Abstract
High-flow nasal cannula (HFNC) therapy is a non-invasive ventilatory support that has gained interest over the last ten years as a valid alternative to nasal continuous positive airway pressure (nCPAP) in children with respiratory failure. Its safety, availability, tolerability, and easy management have resulted its increasing usage, even outside intensive care units. Despite its wide use in daily clinical practice, there is still a lack of guidelines to standardize the use of HFNC. The aim of this review is to summarize current knowledge about the mechanisms of action, safety, clinical effects, and tolerance of HFNC in children, and to propose a clinical practices algorithm for children with respiratory failure.
Keywords: heated humidified high-flow, children, respiratory failure, oxygen therapy, bronchiolitis
1. Introduction
Respiratory diseases are the most frequent cause of hospitalization in pediatric intensive care units (PICUs), of which 15% may require either invasive mechanical ventilation or non-invasive positive pressure ventilation [1].
High-flow nasal cannula (HFNC) is an oxygen therapy that has received growing attention in recent years as an alternative to continuous positive airway pressure (CPAP) for pediatric patients with respiratory failure [2,3]. HFNC was first indicated to treat apnea in premature infants [4], but its use subsequently expanded in recent years to become a popular mode of pediatric respiratory support. The reasons for its increasing application are its tolerability, safety, and easy-to-use devices, as compared to CPAP or other noninvasive ventilation (NIV) devices [5]. HFNC’s ease of use has resulted in its widespread implementation in settings beyond PICUs, such as in emergency departments and in inpatient pediatric wards. Despite the positive feedback from physicians, development of clinical guidelines surrounding the use of HFNC is still lacking.
The methodological heterogeneity of the studies characterized by different settings for vital parameters, definitions of therapeutic failure and environments (i.e., emergency departments vs. general wards vs. intensive care), and the lack of randomized clinical trials (RCTs) in children, make it difficult to implement internationally recognized guidelines.
The aim of this review is to summarize the most recent scientific evidence on the efficacy, indications, and mechanism of action of HFNC, and to propose a possible algorithm for its use in clinical practice with children.
2. Device and Mechanism of Action
A typical HFNC system consists of a flow generator, an active heated humidifier, a single-limb heated circuit, and a nasal cannula [6].
2.1. Flow Generator
There are three types of flow generators: air-oxygen blenders, built-in flow generators, and entrainment systems. Each can provide a flow up to 60 L/min, containing an oxygen concentration from 21% to 100% [2]. The air-oxygen blender consists of a mechanical air-oxygen blender that is connected directly from a wall supply with the source of air and oxygen, with a flow meter that allows a stable delivery of both inspiratory fraction of oxygen (FiO2) and gas flow. The built-in flow generators operate through a turbine that is capable of generating high flows, beginning from the ambient air, without an external source of gas. It guarantees delivery of a fixed FiO2 even if the flow changes, because there is an internal oxygen sensor and a valve which maintains the desired FiO2. However, high oxygen concentrations cannot be supplied with this system. The third type of flow generator uses an air entrainment system to generate air flow, which can be used to administer high concentrations of oxygen.
2.2. Humidifying Devices
It is well known that the use of dry gas has a harmful impact on the respiratory system, leading to mucociliary malfunction, mucus obstruction, mucosa dryness, and ulceration; its use can also induce reactive bronchostruction [7]. For this reason, the use of HFNC requires that heated and humidified air and gas should be administered. Gas conditioning confers several beneficial effects to the patient, such as improving comfort, optimizing mucociliary clearance, and reducing the metabolic effort of the body for gas conditioning. By passing the mixed gas through a narrow bundle of micropored tubes, optimal humidification can be achieved.
2.3. Inspiratory Limb
The main disadvantage of using humidified gas is that part of the humidity is lost as condensation. Inappropriate humidity may negatively impact clinical outcomes by damaging airway mucosa, prolonging the mechanical ventilation, or increasing respiratory effort [8]. Therefore, high-flow devices can have different types of inspiratory circuits, with some of them being equipped with a heating wire embedded in the circuit wall in order to keep the wall temperature sufficiently high to avoid condensation.
2.4. Interface
The circuit is connected to a nasal cannula that is available in different sizes to fit the patient’s nostrils. An important precaution to be adopted is the choice of the cannula based on age and weight. Manufacturers recommend that a cannula should not have a diameter greater than fifty percent of the patient’s nostril, in order to avoid a significant increase in airway pressure, with a subsequent risk of air leakage [9].
3. Clinical Indications
3.1. Acute Bronchiolitis
Bronchiolitis is an infection that affects the lower respiratory tract of infants and children, causing hospitalization in 10% of cases [10], and requiring ventilatory support for respiratory failure and apnea.
Bronchiolitis is the main indication for HFNC in children with moderate to severe respiratory distress. Franklin et al. [11] showed in an RCT that included 1472 infants < 12 months of age with bronchiolitis, that there was a significantly lower failure rate in children treated with HFNC than in children treated with conventional low-flow oxygen therapy (COT) (12% and 23%, respectively). However, no significant differences were observed in the length of hospital stay or in the duration of oxygen therapy between the two groups. The recent pediatric studies regarding the use of HFNC in children with bronchiolitis are summarized in Table 1.
Table 1.
Use of HFNC in children with bronchiolitis.
| Reference | Study Design | Study Population | Comparison | Main Findings |
|---|---|---|---|---|
| Franklin et al. [11] | RCT | 1472 patients < 12 months with bronchiolitis | HFNC vs. COT |
|
| Kepreotes et al. [14] | RCT | 202 patients < 24 months with moderate bronchiolitis | HFNC vs. COT |
|
| Lin et al. [12] | Systematic review | 2121 patients with bronchiolitis | HFNC vs. other oxygen therapies (COT, CPAP) |
|
| Dafydd et al. [13] | Systematic review and meta-analysis | 1159 children up to 24 months of age with bronchiolitis | HFNC vs. other oxygen therapies (COT, nCPAP) |
|
| Moreel et al. [15] | Meta-analysis | 213 infants < 24 months | HFNC vs. nCPAP |
|
COT, conventional oxygen therapy; nCPAP, nasal continuous positive airway pressure; HFNC, high flow nasal cannula; PICU, pediatric intensive care unit; RCT, randomized controlled trial.
Lin et al. [12] carried out a systematic review and meta-analysis that included 2121 children with bronchiolitis; the objective was to compare the efficacy of HFNC with both COT and CPAP. The authors found no evident difference in the length of stay, in the duration of oxygen supplementation, or in transfers to PICU, among the three treatment groups. A significant reduction in the incidence of treatment failure was observed in the HFNC group compared with the COT group, although there was a significant increase in the incidence of treatment failure when the HFNC group was compared with the nasal CPAP group.
More recently, in a systematic review and meta-analysis, including children up to 24 months of age with bronchiolitis, Dafydd et al. [13] showed that HFNC was superior to COT in terms of treatment failure rates, and was not inferior to CPAP in terms of therapeutic failure and intubation rates.
The aforementioned systematic review also included a study by Kepreotes et al. [14] that showed that HFNC is used as a rescue therapy for patients failing on COT. In this latter study, 202 children with bronchiolitis were randomly assigned to either HFNC (101 children) or COT (101 children). The authors observed that HFNC did not significantly reduce the time of oxygen therapy when compared with COT. However, 61% of children who experienced the failure in treatment with low-flow oxygen therapy were rescued using HFNC. These data suggested that the early use of HFNC was not able to modify the natural course in children with moderate bronchiolitis, although it took on a role as a rescue therapy to reduce the PICU admissions.
Studies comparing HFNC with CPAP have led to conflicting results. A recent meta-analysis involved three RCTs that included a total of 213 infants of ages < 24 months [15]. Two studies showed similar failure rates between the HFNC and CPAP patient groups [15,16]. However, the third study which comprised a larger cohort of patients than the aforementioned ones, showed a statistically higher failure rate in the HFNC-treated group [17].
Further studies are necessary to focus on children with bronchiolitis who may better benefit from HFNC, and to identify the ideal cohort of patients who yield the best findings when using HFNC versus COT. It is also important to define the criteria for treatment failure, in order to standardize the study results and maximize the use of HFNC.
3.2. Asthma
Asthma is the most common obstructive respiratory disease in children, and is a frequent cause of visits to emergency and hospital admission [18]. An acute episode of asthma is potentially life-threatening; treatment of an asthma exacerbation requires the use of systemic bronchodilators and inhaled corticosteroids. The most severe asthma exacerbations could benefit from the use of COT. When there is no response to COT, it is possible to use CPAP as rescue oxygen therapy prior to the intubation. The use of CPAP requires careful monitoring for potential complications, such as gastric distension, which increase the risk of vomiting and inhalation [19].
It has been demonstrated that inhalation of cold and dry gas can induce bronchoconstriction and reduce ciliary activity in the airway, thereby worsening an episode of asthma exacerbation [7]. Two retrospective studies showed that the use of HFNC could improve heart rate, respiratory rate, oxygenation, pH, and partial pressure of carbon dioxide (pCO2) [20,21]. Baudin et al. [20] showed that in children with severe asthma treated with HFNC, heart and respiratory rates decreased significantly, and the blood gas improved in the first 24 h. These data were confirmed in a study by Martinez et al. [21] that included children aged 4–5 years with moderate-to-severe asthma exacerbation. It showed that patients treated with HFNC had less PICU admissions compared with those treated using an initial flow of < 15 L/min, and that high flow was well tolerated by children and did not require sedation.
Ballestero et al. [22] carried out a study of 62 children aged 1 to 14 years with asthma exacerbation who were randomly assigned to either HFNC or COT. The authors showed that 53% of the children treated with HFNC had improved respiratory dynamics after two hours of treatment, compared with 28% of children treated with COT [22].
Table 2 summarizes the literature review regarding the use of HFNC in children with asthma.
Table 2.
Use of HFNC in children with asthma.
| Reference | Study design | Study Population | Comparison | Main Results |
|---|---|---|---|---|
| Baudin et al. [20] | Retrospective observational study | 73 PICU patients aged 1 to 18 years with severe asthma | HFNC vs. COT | Lower heart, better respiratory rates and blood gas in the first 24 h in the HFNC group |
| Martinez et al. [21] | Observational study | 536 children aged 4–5 years with moderate-to-severe asthma exacerbation | HFNC vs. COT | Lower PICU admissions in the HFNC group |
| Ballestero et al. [22] | Randomized pilot trial | 62 children aged 1 to 14 years with asthma exacerbation | HFNC vs. COT | Improvement in respiratory dynamics after two hours in the HFNC group compared with the COT group |
COT, conventional oxygen therapy; HFNC, high flow nasal cannula; PICU, pediatric intensive care unit.
Although the current scientific evidence is limited, the use of HFNC appears to play an important role in the treatment of children with acute asthma exacerbation, especially in preventing the escalation of ventilatory support and intubation. Further well-conducted RCTs are necessary to understand what type of patient could better benefit by using high flows during an asthma exacerbation.
3.3. Congenital Heart Diseases
HFNC is an effective respiratory support for patients with precarious hemodynamic balance. In an RCT that included pediatric patients with congenital heart disease, it was shown that the use of HFNC reduced desaturations, risks of escalation to NIV, and hypercapnia, while preserving the hemodynamic balance [23].
It is well known that positive end-expiratory pressure (PEEP) obstructs the venous return to the heart by causing an increase in central venous pressure [24]. Thus, the effectiveness of HFNC is evidenced by the lower PEEP values generated in contrast to CPAP, without significantly modifying the central venous pressure [25].
Furthermore, Shioji et al. [26] carried out a retrospective study which compared HFNC and NIV for the treatment of acute respiratory failure after cardiac surgery in children with congenital heart disease. There was a lower reintubation rate within 28 days (3% vs. 26%; p = 0.04), and a shorter PICU stay (10 vs. 17 days; p = 0.009) in the HFNC group than in the NIV group [26].
3.4. Obstructive Sleep Apnea
Obstructive sleep apnea (OSAS) is another important field of application of HFNC therapy. The current treatment options for OSAS include adenotonsillectomy and nasal-CPAP, which prevent airway collapse.
In view of the risk of facial injury due to nasal CPAP devices, the use of HFNC therapy has been proposed.
In 2009, McGinley et al. showed that the reduction in apnea-hypopnea index (AHI) in 12 children with a mean age of 10 years with mild-to-severe OSAS was similar between HFNC and CPAP [27].
More recently, two studies [28,29] carried out in children with moderate-to-severe OSAS found that HFNC reduced nocturnal respiratory events and improved oxygen saturation (SpO2). Ignatiuk et al. [28] observed a significantly reduced obstructive AHI (28.9 [17.6, 40.2] vs. 2.6 events/h [1.1, 4.0]; p < 0.001) in 22 children who underwent HFNC because of poor surgical candidacy, residual OSAS after surgery, and CPAP intolerance. Hawkins et al. [29] found in 10 school-aged subjects treated with HFNC a reduced median obstructive AHI (11.1 [8.7–18.8] vs. 2.1 events/h [1.7–2.2]; p = 0.002); an increased oxygen saturation mean (91.3% [89.6–93.5%] vs. 94.9% [92.4–96.0%]; p < 0.002); a decreased SpO2 desaturation index (19.2 [12.7–25.8] vs. 6.4 events/h [4.7–10.7]; p = 0.013); and a reduced heart rate (88 [86–91] vs. 74 bpm [67–81]; p = 0.004). In a retrospective report of five patients with OSAS, the authors found clinical improvement and a decreased AHI, with less chronic CO2 retention after treatment with HFNC [30].
Despite this limited evidence, HFNC might be considered as a rescue option in children with OSAS and CPAP intolerance. Further studies comparing therapy with HFNC and CPAP are necessary in order to define a standardized protocol for the use of HFNC in the treatment of OSAS as well.
3.5. Pneumonia
Pneumonia is a leading cause of respiratory failure in children [31].
Respiratory failure may be caused by pneumonia, which leads to hypoxemia and/or hypercapnia and increases respiratory effort. Although COT with high-oxygen concentrations improves hypoxemia, the delivery of continuous positive airway pressure improves the respiratory distress and hypercapnia [15]. However, the use of CPAP is limited, since it requires greater technical and clinical skills for its use than COT [17].
Therefore, in recent years the use of HFNC has spread due to its ease of use and good tolerance. HFNC regulates the flow of oxygen and its concentration, delivers humidified oxygen, and creates a CPAP effect [32].
Currently, few studies have compared HFNC and CPAP treatments in children with pneumonia. A recent RCT, including 84 children < 2 years of age, evaluated the difference between HFNC and CPAP for the treatment of mild to moderate respiratory failure in children with pneumonia [33]. The need for intubation and PICU transfer occurred in 6 out of 43 children in HFNC group and in 4 out of 41 children in CPAP group (14% vs. 10%; p = 0.553). There was no significant difference between the two groups in length of hospital stay (8 [7,8,9] vs. 8 days [7,8,9]; p = 0.461) and in duration of non-invasive treatment (2 [2, 3] vs. 3 days [2, 3]; p = 0.090). Adverse events that were observed in the HFNC group were lower than those experienced in the CPAP group (5% vs. 27%; p < 0.005). Specifically, abdominal distension was less common in the HFNC group compared to the CPAP group (5% vs. 17%; p = 0.066), and trauma of the nasal mucosa was less common in the HFNC group compared to the CPAP group (0% vs. 14%; p = 0.036). No serious adverse events, such as pneumothorax, cardiorespiratory arrest, and asphyxia, were observed in either group. Additionally, sedative use was less frequent in the HFNC group than in the CPAP group (40% vs. 83%; p = 0.000). Recent studies about other clinical indications for the use of HFNC in children are summarized in Table 3.
Table 3.
Other clinical indications for HFNC in pediatric patients.
| Disease | Reference | Study Design | Study Population | Comparison | Main Results |
|---|---|---|---|---|---|
| Congenital heart diseases | Shioji et al. [26] | Retrospective study | 35 children with congenital heart disease surgically corrected and acute respiratory failure | HFNC and NIV |
|
| OSAS | McGinley et al. [27] | Retrospective study | 12 children with a mean age of 10 years with mild-to-severe OSAS | HFNC vs. CPAP | Similar reductions in AHI in both groups |
| Ignatiuk et al. [28] | Retrospective study |
22 children with poor surgical candidacy or residual OSAS after surgery | HFNC vs. no intervention | Significant reduction in AHI with HFNC | |
| Hawkins et al. [29] | Observational study | 10 school-aged patients with OSAS treated with HFNC | HFNC vs. no intervention |
|
|
| Pneumonia | Liu et al. [33] | RCT | 84 children < 2 years with pneumonia and mild to moderate respiratory failure | HFNC and CPAP |
|
AHI, apnea-hypopnea index; COT, conventional oxygen therapy; CPAP, continuous positive airway pressure; HFNC, high-flow nasal cannula; NIV, noninvasive ventilation; PICU, pediatric intensive care unit; OSAS, obstructive sleep apnea.
In 43 adult patients admitted to the intensive care unit with COVID-19 pneumonia who received oxygen via COT or HFNC therapy, a lower short-term mortality (50% vs. 84.2%; p = 0.019) and need for intubation (54.2% vs. 84.2%; p = 0.037) were found in the HFNC group. [34]. Therefore, the authors suggested that HFNC is a safe and effective alternative treatment for acute hypoxic respiratory failure due to COVID-19 pneumonia [35].
4. HFNC Setting: Initiation, Maintenance, and Weaning
Currently, there is no standardized protocol for setting HFNC parameters.
Based on the characteristics of the patient, three parameters must be adjusted independently: the temperature, the gas flow, and FiO2.
-
-
Temperature: between 34–37 °C, with an ideal value of 34 °C for the pediatric patient.
-
-
Flow: can be set up to 60 L/min. In the most of pediatric studies, the flow is set up on the basis of body weight (1–2 L/kg/min). In bronchiolitis, a flow of 2 L/kg/min seems to offer maximum efficacy with minimal risk of adverse events.
-
-
FiO2: set up with the aim of obtaining a saturation of 95–97%.
In order to underline the importance of a shared protocol, a recent study evaluated 584 patients with a median age of 20 months before and after the implementation of a protocol for using HFNC. Two hundred ninety-two patients treated after setting up the protocol had a higher initial flow (14.5 L/min vs. 10.0 L/min; p < 0.001), faster weaning (4.1 L/min/h vs. 2.4 L/min/h; p < 0.001), a lower failure rate of high-flow therapy (10% vs. 17%; p = 0.015), and a shorter hospital stay (5.9 days vs. 6.8 days; p = 0.006). Therefore, with the implementation of a protocol that established an increase in the initial flow, a faster weaning and a lower need for escalation to NIV or mechanical ventilation was obtained [35].
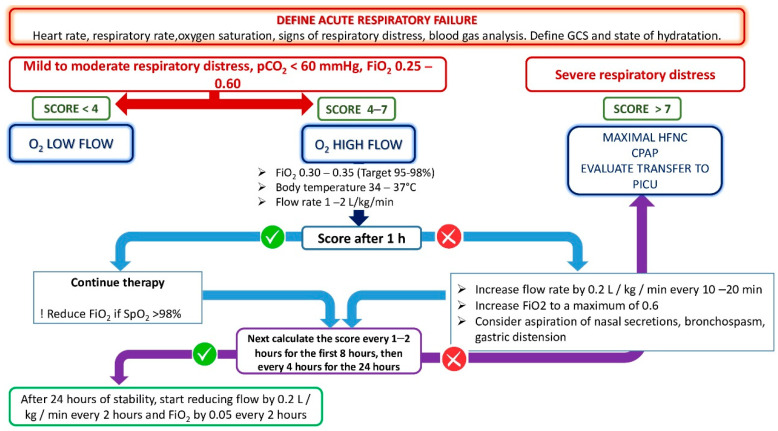
The flow-chart for the management of therapy with HFNC is outlined in Figure 1.
Figure 1.
Decisional flow-chart for the management of high-flow oxygen therapy in children. The Pediatric Early Warning System was considered as the score. GCS = Glasgow Coma Scale; pCO2 = partial pressure of carbon dioxide; FiO2 = fraction of inspired oxygen; O2 = oxygen; SpO2 = blood oxygen saturation; HFNC = high-flow nasal cannula; CPAP = continuous positive airway pressure; PICU = pediatric intensive care unit.
After setting the parameters of the device, it is necessary to constantly monitor the vital signs (oxygen saturation and heart rate) every 1–2 h for the first 8 h, and then every 4 h for the next 24 h. After the first 60–90 min, the flow can be increased by 0.2 L/kg/min every 10–20 min, up to a maximum FiO2 of 0.5 when there is no clinical improvement. It is also important to try to aspirate the gastric secretions. Other methods of ventilation or transfer to the PICU should be considered when there is no clinical response to HFNC therapy.
Weaning can be considered if respiratory dynamics have improved after 24 h.
When a patient’s condition improves, gradually decreasing the set of parameters of the device is recommended, reducing the flow by 0.2 L/kg/min every 2 h and FiO2 by 0.05 every 2 h.
5. Advantages of the Use of HFNC
The use of HFNC has been shown to reduce the respiratory rate and the effort of breathing, while improving alveolar ventilation [36].
Several underlying mechanisms are hypothesized for the observed beneficial effects of HFNC in children, although they are not yet fully understood.
The main physiological mechanism responsible for improving respiratory support in HFNC therapy is the reduction in dead space [20,35,37].
HFNC provides a wash-out of the nasopharyngeal dead space, which is the volume of air localized at the level of the proximal third of the respiratory tract. In particular, the nasopharyngeal dead space does not participate in gas exchange during respiration. For the relatively large head size of infants and children, the total dead space is 50% greater than that of adults [38].
Several studies [37,38,39,40,41,42] showed that increasing the dead space wash-out resulted in an improvement in ventilation by favoring the elimination of CO2. In addition, the reduction in the prosthetic dead space facilitated the pulmonary gas exchange and decreased the ventilator pressure and volume requirements.
Liew et al. [43] carried out a prospective randomized crossover study with 44 preterm infants who received either HFNC or nasal CPAP. The wash-out of the nasopharyngeal dead space was evaluated by measuring the nasopharyngeal end-expiratory CO2 (pEECO2). The authors found that increasing the flow from 2 to 8 L/min led to a significant reduction in pEECO2 and decreased the respiratory rate; the reason for these results was probably because of the reduction in dead space ventilation. The mean nasal-CPAP pEECO2 was higher with CPAP compared with all flow rates of HFNC, although it was only significant at 6–8 L/min (p < 0.05).
The wash-out of the nasopharyngeal dead space determines a better gas exchange, and allows for reductions in the pressure and volume parameters of HFNC [25,41,44].
Several studies [25,45,46] also demonstrated that HFNC significantly reduced the inspiratory resistance at the upper airways, providing a flow of gas that matched or exceeded the inspiratory rate.
Saslow et al. [45] conducted a study that compared the effort of breathing in premature neonates who were supported with HFNC and nasal-CPAP. The authors showed that the effort of breathing with HFNC between 3 and 5 L/min was equivalent to that with nasal-CPAP at 6 cm H2O, although there was a lower esophageal pressure in children who were treated with HFNC instead of CPAP (1.32 ± 0.77 vs. 1.76 ± 1.46 cm H2O; p < 0.05). These data suggest that the use of HFNC may lead to a reduction in gastric distension, with a better tolerance in children.
Although PEEP cannot be measured or controlled as it can with nasal-CPAP because of air leakage around the nostrils and oral breathing, the generation of modest PEEP (2–6 cm H2O with a flow of 8–12 L/min) is also possible with HFNC therapy [9,17]. This “PEEP effect” assists the residual functional capacity, prevents collapse of the pharynx, and reduces the effort of breathing.
6. Adverse Side Effects and Contraindications
High-flow therapy is associated with several adverse events in the pediatric population, such as nasal irritation, epistaxis, and abdominal distension. The latter symptom can be improved with the placement of a nasogastric tube.
The most feared complication is barotrauma, with air-trapping, pneumothorax, and pneumomediastinum. Those complications seem to be associated with the use of inappropriately sized nasal cannulas [5]. A recent meta-analysis of 8 RCTs that included 2259 subjects (1100 assigned to HFNC, 980 to standard oxygen, and 179 to nasal-CPAP) found no difference in the incidence of air leak or pneumothorax among low-flow oxygen therapy, high-flow oxygen therapy, and CPAP groups [43]. Therefore, the occurrence of barotrauma may be related to underlying pathological conditions rather than to the therapy.
However, HFNC is considered less invasive, better tolerated, and associated with fewer complications than mechanical ventilation [44].
Severe hypoxia, which requires invasive ventilation, and hemodynamic instability, represent contraindications for HFNC use. In addition, HFNC is contraindicated where facial trauma or skull base fractures are the mechanical impediment. The presence of pneumothorax contraindicates the use of high flows, which would increase the air trapped in the subpleural space. Lastly, altered consciousness represents a contraindication, since the use of non-invasive ventilatory methods presupposes the presence of spontaneous breathing.
7. Comparison with Other Ventilation Techniques
The use of higher oxygen flows allow differentiation between HFNC and COT, which includes different devices, such as nasal cannulas, with the oxygen delivered at lower flows (at a rate of < 2 L/min in infants and < 6 L/min in children). Low flows also differ from HFNC because they do not require humidification to prevent the drying effect of unhumidified cold oxygen, and discomfort to the nasal mucosa.
A meta-analysis involving 2259 children with respiratory distress and mild hypoxemia caused by bronchiolitis or pneumonia, showed that HFNC significantly reduced the risk of treatment failure compared with COT [47]. More recently, 563 children aged 0–16 years with acute respiratory failure were randomized to high-flow or low-flow oxygen therapy (1:1 ratio). The treatment failure rate was lower in the children treated with high-flows than those treated with low-flow oxygen therapy (11.7% vs. 18.1%; odds ratio 0.62; 95% CI 0.38–1.00). Sixty percent of children who did not benefit from low-flow oxygen therapy successfully responded to high-flow oxygen therapy. No difference in PICU transfer rate nor in the length of hospital stay was observed [11].
Despite these promising findings, 67–87% of children with respiratory distress and hypoxemia respond to low-flow oxygen therapy [47]. Therefore, HFNC is not currently recommended as a first-line therapy in children with respiratory distress and mild hypoxemia, aside from its higher cost and complexity of use compared to low-flow oxygen therapy.
In a recent retrospective study involving 137 children between the ages of 1 month and 2 years, Habra et al. [48] observed a higher failure rate with HFNC compared to BiPAP or CPAP in children with bronchiolitis in PICUs (50.6% vs. 0% for CPAP vs. 8% for BiPAP, p < 0.01). Among those who failed in the HFNC group, 35 patients (90%) were shifted to another mode of noninvasive respiratory support that was therapeutically effective.
These findings suggested that HFNC may be considered as a bridging mode of ventilation between COT and CPAP, reducing the need for nasal-CPAP. However, there is a need for well-executed RCTs that compare HFNC with other modalities of non-invasive respiratory support for the treatment of severe bronchiolitis.
Table 4 describes the main characteristics and differences among COT, HFNC, and CPAP.
Table 4.
Comparison of the characteristics among standard oxygen therapy, high-flow nasal cannula, and continuous positive airway pressure.
| Standard Oxygen Therapy | High-Flow Nasal Cannula | Continuous Positive Airway Pressure | |
|---|---|---|---|
| Optimal gas conditioning | / | +++ | + |
| Generation of positive end-expiratory pressure | / | + | +++ |
| Wash-out of nasopharyngeal dead space | / | +++ | + |
| Improvement in mucociliary clearance | / | +++ | / |
| Flow and oxygen concentration setting | / | +++ | +++ |
| Reduced breathing effort | / | ++ | +++ |
| Reduction in upper airway resistance | / | + | +++ |
| Patient’s comfort | ++ | + | / |
/ no effect; + low effect; ++ medium effect; +++ high effect.
8. Clinical Predictive Scores
Several parameters could be evaluated to predict a good response to HFNC therapy. Patient age and a history of prematurity were not associated with treatment failure [45], although premature birth increases the risk of long-term lung disease [49].
Higher pCO2 values at baseline are associated with HFNC treatment failure. The respiratory rate is another pivotal parameter that is important to consider: non-responder patients were less tachypnoic at baseline, and their respiratory rates did not decrease significantly after therapy. Therefore, patients with hypercapnia and lower respiratory rates should be frequently monitored when beginning HFNC.
According to Mayfield et al. [50], the reductions in respiratory and heart rates by approximately 20% from baseline in the first 90 min of treatment could represent a predictive marker of treatment efficacy.
A score often used in clinical practice to establish the need for HFNC, as well as predict the therapeutic response to HFNC, is the Pediatric Early Warning System (PEWS). A retrospective study involving patients up to 17 years of age documented that a higher and worsening PEWS score 90 min after HFNC onset was predictive of treatment failure [47].
The rate-oxygenation index (ROXI) is the fraction of oxygen saturation, with FiO2 as the numerator and respiratory rate as the denominator. It was also a valid predictor of the need for invasive mechanical ventilation in patients receiving HFNC [51]. Yildizdas et al. [52] created an equivalent pediatric score using the respiratory rate z-score, called the pediatric rate-oxygenation index (p-ROXI). The authors concluded that ROXI and p-ROXI changes could predict treatment failure at 24 and 48 h after HFNC onset.
Lastly, a universally accepted score is not yet available, but would be useful to standardize clinical practice and reduce heterogeneity among studies.
In general, the responder patients present an improvement in respiratory and heart rates, and in respiratory effort within 60–90 min after beginning HFNC. Conversely, an increased oxygen requirement, stable or worsening respiratory and heart rates, or respiratory effort, presuppose the need for a step-up therapy [37].
9. Conclusions
In the literature, current evidence supports the use of HFNC mainly in infants with bronchiolitis. Over the last decade, HFNC therapy has reduced the need for non-invasive and invasive ventilation in these children. However, the cost-benefit assessment indicates that standard oxygen therapy still represents the first-line treatment option. It is reasonable to use HFNC as a second-line treatment in infants with bronchiolitis, using nasal-CPAP in case of HFNC failure.
The ease of use, safety, and availability of HFNC have led to its broader application in other clinical settings and conditions, such as for respiratory failure and distress. Despite its widespread use, shared guidelines are still lacking. The indications for the use of HFNC, the parameters settings, and the treatment response still depend on physician expertise. These factors lead to heterogeneous management in clinical practice and difficulties in comparing related studies. Standardization of HFNC management and the development of predictive scores to identify responder patients are needed as soon as possible.
Author Contributions
Writing, original draft preparation: A.V.; review, editing, supervision: P.D.F.; writing, review: C.S.; supervision: S.D.P.; supervision: F.C.; editing, supervision: M.A. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors disclose no personal or financial support or author involvement with organizations with financial interest in the subject. All authors approved the final version and its submission to the journal. The authors report no conflicts of interest in this study.
Funding Statement
This study received no external funding support.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pierce H.C., Mansbach J.M., Fisher E.S., Macias C.G., Pate B.M., Piedra P.A., Sullivan A.F., Espinola J.A., Camargo C.A. Variability of Intensive Care Management for Children with Bronchiolitis. Hosp. Pediatr. 2015;5:175–184. doi: 10.1542/hpeds.2014-0125. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura M. High-Flow Nasal Cannula Oxygen Therapy Devices. Respir. Care. 2019;64:735–742. doi: 10.4187/respcare.06718. [DOI] [PubMed] [Google Scholar]
- 3.Parke R., McGuinness S., Eccleston M. Nasal High-Flow Therapy Delivers Low Level Positive Airway Pressure. Br. J. Anaesth. 2009;103:886–890. doi: 10.1093/bja/aep280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sreenan C., Lemke R.P., Hudson-Mason A., Osiovich H. High-Flow Nasal Cannulae in the Management of Apnea of Prematurity: A Comparison with Conventional Nasal Continuous Positive Airway Pressure. Pediatrics. 2001;107:1081–1083. doi: 10.1542/peds.107.5.1081. [DOI] [PubMed] [Google Scholar]
- 5.Mikalsen I.B., Davis P., Øymar K. High Flow Nasal Cannula in Children: A Literature Review. Scand. J. Trauma. Resusc. Emerg. Med. 2016;24:93. doi: 10.1186/s13049-016-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura M. High-Flow Nasal Cannula Oxygen Therapy in Adults: Physiological Benefits, Indication, Clinical Benefits, and Adverse Effects. Respir. Care. 2016;61:529–541. doi: 10.4187/respcare.04577. [DOI] [PubMed] [Google Scholar]
- 7.D’Amato M., Molino A., Calabrese G., Cecchi L., Annesi-Maesano I., D’Amato G. The Impact of Cold on the Respiratory Tract and Its Consequences to Respiratory Health. Clin. Transl. Allergy. 2018;8:20. doi: 10.1186/s13601-018-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Ashry H.S., Modrykamien A.M. Humidification during Mechanical Ventilation in the Adult Patient. Biomed Res. Int. 2014;2014:715434. doi: 10.1155/2014/715434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fainardi V., Abelli L., Muscarà M., Pisi G., Principi N., Esposito S. Update on the Role of High-Flow Nasal Cannula in Infants with Bronchiolitis. Children. 2021;8:66. doi: 10.3390/children8020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu J.H., Azadeh N., Samhouri B., Yi E. Recent Advances in the Understanding of Bronchiolitis in Adults. F1000Research. 2020;9:568. doi: 10.12688/f1000research.21778.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin D., Shellshear D., Babl F.E., Hendrickson R., Williams A., Gibbons K., McEnery K., Kennedy M., Pham T.M.T., Acworth J., et al. High Flow in Children with Respiratory Failure: A Randomised Controlled Pilot Trial—A Paediatric Acute Respiratory Intervention Study. J. Paediatr. Child Health. 2021;57:273–281. doi: 10.1111/jpc.15259. [DOI] [PubMed] [Google Scholar]
- 12.Lin J., Zhang Y., Xiong L., Liu S., Gong C., Dai J. High-Flow Nasal Cannula Therapy for Children with Bronchiolitis: A Systematic Review and Meta-Analysis. Arch. Dis. Child. 2019;104:564–576. doi: 10.1136/archdischild-2018-315846. [DOI] [PubMed] [Google Scholar]
- 13.Dafydd C., Saunders B.J., Kotecha S.J., Edwards M.O. Efficacy and Safety of High Flow Nasal Oxygen for Children with Bronchiolitis: Systematic Review and Meta-Analysis. BMJ Open Respir. Res. 2021;8:e000844. doi: 10.1136/bmjresp-2020-000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kepreotes E., Whitehead B., Attia J., Oldmeadow C., Collison A., Searles A., Goddard B., Hilton J., Lee M., Mattes J. High-Flow Warm Humidified Oxygen versus Standard Low-Flow Nasal Cannula Oxygen for Moderate Bronchiolitis (HFWHO RCT): An Open, Phase 4, Randomised Controlled Trial. Lancet. 2017;389:930–939. doi: 10.1016/S0140-6736(17)30061-2. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar M., Sinha R., Roychowdhoury S., Mukhopadhyay S., Ghosh P., Dutta K., Ghosh S. Comparative Study between Noninvasive Continuous Positive Airway Pressure and Hot Humidified High-Flow Nasal Cannulae as a Mode of Respiratory Support in Infants with Acute Bronchiolitis in Pediatric Intensive Care Unit of a Tertiary Care Hospital. Indian J. Crit. Care Med. 2018;22:85–90. doi: 10.4103/ijccm.IJCCM_274_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vahlkvist S., Jürgensen L., la Cour A., Markoew S., Petersen T.H., Kofoed P.E. High Flow Nasal Cannula and Continuous Positive Airway Pressure Therapy in Treatment of Viral Bronchiolitis: A Randomized Clinical Trial. Eur. J. Pediatr. 2020;179:513–518. doi: 10.1007/s00431-019-03533-2. [DOI] [PubMed] [Google Scholar]
- 17.Milési C., Essouri S., Pouyau R., Liet J.M., Afanetti M., Portefaix A., Baleine J., Durand S., Combes C., Douillard A., et al. High Flow Nasal Cannula (HFNC) versus Nasal Continuous Positive Airway Pressure (NCPAP) for the Initial Respiratory Management of Acute Viral Bronchiolitis in Young Infants: A Multicenter Randomized Controlled Trial (TRAMONTANE Study) Intensive Care Med. 2017;43:209–216. doi: 10.1007/s00134-016-4617-8. [DOI] [PubMed] [Google Scholar]
- 18.Dharmage S.C., Perret J.L., Custovic A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019;7:246. doi: 10.3389/fped.2019.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basnet S., Mander G., Andoh J., Klaska H., Verhulst S., Koirala J. Safety, Efficacy, and Tolerability of Early Initiation of Noninvasive Positive Pressure Ventilation in Pediatric Patients Admitted with Status Asthmaticus: A Pilot Study. Pediatr. Crit. Care Med. 2012;13:393–398. doi: 10.1097/PCC.0b013e318238b07a. [DOI] [PubMed] [Google Scholar]
- 20.Baudin F., Buisson A., Vanel B., Massenavette B., Pouyau R., Javouhey E. Nasal High Flow in Management of Children with Status Asthmaticus: A Retrospective Observational Study. Ann. Intensive Care. 2017;7:55. doi: 10.1186/s13613-017-0278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González Martínez F., González Sánchez M.I., Toledo del Castillo B., Pérez Moreno J., Medina Muñoz M., Rodríguez Jiménez C., Rodríguez Fernández R. Treatment with High-Flow Oxygen Therapy in Asthma Exacerbations in a Paediatric Hospital Ward: Experience from 2012 to 2016. An. Pediatr. 2019;90:72–78. doi: 10.1016/j.anpedi.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Ballestero Y., De Pedro J., Portillo N., Martinez-Mugica O., Arana-Arri E., Benito J. Pilot Clinical Trial of High-Flow Oxygen Therapy in Children with Asthma in the Emergency Service. J. Pediatr. 2018;194:204–210.e3. doi: 10.1016/j.jpeds.2017.10.075. [DOI] [PubMed] [Google Scholar]
- 23.Duan X., Wei N., Wei J., Zhu Y., Kang Y., He Y., Huang J., Wang S. Effect of High-Flow Nasal Cannula Oxygen Therapy on Pediatric Patients With Congenital Heart Disease in Procedural Sedation: A Prospective, Randomized Trial. J. Cardiothorac. Vasc. Anesth. 2021;35:2913–2919. doi: 10.1053/j.jvca.2021.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Hong S.H., Choi J.H., Lee J. The Changes of Central Venous Pressure by Body Posture and Positive End-Expiratory Pressure. Korean J. Anesthesiol. 2009;57:723–728. doi: 10.4097/kjae.2009.57.6.723. [DOI] [PubMed] [Google Scholar]
- 25.Dysart K., Miller T.L., Wolfson M.R., Shaffer T.H. Research in High Flow Therapy: Mechanisms of Action. Respir. Med. 2009;103:1400–1405. doi: 10.1016/j.rmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Shioji N., Kanazawa T., Iwasaki T., Shimizu K., Suemori T., Kuroe Y., Morimatsu H. High-Flow Nasal Cannula versus Noninvasive Ventilation for Postextubation Acute Respiratory Failure after Pediatric Cardiac Surgery. Acta Med. Okayama. 2019;73:15–20. doi: 10.18926/AMO/56454. [DOI] [PubMed] [Google Scholar]
- 27.McGinley B., Halbower A., Schwartz A.R., Smith P.L., Patil S.P., Schneider H. Effect of a High-Flow Open Nasal Cannula System on Obstructive Sleep Apnea in Children. Pediatrics. 2009;124:179–188. doi: 10.1542/peds.2008-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ignatiuk D., Schaer B., McGinley B. High Flow Nasal Cannula Treatment for Obstructive Sleep Apnea in Infants and Young Children. Pediatr. Pulmonol. 2020;55:2791–2798. doi: 10.1002/ppul.25009. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins S., Huston S., Campbell K., Halbower A. High-Flow, Heated, Humidified Air via Nasal Cannula Treats CPAP-Intolerant Children with Obstructive Sleep Apnea. J. Clin. Sleep Med. 2017;13:981–989. doi: 10.5664/jcsm.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joseph L., Goldberg S., Shitrit M., Picard E. High-Flow Nasal Cannula Therapy for Obstructive Sleep Apnea in Children. J. Clin. Sleep Med. 2015;11:1007–1010. doi: 10.5664/jcsm.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain S., Williams D.J., Arnold S.R., Ampofo K., Bramley A.M., Reed C., Stockmann C., Anderson E.J., Grijalva C.G., Self W.H., et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Children. N. Engl. J. Med. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon J.W. High-Flow Nasal Cannula Oxygen Therapy in Children: A Clinical Review. Korean J. Pediatr. 2020;63:3–7. doi: 10.3345/kjp.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.33. Liu C., Cheng W.Y., Li J.S., Tang T., Tan P.L., Yang L. High-Flow Nasal Cannula vs. Continuous Positive Airway Pressure Therapy for the Treatment of Children <2 Years With Mild to Moderate Respiratory Failure Due to Pneumonia. Front. Pediatr. 2020;8:590906. doi: 10.3389/fped.2020.590906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiser R.K., Smith A.C., Ma B.B.K., Chen J.G. A Pediatric High-Flow Nasal Cannula Protocol Standardized Initial Flow and Expedites Weaning. Pediatr Pulmonol. 2021;56:1189–1197. doi: 10.1002/ppul.25214. [DOI] [PubMed] [Google Scholar]
- 35.Sayan İ., Altınay M., Çınar A.S., Türk H.Ş., Peker N., Şahin K., Coşkun N., Demir G.D. Impact of HFNC Application on Mortality and Intensive Care Length of Stay in Acute Respiratory Failure Secondary to COVID-19 Pneumonia. Hear. Lung. 2021;50:425–429. doi: 10.1016/j.hrtlng.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreel L., Proesmans M. High Flow Nasal Cannula as Respiratory Support in Treating Infant Bronchiolitis: A Systematic Review. Eur. J. Pediatr. 2020;179:711–718. doi: 10.1007/s00431-020-03637-0. [DOI] [PubMed] [Google Scholar]
- 37.Lodeserto F.J., Lettich T.M., Rezaie S.R. High-Flow Nasal Cannula: Mechanisms of Action and Adult and Pediatric Indications. Cureus. 2018;10:e3639. doi: 10.7759/cureus.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Möller W., Celik G., Feng S., Bartenstein P., Meyer G., Eickelberg O., Schmid O., Tatkov S. Nasal High Flow Clears Anatomical Dead Space in Upper Airway Models. J. Appl. Physiol. 2015;118:1525–1532. doi: 10.1152/japplphysiol.00934.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Subu A.M., Hagen S., Eldridge M., Boriosi J. Aerosol Therapy through High Flow Nasal Cannula in Pediatric Patients. Expert Rev. Respir. Med. 2017;11:945–953. doi: 10.1080/17476348.2017.1391095. [DOI] [PubMed] [Google Scholar]
- 40.Claure N., D’Ugard C., Bancalari E. Elimination of Ventilator Dead Space during Synchronized Ventilation in Premature Infants. J. Pediatr. 2003;143:315–320. doi: 10.1067/S0022-3476(03)00299-3. [DOI] [PubMed] [Google Scholar]
- 41.Dassieu G., Brochard L., Benani M., Avenel S., Danan C. Continuous Tracheal Gas Insufflation in Preterm Infants with Hyaline Membrane Disease: A Prospective Randomized Trial. Am. J. Respir. Crit. Care Med. 2000;162:826–831. doi: 10.1164/ajrccm.162.3.9910063. [DOI] [PubMed] [Google Scholar]
- 42.Danan C., Dassieu G., Janaud J.C., Brochard L. Efficacy of Dead-Space Washout in Mechanically Ventilated Premature Newborns. Am. J. Respir. Crit. Care Med. 1996;153:1571–1576. doi: 10.1164/ajrccm.153.5.8630604. [DOI] [PubMed] [Google Scholar]
- 43.Liew Z., Fenton A.C., Harigopal S., Gopalakaje S., Brodlie M., O’Brien C.J. Physiological Effects of High-Flow Nasal Cannula Therapy in Preterm Infants. Arch. Dis. Child. Fetal Neonatal Ed. 2020;105:87–93. doi: 10.1136/archdischild-2018-316773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nahum A. Animal and Lung Model Studies of Tracheal Gas Insufflation. Respir. Care. 2001;46:149–157. [PubMed] [Google Scholar]
- 45.Saslow J.G., Aghai Z.H., Nakhla T.A., Hart J.J., Lawrysh R., Stahl G.E., Pyon K.H. Work of Breathing Using High-Flow Nasal Cannula in Preterm Infants. J. Perinatol. 2006;26:476–480. doi: 10.1038/sj.jp.7211530. [DOI] [PubMed] [Google Scholar]
- 46.Shepard J.W., Burger C.D. Nasal and Oral Flow-Volume Loops in Normal Subjects and Patients with Obstructive Sleep Apnea. Am. Rev. Respir. Dis. 1990;142:1288–1293. doi: 10.1164/ajrccm/142.6_Pt_1.1288. [DOI] [PubMed] [Google Scholar]
- 47.Luo J., Duke T., Chisti M.J., Kepreotes E., Kalinowski V., Li J. Efficacy of High-Flow Nasal Cannula vs. Standard Oxygen Therapy or Nasal Continuous Positive Airway Pressure in Children with Respiratory Distress: A Meta-Analysis. J. Pediatr. 2019;215:199–208.e8. doi: 10.1016/j.jpeds.2019.07.059. [DOI] [PubMed] [Google Scholar]
- 48.Habra B., Janahi I.A., Dauleh H., Chandra P., Veten A. A Comparison between High-Flow Nasal Cannula and Noninvasive Ventilation in the Management of Infants and Young Children with Acute Bronchiolitis in the PICU. Pediatr. Pulmonol. 2020;55:455–461. doi: 10.1002/ppul.24553. [DOI] [PubMed] [Google Scholar]
- 49.Di Filippo P., Dodi G., Ciarelli F., Di Pillo S., Chiarelli F., Attanasi M. Lifelong Lung Sequelae of Prematurity. Int. J. Environ. Res. Public Health. 2022;19:5273. doi: 10.3390/ijerph19095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayfield S., Jauncey-Cooke J., Hough J.L., Schibler A., Gibbons K., Bogossian F. High-Flow Nasal Cannula Therapy for Respiratory Support in Children. Cochrane Database Syst. Rev. 2014;2014:CD009850. doi: 10.1002/14651858.CD009850.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myers L.C., Mark D., Ley B., Guarnieri M., Hofmeister M., Paulson S., Marelich G., Liu V.X. Validation of Respiratory Rate-Oxygenation Index in Patients With COVID-19–Related Respiratory Failure. Crit. Care Med. 2022;50:e638–e642. doi: 10.1097/CCM.0000000000005474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yildizdas D., Yontem A., Iplik G., Horoz O.O., Ekinci F. Predicting Nasal High-Flow Therapy Failure by Pediatric Respiratory Rate-Oxygenation Index and Pediatric Respiratory Rate-Oxygenation Index Variation in Children. Eur. J. Pediatr. 2021;180:1099–1106. doi: 10.1007/s00431-020-03847-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.