Abstract
A combined cell culture enzyme-linked immunosorbent assay (CCC-ELISA) was developed for measuring the neutralizing antipoliovirus antibodies in human sera. The binding of different concentrations of each of the three poliovirus types to BGM cells in the presence and absence of a constant dilution from each test and reference serum was measured in the CCC-ELISA. The titers of the viruses neutralized by each serum were measured with the titration curves and used for interpretation of neutralizing titers to the three poliovirus types. Analysis of human sera revealed that the sensitivity and specificity of the CCC-ELISA and the microneutralization assay were comparable. The CCC-ELISA is nonsubjective, rapid, and highly reproducible. Furthermore, the CCC-ELISA could potentially be used as a seroepidemiologic tool for assessment of the humoral response to the cell culture infectious viruses.
Poliovirus is a picornavirus of the Enterovirus genus that infects susceptible cells through a specific receptor which binds the virus and changes its conformation (1). Sera from subjects infected or vaccinated with poliovirus contain antibodies to different functional and structural proteins of the virions (4). Polioviruses can be divided into three types on the basis of their neutralization reactions with specific immune sera. Four main antigenic sites have been identified in each of the three poliovirus types (14). Three major neutralization antigenic sites have been mapped to three major polypeptides (VP1, VP2, and VP3) composing the virion surface (11, 15, 17). Detection of the neutralizing antiviral antibodies forms the basis for evaluation of protection. Neutralization of the poliovirus by the antiviral antibodies is believed to involve different mechanisms which block one or more of the viral functions of attachment to cells, penetration, and uncoating (2, 3, 16) or to occur by postadsorption neutralization (13, 18).
In vitro virus neutralization is the main standard assay for the detection and measurement of the neutralizing poliovirus antibodies. Although the polioviruses induce clear visible cytopathic effects (CPE) in susceptible cell cultures, the interpretation of neutralizing titers is influenced by the subjective visual scoring of CPE and the long time required for low virus titers to develop CPE. For these reasons, a number of assays have been described and evaluated as alternatives to the standard assay.
Different nonfunctional assays which avoid the use of cell cultures and living viruses have also been described (5, 6, 7, 9, 10). Although these nonfunctional assays are relatively rapid and less tedious than virus neutralization, they have not replaced neutralization assays for the measurement of poliovirus antibodies. This is mainly because of the low degree of correlation between functional and nonfunctional tests at low antibody concentrations (19, 21), in addition to the fact that nonfunctional tests measure all or some of the neutralizing antibodies, as well as other types of antibody molecules, and require specific monoclonal antibodies. Another sensitive procedure which avoids the visual scoring of CPE but requires special equipment and radioactive isotopes was also described previously (12).
In this article, a relatively rapid and nonsubjective assay for the detection of neutralizing antiviral antibodies is described. The assay was based on establishing an enzyme-linked immunosorbent assay (ELISA) for measuring cell-associated viruses in the presence and absence of a neutralizing antiserum. The assay has been named the combined cell culture (CCC)-ELISA. Also, an evaluation of the CCC-ELISA for the assessment of humoral responses to poliovirus is presented.
MATERIALS AND METHODS
Virus strains.
The poliovirus strains were Sabin types 1, 2, and 3 grown in BGM cell cultures. Virus harvests in tissue culture medium 199 (TCM) were cleared from cell debris by centrifugation for 15 min at 104 × g. The supernatants were distributed into small aliquots and stored at −70°C.
Antisera.
For CCC-ELISA, human serum samples positive for polio (positive human sera [PHS]) were obtained from a previous study for detection of neutralizing antibodies. Fifty human sera were obtained from infants between the ages of 1 and 2 years admitted to local hospitals for reasons other than infectious diseases. The sera were stored frozen at −20°C and heat inactivated before use. Informed consent to use the serum for the study was obtained from each individual or his or her guardians.
Cell cultures.
BGM cells, a continuous cell line of African monkey kidney origin from the American Type Culture Collection, were used for poliovirus propagation and titration. After trypsinization, the BGM cells were suspended in Dulbecco's modified Eagle's medium supplemented with 10% inactivated fetal bovine serum (FBS). Thereafter, 100-μl volumes of BGM cell suspension (105 cells per ml) were dispensed in 96-well tissue culture plates and incubated at 37°C in a CO2 incubator. After overnight incubation, the formed monolayers were washed with TCM and used for performing the titrations in the microneutralization assay (NT) and CCC-ELISA. TCM supplemented with either 1% FBS (TCM-1%) or 5% FBS (TCM-5%) was used as a maintenance medium for cell-virus cultures. TCM-1% was used as a dilution buffer for the sera and viruses.
NT.
The NT was performed according to a standard assay (20), with minor modifications. Test sera were used to prepare twofold dilutions in 96-well transfer plates (Dynatech Laboratories), and a 25-μl volume of each dilution was added to 3 wells. A 25-μl volume containing 75 to 100 50% tissue culture infectious doses (TCID50) of virus was added to each well, and the antigen-antibody mixtures were incubated at 37°C for 2 h. Thereafter, the mixtures were inoculated onto preformed monolayers of BGM cells in a culture plate, and 100 μl of TCM-5% was added to each. The cultures were incubated for 7 days at 37°C in a CO2 incubator and observed periodically for the development of CPE. Titers were determined from serum dilutions in triplicate by the Spearman-Karber procedure (8).
CCC-ELISA.
Serial 10-fold dilutions of each poliovirus type were prepared in transfer plates. A 100-μl volume of each virus dilution was mixed with either 100 μl of a constant serum dilution (neutralization plot) or 100 μl of TCM-1% (virus control plot). Each serum was mixed with an equal volume of TCM-1% for use as a control (serum control plot). The reaction mixtures were incubated overnight at 4°C, and 50 μl of each reaction mixture was transferred to three wells of preformed BGM monolayers. TCM-1% was added to the cell-virus cultures (100 μl/well), and the cultures were incubated overnight at 37°C in a CO2 incubator.
For detection of the cell-associated viruses, the cultures were washed with phosphate-buffered saline (pH 7.4) (PBS) and incubated with the appropriate indicator antibody against the reference serum for the poliovirus type (100 μl/well) for 2 h at 4°C. The cells were washed with PBS, and goat anti-human polyvalent immunoglobulin (G, A, and M) conjugated with peroxidase (Sigma Chemicals, St. Louis, Mo.) was added (100 μl/well) at a dilution of 1:1,000. Plates were incubated for 1 h at 4°C and washed with PBS. The enzyme substrate (0.04% O-phenylenediamine HCl–12% H2O2 in citrate buffer [pH 5] was added (200 μl/well) and incubated for 1 h at 37°C. Thereafter, 150 μl of the supernatant from each well was transferred to an ELISA plate preloaded with 2 M H2SO4 (50 μl/well), and the absorbance was measured at 490 nm.
The titration curves were established by plotting the log of the virus dilutions versus the ELISA optical densities (OD) using a polynomial fitting. The log of the virus dilutions at the intercepts of the serum control plot with the virus neutralization plot and the virus control plot were taken as the endpoints. The difference between the endpoints for a virus titration in the presence and absence of a serum was designated ΔV. The virus neutralized by a constant dilution of a serum was the antilog of ΔV. The neutralization titers were interpreted as relative titers by parallel titration of a reference serum.
Statistical analysis.
Simple regression analysis was used to compute the correlation coefficient (r) between the neutralization titers determined by the NT and the CCC-ELISA (Apple computer software). A probability value of <0.05 was considered significant.
RESULTS
Establishment of the CCC-ELISA.
Ten PHS were screened for antipoliovirus antibodies in the standard NT. Sera with high neutralizing titers were chosen as reference antisera RHS1, RHS2, and RHS3 for serotypes 1, 2, and 3, respectively (Table 1). RHS1 and RHS2 were polyspecific, whereas RHS3 was type specific.
TABLE 1.
Neutralizing titers of the selected reference sera against poliovirus
| Antiserum | NT titer of serum against:
|
||
|---|---|---|---|
| Serotype 1 | Serotype 2 | Serotype 3 | |
| RHS1 | 7,227 | 452 | 56 |
| RHS2 | 891 | 779 | 226 |
| RHS3 | 0 | 0 | 903 |
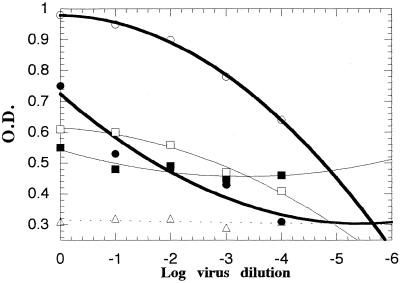
During the process of developing and optimizing the assay conditions, the influence of several factors on the titration curves, and consequently on the CCC-ELISA results, was investigated. The cell-associated viruses were better recognized by the corresponding reference serum, and lower OD values were obtained when a pool of the reference antisera was used for the 24-h time point (Fig. 1). The neutralization plots seemed to be of sigmoid shape, so fitting of the linear part of the plot is theoretically the most appropriate method for analyzing the data. Because it is practically difficult to identify the linear part of the neutralization curve, the smooth and polynomial fitting modes were tried for fitting of the titration curves. The polynomial fitting was the most practical under the experimental conditions used, and the mean value of r for fitting 10 titration curves was 0.98, with a standard deviation of <0.01. The titration curves established for cell-associated viruses at 90 min and 24 h were of different shapes (Fig. 1). The slope of the titration curves at the endpoints increases by increasing the period of cell-virus incubation, which increases the sensitivity in measuring the endpoints for virus titration. However, a decrease in the stability of the cultures was noticed with prolonged incubations and resulted in a less clear endpoint.
FIG. 1.
Recognition of cell-associated virus at different time points from the start of incubation of poliovirus type 2 with BGM cells, using two indicator antibodies. Values were plotted at 90 min (□) and 24 h (○) using RHS2 and at 90 min (■) and 24 h (●) using a pool of the reference sera. The RHS2 control plot was established at 24 h (Δ).
In the standard CCC-ELISA, a constant dilution of the reference serum possessing a titer of 4 to 10 in the NT was used for establishing the virus neutralization curve and as the indicator antibody. Serial (10-fold) dilutions of each poliovirus type initially containing ∼104 to 109 TCID50 were enough to perform the titration when overnight incubation periods were used for measuring the cell-associated virus. The dilutions recommended by the manufacturer for using the horseradish peroxidase-anti-human immunoglobulin conjugate in the conventional ELISA (1/1,000 or 1/2,000) were found to be appropriate for the CCC-ELISA.
Specificity, reproducibility, and sensitivity of the CCC-ELISA.
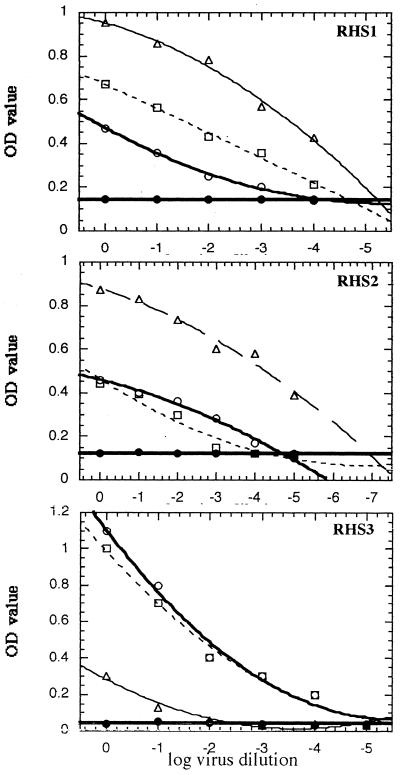
To check the specificity of the CCC-ELISA, the neutralization curves for comparable levels of the three poliovirus types were established with the monospecific reference serum RHS3 (Fig. 2). The neutralization curves demonstrated that the titers of poliovirus types 1 and 2 were close, whereas the titer of type 3 was much lower. Similar results were obtained with the polyspecific antisera RHS1 and RHS2, with the ranking of the neutralizing antibody titers to the three viruses and the observed magnitude of neutralization being exactly the same.
FIG. 2.
Neutralization of poliovirus types 1 (○), 2 (□), and 3 (Δ) with RHS1 (1/1,000), RHS2 (1/100), and RHS3 (1/100) in the equilibrated serum-virus mixtures and as indicator antibodies. The initial concentrations of polioviruses used were ≈104 TCID50 for RHS1 and RHS3 and ≈106 TCID50 for RHS2. A serum control plot was established in the absence of virus (●).
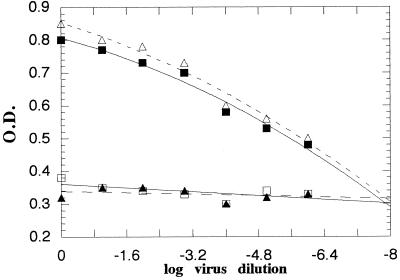
The reproducibility of virus and serum control plots in repeat experiments was checked with a constant set of reagents (Fig. 3). The variation in the endpoints for five repeat experiments was negligible, and the estimated virus titer in five repeated experiments was 107.87 ± 0.025 CCC-ELISA units (mean ± standard deviation), indicating that the plots were highly reproducible.
FIG. 3.
The established virus and serum control plots with poliovirus type 2 and the corresponding RHS2 in two separate experiments. Δ, virus control, experiment 1; ▴, serum control, experiment 1; ■, virus control, experiment 2; □, serum control, experiment 2.
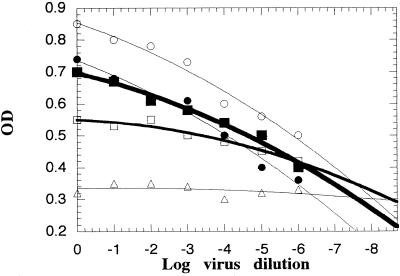
The established neutralization and virus control curves with serum were parallel over a wide range of serum dilutions possessing a titer of 100.6 to 103 in the NT. Neutralization curves of different shapes were obtained with the different test sera (Fig. 4) and when different sera were used as indicator antibodies. The background for the serum control was concentration dependent, and a lower background was obtained with the more diluted sera. However, the magnitude of ΔV decreases by increasing the serum dilution, which decreases the accuracy and sensitivity of the measurements. Furthermore, false negative predictive values were obtained at high serum dilutions. Practically, constant CCC-ELISA neutralization titers were obtained with different indicator antibodies when the test and the reference sera were titrated in parallel in repeated experiments. The mean relative titer ± the standard deviation for a serum (positive serum in Fig. 4) in five repeated experiments was 0.324 ± 0.015. Levels of neutralizing antibodies to any of the three serotypes equivalent to a titer as low as 0.2 in the NT were detected under the stated experimental conditions. Detection of lower levels of neutralizing antibodies was not attempted.
FIG. 4.
Typical titration curves for detection of neutralizing antibodies according to the standard CCC-ELISA. The virus control (○) and neutralization plots were established with poliovirus type 1 (initial titer of ≈109 TCID50) and RHS1. The values of ΔV for the reference (●), negative (□), and positive (■) sera at a dilution of 1:1,000 were 1.71, −0.55, and 0.5, respectively. A serum control plot was established in the absence of virus (Δ).
Comparison of the two assays.
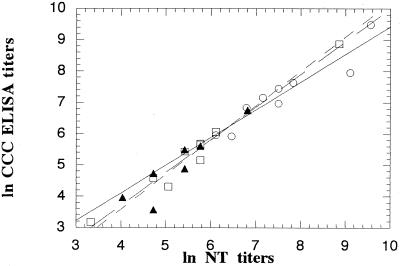
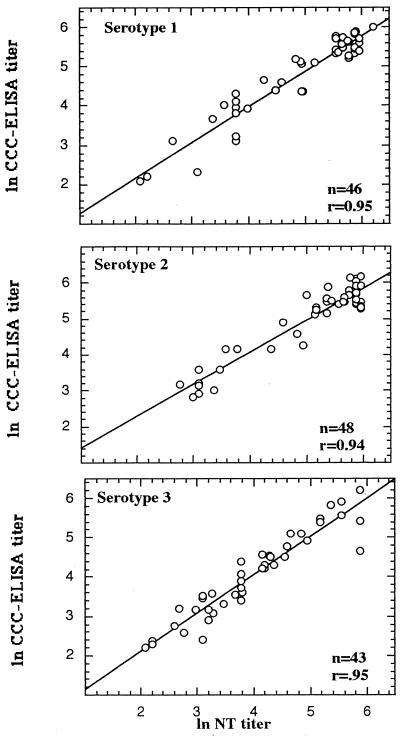
The specificity of the assay was further investigated by comparison of the poliovirus type-specific antibodies obtained by the NT and the CCC-ELISA. The data for PHS (Fig. 5) and infants (Fig. 6) are summarized in Table 2. The data revealed a high correlation for each of the three serotypes over the entire range of antibody concentrations (P < 0.01) for all the comparisons. The data for the sera negative in the NT were excluded when the coefficient of correlation was calculated.
FIG. 5.
Correlation between neutralization titers for poliovirus types 1 (○), 2 (□), and 3 (▴) obtained with standard NT and CCC-ELISA for 10 PHS.
FIG. 6.
Correlation between neutralization titers for poliovirus types 1, 2, and 3 obtained with standard NT and CCC-ELISA for 50 infant sera.
TABLE 2.
Correlation of the CCC-ELISA and NT titers for human sera
| Source | No. of sera | Correlation coefficient for:
|
||
|---|---|---|---|---|
| Serotype 1 | Serotype 2 | Serotype 3 | ||
| PHS | 10 | 0.94 | 0.98 | 0.92 |
| Infants | 50 | 0.95 | 0.94 | 0.95 |
Analyses of 10 PHS revealed that the numbers of sera recognized as positive by both the NT and the CCC-ELISA were 9 of 10, 9 of 10, and 7 of 10 for serotypes 1, 2, and 3, respectively. One serum was negative for serotypes 1 and 2. Two sera of negative predictive value in the serotype 3 NT gave positive signals in the CCC-ELISA.
Analyses of the infant sera revealed that the numbers of sera recognized as positive by both the NT and the CCC-ELISA were 46 of 50, 46 of 50, and 46 of 50 for serotypes 1, 2, and 3, respectively. Three sera of negative predictive value in the serotype 3 NT gave positive signals in the CCC-ELISA.
DISCUSSION
A new procedure for the detection of neutralizing poliovirus antibodies is described. In the first step, equilibrated mixtures containing constant amounts of a test serum and various poliovirus concentrations were prepared. In the second step, quantitative parameters for the infectivity of the equilibrated mixtures in BGM cell cultures were measured in a modified ELISA. The virus titers in the presence and absence of sera were depicted for the determined parameters and used to interpret the neutralized virus quantities. Comparison of the neutralized viruses by reference and test sera allowed accurate determination of the levels of neutralizing antibodies. This new type of neutralization assay differs from the NT in two main aspects. First, virus neutralization was measured at a constant concentration of serum. Second, the CCC-ELISA results are based on a nonsubjective estimation of the residual cytopathic virus.
Therefore, several factors which could potentially influence the sensitivity and reproducibility of the CCC-ELISA neutralization titers, including the cell-virus incubation period, the ELISA setup, and the fitting procedure, were investigated.
Although the cell-associated virus could be measured as early as 90 min after the start of the cell-virus incubation, incubation for 24 h allowed for the detection of low virus concentrations. In addition, it seems that some of the cell-associated viruses were not able to replicate, as described previously (13, 18). The cell-associated poliovirus could be well recognized by the heterotypic antibodies, as demonstrated by neutralization profiles for the three poliovirus types by a monospecific serum in the serotype 3 CCC-ELISA. Since human sera contained different formulas of antibodies to the three-poliovirus types, both the serotype-specific and the heterotypic antibodies could influence each virus neutralization profile. In agreement with this observation, parallel titration curves were obtained when dilutions of a single serum were titrated. A reproducible titer was interpreted from the different neutralization profiles developed by different indicator antibodies for a virus titration. However, the assumption that the heterotypic antibodies do not affect the amount of the neutralized virus was not strictly tested.
One could simply state that the working neutralization mechanisms in both the standard NT and the CCC-ELISA are mainly the same. However, because the endpoint is depicted in a plot for reaction mixtures containing excess virus, it is speculated that some of the proposed interactions (2, 16) are more favorable for each of the assays. Although the amount of neutralized virus in each of the equilibrated mixtures is constant, its determination is always influenced by the setup of the probing assay. Therefore, to avoid such variations, a reference serum was always titrated in parallel with the test sera (19).
Under the conditions used in our experiments, the reproducibility of the CCC-ELISA titers of the neutralizing antibodies was excellent both within and between assays. The use of precisely known virus titers and the reproducible determination of the virus titers were not needed. The use of different sets of reagents did not alter the interpreted neutralization titers. Nine serial dilutions over a range of ∼1 to 108 TCID50 were usually enough for performing the titration of a wide range of neutralizing antibodies.
Analysis of 10 PHS and 50 sera from infants indicated a good correlation between the neutralization titers obtained by the CCC-ELISA and those obtained by the NT. The estimates of seropositive samples for serotypes 1 and 2 were the same. Some sera with predictive negative signals for serotype 3 in the NT gave positive signals in the CCC-ELISA. Three of the PHS and four of the sera from infants were negative by the NT. Some of these negative sera gave positive signals in the serotype 3 CCC-ELISA. Because of the extensive heterotypic cross-reactivity among the three types of poliovirus, the effect of the cross-reactive antibodies on the CCC-ELISA neutralization titers needs to be further investigated with type-specific antipoliovirus antisera. Also, the interference of antibodies to other enteroviruses has to be investigated.
In principle, the CCC-ELISA can be used to measure neutralizing antibodies to a wide range of viruses and is particularly valuable when the viruses do not produce visible CPE. Furthermore, since the proposed assay allowed for the detection of the antibody-neutralized viruses in their equilibrated mixtures, it could be applied to the determination of the avidity of neutralizing monoclonal antibodies when they are used instead of polyvalent antisera.
In conclusion, the newly developed CCC-ELISA is nonsubjective, highly sensitive, and reproducible when used for the detection of neutralizing antibodies to poliovirus. The CCC-ELISA uses NT reagents, an ELISA reader, and a commercially available conjugate. In addition, analysis of the ELISA readings could be automated using software. However, for evaluation of the CCC-ELISA as an alternative for the standard NT in epidemiologic studies, an investigation on a large number of sera from vaccinated humans is essential.
REFERENCES
- 1.Arita M, Koike S, Aoki J, Horie H, Nomoto A. Interaction of poliovirus with its purified receptor and conformational alteration in the virion. J Virol. 1998;72:3578–3586. doi: 10.1128/jvi.72.5.3578-3586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brioen P, Rombaut B, Boeye A. Hit-and-run neutralization of poliovirus. J Gen Virol. 1985;66:2495–2499. doi: 10.1099/0022-1317-66-11-2495. [DOI] [PubMed] [Google Scholar]
- 3.Delaet I, Vrijsen R, Boeye A. Antigenic N to H conversion of poliovirus by a monoclonal antibody at low ionic strength. Virology. 1992;188:93–101. doi: 10.1016/0042-6822(92)90738-b. [DOI] [PubMed] [Google Scholar]
- 4.Edevåg G, Wahren B, Osterhaus A D M E, Sundqvist V-A, Granström M. Enzyme-linked immunosorbent assay-based inhibition test for neutralizing antibodies to polioviruses as an alternative to the neutralization test in tissue culture. J Clin Microbiol. 1995;33:2927–2930. doi: 10.1128/jcm.33.11.2927-2930.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrenfeld E, Brown D, Jia X Y, Summers D F. Antibodies against viral nonstructural proteins in response to infection with poliovirus. J Infect Dis. 1995;171:845–850. doi: 10.1093/infdis/171.4.845. [DOI] [PubMed] [Google Scholar]
- 6.Gary H E, Jr, Freeman C, Penaranda S, Maher K, Anderson L, Pallansch M A. Comparison of a monoclonal antibody-based IgM capture ELISA with a neutralization assay for assessing response to trivalent oral poliovirus vaccine. J Infect Dis. 1997;175:264–267. doi: 10.1093/infdis/175.supplement_1.s264. [DOI] [PubMed] [Google Scholar]
- 7.Hashido M, Horie H, Abe S, Doi Y, Hashizume S, Agboatwalla M, Isomura S, Nishio O, Hagiwara A, Inouye S. Evaluation of an enzyme-linked immunosorbent assay based on binding inhibition for type-specific quantification of poliovirus neutralization relevant antibodies. Microbiol Immunol. 1999;43:73–77. doi: 10.1111/j.1348-0421.1999.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 8.Hawkes R A. General principle underlying laboratory diagnosis of viral infection. In: Lennette E H, editor. Diagnostic procedures for viral, rickettsial and chlamydial infections. 7th ed. Washington, D.C.: American Public Health Association Publications; 1995. pp. 33–35. [Google Scholar]
- 9.Herremans M M P T, van Loon A M, Reimerink J H J, Rümke H C, van der Avoort H G A M, Kimman T G, Koopmans M P G. Poliovirus-specific immunoglobulin A in persons vaccinated with inactivated poliovirus vaccine in The Netherlands. Clin Diagn Lab Immunol. 1997;4:499–503. doi: 10.1128/cdli.4.5.499-503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herremans M M P T, Reimerink J H J, Ras A, Van Der Avoort H G A M, Kimman T G, Van Loon A M, Conyn-Van Spaendonck M A E, Koopmans M P G. Evaluation of a poliovirus-binding inhibition assay as an alternative to the virus neutralization test. Clin Diagn Lab Immunol. 1997;4:659–664. doi: 10.1128/cdli.4.6.659-664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogle J M, Chow M, Filman D J. Three-dimensional structure of poliovirus at 2.9 Å resolution. Science. 1985;229:1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- 12.Hovi T, Roivainen M. Radiometric cytolysis inhibition assay, a new rapid test for neutralizing antibodies to intact and trypsin-cleaved poliovirus. J Clin Microbiol. 1989;27:709–715. doi: 10.1128/jcm.27.4.709-715.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandel B. The interaction of neutralized poliovirus with HeLa cells. I. Adsorption. II. Elution, penetration, uncoating. Virology. 1967;31:238–259. doi: 10.1016/0042-6822(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 14.Minor P D. Antigenic structure of picornaviruses. Curr Top Microbiol Immunol. 1990;161:121–154. doi: 10.1007/978-3-642-75602-3_5. [DOI] [PubMed] [Google Scholar]
- 15.Minor P D, Ferguson M, Evans D M, Almond J W, Icenogle J P. Antigenic structure of polioviruses of serotypes 1, 2 and 3. J Gen Virol. 1986;67:1283–1291. doi: 10.1099/0022-1317-67-7-1283. [DOI] [PubMed] [Google Scholar]
- 16.Thomas A A M, Vrijsen R, Boeyé A. Relationship between poliovirus neutralization and aggregation. J Virol. 1986;59:479–485. doi: 10.1128/jvi.59.2.479-485.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vrijsen R, Mosser A, Boeyé A. Postadsorption neutralization of poliovirus. J Virol. 1993;67:3126–3133. doi: 10.1128/jvi.67.6.3126-3133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiegers K J, Wetz K, Dernick R. Molecular basis for linkage of a continuous and discontinuous neutralization epitope on the structural polypeptide VP2 of poliovirus type 1. J Virol. 1990;64:1283–1289. doi: 10.1128/jvi.64.3.1283-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Expanded Program on Immunization and Division of Communicable Diseases. Geneva, Switzerland: World Health Organization; 1992. Manual for the virological investigation of poliomyelitis, p. C-1211. [Google Scholar]
- 20.World Health Organization. Standard procedures for determining immunity to poliovirus using the micro-neutralization test. WHO/EPI/GEN 93.9. Geneva, Switzerland: World Health Organization; 1993. [Google Scholar]
- 21.World Health Organization. Expanded program on immunization. Certification of poliomyelitis eradication. Am Wkly Epidemiol Rec. 1994;69:293–295. [PubMed] [Google Scholar]