U., Dittrich S. Antimicrobial resistance in Africa: A systematic review. BMC Infectious Diseases. 2017;17:616. doi: 10.1186/s12879-017-2713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egyir B., Dsani E., Owusu-Nyantakyi C., Amuasi G.R., Owusu F.A., Allegye-Cudjoe E., Addo K.K. Antimicrobial resistance and genomic analysis of staphylococci isolated from livestock and farm attendants in Northern Ghana. BMC Microbiol. 2022;22:180. doi: 10.1186/s12866-022-02589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Acelas A.L., Engelman B., Abreu-Almeida M.D. Risk factors for health care–associated infection in hospitalized adults: Systematic review and meta-analysis. Am. J. Infect. Control. 2017;45:e149–e156. doi: 10.1016/j.ajic.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Allegranzi B., Nejad S.B., Combescure C., Graafmans W., Attar H., Donaldson L., Pittet D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet. 2011;377:228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 28.Kluytmans J.A.J.W., Mouton J.W., Ijzerman E.P.F., Vandenbroucke-Grauls C.M.J.E., Maat A.W.J.M., Wagenvoort J.H.T., Verbrugh H.A. Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J. Infect. Dis. 1995;171:216–219. doi: 10.1093/infdis/171.1.216. [DOI] [PubMed] [Google Scholar]
- 29.Harbarth S., Fankhauser C., Schrenzel J., Christenson J., Gervaz P., Bandiera-Clerc C., Renzi G., Vernaz N., Sax H. Universal Screening for Methicillin-Resistant Staphylococcus aureus at Hospital Admission and Nosocomial infection in Surgical Patients. J. Am. Med. Assoc. 2008;299:1149–1157. doi: 10.1001/jama.299.10.1149. [DOI] [PubMed] [Google Scholar]
- 30.Shooter R.A., Smith M.A., Griffiths J.D., Brown M.E.A., Williams R.E.O., Rippon J.E., Jevons M.P. Spread of staphylococci in a surgical ward. Br. Med. J. 1958;1:607–613. doi: 10.1136/bmj.1.5071.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillespie E.H., Devenish E.A., Cowan S.T. Pathogenic staphylococci. Their incidence in the nose and on the skin. Lancet. 1939;234:870–873. doi: 10.1016/S0140-6736(00)62957-4. [DOI] [Google Scholar]
- 32.Van Belkum A., Verkaik N.J., de Vogel C.P., Boelens H.A., Verveer J., Nouwen J.L., Verbrugh H.A., Wertheim H.F.L. Reclassification of Staphylococcus aureus nasal carriage types. J. Infect. Dis. 2009;199:1820–1826. doi: 10.1086/599119. [DOI] [PubMed] [Google Scholar]
- 33.Kaspar U., Kriegeskorte A., Schubert T., Peters G., Rudack C., Pieper D.H., Wos-Oxley M., Becker K. The culturome of the human nose habitats reveals individual bacterial fingerprint patterns. Environ. Microbiol. 2016;18:2130–2142. doi: 10.1111/1462-2920.12891. [DOI] [PubMed] [Google Scholar]
- 34.Von Eiff C., Becker K., Machka K., Stammer H., Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein H.J. The relation between the nasal-staphylococcal-carrier state and the incidence of postoperative complications. N. Engl. J. Med. 1959;260:1303–1308. doi: 10.1056/NEJM195906252602601. [DOI] [PubMed] [Google Scholar]
- 36.Young B.C., Golubchik T., Batty E.M., Fung R., Larner-Svensson H., Votintseva A.A., Miller R.R., Godwin H., Knox K., Everitt R.G., et al. Evolutionary dynamics of Staphylococcus aureus during progression from carriage to disease. Proc. Natl. Acad. Sci. USA. 2012;109:4550–4555. doi: 10.1073/pnas.1113219109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao D., Yu F.Y., Qin Z.Q., Chen C., He S., Chen Z.Q., Zhang L.Q., Wang L.X. Molecular characterization of Staphylococcus aureus isolates causing skin and soft tissue infections (SSTIs) BMC Infect. Dis. 2010;10:133. doi: 10.1186/1471-2334-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shuter J., Hatcher B., Lowy F.D. Staphylococcus aureus Binding to Human Nasal Mucin. Infect. Immun. 1996;64:310–318. doi: 10.1128/iai.64.1.310-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hidron A.I., Kourbatova E.V., Halvosa J.S., Terrell B.J., McDougal L.K., Tenover F.C., Blumberg H.M., King M.D. Risk factors for colonization with methicillin-resistant Staphylococcus aureus (MRSA) in patients admitted to an urban hospital; emergence of community associated MRSA nasal carriage. Clin. Infect. Dis. 2005;41:159–166. doi: 10.1086/430910. [DOI] [PubMed] [Google Scholar]
- 40.Shallcross L.J., Fragaszy E., Johnson A.M., Hayward A.C. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: A systematic review and meta-analysis. Lancet Infect. Dis. 2013;13:43–54. doi: 10.1016/S1473-3099(12)70238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strauß L., Stegger M., Akpaka P.E., Alabi A., Breurec S., Coombs G., Egyir B., Larsen A.R., Laurent F., Monecke S., et al. Origin, evolution, and global transmission of community-acquired Staphylococcus aureus ST8. Proc. Natl. Acad. Sci. USA. 2017;114:E10596–E10604. doi: 10.1073/pnas.1702472114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillet Y., Issartel B., Vanhems P., Fournet J.C., Lina G., Bes M., Vandenesch F., Piemont Y., Brousse M., Floret D., et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotizing pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 43.Novick R.P. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 44.Ritz H.L., Kirkland J.J., Bond G.G., Warner E.K., Petty G.P. Association of high levels of serum antibody to staphylococcal toxic shock antigen with nasal carriage of toxic shock antigen-producing strains of Staphylococcus aureus. Infect. Immun. 1984;43:954–958. doi: 10.1128/iai.43.3.954-958.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaumburg F., Witten A., Flamen A., Stoll M., Alabi A.S., Kremsner P.G., Löffler B., Zipfel P.F., Velavan T.P., Peters G. Complement 5a Receptor Polymorphisms Are Associated with Panton-Valentine Leukocidin-positive Staphylococcus aureus Colonization in African Pygmies. Clin. Infect. Dis. 2019;68:854–856. doi: 10.1093/cid/ciy666. [DOI] [PubMed] [Google Scholar]
- 46.Dinges M.M., Orwin P.M., Schlievert P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000;13:16–34. doi: 10.1128/CMR.13.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou H., Zheng Y. Cell Type-specific Signaling Function of RhoA GTPase: Lessons from Mouse Gene Targeting. J. Biol. Chem. 2013;288:36179–36188. doi: 10.1074/jbc.R113.515486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munro P., Clément R., Lavigne J.P., Pulcini C., Lemichezz E., Landraud L. High prevalence of edin-C encoding RhoA-targeting toxin in Clinical Isolates of Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:965–972. doi: 10.1007/s10096-011-1181-6. [DOI] [PubMed] [Google Scholar]
- 49.Grison C.M., Lambey P., Jeannot S., Del Nero E., Fontanel S., Peysson F., Heuninck J., Sounier R., Durroux T., Leyrat C., et al. Molecular insights into mechanisms of GPCR hijacking by Staphylococcus aureus. Proc. Natl. Acad. Sci. USA. 2021;118:e2108856118. doi: 10.1073/pnas.2108856118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spaan A.N., Schiepers A., de Haas C.J.C., van Hooijdonk D.D.J.J., Badiou C., Contamin H., Vandenesch F., Lina G., Gerard N.P., Gerard C., et al. Differential Interaction of the Staphylococcal Toxins Panton-Valentine Leukocidin and γ-Hemolysin CB with Human C5a Receptors. J. Immunol. 2015;195:1034–1043. doi: 10.4049/jimmunol.1500604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCabe W.R., Jackson G.G. Gram-Negative Bacteremia: I. Etiology and Ecology. JAMA Arch. Intern. Med. 1962;110:847–855. doi: 10.1001/archinte.1962.03620240029006. [DOI] [Google Scholar]
- 52.Köck R., Werner P., Friedrich A.W., Fegeler C., Becker K. Persistence of nasal colonization with human pathogenic bacteria and associated antimicrobial resistance in the German general population. New Microbes New Infect. 2016;9:24–34. doi: 10.1016/j.nmni.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brakstad O.G., Aasbakk K., Maeland J.A. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 1992;30:1654–1660. doi: 10.1128/jcm.30.7.1654-1660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Becker K., Schaumburg F., Fegeler C., Friedrich A.W., Köck R. Staphylococcus aureus from the German general population is highly diverse. Int. J. Med. Microbiol. 2017;307:21–27. doi: 10.1016/j.ijmm.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Idelevich E.A., Schüle I., Grünastel B., Wüllenweber J., Peters G., Becker K. Rapid identification of microorganisms from positive blood cultures by MALDI-TOF mass spectrometry subsequent to very short-term incubation on solid medium. Clin. Microbiol. Infect. 2014;20:1001–1006. doi: 10.1111/1469-0691.12640. [DOI] [PubMed] [Google Scholar]
- 56.Becker K., Pagnier I., Schuhen B., Wenzelburger F., Friedrich A.W., Kipp F., Peters G., von Eiff C. Does nasal cocolonization by methicillin-resistant coagulase-negative staphylococci and methicillin-susceptible Staphylococcus aureus strains occur frequently enough to represent a risk of false-positive methicillin-resistant S. aureus determinations by molecular methods? J. Clin. Microbiol. 2006;44:229–231. doi: 10.1128/JCM.44.1.229-231.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kriegeskorte A., Ballhausen B., Idelevich E.A., Köck R., Friedrich A.W., Karch H., Peters G., Becker K. Human MRSA isolates with novel genetic homolog, Germany. Emerg. Infect. Dis. 2012;18:1016–1018. doi: 10.3201/eid1806.110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Becker K., Friedrich A.W., Lubritz G., Weilert M., Peters G., von Eiff C. Prevalence of genes encoding pyrogenic toxin superantigens and exfoliative toxins among strains of Staphylococcus aureus isolated from blood and nasal specimens. J. Clin. Microbiol. 2003;41:1434–1439. doi: 10.1128/JCM.41.4.1434-1439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Von Eiff C., Friedrich A.W., Peters G., Becker K. Prevalence of genes encoding for members of the staphylococcal leukotoxin family among clinical isolates of Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2004;49:157–162. doi: 10.1016/j.diagmicrobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Blaiotta G., Fusco V., von Eiff C., Villani F., Becker K. Biotyping of enterotoxigenic Staphylococcus aureus by enterotoxin gene cluster (egc) polymorphism and spa typing analyses. Appl. Environ. Microbiol. 2006;72:6117–6123. doi: 10.1128/AEM.00773-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mellmann A., Weniger T., Berssenbrügge C., Keckevoet U., Friedrich A.W., Harmsen D., Grundmann H. Characterization of clonal relatedness among the natural population of Staphylococcus aureus strains by using spa sequence typing and the BURP (based upon repeat patterns) algorithm. J. Clin. Microbiol. 2008;46:2805–2808. doi: 10.1128/JCM.00071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mellmann A., Weniger T., Berssenbrügge C., Rothgänger J., Sammeth M., Stoye J., Harmsen D. Based Upon Repeat Pattern (BURP): An algorithm to characterize the long-term evolution of Staphylococcus aureus populations based on spa polymorphisms. BMC Microbiol. 2007;7:98. doi: 10.1186/1471-2180-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lina G., Boutite F., Tristan A., Bes M., Etienne J., Vandenesch F. Bacterial competition for human nasal cavity colonization: Role of staphylococcal agr alleles. Appl. Environ. Microbiol. 2003;69:18–23. doi: 10.1128/AEM.69.1.18-23.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kondo Y., Ito T., Ma X.X., Watanabe S., Kreiswirth B.N., Etienne J., Hiramatsu K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: Rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 2007;51:264–274. doi: 10.1128/AAC.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in the present study are available within the article and Supplementary Materials section.