Abstract
A complete cDNA sequence encoding a 28-kDa cruzipain-like cysteine protease of adult Paragonimus westermani, termed Pw28CCP, was isolated from an adult cDNA library. The cDNA contained a single open reading frame of 975 bp encoding 325 amino acids, which exhibited the structural motif and domain organization characteristic of cysteine proteases of non-cathepsin Bs including a hydrophobic signal sequence, an ERFNIN motif, and essential cysteine residues as well as active sites in the mature catalytic region. Analysis of its phylogenetic position revealed that this novel enzyme belonged to the cruzipain-like cysteine proteases. The sequence of the first 13 amino acids predicted from the mature domain of Pw28CCP was in accord with that determined from the native 28-kDa enzyme purified from the adult worm. Expression of Pw28CCP was observed specifically in juvenile and adult worms, with a location in the intestinal epithelium, suggesting that this enzyme could be secreted and involved in nutrient uptake and immune modulation. The recombinant protein expressed in Escherichia coli was used to assess antigenicity by immunoblotting with sera from patients with active paragonimiasis and from those with other parasitic infections. The resulting sensitivity of 86.2% (56 of 65 samples) and specificity of 98% (147 of 150 samples) suggest its potential as an antigen for use in immunodiagnosis.
Paragonimus westermani is a trematode parasite that causes chronic inflammatory lung disease as well as systemic infection including cerebral invasion in humans and carnivorous mammals. Human infection occurs by ingesting undercooked freshwater crayfish or crabs containing metacercaria or eating raw boar meat. The metacercariae excyst in the duodenum, penetrate the peritoneal cavity, and finally, migrate to the lung, in which they become adults and are surrounded by a thick granulomatous wall (1, 18). The adult worms can survive for approximately 5 years.
Parasite cysteine proteases are known to play critical roles in parasitic infections. They participate in a broad range of biological processes including egg hatching and subsequent stage transitions, invasion and migration through host tissues, and immune modulation and nutrient uptake (4, 25, 28, 29, 39, 43). Recent studies have shown that these enzymes might be good targets for the development of vaccines (20, 33) and antiparasitic drugs (7, 9).
In P. westermani, at least six different species of cysteine protease with molecular sizes of 53, 34, 28, 27, 17, and 15 kDa, respectively, have been characterized in eggs, metacercariae, juveniles, and adults (4, 5, 14, 22, 45). The 28- and 27-kDa proteases released by the metacercariae have been shown to share biochemical features with cathepsin L of F. hepatica (8) or with cathepsin S of sparganum (25) and are believed to play important roles in metacercarial excystment, tissue migration, and immune evasion (4, 6, 14, 45).
It is of particular interest that P. westermani possesses multiple cysteine proteases of similar sizes ranging from 29 to 27 kDa (4, 6, 14, 45). To date, however, only one gene encoding a 28-kDa neutral thiol protease (NTP) of P. westermani has been well characterized in terms of its molecular and biological properties (45, 46). Recently, a gene encoding a putative cysteine protease (PwCP) was reported (32), but its protein and enzymatic identities have not been described.
In the present study, we elucidate the molecular properties of a new 28-kDa cysteine protease (Pw28CCP) that is expressed specifically in juvenile and adult stages of P. westermani. We characterize it as a 28-kDa cruzipain-like cysteine protease on the basis of its molecular structure. The potential usefulness of a recombinant protein as an immunodiagnostic antigen has also been investigated.
MATERIALS AND METHODS
Parasite materials from different developmental stages of P. westermani.
Cats and dogs were fed metacercariae isolated from crayfish (Cambaroides similis) and were killed 1, 2, 3, 4, 7, and 16 weeks after infection. The 1- and 2-week-old juveniles were harvested from the peritoneal cavity, and 3- and 4-week-old juveniles were harvested from the peritoneal, thoracic, and pleural cavities of infected cats. The 7-week-old juvenile and 16-week-old adult worms were collected from the lungs of infected dogs (5). Eggs were collected by incubating the adult worms in physiological saline overnight at 37°C (22). Eggs, metacercariae, 1-, 2-, 3-, 4-, and 7-week-old juveniles, and adult worms were either used immediately for RNA preparation or stored at −70°C prior to protein extraction.
Purification and N-terminal amino acid sequencing of a 28-kDa native cysteine protease of adult P. westermani.
The 28-kDa cysteine protease of adult P. westermani was purified by Sephacryl S-300 gel filtration (1.6 by 70 cm long), followed by DEAE anion-exchange chromatography (1.6 by 2 cm long), and enzyme activity was monitored with a synthetic substrate, carboxybenzoyl-phenylalanyl-arginyl-7-amino-4-methylcumarin (Cbz-phe-arg-AMC) (5). The purified protein was resolved by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transblotted onto a polyvinylidene difluoride (PVDF) microporous membrane (Millipore, Bedford, Mass.). The enzyme band excised from the membrane was used for protein sequencing. N-terminal sequence determination was carried out on an ABI model 477A sequencer and an ABI model 120A PTH-analyzer (Perkin-Elmer Applied Biosystems, Branchburg, N.J.).
Preparation of RNA samples and construction of adult cDNA library.
Total RNAs of eggs, metacercariae, juveniles 1, 2, 3, 4, and 7 weeks old, and adult worms were extracted by the acid guanidium thiocyanate-phenol-chloroform method (3). Poly(A)+ RNA (5 μg) of adult P. westermani was isolated from total RNA with the Oligotex purification kit (Qiagen, Chatsworth, Calif.) and was used to construct a cDNA library with the λZAP II cDNA synthesis kit according to the manufacturer's instruction (Stratagene, La Jolla, Calif.).
RT-PCR, library screening, and DNA sequencing.
Two primers (sense, 5′-CTGAGCAACAACTGGTCGATTG-3′; antisense, 5′-GATTKGGCAATTCTACCGCATTGCC-3′, where K is G or T) were designed on the basis of two highly conserved regions of helminth cysteine proteases. Total RNA (1 μg) from adult P. westermani was reverse transcribed with oligo(dT)15 primer and Moloney murine leukemia virus reverse transcriptase (Life Technologies, Gaithersburg, Md.). The cDNA was subjected to PCR amplification in a 50-μl reaction mixture containing 5 μl of the reverse transcription (RT)-PCR product, 5 μl of Taq polymerase buffer (10×), 4 μl deoxynucleoside triphosphates (2.5 mM), 25 pM (each) primer, and 5 U of Taq polymerase. The thermal cycle profile consisted of 30 cycles of denaturation at 95°C (30 s), annealing at 55°C (1 min), and extension at 72°C (30 s), with a final extension at 72°C (5 min). The PCR product was subcloned into pT7-Blue vector (Novagen, Madison, Wis.) and, after determination of its sequence, was used to screen the adult P. westermani cDNA library by standard procedures (35). Hybridization to the 32P-labeled probe was done overnight at 42°C. The membrane was washed with high stringency and was exposed to Kodak X-AR film. Positive clones were isolated in vivo with ExAssist helper phage and nonsuppressing Escherichia coli strain SOLR (Stratagene). cDNA inserts were analyzed by restriction mapping and DNA sequencing. DNA sequencing was performed by the use of the dideoxynucleotide chain termination method on an automated DNA sequencer (model 377; Perkin-Elmer Applied Biosystems, Foster City, Calif.) with the ABI Prism Dye Terminator Cycle Sequencing Core kit (Perkin-Elmer) and universal or cDNA-specific primers.
Sequence alignment and phylogenetic analysis.
A search of the GenBank database for sequences with homology allowed us to identify >50 members of the cysteine proteases. Of these, 49 sequences with tertiary structures corresponding to a mature domain were aligned with CLUSTAL W (40). After optimization by manually trimming the sequences and divergence calculation, the neighbor-joining method and the drawgram of PHYLIP, version 3.5c, software were used to calculate the branch length and to draw the unrooted neighbor-joining tree, respectively (11, 21). The phylogram was constructed for only the species that had >40% identity with Pw28CCP. Bootstrapping analysis with 100 replicates was carried out to evaluate the statistical significance of each node.
Southern and Northern hybridizations.
The genomic DNAs (10 μg each), digested with various restriction enzymes including BamHI, EcoRI, HindIII, KpnI, MspI, Sau3AI, and XhoI, were separated on a 0.8% agarose gel and were transferred to a Hybond N+ membrane (Amersham-Pharmacia Biotech, Uppsala, Sweden) by capillary action. Further procedures for Southern blotting with 32P-labeled, full-length cDNA were done by the standard method (35). Northern hybridization was performed with 32P-labeled, full-length cDNA insert as a probe. Total RNAs from the eggs, metacercariae, 3-, 4-, and 7-week-old juveniles, and adults of P. westermani (10 μg for each) were separated on a 1.2% formaldehyde agarose gel and transferred to a Hybond N+ filter (Amersham-Pharmacia Biotech). The labeling of the probe and detection of the hybridization signal were carried out with the 32P-labeled Rediprime DNA Labelling kit under the conditions recommended by the manufacturer (Amersham-Pharmacia Biotech).
Expression of a recombinant protein in E. coli cells.
To construct an expression vector for the production of recombinant Pw28CCP (rPw28CCP) in E. coli, a 642-bp fragment corresponding to the mature enzyme was generated by PCR from a full-length cDNA clone with primers 5′-AGCTCATATGGCCCCGGCAAGTGTTGACTG-3′ and 5′-GAAGTCTCGAGTTAGTGAATGATGGCGG-3′ into which the NdeI and XhoI sites were incorporated (underlined in each sequence). PCR was done as described above, and the PCR product was subcloned into the pET-28a (+) expression vector (Novagen). The recombinant plasmid was transformed into E. coli BL21 (DE3) cells. The identity of the expression construct was verified by DNA sequencing. The expression and purification were done as described in the pET system manual (Novagen). In brief, transformed cells were induced with isopropyl-β-d-thiogalactoside, harvested by centrifugation, and lysed with TE (Tris-EDTA) buffer (pH 8.0) in the presence of 10 μg of lysozyme per ml. Inclusion bodies, which contained most of the recombinant protein, were solubilized with 6 M urea. The recombinant protein was purified with His-Bind metal chelation resin (Novagen). Urea was removed by stepwise dialysis in the presence of 2 mM dithiothreitol.
RT-PCR analysis of the expression levels of Pw28CCP, NTP, and PwCP mRNAs in different developmental stages of P. westermani.
Total RNA samples isolated from different developmental stages of P. westermani were quantified with a spectrophotometer at 260 nm and with a DNA Dipstick (Invitrogen, Carlsbad, Calif.). Two micrograms of total RNA of each sample was reverse transcribed with the oligo(dT)15 primer and Moloney murine leukemia virus reverse transcriptase (Life Technologies). Aliquots of the resulting cDNA were subjected to PCR amplification with three sets of primers specific to genes encoding Pw28CCP (sense, 5′-GTACGTGTCTGAATGCTGGTC-3′; antisense, 5′-GAGAATGATGGCGGACGTAGT-3′), NTP (sense, 5′-GTTGAGTACGCTGCTCAATG-3′; antisense, 5′-ATGATCGCGGATGAAGCCAT-3′) (46), and PwCP (sense, 5′-CGTAGCTCTATCAGACCTTG-3′; antisense, 5′-GCTTAAGTTCGTTGTACGCG-3′) (32), respectively. PCR conditions were identical for all reactions. The cycling parameters were 95°C (2 min), followed by 27 cycles of 94°C (30 s), 58°C (30 s), and 72.5°C (1 min), with a final incubation at 72.5°C (10 min). PCR products were analyzed on a 1.3% agarose gel. The quantities of all samples were standardized by using the PCR mixtures with NTP (1×).
In situ hybridization.
To examine the expression patterns of Pw28CCP and PwCP in the cells and tissue of the worm, in situ hybridization was performed. Sense and antisense RNA probes corresponding to Pw28CCP and PwCP cDNAs were inserted into the pGEM-T easy vector (Promega, Madison, Wis.) and were labeled with digoxigenin (DIG)-labeled UTP (Boehringer Mannheim, Indianapolis, Ind.) by using T7 or SP6 polymerase. The worms, fixed in 4% paraformaldehyde, were embedded in a paraffin block. The worm sections (4 μm), applied to a Probe On slide (Dako, Kyoto, Japan), were incubated at 55°C for 1 h and were stored at 4°C. The slides were further processed for in situ hybridization with Boehringer Mannheim tissue kits. Briefly, sections were dewaxed with xylene, rehydrated through graded ethanol, and coated with 3-amino propyltriethoxysilane (TESTA; Sigma, St. Louis, Mo.), after which the sections were rinsed twice in diethyl pyrocarbonate–phosphate-buffered saline (pH 7.4; 0.137 M NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4) and dehydrated through an alcohol series. Hybridization was performed at 50°C for 16 to 18 h with the application of 100 pM DIG-labeled riboprobe in 100 μl of Hybridsol II solution (Oncor, Gaithersburg, Md.). The hybridization signal was detected by using standard DIG reagents and the conditions recommended by the manufacturer (Boehringer Mannheim). Sense probes were used as negative controls.
Diagnostic evaluation of rPw28CCP by immunoblot analysis.
To observe the immunoreactivity of recombinant protein as an antigen, a total of 85 serum samples from patients with paragonimiasis were used. Of 85 samples, 65 were obtained from patients with active pulmonary paragonimiasis diagnosed by clinical manifestation and typical chest radiological findings, together with positive serum reactions by enzyme-linked immunosorbent assay (ELISA) (19); the other 20 samples were from patients with chronic cerebral paragonimiasis diagnosed by brain computed tomography or magnetic resonance imaging (31). In addition, to determine possible cross-reactivity, 100 serum samples were examined from patients with clonorchiasis (n = 32), fascioliasis (n = 20), schistosomiasis japonicum (n = 5), alveolar (n = 8) and cystic (n = 5) echinococcoses, cysticercosis (n = 15), and sparganosis (n = 15). Sera from 50 healthy blood donors who denied any possible exposure to helminthic infections were investigated. The rPw28CCP resolved by 12% SDS–PAGE was transblotted onto a PVDF membrane. Each serum sample was diluted 1:200 and incubated with a PVDF strip overnight. Peroxidase-conjugated anti-human immunoglobulin G (heavy- and light-chain specific; Cappel, West Chester, Pa.) was diluted 1:1,000 and was incubated for over 3 h. The strips were developed with 0.03% (wt/vol) 1-chloro-4-naphthol chromogen (Sigma). The sensitivity and specificity of a recombinant protein were determined with 65 serum samples from patients with active paragonimiasis and 150 serum samples from patients with other parasitic infections and healthy controls.
Sera from four cats each infected with 50 metacercariae were collected biweekly from 2 to 16 weeks and were used to determine the antibody response against rPw28CCP.
Nucleotide sequence accession number.
The nucleotide sequence data for the cruzipain-like 28-kDa cysteine protease of P. westermani are available in the GenBank database at the National Center for Biotechnology Information under accession no. U70537.
RESULTS
Purification and N-terminal amino acid sequencing of the native 28-kDa protease of adult P. westermani.
The use of gel filtration and DEAE ion-exchange chromatography allowed effective purification of the native 28-kDa protease from adult P. westermani. The purified enzyme showed maximal activity against Cbz-phe-arg-AMC at pH 6.0 in the presence of 2 mM dithiothreitol, with a molecular mass of 28 kDa by SDS-PAGE, as described previously (5). The N-terminal sequence, (?)PA(?)VDWREKGAV, showed significant homology with the cathepsin family of cysteine proteases from parasites to mammals. The five residues including Pro, Asp, Trp, Arg, and Val at positions 2, 6, 7, 8, and 13, respectively, have been shown to be highly conserved among members of this enzyme family (25, 27, 28, 44).
Molecular properties and phylogenetic analysis of the Pw28CCP.
RT-PCR with oligonucleotide primers derived from highly conserved regions of the cathepsin family resulted in a 223-bp fragment. Sequence analysis revealed that this partial fragment clearly encoded a unique cysteine protease which shared a significant homology to, but was different from, known cysteine proteases of the cathepsin family available in the EMBL, GenBank, and DDBJ databases. This fragment was used as a probe to screen the adult worm cDNA library. The largest insert identified (1,052 bp in length) had a 975-bp complete open reading frame encoding a protein of 325 amino acids with an estimated molecular mass of 36,105 Da. The 3′ untranslated region contained a putative polyadenylation signal (AATACA), followed by a short poly(A)+ tail of 17 nucleotides. A sequence similarity search of the GenBank database demonstrated that Pw28CCP was most closely related to the NTP of P. westermani metacercariae (46) with an identity of 63.4%. Significant homology was also observed with the different cysteine proteases from other parasites as well as mammals, with identities ranging from 59.1% with Clonorchis sinensis cysteine protease 1 to 29.8% with human cathepsin L (Table 1).
TABLE 1.
Comparison of homologies between Pw28CCP and other cysteine protease members
| Description (GenBank accession no.) | % Homologya |
|---|---|
| Papain-like, papain (M15203) | 37.5 |
| Cruzipain-like | |
| P. westermani NTP (D21124) | 63.4 |
| C. sinensis cysteine protease 1 (AF093243) | 59.1 |
| P. westermani cysteine proteinase (U56865) | 50.5 |
| S. mansoni Puerto Rican cathepsin L1 (U07345) | 50.5 |
| T. congolense cysteine protease (L25130) | 38.2 |
| L. major cathepsin L (U43706) | 37.8 |
| T. cruzi cruzipain (M84342) | 37.5 |
| Cathepsin L-like | |
| F. hepatica secreted cathepsin L2 (U62289) | 47.4 |
| F. hepatica cathepsin L (AB009306) | 38.1 |
| Fasciola sp. cysteine protease (S70380) | 38.1 |
| Mouse cathepsin L (M20495) | 36.0 |
| Human cathepsin L (M20496) | 29.8 |
The sequence used for comparison included the whole region of preproenzyme.
The deduced amino acid sequence comprised an 18-residue hydrophobic region followed by a 93-residue propeptide region and a 214-residue mature protein domain. The putative recognition site for a signal peptidase (42) was identified between the Ala at position −74 and the Val at position −73 (the first residue of the mature enzyme is designated +1). In the propeptide region, the potential ERFNIN motif, which may constitute the core globular portion of the α-helix and which is characteristic of the non-cathepsin B family of cysteine proteases (12, 23, 41), was found; of seven residues, it conserved four amino acids of Arg, Ile, Phe, and Asn at positions −61, −58, −57, and −54, respectively. The mature peptide started from Ala at position 1 since it immediately preceded Pro 2, which is highly conserved in all family members. It was confirmed by N-terminal amino acid sequencing. The sequence of the first 13 amino acids predicted from the mature domain showed a perfect match with the N-terminal sequence determined from the native enzyme from adult worm except for the first and the fourth residues, which could not be clearly determined with the model 120A PTH-analyzer (Perkin-Elmer Applied Biosystems) due to a high background. The mature domain contained three conserved residues of Cys, His, and Asn at positions 25, 161, and 181, respectively, which are likely to be involved in the formation of catalytic triad, as all other cysteine proteases are. The six cysteine residues (at positions 22, 56, 63, 96, 154, and 202, respectively) proposed to be essential for the composition of the tertiary structure by disulfide bridges were also recognized in the same domain. The putative S1 subsites were located at Try, Pro, Ala, and Ala at positions 67, 68, 136, and 162, respectively, and those corresponding to S2 subsites were Pro, Ser, and Ile (at positions 69, 210, and 212, respectively). The potential S1′ sites were identified at Gln 19 and Trp 177. A single site for substrate binding was present at Gly 65. All these sites might be involved in the formation of the active-site cleft that determines the substrate specificity and active catalysis (2).
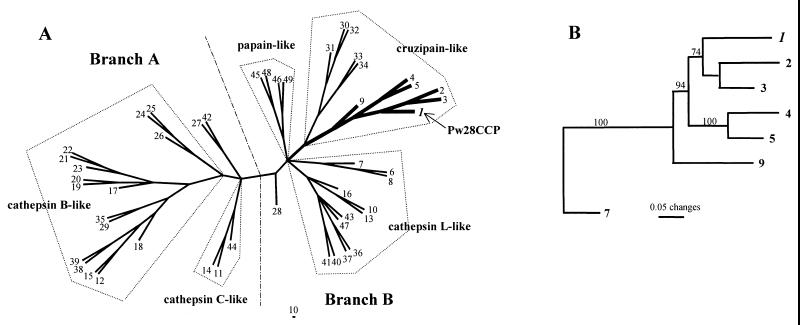
The phylogenetic trees constructed to examine the evolutionary relationship of Pw28CCP to other cysteine proteases revealed that Pw28CCP belonged to the cruzipain-like cysteine protease of branch B (Fig. 1A) (41). It formed a strong clade with the cysteine proteases of P. westermani, C. sinensis, and S. mansoni Puerto Rican preprocathepsin L1 with a 100% bootstrap value (Fig. 1B).
FIG. 1.
Phylogenetic analysis of P. westermani 28-kDa cysteine protease by unrooted neighbor-joining tree (A) and phylogram (B). Analysis shows the position of Pw28CCP as a sister group of the S. mansoni Puerto Rican cathepsin L (branch B), which is one of the ancestral forms of the cruzipain-like cysteine proteases. The alignment set used in the analysis was 115 sites in length in the mature protein domain. The number of each node indicates the bootstrapping value obtained with 100 replicates (GenBank accession nos. are given in parentheses). 1, P. westermani 28-kDa cruzipain-like cysteine protease (Pw28CCP) (U70537); 2, P. westermani NTP (D21124); 3, P. westermani cysteine proteinase (U56865); 4, Clonorchis sinensis cysteine protease (AB020036); 5, C. sinensis cysteine protease 1 (AF093243); 6, Fasciola hepatica cathepsin L (AB009306); 7, F. hepatica secreted cathepsin L2 (U62289); 8, Fasciola sp. cysteine protease (S70380); 9, Schistosoma mansoni Puerto Rican preprocathepsin L1 (U07345); 10, S. mansoni Liberian cathepsin L (Z32529); 11, S. mansoni Liberian cathepsin C (Z32531); 12, S. mansoni cathepsin B (Sm31) (M21309); 13, Schistosoma japonicum preprocathepsin L (U38476); 14, S. japonicum preprocathepsin C (U77932); 15, S. japonicum cathepsin B (X70968); 16, Spirometra erinacei cysteine protease (D63670); 17, Ancylostoma caninum cysteine protease (AcCP-1) (U18911); 18, Caenorhabditis elegans (Bristol N2) cathepsin B (L39927); 19, Haemonchus contortus CP1 (HMCP1, Z69342); 20, HMCP2 (Z69343); 21, HMCP4 (Z69345); 22, HMCP5 (Z69346); 23, H. contortus cysteine protease (Z81327); 24, Giardia muris CP1 (AF006198); 25, G. intestinalis cysteine protease (U83277); 26, Giardia intestinalis cysteine protease (U83275); 27, Toxocara canis cathepsin Z1 (AF143817); 28, T. canis cathepsin L (U53172); 29, Trypanosoma cruzi cathepsin B (AF043246); 30, trypanosome cysteine protease (X54353); 31, T. cruzi cruzipain (M84342); 32, Trypanosoma congolense cysteine protease (L25130); 33, Leishmania donovani chagasi promastigote-specific cysteine protease (AF004593); 34, Leishmania major cathepsin L (U43706); 35, L. major cathepsin B (U43705); 36, mouse cathepsin L (M20495); 37, human cathepsin L (M20496); 38, human cathepsin B (gastric cancer, L16510); 39, human cathepsin B (kidney, M14221); 40, human cathepsin S (M90696); 41, human cathepsin S (alveolar macrophage, M86553); 42, human cathepsin X precursor (ovary) (AF073890); 43, human cathepsin X (osteoclastoma) (U20280); 44, human cathepsin C (X87212); 45, Carica papaya papain (M15203); 46, Zea mays cysteine protease (mir1) (AF019145); 47, Heteroderma glycines cathepsin L (Y09498); 48, C. papaya chymopapain (X97789); 49, Ananas comosus bromelain (D14057).
Further comparison of the ERFNIN motif demonstrated that the cruzipain-like cysteine proteases, including Pw28CCP, tended to share higher levels of sequence homology in the first half of the motif than the posterior half. In cathepsin L-like proteases, the first half of the motif appeared to be less conserved than the posterior half (Fig. 2). In addition, Phe at position −60 and Ala at position −50 were highly conserved in cruzipain-like cysteine proteases but absent from cathepsin L-like enzymes. The Phe at position −57, which was highly conserved in papain- and cruzipain-like proteases, was replaced by Trp in five of seven cathepsin L-like proteases examined. The His residue at position −47 was well conserved in all cathepsin L-like proteases examined. Notably, NTP contained only one residue of the ERFNIN motif on the basis of this alignment. It is likely that NTP did not contain the motif, which further supported the possibility that, in fact, it might be another member of cathepsin family.
FIG. 2.
Alignment of the ERFNIN motif of Pw28CCP with those of other related enzymes. Cruzipain-like cysteine proteases display a high degree of homology in the first half, while cathepsin L-like protease do so in the posterior half. Notably, NTP is not likely to have an ERFNIN motif. The percent conservation is indicated at the end of each sequence. Numbering starts from the N terminus of Pw28CCP. The sequence specific to the ERFNIN motif is set in reverse type. Highly conserved sequences among cruzipain-like cysteine proteases of Phe and Ala are indicated in boxes. His, which is well conserved in cathepsin L-like proteases, is also highlighted. Circled letters show possible replacement of Phe by Trp, which share a high degree of biochemical similarity in cathepsin L-like proteases.
Southern blotting with the full-length cDNA probe showed that the size of Pw28CCP genomic DNA was approximately 2.5 kb on the basis of the BamHI digestion pattern (data not shown). Northern blot analysis with the same probe revealed a single band at ca. 1.0 kb (data not shown), consistent with the length (1,052 bp) of the Pw28CCP cDNA sequence.
Comparison of expression levels of Pw28CCP, NTP, and PwCP among different developmental stages of P. westermani.
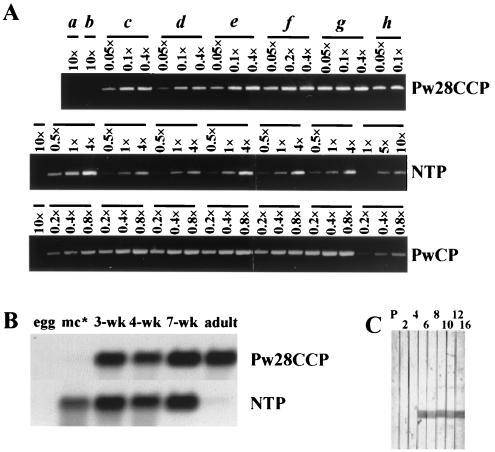
Semiquantitative RT-PCR was performed with primers specific for Pw28CCP, NTP, and PwCP to examine the levels of expression of the mRNAs of these three genes among different life cycles including eggs, metacercariae, juveniles 1, 2, 3, 4, and 7 weeks old, and adults. As shown in Fig. 3A, the expression patterns of the three enzymes appeared to be different from each other. The mRNA of Pw28CCP was absent from eggs and metacercariae but was present in 1- to 7-week-old juveniles and adults. Expression of NTP and PwCP mRNAs was detected in all stages except the egg stage. The levels of expression of Pw28CCP mRNA in juveniles of different ages and adults appeared to be similar. In contrast, the levels of expression of NTP and PwCP mRNAs were shown to be age dependent, with the expression of both mRNAs being down-regulated in the adult. The results of Northern blot analysis of Pw28CCP and NTP mRNA expression were similar to those obtained by RT-PCR, although the expression levels could not be directly compared due to the low sensitivity of the test (Fig. 3B). The expression of NTP declined dramatically in the adult stage as determined by both Northern blot and RT-PCR analyses. Figure 3C shows the immunoreactivity of rPw28CCP with pooled serum collected sequentially from four cats experimentally infected with 50 metacercariae. Specific antibody reactions to rPw28CCP could be detected from 6 weeks after infection.
FIG. 3.
Comparison of the levels of expression of Pw28CCP, NTP, and PwCP genes among different developmental stages of P. westermani. (A) Semiquantitative RT-PCR with total RNAs from eight different stages including eggs (a), metacercariae (b), juveniles 1 (c), 2 (d), 3 (e), 4 (f), and 7 (g) weeks old, and 16-week-old adults (h). (B) Northern blot analysis with full-length cDNAs of Pw28CCP and NTP shows results similar to those shown in panel A. mc∗, metacercariae. (C) Immunoblot of rPw28CCP with pooled serum from four cats experimentally infected with 50 metacercariae shows that the antibody responses are detected from 6 weeks after infection. P, preinfection sera; 2, 4, 6, 8, 10, 12, and 16, weeks after infection.
Localization of Pw28CCP and PwCP by in situ hybridization.
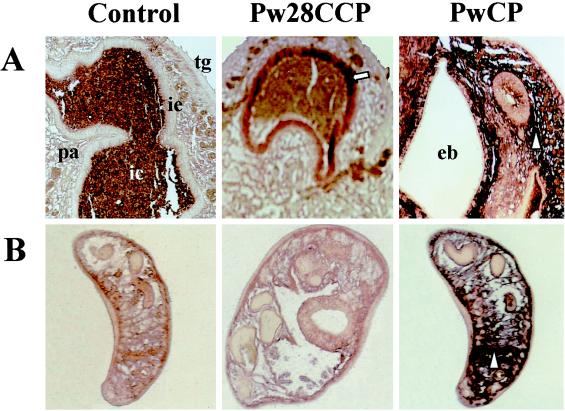
To identify the locations of Pw28CCP and PwCP gene expressions in the cells and tissue of the worm, in situ hybridization was performed. As shown in Fig. 4A and B, the hybridization signals with Pw28CCP were detected in the intestinal epithelium of the adult worm but not in the metacercariae, suggesting that this enzyme may be synthesized and secreted outside only when the worm is grown in the definitive hosts. Positive signals with PwCP occurred in the parenchymal cells of the adult worm in an ill-defined fashion, suggesting that PwCP is a lysosomal enzyme, as reported previously (28). In the metacercarial section, positive signals with PwCP were also seen in the parenchymal cells.
FIG. 4.
Localization of Pw28CCP and PwCP by in situ hybridization. (A) Hybridization signals with cDNAs of Pw28CCP are observed in the intestinal epithelium of the adult worm (arrow), while PwCP shows a positive reaction in the parenchymal cells (arrowhead). (B) In the metacercarial section, Pw28CCP exhibits no reaction, whereas PwCP reveals a positive signal in the parenchymal cells (arrowhead). pa, parenchyme; ic, intestinal contents; ie, intestinal epithelium; tg, tegument; eb, excretory bladder.
In vitro expression and immunological evaluation of rPw28CCP.
We expressed the 642-bp mature protein domain in the pET-28a (+) expression vector, which adds an N-terminal leader sequence with a 6× His tag to aid the purification. Recombinant protein, expressed as inclusion bodies, was solubilized by denaturing in 6 M urea and was purified by Ni-NTA affinity chromatography. The protein migrated as a single band at about 27 kDa, in good accord with the molecular mass calculated from the cDNA sequence by SDS-PAGE analysis (data not shown). However, the refolded rPw28CCP did not exhibit any enzyme activity either by substrate gel electrophoresis or in the fluorogenic molecular substrate (data not shown).
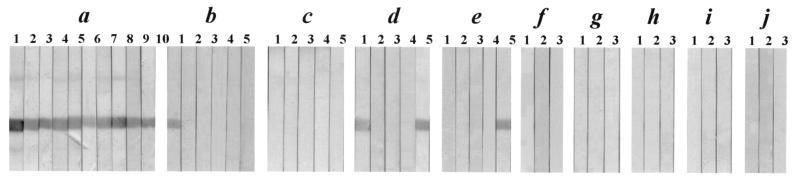
Table 2 summarizes the results of the evaluation of rPw28CCP as a diagnostic antigen by immunoblotting with sera from patients with different parasitic infections. Representative findings are also shown in Fig. 5. Strong antibody reactions against rPw28CCP were observed in 56 of 65 serum samples from patients with active pulmonary paragonimiasis (Fig. 5a), whereas 2 of 20 samples from patients with chronic inactive neuroparagonimiasis showed weak positive reactions (Fig. 5b). Two of five serum samples from patients with schistosomiasis japonicum (Fig. 5d) and 1 of 20 serum samples from patients with fascioliasis (Fig. 5e) exhibited positive reactions. None of the serum samples from patients with clonorchiasis, cystic and alveolar echinococcoses, sparganosis, and cysticercosis or sera from healthy controls reacted positively. The sensitivity of rPw28CCP as a diagnostic antigen was determined to be 86.2% (56 of 65 samples) for active paragonimiasis, and the specificity was 98% (147 of 150 samples).
TABLE 2.
Sensitivity and specificity of rPw28CCP for immunodiagnosis of active paragonimiasis
| Disease or infection | No. of serum samples tested | No. (%) of positive samples |
|---|---|---|
| Paragonimiasis | ||
| Active cases | 65 | 56 (86.2) |
| Chronic cases | 20 | 2 (10) |
| Clonorchiasis | 32 | 0 (0) |
| Schistosomiasis japonicum | 5 | 2 (40) |
| Fascioliasis | 20 | 1 (5) |
| Cystic echinococcosis | 5 | 0 (0) |
| Alveolar echinococcosis | 8 | 0 (0) |
| Sparganosis | 15 | 0 (0) |
| Cysticercosis | 15 | 0 (0) |
| Healthy control | 50 | 0 (0) |
FIG. 5.
Immunoreactivity of rPw28CCP with sera from patients with different parasitic infections as assessed by immunoblotting. Each strip was incubated with an individual serum sample from patients with active pulmonary and chronic calcified cerebral paragonimiasis (a and b, respectively), clonorchiasis (c), schistosomiasis japonicum (d), fascioliasis (e), cystic echinococcosis (f), alveolar echinococcosis (g), sparganosis (h), and cysticercosis (i) and from healthy controls (j). Strong positive reactions are observed in sera from patients with active paragonimiasis, while sera from patients with chronic inactive paragonimiasis exhibit negligible reactions. Some sera from patients with schistosomiasis japonicum (2 of 5) and fascioliasis (1 of 20) show cross-reactions. Sera from patients with other parasitic infections and healthy controls exhibit no positive reaction. Lanes 1 to 10 contain sera from different patients.
DISCUSSION
Despite the significant advances in purification and enzymological characterization of several cysteine proteases of P. westermani in recent years (4–6, 11, 16, 17, 22, 45), the genetic information about these enzymes is poorly understood (32, 46). In this study, we have cloned a cDNA encoding a 28-kDa cysteine protease of adult P. westermani (Pw28CCP) that exhibited a phylogenetic relationship to the cruzipain-like cysteine protease. This enzyme was expressed specifically in the developmental stages of juveniles and adults, when the parasite resides in the definitive hosts. Immunoblot analysis with the recombinant protein showed that it has a strong potential to be an antigen for the serological diagnosis of active paragonimiasis.
To examine whether the isolated cDNA encoded a functional cysteine protease and to provide an abundant source for further biological study of the enzyme, we produced the recombinant enzyme in a bacterial expression system. The mature domain of Pw28CCP cDNA was expressed at a high level as inclusion bodies in E. coli. We solubilized the inclusion bodies using either urea or guanidine and reconstructed the enzyme in soluble form. However, the recombinant enzyme refolded under various conditions did not show any proteolytic activity, which suggested that the native Pw28CCP is synthesized as a precursor molecule and that posttranslational modification is important and is required for conversion of the enzyme to its active form. It has been proposed that many helminth and protozoan parasites, including P. westermani, as demonstrated in the present study, synthesize the cruzipain- and cathepsin-like proteases as an inactive prepro form to protect themselves from autodigestion (23, 27, 37, 41, 44). The lack of catalytic activity of the bacterially expressed recombinant enzyme may be attributable to the lack of the appropriate posttranslational process. On the other hand, the deduced amino acid sequence of the Pw28CCP did not contain any putative N- or O-linked glycosylation sites. Instead, there was Pro at position 2, which is highly conserved in several cysteine proteases and which is believed to be a potential site for posttranslational modification. In F. hepatica cathepsin L, it has been shown that the replacement of Pro at this position with hydroxyproline is essential for enzymatic maturation and excretion (44). It has also been demonstrated in S. mansoni cathepsin B that the conservation of Cys at position 25 is crucial for enzyme processing and catalysis (10). Testing of these hypotheses awaits analysis of site-directed mutagenesis as well as a reliable expression system that yields enzymatically active protein, such as baculovirus vector and insect cells.
It is interesting that NTP had a unique primary structure; it showed a high degree of sequence homology with Pw28CCP and other cruzipain-like cysteine proteases, by which it could be classified as a member of the cruzipain family (Fig. 1). In contrast, it did not contain the ERFNIN motif in the propeptide region (Fig. 2), which hardly allowed it to be categorized as a cruzipain-like protease (23, 41). Enzymologically, it also has a neutral pH optimum, which is an unusual finding for the parasite cysteine proteases. From this point of view, further studies will be necessary to clearly evaluate the characteristics of the enzyme.
Studies of the patterns of expression of Pw28CCP in different developmental stages and in the worm compartment would provide some information about the potential functional significance of this enzyme. We have previously demonstrated that the changes in the activities of five different cysteine proteases during the maturation stages of P. westermani may be associated with the successful adaptation of parasites to the changing environment of the host (5). In this study, semiquantitative RT-PCR and Northern blot analysis revealed that the expression levels of Pw28CCP varied in different stages and appeared to be different from those of other two cysteine proteases, NTP and PwCP. These results suggest strongly that cysteine proteases of P. westermani may consist of very similar but different entities, further supporting the notion that the different species of cysteine protease play different roles in specific stages. Immunoblotting with sera from P. westermani-infected cats showed that specific antibody reactions against rPw28CCP could be detected from 6 weeks after infection, suggesting that Pw28CCP might be expressed from the early juvenile stage shortly after infection. This appeared to coincide well with the observation that antibody-producing B cells directed to a specific antigen could be generated differentially 4 to 6 weeks after priming during parasite infection (13). Histochemical localization of the Pw28CCP in the intestinal epithelium, indicative of excretion-secretion, was consistent with its plausible roles in modulating the host immune responses and nutrient uptake (6).
Although more precise quantitative tests are required to clearly define the changes in enzyme activity, the various levels of expression of each cysteine protease at different stages obtained both by semiquantitative RT-PCR and by Northern blot analysis might reflect the physiological significance of each enzyme during the maturation and migration of P. westermani. Alternatively, it might represent a strategy that has evolved with genetic design for the biological adaptation in a given host (38). Many cysteine protease genes of free-living nematodes and other parasites have also been shown to be developmentally regulated (29, 30, 34, 36).
The successful use of P. westermani cysteine proteases as diagnostic antigens has been documented with the use of partially purified enzymes or the application of cystatin-capture ELISA (15–17). However, its application is limited not only by the availability of parasite materials but also by the difficulties with the purification of the specific antigens. In the present study, the use of rPw28CCP alone in immunoblotting exhibited a sensitivity and a specificity of 86.2 and 98%, respectively, for the detection of the anti-P. westermani antibodies in the patients' sera. The sera from patients with parasitic infections other than schistosomiasis japonicum and fascioliasis used in this study did not cross-react with rPw28CCP. The mechanism of cross-reactivity due either to common epitopes shared by different trematode parasites or to other host factors possibly associated with major histocompatibility complex type II molecules awaits further elucidation to strengthen the diagnostic reliability for paragonimiasis. However, Pw28CCP seems to be an important antigen since its diagnostic value was comparable to those of mixed or partially purified cysteine protease or other protein antigens that have been used in antibody assays (15–17, 26). This study confirmed the usefulness of P. westermani cysteine protease as a potent diagnostic antigen. The availability of rPw28CCP in large quantities may facilitate the development of more effective tests for the diagnosis of human paragonimiasis, which is still endemic in several parts of the world (1).
In conclusion, we have characterized a cDNA encoding the 28-kDa cysteine protease of P. westermani that has structural motifs and a domain organization specific to the cruzipain-like cysteine protease. Our results indicate that cysteine protease species of similar molecular sizes are differentially expressed during the developmental stages of P. westermani. While the metacercarial NTP played critical roles in the early infection stage by suppressing the host immune system (14), Pw28CCP, as a gut-associated enzyme in the juvenile and adult stages, seems to play a major role in nutrient uptake and immune evasion in the definitive host (6). The specific immunoreactivity of rPw28CCP suggests that it could probably be useful for immunodiagnostic applications as well as in the development of a vaccine.
ACKNOWLEDGMENTS
We thank Liang Ma, National Institutes of Health, Bethesda, Md., for critical review of the manuscript. Ui Wook Hwang, Department of Parasitology, Yonsei University College of Medicine, is acknowledged for help with the structural alignment and the phylogenetic analysis.
This work was supported by a research grant for Basic Medical Sciences from the Ministry of Education, Republic of Korea Government (1998).
ADDENDUM
During the revision process, another gene that putatively encodes a cysteine protease of adult P. westermani and that had an open reading frame of 687 bp and a deduced amino acid sequence of 229 (estimated molecular mass of approximately 28.5 kDa) was partially characterized. It shared a significant degree of sequence homology (range, 32 to 74%) with other members of the cathepsin family of cysteine proteases of helminth parasites (24).
REFERENCES
- 1.Blair D, Xu Z B, Agatsuma T. Paragonimiasis and the genus Paragonimus. Adv Parasitol. 1999;42:113–222. doi: 10.1016/s0065-308x(08)60149-9. [DOI] [PubMed] [Google Scholar]
- 2.Brocklehurst K, Brocklehurst S M, Kowlessur D, O'Driscoll M, Patel G, Salih E, Templeton W, Thomas E, Topham C M, Willenbrock F. Supracrystallographic resolution of interactions contributing to enzyme catalysis by use of natural structural variants and reactivity-probe kinetics. Biochem J. 1988;256:543–558. doi: 10.1042/bj2560543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Chung Y B, Kong Y, Joo I J, Cho S Y, Kang S Y. Excystment of Paragonimus westermani metacercariae by endogenous cysteine protease. J Parasitol. 1995;81:137–142. [PubMed] [Google Scholar]
- 5.Chung Y B, Kong Y, Yang H J, Kang S Y, Cho S Y. Changes of cysteine protease activities during the maturation stages of Paragonimus westermani. J Parasitol. 1997;83:902–907. [PubMed] [Google Scholar]
- 6.Chung Y B, Yang H J, Kang S Y, Kong Y, Cho S Y. Activities of different cysteine proteases of Paragonimus westermani in cleaving human IgG. Korean J Parasitol. 1997;35:139–142. doi: 10.3347/kjp.1997.35.2.139. [DOI] [PubMed] [Google Scholar]
- 7.Coombs G H, Mottram J C. Parasite proteinases and amino acid metabolism: possibilities for chemotherapeutic exploitation. Parasitology. 1997;114:S61–S80. [PubMed] [Google Scholar]
- 8.Dowd A J, Smith A M, McGonigle S, Dalton J P. Purification and characterisation of a second cathepsin L proteinase secreted by the parasitic trematode Fasciola hepatica. Eur J Biochem. 1994;223:91–98. doi: 10.1111/j.1432-1033.1994.tb18969.x. [DOI] [PubMed] [Google Scholar]
- 9.Engel J C, Soyle P S, Hsieh I, McKerrow J H. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J Exp Med. 1998;188:725–734. doi: 10.1084/jem.188.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felleisen R, Klinkert M Q. In vitro translation and processing of cathepsin B of Schistosoma mansoni. EMBO J. 1990;9:371–377. doi: 10.1002/j.1460-2075.1990.tb08120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felsenstein J. PHYLIP version 3.5c. Seattle: Department of Genetics, University of Washington; 1995. [Google Scholar]
- 12.Garnier J, Gibrat J F, Robson B. GOR secondary structure prediction method version IV. Methods Enzymol. 1996;266:540–553. doi: 10.1016/s0076-6879(96)66034-0. [DOI] [PubMed] [Google Scholar]
- 13.Garraud O, Perler F B, Bradley J E, Nutman T B. Induction of parasite antigen-specific antibody responses in unsensitized human B cells is dependent on the presence of cytokines after T cell priming. J Immunol. 1997;159:4793–4798. [PubMed] [Google Scholar]
- 14.Hamajima F, Yamamoto M, Tsuru S, Yamakami K, Fujino T, Hamajima H, Katsura Y. Immunosuppression by a neutral thiol protease from parasitic helminth larvae in mice. Parasite Immunol. 1994;16:261–273. doi: 10.1111/j.1365-3024.1994.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda T. Cystatin capture enzyme-linked immunosorbent assay for immunodiagnosis of human paragonimiasis and fascioliasis. Am J Trop Med Hyg. 1998;59:286–290. doi: 10.4269/ajtmh.1998.59.286. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda T. Antibody responses to fluke cysteine proteinases in Paragonimus- and Fasciola-infected rats. J Helminthol. 1998;72:187–191. doi: 10.1017/s0022149x00016424. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda T, Oikawa Y, Nishiyama T. Enzyme-linked immunosorbent assay using cysteine proteinase antigens for immunodiagnosis of human paragonimiasis. Am J Trop Med Hyg. 1996;55:435–437. [PubMed] [Google Scholar]
- 18.Im J G, Kong Y, Shin Y M, Yang S O, Song J G, Han M C, Kim C W, Cho S Y, Ham E K. Pulmonary paragonimiasis: clinical and experimental studies. RadioGraphics. 1993;13:575–586. doi: 10.1148/radiographics.13.3.8316665. [DOI] [PubMed] [Google Scholar]
- 19.Im J G, Whang H Y, Kim W S, Han M C, Shim Y S, Cho S Y. Pleuropulmonary paragonimiasis: radiologic findings in 71 patients. Am J Roentgenol. 1992;159:39–43. doi: 10.2214/ajr.159.1.1609718. [DOI] [PubMed] [Google Scholar]
- 20.Jankovic D, Aslund L, Oswald I P, Caspar P, Champion C, Pearce E, Coligan J E, Strand M, Sher A, James S L. Calpain is the target antigen of a Th1 clone that transfers protective immunity against Schistosoma mansoni. J Immunol. 1996;157:806–814. [PubMed] [Google Scholar]
- 21.Joshua-Tor L, Xu H E, Johnstone S A, Rees D C. Crystal structure of a conserved protease that binds DNA: the bleomycin hydrolase, Gal6. Science. 1995;269:945–950. doi: 10.1126/science.7638617. [DOI] [PubMed] [Google Scholar]
- 22.Kang S Y, Cho M S, Chung Y B, Kong Y, Cho S Y. A cysteine protease of Paragonimus westermani eggs. Korean J Parasitol. 1995;33:323–330. doi: 10.3347/kjp.1995.33.4.323. [DOI] [PubMed] [Google Scholar]
- 23.Karrer K M, Peiffer S L, DiTomas M E. Two distinct gene subfamilies within the family of cysteine protease genes. Proc Natl Acad Sci USA. 1993;90:3063–3067. doi: 10.1073/pnas.90.7.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim T S, Na B K, Park P H, Song K J, Hontzeas N, Song C Y. Cloning and expression of a cysteine proteinase gene from Paragonimus westermani adult worms. J Parasitol. 2000;86:333–339. doi: 10.1645/0022-3395(2000)086[0333:CAEOAC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Kong Y, Chung Y B, Kang S Y, Cho S Y. Cleavage of immunoglobulin G by excretory-secretory cathepsin S-like protease of Spirometra mansoni plerocercoid. Parasitology. 1994;109:611–621. doi: 10.1017/s0031182000076496. [DOI] [PubMed] [Google Scholar]
- 26.Kong Y, Ito A, Yang H J, Chung Y B, Kasuya S, Kobayashi M, Liu Y H, Cho S Y. Immunoglobulin G (IgG) subclass and IgE responses in human paragonimiases caused by three different species. Clin Diagn Lab Immunol. 1998;5:474–478. doi: 10.1128/cdli.5.4.474-478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loukas A, Selzer P M, Maizels R M. Characterisation of Tc-cpl-1, a cathepsin L-like cysteine protease from Toxocara canis infective larvae. Mol Biochem Parasitol. 1998;92:275–289. doi: 10.1016/s0166-6851(97)00245-4. [DOI] [PubMed] [Google Scholar]
- 28.Michel A, Ghoneim H, Resto M, Klinkert M Q, Kunz W. Sequence, characterization and localization of a cysteine proteinase cathepsin L in Schistosoma mansoni. Mol Biochem Parasitol. 1995;73:7–18. doi: 10.1016/0166-6851(95)00092-f. [DOI] [PubMed] [Google Scholar]
- 29.Mottram J C, Brooks D R, Coombs G H. Roles of cysteine proteinases of trypanosomes and Leishmania in host-parasite interactions. Curr Opin Microbiol. 1998;1:455–460. doi: 10.1016/s1369-5274(98)80065-9. [DOI] [PubMed] [Google Scholar]
- 30.Mottram J C, Frame M J, Brooks D R, Tetley L, Hutchison J E, Souza A E, Coombs G H. The multiple cpb cysteine proteinase genes of Leishmania mexicana encode isoenzymes that differ in their stage regulation and substrate preferences. J Biol Chem. 1997;272:14285–14293. doi: 10.1074/jbc.272.22.14285. [DOI] [PubMed] [Google Scholar]
- 31.Nomura M, Nitta H, Nakada M, Yamashima T, Yamashita J. MRI findings of cerebral paragonimiasis in chronic stage. Clin Radiol. 1999;54:622–624. doi: 10.1016/s0009-9260(99)90026-0. [DOI] [PubMed] [Google Scholar]
- 32.Park H, Hong K M, Ryu J S, Shin C H, Lee J B, Soh C T, Paik M K, Min D Y. Cloning of a cysteine proteinase cDNA of adult Paragonimus westermani by polymerase chain reaction. Mol Cell. 1997;7:335–339. [PubMed] [Google Scholar]
- 33.Piacenza L, Acosta D, Basmadjian I, Dalton J P, Carmona C. Vaccination with cathepsin L proteinases and with leucine aminopeptidase induces high levels of protection against fascioliasis in sheep. Infect Immun. 1999;67:1954–1961. doi: 10.1128/iai.67.4.1954-1961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray C, McKerrow J H. Gut-specific and developmental expression of a Caenorhabditis elegans cysteine protease gene. Mol Biochem Parasitol. 1992;51:239–249. doi: 10.1016/0166-6851(92)90074-t. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Skuce P J, Redmond D L, Iiddell S, Stewart E M, Newlands G F J, Smith W D, Knox D P. Molecular cloning and characterization of gut-derived cysteine proteinases associated with a host protective extracts from Haemonchus contortus. Parasitology. 1999;119:405–412. doi: 10.1017/s0031182099004813. [DOI] [PubMed] [Google Scholar]
- 37.Smith A M, Dalton J P, Clough K A, Kilbane C L, Harrop S A, Hole N, Brindley P J. Adult Schistosoma mansoni express cathepsin L proteinase activity. Mol Biochem Parasitol. 1994;67:11–19. doi: 10.1016/0166-6851(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 38.Sukhdeo M V, Sukhdeo S C. Optimal habitat selection by helminths within the host environment. Parasitology. 1994;109:S41–S55. doi: 10.1017/s0031182000085073. [DOI] [PubMed] [Google Scholar]
- 39.Syfrig J, Wells C, Daubenberger C, Musoke A J, Naessens J. Proteolytic cleavage of surface proteins enhances susceptibility of lymphocytes to invasion by Theileria parva sporozoites. Eur J Cell Biol. 1998;76:125–132. doi: 10.1016/S0171-9335(98)80025-3. [DOI] [PubMed] [Google Scholar]
- 40.Thomson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tort J, Brindley P J, Knox D, Wolfe K H, Dalton J P. Proteinases and associated genes of parasitic helminths. Adv Parasitol. 1999;43:161–266. doi: 10.1016/s0065-308x(08)60243-2. [DOI] [PubMed] [Google Scholar]
- 42.von Heijne G. A new method for predicting sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward W, Alvarado L, Rawling N D, Engel J C, Franklin C, McKerrow J H. A primitive enzyme for a primitive cell: the protease required for excystation of Giardia. Cell. 1997;89:437–444. doi: 10.1016/s0092-8674(00)80224-x. [DOI] [PubMed] [Google Scholar]
- 44.Wijffels G L, Panaccio M, Salvatore L, Wilson L, Walker I D, Spithill T W. The secreted cathepsin L-like proteinases of the trematode, Fasciola hepatica, contain 3-hydroxyproline residues. Biochem J. 1994;299:781–790. doi: 10.1042/bj2990781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamakami K, Hamajima F, Akao S, Tadakuma T. Purification and characterization of acid cysteine protease from metacercariae of the mammalian trematode parasite Paragonimus westermani. Eur J Biochem. 1995;233:490–497. doi: 10.1111/j.1432-1033.1995.490_2.x. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto M, Yamakami K, Hamajima F. Cloning of a cDNA encoding a neutral thiol protease from Paragonimus westermani metacercariae. Mol Biochem Parasitol. 1994;64:345–348. doi: 10.1016/0166-6851(94)00027-1. [DOI] [PubMed] [Google Scholar]