Abstract
Purpose
During the COVID-19 pandemic, many radiation oncology departments worldwide adopted the use of shorter and more intense hypofractionated regimens. Hospital foot traffic was reduced through virtual care. This study's primary objective was to assess the collective environmental effect of these strategic changes by identifying sources of carbon dioxide equivalents (CO2e). The rate of radiation-related adverse events from the increased use of hypofractionated treatments was assessed.
Methods and Materials
All patients treated with external beam radiation therapy from April 1, 2019, to March 31, 2021, at our single institution were identified (n = 10,175) along with their radiation therapy visits (176,423 fractions) and unplanned visits to the radiation nursing clinic or emergency department. Out-patient hospital and virtual visits (n = 75,853) during this same period were also analyzed. Environmental effect measures, including linear accelerator power usage, patient travel distances, and personal protection equipment consumption were all converted into CO2e.
Results
The use of curative hypofractionated regimens increased from 17% to 27% during the pandemic year. Carbon footprint was reduced by 39% during the pandemic year (1,332,388 kg CO2e) compared with the prepandemic year (2,024,823 kg CO2e). Comparing patients in the prepandemic versus pandemic year, there was a significant reduction in the proportion of hypofractionated patients who needed a visit to either the radiation nursing clinic (39% vs 25%; P < .001) or emergency department (6% vs 2%; P < .001) during and within 90 days of radiation therapy.
Conclusions
This is the first study to demonstrate the environmental benefits of increased use of hypofractionated regimens and virtual care, while assuring that there was no added acute radiation-related adverse event. Our findings support their continued use as one of many long-term strategies to reduce the environmental footprint of health care delivery.
Introduction
In March 2020, the coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization.1 Health care institutions immediately developed strategies to divert their resources and capacities toward treating patients affected by COVID-19 and reducing virus transmission to patients and staff. Many efforts had been made to “flatten the curve” through various risk mitigation and protection measures, such as wearing face masks, physical distancing, and hand sanitation.2, 3, 4, 5
Within radiation oncology, a proposition was made by Filippi et al to adopt hypofractionated radiation therapy regimens, which consisted of shorter and more intense treatments, to maintain cancer patient care, while reducing foot traffic to hospitals.6, 7, 8 In addition to hypofractionation, many radiation oncology departments also rapidly incorporated the use of virtual care via telephone or videoconferencing to mitigate risks associated with the COVID-19 pandemic.9
The COVID-19 pandemic had revealed opportunities to the health care sector to contribute to reducing the environmental effect through clinical practice.10 , 11 There was an increasing awareness of climate change and its effect on global health, including the development of cancer and cancer care delivery.12, 13, 14 The climate crisis is a global phenomenon, which describes the pressing issues of climate change and global warming. When referring to global warming and greenhouse gas emissions, carbon dioxide equivalent (CO2e) is recognized by the United Nations Framework Convention on Climate Change as the common metric to describe the amount of pure CO2 that would have had an equivalent global warming effect.15
As governments aimed to reduce greenhouse emissions, health care practices need to be examined as the health care industry represents 4% to 5% of the annual carbon footprint.16 The primary objective of this study was to assess the environmental effect of our single radiation oncology department's collective strategic changes implemented during the pandemic to reduce foot traffic to the hospital by identifying sources of CO2e from a health care and patient perspective. Specifically, the main changes that were implemented at our department were an increased use of (1) hypofractionated regimens, (2) virtual patient care, and (3) personal protection equipment during the COVID-19 pandemic. The secondary objective was to assess the rate of patients developing radiation therapy-related serious adverse events (AEs) or toxicities due to the increased use of hypofractionated radiation treatment regimens. With radiation therapy used in 40% to 50% of the annually estimated 19 million new cancer patients worldwide, this real-world evaluation could provide important insights on how to reduce our collective carbon output on a global level.17
Methods and Materials
Study design and data sources
This was a quality improvement study whereby patient records were retrieved from our institutional electronic patient record systems EPR (QuadraMed, TX) and MOSAIQ (Elekta, Stockholm, Sweden) were retrospectively analyzed. In total, 40,134 patients from out-patient visits and 11,912 patients who initiated radiation therapy from April 1, 2019, to March 31, 2021, were identified. Patients who initiated radiation therapy were allocated into 2 cohorts based on their first treatment date: 2019 to 2020 fiscal year (FY) (April 1, 2019, to March 31, 2020) and the 2020 to 2021 FY (April 1, 2020, to March 31, 2021), which represented the prepandemic and pandemic periods, respectively. This timeframe was chosen because it aligned with our institution's FY reporting and coincided with the COVID-19 pandemic timeline in Canada.
Patients were included if they received a radiation treatment using a linear accelerator (LINAC). Patients (n = 1737) treated with nuclear sources (GammaKnife, brachytherapy, or radionuclide) were excluded as their treatments were not associated with the use of photons generated from electricity (Supplementary Materials Figure E1). All patient ages were included for the analysis. A waiver of individual patient consent was granted for this quality improvement study by our institution's Research Ethics Board (REB) or Institutional Review Board. We did not obtain REB exemption or consent in collecting personal information from health care workers and were unable to account for CO2e from health care worker travels and information technology usage.
Patient travel
Postal codes of patients’ residences were retrieved to calculate the travel distance from their home to the Princess Margaret Cancer Centre. Google Maps (Google, Mountain View, CA), an online mapping site, was used to compute travel distances by taking the shortest route for each patient visit. A custom Google Sheets script was developed in-house to automatically process and generate these travel distances from Google Maps. Public transit modeling in the Toronto area was conducted based on public transport ridership data from Google COVID-19 Community Mobility Reports.18 Approximately 26% of the general Toronto population usually took public transportation daily for their travel.19 During the COVID-19 pandemic, however, there was on average, a 54% reduction in public transit ridership in Toronto, which aligned with our study's 2020 to 2021 FY data.18
Multiple assumptions were made for this study: only passenger vehicle distances were computed, tolls and highways were not avoided for the distance calculation. Patient travel was directly from home to hospital and back, one fraction represented one unique hospital visit (patients with multiple treatments in 1 day were accounted for), and the travel distance was under normal driving conditions. Furthermore, 257 patients stayed at our institution's short-term lodge facility during their treatment, and one round trip to the hospital was therefore counted for the entire duration of their treatment. Additionally, patients with a postal code outside of Ontario were excluded from our analysis (n = 44).
Travel distances were calculated for patient consult appointments, computed tomography (CT)–simulation appointments, treatments, follow-up appointments, and any additional visits to the radiation nursing clinic (RNC) or emergency room. CO2e emissions for patient travel were calculated by taking the distance generated and multiplying it by a CO2 emissions conversion factor, which was the average of the 3 most common types of passenger vehicles: Toyota Corolla (0.165 kg CO2e), Honda Civic (0.165 kg CO2e), and Hyundai Elantra (0.157 kg CO2e).20
LINAC power usage
The monitor machine units (MU) and start time of each fraction that was delivered to each patient were retrieved from MOSAIQ. Voltage and current measurements were performed for each LINAC, then converted to total power used per monitor unit delivered radiation (KJ per MU). The total power consumed by the LINAC varied by the beam energy (MV) used for the radiation treatment and treatment start time. The kilojoule per MU was calculated by using an apparent power factor of 0.9 for the LINAC as provided by the manufacturers (Varian, Siemens Healthineers, Germany; and Elekta, Sweden). CO2e from electricity generation in Ontario was retrieved from GridWatch Ontario (Energy Insight, Ottawa) and Ontario's Independent Electricity Systems Operator. The power consumed by the LINAC for each fraction was converted into CO2e based on the hourly average intensity (CO2e/kWh) of electricity in Ontario (Supplementary Materials Appendix E1).
Personal protective equipment
The total amount of personal protective equipment ordered in each FY was retrieved from the department's general ledger, and the units ordered were compared between the 2 FYs. During the pandemic year, every patient and accompanying caregiver were distributed surgical masks at each visit. To determine the environmental effect, the units of PPE ordered were converted into CO2e. The following PPE were included for analysis: bouffant cap, disinfectant wipes, face shields, gloves, disposal gowns, surgical masks and N95 respirators, safety glasses, and shoe covers. PPE CO2e estimates based on life cycle assessments were retrieved from a study conducted by Rizan et al in the United Kingdom and from other relevant studies.21, 22, 23, 24
Treatment intent and regimens
Treatment regimens were categorized into hypofractionation curative, conventional curative, hypofractionation palliative, conventional palliative, or stereotactic body radiation therapy. A treatment regimen was considered hypofractionated if the dose per fraction was ≥240 cGy. If the dose per fraction was ≥600 cGy and the total dose of the treatment regimen was > 2000 cGy, then the treatment course was classified as stereotactic body radiation therapy. A treatment course in radiation therapy consisted of 1 to 35 fractions of radiation, generally with one fraction delivered once per day.
Radiation-related AEs
The RNC was a drop-in clinic (8 AM-6 PM) that helps patients manage the side effects of their radiation treatment. RNC was staffed with nurses that were trained to assess and care for radiation-related side effects in conjunction with the patients’ primary radiation oncologists. At the RNC, patients were either triaged to the emergency room (ER), treated within the RNC and discharged home or admitted to the hospital. The clinic could be used by patients who were currently undergoing radiation therapy or were within 4 weeks of completing treatment. Planned RNC visits, which consisted of patient education visits occurring before RT were not counted as AEs. Patients who had unplanned visits to the RNC or the ER within our hospital institution were identified. These patients were assumed to have developed AEs from radiation therapy, while those presenting to the ER were considered as severe AEs. Patients who visited the RNC within 4 weeks of their last treatment or visited the ER within 90 days of finishing treatment were all included in the analysis.
Statistical analysis
Toxicity evaluations were conducted by comparing the 2 FYs using Pearson's χ2 tests for the proportion of patients who required a RNC or ER visit for each treatment regimen. A Bonferroni correction was applied to account for multiple testing since 5 treatment regimens were assessed; thus, a P value of ≤ .01 was deemed to be statistically significant. The statistical analyses were conducted on Microsoft Excel (Microsoft Corporation, Redmond, WA).
Results
Characteristics of patients treated with radiation therapy between 2019 to 2021
Data from 10,175 patients were included in the LINAC utilization analysis, which consisted of 14,145 treatment courses (176,423 fractions) delivered during the 2 FYs. Table 1 displays the characteristics of the 2 study cohorts categorized by FY and the number of courses delivered by treatment intent. Breast, lung, and genitourinary cancers were the most common sites treated at our institution. The number of treatment course for each cancer histology and treatment intent by FY is displayed in Supplementary Materials Figure E2. Separate analyses were conducted for any out-patients receiving consultation or follow-up appointments at our institution within the same timeframe (75,853 visits). The total number of in-person and virtual consultations and follow-up appointments recorded for this analysis is outlined in Supplementary Materials Figure E3.
Table 1.
Summary of cohort characteristics
| 2019-2020 FY | 2020-2021 FY | P value* | |
|---|---|---|---|
| Demographics | |||
| Age (median [range]), y | 63.6 [1.6-101.9] | 64.5 [1.8-99.3] | .23 |
| Sex (female:male) | 52:48 | 50:50 | |
| Number of patients per treatment course (%) | <.0001 | ||
| Conventional curative | 2175 (41.0%) | 1452 (29.8%) | |
| Hypofractionation curative | 969 (18.3%) | 1,236 (25.4%) | |
| Conventional palliative | 106 (2.0%) | 123 (2.5%) | |
| Hypofractionation palliative | 1552 (29.3%) | 1488 (30.5%) | |
| Stereotactic body radiation therapy | 496 (9.4%) | 578 (11.9%) | |
| Total number of patients treated | 5298 | 4877 | |
| Patient travel | |||
| Median distance traveled from PM (km [range]) | 24.2 [0.5-2686.5] | 24.2 [0.5-1911.8] | .32 |
| Treatment courses (number of treatment courses) | <.0001 | ||
| Conventional curative | 2992 (41%) | 1763 (26%) | |
| Hypofractionation curative | 1275 (17%) | 1834 (27%) | |
| Conventional palliative | 142 (2%) | 152 (2%) | |
| Hypofractionation palliative | 2377 (32%) | 2401 (35%) | |
| Stereotactic body radiation therapy | 558 (8%) | 651 (10%) | |
| Total fractions delivered | 99,391 | 77,032 | |
Abbreviation: FY = fiscal year.
P values: t test for parametric data; χ2 test for nonparametric data.
Radiation therapy treatment related patient travel
Our radiation oncology department was the largest in Canada and provided service to a wide geographic area within Ontario, although most of the patients come from densely populated regions of Toronto and the Greater Toronto Area (Supplementary Materials Figure E4). The total round-trip distance traveled by patients for all types of treatment and hospital visits was approximately 7,416,480km and 5,917,941 km in 2019 to 2020 FY and 2020 to 2021 FY, respectively (Table 2 ). Distances traveled by patients for CT imaging, radiation treatment appointments, as well as RNC and ER visits in 2019 to 2020 and 2020 to 2021 FYs were listed in Table 2. Traveled distances were converted into CO2e. Overall, a decrease in 20% of CO2e from patient transportation alone was observed between the 2020 to 2021 FY and 2019 to 2020 FY; translating to a reduction in approximately 243,263 kg of CO2e. Additionally, total round-trip distances and CO2e were calculated for patients receiving in-person consultation or follow-up appointment in our radiation oncology department (Supplementary Materials Table E1).
Table 2.
Patient travel distances and CO2e for CT simulation and radiation treatment appointments and RNC and ER visits
| Round trip (km) |
CO2e (kg) |
|||||
|---|---|---|---|---|---|---|
| Visit type | 2019-2020 | 2020-2021 | % Change | 2019-2020 | 2020-2021 | Difference |
| CT simulation | 595,961.3 | 562,036.6 | –5.7% | 96,744.4 | 91,237.3 | –5,507.1 |
| Hypofractionation curative | 775,515.9 | 1,584,868.6 | 104.4% | 125,892.1 | 257277.0 | 131,384.9 |
| Conventional curative | 4,696,167.1 | 1,173,711.8 | –75.0% | 762,344.5 | 190,532.6 | –571,811.9 |
| Hypofractionation palliative | 749,039.6 | 1,647,618.7 | 120.0% | 121,594.1 | 267,463.4 | 145,869.3 |
| Conventional palliative | 170,776.9 | 74,960.3 | –56.1% | 27,722.8 | 12,168.6 | –15,554.2 |
| SBRT | 319,038.2 | 786,930.1 | 146.7% | 51,790.5 | 127,745.0 | 75,954.5 |
| RNC visit* | 85,912.5 | 65,108.3 | –24.2% | 13,946.5 | 10,569.3 | –3,377.2 |
| ER | 24,068.3 | 22,706·4 | –5.7% | 3,907.1 | 3,686.0 | –221·1 |
| Total | 7,416,479.9 | 5,917,940.9 | –20% | 1,203,942.0 | 960,679.1 | –243,262.8 |
Abbreviations: CT = computed tomography; ER = emergency room; FY = fiscal year; RNC = radiation nursing clinic; SBRT = stereotactic body radiation therapy.
Occurred beyond the duration of a patient's radiation therapy treatment.
Travel distances in Table 2 and Supplementary Materials Table E1 were calculated without accounting for the use of public transportation by patients. To estimate the effect of public transit, several models based on public transport ridership within the Toronto region were made. If no public transportation was taken by patients in both FYs, the total CO2e reduction would be 750,346 kg. Based on normal public transportation ridership level and reduced ridership during COVID-19, the estimated the CO2e saved from patient travels to be 630,389 to 795,873 kg. However, if the fraction of Toronto patients who used public transport was similar to the typical proportion of ridership within the Toronto region (26% typical public transit ridership), the total CO2e saved was 733,496 kg.
LINAC power usage
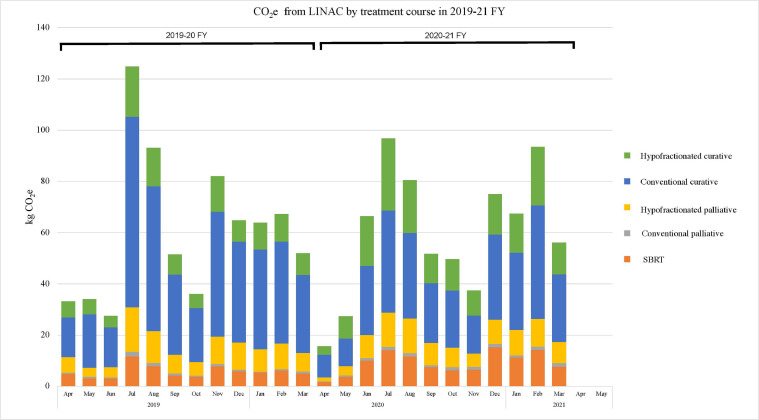
In accordance with an increased proportion of patients treated with hypofractionation, 22,359 fractions less (22.5% decrease) were delivered (Table 1) in the 2020 to 2021 FY. Despite a reduction in the fractions delivered and 421 less patients (7.9% decrease) treated in 2020 to 2021 FY, the reduction in LINAC CO2e emission comparing both FYs was minor. In total, there was a decrease of 12 kg CO2e from all treatments, a 2% decrease compared with the prior year. The total CO2e emitted by electricity generation to power the LINACs was 729.66 kg and 717.30 kg CO2e in the respective 2019 to 2020 and 2020 to 2021 FY (Fig. 1 ). The average CO2e emissions per radiation treatment course range from 0.053-0.178 kg CO2e per treatment.
Fig. 1.
Total carbon dioxide equivalents (CO2e) from power generated for linear accelerators (LINACs). The CO2e expenditures were divided by treatment course intent and month during the 2019 to 2020 and 2020 to 2021 fiscal years (FYs). There was a sustained increase in the use of hypofractionated regimens in the curative setting during the second year. Abbreviation: SBRT = stereotactic body radiation therapy.
Personal protective equipment usage
Compared with 2019 to 2020 FY, 72,651 more units of surgical masks and respirators, as well as 163,020 more units of gloves were purchased in 2020 to 2021 FY (Supplementary Materials Table E2). Furthermore, 14,650 units of face shields were purchased for use during 2020 to 2021 FY compared with none in the previous year. Based on published reports of CO2e from life-cycle assessments of various PPE, PPE utilization in 2020 to 2021 FY resulted in an additional 6716.6 kg of CO2e produced compared with pre–COVID-19 times.21, 22, 23, 24 The aforementioned values likely represented an underestimate of the total CO2e generated from PPE usage as there were equipment (eg, Bouffant caps, disinfectant wipes, shoe covers) for which life-cycle assessments CO2e contributions were unaccounted for due to the lack of information in the current literature.
Virtual patient visits
Before the COVID-19 pandemic, virtual care was an uncommon practice. During 2020 to 2021 FY, the department quickly adopted various virtual care modalities, including the use of videoconferencing (Supplementary Materials Figure E5) platforms and phone calls for patient care and internal communication. Comparing the 2 FYs, there was an 88.5% increase in virtual consult visits, and a 93.1% increase in virtual follow-ups conducted in 2020 to 2021 FY (Supplementary Materials Figure E3).
Radiation-related AEs
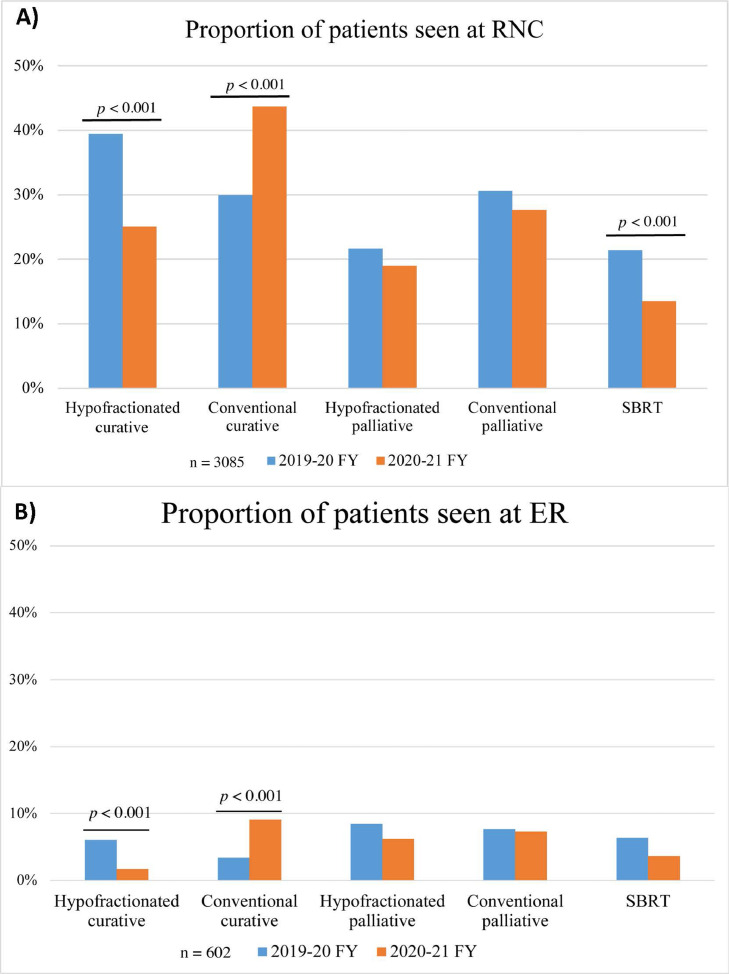
As hypofractionation represents a slight intensification of each radiation therapy fraction, the acute AEs, represented by unplanned visits to the RNC and ER, were assessed. Comparing patients receiving a curative treatment intent in the 2019 to 2020 versus 2020 to 2021 FY, there was a significant decrease in the proportion of hypofractionated patients who needed RNC (39% vs 25%; P < .001; Fig. 2 a) or ER (6% vs 2%; P < .001; Fig. 2b) visits during and within 90 days of radiation therapy completion. Additionally, there were no differences in the rate of patients treated palliatively with hypofractionation (RNC: P = .068, ER: P = .016) or conventional fractionation (RNC: P = .62, ER: P = .92) who required unplanned RNC or ER visits between the 2 FYs (Fig. 2a-b). This suggests that there was no added risk in short-term radiation related AEs or toxicities for patients receiving a hypofractionated radiation, compared with patients receiving a conventional fractionation regimen.
Fig. 2.
Acute adverse events during and after radiation therapy. Proportion of patients treated with radiation therapy during the 2019 to 2020 fiscal year (FY) and 2020 to 2021 FY and seen at the (1) radiation nursing clinic (RNC) and (2) emergency room (ER). Only unplanned visits during and within 90 days of radiation therapy were captured. Abbreviation: SBRT = stereotactic body radiation therapy.
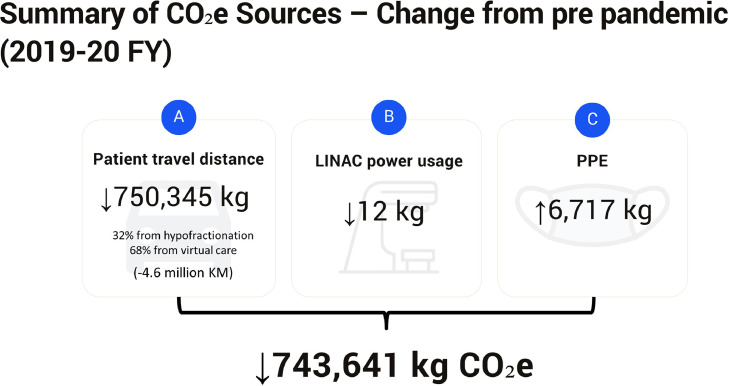
To summarize, the changes in hypofractionation radiation regimens, with the consequent reduction in radiation therapy visits, combined with the rapid switch from in-person to virtual care, even accounting for the slight increase in LINAC power usage, and PPE, translated into a net saving of 743,641 kg CO2e (Fig. 3 ). The CO2e emission from the accounted sources was 1,956,175 kg CO2e in 2019 to 2020 FY and 1,212,534 kg CO2e in 2020 to 2021 FY, representing a 39% reduction in the CO2e emission. The CO2e saving was equivalent to the CO2e sequestered by approximately 12,000 seedling trees planted and grown for 10 years25 or the CO2e from the annual energy consumption of 182 Canadian households.26
Fig. 3.
Summary of the changes in emission of carbon dioxide equivalents (CO2e) from various operational aspects in the department of radiation oncology during the 2019 to 2020 and 2020 to 2021 fiscal years (FYs). CO2e was saved from reduced patient travels (A) due to hypofractionation (32%) and virtual care (68%). These estimates were made assuming that no public transportation was taken by the patients. Linear accelerator (LINAC) power usage (B) and its corresponding CO2e emission was slightly reduced during 2020 to 2021. Conversely, increased personal protective equipment (PPE) (C) usage contributed to the department's CO2e emission. The net reduction in CO2e emission during the pandemic year was 743,641 kg.
Discussion
This was the first study to evaluate the environmental effect of a global shift in how patients were managed in radiation oncology in response to the pandemic. We took advantage of a unique situation to examine the real-world environmental effect of this rapid clinical practice adaptation. We obtained a large amount of data from the year before and during COVID-19 from a large tertiary care institution, allowing us to collect rarer serious AEs to characterize these practice changes. With the ongoing pandemic, patients treated and cared through these new modalities provided an initial glimpse of the real-world effectiveness, safety, and environmental effect of hypofractionated radiation therapy and virtual care. With over 10,000 patients treated, and 170,000 fractions evaluated, we were able to estimate the carbon footprint from changes made within the department's clinical operations.
A summary of the change in various environmental metrics assessed within our study was shown in Figure 3. Owing to the increased usage of hypofractionation during the pandemic, less fractions were delivered in 2020 to 2021 FY. Although this shift to hypofractionated treatments had minimal effect on CO2e from the LINAC power usage, reduction in the number of in-person visits decreased patient travel by 4.6 million km in 2020 to 2021 FY, which equated to 750,346 kg CO2e. From a clinical perspective, our results indicated that hypofractionation had not led to an increase in radiation-related AEs during and within 90 days of radiation therapy completion. Comparing the 2 FYs, patients treated with curative hypofractionation in fact required less RNC or ER visits. This reduction in AE was consistent with findings from the FAST-Forward trial in which patients receiving hypofractionated breast (26-27 Gy in 5 fractions) treatments developed less grade 3 and above toxicities than patients who received 40 Gy in 15 fractions.27 Conversely, we observed more AEs in patients receiving conventional fractionation regimen in 2020 to 2021 FY, which might be from the selection of potentially more advanced or complicated cases to be treated using conventional regimens. Due to the population nature of the present study, individual patient and disease characteristics were not assessed to compare cohorts developing more or less AEs.
Increased PPE usage during the pandemic year costed an additional 6717 kg of CO2e from its manufacturing and downstream lifecycle. This still led to a net reduction in carbon footprint of our radiation oncology department with 743,641 kg of CO2e saved during the pandemic year. In modern day scenarios, this reduction in CO2e would be equivalent to 4.5 million km of car travel, brewing 3.56 million cups of coffee, or on a greater scale, filling the Washington Monument 660 times or the entire CN Tower with CO2.
The present study had several limitations. Due to the population nature of this study, individual patterns of care and traveling could not be assessed. For acute side effects, we could only document patients who checked into the RNC or ER within our institution. We did not assess the reasons and diagnoses of patients who presented to the RNC or ER within 3 months of RT. As retrospective analyses of AEs were often inaccurate, we attributed any unplanned visits to the RNC and ER within 3 months of RT as RT-related AEs. Given the large geographic catchment area of our patient population, those with acute symptoms might well have chosen to be assessed at a local emergency or drop-in clinic, for which we could not account. Moreover, patients preferring to travel shorter distances to local ERs might also have increased during the pandemic in comparison to pre–COVID-19 times. Finally, many patients may have opted to avoid in-person medical visits during the pandemic in fear of COVID-19 transmission, which might have attributed to the reduced the number of RNC and ER visits observed during the pandemic year. As the long-term consequences of radiation therapy could develop many years after the treatments were completed, this study could not capture the long-term complication rates. Similarly, recurrences after radiation therapy typically might occur many years later, this study was unable to assess and compare efficacy. Finally, the current evaluation was based on a tertiary academic institution located in an urban environment in Canada and 48% to 49% of patients lived within the city's transit system. As Canadian nonacademic radiation oncology department are typically located in nonurban areas, patient travels for treatments and visits at nonurban location may emit more greenhouse gas than our estimates. Indeed, the external validity of this study's result was dependent on the distances and mean of transportation used by patients to visit their respective radiation oncology department, which was responsible for 99.95% of the CO2e.
Our study was also unable to evaluate the effect of WFH in CO2e emission as our REB exemption did not include the collection of personal information from health care workers, which were necessary to derive CO2e from commuting and end-user device energy consumptions. The influence of WFH data usage on greenhouse emissions remains unclear as there is a paucity of literature on this topic. Recent studies from the International Energy Agency and Malmodin suggest that the power consumption from increased data usage is minimal at data centers and networks, and mostly tied to the power consumption from end users devices.28 , 29 As summarized by the International Energy Agency and Malmodin, despite a 38% to 50% increase in data traffic observed in major networks during the COVID-19 pandemic, the energy consumption of major network operators increased by <1%.29 These recent findings provided the framework to further investigate the effect of virtual care to deliver CO2e-efficient care.
Conclusions
Our study demonstrated the environmental benefits of shifting a radiation oncology department to increased hypofractionated dose regimens and virtual care, while supporting that there was no added harm in health consequence and acute radiation-related severe AE. Our findings provided insights that could inform radiation oncology departments on how to establish long-term strategies to minimize the environmental footprint of their clinical operations. We hope these data and methodologies will provide a framework for other clinical departments and hospitals interested in conducting similar environmental assessments to support climate action within the health care sector.
Acknowledgments
The authors thank Wendy Issa, Julie Wenz, Veng Chhin, Colin Robertson, Lucy Lu, Nancy Zhang, Wei Zhou, David Lu, Christine Hill, Jeniqua Gribben, and UHN Digital for their support throughout the study.
Footnotes
Disclosures: none.
Data sharing statement: Deidentified data from the patients analyzed are available. Data will be available beginning 3 months and ending 5 years after article publication to researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. Proposals should be directed to philip.wong@rmp.uhn.ca; to gain access, data requestors will need to sign a data access agreement.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ijrobp.2022.04.054.
Appendix. Supplementary materials
References
- 1.World Health Organization (WHO). WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Available at:https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19–11-march-2020. Accessed November 24, 2021.
- 2.World Health Organization (WHO). Coronavirus disease (COVID-19) advice for the public: When and how to use masks. Available at:https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks. Accessed November 24, 2021.
- 3.Fuentes R, Galeotti M, Lanza A, et al. COVID-19 and climate change: A tale of two global problems. Sustainability. 2020;12:8560. [Google Scholar]
- 4.International Energy Agency (IEA). COVID-19: Exploring the impacts of the Covid-19 pandemic on global energy markets, energy resilience, and climate change. 2021. Available at: https://www.iea.org/topics/covid-19. Accessed November 24, 2021.
- 5.Mofijur M, Fattah IMR, Alam MA, et al. Impact of covid-19 on the social, economic, environmental and energy domains: Lessons learnt from a global pandemic. Sustain Prod Consum. 2021;26:343–359. doi: 10.1016/j.spc.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filippi AR, Russi E, Magrini SM, et al. Letter from Italy: First practical indications for radiation therapy departments during COVID-19 outbreak. Int J Radiat Oncol Biol Phys. 2020;107:597–599. doi: 10.1016/j.ijrobp.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jereczek-Fossa BA, Pepa M, Marvaso G, et al. COVID-19 outbreak and cancer radiotherapy disruption in Italy: Survey endorsed by the italian association of radiotherapy and clinical oncology (airo) Radiother Oncol. 2020;149:89–93. doi: 10.1016/j.radonc.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Care Ontario. COVID-19 supplemental clinical guidance for patients with cancer: Cancer care ontario. Available at:https://www.ontariohealth.ca/sites/ontariohealth/files/2020-04/Ontario%20Health%20Cancer%20Care%20Ontario%20COVID-19%20Supplemental%20Clinical%20Guidance%20for%20Patients%20with%20Cancer_29Mar20%20PDF.pdf. Accessed November 30, 2021.
- 9.Knutson NC, Kavanaugh JA, Li HH, et al. Radiation oncology physics coverage during the COVID-19 pandemic: Successes and lessons learned. J Appl Clin Med Phys. 2021;22:4–7. doi: 10.1002/acm2.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salas RN, Maibach E, Pencheon D, et al. A pathway to net zero emissions for healthcare. BMJ. 2020;371:m3785. doi: 10.1136/bmj.m3785. [DOI] [PubMed] [Google Scholar]
- 11.Shankar HM, Ewart G, Garcia E, et al. COVID-19, climate change, and the american thoracic society. A shared responsibility. Ann Am Thorac. 2020;17:1052–1055. doi: 10.1513/AnnalsATS.202002-180VP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie E, Howard C, Buchman S, et al. Acting on climate change for a healthier future: Critical role for primary care in canada. Can Fam Physician. 2021;67:725–730. doi: 10.46747/cfp.6710725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiatt RA, Beyeler N. Cancer and climate change. Lancet Oncol. 2020;21:e519–e527. doi: 10.1016/S1470-2045(20)30448-4. [DOI] [PubMed] [Google Scholar]
- 14.Watts N, Amann M, Arnell N, et al. The 2020 report of the lancet countdown on health and climate change: Responding to converging crises. Lancet. 2021;397:129–170. doi: 10.1016/S0140-6736(20)32290-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United Nations Framework Convention on climate change. Common metrics. 2021. Available at: https://unfccc.int/process-and-meetings/transparency-and-reporting/methods-for-climate-change-transparency/common-metrics. Accessed November 24, 2021.
- 16.Eckelman MJ, Sherman JD, MacNeill AJ. Life cycle environmental emissions and health damages from the Canadian healthcare system: An economic-environmental-epidemiological analysis. PLoS Med. Jul 2018;15 doi: 10.1371/journal.pmed.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 18.Google COVID-19 community mobility reports. Available at: https://www.google.com/covid19/mobility/. Accessed November 24, 2021.
- 19.Savage K. Results from the 2016 census: Commuting within Canada's largest cities. Statistics Canada; 2019 https://www150.statcan.gc.ca/n1/pub/75-006-x/2019001/article/00008-eng.htm Available at: Accessed November 24, 2021. [Google Scholar]
- 20.Canada Go. Fuel consumption ratings. Available at:https://open.canada.ca/data/en/dataset/98f1a129-f628-4ce4-b24d-6f16bf24dd64. Accessed November 30, 2021.
- 21.Klemeš JJ, Fan YV, Jiang P. The energy and environmental footprints of COVID-19 fighting measures: PPE, disinfection, supply chains. Energy (Oxf) 2020;211 doi: 10.1016/j.energy.2020.118701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizan C, Reed M, Bhutta MF. Environmental impact of personal protective equipment distributed for use by health and social care services in england in the first six months of the COVID-19 pandemic. J R Soc Med. 2021;114:250–263. doi: 10.1177/01410768211001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmutz M, Hischier R, Batt T, et al. Cotton and surgical masks—what ecological factors are relevant for their sustainability? Sustainability. 2020;12:10245. [Google Scholar]
- 24.Lee AWL, Neo ERK, Khoo Z-Y, et al. Life cycle assessment of single-use surgical and embedded filtration layer (efl) reusable face mask. Resources, Conservation and Recycling. 2021;170 doi: 10.1016/j.resconrec.2021.105580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States Environmental Protection Agency. Greenhouse gas equivalencies calculator, 2021. Available at: https://www.epa.gov/energy/greenhouse-gas-equivalencies-calculator. Accessed November 30, 2021.
- 26.Statistics Canada. Canadian system of environmental–economic accounts: Energy use and greenhouse gas emissions, 2019. Available at:https://www150.statcan.gc.ca/n1/daily-quotidien/211213/dq211213c-eng.htm?indid=12921-4&indgeo=0. Accessed November 30, 2021.
- 27.Brunt AM, Wheatley D, Yarnold J, et al. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK fast-forward trial. Radiother Oncol. 2016;120:114–118. doi: 10.1016/j.radonc.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Energy Agency (IEA). Data centres and data transmission networks, 2021. Available at: https://www.iea.org/reports/data-centres-and-data-transmission-networks. Accessed November 24, 2021.
- 29.Malmodin J. Paper presented at: Electronics Goes Green 2020 Conference; November 5, 2020. The Royal Swedish Academy of Engineering Sciences; Berlin, Germany: 2020. The power consumption of mobile and fixed network data services: The case of streaming video and downloading large files. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.