Abstract
The objective of this study was to assess the association between patients’ epidemiological characteristics and comorbidities with SARS-CoV-2 infection severity and related mortality risk. An umbrella systematic review, including a meta-analysis examining the association between patients’ underlying conditions and severity (defined as need for hospitalization) and mortality of COVID-19, was performed. Studies were included if they reported pooled risk estimates of at least three underlying determinants for hospitalization, critical disease (ICU admission, mechanical ventilation), and hospital mortality in patients diagnosed with SARS-CoV-2 infection. Evidence was summarized as pooled odds ratios (pOR) for disease outcomes with 95% confidence intervals (95% CI). Sixteen systematic reviews investigating the possible associations of comorbidities with severity or death from COVID-19 disease were included. Hospitalization was associated with age > 60 years (pOR 3.50; 95% CI 2.97–4.36), smoking habit (pOR 3.50; 95% CI 2.97–4.36), and chronic pulmonary disease (pOR 2.94; 95% CI 2.14–4.04). Chronic pulmonary disease (pOR 2.82; 95% CI 1.92–4.14), cerebrovascular disease (pOR 2.74; 95% CI 1.59–4.74), and cardiovascular disease (pOR 2.44; 95% CI 1.97–3.01) were likely to be associated with increased risk of critical COVID-19. The highest risk of mortality was associated with cardiovascular disease (pOR 3.59; 95% CI 2.83–4.56), cerebrovascular disease (pOR 3.11; 95% CI 2.35–4.11), and chronic renal disease (pOR 3.02; 95% CI 2.61–3.49). In conclusion, this umbrella systematic review provides a comprehensive summary of meta-analyses examining the impact of patients’ characteristics on COVID-19 outcomes. Elderly patients and those cardiovascular, cerebrovascular, and chronic renal disease should be prioritized for pre-exposure and post-exposure prophylaxis and early treatment.
Keywords: COVID-19, SARS-CoV-2, meta-analysis, mortality, severe disease, predictors, comorbidities
1. Introduction
By the end of April 2022, the COVID-19 pandemic, caused by the new coronavirus SARS-CoV-2, had caused more than 500 million cases and more than 6 million deaths worldwide [1]. SARS-CoV-2 frequently causes asymptomatic or mild infection; however, some patients progress to a severe disease, which is associated with high mortality [2]. Identifying the patients’ conditions associated with the development of severe forms of COVID-19 and mortality is helpful because it allows identifying the patients who would benefit most from specific preventive interventions, including enhanced transmission-protective measures, being prioritized in vaccination campaigns, and, more recently, receiving antivirals or monoclonal antibodies, which may avoid the progression from mild to severe disease.
Several patient conditions, including age, gender, and several chronic underlying comorbidities, have been associated with worse outcomes [2]. However, the generalizability of the estimates for the relative impact of each of these conditions in the different studies performed may be hampered because the different studies may be affected by selection and information biases and lack of statistical power. As a result, the estimations of the risk associated with underlying conditions of the patients for the development of severe COVID-19 or mortality are frequently heterogeneous, if not contradictory.
The aim of this study was to conduct an umbrella systematic review and meta-analysis in order to assess the association between patients’ epidemiological characteristics and comorbidities with severity and related mortality risk of SARS-CoV-2 infection.
2. Methods
2.1. Design, Data Sources, and Search Strategy
An umbrella literature review was conducted according to the Joanna Briggs Institute recommendations [3] and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [4]. The study protocol was registered in PROSPERO (CRD42021267368). Patients were not involved in the design, conduct, interpretation, and writing up of the results of this study.
For this umbrella review, the PICO question was defined as follows. The patients were outpatients and inpatients diagnosed with SARS-CoV-2 infection; the exposures were the epidemiological characteristics and chronic underlying conditions of the patients; the comparator was the absence of exposure to these characteristics and conditions; and the outcomes considered were hospital admission, severe/critical COVID-19, and in-hospital mortality. Published systematic reviews and meta-analyses on the association of patients’ epidemiological characteristics and comorbidities with hospitalization due to COVID-19, development of severe or critical COVID-19 defined as mechanical ventilation and need of intensive care unit (ICU) admission, and death were considered only in admitted patients with a first episode of infection.
The literature search was conducted in PubMed, MEDLINE, EMBASE, Web of Science, Scopus, Cochrane Library databases, and the JBI database of Systematic Reviews and Implementation Reports, with no language restrictions. The initial search was conducted on 1 August 2021 and updated on 30 September 2021. The full search strategy used is shown in Table S1, Supplementary Materials.
2.2. Inclusion and Exclusion Criteria
Articles were eligible if they were published between December 2019 and August 2021 and if they included a meta-analysis of the association of patients’ epidemiological characteristics and comorbidities with the severity or mortality from COVID-19. Studies had to meet the following criteria: (a) they were conducted on patients diagnosed with SARS-CoV-2 infection by PCR or antigen test in nasopharyngeal or respiratory tract samples; (b) they included the evaluation of at least three epidemiological characteristics and comorbidities in order to be able to assess the confounding effect of one condition on others; (c) they provided quantitative data of patients with and without the conditions and their outcomes; (d) they provided a pooled estimation of the association of the conditions and the outcomes. Studies in which severity or mortality was not the primary outcome, narrative reviews, meta-analyses including fewer than 5 studies, and preprints were excluded. Systematic reviews reporting outcomes in vaccinated patients or in pregnant women and children (aged less than 18 years) were also excluded, as these groups may have specific outcome determinants.
2.3. Article Selection and Data Extraction
All the identified references were managed with a reference management program, and duplicates were removed. The titles and abstracts were screened, and the full texts of the selected articles were then reviewed for eligibility and data extraction by two investigators (ZRP-B and ER-V). A third coauthor (JR-B) resolved any disagreement that could not be resolved by consensus. The data extracted included: author, year of publication, number of participants, number and type of studies included, quality assessment instrument used, method of analysis, patients’ epidemiological characteristics and comorbidities and outcomes assessed, heterogeneity, and the estimated associations between all conditions and the outcomes. The AMSTAR 2 tool [5] was used to assess methodological quality and assign an overall rating for the reviews included Table S2.
The patients’ epidemiological characteristics and comorbidities were grouped into categories. The definitions used in the included systematic review were reviewed for homogeneity. The outcomes considered were hospitalization due to COVID-19, development of severe/critical disease (i.e., need for ICU admission, high-flow oxygen or mechanical ventilation), and mortality. Data of the association of the conditions and outcomes were collected as rate ratios (RR), odds ratios (OR), and hazard ratios (HR), with 95% confidence intervals (CI).
2.4. Data Analysis
The characteristics and results of the included studies were synthesized and presented in tables and forest diagrams. Pooled OR (pOR) with 95% confidence intervals (CI) were calculated for conditions investigated in at least 3 meta-analyses using the DerSimonian and Laird random-effects method, which accounts for inter- and intra-study variance. For dichotomous variables, a summary of estimations was produced by using a logarithmic scale to maintain symmetry in the analysis. An estimate of publication bias was calculated with Egger’s regression test. The I2 statistic was used as an estimate of true variance in the summary estimate and was used as an estimate of the proportion of variance reflecting the true differences in effect size. The degree of overlap of primary studies included in the different meta-analyses was investigated by a citation matrix including the systematic reviews in columns and the primary studies included in rows; the degree of overlap was quantified using the corrected covered area (CCA). The overlap was categorized as very high (>15%), high (11–15%), moderate (6–10%), or light (0–5%) [6]. CCA is a validated method for quantifying the degree of overlap between two or more reviews.
3. Results
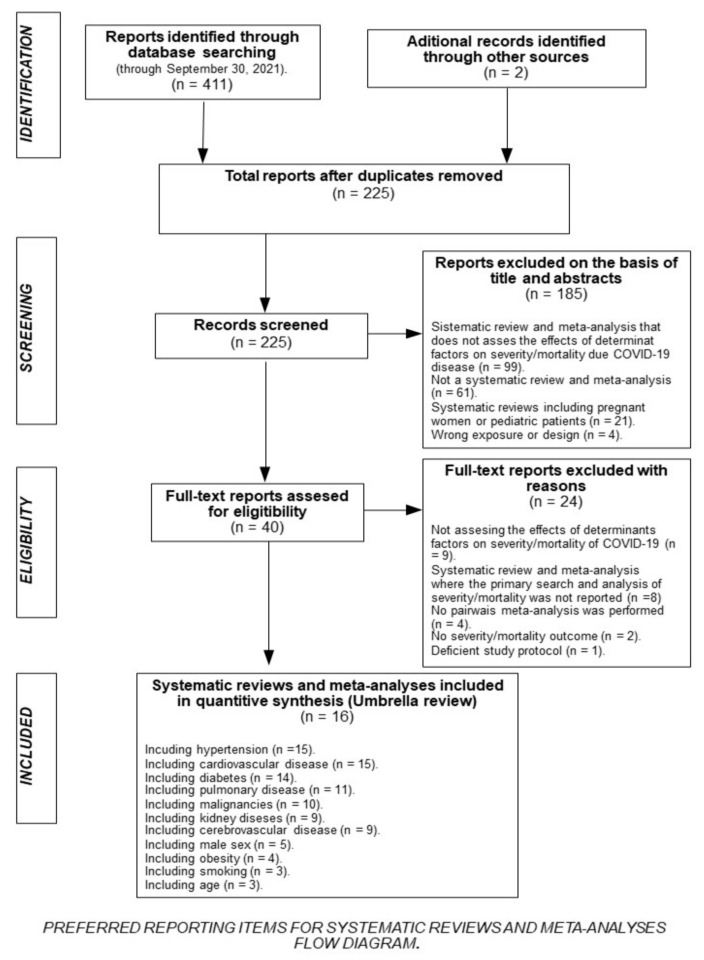
The initial search identified 411 potentially eligible studies. After discarding duplicates, 225 were screened, and finally, 16 systematic reviews and meta-analyses were included [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22] (Figure 1). These systematic reviews included 568 primary studies, with a range of 7 and 77 per meta-analysis.
Figure 1.
Flow chart of included articles according to PRISMA.
The characteristics of the selected studies are shown in Table 1. Overall, the risk estimates for the association of 12 underlying patient conditions with some of the outcomes in patients diagnosed with COVID-19 were available, including: age, sex, smoking status, obesity, hypertension, diabetes mellitus (DM), cardiac disease (CD, including arrhythmia or chronic heart failure), chronic pulmonary diseases (CPD), cancer (hematological cancer, solid cancer, any malignant tumor), cerebrovascular diseases (CVD, including stroke and transient ischemic attack), chronic kidney disease (CKD), and chronic liver disease (CLD). Seven systematic reviews provided information on the risk of hospitalization for 11 characteristics and comorbidities (all except CLD) [7,8,9,10,11,16,22]. Nine reported the risk of severe/critical illness from 10 determinants (all except age and hypertension) [10,11,12,13,14,15,16,17,18], and six reported risk estimates for the mortality ratio of 11 factors (all except age) [14,17,18,19,20,21]. Overall, four considered adjusted estimations for the individual conditions [11,14,18,19].
Table 1.
Summary of features of the systematic reviews and meta-analyses investigating the outcome impact of patients’ characteristics and underlying conditions included in this study.
| Author | Last Date of Search | Names of Databases Searched | Number of Selected Articles | Number of Selected Patients |
Determinant Factors | Outcomes Related to COVID-19 | Instrument of Quality Appraisal | Amstar-2 Score |
|---|---|---|---|---|---|---|---|---|
| Matsushita et al. [19]. | 3 April 2020 | PubMed and Embase | 25 | 76,638 | Age, Male sex, Hypertension, DM, CD | Death | Newcastle Ottawa Quality Assessment Scale | High |
| Dorjee et al. [11] | 31 August 2020 | Medline, Embase, Web of Science, and the WHOOVID-19 database | 77 | 38,906 | Age, Male sex, Smoking, CKD, Hypertension, CLD, DM, CPD, CD | Death, severity | Newcastle Ottawa Quality Assessment Scale | High |
| Khan et al. [21] | 1 May 2020 |
Medline, Web of Science, Scopus, and CINAHL | 41 | 27,670 | Malignancies, CKD, Hypertension, CLD, DM, CPD, CD, CVD | Death | Newcastle Ottawa Quality Assessment Scale | High |
| Zhou et al. [12]. | 25 April 2020 | PubMed, Embase, and Cochrane Library | 34 | 16,110 | Obesity, Malignancies, CKD, Hypertension, CLD, DM, CPD, CD, CVD | Severity/Death | Not reported | High |
| Del Sole et al. [7] | 28 May 2020 | PubMed, ISI Web of Science, SCOPUS, and Cochrane databases | 12 | 2794 | Male sex, Smoking, Hypertension, DM, CPD, CD, CVD | Severity | Not reported | Moderate |
| Yang et al. [13] | 25 February 2020 |
PubMed, EMBASE, and Web of Science | 7 | 1576 | Hypertension, CPD, CD | Severity | Not reported | High |
| Ssentongo et al. [4] | 7 July 2020 |
MEDLINE, SCOPUS, OVID, and Cochrane Library databases and medrxiv.org | 25 | 484 | Malignancies, CKD, Hypertension, DM, CD | Mortality | Newcastle Ottawa Quality Assessment Scale | High |
| Li J et al. [16] | 28 February 2021 |
PubMed, Embase, Web of Science, and Cochrane Library for epidemiological studies | 41 | 21,060 | Male sex, Smoking, Obesity, malignancies, CKD, Hypertension, CLD, DM, CPD, CD, CVD | Severity | Newcastle Ottawa Quality Assessment Scale | High |
| Booth et al. [22] | 9 July 2020 |
PubMed and SCOPUS | 66 | 1,786,001 | Age, Male sex, Obesity, Malignancies | Severity | Newcastle Ottawa Quality Assessment Scale | Moderate |
| Cheng et al. [8] | 1 April 2020 | PubMed, Embase, China National Knowledge Infrastructure (CNKI), and Wanfang Database | 22 | 3286 | Malignancies, Hypertension, DM, CPD, CD, CVD | Severity | Newcastle Ottawa Quality Assessment Scale | High |
| Honardoost et al. [9] | 28 February 2021 |
Electronic literature | 28 | 6270 | Hypertension, DM, CPD, CD, CVD | Severity | Newcastle Ottawa Quality Assessment Scale | Low |
| Yin et al. [10] | 18 January 2021 | PubMed, Web of Science, and CNKI | 41 | 12,526 | Malignancies, CKD, Hypertension, CLD, DM, CPD, CD, CVD | Severity | Not reported | High |
| Sahu et al. [15] | 24 May 2020 | PubMed, Embase, and Web of Science | 22 | 4380 | Obesity, Malignancies, CKD, Hypertension, DM, CPD, CD | Severity | Not reported | High |
| Li X et al. [20] | 14 April 2020 | PubMed, Embase, and Cochrane Library | 12 | 2445 | Malignancies, Hypertension, DM, CPD, CD, CVD | Severity | Newcastle Ottawa Quality Assessment Scale | High |
| Giri et al. [17] | 20 November 2020 |
PubMed, Scopus, Embase, and Web of Science | 41 | 16,495 | Malignancies, Hypertension, DM, CD, CVD | Severity | Methodological Index for Non-Randomized Studies | High |
| Fernández et al. [18] | 28 May 2020 | MEDLINE, bioRXiv, and MedRXiv, | 74 | 44,672 | CKD, Hypertension, CD, CVD | Severity (One parameter for mortality) | ROBINS-I tools | High |
DM: diabetes mellitus; CD: cardiac disease (including arrhythmia or chronic heart failure); CKD: chronic kidney disease; CLD: chronic liver disease; CPD: chronic pulmonary diseases; CVD: cerebrovascular diseases (including stroke and transient ischemic attack).
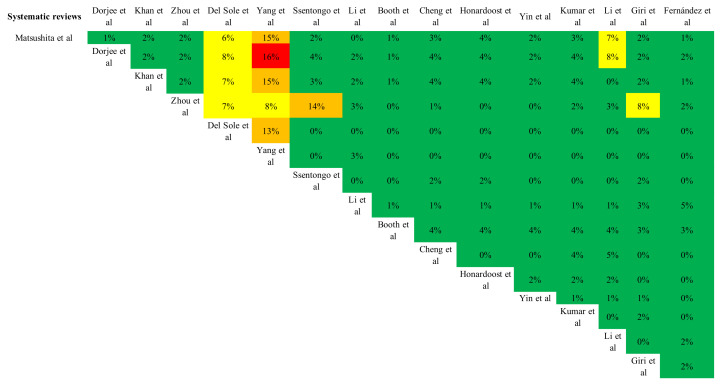
Thirteen of the sixteen systematic reviews and meta-analyses were rated as high quality [8,10,11,12,13,14,15,19,20,21,22], two were considered of moderate quality [7,16], and one was considered of low quality [9], as it did not meet two of the seven critical domains. When overlaps of the primary studies were analyzed (Figure 2), 266 primary studies appeared in at least two reviews. The degree of overlap ranged from 0% to 16% (Figure 2). One study [13] showed high values of overlap with another four studies [7,11,19,21] and moderate values with another one [12]. Another two studies showed cross-overlap, with the value reaching 14% [10,14]. Overall, the CCA showed a degree of overlap of 2.05%, which is considered low.
Figure 2.
Estimation of the overlap across studies included in the umbrella systematic review quantified by using the corrected covered area. Green: light degree of overlapping (0–5%); yellow: moderate degree of overlapping (6–10%), orange: high degree of overlapping (11–15%); and red: very high degree of overlapping (>15%).
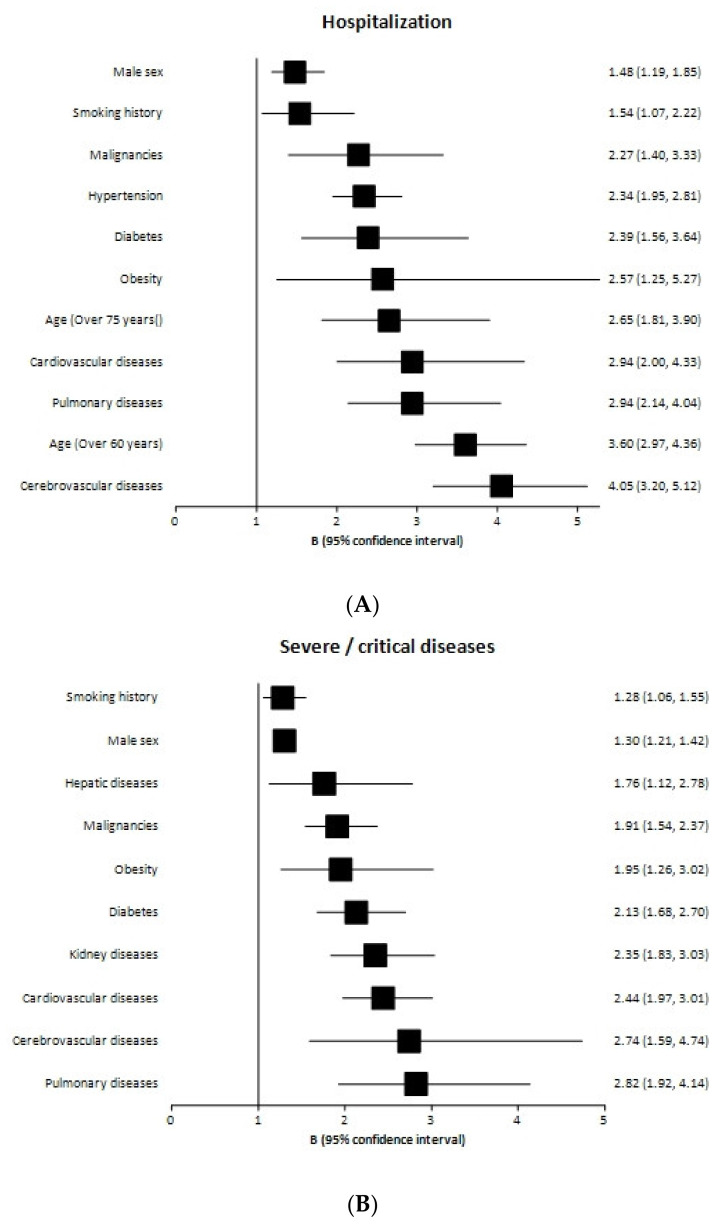
Regarding the risk estimates for COVID-19 hospitalization, all conditions considered in the seven meta-analyses investigating this outcome were found to be associated with increased risk (Table 2 and Figure 3a). For conditions for which we could provide a pOR, CVD showed the strongest association (pOR = 4.05; 95% CI: 3.20–5.12); the estimated pOR for CPD, CD DM, hypertension, and cancer ranged from 2.27 to 2.94, and the pOR for male sex was 1.49. Age, smoking status, obesity, and CKD were studied in <3 meta-analyses, and therefore, we did not calculate a pOR, but all the individual estimations showed an increased risk, which was higher than 2.5 for age, obesity, and CKD. The estimations of the individual meta-analyses were in a similar range for male sex, hypertension, and CVD but were more heterogeneous for cancer, DM, CPD, and CD.
Table 2.
Meta-analysis of the different patients’ characteristics and underlying conditions and the risk of hospitalization due to COVID-19.
| Condition | Study | Number of Primary Studies | Odds Ratio | IC 95% |
|---|---|---|---|---|
| Male sex | De sole et al. | 12 | 1.22 | 1.01–1.49 |
| Xinyian Li et al. | 41 | 1.51 | 1.33–1.71 | |
| Booth et al. | 66 | 2.05 | 1.39–3.04 | |
| pOR | 119 | 1.48 | 1.19–1.85 | |
| Age | Dorjee et al. | 77 | 3.60 (Age > 60 years) |
2.97–4.36 |
| Booth et al. | 66 | 2.65 (Age > 75 years) |
1.81–3.90 | |
| Smoking history | Del Sole et al. | 12 | 1.54 | 1.07–2.22 |
| Obesity | Booth et al. | 66 | 2.57 | 1.25–5.27 |
| Malignancy | Booth et al. | 66 | 1.46 | 1.04–2.04 |
| Cheng et al. | 22 | 3.18 | 2.09–4.82 | |
| Yin et al. | 41 | 2.63 | 1.75–3.93 | |
| pOR | 129 | 2.27 | 1.40–3.33 | |
| Chronic renal disease | Yin et al. | 41 | 3.60 | 2.18–5.94 |
| Hypertension | Del sole et al. | 12 | 2.24 | 1.63–308 |
| Cheng et al. | 22 | 2.79 | 1.66–4.69 | |
| Honardoost et al. | 28 | 2.37 | 1.80–3.13 | |
| Yin et al. | 41 | 2.13 | 1.81–2.51 | |
| pOR | 103 | 2.34 | 1.95–2.81 | |
| Diabetes mellitus | Del Sole et al. | 12 | 2.78 | 2.09–3.72 |
| Cheng et al. | 22 | 1.64 | 1.30–2.08 | |
| Honadoost et al. | 28 | 3.18 | 2.09–4.82 | |
| pOR | 62 | 2.39 | 1.56–3.64 | |
| Chronic pulmonary disease | Del Sole et al. | 12 | 2.39 | 1.10–5.19 |
| Cheng et al. | 22 | 1.98 | 1.26–3.12 | |
| Honadoost et al. | 28 | 4.19 | 2.84–6.19 | |
| Yin et al. | 41 | 3.14 | 2.35–4.19 | |
| pOR | 103 | 2.94 | 2.14–4.04 | |
| Cardiovascular disease | Del Sole et al. | 12 | 2.84 | 1.59–5.10 |
| Cheng et al. | 22 | 1.79 | 1.08–2.96 | |
| Honadoost et al. | 28 | 4.81 | 3.43–6.74 | |
| Yin et al. | 41 | 2.76 | 2.18–3.49 | |
| pOR | 103 | 2.94 | 2.00–4.33 | |
| Cerebrovascular disease | Del Sole et al. | 12 | 3.66 | 1.73–7.72 |
| Cheng et al. | 22 | 3.92 | 2.45–6.28 | |
| Honardoost et al. | 28 | 4.85 | 3.11–7.57 | |
| Yin et al. | 41 | 3.70 | 2.51–5.45 | |
| pOR | 103 | 4.05 | 3.20–5.12 |
pOR: pooled odds ratio of included studies for each condition.
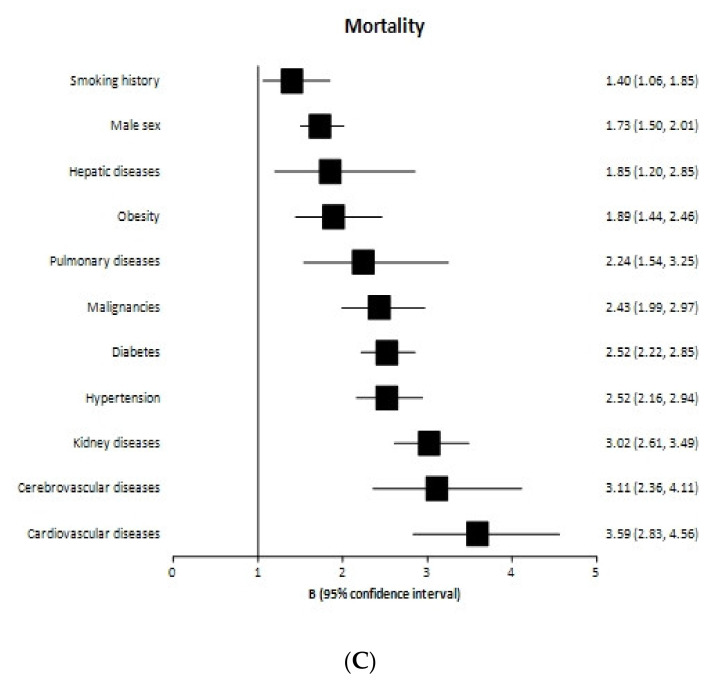
Figure 3.
Forest plot of pooled odds ratio and 95% confidence intervals of the different patients’ characteristics and underlying conditions for (A) Hospitalization (B) Severe/critical condition, and (C) Mortality.
For the development of severe/critical COVID-19, the highest estimated pOR was for CPD (2.82; 95% CI: 1.92–4.14); obesity, cancer, CKD, DM, and CD showed a pOR ranging from 1.91 to 2.44; CLD showed a pOR of 1.76 (95% CI 1.12–2.78); interestingly, this condition was the only one for which some individual meta-analyses could not show a significant association with the outcome. For male sex and smoking status, a pOR could not be calculated; individual studies showed a lower OR than for other epidemiological characteristics and comorbidities, in the range of 1.28–1.30 (Table 3 and Figure 3b). The estimates for each condition in the individual meta-analyses showed some heterogeneity for all of them.
Table 3.
Meta-analysis of the different patients’ characteristics and underlying conditions and the risk of development of severe/critical COVID-19.
| Condition | Study | Number of Primary Studies | Odds Ratio | IC 95% |
|---|---|---|---|---|
| Male sex | Dorjee et al. | 77 | 1.30 | 1.21–1.42 |
| Smoking history | Dorjee et al. | 77 | 1.28 | 1.06–1.55 |
| Obesity | Zhou et al. | 34 | 1.72 | 1.04–2.85 |
| Kumar et al. | 22 | 2.84 | 1.19–6.77 | |
| pOR | 56 | 1.95 | 1.26–3.02 | |
| Malignancy | Zhou et al. | 34 | 2.73 | 1.73–4.21 |
| Ssentongo et al. | 25 | 1.47 | 1.01–2.14 | |
| Kumar et al. | 22 | 2.38 | 1.25–4.52 | |
| Li et al. | 12 | 2.21 | 1.04–4.72 | |
| Giri et al. | 41 | 1.75 | 1.40–2.18 | |
| pOR | 134 | 1.91 | 1.54–2.37 | |
| Chronic renal disease | Dorjee et al. | 77 | 2.5 | 2.09–2.99 |
| Zhou et al. | 34 | 3.02 | 2.23–4.08 | |
| Ssentongo et al. | 25 | 3.25 | 1.13–9.28 | |
| Kumar et al. | 22 | 1.46 | 1.06–2.02 | |
| Fernadez et al. | 74 | 2.5 | 1.82–3.44 | |
| pOR | 232 | 2.35 | 1.83–3.03 | |
| Chronic liver disease | Dorjee et al. | 77 | 2.65 | 1.88–3.75 |
| Zhou et al. | 34 | 1.54 | 0.95–2.49 | |
| Yin et al. | 41 | 1.32 | 0.96–1.82 | |
| pOR | 115 | 1.76 | 1.12–2.78 | |
| Diabetes mellitus | Dorjee et al. | 77 | 1.5 | 1.36–1.65 |
| Zhou et al. | 34 | 2.63 | 2.08–3.33 | |
| Ssentongo et al. | 25 | 1.82 | 1.43–2.23 | |
| Kumar et al. | 22 | 2.29 | 1.56–3.39 | |
| Li et al. | 12 | 3.17 | 2.26–4.45 | |
| Giri et al. | 41 | 2.04 | 1.67–2.50 | |
| pOR | 211 | 2.13 | 1.68–2.70 | |
| Chronic pulmonary disease | Dorjee et al. | 77 | 1.7 | 1.4–2.0 |
| Zhou et al. | 34 | 3.56 | 2.87–4.41 | |
| Yang et al. | 7 | 2.46 | 1.76–3.44 | |
| Kumar et al. | 22 | 2.92 | 1.70–5.02 | |
| Li et al. | 12 | 5.08 | 2.68–9.63 | |
| pOR | 152 | 2.82 | 1.92–4.14 | |
| Cardiovascular disease | Dorjee et al. | 77 | 2.1 | 1.82–2.43 |
| Zhou et al. | 34 | 3.13 | 2.65–3.70 | |
| Ssentongo et al. | 25 | 2.25 | 1.60–3.17 | |
| Kumar et al. | 22 | 1.61 | 1.31–1.98 | |
| Li et al. | 12 | 2.66 | 1.71–4.15 | |
| Giri et al. | 41 | 2.78 | 2.00–3.86 | |
| Fernández et al. | 34 | 3.20 | 2.29–4.48 | |
| pOR | 245 | 2.44 | 1.97–3.01 | |
| Cerebrovascular disease | Zhou et al. | 34 | 2.74 | 1.59–4.74 |
pOR: pooled odds ratio of included studies for each condition.
Regarding mortality, three conditions showed a pOR > 3 (CKD, CVD, CD); pOR ranged from 2.24 to 2.52 for CPD, hypertension, cancer, and DM. A pOR could not be calculated for male sex, smoking status, and obesity; the OR from individual studies was in the range of 1.40 to 1.89 for these conditions (Table 4 and Figure 3c). Overall, the estimated strength of association of the different conditions with mortality was quite homogeneous across studies.
Table 4.
Meta-analysis of the different patients’ characteristics and underlying conditions and the risk of mortality due to COVID-19.
| Condition | Study | Number of Primary Studies | Odds Ratio | IC 95% |
|---|---|---|---|---|
| Male sex | Matsushita et al. | 25 | 1.73 | 1.50–2.01 |
| Smoking history | Xinyang et al. | 41 | 1.40 | 1.06–1.85 |
| Obesity | Xinyang et al. | 41 | 1.89 | 1.44–2.46 |
| Malignancy | Kahn et al. | 41 | 2.22 | 1.63–3.03 |
| Xinyang et al. | 41 | 2.60 | 2.00–3.40 | |
| pOR | 82 | 2.43 | 1.99–2.97 | |
| Chronic kidney disease | Khan et al. | 41 | 3.02 | 2.60–3.51 |
| Li et al. | 41 | 2.97 | 1.63–5.41 | |
| pOR | 82 | 3.02 | 2.61–3.49 | |
| Hypertension | Matsushita et al. | 25 | 2.87 | 2.09–3.93 |
| Li et al. | 41 | 2.42 | 2.03–2.88 | |
| pOR | 66 | 2.52 | 2.16–2.94 | |
| Chronic liver disease | Kahn et al. | 41 | 2.35 | 1.50–3.6 |
| Li et al. | 41 | 1.51 | 1.06–2.17 | |
| pOR | 82 | 1.85 | 1.20–2.85 | |
| Diabetes mellitus | Matsushita et al. | 25 | 3.20 | 2.26–4.53 |
| Kahn et al. | 41 | 2.46 | 2.03–2.85 | |
| Li et al. | 41 | 2.40 | 1.98–2.91 | |
| pOR | 107 | 2.52 | 2.22–2.85 | |
| Chronic pulmonary disease | Khan et al. | 41 | 1.94 | 1.72–2.19 |
| Li et al. | 41 | 2.88 | 1.89–4.38 | |
| pOR | 82 | 2.24 | 1.54–3.25 | |
| Cardiovascular disease | Matsushita et al. | 25 | 4.97 | 2.76–6.58 |
| Ali Khan et al. | 41 | 3.42 | 2.86–4.09 | |
| Yang et al. | 7 | 3.41 | 1.88–6.22 | |
| Li et al. | 41 | 2.87 | 2.22–3.71 | |
| pOR | 114 | 3.59 | 2.83–4.56 | |
| Cerebrovascular disease | Khan et al. | 41 | 4.12 | 3.04–5.58 |
| Li et al. | 41 | 2.47 | 1.54–3.97 | |
| Giri et al. | 41 | 2.68 | 1.29–5.57 | |
| Fernández et al. | 75 | 2.70 | 1.74–4.19 | |
| pOR | 198 | 3.11 | 2.36–4.11 |
pOR: pooled odds ratio of included studies for each condition.
Overall, the exclusion of studies with higher degrees of overlap did not change the results. Twelve of the sixteen selected systematic reviews and meta-analyses had significant heterogeneity, and eleven systematic reviews had I2 > 50%. Individual studies in each meta-analysis differed in terms of geographic location, ethnicity of the selected subjects, frequency of diagnosis of the determinant condition, method of diagnosis, COVID-19 classification, duration of follow-up, and outcome assessment. These studies did not publish the heterogeneity of the primary studies included in the specific risk comparison.
We were unable to establish the possible publication bias according to Egger’s regression test. The test was repeated in 10 studies of meta-analyses because the remaining had insufficient data. Of the ones we reanalyzed, five systematic reviews had statistical evidence of publication bias. For the meta-analyses that could not be reanalyzed, none reported significant publication bias or did not perform or publish any statistical test of publication bias for the specific exposure comparison.
4. Discussion
In this umbrella review, which used restrictive criteria for the inclusion of studies, we found that male sex, age >60 years, being a smoker, and suffering from hypertension, DM, cancer, CD, CPD, CLD, CVD, and CKD are associated with significantly higher risk of hospitalization, severe disease, and death due to COVID-19. The estimations for the risks in the individual meta-analyses showed some differences but were more homogeneous for the mortality predictors.
Previous umbrella reviews also evaluated the impact of underlying conditions on COVID-19 outcomes; however, these studies had important differences with this one, the most important being that the criteria used to select the studies were less restrictive than in ours. Two of them evaluated only one underlying disease or a group of conditions. In a study on obesity, Kristensen et al. found a similar risk estimate as in our study [23]. Kastora et al. studied the impact of DM on COVID-19 outcomes [24]; the risks estimated for ICU admission and mortality in patients with DM in that study were 1.56 (95% CI: 1.28–1.89) and 1.82 (96% CI 1.65–2.02), respectively, which was somewhat lower than in our study. Harrison et al. studied the impact of cardiovascular risk factors on COVID-19 severity [25]; overall, the risk estimates in that study were similar to ours. We found one umbrella review including all the underlying conditions, focusing on whether there were geographical differences [26]. Interestingly, the authors found some regional heterogeneity in the risk estimates.
Some of the chronic conditions associated with worse outcomes in COVID-19, such as hypertension, obesity, DM, CPD, CKD, CLD, and CD, share some characteristics, including chronic proinflammatory states and innate or adaptive immunity dysfunction, which might facilitate a dysregulated immune response against SARS-CoV-2 [27,28]. Some of these conditions and smoking can also increase the expression of ACE2, the viral receptor in respiratory tract cells [29]. Age-related immune system changes (immunosenescence and inflammageing) have been associated with the increased risk of complications and mortality in older persons with COVID-19 [30]. However, from a physiopathogenic perspective, the confounding or modifying effect of age on the underlying conditions and vice versa must be considered when evaluating their independent risk estimates. As an example, male sex is also associated with severity and mortality in patients with COVID-19; although this may be related to the confounding effect of some comorbidities that might be more frequent among men, it might as well be a consequence of the sexual dimorphism in the immune response [31] and lower circulating concentration of ACE in females compared to males [32]. Regarding the smoking status, although the potential confounding effect of some associated comorbidities may play a role in the association found, smoking is known to alter mucosal innate immunity patterns and can increase the expression of ACE2 [33].
The association of cancer with deleterious outcomes in COVID-19 patients found in our study would need some considerations. Early in the pandemic, it was suggested that only patients who had recently received cancer treatment were at increased risk of death, which could be linked to treatment-related immunosuppression [34]. Additionally, patients with active hematologic malignancies seem to be at increased risk of mortality [35]. However, depending on the treatment received, some subsets of patients receiving immunomodulatory drugs, which may help avoiding the deleterious dysregulated immune response in COVID-19, might even have a better prognosis [36]. When interpreting the results of this study, it should be noted that we could only analyze the underlying conditions included in the systematic reviews detected by our strategy. Therefore, we could not evaluate the potential impact of less frequent diseases, including autoimmune conditions being treated with immunosuppressive drugs [37] or rare diseases with neuromuscular involvement [38], among others.
Our study has some limitations; because of the nature of an umbrella review, it was not possible to provide data on specific subgroups within each comorbid condition evaluated, for which the risk may be substantially different. Additionally, the heterogeneity of the systematic reviews and meta-analyses included must be considered when interpreting the data. The possible causes of that heterogeneity are differences in the designs, populations included, and methodological approaches. We could not provide the estimations for publication bias using Egger’s test, since the cumulative assessment did not exceed 10 studies. We included meta-analyses with adjusted and unadjusted estimations. We did not consider the geographical differences in the impact of the conditions. Most of the studies did not include information on in-hospital therapy nor on early treatment. Finally, most of the studies included were performed before vaccines were available and the omicron variant was predominant. However, these results could be useful in case of a rise of new virulent variants with immune-escape capacity. The strengths include studies evaluating several comorbidities and the large number of patients represented in the meta-analysis reported.
5. Conclusions
This umbrella review provides a comprehensive summary of meta-analyses examining the impact of patients’ characteristics on COVID-19 outcomes. Elderly patients and those with cardiovascular, cerebrovascular, and chronic renal diseases should be prioritized for pre-exposure and post-exposure prophylaxis and early treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines10102437/s1, Table S1: Search strategy, and Table S2: AMSTAR-2 Checklist.
Author Contributions
The protocol was prepared by E.R.-V., M.G.C., N.M., E.T., M.G., J.R.-B. and Z.R.P.-B. The literature search and data extraction were performed by E.R.-V., M.G.C., N.M. and P.O. and reviewed by Z.R.P.-B. and J.R.-B. The analyses were performed by E.R.-V. and reviewed by E.R.-V., M.G.C., N.M., P.O., N.C., C.B., B.T., E.C., M.G., E.T., J.R.-B. and Z.R.P.-B. E.R.-V., J.R.-B. and Z.R.P.-B. drafted the manuscript, which was reviewed for scientific content by all authors. E.R.-V. is the guarantor of the review. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This systematic review was developed as part of the ORCHESTRA project (Connecting European Cohorts to increase common and effective SARS-CoV-2 Response), which was funded by the European Union’s Horizon 2020 Research and Innovation Programme (grant agreement no. 101016167).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization COVID-19 Weekly Epidemiological Update. 2022. [(accessed on 19 April 2022)]. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---27-april-2022.
- 2.Cevik M., Bamford C.G.G., Ho A. COVID-19 pandemic-a focused review for clinicians. Clin. Microbiol. Infect. 2020;26:842–847. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moola S., Munn Z., Sears K., Sfetcu R., Currie M., Lisy K., Tufanaru C., Qureshi R., Mattis P., Mu P. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. Int. J. Evid.-Based Healthc. 2015;13:163–169. doi: 10.1097/XEB.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 4.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pieper D., Antoine S.L., Mathes T., Neugebauer E.A., Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014;67:368–375. doi: 10.1016/j.jclinepi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Del Sole F., Farcomeni A., Loffredo L., Carnevale R., Menichelli D., Vicario T., Pignatelli P., Pastori D. Features of severe COVID-19: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2020;50:e13378. doi: 10.1111/eci.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng S., Zhao Y., Wang F., Chen Y., Kaminga A.C., Xu H. Comorbidities’ potential impacts on severe and non-severe patients with COVID-19: A systematic review and meta-analysis. Medicine. 2021;100:e24971. doi: 10.1097/MD.0000000000024971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honardoost M., Janani L., Aghili R., Emami Z., Khamseh M. The Association between Presence of Comorbidities and COVID-19 Severity: A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2021;50:132–140. doi: 10.1159/000513288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin T., Li Y., Ying Y., Luo Z. Prevalence of comorbidity in Chinese patients with COVID-19: Systematic review and meta-analysis of risk factors. BMC Infect. Dis. 2021;21:200. doi: 10.1186/s12879-021-05915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorjee K., Kim H., Bonomo E., Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS ONE. 2020;15:e0243191. doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y., Yang Q., Chi J., Dong B., Lv W., Shen L., Wang Y. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ssentongo P., Ssentongo A.E., Heilbrunn E.S., Ba D.M., Chinchilli V. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE. 2020;15:e0238215. doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahu A.K., Mathew R., Aggarwal P., Nayer J., Bhoi S., Satapathy S., Ekka M. Clinical Determinants of Severe COVID-19 Disease—A Systematic Review and Meta-Analysis. J. Glob. Infect. Dis. 2021;13:13–19. doi: 10.4103/jgid.jgid_136_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., He X., Yuan Y., Zhang W., Li X., Zhang Y., Li S., Guan C., Gao Z., Dong G. Meta-analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. Am. J. Infect. Control. 2021;49:82–89. doi: 10.1016/j.ajic.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giri M., Puri A., Wang T., Guo S. Comparison of clinical manifestations, pre-existing comorbidities, complications and treatment modalities in severe and non-severe COVID-19 patients: A systemic review and meta-analysis. Sci. Prog. 2021;104:368504211000906. doi: 10.1177/00368504211000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernández Villalobos N.V., Ott J.J., Klett-Tammen C.J., Bockey A., Vanella P., Krause G., Lange B. Effect modification of the association between comorbidities and severe course of COVID-19 disease by age of study participants: A systematic review and meta-analysis. Syst. Rev. 2021;10:194. doi: 10.1186/s13643-021-01732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsushita K., Ding N., Kou M.H., Hu X., Chen M.K., Gao Y., Honda Y.M., Zhao D., Dowdy D., Mok Y., et al. The Relationship of COVID-19 Severity with Cardiovascular Disease and Its Traditional Risk Factors: A Systematic Review and Meta-Analysis. Glob. Heart. 2020;15:64. doi: 10.5334/gh.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Zhong X., Wang Y., Zeng X., Luo T., Liu Q. Clinical determinants of the severity of COVID-19: A systematic review and meta-analysis. PLoS ONE. 2021;16:e0250602. doi: 10.1371/journal.pone.0250602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan M.M.A., Khan M.N., Mustagir M.G., Rana J., Islam M.S., Kabir M. Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: A systematic review and meta-analysis. J. Glob. Health. 2020;10:020503. doi: 10.7189/jogh.10.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Booth A., Reed A.B., Ponzo S., Yassaee A., Aral M., Plans D., Labrique A., Mohan D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE. 2021;16:e0247461. doi: 10.1371/journal.pone.0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kristensen N.M., Gribsholt S.B., Andersen A.L., Richelsen B., Bruun J.M. Obesity augments the disease burden in COVID-19: Updated data from an umbrella review. Clin. Obes. 2022;12:e12508. doi: 10.1111/cob.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kastora S., Patel M., Carter B., Delibegovic M., Myint P.K. Impact of diabetes on COVID-19 mortality and hospital outcomes from a global perspective: An umbrella systematic review and meta-analysis. Endocrinol. Diabetes Metab. 2022;5:e00338. doi: 10.1002/edm2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison S.L., Buckley B.J.R., Rivera-Caravaca J.M., Zhang J., Lip G.Y.H. Cardiovascular risk factors, cardiovascular disease, and COVID-19: An umbrella review of systematic reviews. Eur. Heart J.-Qual. Care Clin. Outcomes. 2021;7:330–339. doi: 10.1093/ehjqcco/qcab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treskova-Schwarzbach M., Haas L., Reda S., Pilic A., Borodova A., Karimi K., Koch J., Nygren T., Scholz S., Schönfeld V., et al. Pre-existing health conditions and severe COVID-19 outcomes: An umbrella review approach and meta-analysis of global evidence. BMC Med. 2021;19:212. doi: 10.1186/s12916-021-02058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hameed I., Masoodi S.R., Mir S.A., Nabi M., Ghazanfar K., Ganai B. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J. Diabetes. 2015;6:598–612. doi: 10.4239/wjd.v6.i4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith J.C., Sausville E.L., Girish V., Yuan M.L., Vasudevan A., John K.M., Sheltzer J.M. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev. Cell. 2020;53:514–529.e3. doi: 10.1016/j.devcel.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witkowski J.M., Fulop T., Bryl E. Immunosenescence and COVID-19. Mech. Ageing Dev. 2022;204:111672. doi: 10.1016/j.mad.2022.111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shepherd R., Cheung A.S., Pang K., Saffery R., Novakovic B. Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics. Front. Immunol. 2021;11:604000. doi: 10.3389/fimmu.2020.604000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sama I., Ravera A., Santema B.T., Van Goor H., Ter Maaten J.M., Cleland J.G.F., Rienstra M., Friedrich A.W., Samani N.J., Ng L.L., et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur. Heart J. 2020;41:1810–1817. doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S. Smoking Upregulates Angiotensin-Converting Enzyme-2 Receptor: A Potential Adhesion Site for Novel Coronavirus SARS-CoV-2 (COVID-19) J. Clin. Med. 2020;9:841. doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavez-MacGregor M., Lei X., Zhao H., Scheet P., Giordano S.H. Evaluation of COVID-19 Mortality and Adverse Outcomes in US Patients with or without Cancer. JAMA Oncol. 2022;8:69–78. doi: 10.1001/jamaoncol.2021.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naimi A., Yashmi I., Jebeleh R., Mofrad M.I., Abhar S.A., Jannesar Y., Heidary M., Pakzad R. Comorbidities and mortality rate in COVID-19 patients with hematological malignancies: A systematic review and meta-analysis. J. Clin. Lab. Anal. 2022;36:e24387. doi: 10.1002/jcla.24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martynowicz H., Jodkowska A., Poręba R., Mazur G., Więckiewicz M. Demographic, clinical, laboratory, and genetic risk factors associated with COVID-19 severity in adults: A narrative review. Dent. Med. Probl. 2021;58:115–121. doi: 10.17219/dmp/131795. [DOI] [PubMed] [Google Scholar]
- 37.Akiyama S., Hamdeh S., Micic D., Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: A systematic review and meta-analysis. Ann. Rheum. Dis. 2021;80:384–391. doi: 10.1136/annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- 38.Di Stefano V., Battaglia G., Giustino V., Gagliardo A., D’Aleo M., Giannini O., Palma A., Brighina F. Significant reduction of physical activity in patients with neuromuscular disease during COVID-19 pandemic: The long-term consequences of quarantine. J. Neurol. 2021;268:20–26. doi: 10.1007/s00415-020-10064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.