Abstract
Vaccination to protect against human infectious diseases may be enhanced by using adjuvants that can selectively stimulate immunoregulatory responses. In a murine model, a novel nanoparticulate adjuvant composed of calcium phosphate (CAP) was compared with the commonly used aluminum (alum) adjuvants for its ability to induce immunity to herpes simplex virus type 2 (HSV-2) and Epstein-Barr virus (EBV) infections. Results indicated that CAP was more potent as an adjuvant than alum, elicited little or no inflammation at the site of administration, induced high titers of immunoglobulin G2a (IgG2a) antibody and neutralizing antibody, and facilitated a high percentage of protection against HSV-2 infection. Additional benefits of CAP include (i) an insignificant IgE response, which is an important advantage over injection of alum compounds, and (ii) the fact that CAP is a natural constituent of the human body. Thus, CAP is very well tolerated and absorbed. These studies were performed with animal models. By virtue of the potency of this CAP adjuvant and the relative absence of side effects, we believe that this new CAP formulation has great potential for use as an adjuvant in humans.
Historically, adjuvants have been necessary to improve vaccine efficacy in order to afford protection against infections. A key reason for this is that both attenuated virus preparations and, particularly, recombinant proteins are often poorly antigenic. In the past decade, several adjuvants have been evaluated in clinical trials. Calcium phosphate (CAP), MF59, aluminum (alum) compounds, and virosomes have been approved for human use in several European countries (23). In the United States, alum compounds are the most extensively used adjuvants in licensed vaccines for humans. Although they effectively enhance immune responses, there are several disadvantages associated with their use (3, 5, 14). The disadvantages of alum-based adjuvants include the severity of local tissue irritation, the longer duration of the inflammatory reaction at the injection site, strong Th2 responses, minimal induction of cell-mediated immunity, and a propensity to elicit undesirable immunoglobulin E (IgE) responses (11, 12, 17, 27). Alum compounds have also been shown to increase the levels of potential undesirable homocytotropic antibodies in animal species (9, 21). Furthermore, alum-based vaccines are frequently ineffective for the induction of antiviral immunity (4). For these reasons, new adjuvants are being developed to enhance the immunity against weak antigens. New-generation adjuvants are designed to induce minimal side effects, enhance the duration of the immune response, and concurrently stimulate humoral, cellular, and mucosal immune responses. Furthermore, an ideal adjuvant would be biodegradable, economical, and simple to manufacture. In addition, it would have the potential to selectively trigger a defined class of immune response such as the T-helper 1 (Th1) CD4+ T-cell response and cell-mediated immunity and have equal applicability for any new-generation antigens.
In Europe, CAP has been used as an adjuvant for immunity against diphtheria and tetanus antigens and for allergen desensitization (22). Goto et al. (9) reported that local tissue reactions caused by injection of a CAP gel and suspension completely ceased by the 4th week, while irritation caused by an aluminum hydroxide gel and suspension persisted for 8 weeks. The CAP gel or suspension adjuvants tested did not induce anti-ovalbumin and anti-tetanus toxoid antibodies. They concluded that CAP might not be a good alternative to alum adjuvants. However, they did acknowledge that CAP caused less local tissue irritation.
Here, we describe a unique method of synthesis and the desirable adjuvant properties of a new type of CAP adjuvant. We synthesized CAP nanoparticles with raw materials different from those described by European scientists (20). The results were that the new CAP formulation had different physical and chemical characteristics and adjuvant activities. On the basis of the results of our studies, we report that this CAP formulation provides several outstanding immunological properties, such as the ability to entrap and bind antigens in the CAP adjuvant as well as mediate desirable immune response profiles.
MATERIALS AND METHODS
Preclinical toxicity study of CAP.
The components of a formulation of 12.5 mM calcium chloride, 12.5 mM dibasic sodium phosphate, and 15.6 mM sodium citrate were mixed together and stirred for 48 h. After a 30-min sonication period, particle size was determined with a Coulter N4Plus submicron particle sizer, and the pH of the mixture was monitored with a pH meter (Fisher, Pittsburgh, Pa.). A preclinical acute toxicity study was performed by IITRI Research Institute (Chicago, Ill.) and was conducted in accordance with the U.S. Food and Drug Administration (26). In brief, CAP was administered by oral gavage, by the intramuscular and subcutaneous routes, and by inhalation exposure to four separate groups (each containing five male and five female animals) of adult CRL:(HA) BR Hartley albino guinea pigs (Charles River Laboratories, Wilmington, Mass.) in a single total dose of 1.2 mg/kg of body weight. A fifth group served as an untreated control group for all routes of administration. The guinea pigs were observed daily for mortality or moribundity and adverse clinical signs. Following the 14-day observation period, terminal necropsy was conducted for pathology end point assessment. Tissue biopsy specimens were tested for particle site-specific inflammatory responses. Hematology parameters measured included white blood cell count, erythrocyte count, erythrocyte morphology, and hematocrit. In addition, five more animals from the inhalation and untreated groups underwent bronchoalveolar lavage (BAL) assessment. The PAI pathology computer system (LABCAT) that has a built-in weighing system for calculating the average severity for each tissue assessed was used. Data were analyzed for statistical significance by a one-way analysis of variance (ANOVA), followed by Dunnett's test, using Syatat (version 5.0; SPSS, Inc., Chicago, Ill.). Hematology data were analyzed by ANOVA, followed by Dunnett's test, using LABCAT (version 4.41a; IPA, Princeton, N.J.). A P value of <0.05 was considered significant in all comparisons.
Cells and viruses.
Herpes simplex virus type 2 (HSV-2) and Epstein-Barr virus (EBV) were propagated in Vero and NC37 cells, respectively, all of which were obtained from the American Type Culture Collection. The cells were grown in accordance with American Type Culture Collection recommendations with one modification: the NC37 cells were incubated at 37°C with 10% CO2.
Preparation of viral glycoproteins.
Viral protein was purified from HSV-2 and EBV by the procedure described by Westra et al. (28) with some modifications. Briefly, infected cells were collected and washed with Hanks' balanced salt solution twice at 4°C. After 15 min of sonication (in 5-min intervals), the viral suspension was centrifuged at 5,500 × g for 15 min. The supernatant was collected and treated with 1% IGEPAL (Sigma Chemical Co., St. Louis, Mo.) lysis buffer I for 30 min on ice. The solution was centrifuged at 18,500 × g for 2 h. The supernatant was collected and treated with 1% IGEPAL lysis buffer II (which was the same formulation as lysis buffer I but with the addition of 2 mM phenylmethylsulfonyl fluoride) for 30 min on ice. After centrifugation the supernatant was dialyzed against phosphate-buffered saline (PBS) at 4°C and was stored at −80°C.
Formulation of subunit vaccine.
HSV-2 or EBV protein was added to 12.5 mM calcium chloride, followed by the addition of 12.5 mM dibasic sodium phosphate and 15.6 mM sodium citrate. The solution was stirred until the final particle size was less than 1,000 nm, as determined with a Coulter N4Plus submicron particle sizer. The particle-entrapped HSV-2 or EBV protein was treated with cellobiose overnight (13) and was coated again with HSV-2 or EBV protein. After the unbound protein was washed off with PBS, the particle-protein complex was solubilized with 100 mM EDTA. The concentrations of proteins inside and outside the particles were determined with a Bio-Rad (Hercules, Calif.) protein assay kit.
The alum-based vaccine was prepared by mixing the same quantity of HSV-2 or EBV protein with aluminum hydroxide (the concentration of alum was the same as the concentration of CAP). Thus, the control vaccines were CAP, HSV-2, EBV, and PBS.
Animals.
Female BALB/c mice (age, 6 to 8 weeks; weight, 20 to 25 g) were obtained from Charles River Laboratories. The mice were maintained in standard housing with a normal diet of Purina rodent chow 5001. Each group (i.e., the HSV-2-infected group and the EBV-infected group) consisted of 18 randomly selected mice.
Immunization and sample collection.
Seven groups of six mice each were inoculated intraperitoneally with one of the following vaccine formulations: HSV-2 proteins alone (60 μg/dose/mouse), HSV-2 with alum (60 μg of protein plus 100 μg of alum/dose/mouse), HSV-2 with CAP (60 μg of protein plus 100 μg of CAP/dose/mouse), EBV alone (62.2 μg/dose/mouse), EBV with alum (62.2 μg of protein plus 100 μg of alum/dose/mouse), EBV with CAP (62.2 μg of protein plus 100 μg of CAP/dose/mouse), and CAP alone (100 μg/dose/mouse). An eighth group of mice was immunized with PBS. Mice received three injections at 2-week intervals.
Initial blood samples were collected from mice under metofane anesthesia via the orbital sinus at the end of the 2nd week after primary immunization. Serum was separated by centrifugation and was stored at −20°C. The level of antibodies was monitored every 2 weeks.
Mucosal samples were collected by vaginal lavage with 100 μl of PBS at weeks 6 and 14 after the first immunization. The vaginal lavage sediments were subsequently removed by centrifugation, and 20-μl volumes of each individual sample were pooled and stored −20°C.
ELISA.
Serum and vaginal samples were analyzed for HSV-2- and EBV-specific IgG, IgG2a, IgA, and IgE antibodies by standard enzyme-linked immunosorbent assay (ELISA). In brief, microtiter plates (Corning, Cambridge, Mass.) were coated with HSV-2 or EBV proteins (6 μg/ml) overnight at 4°C and were blocked with PBS containing 0.05% Tween 20 and 0.1% normal goat serum. Serially diluted sera were incubated with antigens at room temperature. After the plates were washed they were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (Chemicon, Temecula, Calif.) and IgG2a, IgA, and IgE (Fisher Scientific Co., Pittsburgh, Pa.) and developed with o-phenylenediamine dihydrochloride (Sigma Chemical Co.) containing H2O2. Optical densities were read at 490 nm with a Benchmark microplate reader (Bio-Rad). Four negative controls (without antigen, without first antibody, without second antibody, and without both antigen and antibody) were set for each plate.
Neutralization assay.
Vero cells were propagated in eight-well culture plates (Becton Dickinson, Franklin Lakes, N.J.). Pooled sera taken from each group of six mice were treated at 56°C for 30 min to inactivate complement. Serially diluted sera were incubated with HSV-2 adjusted to give 100 plaques per well in the absence of neutralizing antibody for 1 h at 37°C. Cells were inoculated with the mixture of virus and serum for 24 h. The HSV-2-specific neutralizing antibody titer was determined by plaque assay.
HSV-2 challenge experiment.
To synchronize the estrous cycle at the progesterone-dominated stage, the mice were injected subcutaneously with medroxyprogesterone (DepoProvera; Upjohn, Kalamazoo, Mich.) at a concentration of 2 mg/mouse in 50 μl of distilled water. Five days following medroxyprogesterone administration, the animals were challenged intravaginally with 103 PFU of HSV-2 (week 15 after primary immunization) as described previously (15). The mice were examined daily for genital pathology. The pathology elicited following intravaginal challenge with HSV-2 was scored as described elsewhere (6).
Statistical analysis.
Pathologic data were analyzed by ANOVA to determine the difference between groups. ELISA data were analyzed by Student's t test.
RESULTS
Table 1 summarizes the inflammatory results seen at the site of delivery in five groups of guinea pigs treated with CAP in a preclinical acute toxicity study. Table 2 shows the results of assessment of BAL specimens from additional animals from the five untreated groups and the inhalation treatment group. No guinea pigs died, and no treatment-related signs of toxicity were observed during the study. There were no statistically significant differences in the hematology parameters for any of the treatment groups compared to those for the untreated control group (data not shown). Upon assessment of BAL specimens, the percent cell viability and the mean lactate dehydrogenase (protein) concentration were statistically significant different between the inhalation and control groups. As expected, histopathological examination indicated that intramuscular and subcutaneous administration of CAP resulted in a minimal to mild inflammatory response localized at the injection sites only.
TABLE 1.
Toxicity study of CAP administration
| Organ | Route of administration | Lesion | Sexa | No. of animals with inflammation/total no. of animals (severity codeb)
|
|
|---|---|---|---|---|---|
| Experimental | Control | ||||
| Skin | Subcutaneous | Interscapular inflammation, granulomatous | M | 2/5 (0.30) | 0/5 |
| F | 2/5 (0.50) | 0/5 | |||
| Skeletal muscle | Intramuscular | Hind limb inflammation, granulomatous | M | 3/5 (1.15) | 0/5 |
| F | 3/5 (0.75) | 0/5 | |||
| Lymph node | Inhalation | Bronchial inflammation | M | 0/5 | 0/5 |
| F | 1/5 (0.40) | 0/5 | |||
| Lymph node | Inhalation | Mediastinal inflammation | M | 0/5 | 0/5 |
| F | 2/5 (0.80) | 0/5 | |||
| Gastrointestinalc | Oral gavage | Inflammation | M | 0/5 | 0/5 |
| F | 0/5 | 0/5 | |||
M, male; F, female.
The severity codes used in this study, as indicated in parentheses, were as follows: 1, minimal; 2, mild; 3, moderate; 4, marked.
The gastrointestinal system organs evaluated were stomach, duodenum, ileum, jejunum, cecum, and colon.
TABLE 2.
Assessment of BAL specimens from guinea pigs that inhaled CAP
| Group and sexa | % Viable cells | LDHa (IU/mg of protein) |
|---|---|---|
| Experimental | ||
| Male | 90.5 ± 2.4b | 0.14 ± 0.052 |
| Female | 88.3 ± 6.5 | 0.13 ± 0.014b |
| Control | ||
| Male | 93.6 ± 1.5 | 0.13 ± 0.04 |
| Female | 92.9 ± 2.9 | 0.10 ± 0.021 |
LDH, lactate dehydrogenase.
Statistically significant differences but no biological differences.
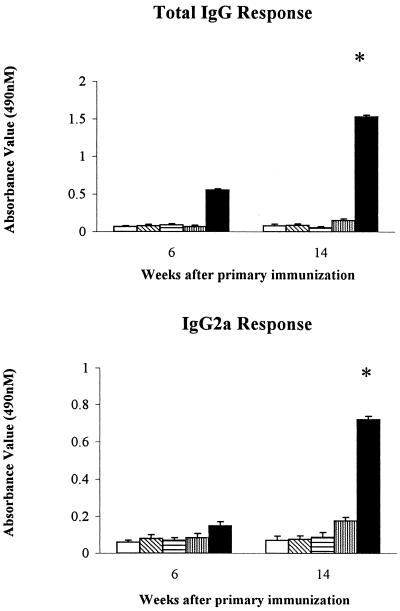
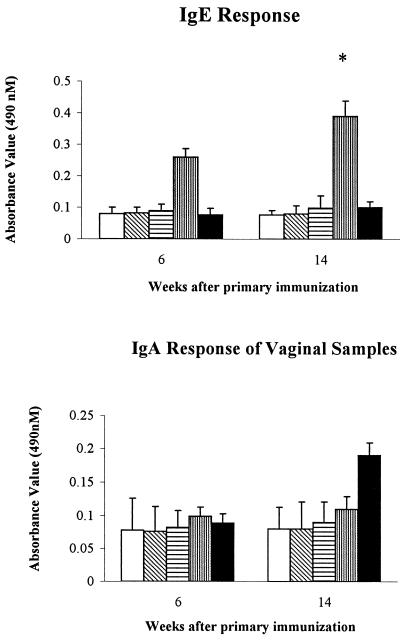
The results presented in Fig. 1 and 2 indicate the level of IgG, IgG2a, IgE, and IgA antibodies in mice immunized with different HSV-2 vaccine formulations at weeks 6 and 14 after primary immunization. Mice injected with HSV-2 plus CAP demonstrated an increase in IgG and IgG2a antibody levels beginning at week 6 after primary immunization, with these increases continuing through the week 14. The other groups of mice failed to develop antibody responses during this same time period. Surprisingly, we saw a higher IgA antibody response in vaginal washes from mice immunized with HSV-2 plus CAP but not in any other group of mice, although this difference was not statistically significant. In contrast, mice vaccinated with HSV-2 plus alum showed a higher level of IgE antibody at week 6 after the primary immunizations and maintained this high level for 14 weeks.
FIG. 1.
Anti-HSV-2 IgG and IgG2a antibody levels at 6 and 14 weeks after the initial immunization among different immunized groups of mice. The antigen concentration and the antibody dilution used in the IgG ELISA were 6 μg/ml and 1:800, respectively. The antigen concentration and the antibody dilution used in the IgG2a ELISA were 12 μg/ml and 1:400, respectively. □, control; ▧, CAP; ▤, HSV-2; ▥, HSV-2 plus alum; ■, HSV-2 plus CAP. ∗, P < 0.05 (HSV-2 plus CAP versus HSV-2 plus alum, HSV-2, and CAP).
FIG. 2.
Anti-HSV-2 IgE and IgA antibody titers for mice immunized with HSV-2 alone, CAP alone, and HSV-2 containing CAP or alum at 6 and 14 weeks after the initial vaccination. The antigen concentration and the antibody dilution used in the IgE ELISA were 6 μg/ml and 1:800, respectively. The antigen concentration used in the IgA ELISA was 100 μg/ml. □, control; ▧, CAP; ▤, HSV-2; ▥, HSV-2 plus alum; ■, HSV-2 plus CAP. ∗, P < 0.05 (HSV-2 plus alum versus HSV-2 plus CAP, HSV-2, CAP, and control).
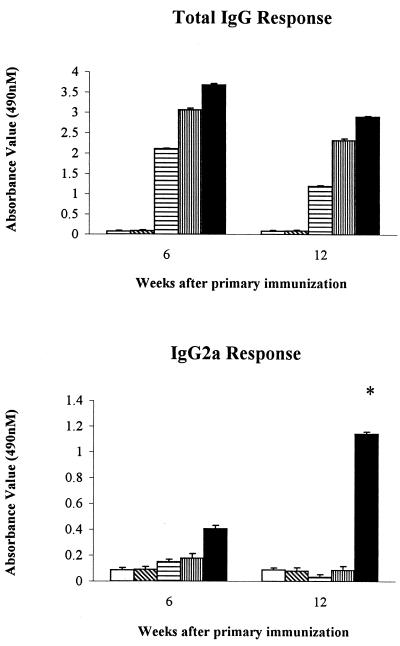
Figure 3 shows the anti-EBV IgG and IgG2a antibody titers in mice immunized with PBS, CAP, EBV alone, and EBV containing CAP or alum, respectively, at weeks 6 and 12 after primary immunization. All groups of vaccinated mice elicited higher IgG titers relative to those elicited by mice treated with CAP alone and PBS alone. Only in the group treated with CAP plus EBV was a high level of IgG2a detected. IgG2a levels remained elevated throughout the study period. None of the preparations caused a significant elevation in IgE levels.
FIG. 3.
Representative anti-EBV IgG and IgG2a antibody titers from mice immunized with EBV alone, CAP alone, EBV and CAP, and EBV and alum at 6 and 12 weeks after the initial immunization. The antigen concentration was 6 μg/ml, and the antibody titer was 1:800. □, control; ▧, CAP; ▤, EBV; ▥, EBV plus alum; ■, EBV plus CAP. ∗, P < 0.05 (EBV plus CAP versus EBV plus alum, EBV, CAP, and control).
A neutralization assay was performed at week 12 after primary immunization with the group of mice vaccinated with different HSV-2 vaccine formulations (Table 3). Both the group treated with HSV plus CAP and the group treated with HSV plus alum had the same neutralizing antibody titer. No detectable antibody titer was observed in mice treated with CAP alone and HSV-2 alone.
TABLE 3.
Neutralizing antibody titer and resistance to low-dose HSV-2 vaginal challenge in immunized micea
| Animal group | Neutralizing antibody titer | Clinical severity at the following days postchallenge (mean ± SD)b
|
||
|---|---|---|---|---|
| 14 | 16 | 20 | ||
| CAP | Undetectable | 0.8 ± 0.837 | 1.2 ± 1.304 | 3.0 ± 2.739 |
| HSV-2 | Undetectable | 0.6 ± 0.548 | 1.2 ± 1.303 | 2.8 ± 2.588 |
| HSV-2 + CAP | 128 | 0.2 ± 0.447 | 0 | 0c |
| HSV-2 + alum | 128 | 0.2 ± 0.447 | 0.2 ± 0.447 | 0c |
The assay performed at 12 weeks after initial immunization.
HSV-2 low-dose challenge was performed at 15 weeks after the initial vaccination. Clinical severity was monitored daily after HSV-2 challenge. Pathology was scored on a five-point scale: 0, no apparent infection; 1, slight redness of external vagina; 2, severe redness and swelling of external vagina; 3, genital ulceration with severe redness, swelling, and loss of hair in genital and surrounding tissue region; 4, severe ulceration of genital and surrounding tissue region and paralysis; 5, death.
P < 0.05 (HSV-2 plus CAP and HSV-2 plus alum versus HSV-2 and CAP).
Experimental mice were challenged at week 15 after the first immunization with a low dose (103 PFU) of HSV-2. Table 3 illustrates the pathologic severity in vaccinated mice on days 14, 16, and 20 after virus infection. While the mean scores were higher in all categories of animals that received CAP alone and HSV-2 alone compared with those in animals treated with HSV-2 plus CAP and HSV-2 plus alum, these differences were not statistically significant on day 14. By days 16 and 20, both groups of mice treated with CAP alone and HSV alone still exhibited significantly severe clinical symptoms; only two of six mice survived in both groups. In contrast, the groups treated with HSV plus CAP and HSV plus alum showed only mild pathologic signs. None of these mice died from HSV-2 infection. By day 20, the differences between the mice vaccinated with adjuvant and those vaccinated without adjuvant were statistically significant.
DISCUSSION
Many adjuvants have routinely been used for research and in veterinary vaccines. However, toxicity and physicochemical properties that affect manufacturability have limited their use in vaccines designed for humans. We have developed a novel formulation of CAP nanoparticles specifically for use as the adjuvant-active components in subunit vaccine formulations. CAP adjuvant is easy to manufacture on an industrial scale and shows less variation in quality and physicochemical properties between batches than alum, which changes significantly with slight alterations in production conditions (5, 7, 14). We have analyzed CAP at 4°C, room temperature, 37°C, and 50°C for a 6-month period and observed no significant changes in pH, size, or surface morphology (data not shown). Earlier studies have reported that CAP adjuvant, in comparison with alum adjuvant, induced a lower level of IgE, decreased the level of local irritation in experiments with animals, and caused fewer variations in human clinical trials (5, 11, 18, 20). Our preclinical toxicity and animal studies confirmed that only minor inflammation occurred at the injection sites in guinea pigs during the first 2 weeks and that CAP did not elicit IgE responses.
From an immunological viewpoint, the most important consideration is whether CAP nanoparticles enhance the immune response to viral antigens and the level of protection against viral infection. Data from this study strongly suggest that CAP nanoparticles significantly enhance the antibody response to HSV-2 antigen, which is normally poorly immunogenic. In this study, we also compared the level of protection against an intravaginal HSV-2 infection after immunization with different HSV-2 vaccine formulations. A total of 40% of nonimmunized mice survived a low-dose (103-PFU) challenge. In contrast, 100% protection was observed in the group of mice immunized with HSV-2 proteins containing adjuvants. The group of mice treated with HSV-2 plus CAP or HSV-2 plus alum showed comparable neutralizing antibody titers that may have protected the animals from low-dose infection.
Particularly high IgG2a titers were observed in the mice immunized with HSV-2 and EBV proteins containing CAP adjuvant but not in mice immunized with HSV-2 and EBV proteins with alum adjuvant or without adjuvants. In the 1980s several groups reported that IgG2a was the major antibody isotype conferring protection against viral infection and the most effective isotype for the induction of macrophage immunity in mice (1, 2, 8, 10, 19). Further studies confirmed that gamma interferon, which is induced by the type 1 CD4+ Th1 response, stimulated the expression of IgG2a and inhibited the production of IgE. Thus, the production of the IgG2a antibody isotype is widely recognized as characteristic of a Th1 CD4+ T-cell response (16, 24, 25). It is also generally accepted that Th1 T-cell responses enhance protective cytotoxic T-lymphocyte responses, thus playing a crucial role in immunity against intracellular viral pathogens like HSV-2. Our data demonstrated that mice immunized with HSV-2 plus CAP had a higher level of IgG2a and could withstand a challenge with live HSV-2. On the basis of these observations it is reasonable to infer that our CAP-based vaccine formulation elicits a strong and effective Th1 response.
Like alum adjuvants, the mechanism behind CAP has not been defined. CAP is believed to act similarly to alum by releasing the antigen slowly over an extended period of time. CAP possesses considerable potential for use in the development of single-dose vaccines with sustained-release capabilities. We have developed a technique in which some antigens are entrapped in the core of the final CAP formulation, which helps to boost immune responses over an extended time and which has the potential to reduce the antigen dose required for immunization. Although we have yet to find a way to show the kinetics of antigen release from CAP, it is reasonable to assume that the surface antigens are released from CAP immediately after injection. Conceivably, then, the antigens from the “core” of the CAP would continue to be released as CAP dissolves, thus making antigen available to stimulate the host's immune system over an extended period of time.
Taken together, these data indicate that (i) CAP adjuvant induces very little inflammation at the site of entry, (ii) CAP-based viral vaccines induce a higher IgG2a response and a lower IgE response relative to the responses induced by alum, and (iii) CAP plus HSV-2 induces protection against live HSV-2 infection. On the basis of these observations, we suggest that CAP nanoparticles present an improved alternative to alum adjuvants, especially for viral antigens.
ACKNOWLEDGMENT
This work was supported by BioSante Pharmaceuticals, Inc.
REFERENCES
- 1.Burton D R. Review: immunoglobulin G functional sites. Mol Immunol. 1985;22:161–206. doi: 10.1016/0161-5890(85)90151-8. [DOI] [PubMed] [Google Scholar]
- 2.Coutelier J P, van der Logt J T M, Heessen F W A, Warnier G, van Sinck J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987;165:64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox J C, Coulter A R. Adjuvants—a classification and review of their modes of action. Vaccine. 1997;15:248–256. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 4.Davenport F M, Hennessy A V, Askin F B. Lack of adjuvant effect of AIPO4 on purified influenza virus haemagglutinins in man. J Immunol. 1968;100:1139–1140. [PubMed] [Google Scholar]
- 5.Feldkamp J R, White J L, Hem S L. Effect of surface charge and particle size on gel structure of aluminum hydroxycarbonate gel. J Pharm Sci. 1982;71:43–46. doi: 10.1002/jps.2600710111. [DOI] [PubMed] [Google Scholar]
- 6.Gallichan W S, Rosenthal K L. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J Infect Dis. 1998;177:1155–1161. doi: 10.1086/515286. [DOI] [PubMed] [Google Scholar]
- 7.Gateff C, Relyveld E H, Le G G. Etude d'une nouvelle association vaccinal quintuple. Ann Microbiol (Inst Pasteur) 1973;124B:387–409. [PubMed] [Google Scholar]
- 8.Germain R N. Antigen presentation. The second class story. Nature. 1991;353:605–607. doi: 10.1038/353605a0. [DOI] [PubMed] [Google Scholar]
- 9.Goto N, Kato H, Maeyama J I. Local tissue irritating effects and adjuvant activities of calcium phosphate and aluminum hydroxide with different physical properties. Vaccine. 1997;15:1364–1370. doi: 10.1016/s0264-410x(97)00054-6. [DOI] [PubMed] [Google Scholar]
- 10.Hocart M J, Mackenzie J S, Stewart G A. The immunoglobulin G subclass response of mice to influenza A virus: the effect of mouse strain, and the neutralizing abilities of individual protein A-purified subclass antibodies. J Gen Virol. 1989;70(Pt. 9):2439–2448. doi: 10.1099/0022-1317-70-9-2439. [DOI] [PubMed] [Google Scholar]
- 11.Ickovic M R, Relyveld E H, Henocq E. Calcium phosphate adjuvanted allergens. Total and specific IgE levels before and after immunotherapy with house dust and mite extracts. Ann Immunol (Inst Pasteur) 1983;134D:385–398. doi: 10.1016/s0769-2625(83)80029-4. [DOI] [PubMed] [Google Scholar]
- 12.Kato H, Shibano M. Relationship between hemolytic activity and absorption capacity of aluminum hydroxide and calcium phosphate as immunological adjuvants for biologicals. Microbiol Immunol. 1994;38:543–548. doi: 10.1111/j.1348-0421.1994.tb01820.x. [DOI] [PubMed] [Google Scholar]
- 13.Kossovsky, N., J. Hnatyszyn, and A. Gelman. 1995. U.S. patent 5462751.
- 14.Kreuter J, Haenzel I. Mode of action of immunological adjuvants: some physicochemical factors influencing the effectivity of polyacrylic adjuvants. Infect Immun. 1978;19:667–675. doi: 10.1128/iai.19.2.667-675.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuklin N, Daheshia M, Rouse B T. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J Virol. 1997;71:3138–3145. doi: 10.1128/jvi.71.4.3138-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mossmann T R, Chervinski H. Two types of murine helper T cell clones. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 17.Nagel J E, White C, Lin M S, Fireman P. IgE synthesis in man. II. Comparison of tetanus and diphtheria IgE antibody in allergic and nonallergic children. J Allergy Clin Immunol. 1979;63:308–314. doi: 10.1016/0091-6749(79)90124-6. [DOI] [PubMed] [Google Scholar]
- 18.Neefjes J J, Mornburg F. Cell biology of antigen presentation. Curr Opin Immunol. 1993;5:27–34. doi: 10.1016/0952-7915(93)90077-6. [DOI] [PubMed] [Google Scholar]
- 19.Neuberger M S, Rajewsky K. Activation of mouse complement by monoclonal mouse antibodies. Eur J Immunol. 1981;11:1012–1016. doi: 10.1002/eji.1830111212. [DOI] [PubMed] [Google Scholar]
- 20.Powell M F, Newman M J, editors. Vaccine design: the subunit and adjuvant approach. New York, N.Y: Plenum Publishing Corp.; 1995. Adjuvant properties of aluminum and calcium compounds; pp. 229–248. [Google Scholar]
- 21.Relyveld E H. Preparation and use of calcium phosphate adsorbed vaccines. Dev Biol Stand. 1986;65:131–136. [PubMed] [Google Scholar]
- 22.Relyveld E H, Ickovic M R, Henocq E, Garcelon M. Calcium phosphate adjuvanted allergens. Ann Allergy. 1985;54:11–19. [PubMed] [Google Scholar]
- 23.Singh M, Carlson J R, Briones M, Ugozzoli M, Kazzaz J, Barackman J, Ott G, O'Hagan D. A comparison of biodegradable microparticles and MF59 as systemic adjuvants for recombinant gD from HSV-2. Vaccine. 1998;16:1822–1827. doi: 10.1016/s0264-410x(98)00179-0. [DOI] [PubMed] [Google Scholar]
- 24.Singh M, O'Hagan D. Advances in vaccine adjuvants. Nat Biotech. 1999;17:1075–1081. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]
- 25.Snapper C M, Paul W E. Interferon-γ and B-cell stimulatory factor 1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration. Code of federal regulations. U.S. Washington, D.C.: Government Printing Office; 1998. Good laboratory practice (GLP) regulations. CFR, Title 21, Part 58. [Google Scholar]
- 27.Vassilev T L. Aluminum phosphate but not calcium phosphate stimulates the specific IgE response in guinea pigs to tetanus toxoid. Allergy. 1978;33:155–159. doi: 10.1111/j.1398-9995.1978.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 28.Westra D F, Glazenburg K L, Harmsen M C, Tiran A, Scheffer A J, Welling G W, Hauw T T, Welling-Wester S. Glycoprotein H of herpes simplex virus type 1 requires glycoprotein L for transport to the surfaces on insect cells. J Virol. 1997;71:2285–2291. doi: 10.1128/jvi.71.3.2285-2291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]