Figure 5.
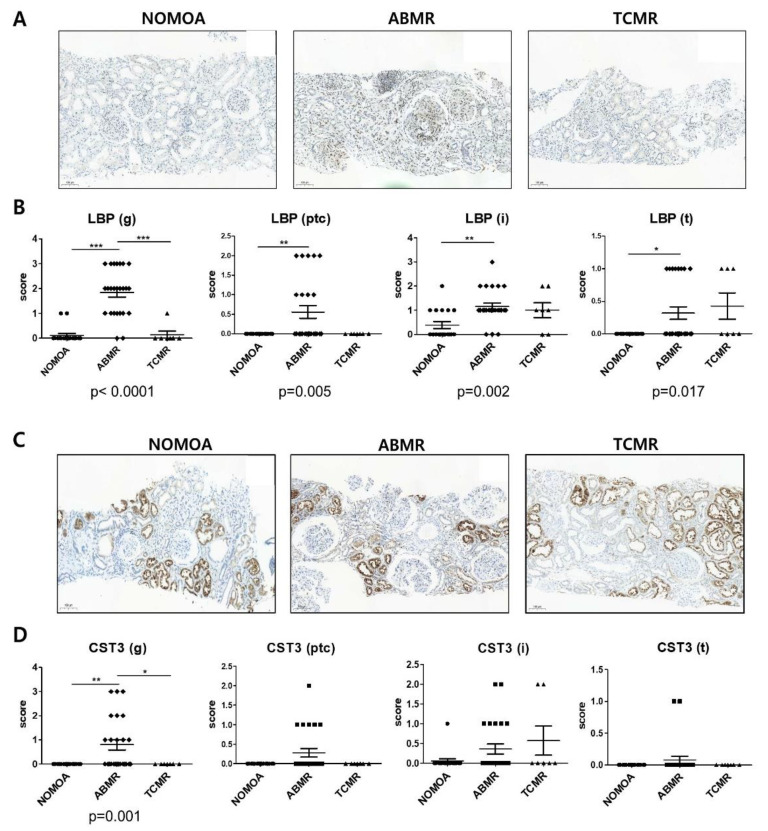
Validation for biomarker candidates in allograft tissues. (A) Immunohistochemistry (IHC) staining for LBP (visible as intense brown deposits) in representative biopsy specimens from the NOMOA (n = 18), ABMR (n = 25), and TCMR (n = 7) groups. (B) Expression of LBP corresponding to histopathologic scores in glomerulus (g), peritubular capillary (ptc), interstitium (i), and tubules (t) (×100 magnification). (C) IHC staining for CST3 (visible as intense brown deposits) in representative biopsy samples from the NOMOA, ABMR, and TCMR groups. (D) Expression of CST3 corresponding to histopathologic scores in g, ptc, i, and t (×100 magnification). Error bars indicate mean ± SEM. Kruskal–Wallis one-way ANOVA p values are indicated for each protein. Dunn’s Multiple Comparison test post hoc test significance values are indicated as * p < 0.05 ** p < 0.01 *** p < 0.001. 95% CI, 95% confidence interval. ABMR, antibody-mediated rejection; NOMOA, no major abnormality; TCMR, T-cell-mediated rejection; CST3, Cystatin-C; LBP, lipopolysaccharide-binding protein; g, glomeruli; ptc, peritubular capillaries; i, interstitium; t, tubules.3.6. Correlation between Banff lesion scores and biomarker scores of allograft.
