Abstract
To clarify the mechanisms underlying the antiproliferative effects of jasplakinolide, a cyclic depsipeptide from marine sponges, we examined whether jasplakinolide induces apoptosis in a variety of transformed and nontransformed cells. Jasplakinolide inhibited proliferation of human Jurkat T cells, resulting in cell death. This was accompanied by chromatin condensation and DNA cleavage at the linker regions between the nucleosomes. When caspase-3-like activity in the cytosolic extracts of Jurkat T cells was examined with a fluorescent substrate, DEVD-MAC (N-acetyl-Asp-Glu-Val-Asp-4-methyl-coumaryl-7-amide), the activity in the cells treated with jasplakinolide was remarkably increased in a time-dependent manner. Pretreatment of Jurkat T cells with the caspase inhibitor zVAD [benzyloxycarbonyl(Cbz)-Val-Ala-β-Asp(OMe)-fluoromethylketone] or DEVD-CHO (N-acetyl-Asp-Glu-Val-Asp-1-aldehyde) prevented the induction of apoptosis by jasplakinolide. Moreover, exposure of various murine transformed cell lines to jasplakinolide resulted in cell death, which was inhibited by zVAD. Although it has been well established that murine immature thymocytes are sensitive to apoptosis when exposed to various apoptotic stimuli, these cells as well as mature T lymphocytes were resistant to jasplakinolide-induced apoptosis. The results suggest that jasplakinolide induces apoptotic cell death through a caspase-3-like protease-dependent pathway. Another important outcome is that transformed cell lines were more susceptible to jasplakinolide-induced apoptosis than normal nontransformed cells.
There has been much interest in the biological properties of jasplakinolide (5) (jaspamide [38]), a cyclic depsipeptide isolated exclusively from marine sponges. The quest to define the bioactive constituents of the sponge extract from Jaspis johnstoni (order Astrophorida) (15), now known as Jaspis splendens (21), partially stimulated the original isolation, characterization, and further study of jasplakinolide's structural properties (13). Jasplakinolide was first described as having anthelminthic (6) and antifungal (23) properties. Quite unexpectedly, it was reencountered during bioassay-guided isolations with extract fractions from Auletta cf. constricta (order Halichondrida) possessing in vitro cytotoxicity to HT-29 cells (7). Jasplakinolide was also shown to possess potent antiproliferative activity in the NCI-60 cell line screen (7), which prompted extensive further investigation of its properties and mechanism of action. Additional studies demonstrated jasplakinolide to be especially active against a number of tumor-derived cell lines, human prostate carcinoma (7), and myeloid leukemia (10). The drug was observed to be active in vivo against Lewis lung carcinoma and human prostate carcinoma xenografts (30).
A number of investigators have found jasplakinolide to be an invaluable tool to probe cytoskeletal proteins. For example, Bubb et al. found that jasplakinolide induces actin polymerization in a similar fashion to phalloidin (2, 3). The results imply that jasplakinolide exerts its cytotoxic effect by inducing actin polymerization and/or inhibiting the depolymerization of actin filaments. In fact, it was demonstrated that exposure of living cells to jasplakinolide results in the formation of multinucleated cells and disruption of actin in these cells (12, 22, 24). Recently, this cell-permeable drug has been used to investigate the involvement of actin microfilaments in a variety of cellular processes, including cell movement, integrin-mediated adhesion, oocyte maturation, and transport in the endocytic pathways, including protein trafficking in the Golgi apparatus (4, 9, 11, 18, 25, 26, 28, 31). Thus, although it is clear that jasplakinolide has strong antiproliferative activity, additional information was needed to link this effect to one or more discrete mechanisms of action.
The results outlined below show that human Jurkat T cells exposed to jasplakinolide exhibit reduced proliferation. This indicates that jasplakinolide-induced cell death occurs by apoptosis via activation of a caspase-3-like protease or proteases. Thus, our findings may account for the antiproliferative properties of jasplakinolide, which have been described in a number of previous studies.
MATERIALS AND METHODS
Isolation of jasplakinolide.
Specimens of the marine sponge, Jaspis splendens, collected in Papua New Guinea, were extracted with 100% methanol (MeOH) to obtain a total polar extract. The general methods for sponge extract work-up are as follows. The crude oil obtained from the total polar extract was successively partitioned between equal volumes of aqueous MeOH, and hexanes followed by CH2Cl2, and the volumes were adjusted to produce a biphasic solution. The CH2Cl2 fraction was subjected to Sephadex LH-20 gel filtration chromatography in 30:70 CH2Cl2-MeOH, yielding eight fractions. The fourth fraction was then subjected to silica gel chromatography and normal-phase high-performance liquid chromatography on a silicon 60 preparative column (10:90 hexanes-ethyl acetate) to afford a pure fraction of jasplakinolide. All of its physical properties match those that have been previously published (5, 13).
Reagents.
Jasplakinolide obtained as described above was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mg/ml. Even with the highest concentration of the drug used in this study, the final concentration of DMSO was no more than 0.1% (vol/vol). Dexamethasone and propidium iodide (PI) were purchased from Sigma Chemical Co. (St. Louis, Mo.). Caspase inhibitors zVAD (benzyloxycarbonyl-Val-Ala-β-methyl-Asp-1-yl-fluoromethane) and DEVD-CHO (N-acetyl-Asp-Glu-Val-Asp-aldehyde) and the fluorogenic substrate DEVD-MAC (N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin) and AMC (7-amino-4-methylcoumarin) were obtained from the Peptide Institute (Osaka, Japan).
Mice.
Six-week-old female C57BL/6 mice were obtained from Clea Japan, Inc. (Tokyo, Japan).
Cells.
Human leukemia Jurkat T cells, murine T lymphoma EL-4 cells, murine myeloma SP-2/0 cells, murine macrophage-like J774.1 cells, and murine fibroblast L cells were maintained in RPMI 1640 medium (GIBCO, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal calf serum (FCS; Hyclone Laboratories, Logan, N.Y.), 5 × 10−5 M 2-mercaptoethanol (2-ME), 1 mM sodium pyruvate, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Thymocytes were prepared from C57BL/6 mice, suspended in RPMI 1640 medium supplemented with 10% FCS, 5 × 10−5 M 2-ME, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml, and then incubated in a 5% CO2 humidified air atmosphere. After C57BL/6 spleen cell suspensions were prepared, splenic T cells were enriched with nylon wool columns and resuspended in the same medium used for thymocytes.
Determination of cell proliferation.
Cells were incubated in the presence of jasplakinolide or DMSO (0.02%) for 24 or 48 h, followed by a 4-h incubation with 1 μCi of [methyl-3H]thymidine. Cells were then harvested on glass filter papers, and the rate of [3H]thymidine uptake was measured by liquid scintillation counting.
Assessment of apoptosis. (i) Trypan blue dye exclusion assay.
Cells were incubated in the presence of jasplakinolide or DMSO (0.02%), with or without caspase inhibitors for the indicated period, and then cell viability was assessed by trypan blue dye exclusion.
(ii) Morphologic analysis of chromatin.
After Jurkat T cells were incubated in the presence or absence of jasplakinolide or DMSO (0.02%) for 48 h, cells were harvested, washed in cold Dulbecco's phosphate-buffered saline (PBS), and fixed with 80% methanol at 4°C for 30 min. Cells were then rinsed with PBS and treated with RNase (10 μg/ml) at 37°C for 45 min. After treatment with RNase, cells were washed with PBS and resuspended in PBS containing PI (2 μg/ml). Observations were performed with an LSM 400 confocal microscope (Carl Zeiss Co., Ltd., Esslingen, Germany).
(iii) Detection of oligonucleosomal DNA.
Jurkat T cells were incubated in the presence of jasplakinolide or DMSO (0.02%) for 24 or 48 h. After the cells were collected, DNA was isolated, and then DNA fragmentation was analyzed by electrophoresis on a 1.5% agarose gel containing ethidium bromide. Ladder formation of oligonucleosomal DNA was detected under UV light.
(iv) LDH release assay.
Cells were cultured in 96-well microplates (5 × 105 cells/well) in the presence or absence of jasplakinolide or DMSO (0.1%) for 24 or 48 h. The lactate dehydrogenase (LDH) release assay was performed by using the CytoTox 96 nonradioactive cytotoxicity assay kit (Promega, Madison, Wis.). The A492 was determined in a microtiter plate reader. The percentage of LDH released was defined as the ratio of LDH activity in the supernatant to that of the sonicate whole-cell suspension.
Measurement of caspase-3-like activity.
A total of 2 × 106 cells were lysed in 100 μl of lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.2], 1% NP-40) containing the protease inhibitors (phenylmethysulfonyl fluoride, leupeptin, antipain, and pepstatin A) centrifuged at 20,000 × g for 15 min, and the supernatant was kept at −70°C. The 50 μg of cytosolic extract was diluted in 200 μl of dilution buffer (10 mM HEPES [pH 7.0], 40 mM β-glycerophosphate, 50 mM NaCl, 2 mM MgCl2, 5 mM EDTA, 1 mM dithiothreitol, supplemented with 0.1% CHAPS {3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate} and 100 μg of bovine serum albumin per ml) and incubated at 30°C for 30 min with 10 μM fluorescent substrate Ac-DEVD-MAC (Peptide Institute, Osaka, Japan). Release of AMC was measured in a fluorospectrophotometer with a filter setting of 380 nm (excitation) and 460 nm (emission). The increase in fluorescence was standardized by using free AMC (Peptide Institute, Osaka, Japan). Values are given as release of AMC in picomoles per minute per milligram of protein.
RESULTS
Jasplakinolide induces growth inhibition, resulting in cell death in Jurkat T cells.
The inhibitory effects of jasplakinolide on proliferation of human Jurkat T cells were examined by [3H]thymidine incorporation. As shown in Fig. 1a, when Jurkat T cells were treated with jasplakinolide at concentrations ranging from 0.25 to 2 μg/ml for 24 h, a dose-dependent decrease in [3H]thymidine incorporation was observed. At 48 h of incubation, the inhibition of cell proliferation was more significant in the cells treated with jasplakinolide.
FIG. 1.
Jasplakinolide induces cell death in human Jurkat T cells. (a) Inhibition of cell proliferation in jasplakinolide-treated Jurkat T cells. Jurkat T cells were cultured in the presence of jasplakinolide or DMSO (0.02%). The cell numbers in 96-well microtiter plates for 24 and 48 h of incubation were 5 × 103 and 2 × 103, respectively. After 24 or 48 h, cells were incubated with [3H]thymidine and harvested after an additional 4 h. Incorporation of [3H]thymidine into cellular DNA was measured in a scintillation counter. (b) Jasplakinolide induces cell death in Jurkat cells. Jurkat T cells were cultured with jasplakinolide or DMSO (0.02%). Twenty-four or 48 h later, cells were harvested, and then cell viability was assessed by trypan blue dye exclusion. The results represent one of three experiments and are expressed as the mean values ± standard deviations obtained from triplicate cultures.
Next, cell viability was examined by trypan blue dye exclusion at a 24-h interval after Jurkat T cells were cultured in the presence of jasplakinolide or DMSO (0.02%). As presented in Fig. 1b, cell death was observed at 24 h with exposure to 0.25 μg of jasplakinolide per ml, and the cell viability was gradually reduced in a dose-dependent manner. When Jurkat T cells were incubated with jasplakinolide (2 μg/ml) for 48 h, the percentage of cell viability was further diminished to approximately 20%.
Jasplakinolide induces apoptosis in Jurkat T cells.
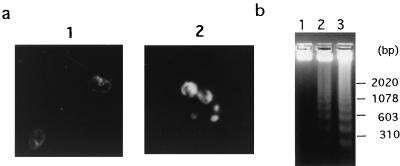
A morphological feature of apoptotic cell death is the condensation of chromatin and its marginalization at the nuclear periphery. The biochemical hallmark of apoptosis is the appearance of a fragmentation pattern in the chromatin, which is indicative of DNA cleavage at the linker regions between nucleosomes (37). Given this background, insights about how jasplakinolide induced cell death via apoptosis were obtained as follows. After exposure of the Jurkat T cells to jasplakinolide (2 μg/ml) or DMSO (0.02%) for 48 h, the cells were analyzed for nuclear morphological changes and/or cleavage of chromatin. The Jurkat T cells treated with jasplakinolide exhibited chromatin condensation, as shown by the morphological observations in Fig. 2a. Furthermore, when DNA from jasplakinolide-treated Jurkat T cells was subjected to agarose gel electrophoresis, DNA fragmentation, as demonstrated by nucleosomal DNA ladder formation, was also observed (Fig. 2b). Overall, these results indicate that jasplakinolide causes apoptotic cell death in Jurkat T cells.
FIG. 2.
Appearance of apoptosis in jasplakinolide-treated Jurkat cells. (a) Morphologic analysis of nuclear chromatin. Jurkat cells were cultured in the presence of jasplakinolide (2 μg/ml) or DMSO (0.02%) for 48 h. The cells were fixed, stained with PI, and then observed under a fluorescence microscope, as described in Materials and Methods. Panel 1, 0.02% DMSO-treated cells; panel 2, jasplakinolide-treated cells. (b) Detection of DNA fragmentation. Jurkat cells were cultured in the presence of jasplakinolide (2 μg/ml) or DMSO (0.02%). Twenty-four or 48 h later, DNA was isolated, and thereafter, 2 μg of each DNA was subjected to electrophoresis on a 1.5% agarose gel. Ladder formation of oligonucleosomal DNA was visualized under UV light. Lanes: 1, cells treated with DMSO (0.02%) for 48 h; 2, cells treated with jasplakinolide (2 μg/ml) for 24 h; 3, cells treated with jasplakinolide (2 μg/ml) for 48 h. The sizes of fragments serving as molecular size markers (HindIII digest of λ phage DNA plus HaeII digest of φX174) are shown to the right.
Enhanced caspase-3 activity is observed in jasplakinolide-treated Jurkat T cells.
In the last few years, a family of cysteine proteases, termed “caspases,” have been implicated in the effector process of apoptosis, which can operate in several systems (1). Although at least 14 members of the caspase family have been identified to date, caspase-3 (also known as CPP32, apopain, or YAMA) is thought to be a key enzyme in mammalian cells during the nuclear changes associated with apoptosis (33). In order to explore this phenomenon, we prepared cytosolic extracts from Jurkat T cells treated with 0.02% DMSO or jasplakinolide (2 μg/ml) and measured caspase-3-like activity by using the fluorescent substrate Ac-DEVD-MAC. Originally, cleavage of the amino acid sequence DEVD had been attributed to caspase-3 (16), but it can likely be catalyzed by several caspases (32). Therefore, the DEVD-cleaving activity observed here is called caspase-3-like activity. Figure 3 illustrates that an increase in caspase-3-like activity was observed in the extracts of Jurkat T cells treated with jasplakinolide for 3 h, unaccompanied by cell death. The latter was assessed by trypan blue dye exclusion (data not shown). Additionally, a significant increase in caspase-3-like activity was observed up to 9 h after treatment with jasplakinolide. These results indicate that jasplakinolide-induced apoptosis is preceded by an increase in caspase-3-like activity.
FIG. 3.
Jasplakinolide induces apoptosis through activation of caspase-3-like proteases in Jurkat T cells. Cultured cells were exposed to jasplakinolide (2 μg/ml) or DMSO (0.02%). At the indicated times, cells were harvested, and cytosolic extracts were prepared as described in Materials and Methods. Caspase-3-like activity was determined by excitation fluorometry of released AMC from the cleaved substrate DEVD-MAC. The results represent one of three experiments and are expressed as the mean values ± standard deviations obtained from triplicate cultures.
It was important to confirm the involvement of caspases in apoptosis as mediated by jasplakinolide. This was accomplished by examining the effect of specific inhibitors of caspases on cell death. The reagent zVAD, an inhibitor of caspases (27, 40), strongly inhibited cell death in Jurkat T cells treated with jasplakinolide, and this occurred in a dose-dependent manner (Fig. 4a). Similarly, DEVD-CHO, a preferential inhibitor of caspase-3-related caspases, also almost completely inhibited jasplakinolide-induced apoptosis in Jurkat T cells. Thus, pretreatment of Jurkat T cells with caspase inhibitors dramatically blocks jasplakinolide-induced cell death. These data suggest that caspase-3-like protease activity is involved in jasplakinolide-induced apoptosis in Jurkat T cells.
FIG. 4.
Caspase inhibitors prevent jasplakinolide-induced apoptosis in Jurkat T cells. Cultured cells were incubated for 1 h either alone or in the presence of the indicated concentrations of zVAD or DEVD-CHO. They were then further incubated for 24 or 48 h in the presence of jasplakinolide (2 μg/ml) or DMSO (0.02%). The percentages of apoptotic cells were assessed by trypan blue dye exclusion. The results represent one of three experiments and are expressed as the mean values ± standard deviations obtained from triplicate cultures.
Susceptibility of various murine transformed lines to jasplakinolide-induced apoptosis.
It was important to probe the likelihood of jasplakinolide-induced apoptosis in murine cell lines from various origins. The relevant experiments employed T-cell lymphoma EL-4 cells, myeloma SP-2/0 cells, macrophage-like J774.1 cells, and fibroblast L cells. The results shown in Fig. 5 demonstrate that cell death was observed when EL-4 cells, SP-2/0 cells, J774.1 cells, or L cells were cultured with jasplakinolide for 24 h. In addition, the DNA isolated from these cells displayed oligonucleosomal fragmentation (data not shown). Finally, pretreatment of these cells with zVAD (300 μM) resulted in a dramatic inhibition of the jasplakinolide-induced cell death.
FIG. 5.
Comparison of sensitivities to jasplakinolide-induced cell death of various murine cell lines. Murine T-cell lymphoma EL-4 cells (a), myeloma SP-2/0 cells (b), macrophage-like J774.1 cells (c), or fibroblast L cells (d) were incubated for 1 h either alone (■) or in the presence of 300 μM zVAD (□). They were then further incubated in the presence or absence of the indicated concentrations of jasplakinolide or DMSO (0.02%). After 24 h, the cells were harvested, and then the cell viability was assessed by trypan blue dye exclusion. The results represent one of three experiments and are expressed as the mean values ± standard deviations obtained from triplicate cultures.
Murine thymocytes and spleen T cells are resistant to jasplakinolide-induced cell death.
It has been well established that CD4+ CD8+ thymocytes are sensitive to apoptosis after treatment with various apoptotic stimuli, including dexamethasone. Alternatively, CD4− CD8−, CD4+CD8−, CD4− CD8+ thymocytes are resistant to apoptosis (29, 36). We examined the susceptibility of different subsets of murine T cells to jasplakinolide-induced apoptosis. Unfractionated thymocytes or splenic T cells of 6-week-old C57BL/6 mice were cultured in the presence or absence of jasplakinolide or 0.1% DMSO. After 24 or 48 h of incubation, cell viability was measured by determining LDH release.
The LDH results obtained during this study are shown in Fig. 6a. The level of cell death of untreated thymocytes or 0.1% DMSO-treated thymocytes was approximately 20% after 24 h of incubation. Cell viability in thymocytes treated with jasplakinolide at concentrations ranging from 1 to 10 μg/ml for 24 h remained at a level approximately that of 0.1% DMSO-treated thymocytes. In accordance with previous studies, treatment of unfractionated thymocytes with dexamethasone (10−7 M) for 24 h resulted in 65% cell death (36). The spontaneous apoptosis rate became higher after an additional 24 h of incubation. Exposure to jasplakinolide at 10 μg/ml resulted in a cell death rate of 40.3% at 48 h of incubation, compared with 42.2% in 0.1% DMSO-treated thymocytes. Thus, no change in cell death was observed in jasplakinolide-treated thymocytes, compared with 0.1% DMSO-treated and untreated thymocytes.
FIG. 6.
Murine thymocytes and splenic T cells are resistant to jasplakinolide-induced cell death. (a) Measurements of cell death in jasplakinolide-treated murine thymocytes. C57BL/6 thymocytes were treated with or without jasplakinolide, DMSO (0.1%), or dexamethasone (Dex) (10−7 M), as indicated. After 24 or 48 h, LDH release was determined as described in Materials and Methods. The results represent one of three experiments and are expressed as the mean values ± standard deviations obtained from triplicate cultures. The difference in cell death between control and Dex-treated cells was statistically significant at P < 0.001 (Student's t test), but the difference in cell death between control and jasplakinolide (5 or 10 μg/ml)-treated cells was not significant (P > 0.05). (b) Measurements of cell death in jasplakinolide-treated murine splenic T cells. Murine splenic T cells were prepared from C57BL/6 spleen cells. The cells were treated with or without jasplakinolide or DMSO (0.1%), as indicated. After 24 or 48 h, LDH release was determined as described in Materials and Methods. The results represent one of three experiments and are expressed as the mean values ± standard deviations obtained from triplicate cultures. The difference in cell death between control and jasplakinolide (5 or 10 μg/ml)-treated cells was not statistically significant (P > 0.05).
Finally, the sensitivity of native peripheral T cells to jasplakinolide was examined. Mature T cells are known to be resistant to the induction of apoptosis (29). The occurrence of spontaneous apoptotic cells in splenic T cells of C57BL/6 mice was not significant even after 48 h of incubation. There was an insignificant difference in LDH release between jasplakinolide-treated splenic T cells and control cells (Fig. 6b). These results suggest that native quiescent T cells may be resistant to jasplakinolide-induced apoptosis.
DISCUSSION
Jasplakinolide exhibits powerful antiproliferative activity in a number of tumor-derived cell lines, especially prostate carcinomas, breast epithelial cells, and myeloid leukemia cells (2, 3, 7, 30). In this study, we observed that jasplakinolide inhibited cell proliferation and subsequent cell death occurred in human leukemia Jurkat T cells. In addition, jasplakinolide-treated cells exhibited DNA cleavage and chromatin condensation, both of which are signature features of apoptosis. It has been reported that jasplakinolide enhances the apoptosis of CTLL-20 cells in response to interleukin 2 deprivation (17). Alternatively, a much simpler situation exists, as illustrated by Rao et al., who found that jasplakinolide alone induces apoptosis in human promyelocytic leukemia cell line HL-60 (20). Similarly, jasplakinolide induced apoptosis in the human leukemia Jurkat T-cell line.
Another relevant circumstance is that members of the caspase protease family, especially caspase-3, play a crucial role in the implementation of apoptosis (33). We found that the exposure of Jurkat T cells to jasplakinolide caused an increased caspase-3-like activity in a time-dependent manner. Furthermore, induction of apoptosis by jasplakinolide in Jurkat T cells was inhibited in the presence of a broad-spectrum caspase inhibitor, zVAD, or a caspase-3-like protease inhibitor, DEVD-CHO. Taken together, these results suggest the involvement of caspase-3-like protease(s) in jasplakinolide-induced apoptosis.
A broad array of experiments have demonstrated that jasplakinolide modulates actin polymerization (2, 12, 22, 24). It is interesting to consider that the well-documented antiproliferative activity of jasplakinolide may correlate with reorganization of the actin cytoskeleton. Perhaps relevant here is our observation of an increase in actin with the Triton-insoluble cytoskeleton in jasplakinolide-treated Jurkat T cells (data not shown). However, how the induction of actin polymerization by jasplakinolide leads to activation of caspase-3-like protease(s) in Jurkat T cells remains unclear.
We observed that jasplakinolide was capable of inducing apoptosis in various murine transformed lines, such as T-cell lymphoma EL-4 cells, myeloma SP-2/0 cells, macrophage-like J774.1 cells, and fibroblast L cells. Moreover, this cell death was inhibited by zVAD. Although murine thymocytes, especially immature double-positive thymocytes, are sensitive to cell death by various apoptotic stimuli, such as dexamethasone, phorbol ester, or calcium ionophore (29, 36), an insignificant increase in cell death was observed in jasplakinolide-treated murine thymocytes versus untreated thymocytes. Murine splenic T lymphocytes were also less susceptible to jasplakinolide-induced apoptosis. Thus, in contrast to the effects of jasplakinolide on various transformed cell lines, murine thymocytes as well as murine naive peripheral T lymphocytes were resistant to jasplakinolide at the concentrations used. These results are significant and indicate that transformed cell lines may be more sensitive to jasplakinolide-induced apoptosis than normal naive cells. It has been demonstrated that transformed cell lines and tumor cells contain less F-actin than normal cell lines and tissues (19, 34, 35). This evidence may explain the discrepancy between susceptibilities to jasplakinolide of various transformed cell lines and normal murine cells. Further experiments will be necessary to determine why various transformed cell lines are more sensitive to jasplakinolide-induced apoptosis than normal cells. It would also be relevant to further examine the relationship between induction of apoptosis and the molecular structure of the reagent by examining natural products related to jasplakinolide (39), such as jasplakinolide B (24), the geodiamolides (8), or the chondramides (14).
In summary, the present study shows that jasplakinolide induces cell death via apoptosis. There is an involvement of caspase-3-like protease action in jasplakinolide-induced apoptosis. In addition, it has been demonstrated that various transformed cell lines were more sensitive to jasplakinolide-induced apoptosis than normal, nontransformed cells. Finally, these results along with complementary findings in the literature indicate that the jasplakinolide pharmacophore has the potential to serve for the development of a novel class of anticancer agents.
REFERENCES
- 1.Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 2.Bubb M R, Senderowicz A M, Sausville E A, Duncan K L, Korn E D. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- 3.Bubb M R, Spector I, Beyer B B, Fosen K M. Effects of jasplakinolide on the kinetics of actin polymerization—an explanation for certain in vivo observations. J Biol Chem. 2000;275:5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- 4.Cramer L P. Role of actin-filament disassembly in lamellipodium protrusion in motile cells revealed using the drug jasplakinolide. Curr Biol. 1999;9:1095–1105. doi: 10.1016/s0960-9822(99)80478-3. [DOI] [PubMed] [Google Scholar]
- 5.Crews P, Manes L V, Boehler M. Jasplakinolide, a cyclodepsipeptide from the marine sponge Jaspis sp. Tetrahedron Lett. 1986;27:2797–2800. [Google Scholar]
- 6.Crews P, Hunter L M. The search for antiparasitic agents from marine animals. In: Zaborsky O R, Attaway D, editors. Marine biotechnology. New York, N.Y: Plenum Press; 1993. pp. 343–389. [Google Scholar]
- 7.Crews P, Farias J J, Emrich R, Keifer P A. Milnamide A, an unusual cytotoxic tripeptide from the marine sponge Auletta cf. constricta. J Org Chem. 1994;59:2932–2936. [Google Scholar]
- 8.de Silva E D, Anderson R J, Allen T M. Geodiamolides C to F, new cytotoxic cyclodepsipeptides from the marine sponge Pseudaxinyssa sp. Tetrahedron Lett. 1990;31:489–492. [Google Scholar]
- 9.di Campli A, Valderrama F, Babia T, De Matteis M A, Luini A, Egea G. Morphological changes in the Golgi complex correlate with actin cytoskeleton rearrangements. Cell Motil Cytoskelet. 1999;43:334–348. doi: 10.1002/(SICI)1097-0169(1999)43:4<334::AID-CM6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Fabian I, Shur I, Bleiberg I, Rudi A, Kashman Y, Lishner M. Growth modulation and differentiation of acute myeloid leukemia cells by jaspamide. Exp Hematol. 1995;23:583–587. [PubMed] [Google Scholar]
- 11.Fabian I, Halperin D, Lefter S, Mittelman L, Altstock R T, Seaon O, Tsarfaty I. Alteration of actin organization by jaspamide inhibits ruffling, but not phagocytosis or oxidative burst, in HL-60 cells and human monocytes. Blood. 1999;93:3994–4005. [PubMed] [Google Scholar]
- 12.Holzinger A, Meindl U. Jasplakinolide, a novel actin targeting peptide, inhibits cell growth and induces actin filament polymerization in the green alga Micrasterias. Cell Motil Cytoskelet. 1997;38:365–372. doi: 10.1002/(SICI)1097-0169(1997)38:4<365::AID-CM6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Inman W D, Crews P. Conformational analysis of jasplakinolide. J Am Chem Soc. 1989;111:2822–2829. [Google Scholar]
- 14.Kunze B, Jansen R, Sasse F, Hoefle G, Reichenbach H. Chondramides A-D, new antifungal and cytostatic depsipeptides from Chondromyces crocatus (Myxobacteria), production, physico-chemical and biological properties. J Antibiot. 1995;48:1262–1266. doi: 10.7164/antibiotics.48.1262. [DOI] [PubMed] [Google Scholar]
- 15.Murray L M, Johnson A, Crews P. Geographic variation in the tropical marine sponge Jaspis cf. johnstoni: an unexpected source of new terpene-benzenoids. J Org Chem. 1997;62:5638–5643. [Google Scholar]
- 16.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, Munday N A, Raju S M, Smulson M E, Yamin T T, Yu V L, Miller D K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 17.Posey S C, Bierer B E. Actin stabilization by jasplakinolide enhances apoptosis induced by cytokine deprivation. J Biol Chem. 1999;274:259–265. doi: 10.1074/jbc.274.7.4259. [DOI] [PubMed] [Google Scholar]
- 18.Poupel O, Tardieux I. Toxoplasma gondii motility and host cell invasiveness are drastically impaired by jasplakinolide, a cyclic peptide stabilizing F-actin. Microbes Infect. 1999;1:653–662. doi: 10.1016/s1286-4579(99)80066-5. [DOI] [PubMed] [Google Scholar]
- 19.Rao J Y, Hurst R E, Bales W D, Jones P L, Bass R A, Archer L T, Bell P B, Hemstreet G P. Cellular F-actin levels as a marker for cellular transformation: relationship to cell division and differentiation. Cancer Res. 1990;50:2215–2220. [PubMed] [Google Scholar]
- 20.Rao J Y, Jin Y S, Zheng Q, Cheng J, Tai J, Hemstreet G P. Alterations of the actin polymerization status as an apoptotic morphological effector in HL-60 cells. J Cell Biochem. 1999;75:686–697. [PubMed] [Google Scholar]
- 21.Sanders M, Diaz M C, Crews P. Taxonomic evaluation of jasplakinolide-containing sponges of the family Coppatiidae. Mem Queensl Mus. 1999;44:525–532. [Google Scholar]
- 22.Sawitzky H, Liebe S, Willingale-Theune J, Menzel D. The anti-proliferative agent jasplakinolide rearranges the actin cytoskeleton of plant cells. Eur J Cell Biol. 1999;78:424–433. doi: 10.1016/S0171-9335(99)80085-5. [DOI] [PubMed] [Google Scholar]
- 23.Scott V R, Boehme R, Matthews T R. New class of antifungal agents: jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis species. Antimicrob Agents Chemother. 1988;32:1154–1157. doi: 10.1128/aac.32.8.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senderowicz A M, Kaur G, Sainz E, Laing C, Inman W D, Rodriguez J, Crews P, Malspeis L, Grever M R, Sausville E A, Duncan K L. Jasplakinolide's inhibition of the growth of prostate carcinoma cells in vitro with disruption of the actin cytoskeleton. J Natl Cancer Inst. 1995;87:46–51. doi: 10.1093/jnci/87.1.46. [DOI] [PubMed] [Google Scholar]
- 25.Sheikh S, Gratzer W B, Pinder J C, Nash G B. Actin polymerisation regulates integrin-mediated adhesion as well as rigidity of neutrophils. Biochem Biophys Res Commun. 1997;238:910–915. doi: 10.1006/bbrc.1997.7407. [DOI] [PubMed] [Google Scholar]
- 26.Shurety W, Stewart N L, Stow J L. Fluid-phase markers in the basolateral endocytic pathway accumulate in response to the actin assembly-promoting drug jasplakinolide. Mol Biol Cell. 1998;9:957–975. doi: 10.1091/mbc.9.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slee E A, Zhu H, Chow S C, MacFarlane M, Nicholson D W, Cohen G M. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD-FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem J. 1996;315:21–24. doi: 10.1042/bj3150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart M P, McDowall A, Hogg N. LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J Cell Biol. 1998;140:699–707. doi: 10.1083/jcb.140.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tadakuma T, Kizaki H, Odaka C, Kubota R, Ishimura Y, Yagita H, Okumura K. CD4+CD8+ thymocytes are susceptible to DNA fragmentation induced by phorbol ester, calcium ionophore and anti-CD3 antibody. Eur J Immunol. 1990;20:779–784. doi: 10.1002/eji.1830200411. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi H, Ara G, Sausville E A, Teicher B. Jasplakinolide: interaction with radiation and hyperthermia in human prostate carcinoma and Lewis lung carcinoma. Cancer Chemother Pharmacol. 1998;42:491–496. doi: 10.1007/s002800050850. [DOI] [PubMed] [Google Scholar]
- 31.Terada Y, Simerly C, Schatten G. Microfilament stabilization by jasplakinolide arrests oocyte maturation, cortical granule exocytosis, sperm incorporation cone resorption, and cell-cycle progression, but not DNA replication, during fertilization in mice. Mol Reprod Dev. 2000;56:89–98. doi: 10.1002/(SICI)1098-2795(200005)56:1<89::AID-MRD11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 33.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 34.Varani J, Wass J A, Rao K M. Actin changes in normal human and rat leukocytes and in transformed human leukocytic cells. JNCI. 1983;70:805–809. [PubMed] [Google Scholar]
- 35.Verderame M, Alcorta D, Egnor M, Smith K, Pollack R. Cytoskeletal F-actin patterns quantitated with fluorescein isothiocyanate-phalloidin in normal and transformed cells. Proc Natl Acad Sci USA. 1980;77:6624–6628. doi: 10.1073/pnas.77.11.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyllie A H. Glucocorticoid-induced thymocytes apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 37.Wyllie A H, Morris R G, Simth A L, Dunpol D. Choromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984;142:66–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- 38.Zabriskie T M, Klocke J A, Ireland C M, Marcus A H, Molinski T F, Faulkner D J, Xu C, Clardy J C. Jaspamide, a modified peptide from a Jaspis sponge, with insecticidal and antifungal activity. J Am Chem Soc. 1986;108:3123–3124. [Google Scholar]
- 39.Zampella A, Giannini C, Debitus C, Roussakis C, D'Auria M V. New jaspamide derivatives from the marine sponge Jaspis splendans collected in Vanuatu. J Nat Prod. 1999;62:332–334. doi: 10.1021/np9803225. [DOI] [PubMed] [Google Scholar]
- 40.Zhu H, Fearnhead H O, Cohen G M. An ICE-like protease is a common mediator of apoptosis induced by diverse stimuli in human monocytic THP.1 cells. FEBS Lett. 1995;374:303–308. doi: 10.1016/0014-5793(95)01116-v. [DOI] [PubMed] [Google Scholar]