Abstract
Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) ST398 is mainly found in Europe and North America, colonizing the nasal cavity of pigs. This study characterized the MRSA isolates recovered from pig nasal swabs (n = 171) by evaluating the antimicrobial susceptibility profile by broth microdilution and characterizing the genetic lineages by spa-typing. Three linezolid-resistant isolates were subjected to Whole-Genome Sequencing (WGS). All strains harbored the mecA gene and were resistant to tetracycline and susceptible to vancomycin. A high frequency of multidrug resistance (97.6%) was evidenced, with 55 different multidrug resistance profiles identified. The MRSA strains were found to belong to 17 spa-types, three being novel. The linezolid-resistant strains appeared to belong to the ST398 type, spa-type t011, and SCCmec_type_Vc and to harbor the cfr, fexA, blaZ, mecA, tetM, and tetK genes. The cfr gene was predicted to be carried in the plasmid, flanked by ISSau9 and the transposon TnpR. MRSA from Portuguese fattening pigs present a high diversity of genetic lineages. The presence of cfr-positive LA-MRSA may represent a risk of transmission to humans, mainly to those in contact with livestock.
Keywords: LA-MRSA, pigs, linezolid resistance, cfr gene, WGS, ST398
1. Introduction
Staphylococcus aureus is a widely disseminated commensal organism colonizing the nasal mucosa and skin of humans and livestock such as pigs, cattle, poultry, pets, and wild animals [1,2,3,4,5,6]. Therefore, S. aureus can also be an opportunistic pathogen responsible for many human infections, from mild skin infections to life-threatening diseases [7]. In animals, it can also cause a variety of infections such as mastitis and udder impetigo, pyoderma in adults and neonates, omphalitis, arthritis, and several other pyemic conditions [8].
Methicillin-resistant S. aureus (MRSA) has been colonizing and infecting humans and animals [1,7,8]. Among MRSA associated with livestock (LA-MRSA) and able to colonize humans, the MRSA ST398 grouping within the clonal complex (CC) 398 is reported globally and is widespread in Europe and North America [9,10]. LA-MRSA CC398 poses a zoonotic risk, particularly for those working in close contact with livestock, such as farmers, veterinarians, abattoir workers, and people living in areas with a high livestock density [9,10,11].
Several studies reported that LA-MRSA CC398 evolved from the human methicillin-susceptible S. aureus (MSSA) CC398 by losing some virulence features (such as ØSa3 prophage and Panton–Valentine leukocidin, PVL) and acquiring new virulence and resistance genes (copper and zinc czrC, tetracycline tet(M) and β-lactam mecA and blaZ) [12]. LA-MRSA CC398 also acquired other antimicrobial resistance genes from Gram-positive and Gram-negative bacteria by plasmid-mediated horizontal gene transfer, including the gene for the phenicol exporter fexA, tetracycline tetL, macrolide, lincosamide, and streptogramin B ermT, trimethoprim dfrK, ABC transporters vgaC and vgaE, and chloramphenicol-florfenicol cfr [12]. The cfr gene encodes an rRNA methyltransferase targeting an adenine residue in the 23S rRNA (A2503), which apart from phenicols, also confers resistance to clindamycin, pleuromutilins, streptogramin A, and oxazolidinones [12,13]. This latter antibiotic class includes linezolid, approved for treating hospital-acquired pneumonia caused by S. aureus, including the MSSA and MRSA strains, multidrug-resistant Streptococcus pneumoniae, and vancomycin-resistant Enterococcus faecium (VRE) infections, among others [13].
In Portugal, MRSA strains belonging to CC398 have been identified in swine farms, wild boars, pets, wild rabbits, quails, and quail meat [3,4,5,6]. In addition, the first detection of linezolid resistance encoded by the cfr gene occurred in 2019 in three MRSA isolates recovered from infected human wounds, but none were included in the CC398 lineage [14]. In 2019, under the scope of MRSA monitoring from healthy pigs at slaughter in Portugal, three linezolid-resistant MRSA isolates belonging to the CC398 cluster and encoded by the acquired resistance cfr gene were detected [15]. Nevertheless, linezolid-resistant MRSA was firstly reported in one dog with severe bilateral otitis, although the genetic basis of resistance was not determined [16].
The emergence of linezolid-resistant MRSA CC398 in food-producing animals carrying the cfr gene poses a significant public health concern due to the possible spread of these bacteria through the animal–human interface by sharing genes through mobile genetic elements. Thus, this study aimed to characterize further the MRSA population previously reported [15], focusing on typing and antibiotic resistance profiles. Moreover, the three linezolid-resistant LA-MRSA isolates carrying the cfr gene were analyzed by Whole-Genome Sequencing (WGS).
2. Results
2.1. Bacterial Isolates and Antimicrobial Resistance Patterns
From 171 samples, 169 isolates (98.8%) were identified as MRSA carrying the mecA gene and being PVL-negative.
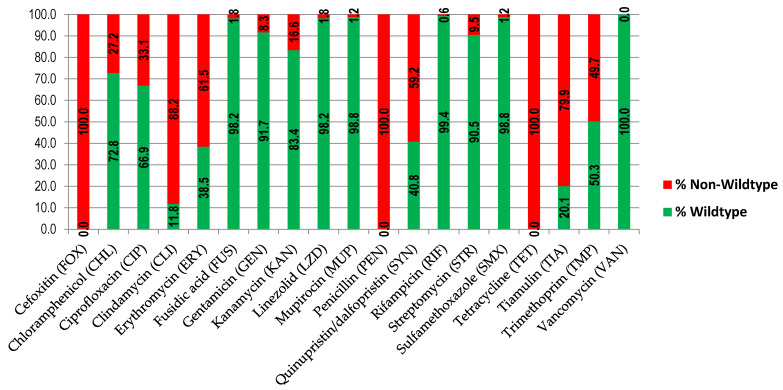
According to antimicrobial susceptibility testing, all isolates were non-wildtype with respect to the genes for cefoxitin, penicillin, and tetracycline resistance and wild-type with respect to the vancomycin resistance gene (Figure 1 and Table 1).
Figure 1.
Antibiotic non-susceptibility resistance distribution of the MRSA isolates (n = 169). Each bar represents the phenotypic result obtained for each antibiotic according to the ECOFF MIC values. The red bars represents the percentages of the non-wildtype isolates, and the green bars represents the percentages of the wild-type isolates for the respective antibiotic.
Table 1.
Clinical and epidemiological breakpoints, MIC50, MIC90, and frequency of resistance of the MRSA isolates.
| Antimicrobial | Clinical MIC Breakpoints [17] (mg/L) | Epidemiological MIC Breakpoints [17] (mg/L) (T) | MIC50 (mg/L) (n = 169) | MIC90 (mg/L) (n = 169) | % DS (N) |
|---|---|---|---|---|---|
| Cefoxitin (FOX) | 4 | NA | >16 | >16 | 100.0 (169) |
| Chloramphenicol (CHL) | 8 | NA | 16 | 64 | 27.2 (46) |
| Ciprofloxacin (CIP) | 1 | 1 | 0.5 | >8 | 33.1 (56) |
| Clindamycin (CLI) | 0.25 | 0.25 | >4 | >4 | 88.2 (149) |
| Erythromycin (ERY) | 2 | 1 | >8 | >8 | 61.5 (104) |
| Fusidic acid (FUS) | 1 | 0.5 | ≤0.5 | ≤0.5 | 1.8 (3) |
| Gentamicin (GEN) | 2 | (2) | ≤1 | ≤1 | 8.3 (14) |
| Kanamycin (KAN) | 8 | NA | ≤4 | 32 | 16.6 (28) |
| Linezolid (LZD) | 4 | 4 | 2 | 4 | 1.8 (3) |
| Mupirocin (MUP) | NA | (1) | ≤0.5 | ≤0.5 | 1.2 (2) |
| Penicillin (PEN) | 0.125 | NA | >2 | >2 | 100.0 (169) |
| Quinupristin/dalfopristin (SYN) | 2 | NA | 2 | 4 | 59.2 (100) |
| Rifampicin (RIF) | 0.06 | (0.03) | ≤0.016 | ≤0.016 | 0.6 (1) |
| Streptomycin (STR) | NA | NA | 8 | 16 | 9.5 (16) |
| Sulfamethoxazole (SMX) | NA | NA | ≤64 | ≤64 | 1.2 (2) |
| Tetracycline (TET) | 2 | 1 | >16 | >16 | 100.0 (169) |
| Tiamulin (TIA) | NA | NA | >4 | >4 | 79.9 (135) |
| Trimethoprim (TMP) | NA | (2) | ≤2 | >32 | 49.7 (84) |
| Vancomycin (VAN) | 2 | 2 | ≤1 | ≤1 | 0.0 (0) |
DS, Decreased susceptibility; NA, Not Applicable; (T), Tentative ECOFF; N, number of DS isolates.
Notably, three isolates (1.8%) showed resistance to linezolid, with MIC = 8 µg/mL. They contained non-wild-type chloramphenicol, clindamycin, quinupristin/dalfopristin, tetracycline, and tiamulin genes, and two of them also contained non-wild-type erythromycin and trimethoprim genes. Besides, 28 (16.6%) wild-type isolates with respect to linezolid had a MIC = 4 µg/mL.
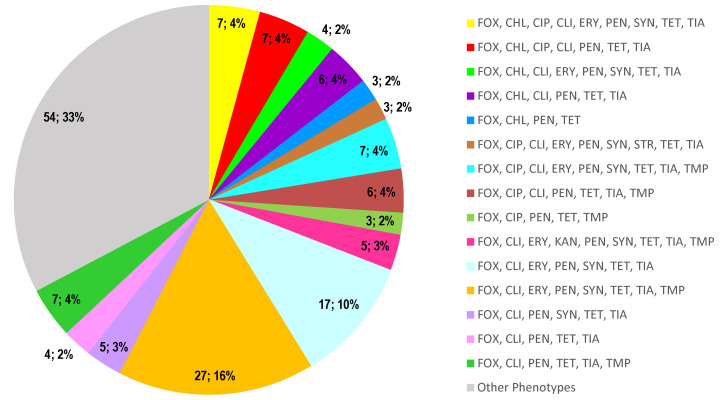
The frequency of multidrug resistance (MDR) was remarkably high (97.6%), with 55 MDR patterns found (Table S1). One isolate exhibited an MDR profile (FOX, CLI, ERY, FUS, GEN, KAN, MUP, PEN, SYN, RIF, STR, SMX, TET, TIA, TMP) comprising 11 antimicrobial classes. The most common profile was FOX, CLI, ERY, PEN, SYN, TET, TIA, and TMP, which include seven antimicrobial classes and was found in 27 isolates (16.4%), followed by the profile including FOX, CLI, ERY, PEN, SYN, TET, TIA, found in 17 isolates (10.3%) (Figure 2).
Figure 2.
Distribution of the MDR profiles, showing the 15 most common phenotypes of the MDR strains (n = 165). The number and the percentage of isolates are indicated in the graph. FOX-Cefoxitin, CHL-Chloramphenicol, CIP-Ciprofloxacin, CLI-Clindamycin, ERY-Erythromycin, FUS-Fusidic acid, GEN-Gentamicin, KAN-Kanamycin, LZD-Linezolid, MUP-Mupirocin, PEN-Penicillin, SYN-Quinupristin/dalfopristin, RIF-Rifampicin, STR-Streptomycin, SMX-Sulfamethoxazole, TET-Tetracycline, TIA-Tiamulin, TMP-Trimethoprim, VAN-Vancomycin.
2.2. Molecular Characterization of the MRSA Population
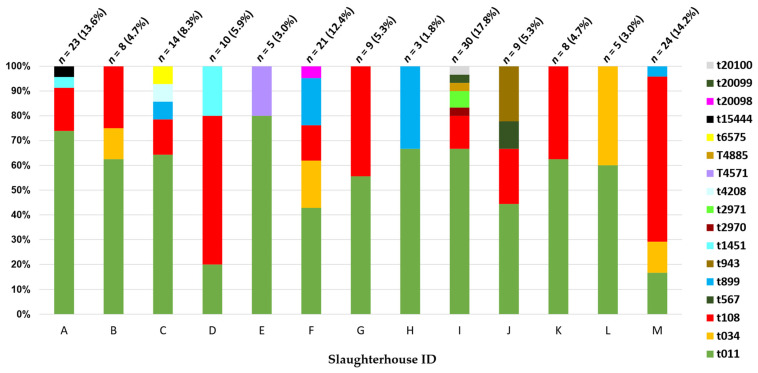
Overall, 17 different spa-types were identified among the MRSA isolates, i.e., t011, t034, t108, t1451, t15444, t2970, t2971, t4208, t4571, t4885, t567, t6575, t899, and t943, including 3 novel types that were submitted to the Ridom SpaServer platform [18], having been classified as t20098, t20099, and t20100 (Figure 3). The spa-type t011 was the most common (52.7%), followed by t108 (27.2%). Furthermore, t011, t034, and t108 belonging to CC398 accounted for 145 isolates (85.8%).
Figure 3.
Distribution of spa-types among the MRSA groups isolated from samples collected from the 13 slaughterhouses (n = 169). Each bar represents the spa-types obtained from a slaughterhouse. The number and the percentage of isolates are shown for each bar.
The three isolates resistant to linezolid (MIC = 8 µg/mL) belonged to t011 and showed amplification of the cfr gene. However, linezolid-non-wild-type isolates with MIC values equal to the cutoff were negative for the gene.
2.3. Whole-Genome Characterization of Linezolid-Resistant MRSA
The genotypic traits of the linezolid-resistant MRSA are summarized in Table 2.
Table 2.
Whole-genome characterization of linezolid-resistant MRSA (n = 3).
| Feature | INIAV_MRSA001 | INIAV_MRSA002 | INIAV_MRSA003 |
|---|---|---|---|
| Sampling date | October 22 | November 7 | November 20 |
| Farm region | AML (Peninsula de Setubal) | Alentejo | Alentejo Litoral |
| Slaughterhouse ID (region) | A (AML, Península de Setúbal) | K (North region, Ave) | K (North region, Ave) |
| Phenotype | FOX, CHL, CLI, ERY, LZD, PEN, SYN, TET, TIA, TMP | FOX, CHL, CLI, ERY, LZD, PEN, SYN, TET, TIA, TMP | FOX, CHL, CLI, LZD, PEN, SYN, TET, TIA, TMP |
| Antibiotic resistance genes | aadD, blaZ, mecA, lsa(B), cfr, fexA, tet(M), tet(L), tet(K) | blaZ, mecA, vga(A)LC, cfr, fexA, tet(M), tet(K), dfrG | blaZ. mecA, erm(B), Isa(B), cfr, vga(A)LC, fexA, tet(M), tet(L), tet(K), dfrK |
| Biocide resistance genes | norA, lmrS, mepA, sepA | norA, lmrS, mepA, sepA | norA, lmrS, mepA, sepA |
| SCCmec elements | SCCmec_type_Vc(5C2&5) | SCCmec_type_Vc(5C2&5) | SCCmec_type_Vc(5C2&5) |
| Virulence genes | aur; hlgA; hlgB; hlgC | aur; hlgA; hlgB; hlgC | aur; hlgA; hlgB; hlgC |
| Plasmid replicons | rep21, rep22, rep7a, repUS43 | rep7a, rep7b, repUS43, repUS5 | rep16, repUS5, rep22, rep7a, rep7b, repUS43 |
| Pathogenicity (%) | 97.9 | 97.7 | 97.7 |
| Spa-type | t011 | t011 | t011 |
| MLST | ST398 | ST398 | ST398 |
FOX-Cefoxitin, CHL-Chloramphenicol, CIP-Ciprofloxacin, CLI-Clindamycin, ERY-Erythromycin, FUS-Fusidic acid, GEN-Gentamicin, KAN-Kanamycin, LZD-Linezolid, MUP-Mupirocin, PEN-Penicillin, SYN-Quinupristin/dalfopristin, RIF-Rifampicin, STR-Streptomycin, SMX-Sulfamethoxazole, TET-Tetracycline, TIA-Tiamulin, TMP-Trimethoprim, VAN-Vancomycin, AML-Área Metropolitana de Lisboa.
Resistome analysis corroborated the presence of the cfr gene and revealed several additional genes in the genome, consistent with the antimicrobial resistance profiles of the isolates. These included β-lactam, encoding the genes blaZ and mecA, the fexA gene, encoding resistance to florfenicol, and genes conferring resistance to tetracycline tet(M) and tet(K). In addition, one isolate (INIAV_MRSA001) also carried the gene aadD conferring resistance to aminoglycosides, and two isolates non-wildtype for the gene for trimethoprim resistance (INIAV_MRSA002 and INIAV_MRSA003) harbored the dfr(G) and dfr(K) genes, respectively (Table 2). Moreover, all isolates carried efflux pump genes responsible for biocide resistance, namely, norA, lmrS, mepA, and sepA. No chromosomal mutations responsible for antimicrobial resistance were found.
All isolates belonged to the ST398 type, t011 spa-type, and SCCmec_type_Vc. VirulenceFinder predicted the presence of four virulence factors (aur; hlgA; hlgB; hlgC) in all. According to PlasmidFinder, the plasmid replicon sequences rep7a and repUS43 were identified in all isolates. Additionally, several other replicons were found, namely, rep21 and rep22 in INIAV_MRSA001; rep7b and repUS5 in INIAV_MRSA002; and rep22, rep7b, repUS5, and rep16 in INIAV_MRSA003. The plasmids identified by having rep22 carried tet(L), aad, and/or dfr(K), rep7a carried tet(K), repUS43 carried tet(M), rep7b carried vga(A)LC, and in INIAV_MRSA003, rep16 carried the erm(B) gene, and repUS5 carried the fex(A) gene.
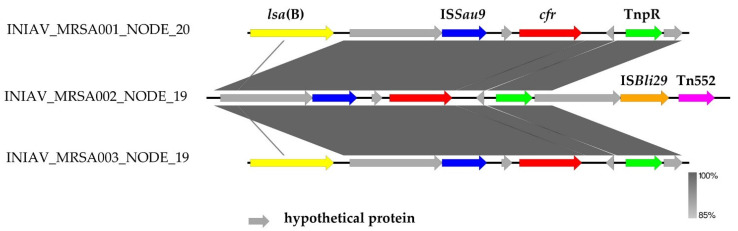
MobileElementFinder did not predict that the fexA gene was carried by MGE in INIAV_MRSA001 and INIAV_MRSA002, while the cfr gene was predicted to be carried by plasmids. The analysis of the genetic vicinity of the cfr gene revealed that all isolates contained the insertion sequence ISSau9 and the transposon TnpR elements flanking the gene. INIAV_MRSA002 also showed the presence of ISBli29 and Tn552 downstream of the TnpR element (Figure 4). The Isa(B) gene encoding resistance to lincosamide was carried in the same contig of the cfr gene in the INIAV_MRSA001 and MRSA003 strains.
Figure 4.
Genetic platforms of the cfr genes in the MRSA isolates determined using EasyFig. This figure represents the genomic environment of the cfr genes, regarding mobilization elements (insertion sequences and transposons) and other antibiotic determinants. The gray area represents the blast identities, and the percentage of identity is indicated in the legend.
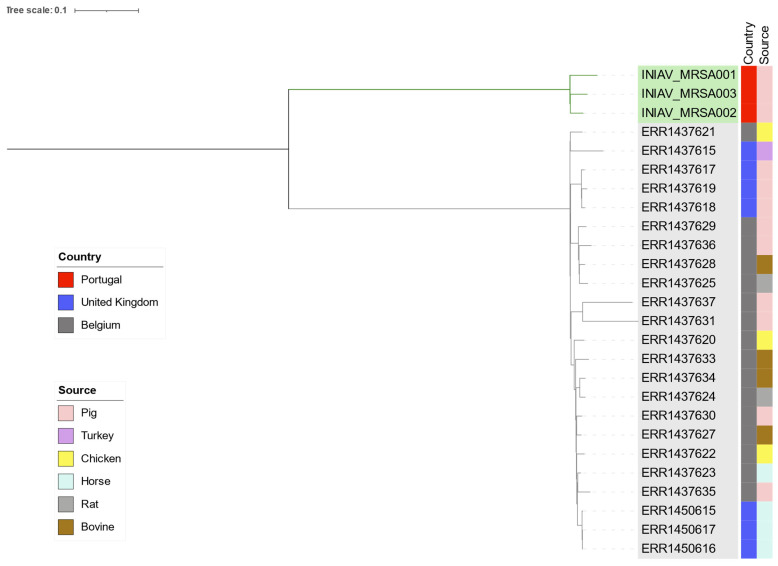
The phylogenetic analysis with the 3 linezolid-resistant isolates from this study and other 23 MRSA ST398 spa-type t011 strains recovered from multiple sources, from Belgium and the United Kingdom, revealed that all strains shared between 1 and 519 SNPs. The strains were arranged in two phylogenetic groups, one composed of the isolates from this study (Figure 5, green label), and the other containing the remaining strains (Figure 5, grey label). Therefore, there was no closed genetic relationship between the isolates from this study and the remaining strains (Figure 5). Most strains from Belgium grouped together despite their source, as well as the main strains isolated from horse samples (Figure 5). The most closely related isolates from this study sharing 39 SNPs were INIAV_MRSA002 and INIAV_MRSA003, obtained from samples collected on different days at the same abattoir from animals from different farms.
Figure 5.
Phylogenetic analysis based on core genome single-nucleotide polymorphisms (SNPs) using CSI Phylogeny v.1.4 of 26 MRSA ST398, t011 strains from multiple sources and different countries. Graphic representation using the iTol interactive tree of life, showing the geographic location, source, and genetic distance of the isolates. In green are the isolates from this study. ERR*******, accession numbers of the strains used in this analysis.
3. Discussion
The present study revealed a very high prevalence of MRSA (98%) in pool nasal swabs collected from healthy, fattening pigs at slaughterhouses in Portugal. Other studies also reported a high prevalence of MRSA in pigs at slaughterhouses and farms in Portugal, ranging from 60 to 99% [3,19,20]. According to the EFSA, the prevalence of MRSA in pigs varies between countries, depending on the sample type, sampling time, antibiotic usage, and legislation implemented at the national level [15].
Globally, 17 different spa-types were identified in the MRSA population, t011 and t108 being the most frequent. The high diversity of spa-types observed in our study may be attributed to the sample type and the significant number of holdings sampled (n = 170) at several slaughterhouses (n = 13). In addition, the high variability can be related to international trade; in fact, countries with high trade levels hold a greater diversity of strains [15].
Previous studies also found t011 and t108 as the most common spa-types [3,19,20,21]. However, besides t011 and t108, t034 was also identified in our study in 5.8% of the isolates (n = 10), following other reports where t034 was one of the most common spa-types found in pigs from animal/herds/holdings/slaughterhouses in other European countries [15]. Additionally, strains belonging to three new spa-types were found from three farms collected at two slaughterhouses (t20098, t20099, and t20100). The spa-type indicates the tandem repeats present in the region X of the spa gene, which is very polymorphic. So far, 20,627 different spa-types have been registered in the Ridom SpaServer, obtained by the random combination of 832 repeats (last accessed on 11 August 2022). New spa-types can be obtained by random rearrangements or by the emergence of new repeats due to genetic mutations or duplication events [22].
Besides the expected resistance to β-lactams, all isolates were also resistant to tetracycline but susceptible to vancomycin, which is in accordance with previous reports [3,19,20]. Overall, 55 different MDR patterns were found; the most common pattern was shared by 27 isolates, with decreased susceptibility to 7 antimicrobial classes, and, notably, one isolate showed resistance to 11 classes. MDR patterns have been observed in LA-MRSA strains originated from the acquisition of genes encoding resistance to several antibiotic families by horizontal gene transfer from other staphylococci and bacteria of human and animal origin [12]. Macrolides, lincosamides, β-lactams, tetracyclines, and sulphonamides are frequently used in pig production as curative, prophylactic, or metaphylactic treatments [23]. The administration of antibiotics can lead to an increase in antimicrobial-resistant bacteria due to their selective pressure exerted on animals and the environment.
The genotype of the linezolid-resistant isolates was in accordance with the susceptibility phenotype, and the resistance genes found, namely, cfr, tet(K), tet(M), tet(L), vga(A)LC, erm(B), fex(A), and dfr(K), were mainly predicted in plasmids. The cfr genes is flanked by ISSau9 (also called IS21-558) and the transposon TnpR. The clindamycin exporter gene Isa(B) was also located upstream of ISSau9 in two strains, as already described [24]. The IS21-558 element, originally identified in the plasmid pSCFS3 recovered from a S. aureus strain of pig origin in Germany, was also found in the plasmid pGMI17-006 from an S. aureus strain from Denmark [25]. Therefore, IS21-558 may be involved in the mobility and dissemination of the multi-resistant gene cfr between different staphylococcal species [24,25]. Regardless of our efforts with the in silico analysis, the short-read sequencing performed in this study should be complemented with long-read sequencing to better understand the location of the resistance genes in plasmids. In addition, further efforts will be made to sequence the plasmid carrying the cfr gene in the strains from this study to provide information for the scientific community.
In addition, cfr-positive MRSA isolates also co-carried some efflux pump genes responsible for biocide resistance (norA, lmrS, mepA, and sepA). Biocides are widely used in animal production as antiseptics and disinfectants, to maintain good hygiene levels in animal holdings and for workers [20]. The three cfr-positive isolates were typed as ST398, spa-type t011, and SCCmec_type_V and harbored four virulence factors, i.e., aur, hlgA, hlgB, and hlgC, encoding aureolysin and gamma-hemolysin, respectively.
Previous studies reported the presence of MRSA from ST398 with the same spa-type observed in pigs, in humans in close contact with pig farms [3,11,20]. The emergence of cfr-mediated linezolid resistance in MRSA from animal origin has also been reported in horses in Germany [26], pigs in Belgium [27], Korea [28], China [29], and Spain [30], and poultry in China [29]. Moreover, cfr-positive MRSA strains have been reported sporadically in humans in several European countries, including Italy [31], Portugal [14], Spain [32], as well in hospital outbreaks in Spain [33] and China [34]. Although cfr-carrying Staphylococcus has already been reported among S. pseudintermedius from a dog in Portugal [35], the detection of cfr-carrying MRSA ST398 in healthy fattening pigs at slaughterhouses is alarming because of the high zoonotic transmissibility reported for ST398 [11].
Using the conservative cutoffs of 25 wgSNPs proposed by Coll et al. (2020) [36], above which transmission of MRSA within the previous 6 months can be ruled out, we can exclude possible transmission events between the closest strains (INIAV_002 and INIAV_003) from different farms collected in the same abattoir on different days. This strain pair does not comply with the criteria established for the occurrence of a direct transmission (<25 SNPs) because the number of SNP differences was 39 SNPs. However, MRSA strains can persist over time [37], and so those isolates are probably related to the same abattoir. Still, the strains from this study belonging to different farm regions and collected from different abattoirs may indicate that the cfr gene is emerging in our country.
Linezolid and vancomycin are the last resource treatments to fight against highly resistant and complicated S. aureus infections in humans [13]. Although all the identified linezolid-resistant MRSA strains harboring the cfr gene were susceptible to vancomycin, these finding is relevant, as they constitute a potential public health risk. Interestingly, we recently found linezolid-resistant enterococci, including optrA- and poxtA-positive Enterococcus faecium and optrA-positive Enterococcus faecalis, in pigs from Portugal [38]. These findings highlight the urgency of monitoring linezolid resistance in selected Gram-positive pathogens from animals in Portugal. The emergence of novel resistance genes poses a major threat to human and animal health due to the possibility of horizontal gene transfer from animals to humans and vice-versa through direct contact or the food chain.
The implementation of surveillance and control strategies in the animal and human sectors under the One Health perspective is crucial to better understand the spread of MRSA ST398 in both reservoirs.
4. Materials and Methods
4.1. Sampling and Bacterial Isolation
One hundred and seventy-one pooled nasal swab samples were collected from pigs sampled at 13 abattoirs across mainland Portugal between October and December 2019. Each pooled sample was composed of nasal swabs from five animals in the same farm, with a total of 170 farms. The samples were sent under refrigeration (4–8 °C) to the National Institute of Agrarian and Veterinary Research (INIAV, IP) for further analyses.
Isolation and identification of MRSA were performed according to the protocol defined by the EU Reference Laboratory for antimicrobial resistance (EURL-AR) [39]. Briefly, the nasal swabs were placed in a pre-enrichment broth containing 6.5% sodium chloride, followed by incubation and plating on the selective chromogenic medium Brilliance MRSA2 agar (Oxoid, Hampshire, UK). The suspected colonies (one single colony by pooled sample) were confirmed to be MRSA by multiplex PCR (mecA, mecC, spa, and pvl genes) [40].
4.2. Antimicrobial Susceptibility Testing
The antimicrobial susceptibility profile of all isolates was assessed considering 19 antimicrobial agents (Clindamycin, Tetracycline, Rifampin, Streptomycin, Fusidic acid, Penicillin, Chloramphenicol, Kanamycin, Tiamulin, Quinupristin/dalfopristin, Vancomycin, Gentamicin, Trimethoprim, Erythromycin, Ciprofloxacin, Cefoxitin, Linezolid, Mupirocin, and Sulfamethoxazole). The determination of the minimum inhibitory concentrations (MIC) was obtained by the broth microdilution method, using the commercially available 96-well microplate assay Sensititre Staphylococci plate-EUST (Sensititre®, Trek Diagnostic Systems, East Grinstead, West Sussex, UK). The results were interpreted according to EUCAST clinical and epidemiological breakpoints (Table 1) [17]. The susceptibility assays used the S. aureus strain ATCC 29213 as a quality control. An isolate is defined as MDR if it shows resistance to three or more classes of antimicrobials. The non-wild-type isolates were considered resistant for the determination of multidrug resistance patterns.
4.3. Molecular Characterization of the Isolates
Molecular characterization of the isolates based on spa-typing was performed. DNA was extracted by the boiling method and tested according to the protocol used by the EURL-AR [41]. The spa-type was determined using Ridom SeqSphere+ software v8 (Ridom GmbH, Münster, Germany).
Isolates with MIC ≥ 4 µg/mL for linezolid were tested for the presence of the cfr gene by standard PCR, using the primers described by Kehrenberg and Schwarz (2006) [26] for the amplification of a 746 bp fragment. The reactions were carried out in a total volume of 25 µL containing 1 × Gel Load Reaction buffer (NzyTech, Lisbon, Portugal), 2 mM MgCl2 (NzyTech), 400 mM dNTPs (NzyTech), 0.4 μM of each primer, 1U of NZYTaq II DNA polymerase (NzyTech), and 2 μL of DNA. Amplification was performed in a Biometra TOne Thermal Cycler (Analytik Jena, Jena, Germany) with an initial denaturation step at 94 °C for 5 min, followed by 30 cycles at 94 °C for 30 s, 54 °C for 90 s, and 72 °C for 60 s, with a final extension at 72 °C for 10 min. The PCR products were visualized, and images were collected using the UVP BioDoc-It® Imaging System (UVP, Cambridge, UK).
4.4. Whole-Genome Sequencing of Linezolid-Resistant Isolates
The genomic DNA of isolates showing resistance to linezolid (MIC > 4 µg/mL) was extracted using the PureLink® Genomic DNA kit, following the Gram-positive bacterial cell lysate protocol (Invitrogen, Carlsbad, CA, USA) and according to the manufacturer’s instructions. DNA was eluted with 50 µL of Tris-HCl buffer, pH 8.5. The quality and quantity of DNA were assessed using a spectrophotometer (Nanodrop® 2000, Thermo Scientific, Waltham, MA, USA) and a QuantusTM Fluorometer with the QuantiFluor® ONE dsDNA Dye kit (Promega, Madison, WI, USA), according to the manufacturer’s recommendations. Library preparation and DNA sequencing were performed by Novogene Europe, UK, using the Illumina HiSeq sequencing technology (NovaSeq 6000 S2 PE150 XP sequencing mode). The nucleotide sequences were deposited in, the European Nucleotide Archive (ENA) [42] with the accession numbers ERS6142034, ERS6142035, and ERS6142036.
Raw data quality was assessed by FastQC [43] and low-quality sequencing data and adapter sequences were removed using Trimmomatic v0.27 [44] with default settings. All pre-processed reads were assembled with SPAdes v3.12.0 [45], and the assembly stats were calculated using QUAST-5.0.2 [46]. Bioinformatics analyses were performed after removing the contigs with sizes lower than 500 bp.
Acquired antimicrobial resistance genes and chromosomal point mutations, plasmid replicons, multi-locus sequence type (MLST), identification of virulence genes, pathogenicity, spa-type, and staphylococcal cassette chromosome mec (SCCmec) elements were assessed using ResFinder v4.0 (command line, 90% threshold for %ID/60% minimum length) [47,48], PlasmidFinder (command line, 95% threshold for %ID) [49], MLST (command line) [50], VirulenceFinder (command line, 90% threshold for %ID/60% minimum length) [51], PathogenFinder v1.1 [52], SpaTyper v1.0 [53], and SCCmecFinder v1.2 (90% threshold for %ID/60% minimum length) [54,55,56], respectively. MobileElementFinder v1.0.3 was used to identify mobile genetic elements and their relation to antimicrobial resistance genes and virulence factors [57]. The Comprehensive Antibiotic Resistance Database (CARD) [58] was used to complement the characterization of the isolates genomic content.
For identifying the genetic platform of the cfr gene, the contigs containing this gene were annotated using Prokka v1.14.6 [59], followed by analysis with Artemis [60], EasyFig v2.2.5 [61], and Basic Local Alignment Search Tool (BLAST) from NCBI website.
A phylogenetic analysis based on single-nucleotide polymorphisms (SNPs) present in the genomes, using CSI Phylogeny v.1.4 (10 reads of minimal depth at SNP positions, 10% minimal relative depth at SNP positions of, 10 bp of minimal distance between SNP, minimal SNP quality of 30, minimal read mapping quality of 25, and a minimal Z-score of 1.96) [62] from the CGE website was conducted with the three MRSA from this study and other 23 MRSA ST398 strains from multiple sources from Belgium and the United Kingdom [63]. Staphylococcus aureus strain ISU926 isolate ST398 (accession number CP017091.1) was used as the reference genome. The graphical representation and tree annotation were performed using iTOL, Interactive Tree Of Life v6. [64].
Acknowledgments
The authors are grateful to all staff involved in sampling and laboratory work for their technical support. The authors also acknowledge the Ridom GmbH, Germany, for the free trial of the Ridom SeqSphere+ software used for spa-type determination. Part of this research was supported by Cost Action CA18217, the European Network for Optimization of Veterinary Antimicrobial Treatment (ENOVAT).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics11101439/s1. Table S1: Antibiotic resistance patterns of the MRSA isolates.
Author Contributions
Conceptualization, L.C., A.A. and M.C.d.; methodology, C.L., T.A. and A.A.; validation, C.L., L.C. and A.A.; formal analysis, C.L., L.C., T.A., M.C.d. and A.A.; investigation, C.L., L.C. and A.A.; data curation, C.L., L.C. and A.A.; writing-original draft preparation, C.L., L.C. and A.A.; writing-review and editing, C.L., L.C. and A.A.; supervision, L.C. and A.A.; funding acquisition, L.C., A.A. and M.C.d. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available within the article. The whole-genome sequence data were deposited in the European Nucleotide Archive with the accession numbers ERS6142034, ERS6142035, and ERS6142036.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Foundation for Science and Technology (FCT), Portugal, grant number PTDC/CVT-CVT/28469/2017; Célia Leão was also funded by the National Funds through FCT under the Project UIDB/05183/2020. The Portuguese National Authority for Food and Veterinary (DGAV) supported this work.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Loncarica I., Lepuschitzb S., Ruppitschb W., Trstana A., Andreadisa T., Bouchlisa N., Marbacha H., Schauera B., Szostaka M., Feßlerc A., et al. Increased genetic diversity of methicillin-resistant Staphylococcus aureus (MRSA) isolated from companion animals. Vet. Microbiol. 2019;235:118–126. doi: 10.1016/j.vetmic.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Hansen J., Ronco T., Stegger M., Sieber R., Fertner M., Martin M., Farre Toft N., Larsen A., Pedersen K. LA-MRSA CC398 in Dairy Cattle and Veal Calf Farms Indicates Spillover From Pig Production. Front. Microbiol. 2019;10:2733. doi: 10.3389/fmicb.2019.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conceição T., Lencastre H., Aires-de-Sousa M. Frequent isolation of methicillin resistant Staphylococcus aureus (MRSA) ST398 among healthy pigs in Portugal. PLoS ONE. 2017;12:e0175340. doi: 10.1371/journal.pone.0175340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couto N., Belas A., Kadlec K., Schwarz S., Pomba S. Clonal diversity, virulence patterns and antimicrobial and biocide susceptibility among human, animal and environmental MRSA in Portugal. J. Antimicrob. Chemother. 2015;70:2483–2487. doi: 10.1093/jac/dkv141. [DOI] [PubMed] [Google Scholar]
- 5.Silva V., Vieira-Pinto M., Saraiva C., Manageiro V., Reis L., Ferreira E., Caniça M., Capelo J.L., Igrejas G., Poeta P. Prevalence and Characteristics of Multidrug-Resistant Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) CC398 Isolated from Quails (Coturnix Coturnix Japonica) Slaughtered for Human Consumption. Animals. 2021;11:2038. doi: 10.3390/ani11072038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sousa M., Silva N., Borges V., Gomes J., Vieira L., Caniça M., Torres C., Igrejas G., Poeta P. MRSA CC398 recovered from wild boar harboring new SCCmec type IV J3 variant. Sci. Total Environ. 2020;722:137845. doi: 10.1016/j.scitotenv.2020.137845. [DOI] [PubMed] [Google Scholar]
- 7.Foster T.J., Geoghegan J.A. Staphylococcus aureus. In: Tang Y.W., Sussman M., Liu D., Poxton I., Schwartzman J., editors. Molecular Medical Microbiology. Elsevier Ltd.; Dublin, Ireland: 2015. pp. 655–674. [Google Scholar]
- 8.Quinn P.J., Carter M.E., Markey B., Carter G.R. Clinical Veterinary Microbiology. Mosby International Limited; London, UK: 2002. Staphylococcus species; pp. 118–126. [Google Scholar]
- 9.Voss A., Loeffen F., Bakker J., Klaassen C., Wulf M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg. Infect. Dis. 2005;11:1965–1966. doi: 10.3201/eid1112.050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinross P., Petersen A., Skov R., Van Hauwermeiren E., Pantosti A., Laurent F., Voss A., Kluytmans J., Struelens M.J. The European Human LA-MRSA Study Group; et al. Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) among human MRSA isolates. European Union/European Economic Area countries. Eurosurveillance. 2017;22:16-00696. doi: 10.2807/1560-7917.ES.2017.22.44.16-00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pirolo M., Visaggio D., Gioffrè A., Artuso I., Gherardi M., Pavia G., Samele P., Ciambrone L., Di Natale R., Spatari G., et al. Unidirectional animal-to-human transmission of methicillin-resistant Staphylococcus aureus ST398 in pig farming; evidence from a surveillance study in southern Italy. Antimicrob. Resist. Infect. Control. 2019;8:187. doi: 10.1186/s13756-019-0650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadlec K., Fessler A.T., Hauschild T., Schwarz S. Novel and uncommon antimicrobial resistance genes in livestock-associated methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2012;18:745–755. doi: 10.1111/j.1469-0691.2012.03842.x. [DOI] [PubMed] [Google Scholar]
- 13.Hashemian S.M.R., Farhadi T., Ganjparvar M. Linezolid: A review of its properties, function, and use in critical care. Drug Des. Dev. Ther. 2018;12:1759–1767. doi: 10.2147/DDDT.S164515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva V., Almeida F., Silva A., Correia S., Carvalho J.A., Castro A.P., Ferreira E., Manageiro V., Caniça M., Igrejas G., et al. First report of linezolid-resistant cfr-positive methicillin-resistant Staphylococcus aureus in humans in Portugal. J. Glob. Antimicrob. Resist. 2019;17:323–325. doi: 10.1016/j.jgar.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 15.European Food Safety Authority. European Centre for Disease Prevention and Control The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA J. Wiley Online Libr. 2021;19:e06490. doi: 10.2903/j.efsa.2021.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seixas R., Monteiro V., Carneiro C., Vilela C.L., Oliveira M. First report of a linezolid-resistant MRSA (methicillin resistant Staphylococcus aureus) isolated from a dog with a severe bilateral otitis in Portugal. Rev. Vet. 2011;22:81–84. doi: 10.30972/vet.2221826. [DOI] [Google Scholar]
- 17.European Committee for Antimicrobial Susceptibility Testing (EUCAST) [(accessed on 23 February 2022)]. Available online: https://www.eucast.org/mic_distributions_and_ecoffs/
- 18.Ridom SpaServer Platform. [(accessed on 30 July 2021)]. Available online: https://spa.ridom.de/submission.shtml.
- 19.Lopes E., Conceição T., Poirel L., de Lencastre H., Aires-de-Sousa M. Epidemiology and antimicrobial resistance of methicillin-resistant Staphylococcus aureus isolates colonizing pigs with different exposure to antibiotics. PLoS ONE. 2019;14:e0225497. doi: 10.1371/journal.pone.0225497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouchami O., Fraqueza M.J., Faria N.A., Alves V., Lawal O.U., de Lencastre H., Miragaia M. Evidence for the Dissemination to Humans of Methicillin-Resistant Staphylococcus aureus ST398 through the Pork Production Chain: A Study in a Portuguese Slaughterhouse. Microorganisms. 2020;8:1892. doi: 10.3390/microorganisms8121892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Food Safety Authority Analysis of the baseline survey on the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU. EJ EFSA J. Wiley Online Libr. 2008;7:1376. doi: 10.2903/j.efsa.2009.1376. [DOI] [Google Scholar]
- 22.Khaled R.A., Suriya R., Amani A., Asim D., Abbas H., Sima T. Molecular typing of MRSA isolates by spa and PFGE. J. King Saud. Univ. Sci. 2019;31:999–1004. doi: 10.1016/j.jksus.2018.07.018. [DOI] [Google Scholar]
- 23.Veterinary Medicines Division . Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018 Trends from 2010 to 2018 Tenth ESVAC Report. Veterinary Medicines Division; Luxembourg: 2020. EMA/24309/2020. [Google Scholar]
- 24.Kehrenberg C., Aarestrup F.M., Schwarz S. IS21-558 insertion sequences are involved in the mobility of the multiresistance gene cfr. Antimicrob. Agents Chemother. 2007;51:483–487. doi: 10.1128/AAC.01340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz S., Zhang W., Du X.-D., Krüger H., Feßler A.T., Ma S., Zhu Y., Wu C., Shen J., Wang Y. Mobile oxazolidinone resistance genes in Gram-positive and Gram-negative bacteria. Clin. Microbiol. Rev. 2021;34:e00188-20. doi: 10.1128/CMR.00188-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kehrenberg C., Schwarz S. Distribution of florfenicol resistance genes fexA and cfr among chloramphenicol-resistant Staphylococcus isolates. Antimicrob. Agents Chemother. 2006;50:1156–1163. doi: 10.1128/AAC.50.4.1156-1163.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peeters L.E.J., Argudín M.A., Azadikhah S., Butaye P. Antimicrobial resistance and population structure of Staphylococcus aureus recovered from pigs farms. Vet. Microbiol. 2015;180:151–156. doi: 10.1016/j.vetmic.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Kang H.Y., Moon D.C., Mechesso A.F., Choi J.H., Kim S.J., Song H.J., Kim M.H., Yoon S.S., Lim S.K. Emergence of cfr-Mediated Linezolid Resistance in Staphylococcus aureus Isolated from Pig Carcasses. Antibiotics. 2020;9:769. doi: 10.3390/antibiotics9110769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S.M., Zhou Y.F., Li L., Fang L.X., Duan J.H., Liu F.R., Liang H.Q., Wu Y.T., Gu W.Q., Liao X.P., et al. Characterization of the Multi-Drug Resistance Gene cfr in Methicillin-Resistant Staphylococcus aureus (MRSA) Strains Isolated From Animals and Humans in China. Front. Microbiol. 2018;9:2925. doi: 10.3389/fmicb.2018.02925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz-Ripa L., Bellés-Bellés A., Fernández-Fernández R., García M., Vilaró A., Zarazaga M., Torres C. Linezolid-resistant MRSA-CC398 carrying the cfr gene, and MRSA-CC9 isolates from pigs with signs of infection in Spain. J. Appl. Microbiol. 2021;131:615–622. doi: 10.1111/jam.14988. [DOI] [PubMed] [Google Scholar]
- 31.Antonelli A., D’Andrea M.M., Galano A., Borchi B., Brenciani A., Vaggelli G., Cavallo A., Bartoloni A., Giovanetti E., Rossolini G.M. Linezolid-resistant cfr-positive MRSA, Italy. J. Antimicrob. Chemother. 2016;71:2349–2351. doi: 10.1093/jac/dkw108. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Ripa L., Bellés A., García M., Torres C. Detection of a cfr-positive MRSA CC398 strain in a pig farmer in Spain. Enferm. Infecc. Microbiol. Clin. Engl. Ed. 2021;39:139–141. doi: 10.1016/j.eimc.2020.03.006. (In English and Spanish) [DOI] [PubMed] [Google Scholar]
- 33.Morales G., Picazo J.J., Baos E., Candel F.J., Arribi A., Peláez B., Andrade R., de la Torre M.A., Fereres J., Sánchez-García M. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin. Infect. Dis. 2010;50:821–825. doi: 10.1086/650574. [DOI] [PubMed] [Google Scholar]
- 34.Jian J., Chen L., Xie Z., Zhang M. Dissemination of cfr-mediated linezolid resistance among Staphylococcus species isolated from a teaching hospital in Beijing, China. J. Int. Med. Res. 2018;46:3884–3889. doi: 10.1177/0300060518781636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couto N., Belas A., Rodrigues C., Schwarz S., Pomba C. Acquisition of the fexA and cfr genes in Staphylococcus pseudintermedius during florfenicol treatment of canine pyoderma. J. Glob. Antimicrob. Resist. 2016;7:126–127. doi: 10.1016/j.jgar.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Coll F., Raven K.E., Knight G.M., Blane B., Harrison E.M., Leek D., Enoch D.A., Brown N.M., Parkhill J., Peacock S.J. Definition of a genetic relatedness cutoff to exclude recent transmission of meticillin-resistant Staphylococcus aureus: A genomic epidemiology analysis. Lancet Microbe. 2020;1:e328–e335. doi: 10.1016/S2666-5247(20)30149-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner N.A., Sharma-Kuinkel B.K., Maskarinec S.A., Eichenberger E.M., Shah P.P., Carugati M., Holland T.L., Fowler V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019;17:203–218. doi: 10.1038/s41579-018-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gião J., Leão C., Albuquerque T., Clemente L., Amaro A. Antimicrobial Susceptibility of Enterococcus Isolates from Cattle and Pigs in Portugal: Linezolid Resistance Genes optrA and poxtA. Antibiotics. 2022;11:615. doi: 10.3390/antibiotics11050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.EURL-AR European Reference Laboratory for Antimicrobial Resistance. Protocols. [(accessed on 23 February 2022)]. Available online: https://www.eurl-ar.eu/CustomerData/Files/Folders/21-protocols/430_mrsa-protocol-final-19-06-2018.pdf.
- 40.Stegger M., Andersen P.S., Kearns A., Pichon B., Holmes M.A., Edwards G., Laurent F., Teale C., Skov R., Larsen A.R. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA(LGA251) Clin. Microbiol. Infect. 2012;18:395–400. doi: 10.1111/j.1469-0691.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- 41.Shopsin B., Gomez M., Montgomery S.O., Smith D.H., Waddington M., Dodge D.E., Bost D.A., Riehman M., Naidich S., Kreiswirth B.N. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 1999;37:3556–3563. doi: 10.1128/JCM.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.European Nucleotide Archive (ENA) [(accessed on 16 March 2021)]. Available online: https://www.ebi.ac.uk/ena/submit/sra/#home.
- 43.Andrews S. FastQC: A quality control tool for high throughput sequence data. 2010. [(accessed on 19 January 2021)]. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- 44.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina Sequence Data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nurk S., Bankevich A., Antipov D., Gurevich A., Korobeynikov A., Lapidus A., Prjibelsky A., Pyshkin A., Sirotkin A., Sirotkin Y., et al. Assembling genomes and mini-metagenomes from highly chimeric reads. In: Deng M., Jiang R., Sun F., Zhang X., editors. Proceedings of the Research in Computational Molecular Biology: 17th Annual International Conference (RECOMB); Beijing, China. 7–10 April 2013; Berlin/Heidelberg, Germany: Springer; 2013. [(accessed on 15 June 2021)]. pp. 158–170. Available online: http://link.springer.com/chapter/10.1007/978-3-642-37195-0_13. [Google Scholar]
- 46.Mikheenko A., Prjibelski A., Saveliev V., Antipov D., Gurevich A. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics. 2018;34:i142–i150. doi: 10.1093/bioinformatics/bty266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bortolaia V., Kaas R.F., Ruppe E., Roberts M.C., Schwarz S., Cattoir V., Philippon A., Allesoe R.L., Rebelo A.R., Florensa A.R., et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020;75:3491–3500. doi: 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zankari E., Hasman H., Cosentino S., Vestergaard M., Rasmussen S., Lund O., Aarestrup F.M., Larsen M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carattoli A., Zankari E., Garcìa-Fernandez A., Larsen M., Lund O., Voldby Villa L., Møller Aarestrup F., Hasman H. PlasmidFinder and pMLST: In silico detection and typing of plasmids. Antimicrob. Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larsen M.V., Cosentino S., Rasmussen S., Friis C., Hasman H., Marvig R.L., Jelsbak L., Sicheritz-Pontéen T., Ussery D.W., Aarestrup F.M., et al. Multilocus Sequence Typing of Total Genome Sequenced Bacteria. J. Clin. Micobiol. 2012;50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joensen K.G., Scheutz F., Lund O., Hasman H., Kaas R.S., Nielsen E.M., Aarestrup F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Micobiol. 2014;52:1501–1510. doi: 10.1128/JCM.03617-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cosentino S., Voldby Larsen M., Møller Aarestrup F., Lund O. PathogenFinder—Distinguishing Friend from Foe Using Bacterial Whole Genome Sequence Data. PLoS ONE. 2013;8:e77302. doi: 10.1371/annotation/b84e1af7-c127-45c3-be22-76abd977600f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartels M.D., Petersen A., Worning P., Nielsen J.B., Larner-Svensson H., Johansen H.K., Andersen L.P., Jarløv J.O., Boye K., Larsen A.R., et al. Comparing whole-genome sequencing with Sanger sequencing for spa typing of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2014;52:4305–4308. doi: 10.1128/JCM.01979-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.International Working Group on The Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC) Classification of staphylococcal cassette chromosome mec (SCCmec): Guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 2009;56:4961–4967. doi: 10.1128/AAC.00579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kondo Y., Ito T., Ma X.X., Watanabe S., Kreiswirth B.N., Etienne J., Hiramatsu K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: Rapid identification system for mec, ccr and major difference in junkyard regions. Antimicrob. Agents Chemother. 2007;51:264–274. doi: 10.1128/AAC.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johansson M.H.K., Bortolaia V., Tansirichaiya S., Aarestrup F.M., Roberts A.P., Petersen T.N. Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: MobileElementFinder. J. Antimicrob. Chemother. 2021;76:101–109. doi: 10.1093/jac/dkaa390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alcock B.P., Raphenya A.R., Lau T.T.Y., Tsang K.K., Bouchard M., Edalatmand A., Huynh W., Nguyen A.V., Cheng A.A., Liu S., et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48:D517–D525. doi: 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 60.Carver T., Harris S.R., Berriman M., Parkhill J., McQuillan J.A. Artemis: An integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics. 2012;28:464–469. doi: 10.1093/bioinformatics/btr703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan M.J., Petty N.K., Beatson S.A. Easyfig: A genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaas R.S., Leekitcharoenphon P., Aarestrup F.M., Lund O. Solving the Problem of Comparing Whole Bacterial Genomes across Different Sequencing Platforms. PLoS ONE. 2014;9:e104984. doi: 10.1371/journal.pone.0104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma M., Nunez-Garcia J., Kearns A.M., Doumith M., Butaye P.R., Argudín M.A., Lahuerta-Marin A., Pichon B., AbuOun M., Rogers J., et al. Livestock-Associated Methicillin Resistant Staphylococcus aureus (LA-MRSA) Clonal Complex (CC) 398 Isolated from UK Animals belong to European Lineages. Front. Microbiol. 2016;7:1741. doi: 10.3389/fmicb.2016.01741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ciccarelli F.D., Doerks T., von Mering C., Creevey C.J., Snel B., Bork P. Toward automatic reconstruction of a highly resolved tree of life. Science. 2006;311:1283–1287. doi: 10.1126/science.1123061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available within the article. The whole-genome sequence data were deposited in the European Nucleotide Archive with the accession numbers ERS6142034, ERS6142035, and ERS6142036.