Abstract
We evaluated the hypothesis that phosphodiesterase-5 inhibitors, including sildenafil and tadalafil, may be associated with reduced incidence of Alzheimer’s disease and related dementia using a patient-level cohort study of Medicare claims and cell culture-based phenotypic assays. We compared incidence of Alzheimer’s disease and related dementia after phosphodiesterase-5 inhibitor initiation versus endothelin receptor antagonist initiation among patients with pulmonary hypertension after controlling for 76 confounding variables through propensity score matching. Across four separate analytic approaches designed to address specific types of biases including informative censoring, reverse causality, and outcome misclassification, we observed no evidence for a reduced risk of Alzheimer’s disease and related dementia with phosphodiesterase-5 inhibitors;hazard ratio (95% confidence interval): 0.99 (0.69–1.43), 1.00 (0.71–1.42), 0.67 (0.43–1.06), and 1.15 (0.57–2.34). We also did not observe evidence that sildenafil ameliorated molecular abnormalities relevant to Alzheimer’s disease in most cell culture-based phenotypic assays. These results do not provide support to the hypothesis that phosphodiesterase-5 inhibitors are promising repurposing candidates for Alzheimer’s disease and related dementia.
Keywords: PDE5 inhibitors, Alzheimer’s disease, dementia, cohort study, repurposing
Desai et al. report no association between initiation of phosphodiesterase-5 inhibitors and risk of dementia in a rigorous pharmacoepidemiologic analysis using robust design principles including restriction by the same underlying indication of pulmonary hypertension and an equivalent comparator group of endothelin receptor antagonists to address measured and unmeasured confounding.
See Newby (https://doi.org/10.1093/braincomms/fcac260) for a scientific commentary on this article.
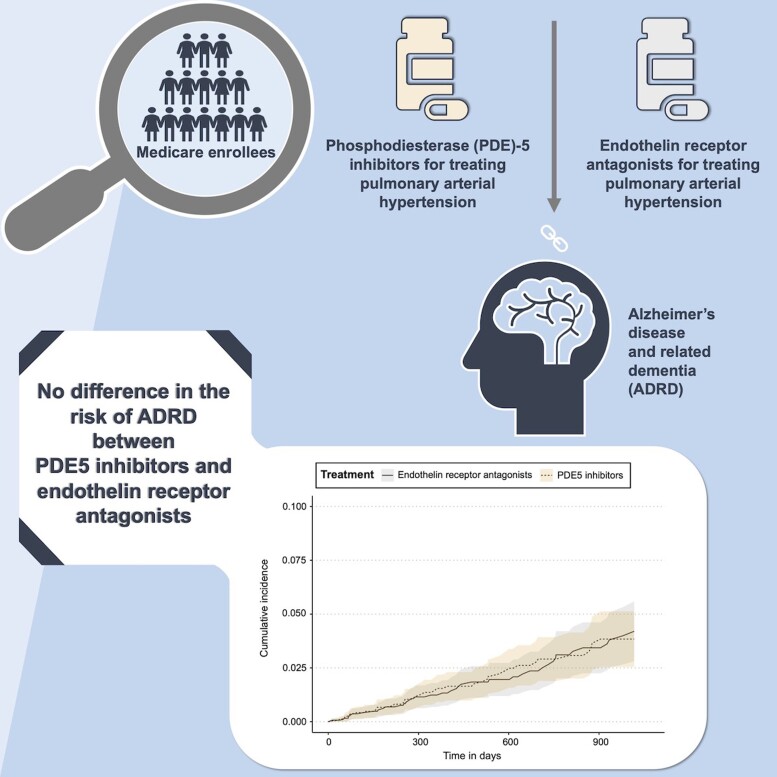
Graphical Abstract
Graphical abstract.
See Newby (https://doi.org/10.1093/braincomms/fcac260) for a scientific commentary on this article.
Introduction
Traditional drug discovery approaches using experimental animal models that recapitulate pathological features of Alzheimer’s disease and related dementia (ADRD) have met with limited success.1–3 These approaches have relied mainly on inhibiting the formation of or removal of Aβ plaques in the brain.4 Alternative treatment approaches include inhibition of tau aggregation5 and attenuating neuroinflammation.6
In recent years, there has been significant interest in using computational biology-based approaches to identify novel drug repurposing candidates for ADRD.7–10 These include the use of network proximity analyses,7 machine learning9 and unbiased analyses of large clinical data sets.8 In the Drug Repurposing for Effective Alzheimer’s Medicines (DREAMs) initiative,10 we have generated testable hypotheses based on multi-omics phenotyping of Alzheimer’s disease. We first identify genetic regulators of abnormal metabolic pathways associated with ADRD neuropathology. In contrast to unbiased computational approaches, these abnormal metabolic pathways are a priori hypothesized to be linked to dysregulation in brain glycolysis and are broadly defined as the Alzheimer’s Disease Aberrant Metabolism (ADAM) network10 that includes plausible candidate drug targets in ADRD. Second, we identify existing FDA-approved medications that act upon these targets, nominating them as repurposing candidates. Consistent with an open science approach in these studies, our prior publication describing the design and rationale of the DREAM study includes a list of all candidate ADRD treatments that we propose to evaluate.10 To test these hypotheses, we then conduct rigorous patient-level pharmacoepidemiologic analyses using population-based healthcare data collected during routine care to evaluate the association between exposure to candidate drugs and incident ADRD. In a recent publication arising from the DREAM study, we have tested drugs targeting the JAK/STAT signalling pathway as candidate ADRD treatments.11 In a prior publication, we have used a similar approach to test bile acid signalling as a an ADRD drug target.12 In order to explore molecular mechanisms modulated by our candidate Alzheimer’s disease drugs, we additionally assess them in cell culture-based phenotypic assays that provide read-outs of diverse molecular abnormalities relevant to Alzheimer’s disease.13 Recent studies have used multi-omics analyses incorporating metabolomic, transcriptomic and proteomic data in human tissue samples to identify structural variants14 and pathway alterations in Alzheimer’s disease.15 Our approach is unique in the linking of multi-omics analyses in human brain tissue to rigorous pharmacoepidemiologic analyses and experimental models in a drug discovery ‘pipeline’ to discover plausible drug repurposing candidates for ADRD. Additionally, we prespecify all our design choices and hypotheses in our prior publication10 to safeguard against publication bias.16 Within the recently proposed ADAM network, we hypothesized that the ATP-dependent efflux transporter, ABCC4, may be an ADRD drug target.10 Phosphodiesterase (PDE)-5 inhibitors have been shown to inhibit ABCC4.17,18 Additionally, prior evidence mainly in animal models has shown that PDE5 inhibitors, including sildenafil and tadalafil, may be potential Alzheimer’s disease treatments.19
In rodents, sildenafil has been shown to improve memory and cognitive function;20–23 rescue impairment in synaptic plasticity;24 attenuate learning impairment;25 rescue impaired long-term potentiation26 and reduce amyloid burden.20 In rodents, tadalafil has also been shown to improve memory and learning20,27,28 and reduce amyloid burden.20 However, results in human studies have been inconsistent.29 Prior studies in cognitively normal, healthy individuals showed that sildenafil may improve reaction time30 and attention based on auditory event-related potentials 26 although beneficial effects on memory were not observed. In Alzheimer’s disease patients, sildenafil decreased spontaneous neural activity, increased cerebral metabolic rate of oxygen and cerebral blood flow (CBF) and decreased cerebrovascular reactivity.31 Tadalafil has been shown to increase regional CBF and improve cognitive function in patients with erectile dysfunction (ED) and mild cognitive impairment;32 improve cerebral perfusion in patients with small-vessel disease stroke;33 and improve cognitive function in patients with low urinary tract symptoms and ED.34 However, a recent randomized clinical trial testing tadalafil failed to detect treatment effects on CBF in non-demented older adults.35 A recently published study by Fang and colleagues proposed sildenafil as a promising repurposing candidate for Alzheimer’s disease based on endophenotype network analysis supported by analyses of insurance claims data as well as in vitro studies in induced pluripotent stem cells (iPSCs) derived from Alzheimer’s disease patients.36
In this report, we describe results from the DREAM study comparing the risk of ADRD in Medicare beneficiaries with pulmonary arterial hypertension (PAH) treated with the PDE5 inhibitors, sildenafil or tadalafil, compared with an active comparator for the same indication, endothelin receptor antagonists (ERAs). By restricting to a homogenous population with the same underlying indication and theselection of an alternative treatment for the same indication as a comparator, our study explicitly attempts to address measured and unmeasured confounding37 and is therefore, a unique addition to the literature. In addition to our pharmacoepidemiologic analyses, we also tested whether sildenafil affects multiple molecular endophenotypes of Alzheimer’s disease in cell culture-based phenotypic assays. These included Aβ secretion, clearance and toxicity, as well as several other outcomes such as neuroinflammation, cell death, neurite outgrowth and tau phosphorylation. Our primary motivation in designing this study was to test the hypothesis that PDE5 inhibition may be a plausible treatment strategy in ADRD and compare our results with those recently reported by Fang et al.36
Materials and methods
Pharmacoepidemiologic analyses
The full study protocols for patient-level analyses in Medicare claims were pre-registered on clinicaltrials.gov prior to data analysis (NCT05039086) and contain detailed information on implementation including all codes that were used to identify study variables to allow for independent replication and validation.
Data source
We used Medicare Fee-For-Service claims data from 2007 through 2018. The starting period (2007) aligns with introduction of Pharmacy benefits (part D) under Medicare and the end date (2018) aligns with the most recently available data from CMS at the time this analysis was initiated. Medicare Part A (hospitalizations), B (medical services) and D (prescription medications) claims are available for research purposes through the Centers for Medicare and Medicaid Services. A signed data use agreement with the CMS was available and the Brigham and Women’s Hospital’s Institutional Review Board approved this study (Protocol # 2019P003607). Data analyzed in this study represent patient-level information and are not allowed for public sharing based on the data use agreement with the Centers for Medicare and Medicaid Services.
Study cohort
A new user, active comparator cohort study design was implemented. Patients were required to have 365-day of continuous enrolment in Medicare parts A, B and D (i.e. the baseline period) before cohort entry, which was defined as the date of starting either a PDE5 inhibitor (sildenafil, tadalafil) or ERA (bosentan, ambrisentan, macitentan). Patients were required to have ≥2 claims with PAH diagnosis during the baseline period including the cohort entry date.38 We excluded patients with existing diagnoses of ADRD any time prior to and including cohort entry date to exclude patients with prevalent ADRD. We also excluded patients with a nursing home admission prior to cohort entry as medication records for short nursing home stays are unavailable in Medicare claims. Supplementary Figure 1 summarizes the study design.
Outcome measurement
We defined incident ADRD based on diagnosis codes recorded on one inpatient or two outpatient claims of Alzheimer’s disease, vascular dementia, senile, presenile, or unspecified dementia or dementia in other diseases classified elsewhere (codes provided in Supplementary Table 1). Medicare claims-based dementia identification is reported to have a positive predictive value (PPV) in the range of 65–78% when validated against a structured in-home dementia assessment.39
Alternative analytic approaches
We employed the following alternative analyses with equal priority where we varied key assumptions involved in design of this pharmacoepidemiologic analysis based on epidemiological principles.40Supplementary Fig. 2 provides a visual summary of all four analytic approaches.
Analysis 1—‘as-treated’ follow-up approach
In this approach, we followed patients from cohort entry until treatment discontinuation or switch to the comparator treatment, insurance disenrolment, death, or administrative endpoint (December 2018). Treatment discontinuation was defined as occurring 90 days after the expected days supply of the most recently filled prescription to accommodate suboptimal adherence during treatment periods.
Analysis 2—‘as-started’ follow-up approach incorporating a 6-month ‘induction’ period
In this approach, we varied two assumptions: (i) we incorporated a 6-month induction period after the cohort entry date and disregarded ADRD events diagnosed in this period to address reverse causation concerns if treatment decisions were driven by early disease symptoms and disease diagnosis was recorded shortly after treatment initiation; and (2) we followed patients for a maximum of 3 years after the 6-month induction period regardless of subsequent treatment changes or discontinuation, similar to an intention-to-treat approach in randomized controlled trials.41 This assumption addresses concerns related to informative censoring, which would occur if patients discontinued (by choice or through de-prescribing) treatment because of unrecorded memory problems associated with ADRD.
Analysis 3—incorporating a 6-month ‘symptoms to diagnosis’ period
As ADRD symptoms may appear some time before a diagnosis is recorded in insurance claims, we assigned an outcome date in this analysis that was 6 months before the first recorded ADRD date and excluded the last 6 months of follow-up for those who are censored without an event.
Analysis 4—high-specificity outcome definition
As diagnosis codes-based outcome definition may have limited specificity, we defined
the outcome in this analysis using a combination of diagnosis code and ≥1 prescription claims for a symptomatic treatment (donepezil, galantamine, rivastigmine and memantine) occurring within 6 months of each other, with the outcome date assigned to the second event in the sequence. Use of medication records to identify dementia has a PPV exceeding 95% in a previous validation study.42
Patient characteristics
We measured several sets of covariates in the 365-day baseline period preceding cohort entry: (i) sociodemographic factors including age, gender, race, receipt of low-income subsidy, (ii) risk factors for ADRD including diabetes, stroke and depression,43–45 (3) lifestyle factors, such as smoking, as well as use of preventive services such as screening mammography and vaccinations to account for healthy-user effect;46 measures of healthcare services use before cohort entry including number of prescriptions filled, number of emergency department visits, hospitalizations, and physician office visits to minimize the impact of differential surveillance, and a frailty score47 (iv) PAH-related factors including other treatments including calcium channel blockers, riociguat and prostanoids (beraprost, epoprostenol, iloprost, treprostinil), as well as number of hospitalizations for PAH and (v) other comorbid conditions and co-medications (Supplementary Table 2).
Cell culture-based phenotypic assays
We tested whether sildenafil could rescue molecular phenotypes relevant to Alzheimer’s disease including tau phosphorylation, Aβ1-42 clearance, Aβ secretion, Aβ toxicity, lipopolysaccharide (LPS)-induced neuroinflammation, cell death due to trophic factor withdrawal and neurite outgrowth and neurogenesis. Supplementary Table 3 includes detailed descriptions of the phenotypic assays and sildenafil concentrations tested. In all assays described below, cells treated with sildenafil were compared to either the vehicle control (VC) or lesion control.
MTT viability assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) viability assay was used to establish the effects of various concentrations of sildenafil on cell viability. Differentiated SH-SY5Y cells were seeded onto uncoated 96-well plates at a cell density of 2.5 × 104 cells per well (DIV1) in culture medium (DMEM medium, 10% FCS, 1% NEAA, 1% L-Glutamine, 100 µg/ml Gentamycin) containing 10 µM RA at 37°C; 95% humidity and 5% CO2. Next day (DIV2), sildenafil (TargetMol, T0467) was applied at 50, 10, 3, 1, 0.1 and 0.01 µM final concentration. Additionally, the appropriate vehicle control of the test items (0.1% DMSO) was investigated. After 24 h of incubation (DIV3), treated cells were subject to MTT assay. Additional details are included in Roberts et al.13
Tau phosphorylation
Details are included in Roberts et al.13
Aβ1-42 clearance
Details are included in Roberts et al.13
Aβ secretion
Details are included in Roberts et al.13
Aβ toxicity
Primary hippocampal neurons were prepared from E18.5 timed pregnant C57BL/6JRccHsd mice as previously described. Cells were seeded in poly-D-lysine pre-coated 96-well plates at a density of 4 × 104 cells/well and cultivated until DIV10 (Neurobasal, 2% B-27, 0.5 mM glutamine, 25 μM glutamate, 1% Penicillin–Streptomycin). On DIV10 pre-aggregated Aβ1-42 (Bachem 4061966, final concentration 10 µM, 48 h at 4°C) was added to the cells in the presence or absence of sildenafil (10, 1 and 0.1 µM). On DIV16, cells were subject to MTT assay to determine cell viability.
Lipopolysaccharide-induced neuroinflammation
Details are included in Roberts et al.13
Cell death due to trophic factor withdrawal
Details are included in Roberts et al.13
Neurite outgrowth and neurogenesis
Primary hippocampal neurons were prepared from E18.5 timed pregnant C57BL/6JRccHsd mice as previously described. Cells were seeded in poly-D-lysine pre-coated 96-well plates at a density of 2.6 × 104 cells/well in medium (Neurobasal, 2% B-27, 0.5 mM glutamine, 25 μM glutamate, 1% Penicillin–Streptomycin). Directly on DIV1, sildenafil (10, 1 and 0.1 µM) or VC was applied. On DIV2, 10 µM Bromodeoxyuridine (BrdU; B5002 Sigma Aldrich) was added and cells were fixed after additional 24h. Cells were permeabilized with 0.1% Triton-X and incubated with primary Beta Tubulin Isotype III (T8660, Sigma Aldrich) and BrdU antibodies (MAS25°c, AHrlan-Sera Lab) overnight at 4°C. Afterwards, cells were washed two times with PBS and incubated with fluorescently labelled secondary antibodies and DAPI for 1.5 h at room temperature (RT) in the dark. Cells were rinsed three times with PBS and imaged with the Cytation 5 Multimode reader (BioTek) at 10 × magnification (six images per well). BrdU-positive cells were counted as a marker of neurogenesis and Beta Tubulin Isotype III signal was used for macro-based quantification of neurite outgrowth.
Statistical analyses
Pharmacoepidemiologic analyses
Propensity score (PS)-matching was used to account for measured confounding in this study.48 The PS were calculated as the predicted probability of initiating PDE5 inhibitors versus ERA conditional on baseline covariates using multivariable logistic regression. Matching used a nearest-neighbor algorithm within a caliper of 0.025 on the natural scale of the PS.49,50 We evaluated PS distributional overlap before and after matching to ensure comparability of these groups51 and balance in each individual covariate between two treatment groups using standardized differences.52
Incidence rates of the outcome with 95% confidence intervals were estimated for both treatment groups in the PS-matched cohort. To account for the competing risk of mortality, we calculated the cumulative incidence of ADRD using cumulative incidence functions and provided cause-specific hazard ratios from Cox proportional hazards regression models.53 Pre-specified subgroup analyses were conducted based on age, sex and baseline cardiovascular disease as there is evidence of potentially heterogenous etiology of ADRD based on these factors.54–56 A commonly used age cut off of 75 years from prior epidemiologic literature was used to define the age subgroup as the incidence appears to sharply increase after this age.57
All analyses of the claims database was conducted using the Aetion Evidence Platform v4.30 (incl. R v3.4.2), which has been scientifically validated by accurately repeating a range of previously-published studies58 and by replicating59 or predicting clinical trial findings.60
Cell culture-based phenotypic assays
Statistical analysis for cell culture-based phenotypic assays was performed in GraphPad Prism 9.1.2. Group differences were evaluated separately for each test item versus VC or lesion control by one-way ANOVA followed by Dunnett’s multiple comparison test.
Data availability
Data analyzed in this study represent patient-level information and are not allowed for public sharing based on the data use agreement with the Centers for Medicare and Medicaid Services.
Results
To test whether exposure to PDE5 inhibitors lowers ADRD risk in older individuals, we used longitudinal insurance claims data from Medicare beneficiaries. The cohort of patients (described below) included PDE5 inhibitor initiators and an active comparator, ERA initiators. We estimated treatment effects in four alternative analyses designed to address various uncertainties associated with claims-based analyses of dementia risk including exposed person-time misclassification, reverse causation, informative censoring and misclassification of outcome onset as described previously (Supplementary Figure 2).10
Cohort characteristics
We identified 9968 PDE5 inhibitor initiators and 3053 ERA initiators who met our inclusion criteria (Supplementary Figure 3—CONSORT diagram). For confounding adjustment, 2888 PDE5 inhibitor initiators (73.6% sildenafil; 26.4% tadalafil) were pair-matched to 2888 comparable ERA initiators (Table 1, Supplementary Table 2). The average age of included patients was 74 years (range 65–96 years), and 69% were women. Prevalence of comorbid conditions was high in this cohort, with 90% with hypertension, 72% with heart failure, and 43% with atrial fibrillation. In our cohort, more than 99% of sildenafil initiators (2107/2123) were treated with 20 mg thrice a day regimen and 98% of tadalafil initiators (745/761) were treated with 40 mg daily regimen in accordance with recommended dosing for PAH.
Table 1.
Select baseline characteristics of patients included in the study cohort before and after 1:1 propensity score matching, Medicare data 2007–2018
| Variable | Unmatched | Endothelin receptor antagonists | St. Diff | PS-matched | Endothelin receptor antagonists | St. Diff |
|---|---|---|---|---|---|---|
| PDE5 inhibitors | PDE5 inhibitors | |||||
| (N = 9968) | (N = 3053) | (N = 2888) | (N = 2888) | |||
| Demographics | ||||||
| Age, mean (SD) | 74.74 (6.78) | 73.79 (6.14) | 0.15 | 73.87 (6.43) | 73.84 (6.15) | 0.00 |
| Female, n (%) | 6069 (60.9%) | 2117 (69.3%) | −0.18 | 1999 (69.2%) | 1990 (68.9%) | 0.01 |
| White, n (%) | 8031 (80.6%) | 2435 (79.8%) | 0.02 | 2293 (79.4%) | 2311 (80.0%) | −0.01 |
| Low-income subsidy, n (%) | 2382 (23.9%) | 713 (23.4%) | 0.01 | 706 (24.4%) | 682 (23.6%) | 0.02 |
| Dementia risk factors, n (%) | ||||||
| Diabetes | 4486 (45.0%) | 1204 (39.4%) | 0.11 | 1180 (40.9%) | 1145 (39.6%) | 0.03 |
| Obesity | 3351 (33.6%) | 968 (31.7%) | 0.04 | 911 (31.5%) | 918 (31.8%) | −0.01 |
| Hypertension | 8877 (89.1%) | 2729 (89.4%) | −0.01 | 2587 (89.6%) | 2595 (89.9%) | −0.01 |
| Coronary artery disease | 7445 (74.7%) | 2273 (74.5%) | 0.00 | 2136 (74.0%) | 2150 (74.4%) | −0.01 |
| Depression | 1995 (20.0%) | 571 (18.7%) | 0.03 | 515 (17.8%) | 537 (18.6%) | −0.02 |
| Anxiety | 1824 (18.3%) | 494 (16.2%) | 0.06 | 466 (16.1%) | 465 (16.1%) | 0.00 |
| Bipolar disorder | 88 (0.9%) | 33 (1.1%) | −0.02 | 28 (1.0%) | 32 (1.1%) | −0.01 |
| Schizophrenia | 26 (0.3%) | 12 (0.4%) | −0.02 | 12 (0.4%) | 12 (0.4%) | 0.00 |
| Markers for healthy behaviour, frailty, healthcare use, n (%) | ||||||
| Smoking | 4286 (43.0%) | 1126 (36.9%) | 0.12 | 1056 (36.6%) | 1068 (37.0%) | −0.01 |
| Mammography | 1743 (17.5%) | 630 (20.6%) | −0.08 | 593 (20.5%) | 597 (20.7%) | 0.00 |
| Colonoscopy | 1239 (12.4%) | 345 (11.3%) | 0.03 | 327 (11.3%) | 317 (11.0%) | 0.01 |
| Fecal occult blood test | 774 (7.8%) | 238 (7.8%) | 0.00 | 232 (8.0%) | 223 (7.7%) | 0.01 |
| Influenza vaccination | 6953 (69.8%) | 2150 (70.4%) | −0.01 | 2037 (70.5%) | 2036 (70.5%) | 0.00 |
| Other PAH treatments and PAH severity indicators | ||||||
| Use of calcium channel blockers, n (%) | 4652 (46.7%) | 1506 (49.3%) | −0.05 | 1462 (50.6%) | 1422 (49.2%) | 0.03 |
| Riociguat, n (%) | 26 (0.3%) | 90 (2.9%) | −0.21 | 26 (0.9%) | 25 (0.9%) | 0.00 |
| Prostanoids, n (%) | 96 (1.0%) | 55 (1.8%) | −0.07 | 54 (1.9%) | 48 (1.7%) | 0.02 |
| Number of PAH hospitalizations, mean (SD) | 0.10 (0.34) | 0.11 (0.35) | −0.03 | 0.12 (0.37) | 0.11 (0.34) | 0.03 |
| Comorbid conditions, n (%) | 0.00 | |||||
| Atrial fibrillation | 5321 (53.4%) | 1282 (42.0%) | 0.23 | 1232 (42.7%) | 1228 (42.5%) | 0.00 |
| Heart failure | 8064 (80.9%) | 2197 (72.0%) | 0.21 | 2088 (72.3%) | 2088 (72.3%) | 0.00 |
| Stroke or transient ischaemic attack | 1020 (10.2%) | 276 (9.0%) | 0.04 | 254 (8.8%) | 260 (9.0%) | −0.01 |
| Chronic obstructive pulmonary disease | 6521 (65.4%) | 2020 (66.2%) | −0.02 | 1941 (67.2%) | 1915 (66.3%) | 0.02 |
PAH, pulmonary arterial hypertension, SD, standard deviation, Std diff, standardized difference.
Incidence rates of ADRD
Incidence rates of ADRD varied across analysis schemes, ranging 15 to 23 per 1000 person years based on diagnosis codes alone (analyses 1, 2 and 3) and 4.7 to 5.2 per 1000 person years based on a more specific definition combining diagnosis codes and prescription claims (Analysis 4) (Table 2, unmatched/crude data included in Supplementary Table 4).
Table 2.
Incidence rates for the outcome of Alzheimer’s disease and related dementia across four analysis schemes
| Analysis schemea | Exposure | n of patients | N outcomes | N person years | Median follow-up (IQR), days | Incidence rate (95% CI)/1000 person years |
|---|---|---|---|---|---|---|
| Analysis 1 | PDE5 inhibitors | 2888 | 55 | 3065 | 168 (37, 530) | 17.9 (13.5–23.4) |
| ERA | 2888 | 59 | 3136 | 151 (47, 508) | 18.8 (14.3–24.3) | |
| Analysis 2 | PDE5 inhibitors | 1706 | 62 | 3125 | 693 (320, 1095) | 19.8 (15.2–25.4) |
| ERA | 1706 | 63 | 3168 | 720 (328, 1095) | 19.9 (15.3–25.4) | |
| Analysis 3 | PDE5 inhibitors | 1292 | 31 | 2060 | 382 (150, 794) | 15.1 (10.2–21.4) |
| ERA | 1292 | 46 | 2037 | 358 (124, 810) | 22.6 (16.5–30.1) | |
| Analysis 4 | PDE5 inhibitors | 2888 | 16 | 3099 | 169 (38, 533) | 5.2 (3–8.4) |
| ERA | 2888 | 15 | 3167 | 151 (47, 517) | 4.7 (2.7–7.8) |
Analysis 1: ‘As-treated’ follow-up approach; Analysis 2: ‘As-started’ follow-up approach incorporating a 6-month induction period; Analysis 3: Incorporating a 6-month ‘symptom to diagnosis’ period’ and Analysis 4: Alternate outcome definition (See Methods for additional description of analytic approach). ERAs, endothelin receptor antagonists, IQR, interquartile range, PDE, phosphodiesterase.
Comparative risk of ADRD
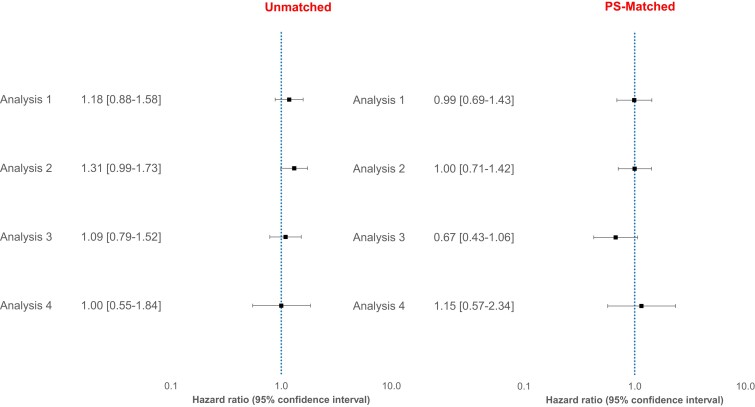
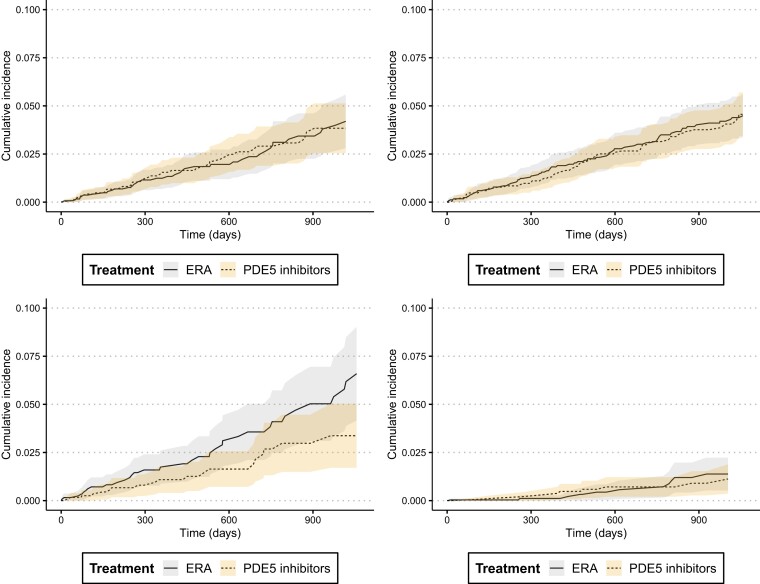
Across the four analysis schemes, we did not find evidence for a difference in the risk of incident ADRD in patients treated with PDE5 inhibitors (sildenafil/tadalafil) versus ERA in the PS-matched sample. HRs (95% CI) were 0.99 (0.69–1.43), 1.00 (0.71–1.42), 0.67 (0.43–1.06) and 1.15 (0.57–2.34), respectively, for analysis 1–4 (Fig. 1). We also noted that the cumulative incidence of ADRD was similar in the two treatment groups with overlapping confidence intervals in all four analyses (Fig. 2). Cumulative incidence curves were approximately parallel and did not indicate gross violation of the proportional hazard assumption.
Figure 1.
Relative risk of Alzheimer’s disease and related dementia in patients treated with PDE5 inhibitors versus ERAs before and after 1:1 propensity score matching, Medicare data 2007–2018. Analysis 1: ‘As-treated’ follow-up approach; Analysis 2: ‘As-started’ follow-up approach incorporating a 6-month induction period; Analysis 3: Incorporating a 6-month ‘symptom to diagnosis’ period’ and Analysis 4: Alternate outcome definition (See Methods for additional description of analytic approach).
Figure 2.
Cumulative incidence of Alzheimer’s disease and related dementia in patients treated with PDE5 inhibitors versus ERAs after 1:1 propensity score matching, Medicare data 2007–2018. Analysis 1: ‘As-treated’ follow-up approach; Analysis 2: ‘As-started’ follow-up approach incorporating a 6-month induction period; Analysis 3: Incorporating a 6-month ‘symptom to diagnosis’ period’ and Analysis 4: Alternate outcome definition (See Methods for additional description of analytic approach).
Subgroup analyses
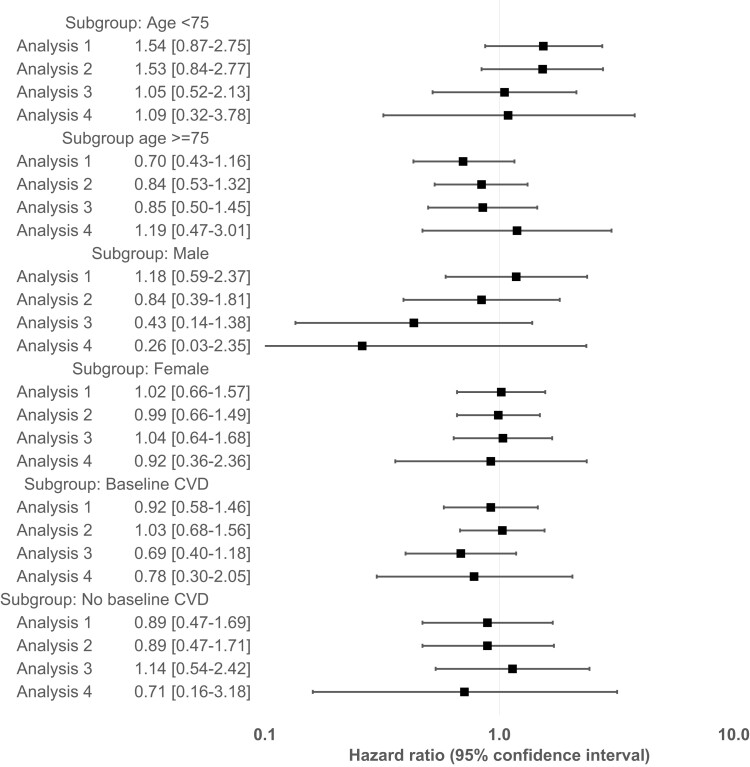
We found no evidence of heterogeneity of treatment effect by pre-specified subgroups of age, gender, and baseline cardiovascular disease; however, confidence intervals were generally wide due to small event counts in subgroups (Fig. 3).
Figure 3.
Relative risk of Alzheimer’s disease and related dementia in patients treated with PDE5 inhibitors versus ERAs after 1:1 propensity score matching within pre-specified subgroups, Medicare data 2007–2018. Analysis 1: ‘As-treated’ follow-up approach; Analysis 2: ‘As-started’ follow-up approach incorporating a 6-month induction period; Analysis 3: Incorporating a 6-month ‘symptom to diagnosis’ period’ and Analysis 4: Alternate outcome definition (See Methods for additional description of analytic approach).
Effect of sildenafil on cell culture-based phenotypic assays
We first established the effects of various concentrations of sildenafil on cell viability using the MTT assay in SH-SY5Y cells. At concentrations of 0.01, 0.1, 1.0, 3.0, 10 and 50 µM, there were no adverse effects of sildenafil on cell viability. In subsequent phenotypic assays, we used a range of 0.1, 1.0 and 10 µM concentrations of sildenafil to test whether the drug exerted dose-dependent rescue of molecular abnormalities associated with Alzheimer’s disease.
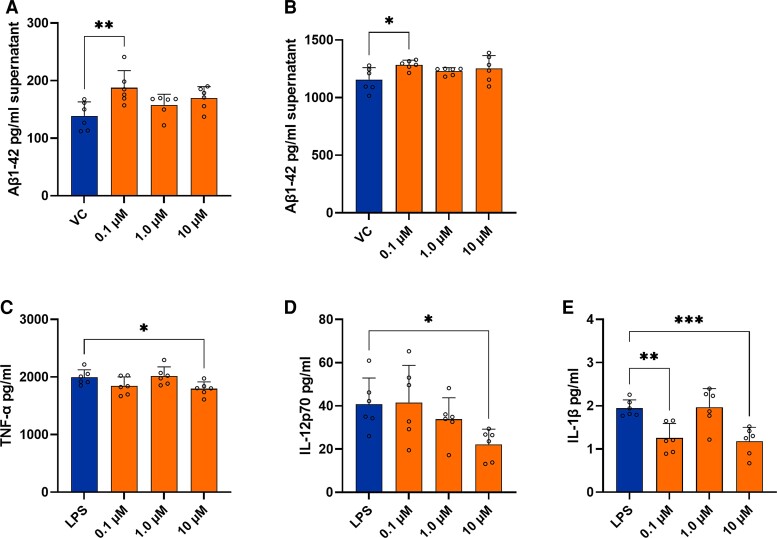
Sildenafil was not associated with a protective effect across the majority of phenotypic assays. Sildenafil had an adverse effect on clearance of exogenous Aβ1-42; at the lowest concentration (0.10 µM) we observed increased levels of Aβ1-42 in the supernatant indicating inhibition of phagocytotic activity in BV2 microglial cells (Fig. 4A). Similarly, in human APP overexpressing H4-hAPP neuroglioma cells, sildenafil had an adverse effect on secretion of Aβ1-42; at the lowest concentration (0.10 µM) we observed increased secretion of Aβ1-42 (Fig. 4B) and no significant effects at 1.0 and 10 µM concentrations. We observed that sildenafil had a modest anti-inflammatory effect in the LPS-associated neuroinflammation assay. Sildenafil reduced TNF-α secretion (Fig. 4C) and IL-12p70 (Fig. 4D) at the highest concentration (10 µM) and reduced IL-1β secretion (Fig. 4E) at the highest and lowest concentrations (10 µM and 0.10 µM, respectively). A summary of results across all Alzheimer’s disease-related phenotypic assays is included in Supplementary Table 5.
Figure 4.
Effect of sildenafil on cell culture-based phenotypic assays. (A) Levels of exogenous Aβ1–42 in the supernatant in BV2 microglial cells and (B) in H4-hAPP overexpressing neuroglioma cells after 24 h treatment with sildenafil. Sildenafil at the lowest concentration (0.10 µM) significantly increased levels of Aβ1-42. Levels of inflammatory cytokines (C) TNF-α, (D) IL-12p70, (E) IL-1β in the supernatant of BV2 (microglial) cells after 24 h LPS stimulation and sildenafil treatment. Sildenafil at the highest concentration (10 µM) significantly reduced secretion of TNF-α and IL-12p70; sildenafil at the highest and lowest concentrations (10 and 0.10 µM, respectively) significantly reduced secretion of IL-1β. Error bars in all bar graphs indicate group mean + standard deviation (SD). Individual values are shown as dots (n = 6 per group). Each dot represents a technical replicate. Group differences comparing sildenafil-treated cells to the VC or LPS control were evaluated using the one-way ANOVA test followed by Dunnett’s multiple comparison test. Asterisks indicate significant differences between groups: * P < 0.05; ** P < 0.01; *** P < 0.001. VC, vehicle control (0.1% DMSO); LPS, lipopolysaccharide.
Discussion
In this population-based cohort study, we did not find evidence to suggest a decreased risk of incident ADRD in patients treated with PDE5 inhibitors versus ERA. These results were consistent across various analyses and subgroups. Consistent with the findings from our pharmacoepidemiologic analyses, we also did not observe evidence of amelioration in molecular abnormalities relevant to Alzheimer’s disease in most cell culture-based phenotypic assays.
Our results are at odds with a recent study that indicated a substantial (69%) reduction in Alzheimer’s disease incidence after sildenafil treatment.36 Major differences in the design of these two investigations likely explain this inconsistency. In the previous study, Fang et al. compared the risk of incident Alzheimer’s disease in sildenafil users with non-users or users of various cardiometabolic medications, including diltiazem, glimepiride, losartan and metformin, without restriction to any specific indication. This design choice is likely to result in comparison of individuals with ED, which is by far the most common indication for sildenafil, to generally older individuals with diabetes or hypertension. Since worse cardiometabolic health is a well-recognized risk factor for AD43 and seeking treatment for ED is likely reflective of a certain level of preserved physical and cognitive function, we believe that results from Fang et al. can be at least partly be explained by potential confounding by indication. While statistical approaches, such as regression or PS-matching, can account for measured differences in characteristics between exposure group, they do not account for unmeasured differences such as frailty, blood pressure control, or glycemic control which are not available in insurance claims.61 One widely accepted strategy to reduce the threat of such unmeasured confounding is restriction to a homogenous population with the same underlying indication and selection of an alternative treatment for the same indication as a comparator.37 Therefore, we restricted our analyses to an alternative indication for PDE5 inhibitors (sildenafil/tadalafil)—PAH—and selected an equivalent comparator drug that is used for the same indication to minimize confounding by indication.
In their attempt to derive insight into mechanisms underlying their pharmacoepidemiologic findings, Fang et al. performed phenotypic assays using iPSCs derived from human Alzheimer’s disease patient neurons in cell culture-based experiments. They observed that at a concentration of 30 µM, sildenafil treatment was associated with an increase in neurite outgrowth and a reduction in tau phosphorylation. Our studies used primary mouse hippocampal and cortical neurons, as well as immortalized microglia-like, neuroblastoma, neuroglioma cell lines in assays to assess the effects of sildenafil on several distinct Alzheimer’s disease-related phenotypes. We did not show a beneficial effect of sildenafil on the majority of Alzheimer’s disease relevant outcomes, although sildenafil treatment was associated with a reduction in secretion of some pro-inflammatory cytokines after LPS stimulation of microglial cells. An important difference in the design of these experiments is the use of primary, iPSC derived neurons from Alzheimer’s disease patients in Fang et al compared to our use of multiple cell lines including those that overexpress the phenotype of interest (i.e. tau phosphorylation).
Prior evidence mainly from rodent and other animal models suggest that PDE5 inhibitors including both sildenafil and tadalafil may improve memory and cognitive function and reduce Alzheimer’s disease-related pathology including Aβ plaques.19 However, human observational and clinical trial evidence is limited. A recent randomized clinical trial showed no effect of a single administration of tadalafil on CBF.35 Fang et al.’s study36 is the first to report that sildenafil may protect against Alzheimer’s disease in a large clinical data set. The evidence in our current report is contrary to those findings.
There are several methodologic differences between the pharmacoepidemiologic design used by Fang et al and our study. First, Fang et al. did not restrict their analyses to a specific indication, while our study examined the use of PDE5 inhibitors compared to ERA for the same indication—i.e. PAH. We believe this difference, which likely resulted in significant confounding by indication in Fang et al.’s study, at least partially explain the significant reduction in Alzheimer’s disease incidence reported after sildenafil treatment. Second, as the use of PDE5 inhibitors in ED is substantially more prevalent than PAH, it is reasonable to assume that the Fang et al. cohort included a large majority of patients with ED. The dose and frequency of PDE5 use for ED is very different than that for PAH. For example, the recommended dose of sildenafil for ED is 50 mg for adults under 65 and 25 mg for adults 65 years or older no more than once per day, whereas the recommended dose for PAH is 5 or 20 mg three times a day for all adults.62 Notably, the optimal dose and frequency to derive potential cognitive benefits, and the difference between lower, more frequent exposure versus higher and less frequent exposure is unknown. In Fang et al.’s study, the reported average daily dose of sildenafil was 75 mg/day among males and 22 mg/day among females, while 99% of sildenafil initiators in our cohort were treated with 20 mg/thrice a day regimen in accordance with recommended dose for PAH. It is important to note however that Fang et al. did not find a dose effect (‘average daily dosage of sildenafil was not associated with incidence of Alzheimer’s disease’36) and subgroup analyses indicated similar effect estimates for patients in both ED and PAH populations. Nonetheless, in future studies, it would be important to compare brain tissue accumulation of PDE5 inhibitors after chronic low dose exposure to less frequent high dose exposure. Third, Fang et al. restricted PDE5 use to sildenafil, whereas our study included both sildenafil and tadalafil users (73.6% sildenafil; 26.4% tadalafil). There is pharmacokinetic evidence that sildenafil63 can cross the blood–brain barrier (BBB). While tadalafil can also cross the BBB,64 its permeability may be lower than that of sildenafil. Additionally, the half-life of tadalafil is significantly longer than that of sildenafil (17.5 hrs/4 hrs).65 Moreover, tadalafil and sildenafil vary in selectivity to PDE5 relative to other PDEs.66 Future comparative studies are needed to determine how BBB permeability, differences in half-life and PDE selectivity may impact any potential neuroprotective benefits of these drugs.
Our investigation has certain limitations. First, it is possible that our study may have been underpowered to detect differences that are of small magnitude. Reflecting treatment adherence from routine care, our average length of follow-up was also short, which could lead to underestimation of effects that require longer treatment. Next, despite the care in design of this study with a homogenous patient population and equivalent comparator, we cannot rule out confounding by indication. Also, it should be noted that while restriction to a homogenous population with diagnosis of PAH enables unbiased comparisons of treatment effects, this population is also atypical with a high underlying cardiometabolic disease burden and may not be representative of all older individuals at risk for ADRD in routine care. Finally, cell culture-based phenotypic assays performed in our study only reflect discrete aspects of Alzheimer’s disease pathogenesis and do not recapitulate complex gene–environment interactions that underlie the disease in older individuals.
In conclusion, our study did not provide evidence to support the hypothesis that PDE5 inhibitor use reduces risk of incident ADRD. While wider use of routinely collected healthcare data to evaluate biological hypotheses for drug repurposing is a welcome development, caution is warranted to avoid common pitfalls and consequent overinterpretation of estimates generated from these data.
Supplementary Material
Abbreviations
- ADAM =
Alzheimer’s Disease Aberrant Metabolism
- ADRD =
Alzheimer’s disease and related dementia
- BBB =
blood–brain barrier
- CBF =
cerebral blood flow
- DREAM =
Drug Repurposing for Effective Alzheimer’s Medicines
- ERAs =
endothelin receptor antagonists
- ED =
erectile dysfunction
- HR =
hazzard ratio
- iPSCs =
induced pluripotent stem cells
- LDH =
lactate dehydrogenase
- PDE =
phosphodiesterase
- PS =
propensity score
- PAH =
pulmonary arterial hypertension
Contributor Information
Rishi J Desai, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital & Harvard Medical School, Boston, MA 02115, USA.
Mufaddal Mahesri, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital & Harvard Medical School, Boston, MA 02115, USA.
Su Been Lee, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital & Harvard Medical School, Boston, MA 02115, USA.
Vijay R Varma, Clinical & Translational Neuroscience Section, Laboratory of Behavioral Neuroscience, National Institute on Aging, Baltimore, MD 21224, USA.
Tina Loeffler, QPS Austria GmbH, Parkring 12, 8074 Grambach, Austria.
Irene Schilcher, QPS Austria GmbH, Parkring 12, 8074 Grambach, Austria.
Tobias Gerhard, Rutgers Center for Pharmacoepidemiology and Treatment Science, New Brunswick, NJ 08901, USA; Ernest Mario School of Pharmacy, Rutgers University, Piscataway, NJ 08854, USA.
Jodi B Segal, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Mary E Ritchey, Rutgers Center for Pharmacoepidemiology and Treatment Science, New Brunswick, NJ 08901, USA.
Daniel B Horton, Rutgers Center for Pharmacoepidemiology and Treatment Science, New Brunswick, NJ 08901, USA; Rutgers Robert Wood Johnson Medical School, Rutgers University, Piscataway, NJ 08901, USA.
Seoyoung C Kim, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital & Harvard Medical School, Boston, MA 02115, USA; Division of Rheumatology, Inflammation, and Immunity, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA.
Sebastian Schneeweiss, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital & Harvard Medical School, Boston, MA 02115, USA.
Madhav Thambisetty, Clinical & Translational Neuroscience Section, Laboratory of Behavioral Neuroscience, National Institute on Aging, Baltimore, MD 21224, USA.
Funding
The DREAM study is funded by the intramural programme of the National Institute on Aging. M.T. is grateful for funding support from the Andrew and Lillian A. Posey Foundation to the Clinical and Translational Neuroscience Section, Laboratory of Behavioral Neuroscience, NIA.
Competing interests
R.J.D. reports serving as Principal Investigator on research grants from Bayer, Vertex and Novartis to the Brigham & Women’s Hospital for unrelated projects. T.G. reports grants from NIA during the conduct of the study; grants and personal fees from Bristol-Myers Squibb; personal fees from Merck, Pfizer, Lilly, IntraCellular Therapies, and Eisai, all outside the submitted work. S.S. is co-principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Boehringer Ingelheim unrelated to the topic of this study. He is a consultant to Aetion Inc., a software manufacturer of which he owns equity. His interests were declared, reviewed and approved by the Brigham and Women’s Hospital and Partners HealthCare System in accordance with their institutional compliance policies. S.C.K. has received research grants to the Brigham and Women’s Hospital from Pfizer, Roche, AbbVie and Bristol-Myers Squibb for unrelated topics. D.B.H. has received research grants to Rutgers University from Danisco USA, Inc. for unrelated topics. M.E.R. is principal and owner of Med Tech Epi, LLC, a consultancy which conducts contracted research on behalf of pharmaceutical and medical device clients for unrelated topics. Her interests are declared and reviewed annually by Rutgers University Ethics and Compliance in accordance with institutional compliance policies. Other authors declare no conflict of interest.
Supplementary material
Supplementary material is available at Brain Communications online.
References
- 1. Godyn J, Jonczyk J, Panek D, Malawska B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol Rep. 2016;68(1):127–138. [DOI] [PubMed] [Google Scholar]
- 2. Iqbal K, Liu F, Gong CX. Alzheimer Disease therapeutics: Focus on the disease and not just plaques and tangles. Biochem Pharmacol. 2014;88(4):631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panza F, Logroscino G, Imbimbo BP, Solfrizzi V. Is there still any hope for amyloid-based immunotherapy for Alzheimer’s disease? Curr Opin Psychiatry. 2014;27(2):128–137. [DOI] [PubMed] [Google Scholar]
- 4. Giacobini E, Gold G. Alzheimer Disease therapy–moving from amyloid-β to tau. Nature Rev Neurol. 2013;9(12):677–686. [DOI] [PubMed] [Google Scholar]
- 5. Takashima A. Tau aggregation is a therapeutic target for Alzheimer’s disease. Curr Alzheimer Res. 2010; 7(8):665–669. [DOI] [PubMed] [Google Scholar]
- 6. Yiannopoulou KG, Papageorgiou SG. Current and future treatments in Alzheimer disease: An update. J Cent Nerv Syst Dis. 2020;12:1179573520907397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Y, Fang J, Bekris LM, et al. . AlzGPS: A genome-wide positioning systems platform to catalyze multi-omics for Alzheimer’s drug discovery. Alzheimers Res Ther. 2021;13(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pushpakom S, Iorio F, Eyers PA, et al. . Drug repurposing: Progress, challenges and recommendations. Nat Rev Drug Discov. 2019; 18(1):41–58. [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez S, Hug C, Todorov P, et al. . Machine learning identifies candidates for drug repurposing in Alzheimer’s disease. Nat Commun. 2021;12(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desai RJ, Varma VR, Gerhard T, et al. . Targeting abnormal metabolism in Alzheimer’s disease: The drug repurposing for effective Alzheimer’s medicines (DREAM) study. Alzheimers Dementia. 2020;6(1):e12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desai RJ, Varma VR, Gerhard T, et al. . Comparative risk of Alzheimer disease and related dementia among medicare beneficiaries with rheumatoid arthritis treated with targeted disease-modifying antirheumatic agents. JAMA Netw Open. 2022;5(4):e226567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varma VR, Wang Y, An Y, et al. . Bile acid synthesis, modulation, and dementia: A metabolomic, transcriptomic, and pharmacoepidemiologic study. PLoS Med. 2021;18(5):e1003615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts JA, Varma VR, An Y, et al. . A brain proteomic signature of incipient Alzheimer’s disease in young APOE ε4 carriers identifies novel drug targets. Sci Adv. 2021;7(46):eabi8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vialle RA, de Paiva Lopes K, Bennett DA, Crary JF, Raj T. Integrating whole-genome sequencing with multi-omic data reveals the impact of structural variants on gene regulation in the human brain. Nat Neurosci. 2022;25(4):504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clark C, Dayon L, Masoodi M, Bowman GL, Popp J. An integrative multi-omics approach reveals new central nervous system pathway alterations in Alzheimer’s disease. Alzheimer’s Res Ther. 2021;13(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith GD, Ebrahim S, eds. Data dredging, bias, or confounding: They can all get you into the BMJ and the Friday papers. British Medical Journal Publishing Group; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tiwari AK, Chen Z-S. Repurposing phosphodiesterase-5 inhibitors as chemoadjuvants. Front Pharmacol. 2013; 4:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haider M, Elsherbeny A, Pittalà V, Fallica AN, Alghamdi MA, Greish K. The potential role of sildenafil in cancer management through EPR augmentation. J Pers Med. 2021;11(6):585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El-Bakly W, Wagdy O, Sobhy A, et al. . The efficacy and underlying mechanism of phosphodiesterase- 5 inhibitors in preventing cognitive impairment and Alzheimer pathology: A systematic review of animal studies. Behav Brain Res. 2019;372:112004. [DOI] [PubMed] [Google Scholar]
- 20. Puzzo D, Staniszewski A, Deng SX, et al. . Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer’s disease mouse model. J Neurosci. 2009;29(25):8075–8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cuadrado-Tejedor M, Hervias I, Ricobaraza A, et al. . Sildenafil restores cognitive function without affecting β-amyloid burden in a mouse model of Alzheimer's disease. Br J Pharmacol. 2011;164(8):2029–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hosseini-Sharifabad A, Ghahremani MH, Sabzevari O, et al. . Effects of protein kinase A and G inhibitors on hippocampal cholinergic markers expressions in rolipram- and sildenafil-induced spatial memory improvement. Pharmacol Biochem Behav. 2012;101(3):311–319. [DOI] [PubMed] [Google Scholar]
- 23. Prickaerts J, van Staveren WC, Sik A, et al. . Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience. 2002;113(2):351–361. [DOI] [PubMed] [Google Scholar]
- 24. Palmeri A, Privitera L, Giunta S, Loreto C, Puzzo D. Inhibition of phosphodiesterase-5 rescues age-related impairment of synaptic plasticity and memory. Behav Brain Res. 2013;240:11–20. [DOI] [PubMed] [Google Scholar]
- 25. Devan BD, Bowker JL, Duffy KB, et al. . Phosphodiesterase inhibition by sildenafil citrate attenuates a maze learning impairment in rats induced by nitric oxide synthase inhibition. Psychopharmacology (Berl). 2006;183(4):439–445. [DOI] [PubMed] [Google Scholar]
- 26. Schultheiss D, Müller SV, Nager W, et al. . Central effects of sildenafil (viagra) on auditory selective attention and verbal recognition memory in humans: A study with event-related brain potentials. World J Urol. 2001;19(1):46–50. [DOI] [PubMed] [Google Scholar]
- 27. Al-Amin MM, Hasan SM, Alam T, et al. . Tadalafil enhances working memory, and reduces hippocampal oxidative stress in both young and aged mice. Eur J Pharmacol. 2014;745:84–90. [DOI] [PubMed] [Google Scholar]
- 28. Hasan N, Zameer S, Najmi AK, Parvez S, Yar MS, Akhtar M. Roflumilast and tadalafil improve learning and memory deficits in intracerebroventricular Aβ1-42 rat model of Alzheimer’s disease through modulations of hippocampal cAMP/cGMP/BDNF signaling pathway. Pharmacol Rep. 2021;73(5):1287–1302. [DOI] [PubMed] [Google Scholar]
- 29. Zuccarello E, Acquarone E, Calcagno E, et al. . Development of novel phosphodiesterase 5 inhibitors for the therapy of Alzheimer’s disease. Biochem Pharmacol. 2020;176:113818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Denny JC, Bastarache L, Ritchie MD, et al. . Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotechnol. 2013;31(12):1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanders O. Sildenafil for the treatment of Alzheimer’s disease: A systematic review. J Alzheimers Dis Rep. 2020;4(1):91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi JB, Cho KJ, Kim JC, et al. . The effect of daily low dose tadalafil on cerebral perfusion and cognition in patients with erectile dysfunction and mild cognitive impairment. Clin Psychopharmacol Neurosci. 2019;17(3):432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ölmestig J, Marlet I, Hansen R, et al. . Tadalafil may improve cerebral perfusion in small-vessel occlusion stroke—A pilot study. Brain Commun. 2020;2(1):fcaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Urios A, Ordono F, Garcia-Garcia R, et al. . Tadalafil treatment improves inflammation, cognitive function, and mismatch negativity of patients with low urinary tract symptoms and erectile dysfunction. Sci Rep. 2019;9(1):17119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pauls MMH, Binnie LR, Benjamin P, et al. . The PASTIS trial: Testing tadalafil for possible use in vascular cognitive impairment. Alzheimers Dementia. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fang J, Zhang P, Zhou Y, et al. . Endophenotype-based in silico network medicine discovery combined with insurance record data mining identifies sildenafil as a candidate drug for Alzheimer’s disease. Nat Aging. 2021;1:1175–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Psaty BM, Siscovick DS. Minimizing bias due to confounding by indication in comparative effectiveness research: The importance of restriction. JAMA. 2010;304(8):897–898. [DOI] [PubMed] [Google Scholar]
- 38. Sprecher VP, Didden E-M, Swerdel JN, Muller A. Evaluation of code-based algorithms to identify pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension patients in large administrative databases. Pulm Circ. 2020;10(4):2045894020961713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taylor Jr DH, Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of medicare claims as an epidemiological tool: The case of dementia revisited. J Alzheimer’s Dis. 2009;17(4):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schneeweiss S. A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf. 2010;19(8):858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Detry MA, Lewis RJ. The intention-to-treat principle: How to assess the true effect of choosing a medical treatment. JAMA. 2014;312(1):85–86. [DOI] [PubMed] [Google Scholar]
- 42. Solomon A, Ngandu T, Soininen H, Hallikainen MM, Kivipelto M, Laatikainen T. Validity of dementia and Alzheimer’s disease diagnoses in Finnish national registers. Alzheimer’s Dement. 2014;10(3):303–309. [DOI] [PubMed] [Google Scholar]
- 43. Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurol. 2006;5(9):735–741. [DOI] [PubMed] [Google Scholar]
- 44. Barnes DE, Beiser AS, Lee A, et al. . Development and validation of a brief dementia screening indicator for primary care. Alzheimers Dement. 2014;10(6):656–665.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Albrecht JS, Hanna M, Kim D, Perfetto EM. Predicting diagnosis of Alzheimer’s disease and related dementias using administrative claims. J Manag Care Spec Pharm. 2018;24:1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brookhart MA, Patrick AR, Dormuth C, et al. . Adherence to lipid-lowering therapy and the use of preventive health services: An investigation of the healthy user effect. Am J Epidemiol. 2007;166(3):348–354. [DOI] [PubMed] [Google Scholar]
- 47. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in medicare data: Development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 49. Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 2:69–80. [DOI] [PubMed] [Google Scholar]
- 50. Austin PC. Some methods of propensity-score matching had superior performance to others: Results of an empirical investigation and monte carlo simulations. Biometrical J. 2009;51(1):171–184. [DOI] [PubMed] [Google Scholar]
- 51. Walker AM AM, Patrick A, Lauer M, et al. . Tool for assessing the feasibility of comparative effectiveness research. Comp Effect Res. 2013;3:11–20. [Google Scholar]
- 52. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–1234. [Google Scholar]
- 53. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Andrew MK, Tierney MC. The puzzle of sex, gender and Alzheimer’s disease: Why are women more often affected than men? Womens Health. 2018;14:1745506518817995. [Google Scholar]
- 55. Tublin JM, Adelstein JM, Del Monte F, Combs CK, Wold LE. Getting to the heart of Alzheimer disease. Circ Res. 2019;124(1):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fayosse A, Nguyen D-P, Dugravot A, et al. . Risk prediction models for dementia: Role of age and cardiometabolic risk factors. BMC Med. 2020;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer Disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang SV, Verpillat P, Rassen JA, Patrick A, Garry EM, Bartels DB. Transparency and reproducibility of observational cohort studies using large healthcare databases. Clin Pharmacol Ther. 2016;99(3):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fralick M, Kesselheim AS, Avorn J, Schneeweiss S. Use of health care databases to support supplemental indications of approved medications. JAMA Intern Med. 2018;178(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patorno E, Schneeweiss S, Gopalakrishnan C, Martin D, Franklin JM. Using real-world data to predict findings of an ongoing phase IV cardiovascular outcome trial: Cardiovascular safety of linagliptin versus glimepiride. Diabetes Care. 2019;42(12):2204–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005; 58(4):323–337. [DOI] [PubMed] [Google Scholar]
- 62. Mayo Clinic . Sildenafil (Oral Route). Accessed 4 January 2022. https://www.mayoclinic.org/drugs-supplements/sildenafil-oral-route/proper-use/drg-20066989. [Google Scholar]
- 63. Gómez-Vallejo V, Ugarte A, García-Barroso C, et al. . Pharmacokinetic investigation of sildenafil using positron emission tomography and determination of its effect on cerebrospinal fluid cGMP levels. J Neurochem. 2016;136(2):403–415. [DOI] [PubMed] [Google Scholar]
- 64. García-Barroso C, Ricobaraza A, Pascual-Lucas M, et al. . Tadalafil crosses the blood-brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology. 2013;64:114–123. [DOI] [PubMed] [Google Scholar]
- 65. Coward RM, Carson CC. Tadalafil in the treatment of erectile dysfunction. Ther Clin Risk Manag. 2008;4(6):1315–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wright PJ. Comparison of phosphodiesterase type 5 (PDE5) inhibitors. Int J Clin Pract. 2006;60(8):967–975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data analyzed in this study represent patient-level information and are not allowed for public sharing based on the data use agreement with the Centers for Medicare and Medicaid Services.