Abstract
An accurate and reliable susceptibility testing method for polymyxins is urgently needed not only for the clinical laboratory but also for new polymyxin-like lipopeptide development. Reference broth microdilution (rBMD), which was the recommended method by CLSI-EUCAST in clinics, has been proven not to be ideal, while the agar dilution (AD) method that was widely used in new antibiotics discovery has been neglected. In the present study, the AD method was compared with rBMD and broth macrodilution (BMAD) in susceptibility testing of polymyxin B and colistin against >200 Gram-negative isolates. AD showed strong agreement with BMAD for colistin (except for Klebsiella aerogenes and Pseudomonas aeruginosa); however, its performance was poor for polymyxin B or compared to rBMD. MICs of AD method were not affected when different types of Petri dishes were used, while glass-bottom microtiter plates could lower the MIC of polymyxins 2–8 times compared to tissue-culture-treated polystyrene plates when using rBMD, which demonstrated that tissue-culture-treated plates were not suitable. It was then validated with non-tissue-culture-treated plates. The culture volume was another influencing factor of accuracy for rBMD, and 200 μL seemed to be the most suitable volume for MIC detection of polymyxins. Additionally, no lack of growth phenomenon (skipped well) was observed for AD when it frequently occurred for both BMAD and rBMD. As for strains carrying mcr-1 gene, 100% of AD results were in essential agreement (EA) and categorical agreement (CA) with both rBMD and BMAD. Overall, rBMD is convenient and widely accepted for susceptibility testing of polymyxins. Although it may be too early to say that AD is superior compared to rBMD and BMAD, it did show some advantages in repeatability and anti-interference ability.
Keywords: polymyxins, antimicrobial susceptibility testing, agar dilution, broth microdilution
1. Introduction
Polymyxins represent a class of non-ribosomal synthesized, cationic, cyclic-lipopeptide antibiotics that can interact with the lipid A moiety of lipopolysaccharide [1,2]. To date, nine polymyxin superfamily members have been identified: polymyxins A, B, C, D, E, F, K, M, P, S, and T [3,4]. Polymyxin B and colistin (polymyxin E) are the only polymyxins available in clinical practice [5], which were discovered in the 1940s from the soil bacterium Paenibacillus polymyxa [6] and were widely used to treat severe infections till the mid-1980s. The rate of polymyxin-associated neurotoxicity and nephrotoxicity was reported as high as 27% and 60% then, which restricted their use. However, neurotoxicity was proved not to be a major concern according to later studies, and nephrotoxicity was reversible in most patients and can be managed through close monitoring [7,8]. Over the last decades, with the growing prevalence of multidrug-resistant bacteria, the paucity or unbearable high cost of new effective antibiotics, polymyxins have re-emerged as the last resort therapeutic option. Although there are international consensus guidelines for use and susceptibility testing of colistin, improvements to susceptibility testing are still required.
Low accuracy and reliability of polymyxin susceptibility testing led to misuse of polymyxins and hindered the development of new polymyxin drugs [9,10]. In 2016, a CLSI-EUCAST working group recommended ISO-standard BMD (20776-1) as the reference method when testing MIC of colistin on Enterobacteriaceae, P. aeruginosa, and Acinetobacter spp. [11]. However, adherence of polymyxin molecules onto the plastic-surface plates used in BMD has raised concerns about the reliability and inter-laboratory comparison of MIC results [12]. As for the AD and BMAD methods, there were relatively few studies to analyze their performance [13,14,15,16,17,18]. Thus, there is still a long way to go to find a practical method for polymyxin susceptibility testing. In the current work, we compared the AD with the rBMD and BMAD methods to determine the feasibility of AD to be used in the susceptibility testing of polymyxins.
2. Results
2.1. MICs of Polymyxin B and Colistin by Three Susceptibility Methods
220 Gram-negative isolates, including 40 E. coli, 38 Klebsiella pneumoniae, 37 Enterobacter cloacae, 30 K. aerogenes, 38 Acinetobacter baumannii, and 37 P. aeruginosa, were tested. The MICs of quality control strains were within the acceptable range for all three methods. The MIC50 and MIC90 are shown in Table 1; MIC distributions for polymyxin B and colistin are presented in Supplementary Materials Tables S1–S3. Generally, AD resulted in moderate MICs which were lower than rBMD but higher than BMAD for both polymyxin B and colistin. The MIC50 ranges were 0.25–4.0 μg/mL for AD, 0.5–8.0 μg/mL for rBMD, and 0.125–4.0 μg/mL for BMAD.
Table 1.
MIC50 and MIC90 of polymyxins.
| Species | PMB | CST | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | rBMD* | BMAD | AD | rBMD* | BMAD | |||||||
| μg/mL | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 |
| E. coli | 0.5 | 1 | 1 | 2 | 0.5 | 1 | 0.5 | 0.5 | 1 | 2 | 0.25 | 0.5 |
| K. pneumoniae | 1 | 2 | 2 | 2 | 1 | 2 | 0.5 | 1 | 1 | 2 | 0.5 | 1 |
| E. cloacae | 1 | 1 | 2 | 4 | 0.5 | 1 | 1 | 1 | 1 | 2 | 0.5 | 1 |
| K. aerogenes | 1 | 1 | 2 | 2 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 2 | 0.25 | 0.5 |
| A. baumannii | 1 | 1 | 2 | 2 | 0.5 | 1 | 0.5 | 0.5 | 1 | 2 | 0.5 | 1 |
| P. aeruginosa | 2 | 2 | 2 | 4 | 1 | 1 | 2 | 2 | 2 | 4 | 1 | 2 |
PMB: polymyxin B; CST: colistin; AD: agar dilution; rBMD: reference broth microdilution; BMAD: broth macrodilution. *: using tissue-culture-treated microtiter plates.
Of the 220 isolates, only a single strain (0.5%) was resistant to polymyxin B and all isolates were sensitive to colistin when tested by AD; the corresponding MICs were in the ranges of 0.5–4.0 μg/mL and 0.25–2.0 μg/mL, respectively (Supplementary Material Table S1). Of these, 27 (12%) were resistant to polymyxin B and 20 (9%) to colistin for rBMD method; the corresponding MICs ranged around 0.5–8.0 μg/mL and 0.5–4.0 μg/mL (Supplementary Material Table S2). As for BMAD, 3 (1%) strains were resistant to polymyxin B and 8 (4%) to colistin, with corresponding MICs ranging from 0.25–4.0 μg/mL and 0.125–4.0 μg/mL (Supplementary Material Table S3).
As for 7 mcr-1-positive strains (not included in the 220 isolates) which were used to test the impact of resistance genes carried by plasmids, the MICs (Table 2) showed that AD, rBMD, and BMAD correlated very well with each other (100% EA and 100% CA, no VME or ME). All strains were resistant to both polymyxins B and colistin using AD, rBMD, and BMAD; MICs ranged from 4–16 μg/mL, 4–8 μg/mL, and 4–8 μg/mL for polymyxin B, respectively, and 8–32 μg/mL, 8–16 μg/mL, and 8–32 μg/mL for colistin, respectively.
Table 2.
MIC values (μg/mL) of polymyxin B and colistin for mcr-1 positive isolates.
| No. | Strains | PMB | CST | ||||
|---|---|---|---|---|---|---|---|
| AD | rBMD* | BMAD | AD | rBMD* | BMAD | ||
| 1 | E. coli NCTC13846 | 4 | 4 | 4 | 8 | 8 | 8 |
| 2 | E. coli CCPM(A)-P-070885 | 8 | 4 | 4 | 16 | 16 | 8 |
| 3 | E. coli CCPM(A)-P-071343 | 8 | 4 | 4 | 16 | 16 | 8 |
| 4 | E. coli CCPM(A)-P-071366 | 8 | 4 | 4 | 16 | 8 | 8 |
| 5 | E. coli CCPM(A)-P-071368 | 8 | 8 | 4 | 8 | 16 | 8 |
| 6 | E. coli CCPM(A)-P-0717R14 | 8 | 4 | 4 | 8 | 8 | 8 |
| 7 | K. pneumoniae CCPM(A)-P-080920 | 16 | 8 | 8 | 32 | 16 | 32 |
No.: number; PMB: polymyxin B; CST: colistin; AD: agar dilution; rBMD: reference broth microdilution; BMAD: broth macrodilution. *: using tissue-culture-treated microtiter plates.
2.2. Comparison of AD with Other Susceptibility Testing Methods
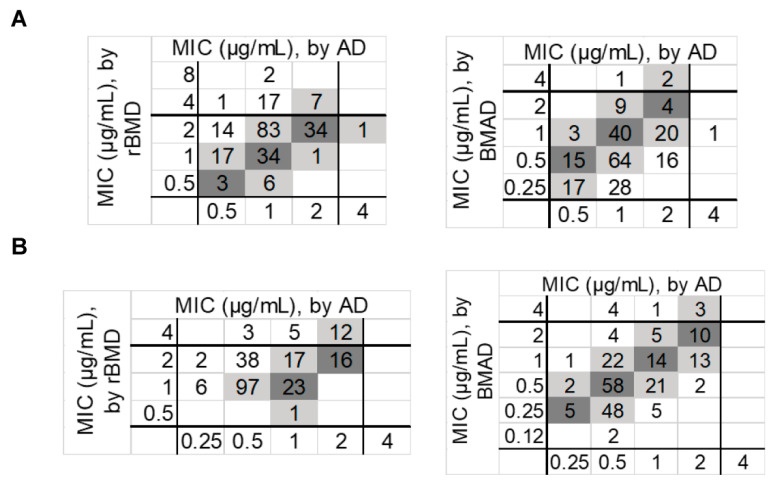
Comparison to rBMD as the test method. The comparative result between AD and rBMD is shown in Table 3 and Figure 1. The correlation of AD and rBMD was especially poor, which revealed VMEs of 12.2% and 9.1% for polymyxin B and colistin, respectively. Acceptable MEs of polymyxin B (0.4%) and colistin (0%) were observed. CA for polymyxin B (87.3%) was a little bit lower than that of colistin (90.9%), whereas EAs of 85% and 75.1% were observed for polymyxin B and colistin, respectively. It was worthy of note that VMEs for P. aeruginosa were 29.7% and 35.1%, respectively, for polymyxin B and colistin.
Table 3.
AD Compared with rBMD and BMAD for polymyxin B and colistin.
| Species | Tested Agents | rBMD* | BMAD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % | VME | ME | CA | EA | VME | ME | CA | EA | |
| E. coli | PMB | 2.5 | 0 | 97.5 | 80 | 0 | 0 | 100 | 87.5 |
| K. pneumoniae | 10.5 | 0 | 89.5 | 94.7 | 5.3 | 0 | 94.7 | 89.5 | |
| E. cloacae | 10.8 | 0 | 89.2 | 86.5 | 0 | 0 | 100 | 62.2 | |
| K. aerogenes | 3.3 | 0 | 96.7 | 90 | 0 | 0 | 100 | 73.3 | |
| A. baumannii | 15.8 | 0 | 84.2 | 76.3 | 2.6 | 0 | 97.4 | 86.8 | |
| P. aeruginosa | 29.7 | 2.7 | 67.6 | 81.1 | 0 | 2.7 | 97.3 | 73 | |
| Total (%) | 12.2 | 0.4 | 87.3 | 85 | 1.4 | 0.4 | 98.2 | 79.1 | |
| E. coli | CST | 2.5 | 0 | 97.5 | 67.5 | 0 | 0 | 100 | 92.5 |
| K. pneumoniae | 7.9 | 0 | 92.1 | 68.4 | 2.6 | 0 | 97.4 | 86.8 | |
| E. cloacae | 0 | 0 | 100 | 78.4 | 2.7 | 0 | 97.3 | 89.2 | |
| K. aerogenes | 0 | 0 | 100 | 73.3 | 6.7 | 0 | 93.3 | 90 | |
| A. baumannii | 7.9 | 0 | 92.1 | 71.1 | 0 | 0 | 100 | 100 | |
| P. aeruginosa | 35.1 | 0 | 64.9 | 94.6 | 10.8 | 0 | 89.2 | 89.2 | |
| Total (%) | 9.1 | 0 | 90.9 | 75.1 | 3.6 | 0 | 96.4 | 91.8 | |
VME: very major errors; ME: major errors; CA: categorical agreement; EA: essential agreement; PMB: polymyxin B; CST: colistin; BMAD: broth macrodilution; AD: agar dilution; rBMD: reference broth microdilution. *: using tissue-culture-treated microtiter plates.
Figure 1.
Scatterplot of MIC values for polymyxin B (A) and colistin (B) measured by AD versus rBMD, and AD versus BMAD using all study isolates (n = 220). The coordinate axis shows the MIC range of the test method. Dark gray, absolute agreement; light gray, essential agreement. BMAD: broth macrodilution; rBMD: reference broth microdilution; AD: agar dilution.
Comparison to BMAD as the test method. Overall, AD showed a high level of agreement with BMAD for colistin when testing E. coli and A. baumannii, and the results of K. pneumonia and E. cloacae were barely acceptable. However, the VMEs of K. aerogenes (6.7%) and P. aeruginosa (10.8%) were extremely high. Although the overall VME, ME and CA were within the acceptance criteria for polymyxin B, the EA was much too low (79.1%).
2.3. Differences of MICs between Polystyrene and Glass-Bottom Microtiter Plates
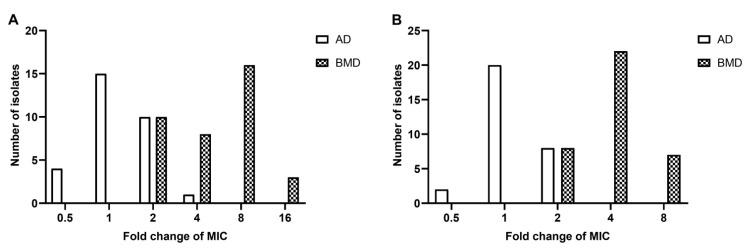
It was noted that glass-bottom microtiter plates would bring 2–8 times lower MICs of polymyxin B and colistin than tissue culture-treated polystyrene plates for all 6 isolates tested (Table 4). The results indicate that polymyxins were adsorbed onto surface of the plastic plates, which led to a higher MIC; whereas the degrees of polymyxin loss were much lower in glass-bottom plates. When using non-tissue culture-treated polystyrene plates, the MICs correspond well with glass-bottom ones (Table 4). When comparing MICs of tissue culture-treated polystyrene and glass-bottom plates with 37 clinical isolates, it validated what we saw in standard strains, i.e., that tissue culture-treated polystyrene plates were unreliable for rBMD (Figure 2).
Table 4.
Differences of MICs of polymyxin B and colistin on different plate materials.
| Tested Agents | Strains | rBMD | AD | ||||
|---|---|---|---|---|---|---|---|
| TC-PS | GB | nTC-PS | TC Plates | GA | nTC Plates | ||
| PMB | E. coli ATCC 25922 | 2 | 0.5 | 1 | 2 | 1 | 1 |
| E. coli ATCC 2469 | 2 | 0.5 | 0.5 | 1 | 0.5 | 1 | |
| K. pneumoniae ATCC 700603 | 4 | 0.5 | 0.5 | 2 | 1 | 1 | |
| K. pneumoniae ATCC 2146 | 4 | 0.5 | 0.5 | 2 | 1 | 1 | |
| A. baumannii ATCC 19606 | 2 | 0.5 | 0.5 | 1 | 0.5 | 1 | |
| P. aeruginosa ATCC 27853 | 2 | 0.5 | 1 | 2 | 1 | 2 | |
| CST | E. coli ATCC 25922 | 2 | 1 | 1 | 1 | 0.5 | 1 |
| E. coli ATCC 2469 | 2 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | |
| K. pneumoniae ATCC 700603 | 4 | 0.5 | 0.5 | 1 | 0.5 | 1 | |
| K. pneumoniae ATCC 2146 | 2 | 0.5 | 0.5 | 1 | 0.5 | 1 | |
| A. baumannii ATCC 19606 | 2 | 1 | 1 | 1 | 0.5 | 0.5 | |
| P. aeruginosa ATCC 27853 | 2 | 1 | 1 | 2 | 1 | 1 | |
PMB: polymyxin B; CST: colistin; TC-PS: tissue-culture-treated polystyrene microtiter plates; GB: glass-bottom microtiter plates; nTC-PS: non-tissue-culture-treated polystyrene microtiter plates; TC plates: tissue-culture-treated polystyrene plates; GA: glass plates; nTC plates: non-tissue culture-treated polystyrene plates; rBMD: reference broth microdilution; AD: agar dilution.
Figure 2.
Comparison of MIC values for polymyxin B (A) and colistin (B) using polystyrene and glass plates. Fold change of MIC means the ratio of the MIC values obtained from polystyrene to those obtained from glass plates (AD: non-tissue-culture-treated plates vs. glass plates; rBMD: tissue-culture-treated microtiter plates vs. glass-bottom microtiter plates).
To further investigate whether the adsorption of polymyxins was affected by the culture volume, two types of 96-well plates (glass-bottom and tissue-culture-treated polystyrene plates) and three different volumes were used to determine MICs of polymyxin B and colistin (Table 5). Compared with 100 μL culture volume, the MICs of polymyxins were 2–4 times lower when using 200 μL or 300 μL broth per well, while the MICs of levofloxacin (negative control) were identical in all three volumetric systems. All of these results revealed that as the volume of the test system increased, the loss of polymyxins due to adsorption decreased, and the measured MICs were more accurate. Hence, when rBMD is applied for polymyxins MIC determination, reliability can be improved by using a 200 uL culture volume.
Table 5.
MIC values (μg/mL) measured with three volumes and two plate materials by rBMD.
| Tested Agents | Strains | 100 μL | 200 μL | 300 μL | |||
|---|---|---|---|---|---|---|---|
| TC-PS | GB | TC-PS | GB | TC-PS | GB | ||
| PMB | E. coli ATCC 25922 | 2 | 0.5 | 1 | 0.25 | 1 | 0.25 |
| E. coli ATCC 2469 | 2 | 0.5 | 0.5 | 0.25 | 0.5 | 0.12 | |
| K. pneumoniae ATCC 700603 | 4 | 0.5 | 1 | 0.25 | 1 | 0.25 | |
| K. pneumoniae ATCC 2146 | 4 | 0.5 | 1 | 0.12 | 1 | 0.12 | |
| A. baumannii ATCC 19606 | 2 | 0.5 | 1 | 0.12 | 1 | 0.12 | |
| P. aeruginosa ATCC 27853 | 2 | 0.5 | 1 | 0.5 | 1 | 0.5 | |
| CST | E. coli ATCC 25922 | 2 | 1 | 1 | 0.25 | 1 | 0.25 |
| E. coli ATCC 2469 | 2 | 0.5 | 1 | 0.25 | 0.5 | 0.25 | |
| K. pneumoniae ATCC 700603 | 4 | 0.5 | 1 | 0.25 | 1 | 0.25 | |
| K. pneumoniae ATCC 2146 | 2 | 0.5 | 1 | 0.12 | 0.5 | 0.25 | |
| A. baumannii ATCC 19606 | 2 | 1 | 1 | 0.5 | 1 | 0.5 | |
| P. aeruginosa ATCC 27853 | 2 | 1 | 1 | 0.5 | 1 | 0.5 | |
PMB: polymyxin B; CST: colistin; TC-PS: tissue-culture-treated polystyrene microtiter plates; GB: glass-bottom microtiter plates; rBMD: reference broth microdilution. Levofloxacin was used as a control and MIC values of levofloxacin were consistent with three volumes and two plate materials.
2.4. Differences of MICs between Polystyrene and Glass Petri Dishes
Testing was conducted with 6 ATCC isolates to compare the MICs between glass, tissue-culture-treated and non-tissue-culture-treated polystyrene dishes. The results showed that MICs differed within two-fold, which were in acceptable ranges [19,20] (Table 4). MICs of non-tissue-culture-treated polystyrene dishes and glass dishes were then compared with 30 clinical isolates. It showed that most isolates exhibited the same MICs on two types of dishes, and MICs of the remaining strains differed by an acceptable two-fold only (Figure 2). Overall, AD showed great consistency on different materials or types of dishes.
2.5. The Ratio of Lack of Growth
Lack of growth phenomenon is equivalent to skip well for rBMD and BMAD, which means bacteria exhibit no growth at a lower antibiotic concentration, whereas growth is observed with higher concentrations [9]. In our studies, no lack of growth occurred for AD, while skip wells in 4 of 220 strains for polymyxin B and 9 of 220 strains for colistin when testing with rBMD and in 17 of 220 strains for polymyxin B and colistin when testing with BMAD were observed.
3. Discussion
Although several potent antibiotics (such as cefiderocol and meropenem–vaborbactam) were recommended to deal with severe Gram-negative infections, polymyxins remain to be the only option for infections caused by carbapenem-resistant isolates in many countries where these costly new antibiotics are unavailable [21]. Colistin also has an important role as the salvage therapy for cystitis and HAP/VAP, which is endorsed by Infectious Diseases Society of America (IDSA), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and Chinese Research Hospital Association of Critical Care Medicine [22,23,24]. Therefore, optimization and standardization of in vitro polymyxin susceptibility testing, as well as the definition of accurate breakpoints, are critical issues for both patient care and epidemiological surveillance purposes, particularly in view of the increased clinical use of polymyxins.
CLSI and EUCAST had a consensus that rBMD should be used as a reference method, but they had no agreement on some other issues. For CLSI, rBMD, CBDE, and CAT, MIC methods are all acceptable now for colistin and polymyxin B when testing Enterobacterals and P. aereginosa, but rBMD is the only approved method for Acinetobacter spp. [25]. In contrast to CLSI, ECUAST only approved rBMD method for colistin susceptibility testing [26]. CLSI deleted the breakpoint of susceptible category (S) after reviewing the preclinical PK/PD, clinical PK/TD, and MIC distribution data, and classified the strains with MIC ≤ 2 μg/mL into intermediate category in 2020, while EUCAST maintains ≤2 μg/mL as the breakpoint of the susceptible category.
Numerous studies have shown that commercial disk diffusion, gradient strips, and automated detection systems were unreliable for assessing colistin susceptibility, exhibiting unacceptable high rates of very major errors (VME) and low levels of reproducibility [1]. rBMD is the only co-validated susceptibility testing method for polymyxins by CLSI and EUCAST, although it is not an ideal one. Mariana et al. reported that when retesting 200 K. pneumonia with rBMD, 40% of the MICs showed differences greater than the ±1 dilution accepted variability, indicating the low reproducibility of rBMD [27]. Romney et al. found CAMHB from Oxoid would yield unacceptable low MICs for QC strain P. aeruginosa ATCC 27853 [28]. We used Corning CostarTM 3799 plates for rBMD, and MICs of the QC strains were all within the QC range. It is the most commonly used plate for rBMD, but Mahablleshwar et al. discovered that this type of plate, which are treated with corona discharge (so-called tissue-culture-treated), yielded a much higher MIC comparing to non-coated ones [29]. Then we compared the MIC results of CostarTM 3799 plates with glass-bottom plates and non-tissue culture-treated plates and found that the MICs of the latter two plates were 2–4 times lower, which meant a low adsorption rate. It demonstrated that tissue culture treatment played an important role in binding ability of polymyxins to plate surface, and the current QC range is not appropriate [30]. On the other hand, neither CLSI nor EUCAST specified the types or brands of microtiter plates that should be chosen for rBMD, which may cause many uncertainties and problems [25,26]. In some published literature, the authors used customized rBMD panels that could ensure accurate results [31,32], while the tissue-culture-treated plates may be misused to get higher MICs with rBMD [33]. The adsorption of polymyxins to plastic plates has been widely discussed; Matti et al. characterized the extent of colistin loss in plates of different types and brands [12]. They found both polystyrene and polypropylene microtiter plates could adsorb colistin in different intensities; in addition, two brands of plates yielded a significant difference in the measured concentrations. Low-protein-binding polypropylene plates or glass-coated plates (similar to glass-bottom plates) were recommended for measuring MIC of colistin, but the cost of these plates is much too high [1,12].
Furthermore, we measured the MICs with polystyrene and glass-bottom plates using different volumes of broth and found out the MICs would decrease with larger culture volume regardless of types of the plates. It could be explained by the adsorption characteristics of polymyxins because the surface area to volume ratio of 100 μL system is bigger than the 200 μL one. In addition, the surface-area-to-volume ratio of AD on 9 cm Petri dishes is close to 200μL system, which could explain the comparability of the results of these two methods. CLSI and EUCAST recommended 100 μL as the standard volume of rBMD, while in our opinion, 200 μL seems to be better considering the capacity of the microtiter plates.
AD is thought to be the solid equivalent of broth dilution, either in the rBMD or BMAD format [34], which relies on various concentrations of 2-fold dilution of antibiotics in agar plates. AD is rarely used because it is time- and labor-consuming compared to Etest and disk diffusion in clinical use, although it could test as many as 37, 60, 70, or even more isolates on one plate simultaneously with a semi-auto multipoint inoculator. The CAT method, which is approved by CLSI in 2020, is actually a modified AD method [35]. Operators need to streak 10 μL suspension onto an agar plate with a pipette or loop instead of multipoint inoculators which are uncommon in clinical use. The AD method, consistent with CAT, has no diffusion problem as Etest and disk diffusion and may theoretically avoid the adsorption of polymyxins to the plate surface [1], which was proved by our experiments. Further, Fereshteh et al. reported that the MICs showed no difference when testing ATCC strains with 1-week-old colistin agar plate or freshly made ones [36]. We also noted that no lack of growth phenomenon was observed for AD, whereas it happened at 1.8% or 4.1% for rBMD when testing polymyxin B or colistin, respectively, and 7.7% for BMAD when testing polymyxin B and colistin. It is related to heteroresistance [37], and small inoculation volume (1–2 μL for AD, 10 μL for rBMD and 100 μL for BMAD) may be the reason for low incidence [1]. We could not say it is an advantage of AD, but it did facilitate the reading and results analysis.
Binding of polymyxins to labware is concentration-dependent and saturable [38]. It has been proved that the proportion of free colistin would decrease with lower drug concentration. The same conclusion has been revealed from our result. We tested all the isolates with AD, rBMD, and BMAD and found out that only at high concentrations of polymyxins (≥4 μg/mL) when binding was saturated, the MICs of these three methods could agree well with each other.
CLSI and EUCAST considered colistin and polymyxin B as equivalent, and the MIC of one agent could predict that of the other one, but our result showed MICs of colistin was lower than polymyxin B, indicating a stronger activity of colistin.
4. Materials and Methods
4.1. Bacterial Strains
Briefly, 212 clinical isolates collected from Peking Union Medical College Hospital and The First Affiliated Hospital of Hebei North University and 8 strains from the American Type Culture Collection (ATCC) were used to evaluate the antibacterial susceptibility of colistin and polymyxin B with three testing methods: AD, rBMD, and BMAD. The characteristics of the isolates used were summarized in Supplementary Material Table S4. To evaluate the impact of resistance genes carried by plasmids, 7 mcr-1-positive strains not included in the 220 isolates (1 standard strain NCTC 13846, 5 E. coli clinical isolates, and 1 K. pneumonia clinical isolate) were tested. All strains were stored at the CAMS Collection Center of Pathogen Microorganisms (CAMS-CCPM) in Beijing, China.
4.2. Antibacterial Agents and Susceptibility Testing
Polymyxin B sulfate and colistin sulfate were purchased from the National Institutes for Food and Drug Control (Beijing, China). The powdered drugs were dissolved with sterilized double-distilled water into a concentration of 10 mg/mL and stored at −80 °C until use. rBMD followed the CLSI guidelines, AD and BMAD were performed according to the existing procedures [39]. Tubes and plates were incubated at 35 ± 2 °C for 16–18 h, followed by a visual assessment of turbidity. E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as quality control strains in the susceptibility tests.
Agar dilution. DifcoTM Mueller–Hinton agar (BD, Franklin Lakes, NJ, USA) plates containing 0.125–256 μg/mL polymyxins were prepared in Petri dishes. A 1 μL inoculum of 1:10 dilution of a 0.5 McFarland suspension was inoculated onto agar plates using a Denley® (Denley Instruments Ltd, Sussex, UK) multipoint inoculator (37 spots per plate). The final bacterial inoculum amounted to 1 × 104 CFU/spot. Non-tissue culture-treated polystyrene dishes are most accessible; therefore, experiments were carried out on this type of dish. To investigate the variance of binding ability of different materials, glass and tissue-culture-treated polystyrene dishes were used, and results were compared.
Broth dilution. To minimize the loss of polymyxins in dilution procedure, incremental dilution was carried out. Briefly, polymyxin B and colistin stock solutions were diluted into working solutions (0.125 μg/mL to 16 μg/mL) using BBLTM cation-adjusted Mueller–Hinton II broth (BD, Franklin Lakes, NJ, USA). Antibiotic concentrations were the same for BMAD and rBMD, and 1 mL or 100 μL of each dilution was dispensed to glass tubes or microtiter plates. Bacterial suspensions were adjusted to 0.5 McFarland and the final inoculum amounted to 5 × 105 CFU/mL for both methods. Tissue-culture-treated microtiter plates were from Corning (CostarTM 3799; Corning, ME, USA), which are the most commonly used plates for rBMD in China and some other countries. To further explore the binding ability of polymyxin to the surface of microtiter plates, glass-bottom plates (Cellvis, Mountain View, CA, USA) and non-tissue-culture-treated plates (Nest, China) were used for comparison.
4.3. Interpretation of Antimicrobial Susceptibility Testing Methods
EUCAST-recommended MIC breakpoints for colistin were used to assess differences between the tested methods. For Enterobacteriaceae, P. aeruginosa, and Acinetobacter spp., EUCAST recommends a breakpoint ≤2 μg/mL for susceptible and ≥4 μg/mL for resistant (EUCAST, 2021). CLSI mandates the quality control range of polymyxin B and colistin for E. coli ATCC 25922 to be 0.25–2.0 μg/mL, while for P. aeruginosa ATCC 27853 those values are 0.5–2.0 μg/mL and 0.5–4.0 μg/mL.
Testing would be repeated for isolates when >1 skipped well occurred with broth dilution method, and if it happened again, the isolates would be excluded. It was the same for AD when isolates did not grow on low concentration plates but grew on plates with higher concentrations of polymyxins.
4.4. Data Analysis
MIC50 and MIC90 values were calculated for all susceptibility testing methods. Essential agreement (EA) and categorical agreement (CA) were evaluated; very major errors (VME) and major errors (ME) were analyzed. EA was defined as MICs of the two methods were within a single doubling dilution; CA was the proportion of isolates classified in the same susceptibility category by the methods evaluated; VME was defined as that the result of the test method was susceptible while the result of the reference method was resistant; ME was defined as that the result of the test method was resistant while the result of the reference method was susceptible.
5. Conclusions
The rBMD method which CLSI-EUCAST recommended for susceptibility testing of polymyxins is convenient and widely accepted, although it has quite a lot of limitations. It is still challenging for both clinical and research labs to carry out the experiment without an ideal method. According to the results of this study, we think the reference method should be improved further, such as clearly specifying the brand of CAMHB and plate, using 200 μL culture volumes, and reevaluating the interchangeability of polymyxin B and colistin. BMAD developed by Fleming a century ago is a traditional method. It can be seen as the enlarged BMD except that it is very laborious. AD has some advantages in repeatability and anti-interference ability compared to rBMD and BMAD, but it is time- and labor-consuming and is rarely used by clinical laboratories. All in all, the feasibility to use AD as a reference method is still an open question.
Acknowledgments
We are grateful to the Peking Union Medical College Hospital and The First Affiliated Hospital of Hebei North University for providing clinical isolates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11101392/s1, Table S1: MIC distributions of isolates tested by AD method; Table S2: MIC distributions of isolates tested by rBMD method; Table S3: MIC distributions of isolates tested by BMAD method; Table S4: Characteristics of the 220 isolates used in the study.
Author Contributions
Methodology and data curation, X.H.; writing—original draft preparation, L.S. and G.L.; investigation, T.N. and Y.Y.; resources, X.W., J.P. and X.L. (Xi Lu); formal analysis, X.L. (Xue Li), Y.L., C.L. and Y.M.; supervision, X.Y. (Xinyi Yang); writing—review and editing, G.L. and X.Y. (Xuefu You); funding acquisition, X.Y. (Xuefu You). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by research grants from the Major Research Plan of National Natural Science Foundation of China (32141003), the CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-026), the Fundamental Research Funds for the Central Universities (2021-PT350-001), and the National Science and Technology Infrastructure of China (National Pathogen Resource Center-NPRC-32).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ezadi F., Ardebili A., Mirnejad R. Antimicrobial Susceptibility Testing for Polymyxins: Challenges, Issues, and Recommendations. J. Clin. Microbiol. 2019;57:e01390-18. doi: 10.1128/JCM.01390-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun J., Zhang H., Liu Y.H., Feng Y. Towards Understanding MCR-like Colistin Resistance. Trends Microbiol. 2018;26:794–808. doi: 10.1016/j.tim.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Blaskovich M.A.T., Pitt M.E., Elliott A.G., Cooper M.A. Can octapeptin antibiotics combat extensively drug-resistant (XDR) bacteria? Expert Rev. Anti. Infect. Ther. 2018;16:485–499. doi: 10.1080/14787210.2018.1483240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui A.L., Hu X.X., Gao Y., Jin J., Yi H., Wang X.K., Nie T.Y., Chen Y., He Q.Y., Guo H.F., et al. Synthesis and Bioactivity Investigation of the Individual Components of Cyclic Lipopeptide Antibiotics. J. Med. Chem. 2018;61:1845–1857. doi: 10.1021/acs.jmedchem.7b01367. [DOI] [PubMed] [Google Scholar]
- 5.Falagas M.E., Kasiakou S.K. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005;40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 6.Gallardo-Godoy A., Hansford K.A., Muldoon C., Becker B., Elliott A.G., Huang J.X., Pelingon R., Butler M.S., Blaskovich M.A.T., Cooper M.A. Structure-Function Studies of Polymyxin B Lipononapeptides. Molecules. 2019;24:553. doi: 10.3390/molecules24030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dijkmans A.C., Wilms E.B., Kamerling I.M., Birkhoff W., Ortiz-Zacarias N.V., Van Nieuwkoop C., Verbrugh H.A., Touw D.J. Colistin: Revival of an Old Polymyxin Antibiotic. Ther. Drug Monit. 2015;37:419–427. doi: 10.1097/FTD.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 8.Tran T.B., Velkov T., Nation R.L., Forrest A., Tsuji B.T., Bergen P.J., Li J. Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: Are we there yet? Int. J. Antimicrob. Agents. 2016;48:592–597. doi: 10.1016/j.ijantimicag.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poirel L., Jayol A., Nordmann P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017;30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaara M. Polymyxins and Their Potential Next Generation as Therapeutic Antibiotics. Front. Microbiol. 2019;10:1689. doi: 10.3389/fmicb.2019.01689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.EUCAST Recommendations for MIC Determination of Colistin (Polymyxin E) as Recommended by the Joint CLSI-EUCAST Polymyxin Breakpoints Working Group. 2016. [(accessed on 22 March 2016)]. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf.
- 12.Karvanen M., Malmberg C., Lagerback P., Friberg L.E., Cars O. Colistin Is Extensively Lost during Standard In Vitro Experimental Conditions. Antimicrob. Agents Chemother. 2017;61:e00857-17. doi: 10.1128/AAC.00857-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan T.Y., Ng L.S. Comparison of three standardized disc susceptibility testing methods for colistin. J. Antimicrob. Chemother. 2006;58:864–867. doi: 10.1093/jac/dkl330. [DOI] [PubMed] [Google Scholar]
- 14.Lo-Ten-Foe J.R., De Smet A.M., Diederen B.M., Kluytmans J.A., Van Keulen P.H. Comparative evaluation of the VITEK 2, disk diffusion, etest, broth microdilution, and agar dilution susceptibility testing methods for colistin in clinical isolates, including heteroresistant Enterobacter cloacae and Acinetobacter baumannii strains. Antimicrob. Agents Chemother. 2007;51:3726–3730. doi: 10.1128/AAC.01406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Heijden I.M., Levin A.S., De Pedri E.H., Fung L., Rossi F., Duboc G., Barone A.A., Costa S.F. Comparison of disc diffusion, Etest and broth microdilution for testing susceptibility of carbapenem-resistant P. aeruginosa to polymyxins. Ann. Clin. Microbiol. Antimicrob. 2007;6:8. doi: 10.1186/1476-0711-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behera B., Mathur P., Das A., Kapil A., Gupta B., Bhoi S., Farooque K., Sharma V., Misra M.C. Evaluation of susceptibility testing methods for polymyxin. Int. J. Infect. Dis. 2010;14:e596–e601. doi: 10.1016/j.ijid.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Moskowitz S.M., Garber E., Chen Y., Clock S.A., Tabibi S., Miller A.K., Doctor M., Saiman L. Colistin susceptibility testing: Evaluation of reliability for cystic fibrosis isolates of Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 2010;65:1416–1423. doi: 10.1093/jac/dkq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maalej S.M., Meziou M.R., Rhimi F.M., Hammami A. Comparison of disc diffusion, Etest and agar dilution for susceptibility testing of colistin against Enterobacteriaceae. Lett. Appl. Microbiol. 2011;53:546–551. doi: 10.1111/j.1472-765X.2011.03145.x. [DOI] [PubMed] [Google Scholar]
- 19.Kowalska-Krochmal B., Dudek-Wicher R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens. 2021;10:165. doi: 10.3390/pathogens10020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EUCAST of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth microdilution. EUCAST Discussion Document E. Def 2003, 5.1. Clin. Microbiol. Infect. 2003;9:1–7. [Google Scholar]
- 21.Satlin M.J., Lewis J.S., Weinstein M.P., Patel J., Humphries R.M., Kahlmeter G., Giske C.G., Turnidge J. Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing Position Statements on Polymyxin B and Colistin Clinical Breakpoints. Clin. Infect. Dis. 2020;71:e523–e529. doi: 10.1093/cid/ciaa121. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji B.T., Pogue J.M., Zavascki A.P., Paul M., Daikos G.L., Forrest A., Giacobbe D.R., Viscoli C., Giamarellou H., Karaiskos I., et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP) Pharmacotherapy. 2019;39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamma P.D., Aitken S.L., Bonomo R.A., Mathers A.J., Van Duin D., Clancy C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum beta-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa) Clin. Infect. Dis. 2021;72:1109–1116. doi: 10.1093/cid/ciab295. [DOI] [PubMed] [Google Scholar]
- 24.Chinese Research Hospital Association of Critical Care Medicine. Chinese Research Hospital Association of Evidence Base. Translational Infectious Diseases Chinese expert consensus on polymyxins in the clinical practice. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31:1194–1198. doi: 10.3760/cma.j.issn.2095-4352.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 25.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2021. [(accessed on 29 March 2021)]. CLSI Supplement M100. Available online: https://www.treata.academy/wp-content/uploads/2021/03/CLSI-31-2021.pdf. [Google Scholar]
- 26.EUCAST The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2021. [(accessed on 1 January 2020)]. Available online: http://www.eucast.org.
- 27.Castanheira M., Doyle T.B., Carvalhaes C.G., Roth B.M., Rhomberg P.R., Mendes R.E. Media for colistin susceptibility testing does not improve the detection of Klebsiella pneumoniae isolates carrying MgrB disruption and other mutation driven colistin resistance mechanisms. Diagn. Microbiol. Infect. Dis. 2020;98:115077. doi: 10.1016/j.diagmicrobio.2020.115077. [DOI] [PubMed] [Google Scholar]
- 28.Humphries R.M., Green D.A., Schuetz A.N., Bergman Y., Lewis S., Yee R., Stump S., Lopez M., Macesic N., Uhlemann A.C., et al. Multicenter Evaluation of Colistin Broth Disk Elution and Colistin Agar Test: A Report from the Clinical and Laboratory Standards Institute. J. Clin. Microbiol. 2019;57:e01269-19. doi: 10.1128/JCM.01269-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albur M., Noel A., Bowker K., Macgowan A. Colistin susceptibility testing: Time for a review. J. Antimicrob. Chemother. 2014;69:1432–1434. doi: 10.1093/jac/dkt503. [DOI] [PubMed] [Google Scholar]
- 30.Matuschek E., Ahman J., Webster C., Kahlmeter G. Antimicrobial susceptibility testing of colistin-evaluation of seven commercial MIC products against standard broth microdilution for Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. Clin. Microbiol. Infect. 2018;24:865–870. doi: 10.1016/j.cmi.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Simner P.J., Bergman Y., Trejo M., Roberts A.A., Marayan R., Tekle T., Campeau S., Kazmi A.Q., Bell D.T., Lewis S., et al. Two-Site Evaluation of the Colistin Broth Disk Elution Test To Determine Colistin In Vitro Activity against Gram-Negative Bacilli. J. Clin. Microbiol. 2019;57:e01163-18. doi: 10.1128/JCM.01163-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green D.A., Macesic N., Uhlemann A.C., Lopez M., Stump S., Whittier S., Schuetz A.N., Simner P.J., Humphries R.M. Evaluation of Calcium-Enhanced Media for Colistin Susceptibility Testing by Gradient Agar Diffusion and Broth Microdilution. J. Clin. Microbiol. 2020;58:e01522-19. doi: 10.1128/JCM.01522-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bardet L., Okdah L., Le Page S., Baron S.A., Rolain J.M. Comparative evaluation of the UMIC Colistine kit to assess MIC of colistin of gram-negative rods. BMC Microbiol. 2019;19:60. doi: 10.1186/s12866-019-1424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turnidge J.D., Bell J.M. Antimicrobial susceptibility on solid media. In: Amsterdam D., editor. Antibiotics in Laboratory Medicine. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2015. [Google Scholar]
- 35.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2020. [(accessed on 19 April 2020)]. CLSI Supplement M100. Available online: https://clsi.org/media/3481/m100ed30_sample.pdf. [Google Scholar]
- 36.Turlej-Rogacka A., Xavier B.B., Janssens L., Lammens C., Zarkotou O., Pournaras S., Goossens H., Malhotra-Kumar S. Evaluation of colistin stability in agar and comparison of four methods for MIC testing of colistin. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:345–353. doi: 10.1007/s10096-017-3140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landman D., Salamera J., Quale J. Irreproducible and uninterpretable Polymyxin B MICs for Enterobacter cloacae and Enterobacter aerogenes. J. Clin. Microbiol. 2013;51:4106–4111. doi: 10.1128/JCM.02129-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turnidge J., Sei K., Mouton J. Polymyxin Susceptibility Testing and Breakpoint Setting. Adv. Exp. Med. Biol. 2019;1145:117–132. doi: 10.1007/978-3-030-16373-0_9. [DOI] [PubMed] [Google Scholar]
- 39.Amsterdam D. Susceptibility Testing of Antimicrobials in Liquid Media. In: Amsterdam D., editor. Antibiotics in Laboratory Medicine. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.