Abstract
Nitrobindins (Nbs) are all-β-barrel heme proteins and are present in prokaryotes and eukaryotes. Although their function(s) is still obscure, Nbs trap NO and inactivate peroxynitrite. Here, the kinetics of peroxynitrite scavenging by ferric Danio rerio Nb (Dr-Nb(III)) in the absence and presence of CO2 is reported. The Dr-Nb(III)-catalyzed scavenging of peroxynitrite is facilitated by a low pH, indicating that the heme protein interacts preferentially with peroxynitrous acid, leading to the formation of nitrate (~91%) and nitrite (~9%). The physiological levels of CO2 dramatically facilitate the spontaneous decay of peroxynitrite, overwhelming the scavenging activity of Dr-Nb(III). The effect of Dr-Nb(III) on the peroxynitrite-induced nitration of L-tyrosine was also investigated. Dr-Nb(III) inhibits the peroxynitrite-mediated nitration of free L-tyrosine, while, in the presence of CO2, Dr-Nb(III) does not impair nitro-L-tyrosine formation. The comparative analysis of the present results with data reported in the literature indicates that, to act as efficient peroxynitrite scavengers in vivo, i.e., in the presence of physiological levels of CO2, the ferric heme protein concentration must be higher than 10−4 M. Thus, only the circulating ferric hemoglobin levels appear to be high enough to efficiently compete with CO2/HCO3− in peroxynitrite inactivation. The present results are of the utmost importance for tissues, like the eye retina in fish, where blood circulation is critical for adaptation to diving conditions.
Keywords: Danio rerio heme-protein, effect of CO2, kinetics, peroxynitrite detoxification, tyrosine protection, zebrafish nitrobindin
1. Introduction
Nitrosative stress plays a key role in the etiology of human diseases, such as atherosclerosis, inflammation, cancer, and neurological diseases, being particularly relevant in the onset of retinopathies and glaucoma [1,2,3]. In fact, reactive nitrogen species (RNS) can cause protein, DNA, and lipid nitration, impairing their functions [4,5,6,7,8,9,10,11]. One of the most potent biological nitrosative agents is peroxynitrite (ONOO−), which is produced when nitric oxide (●NO) and superoxide (●O2−) are combined at extremely rapid rates [4,5,9,10,11,12,13]. ONOO− diffuses through membrane anion channels as well as the conjugated peroxynitrous acid ONOOH (pKa = 6.9) [13]. ONOO− is relatively stable, while ONOOH decays rapidly, with an apparent half-life of 1-2 s at physiological pH, yielding ~70% nitrate (NO3−) and H+, and ~30% nitrogen dioxide (●NO2) and hydroxyl (●OH) radicals via the homolysis of the O–O bond. The secondary reactions of ●NO2 and O●− lead to NO2− and O2 [4,9,10,11,12,13]. ONOO− reacts with biomolecules mainly by a direct reaction or immediately after ONOOH is homolyzed to ●NO2 and ●OH [4,9,10,11,12,13].
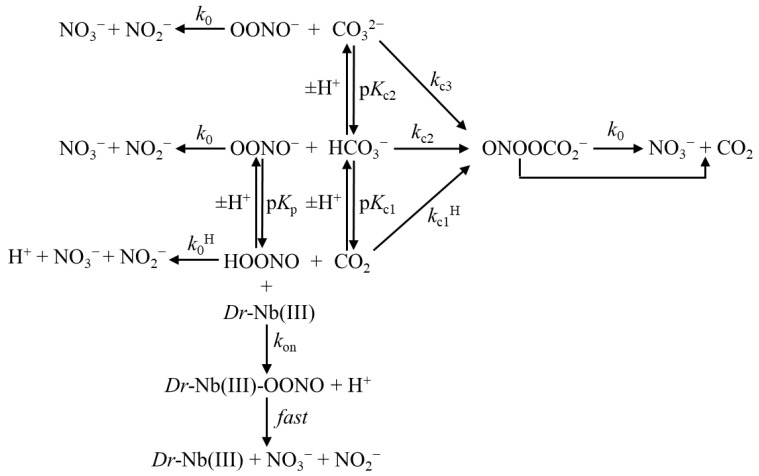
At the end of the last century, carbon dioxide (CO2) was reported to react with ONOO− [14,15]. Since the CO2 concentration is relatively high in vivo (~1.2 × 10−3 M at physiological pH in equilibrium with ~2.4 × 10−2 M HCO3− with a pKa ≈ 6.3 at 25.0 °C), most of the ONOO− rapidly reacts with the CO2/HCO3− species (depending on pH), leading mostly to the formation of 1-carboxylato-2-nitrosodioxidane adduct (ONOOCO2−), which displays an apparent half-life of 0.5–3 ms. This compound further decays (via homolysis of the O–O bond), yielding the reactive species trioxocarbonate●− (CO3●−) and ●NO2 (3% to 35%), which then proceed towards CO2 and NO3− (or by direct yielding of NO3− and CO2 (65% to 90%) [9,11,12,13,14,15,16,17,18,19,20,21]). As a whole, the complexity of the peroxynitrite inactivation mechanism can be sketched, as is shown in Scheme 1 [13,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41], where Dr-Nb(III) represents the contribution of heme proteins.
Scheme 1.
Dr-Nb(III)-mediated isomerization of peroxynitrite in the absence and presence of CO2 (representing the CO2/HCO3−/CO32− system). The disappearance of the pH-dependent ONOO−/ONOOH species is characterized by the absorption decrease at 302 nm. For the sake of clarity, only the final products of the peroxynitrite inactivation mechanisms (indicated by k0 and k0H) are reported, while the intermediate species are described in the text.
Moreover, the reaction of ONOO− with CO2/HCO3−/CO32− redirects the ONOO−/ONOOH-based oxidation of aromatic and aliphatic amino acid residues, facilitating the nitration of tyrosine and tryptophan and limiting the oxidation of methionine and cysteine [5,9,10,11,12,13,14,15,20,21,42,43,44,45]. Remarkably, ●NO2 and CO3●− radicals are much stronger oxidant species than their precursors ●NO, O2●−, and ONOO−/ONOOH [9,10,11,12,13,20,21,46].
Tyrosine nitration through the peroxynitrite pathway (see Scheme 1) is operative in most fish, including zebrafish (Danio rerio), under stressed conditions [47,48]. Further, the effect of the enrichment of CO2 in the atmosphere brings about the raising of CO2 levels also in the oceans [49]. Although the increased levels of CO2 create only limited behavioral effects, they may significantly affect the body response to external insults. In addition, the increased concentration of CO2 in the blood is of particular relevance in fish, for which retinal circulation is of crucial importance due to the high hydrostatic pressure of the environment where they live [50,51].
Here, the kinetics of ONOO−/ONOOH (hereafter peroxynitrite) inactivation by all-β-barrel ferric Danio rerio nitrobindin (Dr-Nb(III)), in the absence and presence of CO2, are reported and analyzed in parallel with those of all-α-helical globins. The present results open a question on the competition between the protecting activity of heme proteins (which inactivate the damaging reactions of peroxynitrite on L-tyrosine nitration) and the non-protecting action of CO2/HCO3−, which indeed depends on the levels of ferric heme-proteins. Thus, the levels (and the scavenging activity) of most heme proteins might be too low to act as peroxynitrite scavengers in vivo; only circulating ferric hemoglobin (Hb(III)) levels appear to be high enough to efficiently compete with CO2/HCO3−/CO32- in peroxynitrite inactivation.
2. Materials and Methods
2.1. Materials
Dr-Nb(III) was cloned, expressed, and purified as already reported [52]. The Dr-Nb(III) concentration was determined spectrophotometrically by measuring the absorbance at 407 nm (ε = 1.57 × 105 M−1 cm−1) [52]. Apo-Dr-Nb was prepared, as already reported [53]. Peroxynitrite was obtained from Cayman Chemical (Ann Arbor, MI, USA). The concentration of peroxynitrite was determined spectrophotometrically at 302 nm (ε = 1.705 × 103 M−1 cm−1) [12]. L-tyrosine (obtained from Merck KGaA, Darmstadt, Germany) was dissolved in 5.0 × 10−2 M Bis-Tris propane buffer, at pH 7.0 and 22.0 °C; the final L-tyrosine concentration was 1.0 × 10−4 M [25,30,36,39]. All the other chemicals were purchased from Merck KGaA (Darmstadt, Germany). All chemicals were of analytical grade and were used without further purification.
2.2. Methods
In the absence and presence of CO2, peroxynitrite isomerization by Dr-Nb(III) and apo-Dr-Nb was investigated at pH 5.8, 7.0, and 8.5 (5.0 × 10−2 M Bis-Tris propane buffer) and 22.0 °C, under anaerobic conditions. In the presence of CO2, peroxynitrite isomerization by Dr-Nb(III) and apo-Dr-Nb was investigated by adding NaHCO3 (final concentration, 5.0 × 10−1 M) to the Dr-Nb(III) and apo-Dr-Nb solutions; this NaHCO3 concentration corresponds to different concentrations of CO2, HCO3−, and CO32- depending on pH. After the addition of NaHCO3, the Dr-Nb(III) and apo-Dr-Nb solutions were allowed to equilibrate for at least 5 min; then, the pH was readjusted to the desired pH if needed [25,27,28,30,36,39].
Peroxynitrite isomerization by Dr-Nb(III) and apo-Dr-Nb was investigated via rapid mixing of the Dr-Nb(III) and apo-Dr-Nb solutions (final concentration ranging between 5.0 × 10−6 M and 3.5 × 10−5 M) with the peroxynitrite solution (final concentration ranging between 2.5 × 10−5 M and 2.0 × 10−4 M); no gaseous phase was present. Peroxynitrite isomerization was monitored at 302 nm [9,12,25,26,27,30,36,39] by the SFM-20/MOS-200 rapid-mixing stopped-flow apparatus (BioLogic Science Instruments, Claix, France); the light path of the observation chamber was 10 mm and the dead-time was 1.3 ms.
Values of the pseudo-first-order rate constant for peroxynitrite isomerization by Dr-Nb(III) and apo-Dr-Nb (i.e., kobs) were determined according to Equation (1) [13,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,54,55]:
| [peroxynitrite]t = [peroxynitrite]i × e − kobs × t | (1) |
Values of the second-order rate constant for peroxynitrite isomerization by Dr-Nb(III) (i.e., kon), and of the first-order rate constant for the spontaneous decay of peroxynitrite (i.e., k0), were obtained from the dependence of kobs on the Dr-Nb(III) concentration, according to Equation (2) [13,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,54,55]:
| kobs = kon × [Dr-Nb(III)] + k0 | (2) |
The reaction of peroxynitrite with L-tyrosine was investigated at pH 7.0 and 22.0 °C, as reported elsewhere. The relative nitro-L-tyrosine yield (%) corresponds to: (yield with added Dr-Nb(III) or apo-Dr)/(yield with no Dr-Nb(III) or apo-Dr-Nb) × 100 [13,25,30,34,35,36,39,55,56].
The percentage of NO3− and NO2− obtained from peroxynitrite isomerization was determined spectrophotometrically at 543 nm by using the Griess reagent and vanadium(III) chloride (VCl3) to catalyze the conversion of NO3− to NO2−, according to literature [25,30,36,39,56].
Kinetic data were analyzed using the GraphPad Prism program, version 5.03 (GraphPad Software, San Diego, CA, USA). The results are given as the mean values of at least four experiments plus and minus the standard deviation. Data were analyzed using GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA) and were expressed as mean values ± standard deviation (SD). Statistical analysis was performed using either the Student’s t-test or the one-way ANOVA comparison test (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001).
3. Results and Discussion
In the absence and presence of CO2, the absorbance at 302 nm decreases upon mixing the Dr-Nb(III), apo-Dr-Nb, and peroxynitrite solutions over the whole pH range explored. According to the literature [13,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,54,55], this reflects peroxynitrite isomerization. No absorbance spectroscopic changes were observed in the Soret region.
Under all the experimental conditions, the time course of peroxynitrite isomerization in the absence and presence of Dr-Nb(III), apo-Dr-Nb, and CO2 was fitted to a single-exponential decay for more than 93% of its course, according to Equation (1) (Figure 1, Figure 2 and Figure 3, panels A and B). The values of the pseudo-first-order rate constant for peroxynitrite isomerization catalyzed by Dr-Nb(III) (i.e., kobs) increase with the heme protein concentration (Figure 1, Figure 2 and Figure 3, panels C and D). Previous experiments have shown that, once bound to a heme protein (likely as Fe(III)-ONOOH), peroxynitrite isomerizes to NO3− at a rate of ~70 s−1 [13]. Therefore, since the values of kobs range between 1 × 10−1 s−1 and 8 s−1 (Figure 1, Figure 2 and Figure 3, panel C), they indicate that, under our conditions, the formation of the Dr-Nb(III)-OONOH species represents the rate-limiting step of the catalytic process. Notably, the Dr-Nb(III)-OONOH conversion to Dr-Nb(III) and NO3− is faster by at least 10-fold than the formation of the Dr-Nb(III)-OONOH complex.
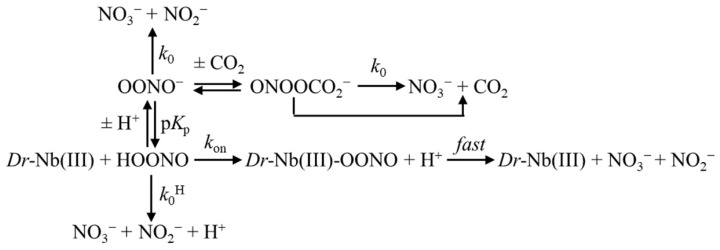
Figure 1.
Effect of Dr-Nb(III) on the kinetics for peroxynitrite isomerization, at pH 5.8 and 22.0 °C. (A) Averaged time courses of the Dr-Nb(III)-catalyzed isomerization of peroxynitrite in the absence of CO2. The time course analysis, according to Equation (1), allowed us to determine the following values of kobs: trace a, 3.1 s−1, R = 0.998; trace b, 7.6 s−1, R = 0.998. The Dr-Nb(III) concentration was: trace a, 1.0×10−5 M; trace b, 3.0 × 10−5 M. (B) Averaged time courses of the Dr-Nb(III)-catalyzed isomerization of peroxynitrite in the presence of CO2. The time course analysis, according to Equation (1), allowed us to determine the following values of kobs: trace a, 7.4 s−1, R = 0.999; trace b, 1.3 × 101 s−1, R = 0.998. The Dr-Nb(III) concentration was: trace a, 1.0 × 10−5 M; trace b, 3.0 × 10−5 M. The CO2 concentration was 1.2 × 10−3 M. (C) Dependence of kobs on the concentration of Dr-Nb(III) in the absence of CO2. Data were analyzed according to Equation (2) with kon = 2.2 × 105 M−1 s−1 and k0 = 8.1 × 10−1 s−1; R = 0.989, p = 0.0006. (D) Dependence of kobs on the concentration of Dr-Nb(III) in the presence of CO2. Data obtained were analyzed according to Equation (2) with kon = 2.8 × 105 M−1 s−1 and k0 = 4.1 s−1; R = 0.991, p = 0.0005. (E) Averaged time courses of peroxynitrite isomerization by apo-Dr-Nb in the absence of CO2. The time course analysis, according to Equation (1), allowed us to determine the following values of kobs: trace a, 8.3 × 10−1 s−1; trace b, 7.7 × 10−1 s−1. The apo-Dr-Nb concentration was: trace a, 1.0 × 10−5 M; trace b, 3.0 × 10−5 M. For clarity, trace b has been up-shifted of 0.3 units. (F) Averaged time courses of peroxynitrite isomerization by apo-Dr-Nb in the presence of CO2. The time course analysis, according to Equation (1), allowed us to determine the following values of kobs: trace a, 4.3 s−1; trace b, 3.9 s−1. The apo-Dr-Nb concentration was: trace a, 1.0 × 10−5 M; trace b, 3.0 × 10−5 M. For clarity, trace b has been up-shifted of 0.3 units. The CO2 concentration was 1.2 × 10−3 M. (G) Dependence of kobs on the concentration of apo-Dr-Nb in the absence of CO2. The symbol on the ordinate indicates the value of k0: 8.0 × 10−1 s−1. The average value of kobs obtained in the presence of apo-Dr-Nb is: 8.1 × 10−1 s−1. (H) Dependence of kobs on the concentration of apo-Dr-Nb in the presence of CO2. The symbol on the ordinate indicates the value of k0: 4.1 s−1. The average value of kobs obtained in the presence of apo-Dr-Nb and CO2 is: 4.1 s−1. In panels G and H, the differences among the kobs values are not statistically significant. The peroxynitrite concentration was 2.0 × 10−4 M. The HCO3− concentration was 5.0 × 10−1 M. Where not shown, the standard deviation is smaller than the symbol.
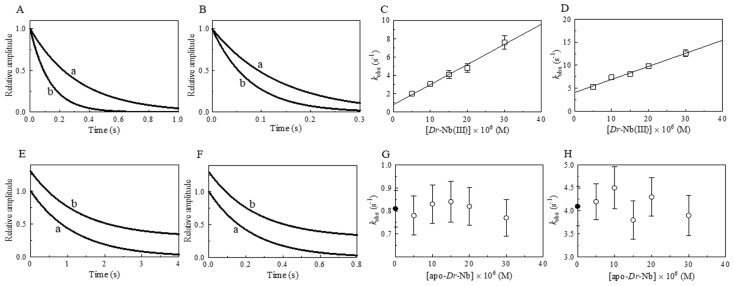
Figure 2.
Effect of Dr-Nb(III) on the kinetics for peroxynitrite isomerization, at pH 7.0 and 22.0 °C. (A) Averaged time courses of the Dr-Nb(III)-catalyzed isomerization of peroxynitrite in the absence of CO2. The time course analysis according to Equation (1) allowed us to determine the following values of kobs: trace a, 7.3 × 10−1 s−1, R = 0.998; trace b, 1.8 s−1, R = 0.999. The Dr-Nb(III) concentration was: trace a, 1.0 × 10−5 M; trace b, 3.0 × 10−5 M. (B) Averaged time courses of the Dr-Nb(III)-catalyzed isomerization of peroxynitrite in the presence of CO2. The time course analysis according to Equation (1) allowed us to determine the following values of kobs: trace a, 1.5 × 101 s−1, R = 0.999; trace b, 1.7 × 101 s−1, R = 0.998. The Dr-Nb(III) concentration was: trace a, 1.0 × 10−5 M; trace b, 3.0 × 10−5 M. The CO2 concentration was 1.2 × 10−3 M. For clarity, trace b has been up-shifted of 0.3 units. (C) Dependence of kobs on the concentration of Dr-Nb(III) in the absence of CO2. Data were analyzed according to Equation (2) with kon = 4.7 × 104 M−1 s−1 and k0 = 3.1 × 10−1 s−1; R = 0.989, p = 0.0006. (D) Dependence of kobs on the concentration of Dr-Nb(III) in the presence of CO2. (E) Averaged time courses of peroxynitrite isomerization by apo-Dr-Nb in the absence of CO2. The time course analysis according to Equation (1) allowed us to determine the following values of kobs: trace a, 3.1 × 10−1 s−1; trace b, 2.7 × 10−1 s−1. The apo-Dr-Nb concentration was: trace a, 1.0 × 10−5 M; trace b, 3.0 × 10−5 M. For clarity, trace b has been up-shifted of 0.3 units. (F) Averaged time courses of peroxynitrite isomerization by apo-Dr-Nb in the presence of CO2. The time course analysis according to Equation (1) allowed us to determine the following values of kobs: trace a, 1.5 × 101 s−1; trace b, 1.6 × 101 s−1. The apo-Dr-Nb concentration was: trace a, 1.0 × 10−5 M; trace b, 3.0 × 10−5 M. For clarity, trace b has been up-shifted of 0.3 units. The CO2 concentration was 1.2 × 10−3 M. (G) Dependence of kobs on the concentration of apo-Dr-Nb in the absence of CO2. The symbol on the ordinate indicates the value of k0: 3.1 × 10−1 s−1. The average value of kobs obtained in the presence of apo-Dr-Nb is: 3.0 × 10−1 s−1. (H) Dependence of kobs on the concentration of apo-Dr-Nb in the presence of CO2. The symbol on the ordinate indicates the value of k0: 1.5 × 101 s−1. The average value of kobs obtained in the presence of apo-Dr-Nb and CO2 is: 1.5 × 101 s−1. In panels D, G, and H, the differences among the kobs values are not statistically significant. The peroxynitrite concentration was 2.0 × 10−4 M. The HCO3− concentration was 5.0 × 10−1 M. Where not shown, the standard deviation is smaller than the symbol.
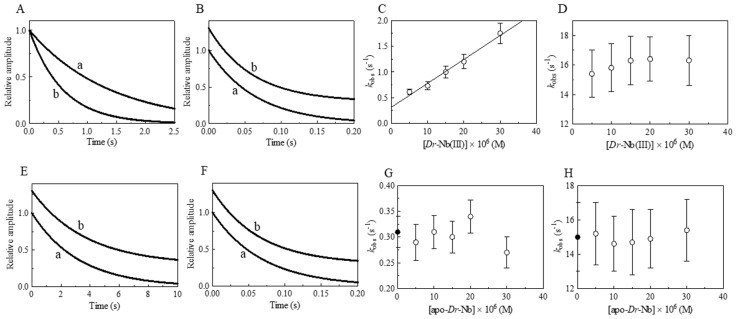
Figure 3.
Effect of Dr-Nb(III) on the kinetics for peroxynitrite isomerization, at pH 8.5 and 22.0 °C. (A) Averaged time courses of the Dr-Nb(III)-catalyzed isomerization of peroxynitrite in the absence of CO2. The time course analysis according to Equation (1) allowed us to determine the following values of kobs: trace a, 1.0 × 10−1 s−1, R = 0.998; trace b, 3.7 × 10−1 s−1, R = 0.999. The Dr-Nb(III) concentration was: trace a, 1.0 × 10−5 M; trace b, 3.0 × 10−5 M. (B) Averaged time courses of the Dr-Nb(III)-catalyzed isomerization of peroxynitrite in the presence of CO2. The time course analysis according to Equation (1) allowed us to determine the following values of kobs: trace a, 4.8 × 101 s−1, R = 0.999; trace b, 4.8 × 101 s−1, R = 0.998. The Dr-Nb(III) concentration was: trace a, 1.0 × 10−5 M; trace b, 3.0 × 10−5 M. The CO2 concentration was 1.2 × 10−3 M. For clarity, trace b has been up-shifted of 0.3 units. (C) Dependence of kobs on the concentration of Dr-Nb(III) in the absence of CO2. Data were analyzed according to Equation (2) with values of kon = 1.1 × 104 M−1 s−1 and k0 = 4.9 × 10−2; R = 0.995, p = 0.0002. (D) Dependence of kobs on the concentration of Dr-Nb(III) in the presence of CO2. (E) Averaged time courses of peroxynitrite isomerization by apo-Dr-Nb in the absence of CO2. The time course analysis according to Equation (1) allowed us to determine the following values of kobs: trace a, 5.3 × 10−2 s−1; trace b, 5.2 × 10−2 s−1. The apo-Dr-Nb concentration was: trace a, 1.0 × 10−5 M; trace b, 3.0 × 10−5 M. For clarity, trace b has been up-shifted of 0.3 units. (F) Averaged time courses of peroxynitrite isomerization by apo-Dr-Nb in the presence of CO2. The time course analysis according to Equation (1) allowed us to determine the following values of kobs: trace a, 5.3 × 101 s−1; trace b, 4.5 × 101 s−1. The apo-Dr-Nb concentration was: trace a, 1.0 × 10−5 M; trace b, 3.0 × 10−5 M. For clarity, trace b has been up-shifted of 0.3 units. The CO2 concentration was 1.2 × 10−3 M. (G) Dependence of kobs on the concentration of apo-Dr-Nb in the absence of CO2. The symbol on the ordinate indicates the value of k0: 4.9 × 10−2 s−1. The average value of kobs obtained in the presence of apo-Dr-Nb is: 4.9 × 10−2 s−1. (H) Dependence of kobs on the concentration of apo-Dr-Nb in the presence of CO2. The symbol on the ordinate indicates the value of k0: 4.8 × 101 s−1. The average value of kobs obtained in the presence of apo-Dr-Nb and CO2 is 4.9 × 101 s−1. In panels D, G, and H the differences among the kobs values are not statistically significant. The peroxynitrite concentration was 2.0 × 10−4 M. The HCO3− concentration was 5.0 × 10−1 M. Where not shown, the standard deviation is smaller than the symbol.
The analysis of the data shown in Figure 1, Figure 2 and Figure 3 (panels C and D), according to Equation (2), allowed us to determine the values of kon and k0, corresponding to the slope and the y-intercept of the linear plots, respectively (Table 1). Moreover, the values of k0 were measured in the absence of Dr-Nb(III) via the rapid mixing of the peroxynitrite solution with the appropriate Bis-Tris propane buffer solution (Figure 1, Figure 2 and Figure 3, panels E and F). Both in the absence and presence of CO2, the values of k0 obtained by the different methods match well with each other (Table 1) and agree well with those previously reported [12,13,25,27,28,30,33,34,36,39,54] (see Table 2).
Table 1.
Effect of pH and CO2 on the values of kon and k0 for Dr-Nb(III)-induced isomerization of peroxynitrite at 22.0 °C a.
| pH | − CO2 | + CO2 | ||
|---|---|---|---|---|

|

|
|||
| kon (M−1 s−1) | k0 (s−1) | kon (M−1 s−1) | k0 (s−1) | |
| 5.8 | (2.2 ± 0.3) × 105 | (8.1 ± 0.8) × 10−1 | (2.8 ± 0.3) × 105 | 4.1 ± 0.4 |
| 7.0 | (4.7 ± 0.5) × 104 | (3.1 ± 0.3) × 10−1 | n.d. | (1.5 ± 0.2) × 101 |
| 8.5 | (1.1 ± 0.1) × 104 | (4.9 ± 0.5) × 10−2 | n.d. | (4.8 ± 0.5) × 101 |
a The CO2 concentration was 1.2 × 10−3 M. 5.0 × 10−2 M Bis-Tris propane buffer. n.d., not determined.
Table 2.
Effect of pH and CO2 on values of kon and k0 for heme-protein-induced isomerization of peroxynitrite.
| Heme-Protein | − CO2 | + CO2 | ||
|---|---|---|---|---|

|

|
|||
| kon (M−1 s−1) | k0 (s−1) | kon (M−1 s−1) | k0 (s−1) | |
| Ma-Pgb(III) a | 3.8 × 104 | 2.8 × 10−1 | n.d. | n.d. |
| Mt-trHbN(III) b | 6.2 × 104 | 2.7 × 10−1 | n.d. | n.d. |
| Ph-trHbO(III) c | 2.9 × 104 | 2.8 × 10−1 | n.d. | n.d. |
| Cj-trHbP(III) d | 9.6 × 105 | 3.0 × 10−1 | 8.8 × 105 | 2.1 × 101 |
| Efc-Mb(III) e | 2.9 × 104 | 3.5 × 10−1 | 7.7 × 104 | 1.7 × 101 |
| Pc-Mb(III) f | 1.6 × 104 | n.d. | n.d. | n.d. |
| Hs-Hb(III) e | 1.2 × 104 | 3.0 × 10−1 | 3.9 × 104 | 1.7 × 101 |
| Mt-Nb(III) g | 6.9 × 104 | 2.6 × 10−1 | n.d. | n.d. |
| At-Nb(III) g | 3.7 × 104 | 3.0 × 10−1 | n.d. | n.d. |
| Dr-Nb(III) h | 4.7 × 104 | 3.1 × 10−1 | n.d. | 1.5 × 101 |
| Hs-Nb(III) i | 3.4 × 104 | 2.6 × 10−1 | n.d. | n.d. |
a pH 7.4 and 20.0 °C. From [55]. b pH 7.0 and 20.0 °C. From [34]. c pH 7.0 and 20.0 °C. From [35]. d pH 7.3 and 25.0 °C. From [36]. e pH 7.0 and 20.0 °C. From [25]. f pH 7.5 and 20.0 °C. From [27]. g pH 7.2 and 25.0 °C. From [40]. h pH 7.0 and 22.0 °C. Present study. i pH 7.1 and 25.0 °C. From [39]. n.d., not determined.
As shown in Table 1, the values of kon for the interaction between Dr-Nb(III) and peroxynitrite were unaffected by CO2/HCO3−/CO32−, suggesting that CO2/HCO3−/CO32− does not alter the binding properties of Dr-Nb(III). The values of kon for peroxynitrite isomerization via all-α-helical globins and all-β-barrel nitrobindins ranged between 1.2 × 104 M−1 s−1 for Hs-Hb(III) [25] and 6.9 × 104 M−1 s−1 for Mt-Nb(III) [40] (Table 2), suggesting that the very different structural organization [40,57,58,59,60,61] is not at the root of the different rate of peroxynitrite isomerization. However, the coordination of the heme-Fe(III) atom, the in- or out-of-plane position of the metal with respect to the pyrrole nitrogen atoms of the porphyrin, the ligand accessibility of the heme-Fe(III) atom, and its Lewis acidity may tune the kinetics of the related peroxynitrite decomposition [36].
To outline the role of the metal center, the values of kobs have been determined as a function of (i) apo-Dr-Nb concentration (at a fixed peroxynitrite concentration) and (ii) peroxynitrite concentration (at fixed Dr-Nb(III) concentration). Apo-Dr-Nb does not induce the isomerization of peroxynitrite; in fact, the values of kobs and k0 match each other in the presence of apo-Dr-Nb (Figure 1, Figure 2 and Figure 3, panels E–H), as reported for apo-Efc-Mb, apo-Hs-Hb, and apo-Hs-Nb [25,39]. As shown in Figure 4, the values of kobs slightly decrease with an increasing peroxynitrite concentration, reflecting either the slow isomerization process of peroxynitrite-peroxynitrous acid dimers or their slow dissociation preceding the Dr-Nb(III)-catalyzed isomerization of peroxynitrite [30,36].
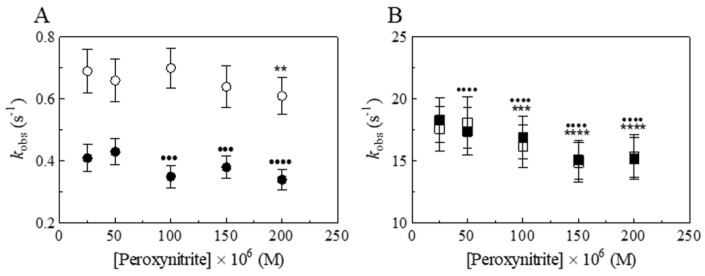
Figure 4.
Effect of peroxynitrite concentration on the values of kobs for the isomerization of peroxynitrite by Dr-Nb(III) and apo-Dr-Nb in the absence and presence of CO2, at pH 7.0 and 22.0 °C. (A) Dependence of kobs on the peroxynitrite concentration in the presence of Dr-Nb(III) (open circles) and apo-Dr-Nb (filled circles), in the absence of CO2 (One-way ANOVA: open circles, ** p < 0.01, 25 × 10−6 M versus 200 × 10−6 M; filled circles, ●●● p < 0.001, 25 × 10−6 M versus 100 × 10−6 M and versus 150 × 10−6 M, ●●●● p < 0.0001, 25 × 10−6 M versus 200 × 10−6 M). (B) Dependence of kobs on the peroxynitrite concentration in the presence of Dr-Nb(III) (open squares) and apo-Dr-Nb (filled squares), in the presence of CO2 (One-way ANOVA: open squares, *** p < 0.001, 25 × 10−6 M versus 100 × 10−6 M, **** p < 0.0001, 25 × 10−6 M versus 150 × 10−6 M and versus 200 × 10−6 M; filled squares, ●●●● p < 0.0001, 25 × 10−6 M versus 50 × 10−6 M, 100 × 10−6 M, 150 × 10−6 M, and 200 × 10−6 M). The Dr-Nb(III) and apo-Dr-Nb(III) concentration was 5.0 × 10−6 M. The HCO3− concentration was 5.0 × 10−1 M.
As shown in Figure 1, Figure 2 and Figure 3 (panel D) and in Figure 4 (panel B) it turns out that, in the presence of 5.0 × 10−1 M CO2/HCO3−/CO32−, the role of Dr-Nb(III) in characterizing the rate constant of peroxynitrite isomerization becomes progressively less relevant as the pH is raised since values of k0 for peroxynitrite isomerization obtained in the presence of CO2/HCO3−/CO32− are faster by about two orders of magnitude than those obtained in its absence (Table 1). This indicates that CO2/HCO3−/CO32− dramatically speeds up the decay of peroxynitrite through direct interaction, without interfering with peroxynitrite binding to Dr-Nb(III) [9,12,13,25,27,28,30,32,33,34,36].
To clarify the differential roles of various ferric heme proteins and CO2/HCO3−/CO32− levels in peroxynitrite detoxification, the overall effect of pH and CO2/HCO3−/CO32− on the observed rate of peroxynitrite isomerization was investigated. Thus, the protonation equilibria of OONO−/HOONO and CO2/HCO3−/CO32− were considered. The effect of CO2 on peroxynitrite isomerization in the absence of heme proteins (i.e., kobs) can be described by Equation (3):
| (3) |
where k0 and k0H are the intrinsic degradation rates of OONO− and HOONO, respectively, [H+] (= 10−pH) is the proton concentration, KP (= ([OONO−] × [H+])/[HOONO]) is the protonation constant of peroxynitrite, [L] is the reactant concentration (in our case [HCO3−] = 5.0 × 10−1 M), Kc1 ([H2O]) (= ([HCO3−] × [H+])/[CO2] = 10−6.34) is the protonation equilibrium constant between HCO3− and CO2, Kc2 (= ([CO32−] × [H+])/[HCO3−] = 10−10.25) is the protonation equilibrium constant between CO32− and HCO3−; kc1 and kc1H are the second-order rate constants for the reaction of CO2 with OONO− and HOONO, respectively, kc2 and kc2H are the second-order rate constants for the reaction with HCO3− of OONO− and HOONO, respectively, and kc3 and kc3H are the second-order rate constants for the reaction with CO32− of OONO− and HOONO, respectively.
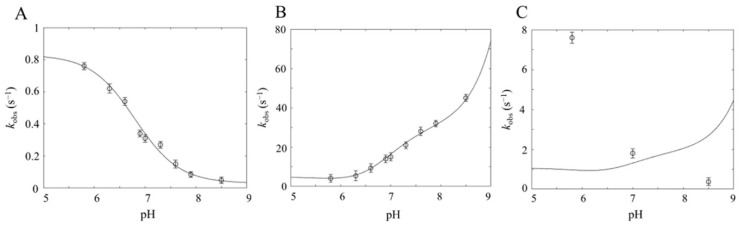
The pH-dependence of peroxynitrite degradation in the absence of CO2/HCO3−/CO32− (i.e., [L] = 0, see Equation (3)), allowed us to determine the values of k0 (= 3.0 × 10−2 s−1), k0H (= 8.3 × 10−1 s−1) and KP (= 10−6.8) (Figure 5, panel A), showing that HOONO decays to NO3− faster than OONO−, with a pKa ≈ 6.8 [13,36]. On the other hand, as already outlined before [9,13,22,27,28,30,36,39], when CO2/HCO3−/CO32− is present (i.e., [L] = 5.0 × 10−1 M), the rate of peroxynitrite degradation becomes much faster and increases with a rising pH (see Table 1 and [36]). Therefore, employing Equation (3) and knowing the values of Kc1 and Kc2 (see above), the pH dependence of peroxynitrite degradation (Figure 5, panel B) gives information on the different values of kci and kciH (with i = 1, 2, 3). The inspection of Table 3 allows for the following considerations:
Figure 5.
pH dependence of the peroxynitrite isomerization rate constant in the absence of CO2/HCO3−/CO32− (A), in the presence of 5.0 × 10−1 M CO2/HCO3−/CO32− (B), and in the presence of 3.0 × 10−2 M CO2/HCO3−/CO32− (C). In all three panels, the continuous lines correspond to kobs, as defined in Equation (3), under different conditions (i.e., [L] = 0 (A), [L] = 5.0 × 10−1 M (B), [L] = 3.0×10−2 M (C), employing the parameters reported in Table 3. In (A,B), the open circles correspond to experimental data obtained from the literature [36] on the peroxynitrite isomerization rate constants as a function of pH under the conditions described above. The continuous lines were obtained according to Equation (3) by the non-linear least-squares fitting of data. In (C), the open circles correspond to values of kobs in the presence of 3.0 × 10−5 M Dr-Nb(III) in the absence of CO2/HCO3−/CO32− at different pH values (present study). Data shown in (C) indicate that, at pH 5.8, the efficiency of the heme protein (at the indicated concentration) is higher than that of the CO2/HCO3−/CO32− system, while at pH 8.5, the efficiency is lower.
Table 3.
Values of the parameters fitting data with Equation (3).
| Parameter | Value |
|---|---|
| k0 (s−1) | (3.0 ± 0.5) × 10−2 |
| k0H (s−1) | (8.3 ± 1.0) × 10−1 |
| KP (M) | (1.6 ± 0.3) × 10−7 |
| Kc1 (M) | (4.6 ± 0.9) × 10−7 |
| Kc2 (M) | (5.6 ± 1.2) × 10−11 |
| kc2 (M−1s−1) | (6.2 ± 1.3) × 101 |
| kc3 (M−1s−1) | (1.7 ± 0.4) × 103 |
| kc1H (M−1s−1) | 8.0 ± 2.1 |
(i) kc1, kc2H, and kc3H are not playing any role, likely because either one or both reactants are too scarcely populated over the pH range investigated for the pseudo-first-order rate constant to have a detectable value, which can then be considered as ≈0 s−1;
(ii) At pH ≤ 6.2, the peroxynitrite degradation rate is mostly characterized by the reaction between HOONO and CO2, corresponding to kc1H = 8.0 M−1 s−1);
(iii) For 6.2 < pH < 8.2, the peroxynitrite degradation rate is mostly characterized by the reaction between OONO− and HCO3−, corresponding to kc2 = 6.2 × 101 M−1 s−1;
(iv) For pH ≥ 8.2, the peroxynitrite degradation rate is mostly characterized by the reaction between OONO− and CO32−, corresponding to kc3 = 1.8 × 103 M−1 s−1.
Obviously, the value of kobs and its pH dependence depend on the CO2/HCO3−/CO32− levels (i.e., [L]), envisaging that the relative levels of the ferric heme protein and CO2/HCO3−/CO32− are crucial in defining the respective role for peroxynitrite detoxification. Of note, data reported in Figure 1, Figure 2 and Figure 3 (panel D) refer to [CO2/HCO3−/CO32−] = 5.0 × 10−1 M, while in the bloodstream, the physiological levels of CO2/HCO3−/CO32− (~3.0 × 10−2 M) are about 10-20 fold lower. Therefore, under these conditions, kobs decreases significantly even in the presence of CO2/HCO3−/CO32−, as indicated by Equation (3) (Figure 5, panel C). The pH-dependent mechanism of peroxynitrite degradation can be described by Scheme 2, which reports the different pathways for peroxynitrite degradation.
Scheme 2.
Effect of pH on peroxynitrite degradation. The scheme reproduces the mechanism described by Equation (3), clarifying the meaning of the different parameters; only the significant parameters are reported (see text).
According to Equation (3), the effects of the heme protein and CO2/HCO3−/CO32− levels were simulated (Figure 5, panel C), with the values of the rates at [CO2/HCO3−/CO32−] = 3.0 × 10−2 M and [Dr-Nb(III)] = 3.0 × 10−5 M (see Figure 1, Figure 2 and Figure 3, panels C). At acidic pH values, the role of Dr-Nb(III) (= 3.0 × 10−5 M) is prevalent for peroxynitrite isomerization, while at a neutral pH the role of Dr-Nb(III) (= 3.0 × 10−5 M) is equivalent to that of the CO2/HCO3−/CO32− system (Figure 5C). For alkaline pH values, Dr-Nb(III) levels higher than 3.0 × 10−5 M would be required to play a relevant role in peroxynitrite detoxification (see Figure 5, panel C).
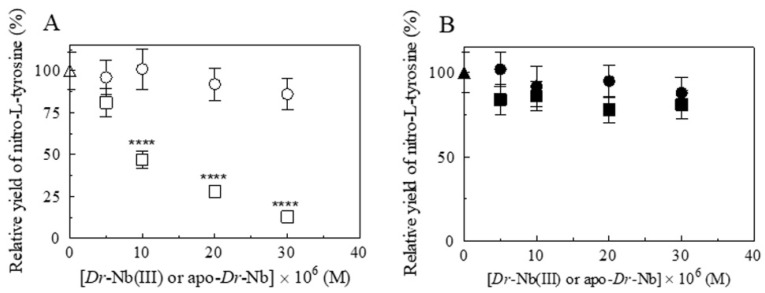
The relative importance of the heme proteins and CO2/HCO3−/CO32− catalyzing the peroxynitrite isomerization is crucial since, according to the literature [9,12,25,30,36,62,63], ferric heme proteins prevent L-tyrosine nitration, which, instead, occurs either in the presence of apo-Dr-Nb and/or of CO2/HCO3−/CO32− (Figure 6) (see also Scheme 1). In fact, the relative yield of NO3− and NO2−, obtained from peroxynitrite isomerization catalyzed by Dr-Nb(III), ranged between 89 and 92%, and between 7 and 12%, respectively. However, in the absence of Dr-Nb(III) and/or in the presence of apo-Dr-Nb and/or CO2/HCO3−/CO32−, the values of the relative yield of NO3− and NO2− ranged between 69 and 74%, and between 7 and 12%, respectively (Table 4). The great relevance of these observations is confirmed by the fact that, under stressed conditions, zebrafish triggers a defense mechanism through nitrosative stress and peroxynitrite production for which its degradation may be, on one side, enhanced by the elevated levels of CO2 [48,49], but, on the other side, inhibited by the consequent lowering of the pH level. In any event, the correlation between the peroxynitrite degradation by ferric heme proteins and CO2 levels appears to be relevant within in vivo models of zebrafish, and it may have important consequences for the O2 supply to poorly oxygenated tissues, such as the retina [50,51].
Figure 6.
Effect of Dr-Nb on L-tyrosine nitrosylation induced from peroxynitrite, at pH 7.0 and 22.0 °C. (A) Dependence of the nitro-L-tyrosine yield on the Dr-Nb(III) (open squares) and apo-Dr-Nb (open circles) concentration in the absence of CO2 (One-way ANOVA: open squares, **** p < 0.0001, 0 M versus 10 × 10−6 M, 20 × 10−6 M, and 30 × 10−6 M). (B) Dependence of the nitro-L-tyrosine yield on the Dr-Nb(III) (filled squares) and apo-Dr-Nb (filled circles) concentration in the presence of CO2. The symbols on the y-axis (open and filled triangle) indicate the nitro-L-tyrosine yield in the absence and presence of CO2, respectively. The L-tyrosine concentration was 1.0 × 10−4 M. The peroxynitrite concentration was 2.0 × 10−4 M. Where not shown, the standard deviation is smaller than the symbol.
Table 4.
Percentage of NO3− and NO2− obtained from peroxynitrite isomerization at pH 7.0 and at 22.0 °C a.
| Dr-Nb(III) | Apo-Dr-Nb | CO2 | NO3− | NO2− |
|---|---|---|---|---|
| (M) | (M) | (M) | (%) | (%) |
| 0.0 | 0.0 | 0.0 | 73 ± 8 | 28 ± 3 |
| 0.0 | 0.0 | 1.2 × 10−3 | 69 ± 7 | 30 ± 3 |
| 0.0 | 3.5 × 10−5 | 0.0 | 71 ± 8 | 29 ± 3 |
| 0.0 | 3.5 × 10−5 | 1.2 × 10−3 | 74 ± 7 | 25 ± 2 |
| 3.5 × 10−5 | 0.0 | 0.0 | 89 ± 9 | 12 ± 2 |
| 3.5 × 10−5 | 0.0 | 1.2 × 10−3 | 93 ± 8 | 7 ± 1 |
| 3.5 × 10−5 | 3.5 × 10−5 | 0.0 | 91 ± 9 | 10 ± 1 |
| 3.5 × 10−5 | 3.5 × 10−5 | 1.2 × 10−3 | 92 ± 9 | 8 ± 1 |
a The peroxynitrite concentration was 2.0 × 10−4 M; 5.0 × 10−2 M Bis-Tris propane buffer.
4. Conclusions
Ferric heme proteins and the CO2/HCO3−/CO32− system are the major players in peroxynitrite detoxification, for which efficiency depends on both the concentration of these two actors and the pH level. CO32− is much more effective at peroxynitrite inactivation, as compared to CO2, with HCO3− displaying an intermediate activity. Of note, the CO32− levels were much lower than those of the other two components in the system under physiological conditions. Although ferric heme proteins are intrinsically much more effective than the CO2/HCO3−/CO32− system, their levels are usually significantly lower than CO2/HCO3−/CO32−. Since over the physiological pH range the rate of peroxynitrite detoxification by CO2/HCO3−/CO32− (i.e., ~3.0 × 10−2 M) is ~1.5 s−1, values of kobs (= kon × [heme-Fe(III)]), for peroxynitrite scavenging by ferric heme-proteins must be larger than 2 s−1. Overall, only the level of the circulating Hs-Hb(III) (~2.0 × 10−4 M; corresponding to about 2–3% of the total Hs-Hb in red cells) appears to be sufficient to play a relevant role in the detoxification by peroxynitrite under physiological conditions.
Furthermore, it must be remarked that acidification, which often occurs in the bloodstream of fish when they dive to depth [64,65,66] and can even be magnified by increased levels of CO2 [49], decreases the effect of the CO2/HCO3−/CO32− system, rendering the role of ferric heme proteins even more crucial. This is especially important in the ocular system and in the protection of the retina against oxidative stress linked to peroxynitrite [3,50]. Therefore, under specific environmental conditions (typical of diving fish), Dr-Nb(III) indeed may play a relevant physiological role in peroxynitrite scavenging from poorly oxygenated tissues, such as the retina.
Author Contributions
A.C. and G.D.S. performed experiments; A.d.M. supervised molecular biology experiments; P.A. and M.C. conceptualized and supervised biochemical experiments, formally analyzed the data, wrote reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data will be available upon request to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
G.D.S., A.d.M. and P.A. gratefully acknowledge the grant of Dipartimento di Eccellenza, MIUR, Roma Tre University (Legge 232/2016, Articolo 1, Comma 314-337). M.C. gratefully acknowledges the support of the Italian Ministry of Health and of Fondazione Roma.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cantó A., Olivar T., Romero F.J., Miranda M. Nitrosative Stress in Retinal Pathologies: Review. Antioxidants. 2019;8:543. doi: 10.3390/antiox8110543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad A., Ahsan H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J. Immunoass. Immunochem. 2020;41:257–271. doi: 10.1080/15321819.2020.1726774. [DOI] [PubMed] [Google Scholar]
- 3.Lei Y., Gao Y., Song M., Cao W., Sun X. Peroxynitrite is a novel risk factor and treatment target of glaucoma. Nitric Oxide. 2020;99:17–24. doi: 10.1016/j.niox.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Beckman J.S., Koppenol W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 5.Ducrocq C., Blanchard B., Pignatelli B., Ohshima H. Peroxynitrite: An endogenous oxidizing and nitrating agent. Cell. Mol. Life Sci. 1999;55:1068–1077. doi: 10.1007/s000180050357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clementi E., Nisoli E. Nitric oxide and mitochondrial biogenesis: A key to long-term regulation of cellular metabolism. Comp. Biochem. Physiol. 2005;142:102–110. doi: 10.1016/j.cbpb.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 7.DeNicola A., Radi R. Peroxynitrite and drug-dependent toxicity. Toxicology. 2005;208:273–288. doi: 10.1016/j.tox.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Pacher P., Beckman J.S., Liaudet L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein S., Merényi G. The Chemistry of Peroxynitrite: Implications for Biological Activity. Methods Enzymol. 2008;436:49–61. doi: 10.1016/s0076-6879(08)36004-2. [DOI] [PubMed] [Google Scholar]
- 10.Jones L.H. Chemistry and Biology of Biomolecule Nitration. Chem. Biol. 2012;19:1086–1092. doi: 10.1016/j.chembiol.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA. 2018;115:5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein S., Lind J., Merényi G. Chemistry of peroxynitrites and peroxynitrates. Chem. Rev. 2005;105:2457–2470. doi: 10.1021/cr0307087. [DOI] [PubMed] [Google Scholar]
- 13.Ascenzi P., Leboffe L., Santucci R., Coletta M. Ferric microperoxidase-11 catalyzes peroxynitrite isomerization. J. Inorg. Biochem. 2015;144:56–61. doi: 10.1016/j.jinorgbio.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Lymar S.V., Hurst J.K. Rapid reaction between peroxynitrite ion and carbon dioxide: Implications for biological activity. J. Am. Chem. Soc. 1995;117:8867–8868. doi: 10.1021/ja00139a027. [DOI] [Google Scholar]
- 15.DeNicola A., Freeman B.A., Trujillo M., Radi R. Peroxynitrite Reaction with Carbon Dioxide/Bicarbonate: Kinetics and Influence on Peroxynitrite-Mediated Oxidations. Arch. Biochem. Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 16.Bonini M.G., Radi R., Ferrer-Sueta G., Ferreira A.M.D.C., Augusto O. Direct EPR Detection of the Carbonate Radical Anion Produced from Peroxynitrite and Carbon Dioxide. J. Biol. Chem. 1999;274:10802–10806. doi: 10.1074/jbc.274.16.10802. [DOI] [PubMed] [Google Scholar]
- 17.Radi R., Denicola A., Freeman B.A. Peroxynitrite reactions with carbon dioxide-bicarbonate. Methods Enzymol. 1999;301:353–367. doi: 10.1016/s0076-6879(99)01099-x. [DOI] [PubMed] [Google Scholar]
- 18.Meli R., Nauser T., Latal P., Koppenol W.H. Reaction of peroxynitrite with carbon dioxide: Intermediates and determination of the yield of CO3− and NO2●. J. Biol. Inorg. Chem. 2002;7:31–36. doi: 10.1007/s007750100262. [DOI] [PubMed] [Google Scholar]
- 19.Squadrito G.L., Pryor W.A. Mapping the reaction of peroxynitrite with CO2: Energetics, reactive species, and biological im-plications. Chem. Res. Toxicol. 2002;15:885–895. doi: 10.1021/tx020004c. [DOI] [PubMed] [Google Scholar]
- 20.Augusto O., Goldstein S., Hurst J.K., Lind J., Lymar S.V., Merenyi G., Radi R. Carbon dioxide-catalyzed peroxynitrite reactivity—The resilience of the radical mechanism after two decades of research. Free Radic. Biol. Med. 2019;135:210–215. doi: 10.1016/j.freeradbiomed.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Augusto O., Truzzi D.R. Carbon dioxide redox metabolites in oxidative eustress and oxidative distress. Biophys. Rev. 2021;13:889–891. doi: 10.1007/s12551-021-00860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehl M., Daiber A., Herold S., Shoun H., Ullrich V. Peroxynitrite reaction with heme proteins. Nitric Oxide. 1999;3:142–152. doi: 10.1006/niox.1999.0217. [DOI] [PubMed] [Google Scholar]
- 23.Shimanovich R., Groves J.T. Mechanisms of Peroxynitrite Decomposition Catalyzed by FeTMPS, a Bioactive Sulfonated Iron Porphyrin. Arch. Biochem. Biophys. 2001;387:307–317. doi: 10.1006/abbi.2000.2247. [DOI] [PubMed] [Google Scholar]
- 24.Jensen M.P., Riley D.P. Peroxynitrite Decomposition Activity of Iron Porphyrin Complexes. Inorg. Chem. 2002;41:4788–4797. doi: 10.1021/ic011089s. [DOI] [PubMed] [Google Scholar]
- 25.Herold S., Shivashankar K. Metmyoglobin and Methemoglobin Catalyze the Isomerization of Peroxynitrite to Nitrate. Biochemistry. 2003;42:14036–14046. doi: 10.1021/bi0350349. [DOI] [PubMed] [Google Scholar]
- 26.Herold S., Fago A., Weber R.E., Dewilde S., Moens L. Reactivity studies of the Fe(III) and Fe(II)NO forms of human neu-roglobin reveal a potential role against oxidative stress. J. Biol. Chem. 2004;279:22841–22847. doi: 10.1074/jbc.M313732200. [DOI] [PubMed] [Google Scholar]
- 27.Herold S., Kalinga S., Matsui A.T., Watanabe Y. Mechanistic Studies of the Isomerization of Peroxynitrite to Nitrate Catalyzed by Distal Histidine Metmyoglobin Mutants. J. Am. Chem. Soc. 2004;126:6945–6955. doi: 10.1021/ja0493300. [DOI] [PubMed] [Google Scholar]
- 28.Herold S., Puppo A. Kinetics and mechanistic studies of the reactions of metleghemoglobin, ferrylleghemoglobin, and nitrosylleghemoglobin with reactive nitrogen species. J. Biol. Inorg. Chem. 2005;10:946–957. doi: 10.1007/s00775-005-0047-8. [DOI] [PubMed] [Google Scholar]
- 29.Ascenzi P., Visca P. Scavenging of Reactive Nitrogen Species by Mycobacterial Truncated Hemoglobins. Methods Enzymol. 2008;436:317–337. doi: 10.1016/s0076-6879(08)36018-2. [DOI] [PubMed] [Google Scholar]
- 30.Ascenzi P., di Masi A., Coletta M., Ciaccio C., Fanali G., Nicoletti F.P., Smulevich G., Fasano M. Ibuprofen impairs allo-sterically peroxynitrite isomerization by ferric human serum heme-albumin. J. Biol. Chem. 2009;284:31006–31017. doi: 10.1074/jbc.M109.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ascenzi P., Bolli A., Di Masi A., Tundo G.R., Fanali G., Coletta M., Fasano M. Isoniazid and rifampicin inhibit allosterically heme binding to albumin and peroxynitrite isomerization by heme–albumin. J. Biol. Inorg. Chem. 2010;16:97–108. doi: 10.1007/s00775-010-0706-2. [DOI] [PubMed] [Google Scholar]
- 32.Ascenzi P., Ciaccio C., Sinibaldi F., Santucci R., Coletta M. Cardiolipin modulates allosterically peroxynitrite detoxification by horse heart cytochrome c. Biochem. Biophys. Res. Commun. 2011;404:190–194. doi: 10.1016/j.bbrc.2010.11.091. [DOI] [PubMed] [Google Scholar]
- 33.Ascenzi P., Ciaccio C., Sinibaldi F., Santucci R., Coletta M. Peroxynitrite detoxification by horse heart carboxymethylated cytochrome c is allosterically modulated by cardiolipin. Biochem. Biophys. Res. Commun. 2011;415:463–467. doi: 10.1016/j.bbrc.2011.10.094. [DOI] [PubMed] [Google Scholar]
- 34.Ascenzi P., Coletta A., Cao Y., Trezza V., Leboffe L., Fanali G., Fasano M., Pesce A., Ciaccio C., Marini S., et al. Isoniazid Inhibits the Heme-Based Reactivity of Mycobacterium tuberculosis Truncated Hemoglobin N. PLoS ONE. 2013;8:e69762. doi: 10.1371/journal.pone.0069762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppola D., Giordano D., Tinajero-Trejo M., di Prisco G., Ascenzi P., Poole R.K., Verde C. Antarctic bacterial haemoglobin and its role in the protection against nitrogen reactive species. Biochim. Biophys. Acta. 2013;1834:1923–1931. doi: 10.1016/j.bbapap.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Ascenzi P., Pesce A. Peroxynitrite scavenging by Campylobacter jejuni truncated hemoglobin P. J. Biol. Inorg. Chem. 2017;22:1141–1150. doi: 10.1007/s00775-017-1490-z. [DOI] [PubMed] [Google Scholar]
- 37.Ascenzi P., Coletta M. Peroxynitrite Detoxification by Human Haptoglobin:Hemoglobin Complexes: A Comparative Study. J. Phys. Chem. B. 2018;122:11100–11107. doi: 10.1021/acs.jpcb.8b05340. [DOI] [PubMed] [Google Scholar]
- 38.Coppola D., Giordano D., Milazzo L., Howes B.D., Ascenzi P., di Prisco G., Smulevich G., Poole R.K., Verde C. Coex-istence of multiple globin genes conferring protection against nitrosative stress to the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125. Nitric Oxide. 2018;73:39–51. doi: 10.1016/j.niox.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 39.De Simone G., di Masi A., Polticelli F., Ascenzi P. Human nitrobindin: The first example of an all-β-barrel ferric heme-protein that catalyzes peroxynitrite detoxification. FEBS Open Bio. 2018;8:2002–2010. doi: 10.1002/2211-5463.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Simone G., Di Masi A., Vita G.M., Polticelli F., Pesce A., Nardini M., Bolognesi M., Ciaccio C., Coletta M., Turilli E.S., et al. Mycobacterial and Human Nitrobindins: Structure and Function. Antioxidants Redox Signal. 2020;33:229–246. doi: 10.1089/ars.2019.7874. [DOI] [PubMed] [Google Scholar]
- 41.Giordano D., Pesce A., Vermeylen S., Abbruzzetti S., Nardini M., Marchesani F., Berghmans H., Seira C., Bruno S., Luque F.J., et al. Structural and functional properties of Antarctic fish cytoglobins-1: Cold-reactivity in multi-ligand reactions. Comput. Struct. Biotechnol. J. 2020;18:2132–2144. doi: 10.1016/j.csbj.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radi R., Beckman J.S., Bush K.M., Freeman B.A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991;266:4244–4250. doi: 10.1016/S0021-9258(20)64313-7. [DOI] [PubMed] [Google Scholar]
- 43.Gow A., Duran D., Thom S.R., Ischiropoulos H. Carbon dioxide enhancement of peroxynitrite mediated protein tyrosine nitration. Arch. Biochem. Biophys. 1996;333:42–48. doi: 10.1006/abbi.1996.0362. [DOI] [PubMed] [Google Scholar]
- 44.Scorza G., Minetti M. One electron oxidation pathway of thyols by peroxynitrite in biological fluids: Bicarbonate and ascorbate promote the formation of albumin disulphide dimers in human blood plasma. Biochem. J. 1998;329:405–413. doi: 10.1042/bj3290405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonini M.G., Augusto O. Carbon Dioxide Stimulates the Production of Thiyl, Sulfinyl, and Disulfide Radical Anion from Thiol Oxidation by Peroxynitrite. J. Biol. Chem. 2001;276:9749–9754. doi: 10.1074/jbc.M008456200. [DOI] [PubMed] [Google Scholar]
- 46.Augusto O., Bonini M.G., Amanso A.M., Linares E., Santos C.C., De Menezes S.L. Nitrogen dioxide and carbonate radical anion: Two emerging radicals in biology. Free Radic. Biol. Med. 2002;32:841–859. doi: 10.1016/S0891-5849(02)00786-4. [DOI] [PubMed] [Google Scholar]
- 47.Elks P.M., van der Vaart M., van Hensbergen V., Schutz E., Redd M.J., Murayama E., Spaink H.P., Meijer A.H. Myco-bacteria counteract a TLR-mediated nitrosative defense mechanism in a zebrafish infection model. PLoS ONE. 2014;9:e100928. doi: 10.1371/journal.pone.0100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shahab M., Rosati R., Meyer D.N., Shields J.N., Crofts E., Baker T.R., Jamesdaniel S. Cisplatin-induced hair cell loss in zebrafish neuromasts is accompanied by protein nitration and Lmo4 degradation. Toxicol. Appl. Pharmacol. 2020;410:115342. doi: 10.1016/j.taap.2020.115342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vossen L.E., Jutfelt F., Cocco A., Thörnqvist P.-O., Winberg S. Zebrafish (Danio rerio) behavior is largely unaffected by el-evated pCO2. Conserv. Physiol. 2016;4:cow065. doi: 10.1093/conphys/cow065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Damsgaard C. Physiology and evolution of oxygen secreting mechanism in the fisheye. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020;252:110840. doi: 10.1016/j.cbpa.2020.110840. [DOI] [PubMed] [Google Scholar]
- 51.Tummanapalli S.S., Kuppusamy R., Yeo J.H., Kumar N., New E.J., Willcox M.D. The role of nitric oxide in ocular surface physiology and pathophysiology. Ocul. Surf. 2021;21:37–51. doi: 10.1016/j.jtos.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 52.De Simone G., Tundo G.R., Coletta A., Coletta M., Ascenzi P. Hydroxylamine-induced oxidation of ferrous nitrobindins. J. Biol. Inorg. Chem. 2022;27:443–453. doi: 10.1007/s00775-022-01940-9. [DOI] [PubMed] [Google Scholar]
- 53.Antonini E., Brunori M. Hemoglobin and Myoglobin in Their Reactions with Ligands. North Holland Publishing Co.; Amsterdam, The Netherlands: London, UK: 1971. [Google Scholar]
- 54.Herold S., Exner M., Boccini F. The Mechanism of the Peroxynitrite-Mediated Oxidation of Myoglobin in the Absence and Presence of Carbon Dioxide. Chem. Res. Toxicol. 2003;16:390–402. doi: 10.1021/tx025595l. [DOI] [PubMed] [Google Scholar]
- 55.Ascenzi P., Leboffe L., Pesce A., Ciaccio C., Sbardella D., Bolognesi M., Coletta M. Nitrite-Reductase and Peroxynitrite Isomerization Activities of Methanosarcina acetivorans Protoglobin. PLoS ONE. 2014;9:e95391. doi: 10.1371/journal.pone.0095391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miranda K.M., Espey M.G., Wink D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 57.Perutz M.F. Regulation of Oxygen Affinity of Hemoglobin: Influence of Structure of the Globin on the Heme Iron. Annu. Rev. Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- 58.Bolognesi M., Bordo D., Rizzi M., Tarricone C., Ascenzi P. Nonvertebrate hemoglobins: Structural bases for reactivity. Prog. Biophys. Mol. Biol. 1997;68:29–68. doi: 10.1016/S0079-6107(97)00017-5. [DOI] [PubMed] [Google Scholar]
- 59.Bianchetti C.M., Blouin G.C., Bitto E., Olson J.S., Phillips G.N., Jr. The structure and NO binding properties of the nitro-phorin-like heme-binding protein from Arabidopsis thaliana gene locus At1 g79260.1. Proteins. 2010;78:917–931. doi: 10.1002/prot.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bianchetti C.M., Bingman C.A., Phillips G.N., Jr. Structure of the C-terminal heme-binding domain of THAP domain con-taining protein 4 from Homo sapiens. Proteins. 2011;79:1337–1341. doi: 10.1002/prot.22944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ascenzi P., Brunori M. A molecule for all seasons: The heme. J. Porphyrins Phthalocyanines. 2016;20:134–149. doi: 10.1142/S1088424616300081. [DOI] [Google Scholar]
- 62.Kissner R., Koppenol W.H. Product Distribution of Peroxynitrite Decay as a Function of pH, Temperature, and Concentration. J. Am. Chem. Soc. 2001;124:234–239. doi: 10.1021/ja010497s. [DOI] [PubMed] [Google Scholar]
- 63.Ascenzi P., Bocedi A., Visca P., Minetti M., Clementi E. Does CO2 modulate peroxynitrite specificity? IUBMB Life. 2006;58:611–613. doi: 10.1080/15216540600746344. [DOI] [PubMed] [Google Scholar]
- 64.Brunori M. Molecular Adaptation to Physiological Requirements: The Hemoglobin System of Trout. Curr. Top. Cell. Regul. 1975;9:1–39. doi: 10.1016/b978-0-12-152809-6.50008-1. [DOI] [PubMed] [Google Scholar]
- 65.Brunori M., Coletta M., Giardina B., Wyman J. A macromolecular transducer as illustrated by trout hemoglobin IV. Proc. Natl. Acad. Sci. USA. 1978;75:4310–4312. doi: 10.1073/pnas.75.9.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ciaccio C., Coletta A., Coletta M. Role of hemoglobin structural-functional relationships in oxygen transport. Mol. Asp. Med. 2021;84:101022. doi: 10.1016/j.mam.2021.101022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data will be available upon request to the corresponding authors.