Abstract
Based on the observation that administration of clarithromycin led to an attenuation of the inflammatory response induced by surgical trauma in a guinea pig model, we investigated the potential beneficial effects of clarithromycin on the local and systemic inflammatory response in patients undergoing mastectomy in an open-label prospective study. During a 16-month period, 54 patients who underwent mastectomy were randomly divided into two groups. In one group, the patients received oral clarithromycin at a dose of 500 mg twice a day, from the day before to 3 days after mastectomy. There was no significant difference in the incidence of antibiotic prophylaxis-related toxicities or postoperative infections between the patients who received clarithromycin and those who did not. Clarithromycin treatment was significantly associated with an attenuation of febrile response, tachycardia, tachypnea, and an increase in monocyte counts (P, <0.0001, <0.01, <0.05, and <0.01, respectively). Clarithromycin also reduced the intensity and duration of postoperative pain (P, <0.05 and <0.005, respectively) and increased the range of motion of the involved shoulder (P < 0.05 for abduction and flexion). We conclude that clarithromycin effectively modulates the acute inflammatory response associated with mastectomy and produces a better clinical outcome.
Surgical operations induce major physiological and immunological changes. These changes are activated by various stimuli, such as nociceptive stimulation, tissue injury, tissue ischemia, reperfusion, and hemodynamic disturbances. The clinical response is the result of complex changes, which include dysregulation of T-cell function, changes in the balance of cytokines and counterregulatory hormones, and increased hepatic synthesis of acute-phase reactants. In most patients, the systemic changes are minimal and self-limiting. However, in patients with complicated surgery or major trauma, the response becomes extensive and prolonged, resulting in systemic inflammatory response syndrome (SIRS), which is characterized by increased or suppressed body temperature, increased heart rate, hyperventilation, and an abnormal peripheral leukocyte count (2). Although an inflammatory response is an inevitable and essential part of the repair process and a natural defensive reaction to prevent infections associated with trauma, such undesirable fallout from surgical treatment is associated with significant morbidity and even mortality.
The macrolide group of antibiotics is associated with in vitro and in vivo immunomodulating activities (18, 31–33). Erythromycin, one of the macrolides, has been used extensively for antimicrobial prophylaxis in colorectal surgery (22). Recently, it has also been shown that clarithromycin attenuates the inflammatory response induced by surgical trauma in a guinea pig model (32).
Mastectomy is a suitable surgical procedure for the study of the acute inflammatory response to surgery because the extensive dissection causes significant tissue damage and bacterial contamination of the surgical site is uncommon. In this study, we report the clinical profile of the local and systemic inflammatory responses due to mastectomy and the effects of clarithromycin on the mastectomy-induced acute inflammatory response in an open-label, prospective, randomized-control trial.
MATERIALS AND METHODS
Preoperative protocol.
Approval from the local ethics committee was obtained for this trial. Patients were recruited between February 1997 and May 1998. The diagnosis of breast cancer was confirmed by mammographic examinations and cytological examinations of fine-needle aspirates before the operation. Patients who were pregnant and those with diabetes mellitus, cirrhosis of the liver, chronic renal failure, myasthenia gravis, a bleeding tendency, history of allergy to macrolides, antibiotic treatment within 2 weeks of the study, or long-term immunosuppression were excluded from the study. If there were no reasons for exclusion, the nature and purpose of the trial were explained to the patients and informed consent was obtained for inclusion in the trial. Fifty-six patients with breast cancer were recruited for the randomized trial. Two patients in the control group dropped out due to refusal of venipuncture. One patient from each group underwent additional transrectus abdominal myocutaneous (TRAM) flap surgery for breast reconstruction.
Blood was drawn from patients the day before the operation for a preanesthetic workup that included complete blood counts, differential white cell counts, renal and liver function tests, coagulation profiles, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) level. Levels of interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) in serum were also determined.
Randomization.
Consecutive patients (except those excluded) were enrolled and randomized into two groups by computer. Patients in the study group were given oral clarithromycin (500 mg twice a day) from the day before to 3 days after the operation. Patients in the control group did not receive any clarithromycin. All surgeons and medical staff responsible for assessing the outcome were unaware of the randomization results because separate prescription sheets were given for the clarithromycin prescription.
Anesthesia.
All patients underwent standard general anesthesia. They were not given any premedication except for the single morning dose of clarithromycin. Induction was done with thiopenal, fentanyl, and vecuronium, and maintenance was achieved with nitrous oxide and isoflurane. Fentanyl and vecuronium were given as required.
Surgical procedures.
The operation involved the total removal of the breast and axillary dissection up to the level II lymph nodal station. The raising of the flaps and tissue dissection during mastectomy were performed by electric cautery. Closed-suction drains were placed at the chest and axilla after the mastectomy and a deep culture swab was taken from the surgical wound before closure. The wound was closed by interrupted subcuticular sutures.
Two patients underwent additional TRAM flap reconstruction. This involved the raising by electric cautery of extensive abdominal skin and subcutaneous tissue from the underlying muscle and fascia. The rectus muscles and the overlying flap were harvested and placed at the mastectomy site. The defect in the abdominal wall was closed by primary closure.
Patients were closely monitored during the operation. Blood loss, operative time, administration of anesthetic drugs, and intravenous fluid were recorded. A deep wound swab for bacterial culture was taken at the operation site. The area of dissection for mastectomy and TRAM flap reconstruction was measured by placing the specimen or the harvested flap on a piece of paper and marking out the surface area.
Postoperative management.
The patients were monitored at 4-h intervals after the operation for elevation of temperature, respiratory rate, and heart rate. Postoperative pain was treated with oral acetaminophen and intravenous propoxyphene hydrochloride, given on an as-needed basis every 4 h. The intensity of pain was charted every 4 h and whenever analgesics were given, using a visual log scale from 0 to a maximum of 10 (1). The intravenous-fluid requirement and the urine output for the first 48 h were monitored. The amount of drain fluid was charted daily until the drains were removed. The wound was inspected daily and any development of flap necrosis, infection (cellulitis with a purulent discharge), or blister formation was charted. Blood samples were taken at the peak of the febrile episodes and cultured to monitor for any possible transient bacteremia. Each set of blood cultures consisted of an aerobic bottle with resin and an anaerobic bottle, and the BACTEC 9240 blood culture system (Becton Dickinson, Gaithersburg, Md.) was used. The range of movement of the shoulder on the corresponding side of the surgery was charted on postoperative day 5 by an experienced physiotherapist who was not aware of the randomization results.
Blood was drawn immediately after the operation in the recovery room and on postoperative days 1, 2, and 5. Except for the renal and liver function tests, the samples were tested for the parameters listed in the preoperative protocol. Drain fluid was also collected on postoperative days 1, 2, and 5. The drain fluid was sent for bacterial culture and tested for total cell and differential counts. Biochemical assays of serum and drain fluid were also performed to analyze the levels of CRP, IL-6, and TNF-α.
Measurement of IL-6 and TNF-α by enzyme-linked immunosorbent assays.
Commercial kits (Boehringer GmbH, Mannheim, Germany) were used for all enzyme-linked immunosorbent assays. Serum samples were centrifuged at high speed to remove turbidity and particles. Immunoreagent solution was prepared by adding 50 μl of peroxidase-conjugated detection antibody to 0.9 ml of incubation buffer, and 50 μl of biotin antibody was added after mixing. Twenty microliters of each supernatant was added into the microtiter plate provided, and 200 μl of immunoreagent was pipetted into all wells containing the test samples. The microtiter plate was then covered tightly with adhesive foil and incubated for 2 h at room temperature on a shaker at 250 rpm. The incubation buffer was removed by tapping and the wells were rinsed three times with washing buffer. The washing solution was removed and 200 μl of substrate solution was added to the wells. The microtiter plate was covered again with the adhesive foil and incubated in the same manner for another 20 to 30 min at room temperature. Fifty microliters of stop solution was added to each well, and after incubation for 1 min the plate was read by using a spectrophotometer at 450 nm (reference wavelength, 690 nm).
Statistical analysis.
Parameters were compared using SPSS software, release 6.0 (SPSS Inc., Chicago, Ill.). Interval changes in the physiological and hematological parameters, acute-phase proteins, and cytokines were analyzed with reference to preoperative values. One-way analysis-of-variance tests were used to compare means in each group over the time period studied. Fisher's exact test or the chi-square test was used to compare the number of events between groups. Bivariate correlation was performed for univariate analysis. All values are means and standard errors of the means (SEM), unless otherwise stated. A P value of <0.05 was considered statistically significant.
RESULTS
Patients.
There were no significant differences between the two groups in terms of age, area of dissection, blood loss, operation time, and the amount of parenteral fluid administered during the perioperative period (Table 1). The culture swabs taken during the operation were negative for bacterial growth for all 54 patients.
TABLE 1.
Patient characteristics of control and clarithromycin groups
| Characteristic | Control groupa(n = 26) | Clarithromycin groupa(n = 28) |
|---|---|---|
| Age (yr) | 52.1 ± 2.1 | 51.1 ± 2.1 |
| Area of dissection (cm2) | 575.0 ± 51.5 | 581.9 ± 45.0 |
| Blood loss (ml) | 336.5 ± 55.4 | 330.4 ± 29.5 |
| Operation time (min) | 141.4 ± 12.0 | 134.1 ± 7.3 |
| Amt of parenteral fluid (ml) | 1,380.8 ± 207.8 | 1,600.0 ± 134.3 |
Values are means ± SEM. P values, calculated by using Student's t test, were not significant.
Acute inflammatory response after mastectomy.
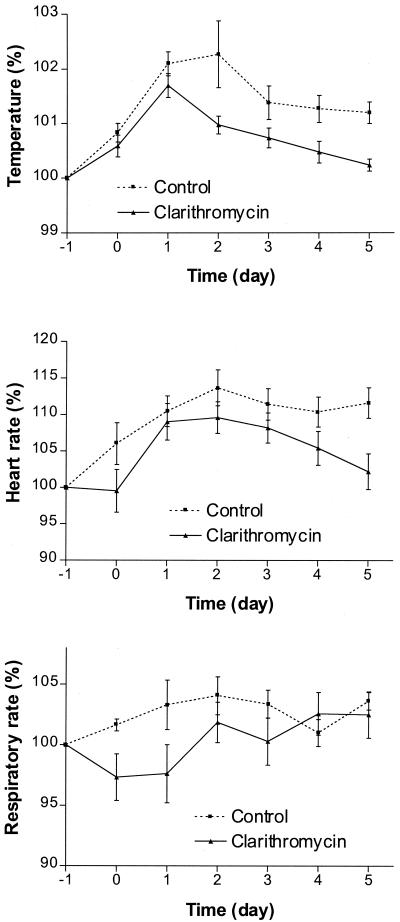
The parameters reflecting a systemic inflammatory response were analyzed in the control group. There was a progressive elevation of temperature, heart rate, and respiratory rate above preoperative levels (Fig. 1) until postoperative day 5. Mastectomy produced significant changes in temperature (P < 0.0001) and heart rate (P < 0.001). The changes in respiratory rate did not reach statistical significance.
FIG. 1.
Changes in physiological parameters for control and clarithromycin groups.
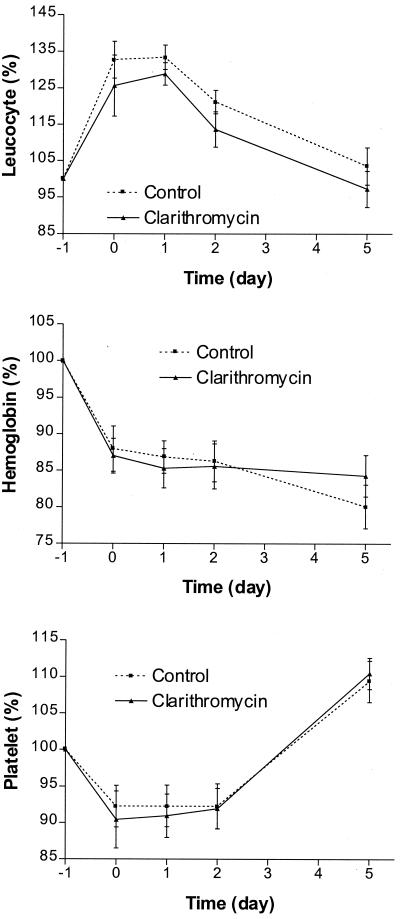
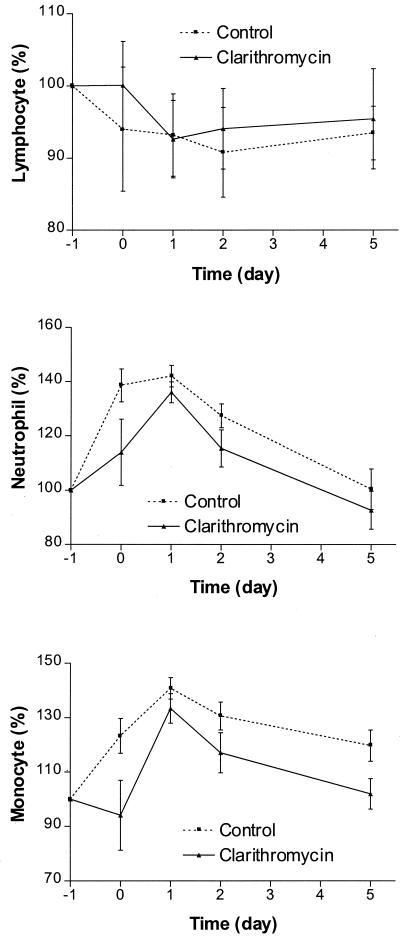
The hematological parameters after mastectomy are shown in Fig. 2. There was a drop in hemoglobin and the interval changes were statistically significant (P < 0.0001). The changes in white blood cell and platelet counts, on the other hand, were more marked. There was an elevation of white blood cell counts almost immediately after the operation. This returned to the preoperative level on day 5. The interval changes were statistically significant (P < 0.0001). The platelet counts showed a different time trend. There was an immediate drop after mastectomy and this persisted until 48 h after surgery. However, the counts returned to the preoperative level on postoperative day 5. The interval changes were not statistically significant. The elevation of neutrophil counts was greatest on the day of the operation and monocyte counts reached a peak 48 h after the operation (Fig. 3). The interval changes in monocyte and neutrophil counts were statistically significant (P < 0.0001 for both parameters). On the other hand, there was progressive lymphopenia after the operation; the percentage changes increased on the day of the operation but dropped below the basal level from postoperative day 1 to 5.
FIG. 2.
Changes in hematological parameters for control and clarithromycin groups.
FIG. 3.
Changes in differential cell counts for control and clarithromycin groups.
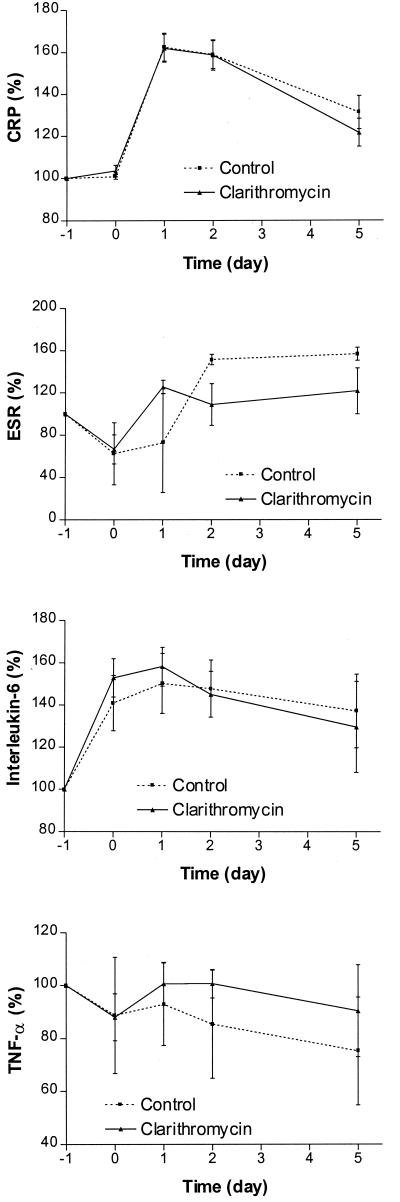
The other parameters reflecting acute inflammatory reactions are shown in Fig. 4. The interval changes in CRP and ESR were statistically significant (P, <0.005 and <0.0001). Statistical significance was not reached for changes in levels of IL-6 and TNF-α.
FIG. 4.
Changes in CRP, ESR, IL-6, and TNF-α for control and clarithromycin groups.
Effects of clarithromycin on the mastectomy-induced acute inflammatory response.
The results of the comparison between the clarithromycin and control groups for the mean interval changes in physiological, hematological, and other laboratory parameters measured for the acute inflammatory response are summarized in Table 2. The differences between the two groups were statistically significant for changes in temperature, heart rate, respiratory rate, and monocyte count. The differences in changes in neutrophil count and ESR did not reach statistical significance.
TABLE 2.
Comparison of mean interval changesa of parameters for control and clarithromycin groups
| Parameter | Control groupb | Clarithromycin groupb | P value |
|---|---|---|---|
| Temp (°C) | 0.48 ± 0.05 | 0.25 ± 0.03 | <0.0001 |
| Heart rate (beats/min) | 8.59 ± 0.84 | 5.56 ± 0.74 | <0.01 |
| Respiratory rate (counts/min) | 0.62 ± 0.10 | 0.19 ± 0.16 | <0.05 |
| White cell count (109/liter) | 2.21 ± 0.28 | 1.69 ± 0.24 | NSc |
| Hemoglobin (g/dl) | −1.30 ± 0.14 | −1.19 ± 0.12 | NS |
| Platelet count (109/liter) | −2.19 ± 2.67 | −3.02 ± 2.76 | NS |
| Lymphocyte count (109/liter) | 0.01 ± 0.04 | 0.11 ± 0.06 | NS |
| Monocyte count (109/liter) | 0.17 ± 0.02 | 0.10 ± 0.02 | <0.01 |
| Neutrophil count (109/liter) | 2.06 ± 0.27 | 1.49 ± 0.21 | NS |
| CRP (109/liter) | 1.00 ± 0.22 | 1.06 ± 0.23 | NS |
| ESR (mm/h) | 8.35 ± 1.74 | 5.19 ± 0.93 | NS |
| IL-6 (pg/ml) | 5.25 ± 1.67 | 4.21 ± 0.81 | NS |
| TNF-α (pg/ml) | 0.73 ± 1.91 | −1.56 ± 1.75 | NS |
The mean interval change for a parameter is defined as the mean of the difference between each day and the day before the operation for the corresponding parameter.
Values are means ± SEM.
NS, not significant.
When the total amount of drain fluid was analyzed, the mean amount (±SEM) of drain fluid for the control group was 926 (±88.1) ml, whereas that for the clarithromycin group was 1,052 (±145.0) ml. There was no statistically significant difference between the two groups. The durations of drainage output were 7.8 (±0.53) days and 7.9 (±0.54) days for the control and clarithromycin groups, and again there was no statistically significant difference. The total white blood cell count and differential counts, as well as the levels of CRP, IL-6, and TNF-α in the drain fluid, showed no statistically significant difference between the two groups.
The intensity of pain from mastectomy was higher for the control group of patients. The mean score was 4.2 (±0.43) for the control group, and the corresponding value for the clarithromycin group was 3.2 (±0.35). The difference was statistically significant (P < 0.05). The duration of pain was also longer for the control group. The mean durations for the control and clarithromycin groups were 3.98 (±0.34) days and 2.63 (±0.31) days, respectively. The difference was statistically significant (P < 0.005). The patients of the control group consumed more analgesics for relief of pain. The mean frequencies of analgesic consumption for the control and clarithromycin groups were 2.77 (±0.41) and 1.35 (±0.24) times, respectively, and the difference was statistically significant (P < 0.005).
Postoperative functional status in terms of range of shoulder abduction and flexion was better for the clarithromycin group. The mean range of abduction for the control group was 66.2° (±4.2°), whereas the mean value for the clarithromycin group was 80.0° (±5.2°) (P < 0.05). The corresponding values for the range of flexion were 76.5° (±4.2°) and 91.2° (±4.7°) for the respective groups (P < 0.05).
No patients developed a wound infection. Wound flap necrosis for both groups was minor and limited to the wound edge. Five and two patients of the control and clarithromycin groups developed flap necrosis, respectively.
DISCUSSION
Surgery induces both inflammation and immunosuppression, resulting in the development of local and systemic inflammatory responses (8). These responses usually abate spontaneously. However, when they are excessive, physiological homeostasis is upset and when challenged by additional insults, deleterious effects, such as severe SIRS and multiorgan dysfunction, may occur.
Clinical researchers have been looking for ways to modulate the physiological responses after surgery. Anesthetic agents have effects on the immune system (12, 19, 23). The cell number and activity of natural killer cells are diminished during induction and there is a change in the balance of pro- and anti-inflammatory cytokines. Corticosteroids are powerful drugs for inflammatory conditions, and parenteral administration of betamethasone, for example, can significantly reduce postoperative swelling and pain (11, 28). Nonsteroidal anti-inflammatory drugs (NSAIDs) are effective in reducing postoperative pain, but their modulating effect on the systemic inflammatory response after surgery is not satisfactory. Epidural analgesia in combination with systemic indomethacin markedly reduces postoperative pain (27). However, the elevation of plasma cortisol, acute-phase proteins, and leukocyte and differential counts, which are changes typical of the acute-phase response, are only minimally modulated. Moreover, when the effect of an NSAID was compared with that of glucocorticoid, the steroid was found to have a greater effect than the NSAID on suppression of postoperative inflammation (30). Furthermore, when the combined effect of prednisolone, epidural analgesia, and systemic indomethacin was studied, the leukocytic reaction and acute-phase response were unmodified (7, 10). Another approach utilizes synthetic analogues of thymic hormones, such as thymopentin, to combat the immunosuppression associated with major surgery (3). It has been shown that thymopentin, in combination with indomethacin, can adequately preserve and/or restore intact macrophage and T-cell interaction and thus appears to be a feasible approach to maintain normal host defense activity in patients undergoing major surgery (14). In addition, thymopentin has been shown to reduce mortality in experimental thermal injury (5). The improvement in host survival is related to the reduction in levels of IL-4 and increased production of IL-2.
With the advent of laparoscopic technology, clinical studies have shown that the reduction in the extent of surgical dissection by laparoscopic surgery reduces nociceptive stimulation and consequently the neuroendocrine response. This is evident by the reduction in postoperative pain and early return to normal function. The granulocyte count and IL-6 level were lower in patients undergoing laparoscopic surgery (16, 17, 20), and the immunocompetence measured by phytohemagglutinin responsiveness was also stronger for the laparoscopic group (17). Laparoscopic surgery is associated with a significant decrease in monocyte and neutrophil release of O2−, TNF-α, chemotaxis, and white blood cell count (29). However, CD4/CD8 ratios were still decreased after laparoscopic surgery but reverted back to normal 1 week later (15). When laparoscopic surgery has been compared with minilaparotomy, earlier studies reported that laparoscopic surgery was superior in reducing hospital stay and postoperative dysfunction as well as leading to a quicker return to normal activities. However, subsequent reports did not show any improvement in postoperative recovery and respiratory impairment or any reduction in neuroendocrine response and cortisol production (4, 24, 34). When laparoscopic surgery involving a larger extent of surgical dissection, such as laparoscopic adrenalectomy, was compared with open surgery, white blood cell count and CRP level, two parameters that reflect acute inflammation, did not differ significantly between groups (6). Therefore, laparoscopic surgery is not the complete answer for reducing the harmful effects caused by surgical trauma.
Studies comparing mastectomy with other major surgeries, such as esophagectomy and pulmonary lobectomy, demonstrated that mastectomy could induce higher neutrophil, adrenocorticotropin, and cortisol levels (9). In the present study, we also found slight lymphopenia after mastectomy, in addition to the elevation of neutrophil and monocyte counts. Therefore, mastectomy provides a good opportunity for the study of immunomodulation after surgical trauma. The amount of energy applied to the whole operative field is adequate to produce a significant local inflammatory response at the operative site in all patients and SIRS in a certain proportion of patients. Twenty-four of 26 patients in the control group (92.3%) developed one or more features of an inflammatory response after mastectomy. Nine patients (34.6%) met the criteria for SIRS (2).
An effective antibiotic, besides having an antimicrobial effect, may also be a potent immunomodulator to combat the alteration of the immune system. The modulation of the immune system by antibiotics was first observed by Metchnikoff and Helmholz (cited in reference 13). They reported the contradictory results of enhancement and depression of phagocyte functions by quinine. Antibiotics of the macrolide group, such as azithromycin, erythromycin, and roxithromycin, have also been reported to have immunomodulating effects (18, 21, 25).
In a previous study on the effects of clarithromycin on surgical trauma in an animal model, the neutrophil and monocyte counts of the clarithromycin-treated group were significantly lower than those of the control group (32). The platelet counts were also lower for the clarithromycin group. In addition, there was a trend towards a milder elevation of temperature and respiratory rate in the group of animals pretreated with clarithromycin. Based on these observations, it was concluded that clarithromycin suppresses both the local and systemic inflammatory responses after surgical trauma.
In the present study, we found that there was a marked reduction in the acute-phase response in the clarithromycin group. The important parameters, such as temperature, heart rate, and respiratory rate, showed significantly smaller changes in the clarithromycin group than in the control group. When the parameters were analyzed on interval scales, there was a greater reduction in the elevation of temperature for the clarithromycin group. The changes in the heart and respiratory rates were also significantly less than in the control group. Although the changes in white cell counts did not reach statistical significance, the increase in monocyte count in the clarithromycin group was significantly less than that in the control group.
The clinical effects of clarithromycin at the surgical site were assessed. The intensity as well as the duration of pain was lower in the clarithromycin group than in the control group. The frequency of analgesic use was also lower. The range of movement was significantly greater than in the control group. Moreover, the odds ratios for wound infection and necrosis were lower for patients treated with clarithromycin. These parameters reflect that this macrolide has a considerable “cleansing” effect at the operative site, leading to a much weaker local inflammatory response and a much better clinical outcome. In addition to the immunomodulatory effects of clarithromycin on surgical trauma, this drug has also been reported to be a potent inhibitor of tumor-induced angiogenesis in a mouse cancer model (35). A clinical study also demonstrated that the drug prolonged survival of patients with unresectable non-small-cell lung cancer by increasing the bioactivity of IL-12 and natural killer cell activity (26). One important limitation of using clarithromycin for immunomodulatory prophylaxis in surgical trauma is the possibility of inducing antimicrobial resistance. This could be overcome by developing clarithromycin analogues which do not have antimicrobial activities. In the meantime, further studies should be conducted to assess if a single dose or 48-h doses of clarithromycin would also be effective.
These observations reflect that clarithromycin has the capacity to modulate the inflammatory response resulting from surgical trauma. Although it was not apparent statistically that the modulation was mediated through changes in IL-6, CRP, or TNF-α, the mean values for these variables in the clarithromycin group were lower than in the control group. Although the mechanism of action of clarithromycin remains to be elucidated, it is likely that the drug acts on the function and number of monocytes and macrophages, in a manner similar to that of erythromycin. Of the leukocyte populations studied, we found that there is a significant modulation by clarithromycin of the increase in monocytes. The lymphopenia associated with surgical operations was not nullified and the increase in neutrophils was not reduced. The drug may not act on T cells directly, but when the antigen-presenting capacity of monocytes is affected, T-cell function may also be altered. The other possible mode of action could be related to the augmentation of the capacity of phagocytosis by macrophages to clean up the inflammatory debris from surgical operations. The phagocytic augmentation is strong enough locally to reduce the intensity of pain and shorten its duration. These actions enable the drug to attenuate the development of SIRS. In conclusion, this randomized-control, open-label, prospective study provided sufficient evidence of macrolide modulation of surgical-trauma-related inflammation. Further placebo-controlled trials with larger numbers of patients and different operative procedures should be conducted to ascertain the beneficial effects of immunomodulation in patients with surgical trauma.
REFERENCES
- 1.Ad Hoc Committee on Cancer Pain of the American Society of Clinical Oncology. Cancer pain assessment and treatment curriculum guidelines. J Clin Oncol. 1992;10:1976–1982. doi: 10.1200/JCO.1992.10.12.1976. [DOI] [PubMed] [Google Scholar]
- 2.Bone R C, Balk R A, Cerra F B, Dellinger R P, Fein A M, Knaus W A, Schein R M, Sibbald W J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 3.Braga M, Gianotti L, Gentilini O, Fortis C, Consogno G, DiCarlo V. Thymopentin modulates Th1 and Th2 cytokine response and host survival in experimental injury. J Surg Res. 1996;62:197–200. doi: 10.1006/jsre.1996.0195. [DOI] [PubMed] [Google Scholar]
- 4.Carlone N A, Cuffini A M, Tullio V, Sassella D. Comparative effects of roxithromycin and erythromycin on cellular immune functions in vitro. 2. Chemotaxis and phagocytosis of 3H-Staphylococcus aureus by human macrophages. Microbios. 1989;58:17–25. [PubMed] [Google Scholar]
- 5.Cho J M, LaPorta A, Clark J R, Schofield M J, Hammond S L, Mallory P L., 2nd Response of serum cytokines in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 1994;8:1380–1383. doi: 10.1007/BF00187340. [DOI] [PubMed] [Google Scholar]
- 6.Cuffini A M, Carlone N A, Tullio V, Borsotto M. Comparative effects of roxithromycin and erythromycin on cellular immune functions in vitro. 3. Killing of intracellular Staphylococcus aureus by human macrophages. Microbios. 1989;58:27–33. [PubMed] [Google Scholar]
- 7.Faist E, Markewitz A, Fuchs D, Lang S, Zarius S, Schildberg F W, Wachter H, Reichart B. Immunomodulatory therapy with thymopentin and indomethacin. Successful restoration of interleukin-2 synthesis in patients undergoing major surgery. Ann Surg. 1991;214:264–273. doi: 10.1097/00000658-199109000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faist E, Faist E, Kim C. Therapeutic immunomodulatory approaches for the control of systemic inflammatory response syndrome and the prevention of sepsis. New Horiz. 1998;2(Suppl.):S97–S102. [PubMed] [Google Scholar]
- 9.Fujii T, Kadota J, Kawakami K, Iida K, Shirai R, Kaseda M, Kawamoto S, Kohno S. Long term effect of erythromycin therapy in patients with chronic Pseudomonas aeruginosa infection. Thorax. 1995;50:1246–1252. doi: 10.1136/thx.50.12.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein G, Audhya T. Thymopoietin to thymopentin: experimental studies. Surv Immunol Res. 1985;4:1–10. doi: 10.1007/BF02919050. [DOI] [PubMed] [Google Scholar]
- 11.Higgs G A, Moncada S, Vane J R. The role of arachidonic acid metabolites in inflammation. In: Weissmann G, Samuelsson B, Paoletti R, editors. Advances in inflammatory research. Vol. 1. New York, N.Y: Raven Press; 1979. pp. 419–430. [Google Scholar]
- 12.Hjortso N C, Neumann P, Frosig F, Andersen T, Lindhard A, Rogon E, Kehlet H. A controlled study on the effect of epidural analgesia with local anaesthetics and morphine on morbidity after abdominal surgery. Acta Anaesthesiol Scand. 1985;29:790–796. doi: 10.1111/j.1399-6576.1985.tb02302.x. [DOI] [PubMed] [Google Scholar]
- 13.Keicho N, Kudoh S, Yotsumoto H, Akagawa S. Erythromycin promotes monocyte to macrophage differentiation. J Antibiot. 1994;47:80–89. doi: 10.7164/antibiotics.47.80. [DOI] [PubMed] [Google Scholar]
- 14.Kloosterman T, von-Blomberg B M, Borgstein P, Cuesta M A, Scheper R J, Meijer S. Unimpaired immune functions after laparoscopic cholecystectomy. Surgery. 1994;115:424–428. [PubMed] [Google Scholar]
- 15.Kollmorgen C F, Thompson G B, Grant C S. Laparoscopic versus open posterior adrenalectomy: comparison of acute-phase response and wound healing in the cushingoid porcine model. World J Surg. 1998;22:613–620. doi: 10.1007/s002689900443. [DOI] [PubMed] [Google Scholar]
- 16.Majeed A W, Troy G, Nicholl J P, Reed M W R, Stoddard C J, Johnson A G. Randomized, prospective, single-blind comparison of laparoscopic versus small-incision cholecystectomy. Lancet. 1996;347:989–994. doi: 10.1016/s0140-6736(96)90143-9. [DOI] [PubMed] [Google Scholar]
- 17.Maruszynski M, Pojda Z. Interleukin 6 (IL-6) levels in the monitoring of surgical trauma. A comparison of serum IL-6 concentrations in patients treated by cholecystectomy via laparotomy or laparoscopy. Surg Endosc. 1995;9:882–885. doi: 10.1007/BF00768883. [DOI] [PubMed] [Google Scholar]
- 18.Matsuoka N, Eguchi K, Kawakami A, Tsuboi M, Kawabe Y, Aoyagi T, Nagataki S. Inhibitory effect of clarithromycin on costimulatory molecule expression and cytokine production by synovial fibroblast-like cells. Clin Exp Immunol. 1996;104:501–508. doi: 10.1046/j.1365-2249.1996.46752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McBride W T, Armstrong M A, McBride S J. Immunomodulation: an important concept in modern anesthesia. Anesthesia. 1996;51:465–473. doi: 10.1111/j.1365-2044.1996.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 20.McMahon A J, Russell I T, Baxter J N, Ross S, Anderson J R, Morran C G, Sunderland G, Galloway D, Ramsay G, O'Dwyer P J. Laparoscopic versus minilaparotomy cholecystectomy: a randomized trial. Lancet. 1994;343:135–138. doi: 10.1016/s0140-6736(94)90932-6. [DOI] [PubMed] [Google Scholar]
- 21.Nagai H, Shishido H, Yoneda R, Yamaguchi E, Tamura A, Kurashima A. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration. 1991;58:145–149. doi: 10.1159/000195915. [DOI] [PubMed] [Google Scholar]
- 22.Nichols R L, Smith J W, Garcia R Y, Waterman R S, Holmes W C. Current practices of preoperative bowel preparation among North American colorectal surgeons. Clin Infect Dis. 1997;24:609–619. doi: 10.1093/clind/24.4.609. [DOI] [PubMed] [Google Scholar]
- 23.Norman J G, Fink G W. The effects of epidural anesthesia on the neuroendocrine response to major surgical stress: a randomized prospective trial. Am Surg. 1997;63:75–80. [PubMed] [Google Scholar]
- 24.Panteix G, Guillaumond B, Harf R, Desbos A, Sapin V, Leclercq M, Perrin-Fayolle M. In-vitro concentration of azithromycin in human phagocytic cells. J Antimicrob Chemother. 1993;31(Suppl. E):1–4. doi: 10.1093/jac/31.suppl_e.1. [DOI] [PubMed] [Google Scholar]
- 25.Roche Y, Gougerot-Pocidalo M A, Fay A, Forest N, Pocidalo J J. Macrolides and immunity: effects of erythromycin and spiramycin on human mononuclear cell proliferation. J Antimicrob Chemother. 1986;17:195–203. doi: 10.1093/jac/17.2.195. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto M, Mikasa K, Hamada K, Konishi M, Maeda K, Yoshimoto E, Ueda K, Majima T, Sawaki M, Kita E, Narita N. Effect of clarithromycin treatment on natural killer cell activity in patients with advanced non-small cell lung cancer. Gan To Kagaku Ryoho. 1998;25:2259–2266. [PubMed] [Google Scholar]
- 27.Schulze S, Roikjaer O, Hasselstrom L, Jensen N H, Kehlet H. Epidural bupivacaine and morphine plus systemic indomethacin eliminates pain but not systemic response and convalescence after cholecystectomy. Surgery. 1988;103:321–327. [PubMed] [Google Scholar]
- 28.Skjelbred P, Lokken P. Post-operative pain and inflammatory reaction reduced by injection of a corticosteroid—a controlled trial in bilateral oral surgery. Eur J Clin Pharmacol. 1982;21:391–396. doi: 10.1007/BF00542325. [DOI] [PubMed] [Google Scholar]
- 29.Squirrell D M, Majeed A W, Troy G, Peacock J E, Nicholl J P, Johnson A G. A randomized, prospective, blinded comparison of postoperative pain, metabolic response, and perceived health after laparoscopic and small incision cholecystectomy. Surgery. 1998;123:485–495. doi: 10.1067/msy.1998.87552. [DOI] [PubMed] [Google Scholar]
- 30.Troullos E S, Hargreaves K M, Butler D P, Dionne R A. Comparison of nonsteroidal anti-inflammatory drugs, ibuprofen and fluprofen, with methylprednisolone and placebo for acute pain, swelling, and trismus. J Oral Maxillofac Surg. 1990;48:945–952. doi: 10.1016/0278-2391(90)90007-o. [DOI] [PubMed] [Google Scholar]
- 31.Woo P C Y, Tsoi H-W, Wong L-P, Leung H C H, Yuen K-Y. Antibiotics modulate vaccine-induced humoral immune response. Clin Diagn Lab Immunol. 1999;6:832–837. doi: 10.1128/cdli.6.6.832-837.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo P C Y, Chow L W C, Ma E S K, Yuen K Y. Clarithromycin attenuates the inflammatory response induced by surgical trauma in a guinea pig model. Pharmacol Res. 1999;39:49–54. doi: 10.1006/phrs.1998.0400. [DOI] [PubMed] [Google Scholar]
- 33.Woo P C Y, Ng W F, Leung H C H, Tsoi H W, Yuen K Y. Clarithromycin attenuates cyclophosphamide-induced mucositis in mice. Pharmacol Res. 2000;41:527–532. doi: 10.1006/phrs.1999.0613. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi H, Kobayashi E, Yoshida T, Kiyozaki H, Hozumi Y, Kohiyama R, Suminaga Y, Sakurabayashi I, Fujimura A, Miyata M. Changes in immune-endocrine response after surgery. Cytokine. 1998;10:549–554. doi: 10.1006/cyto.1997.0322. [DOI] [PubMed] [Google Scholar]
- 35.Yatsunami J, Turuta N, Wakamatsu K, Hara N, Hayashi S. Clarithromycin is a potent inhibitor of tumor-induced angiogenesis. Res Exp Med. 1997;197:189–197. doi: 10.1007/s004330050068. [DOI] [PubMed] [Google Scholar]