Abstract
To investigate pathophysiologies of Mycoplasma pneumoniae infection from an immunological point of view, we measured the levels of interleukin-18 (IL-18) (originally designated gamma interferon [IFN-γ]-inducing factor) in 19 serum samples from 10 patients with pneumonia without pleural effusion (ages 1 to 16 years), 3 serum and 13 pleural fluid samples from 11 patients with pleural effusions (ages 11 months to 15 years), and 18 serum and 27 cerebrospinal fluid samples from 24 patients with central nervous system complications (ages 1 to 15 years). IL-18 was measured by a commercially available enzyme-linked immunosorbent assay kit (MBL, Nagoya, Japan). In addition, the levels of tumor necrosis factor alpha, IFN-γ, IL-6, IL-12, and KL-6 (a mucin-like glycoprotein expressed on type 2 pneumocytes) were measured in selected samples. The results concerning pleural effusions showed that elevated levels of IL-18 in pleural fluid, but not in serum, were solely associated with a sustained fibrotic change of the lung on chest roentgenography which might represent a pathological feature of intraluminal organization. All the pleural fluid samples with elevated levels of IL-18 were positive by PCR for M. pneumoniae DNA. There was no association between IL-18 and IFN-γ levels in serum or in the pleural fluid. On the other hand, elevated levels of IL-18 in serum, but not in cerebrospinal fluid samples, were observed in the cases complicated by central nervous system involvement, including profound brain dysfunction with seizures. Our study demonstrated that M. pneumoniae can induce IL-18 and that the enhanced local production of IL-18 in the lung is closely associated with pulmonary disease manifestation.
Mycoplasma pneumoniae is a common cause of community-acquired pneumonia, mainly in children and young adults, and is well known to cause a wide variety of respiratory and extrapulmonary diseases (2). However, rather little is known about the pathogenesis of this organism. This must be in part because of its unique genomic composition, cellular biology, and fastidious nature as the smallest cell-free living organism which lacks a cell wall (30). One point which seems to be sure is that pneumonia, the hallmark of M. pneumoniae infection, is a consequence of a host immune response, particularly of cellular immunity (2, 4, 6). In this respect, previous studies have suggested that a T-helper 1 (Th1)-type response of the host may play an important role in developing pathologic features as well as in radiographical patterns of M. pneumoniae pneumonia (34, 35). At the same time, it has been reported that a local immune response on the pulmonary surface of the host may play an important role in developing extrapulmonary diseases associated with M. pneumoniae (23; M. Narita, Letter, Clin. Infect. Dis. 30:405, 2000). In this context, we postulated a critical role for Th1-type cytokines in the pathogenesis of M. pneumoniae infection in terms of both pulmonary and extrapulmonary diseases.
Interleukin-18 (IL-18) is a new member of Th1-type cytokines which was originally designated gamma interferon (IFN-γ)-inducing factor (26, 38). This cytokine was at first reported to be produced by Kupffer cells and activated macrophages and to be a critical factor in the induction of liver injury in mice (26). In humans, elevated concentrations of IL-18, along with IFN-γ, in plasma were reported for patients with severe melioidosis (15). On the other hand, IL-18 was shown to prevent the dissemination of Cryptococcus neoformans from the lung to the brain by inducing IFN-γ in mice (9). IL-18 is constitutively produced in the lung and liver and has different effects in these organs against Escherichia coli and Salmonella enterica serovar Typhimurium infections in mice through the different functions of active mediators, such as IL-1β, IL-8, tumor necrosis factor alpha (TNF-α), and macrophage inflammatory proteins (25, 29). Moreover, IL-18 was reported to induce granulocyte-macrophage colony-stimulating factor and to participate in the local control of osteoclastogenesis in mice (37). Thus, the effects of IL-18 on disease conditions are not restricted to those mediated through IFN-γ induction and must be variable in terms of being beneficial or detrimental, depending on the organs involved and on the mediators involved (25). In addition, IL-18 induces IFN-γ more potently than does IL-12 (26). Because of the above observations taken together, we focused the present study on IL-18.
In a previous study concerning patients with massive (i.e., requiring removal) pleural effusions due to M. pneumoniae infection, M. pneumoniae DNA was detected in the acute-phase pleural fluid (PF) of 4 of 10 patients by PCR, and 3 of the 4 PF-PCR-positive patients showed a sustained fibrotic change on chest roentgenography remaining for more than 4 weeks (24). Despite being similarly massive, the effusions were transient and roentgenography showed that they became normal within 4 weeks in the 6 PF-PCR-negative patients. To further characterize these two distinct conditions, we tested in the present study the contribution of IL-18 to pulmonary manifestation.
In another study concerning patients with central nervous system (CNS) complications due to M. pneumoniae infection, the M. pneumoniae DNA was detected in cerebrospinal fluid (CSF) by PCR at a significantly higher rate for patients with early-onset encephalitis, defined as a CNS disease onset of ≤7 days from the onset of fever, than for patients with late-onset encephalitis, defined as a CNS disease onset of ≥8 days (22). Recently, this finding has been supported by both early-onset, PCR-positive (5, 7, 36) and late-onset, PCR-negative (27) cases from institutions other than ours. To further characterize these two conditions, we also tested in the present study the contribution of IL-18 to CNS manifestation.
MATERIALS AND METHODS
Pneumonia without pleural effusion.
Nineteen serum samples were obtained from 10 patients (ages 1 to 16 years) with radiographically confirmed pneumonia without pleural effusion. Mycoplasmal infection was confirmed serologically by either a ≥4-fold rise in titer (n = 6) or an initial high titer of >1:320 (n = 4) of antimycoplasma immunoglobulin antibody measured by a microparticle agglutination (MPA) test (Serodia Myco II; Fujirebio, Tokyo, Japan), and all patients had a positive reaction for antimycoplasma immunoglobulin M antibody by an enzyme-linked immunosorbent assay (ELISA) kit (Zeus Scientific, Raritan, N.J.). For some patients, sequential serum samples were obtained at intervals of 4 to 7 days. In one patient (a 7-year-old male), the pneumonia was complicated by fulminant hepatitis with hepatic coma, which resolved with plasma exchanges. One serum sample was collected before and two subsequent samples were collected after the plasma exchanges.
Pneumonia with pleural effusion.
Thirteen PF and three serum samples were obtained from 11 patients (ages 11 months to 15 years). Of these, nine patients had already been characterized in a previous study (cases 2 to 10) (24). The other two patients were an 8-year-old boy (case 11) and a 2-year-old girl (case 12). Mycoplasmal infection was confirmed serologically by the MPA test (an initial titer of 1:2,560 for case 11 and a rise from 1:<40 to 1:5,120 for case 12). Adenovirus (Ad) type 7, which is known to cause severe respiratory disease (19) and to induce IL-18 (39), was isolated from the PF of patient 12, which indicated a coinfection with this virus. M. pneumoniae DNA was detected by PCR in the PF of patient 11, and this patient showed a sustained fibrotic change on chest roentgenography. M. pneumoniae DNA was not detected in the PF of patient 12, and the effusion was transient without any remaining fibrotic change. Effusions were bilateral in two patients (cases 7 and 10). In case 7, while a sustained fibrotic change was observed in the right lung, which was positive for M. pneumoniae DNA by PF-PCR, the left effusion was transient and yielded a negative PCR result. In case 10, both effusions were transient, and PF was removed only from the right lung. The only patient with a positive PF-PCR result but without a sustained fibrotic change on chest roentgenography was an 11-month-old infant (case 8) with complications from profound hypoxemia requiring mechanical ventilation. While the lung disease was transient, case 5 was complicated by skin rash, liver dysfunction, and syndrome of inappropriate secretion of antidiuretic hormone.
CNS complications.
Eighteen serum samples and 27 CSF samples were obtained from 24 patients with CNS complications (ages 1 to 15 years). They comprised 15 patients with early-onset encephalitis, 5 patients with late-onset encephalitis, and 4 patients with aseptic meningitis. Of these, serum and/or CSF samples from eight patients with early-onset encephalitis, two patients with late-onset encephalitis, and three patients with aseptic meningitis had already been included in previous studies concerning the PCR detection of M. pneumoniae DNA (22, 23) or the determination of IL-6 levels (18). “Encephalitis” represented a clinical diagnosis which comprised encephalitis, meningoencephalitis, cerebellitis, and acute disseminated encephalomyelitis. Serological diagnosis of mycoplasmal infection was made for most of the patients by the MPA test as mentioned above and in a few cases by other methods. No patients died.
Pneumonia associated with other respiratory infections.
The following samples from patients with pneumonia associated with viral infections were tested for comparison of IL-18 results. Four serum and two PF samples were collected from six patients with adenoviral pneumonia, of whom two patients had pleural effusions. All six patients had Ad type 3 or type 7 isolated from throat swab or PF samples. A serum sample was obtained from one patient with influenza pneumonia for whom type H3N2 virus had been isolated from a throat swab sample. Four serum samples were collected from four patients with pneumonia-associated serologically confirmed measles. Of these 11 patients, 3 patients with Ad type 7 infection without pleural effusion had a fibrotic change similar to those observed in M. pneumoniae infections.
ELISAs.
IFN-γ (Amersham International, Amersham, United Kingdom), TNF-α (Amersham International), IL-6 (Fujirebio), and IL-18 (MBL, Nagoya, Japan) were measured by commercially available ELISA kits, and all assays were performed according to each supplier's recommendations. The minimal significant level of detection (i.e., sensitivity) in serum for each cytokine is set by the suppliers at 0.1 pg/ml for IFN-γ, 4.4 pg/ml for TNF-α, 2.5 pg/ml for IL-6, and 12.5 pg/ml for IL-18. Values above the following were arbitrarily taken as abnormally elevated, irrespective of the kind of samples (i.e., serum, PF, or CSF): 1.5 pg/ml for IFN-γ, which is recommended as a normal upper limit for serum samples (high-sensitivity human IFN-γ ELISA system instruction manual, Amersham Life Science, Amersham, United Kingdom); 4.5 pg/ml for TNF-α, which is just above the detection limit; 5.0 pg/ml for IL-6 (18); and 260 pg/ml for IL-18 (a mean plus 3 standard deviations of serum samples from healthy controls was 259.4 pg/ml [human IL-18 ELISA kit instruction manual, MBL, Nagoya, Japan]). IL-12 was measured by an in-house ELISA method which was constructed using a mouse anti-human IL-12 (p35-p70) monoclonal antibody cocktail (Pharmingen, San Diego, Calif.), a biotinylated mouse anti-human IL-12 (p70) monoclonal antibody (Pharmingen), and a recombinant human IL-12 (p70) standard (Pharmingen). The minimal significant level of detection and the normal upper limit for serum with this system are set at 15 pg/ml (M. Kubota, unpublished data).
KL-6 was also measured by a commercially available ELISA kit (Eizai, Tokyo, Japan) according to the supplier's recommendations (see Results for details of KL-6). A normal upper limit of KL-6 for serum is set at 500 U/ml (Eitest KL-6 instruction manual, Eizai Co., Tokyo, Japan).
RESULTS
IL-18 in serum and PF.
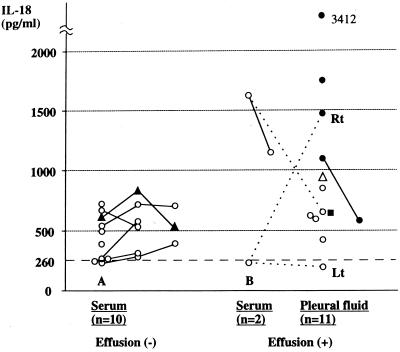
Figure 1 shows the levels of IL-18 in serum and/or PF samples from patients with or without pleural effusions. Patients with pneumonia without effusions showed normal upper to mildly elevated levels of serum IL-18 which did not exceed 1,000 pg/ml.
FIG. 1.
Levels of IL-18 in patients with M. pneumoniae pneumonia. Samples were obtained from patients with no (A) or massive (B) pleural effusions. A solid line within the same kind of samples and a dotted line between serum and PF samples indicate that the samples were from the same individual. A broken line at 260 pg/ml denotes the normal upper limit of IL-18 in serum. ▴, a patient with fulminant hepatitis; ▵, a patient with coinfection by Ad type 7; ●, a patient with a positive PCR result for M. pneumoniae DNA with a sustained fibrotic change on chest roentgenography; ■, a patient with a positive PCR result for M. pneumoniae DNA without fibrotic change; Rt, PF sample taken from the right lung; Lt, PF sample taken from the left lung.
Most notably, only the four patients with a positive PF-PCR result and a sustained fibrotic change of the lung on chest roentgenography showed a substantial increase in the levels of PF IL-18 which exceeded 1,000 pg/ml. Interestingly, for patient 7, from whom bilateral PF samples were obtained, while the PF sample from the right lung (with the fibrotic change) showed an elevated level of IL-18 (1,475 pg/ml), the PF sample from the left lung (without the fibrotic change) and the patient's serum sample showed normal levels of IL-18 (192 and 228 pg/ml, respectively). Moreover, for patient 5, who had pneumonia without a fibrotic change of the lung but with severe systemic disease, despite the serum sample at the time of PF collection showing a high level of IL-18 (1,628 pg/ml), the PF sample showed an IL-18 level of 625 pg/ml, which was comparable to those of the other PF samples from the patients without fibrotic change. In addition, patient 8, the only one with positive PF-PCR but without a fibrotic change of the lung, showed a PF IL-18 level of 640 pg/ml, which was comparable to those of the PF samples from the patients without fibrotic change. These results strongly suggested that the enhanced local production of IL-18 plays a significant role in the formation of fibrotic change in the PF-PCR-positive lungs.
Association of IL-18 levels with those of Th1 cytokines.
We next sought to determine whether the effect of IL-18 was through the function of inducing IFN-γ or other Th1-type cytokines. The results for the PF samples are shown in Table 1. Rather unexpectedly, the values for IFN-γ had no association with those for IL-18. For example, while IL-18 levels were high in the right lung of patient 7 (1,475 pg/ml) and in the lung of patient 9 (1,739 pg/ml), both of which had fibrotic change, IFN-γ levels were only slightly elevated (7.5 and 6.95 pg/ml, respectively). On the other hand, TNF-α was detected in only two of the six patients tested, one with fibrotic change and the other without. Levels of IL-6 were remarkably high in all PF samples, and the degree of elevation had no relevance to fibrotic change. Also, the levels of IL-12 did not have any association with those of IL-18 or IFN-γ. These results suggested that the effect of IL-18 on fibrotic change of the lung is independent of the functions of these cytokines.
TABLE 1.
Levels of TNF-α, IL-6, IL-12, IL-18, and IFN-γ in PF samples from patients with M. pneumoniae infection
| Case no.a | Level of cytokine (pg/ml)
|
PCR result | Lung changeb | ||||
|---|---|---|---|---|---|---|---|
| TNF-α | IL-6 | IL-12 | IL-18 | IFN-γ | |||
| 2 | <7.5 | 7,217 | <15 | 420 | 95 | − | Transient |
| 3 | <7.5 | 6,265 | 897 | 848 | 92 | − | Transient |
| 4 | |||||||
| Sample 1 | 210.3 | 3,851 | 804 | 1,100 | 81 | + | Fibrotic change |
| Sample 2 | 32.5 | 55,450 | 405 | 583 | 73 | + | Fibrotic change |
| 5 | |||||||
| PF | <7.5 | 2,354 | <15 | 625 | 80 | − | Transient |
| Serum | NTc | NT | <15 | 1,628 | 5.0 | − | Transient |
| 6 | 24.2 | 3,851 | 82 | 598 | 52 | − | Transient |
| 7 | |||||||
| Right lung | <7.5 | 3,755 | 96 | 1,475 | 7.5 | + | Fibrotic change |
| Left lung | <7.5 | 2,392 | 217 | 192 | <0.1 | − | Transient |
| Serum | <7.5 | 104 | 484 | 228 | <0.1 | − | Transient |
| 8 | NT | NT | 58 | 640 | <0.1 | + | Transient |
| 9 | NT | NT | 194 | 1,739 | 6.95 | + | Fibrotic change |
| 10 | NT | NT | <15 | 597 | 46 | − | Transient |
| 11 | NT | NT | <15 | 3,412 | 120 | + | Fibrotic change |
| 12 | NT | NT | 58 | 941 | 28 | − | Transient |
Case number corresponds to that of a previous report (24). For case 4, sample 2 was obtained 4 days after sample 1.
Transient means that the retention of PF was transient and a chest roentgenography became normal within 4 weeks; fibrotic change means that a sustained fibrotic change on chest roentgenography was observed for more than 4 weeks.
NT, not tested.
To further clarify the relationship between IL-18 and IFN-γ in M. pneumoniae infection, we tested sequential serum samples from five patients with pneumonia, one of whom had late-onset encephalitis. The results are shown in Table 2. The sharp rise of MPA titers indicated that these patients had been infected by M. pneumoniae very recently. While the level of IFN-γ was always the highest in the first serum sample, except for that of patient Pn-5, in which it was not detected throughout, the level of IL-18 was always higher in the second serum sample than in the first sample. These results suggested that IFN-γ levels rise in serum during the very acute phase of M. pneumoniae infection but that this is not through the function of IL-18.
TABLE 2.
Time course of IL-18 and IFN-γ in sera from patients with M. pneumoniae pneumoniaa
| Case no. (status) and protein | Level of proteinb in
|
||
|---|---|---|---|
| 1st sample | 2nd sample | 3rd sample | |
| Pn-1 (late-onset encephalitisc) | |||
| IL-18 | 622 | 1,240 | 305 |
| IFN-γ | 66 | 38 | <0.1 |
| Antibody | 80 | 640 | 1,280 |
| Pn-2 (no complications) | |||
| IL-18 | 265 | 305 | |
| IFN-γ | 14.6 | <0.1 | |
| Antibody | 80 | 2,560 | |
| Pn-3 (no complications) | |||
| IL-18 | 229 | 280 | 389 |
| IFN-γ | 6.7 | 0.5 | 0.23 |
| Antibody | 80 | 640 | 5,120 |
| Pn-4 (no complications) | |||
| IL-18 | 240 | 575 | |
| IFN-γ | 5.0 | <0.1 | |
| Antibody | 320 | 20,480 | |
| Pn-5 (no complications) | |||
| IL-18 | 540 | 715 | 704 |
| IFN-γ | <0.1 | <0.1 | <0.1 |
| Antibody | 320 | 20,480 | 40,960 |
Intervals between sample collections were 4 to 7 days.
IL-18 and IFN-γ levels are shown in picograms per milliliter. Values of >260 pg/ml for IL-18 and 1.5 pg/ml for IFN-γ can be considered significantly elevated. Anti-M. pneumoniae antibody titers were measured by the MPA.
A CNS disease onset of ≥8 days from the onset of fever (22).
Association of IL-18 levels with KL-6.
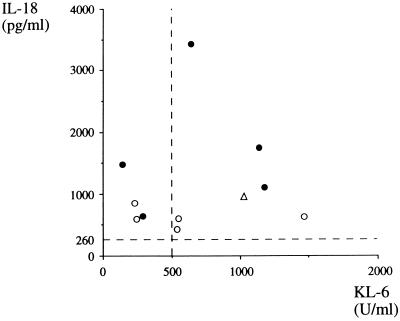
KL-6 is a mucin-like, high-molecular-weight glycoprotein which is normally expressed on type 2 pneumocytes and on respiratory bronchiolar epithelial cells of the lung. Since this molecule is strongly expressed on regenerating type 2 pneumocytes, elevated levels of KL-6 in serum (12, 14) or bronchoalveolar lavage fluid (13) have been reported for patients with pneumonias of an interstitial nature, such as idiopathic interstitial pneumonia or hypersensitive pneumonitis. Thus, KL-6 is a useful marker for the disease activity of interstitial pneumonia. If the fibrotic change of the lung is a consequence of lung fibrosis, a significant correlation can be expected between IL-18 and KL-6. As shown in Fig. 2, however, although elevated levels of KL-6 were found in some patients, they had no association with the fibrotic change of the lung. Also, no correlation was found between the levels of IL-18 and KL-6 (r = 0.06, P > 0.1, n = 11, Pearson's correlation coefficient).
FIG. 2.
Levels of IL-18 and KL-6 in PF samples from patients with M. pneumoniae infections. ●, PCR positive; ○, PCR negative; ▵, patient was coinfected by Ad type 7. Broken lines at 260 pg/ml on the vertical scale and at 500 U/ml on the horizontal scale denote the normal upper limits of IL-18 and KL-6 in serum, respectively.
IL-18 in serum and CSF.
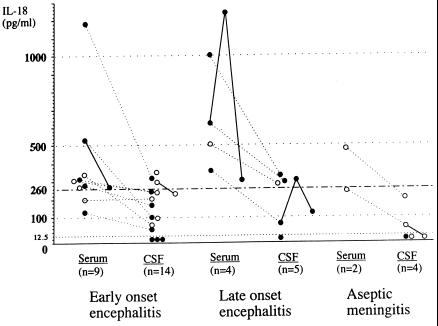
Next we investigated serum and CSF samples from patients with CNS complications for levels of IL-18. As shown in Fig. 3, CSF IL-18 levels were below the detection limit in seven samples and only slightly above the tentative CSF normal upper limit of 260 pg/ml in seven samples (at maximum, 377 pg/ml), out of a total of 27 CSF samples. Moreover, the fact that there was a close correlation between the IL-18 levels in serum and CSF (r = 0.90, P < 0.001, n = 14, Pearson's correlation coefficient) strongly suggested that IL-18 in CSF was a result of simple diffusion from serum. There was no association between the levels of CSF IL-18 and CSF-PCR positivity (data not shown). These results suggested that levels of IL-18 in CSF are of virtually no pathognomonic importance. On the other hand, three patients with pneumonia, one with early-onset encephalitis and two with late-onset encephalitis, showed a substantial increase in serum IL-18 levels, exceeding 1,000 pg/ml.
FIG. 3.
Levels of IL-18 in patients with CNS complications due to M. pneumoniae infection. Early-onset encephalitis is defined as a CNS disease onset of ≤7 days from the onset of fever; late-onset encephalitis is defined as a CNS disease onset of ≥8 days (22). ●, with pneumonia; ○, without pneumonia. A solid line within the same kind of samples and a dotted line between serum and CSF samples indicate that the samples were from the same individual. The broken lines at 12.5 and 260 pg/ml denote a minimal significant level of detection and the normal upper limit of IL-18 in serum, respectively.
Interestingly, only these three patients had both disturbance of consciousness and seizures. These results suggested that IL-18 may be associated with systemic, but not with intrathecal, inflammatory responses in CNS complications. There was no association between the levels of serum IL-18 and serum-CSF PCR positivity (data not shown).
IL-18 in pneumonia associated with other respiratory infections.
Levels of IL-18 in serum or PF samples from patients with viral infections are shown in Table 3. Serum levels of IL-18 were distributed widely within the same kind of viral infection, and a consistent tendency could not be found in relation to the disease severity or duration of illness. Although one PF sample of Ad type 7 infection (case Ad-6) showed an increase in the IL-18 level which was comparable to those of the cases with fibrotic change during M. pneumoniae infection, this patient did not have the fibrotic change or any other remaining abnormality.
TABLE 3.
Levels of IL-18 in serum or PF samples from pneumonia patients with viral infections
| Case no. | Age (yr, except as noted) | Sexa | Kind of virusb | Presence of pleural effusion | Presence of fibrotic change | Kind of sample | IL-18c (pg/ml) |
|---|---|---|---|---|---|---|---|
| Ad-1 | 15 | F | Ad type 3 | Absent | Absent | Serum | 1,048 |
| Ad-2 | 1 | F | Ad type 7 | Absent | Present | Serum | 608 |
| Ad-3 | 7 mo | F | Ad type 7 | Absent | Present | Serum | 799 |
| Ad-4 | 1 | M | Ad type 7 | Absent | Present | Serum | 372 |
| Ad-5 | 4 | F | Ad type 7 | Present | Absent | PF | 833 |
| Ad-6 | 1 | M | Ad type 7 | Present | Absent | PF | 1,391 |
| Inf-1 | 3 mo | M | H3N2 | Absent | Absent | Serum | 212 |
| Me-1 | 1 | M | Measles | Absent | Absent | Serum | 204 |
| Me-2 | 4 | F | Measles | Absent | Absent | Serum | 279 |
| Me-3 | 1 | F | Measles | Absent | Absent | Serum | 933 |
| Me-4 | 5 | M | Measles | Absent | Absent | Serum | 858 |
F, female; M, male.
H3N2, influenza virus type H3N2.
Values of >260 pg/ml can be considered significantly elevated for serum samples.
DISCUSSION
To date, some clinical as well as experimental studies have focused on cytokines as candidates responsible for the mechanism of cell injury by M. pneumoniae or as markers of disease severity in M. pneumoniae infection. These include studies with TNF-α (1, 11, 28), IFN-α (20, 21), IFN-γ (20, 28), IL-1β (11, 28), IL-2 (11, 28), IL-6 (11, 28), and IL-10 (28). Previous studies revealed that various cytokines are certainly induced during M. pneumoniae infection and that mycoplasmal cell components contain a potent inducer(s) of cytokines different from but comparable to bacterial lipopolysaccharides (1, 32, 33). However, conclusive evidence that a certain cytokine is responsible for a certain clinical picture of M. pneumoniae infection has not been obtained, due to the complexity of the cytokine network and possibly due to the fact that the above-listed cytokines do not actually play a pivotal role in disease development in M. pneumoniae infection. In this respect, our results concerning PF must have different meanings.
Our study concerning patients with pleural effusions at first demonstrated that the elevated levels of IL-18 in PF but not in serum were associated with fibrotic change of the lung on chest roentgenography. Moreover, all of the PF samples from the lungs with fibrotic change and elevated levels of IL-18 were positive by PCR for M. pneumoniae DNA. It was less likely that the effect of IL-18 on the lung was through the function of inducing IFN-γ. Other cytokines, including TNF-α, IL-6, and IL-12, did not have any direct association with fibrotic change like IL-18 did. These results strongly suggest that IL-18 induced by M. pneumoniae cells above a certain level within the lung cavity plays a central role in the formation of fibrotic change.
Originally, IL-18 was reported to be produced by Kupffer cells of the liver and activated macrophages but not by T and B lymphocytes (26, 38). In another study, IL-18 was found to be expressed primarily on airway epithelium and mononuclear cells of the lungs of mice (3). On the other hand, according to previous histological studies (8, 10, 16, 17, 31), infiltrating cells in the lungs of M. pneumoniae pneumonia patients were mainly macrophages/monocytes, lymphocytes, and polymorphonuclear leukocytes, with a different order of frequency in each report (8, 16, 17, 31). From these facts, macrophages, which are proved to produce IL-18, are most likely to be responsible for the IL-18 production in the lung. Interestingly, those histological studies revealed that diffuse and extensive interstitial fibrosis was rather exceptional and was observed exclusively in one fatal case (8), and peribronchiolar type 2 pneumocyte hyperplasia was an occasional finding (31). The latter finding is fairly consistent with our results of an occasional increase in KL-6 levels in PF. In this respect, a more common and remarkable finding was intraluminal organization with fibroblastic or granulation tissues within alveolar ducts (10, 16, 17, 31). Given that the “organizing pneumonia” (the term which was used by Rollins et al. in their excellent review [31]) of various degrees is a common pathologic feature of M. pneumoniae pneumonia, with or without pleural effusions, the change we call fibrotic in this report must represent a roentgenographic appearance mainly of the intraluminal organization with lesser contribution of interstitial fibrosis. In this context, it is tempting to assume that IL-18 plays a central role in the recruitment of inflammatory cells.
With regard to other respiratory infections, the results shown in Table 3 indicate that serum IL-18 levels are in some cases elevated in viral infections. On the other hand, the absence of remaining abnormality despite the elevated level of IL-18 in PF in case Ad-6 suggests that the close association between the enhanced local production of IL-18 and the fibrotic change of the lung is a phenomenon specific to M. pneumoniae pneumonia with pleural effusion. An elevated level of IL-18 in PF with the invasion of M. pneumoniae into the pleura, which is evidenced by PCR, might be a prerequisite for this remaining abnormality.
Lastly, although it is quite difficult within the limited amount of available information to determine the mode of action of IL-18, if it becomes clear, steroid therapy for severe cases of M. pneumoniae pneumonia (8, 16) might be further warranted.
In conclusion, our clinical study clearly demonstrated that the enhanced local production of IL-18 in the lung was closely associated with the formation of a sustained fibrotic change on chest roentgenography which might represent a pathological feature of intraluminal organization. This effect of IL-18 was not through the induction of IFN-γ. In addition, serum IL-18 levels were apparently associated with systemic disease severity, such as profound brain dysfunction with seizures. Further experimental studies will be needed to clarify which are the IL-18-producing cells and what the mode of action of IL-18 is in M. pneumoniae infection.
ACKNOWLEDGMENTS
We thank Kunihiko Kobayashi of the Department of Pediatrics, Hokkaido University School of Medicine, for his critical review of the manuscript. We also thank Hiroyuki Naito of the Department of Pediatrics, Sapporo City General Hospital, for providing us with necessary samples.
REFERENCES
- 1.Arai S, Furukawa M, Munakata T, Kuwano K, Inoue H, Miyazaki T. Enhancement of cytotoxicity of active macrophages by mycoplasma: role of mycoplasma-associated induction of tumor necrosis factor-α (TNF-α) in macrophages. Microbiol Immunol. 1990;34:231–243. doi: 10.1111/j.1348-0421.1990.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 2.Broughton R A. Infections due to Mycoplasma pneumoniae in childhood. Pediatr Infect Dis J. 1986;5:71–85. doi: 10.1097/00006454-198601000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Cameron L A, Taha R A, Tsicopoulos A, Kurimoto M, Olivenstein R, Wallaert B, Minshall E M, Hamid Q A. Airway epithelium expresses interleukin-18. Eur Respir J. 1999;14:553–559. doi: 10.1034/j.1399-3003.1999.14c12.x. [DOI] [PubMed] [Google Scholar]
- 4.Cole B C. Mycoplasma interactions with the immune system: implications for disease pathology. ASM News. 1996;62:471–475. [Google Scholar]
- 5.Dionisio D, Valassina M, Mata S, Rossetti R, Vivarelli A, Esperti F C, Benvenuti M, Catalani C, Uberti M. Encephalitis caused directly by Mycoplasma pneumoniae. Scand J Infect Dis. 1999;31:506–509. doi: 10.1080/00365549950164067. [DOI] [PubMed] [Google Scholar]
- 6.Foy H M, Ochs H, Davis S D, Kenny G E, Luce R R. Mycoplasma pneumoniae infections in patients with immunodeficiency syndromes: report of four cases. J Infect Dis. 1973;127:388–393. doi: 10.1093/infdis/127.4.388. [DOI] [PubMed] [Google Scholar]
- 7.Ieven M, Demey H, Ursi D, van Goethem G, Cras P, Goossens H. Fatal encephalitis caused by Mycoplasma pneumoniae diagnosed by the polymerase chain reaction. Clin Infect Dis. 1998;27:1552–1553. doi: 10.1086/517753. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman J M, Cuvelier C A, van der Straeten M. Mycoplasma pneumonia with fulminant evolution into diffuse interstitial fibrosis. Thorax. 1980;35:140–144. doi: 10.1136/thx.35.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawakami K, Qureshi M H, Zhang T, Okamura H, Kurimoto M, Saito A. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-γ production. J Immunol. 1997;159:5528–5534. [PubMed] [Google Scholar]
- 10.Kim C K, Chung C Y, Kim J S, Kim W S, Park Y, Koh Y Y. Late abnormal findings on high-resolution computed tomography after Mycoplasma pneumonia. Pediatrics. 2000;105:372–378. doi: 10.1542/peds.105.2.372. [DOI] [PubMed] [Google Scholar]
- 11.Kita M, Ohmoto Y, Hirai Y, Yamaguchi N, Imanishi J. Induction of cytokines in human peripheral blood mononuclear cells by mycoplasmas. Microbiol Immunol. 1992;36:507–516. doi: 10.1111/j.1348-0421.1992.tb02048.x. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi J, Kitamura S. KL-6: a serum marker for interstitial pneumonia. Chest. 1995;108:311–315. doi: 10.1378/chest.108.2.311. [DOI] [PubMed] [Google Scholar]
- 13.Kohno N, Awaya Y, Oyama T, Yamakido M, Akiyama M, Inoue Y, Yokoyama A, Hamada H, Fujioka S, Hiwada K. KL-6, a mucin-like glycoprotein, in bronchoalveolar lavage fluid from patients with interstitial lung disease. Am Rev Respir Dis. 1993;148:637–642. doi: 10.1164/ajrccm/148.3.637. [DOI] [PubMed] [Google Scholar]
- 14.Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest. 1989;96:68–73. doi: 10.1378/chest.96.1.68. [DOI] [PubMed] [Google Scholar]
- 15.Lauw F N, Simpson A J H, Prins J M, Smith M D, Kurimoto M, van Deventer S J H, Speelman P, Chaowagul W, White N J, van der Poll T. Elevated plasma concentrations of interferon (IFN)-γ and the IFN-γ-inducing cytokines interleukin (IL)-18, IL-12, and IL-15 in severe melioidosis. J Infect Dis. 1999;180:1878–1885. doi: 10.1086/315155. [DOI] [PubMed] [Google Scholar]
- 16.Llibre J M, Urban A, Garcia E, Carrasco M A, Murcia C. Bronchiolitis obliterans organizing pneumonia associated with acute Mycoplasma pneumoniae infection. Clin Infect Dis. 1997;25:1340–1342. doi: 10.1086/516124. [DOI] [PubMed] [Google Scholar]
- 17.Maisel J C, Babbitt L H, John T J. Fatal Mycoplasma pneumoniae infection with isolation of organisms from lung. JAMA. 1967;202:139–142. [PubMed] [Google Scholar]
- 18.Matsuzono Y, Narita M, Akutsu Y, Togashi T. Interleukin-6 in cerebrospinal fluid of patients with central nervous system infections. Acta Paediatr. 1995;84:879–883. doi: 10.1111/j.1651-2227.1995.tb13784.x. [DOI] [PubMed] [Google Scholar]
- 19.Murtagh P, Cerqueiro C, Halac A, Avila M, Kajon A. Adenovirus type 7h respiratory infections: a report of 29 cases of acute lower respiratory disease. Acta Paediatr. 1993;82:557–561. doi: 10.1111/j.1651-2227.1993.tb12753.x. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama T, Sonoda S, Urano T, Osano M, Maehara N, Sasaki K, Hayatsu E, Makino S. Interferon production during the course of Mycoplasma pneumoniae infection. Pediatr Infect Dis J. 1992;11:72–77. doi: 10.1097/00006454-199202000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama T, Urano T, Osano M, Maehara N, Makino S. α interferon in the sera of patients infected with Mycoplasma pneumoniae. J Infect Dis. 1986;154:904–906. doi: 10.1093/infdis/154.5.904. [DOI] [PubMed] [Google Scholar]
- 22.Narita M, Itakura O, Matsuzono Y, Togashi T. Analysis of mycoplasmal central nervous system involvement by polymerase chain reaction. Pediatr Infect Dis J. 1995;14:236–237. doi: 10.1097/00006454-199503000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Narita M, Matsuzono Y, Itakura O, Togashi T, Kikuta H. Survey of mycoplasmal bacteremia detected in children by polymerase chain reaction. Clin Infect Dis. 1996;23:522–525. doi: 10.1093/clinids/23.3.522. [DOI] [PubMed] [Google Scholar]
- 24.Narita M, Matsuzono Y, Itakura O, Yamada S, Togashi T. Analysis of mycoplasmal pleural effusion by the polymerase chain reaction. Arch Dis Child. 1998;78:67–69. doi: 10.1136/adc.78.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Netea M G, Fantuzzi G, Kullberg B J, Stuyt R J L, Pulido E J, McIntyre R C, Jr, Joosten L A B, Van der Meer J W M, Dinarello C A. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J Immunol. 2000;164:2644–2649. doi: 10.4049/jimmunol.164.5.2644. [DOI] [PubMed] [Google Scholar]
- 26.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 27.Pellegrini M, O'Brien T J, Hoy J, Sedal L. Mycoplasma pneumoniae infection associated with an acute brainstem syndrome. Acta Neurol Scand. 1996;93:203–206. doi: 10.1111/j.1600-0404.1996.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 28.Pietsch K, Ehlers S, Jacobs E. Cytokine gene expression in the lungs of BALB/c mice during primary and secondary intranasal infection with Mycoplasma pneumoniae. Microbiology. 1994;140:2043–2048. doi: 10.1099/13500872-140-8-2043. [DOI] [PubMed] [Google Scholar]
- 29.Puren A J, Fantuzzi G, Gu Y, Su M S-S, Dinarello C A. Interleukin-18 (IFNγ-inducing factor) induces IL-8 and IL-1β via TNFα production from non-CD14+ human blood mononuclear cells. J Clin Investig. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rollins S, Colby T, Clayton F. Open lung biopsy in Mycoplasma pneumoniae pneumonia. Arch Pathol Lab Med. 1986;110:34–41. [PubMed] [Google Scholar]
- 32.Sher T, Rottem S, Gallily R. Mycoplasma capricolum membranes induce tumor necrosis factor α by a mechanism different from that of lipopolysaccharide. Cancer Immunol Immunother. 1990;31:86–92. doi: 10.1007/BF01742371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugama K, Kuwano K, Furukawa M, Himeno Y, Satoh T, Arai S. Mycoplasmas induce transcription and production of tumor necrosis factor in a monocytic cell line, THP-1, by a protein kinase C-independent pathway. Infect Immun. 1990;58:3564–3567. doi: 10.1128/iai.58.11.3564-3567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka H, Honma S, Abe S, Tamura H. Effects of interleukin-2 and cyclosporin A on pathologic features in Mycoplasma pneumonia. Am J Respir Crit Care Med. 1996;154:1908–1912. doi: 10.1164/ajrccm.154.6.8970385. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka H, Koba H, Homma S, Sugaya F, Abe S. Relationships between radiological pattern and cell-mediated immune response in Mycoplasma pneumoniae pneumonia. Eur Respir J. 1996;9:669–672. doi: 10.1183/09031936.96.09040669. [DOI] [PubMed] [Google Scholar]
- 36.Tjhie J H T, van de Putte E M, Haasnoot K, van den Brule A J C, Vandenbroucke-Grauls C M J E. Fatal encephalitis caused by Mycoplasma pneumoniae in a 9-year-old girl. Scand J Infect Dis. 1997;29:424–425. doi: 10.3109/00365549709011844. [DOI] [PubMed] [Google Scholar]
- 37.Udagawa N, Horwood N J, Elliott J, Mackay A, Qwens J, Okamura H, Kurimoto M, Chambers T J, Martin T J, Gillespie M T. Interleukin-18 (interferon-γ-inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-γ to inhibit osteoclast formation. J Exp Med. 1997;185:1005–1012. doi: 10.1084/jem.185.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ushino S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, Tanabe F, Konishi K, Micallef M, Fujii M, Torigoe K, Tanimoto T, Fukuda S, Ikeda M, Okamura H, Kurimoto M. Cloning of the cDNA for human IFN-γ-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]
- 39.Xing Z, Zganiacz A, Wang J, Divangahi M, Nawaz F. IL-12-independent Th1-type immune responses to respiratory viral infection: requirement of IL-18 for IFN-γ release in the lung but not for the differentiation of viral-reactive Th1-type lymphocytes. J Immunol. 2000;164:2575–2584. doi: 10.4049/jimmunol.164.5.2575. [DOI] [PubMed] [Google Scholar]