ABSTRACT
Peste des petits ruminants virus (PPRV) infection leads to autophagy, and the molecular mechanisms behind this phenomenon are unclear. Here, we demonstrate that PPRV infection results in morphological changes of the endoplasmic reticulum (ER) and activation of activating transcription factor 6 (ATF6) of the ER stress unfolded protein response (UPR). Knockdown of ATF6 blocked the autophagy process, suggesting ATF6 is necessary for PPRV-mediated autophagy induction. Further study showed that PPRV infection upregulates expression of the ER-anchored adaptor protein stimulator of interferon genes (STING), which is well-known for its pivotal roles in restricting DNA viruses. Knockdown of STING suppressed ATF6 activation and autophagy induction, implying that STING functions upstream of ATF6 to induce autophagy. Moreover, the STING-mediated autophagy response originated from the cellular pattern recognition receptor melanoma differentiation-associated gene 5 (MDA5). The absence of MDA5 abolished the upregulation of STING and the activation of autophagy. The deficiency of autophagy-related genes (ATG) repressed the autophagy process and PPRV replication, while it had no effect on MDA5 or STING expression. Overall, our work revealed that MDA5 works upstream of STING to activate ATF6 to induce autophagy.
IMPORTANCEPPRV infection induces cellular autophagy; however, the intracellular responses and signaling mechanisms that occur upon PPRV infection are obscure, and whether innate immune responses are linked with autophagy to regulate viral replication is largely unknown. Here, we uncovered that the innate immune sensor MDA5 initiated the signaling cascade by upregulating STING, which is best known for its role in anti-DNA virus infection by inducing interferon expression. We first provide evidence that STING regulates PPRV replication by activating the ATF6 pathway of unfolded protein responses (UPRs) to induce autophagy. Our results revealed that in addition to mediating responses to foreign DNA, STING can cross talk with MDA5 to regulate the cellular stress response and autophagy induced by RNA viruses; thus, STING works as an adaptor protein for cellular stress responses and innate immune responses. Modulation of STING represents a promising approach to control both DNA and RNA viruses.
KEYWORDS: STING, RNA virus, autophagy, PPRV, MDA5, UPR, ATF6
INTRODUCTION
Peste des petits ruminants (PPR) is a highly infectious, fatal viral disease in domestic and wild small ruminants, especially in goats and sheep (1–3). It has been listed by the World Organization for Animal Health (OIE) as an important and notifiable animal disease (4). The causative agent, Peste des petits ruminants virus (PPRV), is an enveloped negative-stranded RNA virus belonging to the family Paramyxoviridae, genus Morbillivirus (5). PPRV infection leads to high morbidity (up to 100%) and mortality (up to 90%) and is considered to cause great economic losses in developing countries (6). PPR is identified as one of the priority animal diseases for poverty alleviation in Western Africa and South Asia (4). The clinical syndromes caused by PPRV include pyrexia, stomatitis, diarrhea, and pneumonia. Abortion could occur in pregnant animals (7, 8). PPR was first detected in West Africa in 1942 (4). Currently, the disease is endemic in most of Africa, the Middle East, South Asia, and China (4, 9). For the control of PPR, vaccination is considered the most effective way, and several attenuated live vaccines have been developed (10–13); however, the vaccine is thermo-labile in subtropical climates and has high costs for production (4). Therefore, an improved understanding of the protective and detrimental responses elicited by PPRV is necessary for the successful control of this disease.
The innate immune response is rapidly activated when host pattern recognition receptors (PRRs) detect the pathogen-associated molecular patterns (PAMPs) of invading pathogens, including viruses, viral proteins, DNA, and RNA. It has been well-demonstrated that the cyclic GMP-AMP (cGAMP) synthase (cGAS) and retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) are the principal sensors for cytosolic DNA and RNA, respectively (14, 15). Upon DNA binding, cGAS is activated and catalyzes the production of the second messenger, cGAMP, which engages the endoplasmic reticulum (ER)-located protein the stimulator of interferon genes (STING) (16–18). Alternatively, upon RIG-I and MDA5 detection of viral RNA, these activated receptors engage and activate the mitochondrial antiviral signaling protein (MAVS) (19–22). The two adaptor proteins, STING and MAVS, subsequently recruit and phosphorylate the protein kinase TANK-binding kinase 1 (TBK1); in turn, the activated TBK1 phosphorylates these adaptors. Phosphorylated STING and MAVS then recruit interferon regulatory factor 3 (IRF3), which is phosphorylated by the activated TBK1 (23). Finally, phosphorylated IRF3 translocates to the nucleus and induces the production of type I interferon (IFN) to provide broad protection against DNA and RNA viruses and establish an antiviral cellular state (18, 24–26). Thus, the IFN responses induced by DNA and RNA viruses are generally considered to be regulated by the cGAS-STING and RIG-I/MDA5-MAVS pathway, respectively. Despite the distinctions between the RNA and DNA virus-sensing pathways when taking IFN expression into consideration, the absence of STING also increases sensitivity of cells or mice to several RNA viruses (27–30). Compared with wild-type cells, the replication of vesicular stomatitis virus (VSV) and dengue virus (DENV) were enhanced in fibroblasts lacking STING (18, 25, 31, 32), indicating that STING also displays activities during RNA virus infection. However, how STING affects the host response during RNA virus infection is unclear.
Autophagy is an evolutionarily conserved lysosomal degradation pathway by which the cell self-digests its own components (such as excessive or damaged organelles), misfolded proteins, and invading microorganisms to maintain cellular hemostasis and prevent nutritional, metabolic, and infection-mediated stresses (33–35). The process has three main steps, i.e., initiation, maturation, and elongation (36), and the key process depends on the formation of double-membrane vesicles called autophagosomes, which engulf and deliver cell components to the lysosome for degradation. The whole process is fine-tuned by over 20 conserved autophagy-related (ATG) proteins, and upon autophagy induction, ATGs are recruited to specific ER subdomains to play essential roles during autophagosome formation (33–35). The unfolded protein response (UPR) is characterized by three major proteins that lead to specific changes in transcriptional and translational programs in stressed cells. Activation of UPR can induce the autophagy program. There is increasing evidence for the role that UPR-induced autophagy plays in virus infection (37). Although several reports have demonstrated that PPRV infection can induce autophagy (38–40), the mechanism of autophagy induction and the impact of UPR on PPRV-mediated autophagy remain unknown.
In this study, we investigated the signaling mechanism of autophagy induction during PPRV infection, and we determined that the ER-anchored innate immune sensor STING triggered autophagy program via activating ATF6 of UPR. Furthermore, MDA5 is the essential prerequisite for initiating the PPRV-induced signaling cascades. As a functional consequence of autophagy induction by the MDA5-STING pathway, which has not been reported in previous studies, uncovering convergence of these intracellular PAMP recognition pathways would provide promising insights into the control of both DNA and RNA virus infections.
RESULTS
PPRV infection triggers disturbance of ER homeostasis.
It has been reported that PPRV infection results in autophagy, but the underlying mechanisms that trigger this conserved phenomenon have not been clarified. The multifunctional organelle ER is associated with several pathways involved in cellular homeostasis and survival (37). To explore whether PPRV affects ER homeostasis, morphology of ER was examined in PPRV-infected Vero cells. We observed that significant dilation was generated in the ER of infected cells at 48 h postinfection (hpi) (Fig. 1A). To further confirm that the ER swelling was induced by PPRV, we stained the ER in control and infected cells and found that the ER generated a fine reticular staining in infected Vero cells (Fig. 1B and C). As the protein synthesis factory, when the misfolded or mutant proteins accumulate, ER stress occurs (41). The major ER stress pathway is the UPR (42, 43), which is a potent stimulus of autophagy and has three branches that are mediated by three corresponding ER membrane-associated sensor proteins, PKR-like eukaryotic initiation factor 2 subunit α (eIF2α) kinase (PERK, also known as EIF2AK3), activating transcription factor-6 (ATF6), and inositol-requiring enzyme 1 (IRE1) (42). Next, we tested if infection with PPRV activates the typical ER stress response, UPR. As shown in Fig. 1D, with the increase of PPRV N, the PERK-eIF2α-ATF4 and IRE1-XBP1 pathways were not changed; however, the cleaved ATF6 increased, suggesting the activation of the ATF6 branch of UPR. Recently, the ER-transmembrane protein STING was identified as an important signaling mediator during RNA virus infection. Considering that ATF6 and STING are both ER-located proteins, we wondered whether STING exerts a role during PPRV infection. We found that infection with PPRV upregulated the protein level of STING from 48 hpi (Fig. 1E), indicating that STING has functions during PPRV replication. It has been reported that autophagy induction is a primordial function of the STING pathway, and we therefore analyzed the autophagy level and found that the onset of LC3-II production was at 48 hpi, which was coincident with STING upregulation and ATF6 activation (Fig. 1E). As the cGAS-STING pathway plays important roles in restricting DNA viruses, we naturally questioned whether the infection of PPRV also affects the expression of cGAS. To test this possibility, we monitored the expression of cGAS from 24 hpi to 72 hpi; interestingly, PPRV infection made no difference in cGAS expression (Fig. 1F). Recently, it has been demonstrated that STING inhibits global translation in a RIG-I/MDA5-dependent manner (44). We therefore examined the RNA virus pattern recognition receptors RIG-I and MDA5 by Western blotting; however, MDA5 but not RIG-I was upregulated at 48 hpi in infected cells (Fig. 1F). Taken together, these findings indicate that PPRV infection elicits cellular stress responses and disturbances of ER homeostasis.
FIG 1.

Infection of PPRV leads to ATF6 activation and STING upregulation. (A) Transmission electron microscopy (TEM) detection of morphological changes in ER. Vero cells were mock infected or infected with PPRV at an MOI of 1 for 48 h. (B) PPRV-infected (MOI = 1) and uninfected Vero cells were stained with ER Tracker Red at 48 hpi. The fluorescent signals were visualized by confocal immunofluorescence microscopy. Scale bar, 10 μm. (C) PPRV-infected (MOI = 1) and uninfected Vero cells were stained with ER Tracker Red at 48 hpi. The fluorescent signals were visualized by confocal immunofluorescence microscopy. Scale bar, 5 μm. (D) Western blot detection of N, phosphorylated PERK and eIF2α, XBP1, and cleaved ATF6 levels in PPRV-infected (MOI = 1) and uninfected control cells at the indicated time points. β-Tubulin was used as a loading control. (E) Western blot analysis of STING and LC3 in PPRV-infected (MOI = 1) and uninfected Vero cells. β-Tubulin was used as a loading control. (F) Western blot analysis of cGAS, RIG-I, and MDA5 in PPRV-infected (MOI = 1) and uninfected Vero cells (24, 48, and 72 hpi). β-Tubulin was used as a loading control.
PPRV activates autophagy through the ATF6 pathway of UPR.
Though UPR and autophagy are two different cellular programs which either work independently or coordinate to maintain cellular homeostasis against a diverse range of stresses. Autophagy is associated with the UPR by restricting protein production or removing misfolded proteins (37). Taking into consideration that ATF6 was activated during PPRV infection, we reasoned that ATF6 is involved in PPRV-induced autophagy. To test this hypothesis, we generated ATF6 stable knockdown Vero cells and verified our findings through Western blotting (Fig. 2A). As expected, knockdown of ATF6 abolished the PPRV-induced autophagy process, indicating the essential role of ATF6 in initiating autophagy (Fig. 2B and Fig. S1A in the supplemental material). Whereas the absence of ATF6 showed no effect on STING or MDA5 expression (Fig. 2B), one possible explanation for this is that ATF6 activation and STING/MDA5 upregulation are two independent events during PPRV infection, or that ATF6 works downstream of either or both of them. Next, viral replication was monitored, and we observed higher expression of N (Fig. 2C), accompanied by promoted PPRV mRNA level and viral titer (Fig. 2D and E). Considering PPRV takes advantage of autophagy to facilitate its replication (40), these results suggest that autophagy induction is at least not the mere function of ATF6 during PPRV infection and autophagy is not the only primary strategy manipulated by PPRV to favor its replication. The main function that UPR effectors share is to severely restrict global mRNA translation when transcription and translation of very specific cell fate effectors are upregulated (45). Moreover, although Vero cells have an inherent deficiency in producing IFN when infected with viruses, antiviral responses to PPRV infection through activation of the IFN response have been reported in HEK 293T cells (46). Indeed, we found that PPRV infection could activate TBK1 and NF-κB in wild-type cells (see Fig. S1B), and inactivation of TBK1, IRF3, and NF-κB in PPRV-infected Vero cells is one of the rational explanations for the increased susceptibility of ATF6 knockdown cells to PPRV (Fig. 2F).
FIG 2.
ATF6 is required for PPRV-mediated autophagy. (A) ATF6 silencing efficiency was verified by Western blotting. β-Tubulin was used as a loading control. (B) Western blot analysis of LC3, STING, and MDA5 levels in PPRV-infected (MOI = 1) and uninfected ATF6 KD cells (48 hpi). β-Tubulin was used as a loading control. (C) Western blot analysis of PPRV N protein in PPRV-infected wild-type and ATF6 KD cells (MOI = 1, 48 hpi). (D) PPRV mRNA level in PPRV-infected wild-type and ATF6 KD cells (MOI = 1, 48 hpi), measured by qPCR. The data show means ± standard deviations (SD; n = 3). **, P < 0.01. (E) Wild-type and ATF6 KD cells were infected with PPRV (MOI = 1), and virus titers were measured based on the TCID50 (48 hpi). The data show the means ± SD (n = 3). ***, P < 0.001. (F) Western blot detection of phosphorylated TBK1, IRF3, and NF-κB levels in PPRV-infected (MOI = 1) and uninfected ATF6 KD cells (48 hpi). β-Tubulin was used as a loading control.
STING functions upstream of ATF6 to induce autophagy.
To determine if the increased expression of STING in infected cells is associated with the PPRV-mediated autophagy, RNA interference technology was used to construct STING gene stable silencing cells that were confirmed by Western blot analysis (Fig. 3A). We assayed activation of autophagy, MDA5, and ATF6 in STING-deficient cells. At 48 hpi in PPRV-infected cells, Western blotting detected blockage of autophagy and inhibition of ATF6 activation, while the upregulation of MDA5 occurred when compared with mock-infected cells (Fig. 3B and Fig. S2). These results imply that STING is the essential upstream element for ATF6 activation and autophagy induction. To determine the role of STING in PPRV replication, wild-type and STING-deficient cells were infected with PPRV. The viral replication was then quantified by measuring the expression of PPRV structural protein N. The Western blot analysis showed a significant abundance of the viral protein in STING-deficient cells compared with the control (wild-type) cells (Fig. 3C). Similarly, the viral mRNA level and the production of the infectious virus were higher in STING-deficient cells than in control (wild-type) cells (Fig. 3D and E).
FIG 3.
STING serves as the upstream target of ATF6 to induce autophagy. (A) STING silencing efficiency was verified by Western blotting. β-Tubulin was used as a loading control. (B) Western blot analysis of LC3, cleaved ATF6, and MDA5 levels in PPRV-infected (MOI = 1) and uninfected STING KD cells (48 hpi). β-Tubulin was used as a loading control. (C) Western blot analysis of PPRV N protein in PPRV-infected (MOI = 1) wild-type and STING KD cells (48 hpi). (D) PPRV mRNA levels in PPRV-infected (MOI = 1) wild-type and STING KD cells (48 hpi) were measured by qPCR. The data show the means ± SD (n = 3). **, P < 0.01. (E) Wild-type and STING KD cells were infected with PPRV (MOI = 1), and virus titers were measured based on the TCID50 (48 hpi). The data show the means ± SD (n = 3). ***, P < 0.001.
Activation of STING-dependent autophagy by PPRV requires MDA5.
Giving the crucial roles of cGAS in the innate STING-dependent DNA-sensing pathway, we reasoned that the activation of cGAS may be a prerequisite for STING-mediated autophagy induction. To address this possibility, Vero cells were transduced with short hairpin RNAs (shRNAs) targeting cGAS (sh-cGAS) to the fully depleted cGAS protein in sh-cGAS-transduced cells, as validated by Western blot analysis (Fig. 4A). If the hypothesis were true, the activation of the STING-ATF6 pathway and the induction of autophagy flux would be silenced in the cGAS-deficient cells. Contrary to the expectation, Western blot analysis detected the formation of LC3-II, and immunofluorescence showed accumulated green LC3 punctate forms in PPRV-infected cGAS-deficient cells, whereas the mock-infected cells displayed a significant faint LC3-II band and exhibited no LC3 punctate accumulation (Fig. 4B and C). Meanwhile, the absence of cGAS exerted no impact on the expression of STING and MDA5, or on the activation of ATF6 (Fig. 4B). Western blotting revealed an equal amount of N protein in PPRV-infected wild-type and cGAS knockdown cells (Fig. 4D). Taken together, these results suggest that although cGAS is essential in initiating DNA virus-dependent innate responses, it is not required in the PPRV (an RNA virus)-induced STING-dependent autophagy response and viral infection.
FIG 4.

cGAS is dispensable for STING-dependent autophagy. (A) cGAS silencing efficiency was verified by Western blotting. β-Tubulin was used as a loading control. (B) Western blot analysis of LC3, STING, cleaved ATF6, and MDA5 levels in PPRV-infected (MOI = 1) and uninfected cGAS KD cells (48 hpi). β-Tubulin was used as a loading control. (C) PPRV-infected (MOI = 1) and uninfected cGAS KD cells were analyzed by immunofluorescence using anti-LC3 antibodies (green) at 48 hpi. The nuclei were stained with DAPI (blue). The fluorescent signals were visualized by confocal immunofluorescence microscopy. (D) Western blot analysis of PPRV N protein in PPRV-infected (MOI = 1) wild-type and cGAS KD cells (48 hpi).
Recently, interaction of RIG-I and MAVS with STING during RNA virus infection has been reported (44). We therefore attempted to inhibit RIG-I and MAVS expression by using the RNA interference technology described above to determine their roles in STING-mediated autophagy. Western blot analysis of RIG-I and MAVS indicated a significant decrease in corresponding shRNA-transduced cells, compared with control cells (Fig. 5A and 6A). Conversion from LC3-I to LC3-II and accumulation of green LC3 punctate forms were detected in both PPRV-infected RIG-I- and MAVS-deficient cells (Fig. 5B and C and Fig. 6B and C). Moreover, PPRV infection still upregulated the expression of MDA5 and STING and activated ATF6 under RIG-I- or MAVS-deficient conditions (Fig. 5B and 6B). To determine the role of RIG-I and MAVS during virus infection, wild-type, RIG-I-deficient, or MAVS-deficient cells were infected with PPRV and the viral replication was determined by using Western blotting to quantify the level of PPRV N. As expected, the absence of either RIG-I or MAVS made these cells more permissive for PPRV replication than counterparts (Fig. 5D and 6D), and these phenomena were in line with the fundamental and essential roles of RIG-I and MAVS in sensing and initiating the RNA virus-induced innate antiviral responses. These results rule out the involvement of RIG-I and MAVS in STING-dependent autophagy.
FIG 5.

STING-dependent autophagy is independent of RIG-I. (A) RIG-I silencing efficiency was verified by Western blotting. β-Tubulin was used as a loading control. (B) Western blot analysis of LC3, STING, cleaved ATF6, and MDA5 levels in PPRV-infected (MOI = 1) and uninfected RIG-I KD cells (48 hpi). β-Tubulin was used as a loading control. (C) PPRV-infected (MOI = 1) and uninfected RIG-I KD cells were analyzed by immunofluorescence using anti-LC3 antibodies (green) at 48 hpi. The nuclei were stained with DAPI (blue). The fluorescent signals were visualized by confocal immunofluorescence microscopy. (D) Western blot analysis of PPRV N protein in PPRV-infected (MOI = 1) wild-type and RIG-I KD cells (48 hpi).
FIG 6.

MAVS is not required for STING-dependent autophagy. (A) MAVS silencing efficiency was verified by Western blotting. β-Tubulin was used as a loading control. (B) Western blot analysis of LC3, STING, cleaved ATF6, and MDA5 levels in PPRV-infected (MOI = 1) and uninfected MAVS KD cells (48 hpi). β-Tubulin was used as a loading control. (C) PPRV-infected (MOI = 1) and uninfected MAVS KD cells were analyzed by immunofluorescence using anti-LC3 antibodies (green) at 48 hpi. The nuclei were stained with DAPI (blue). The fluorescent signals were visualized by confocal immunofluorescence microscopy. (D) Western blot analysis of PPRV N protein in PPRV-infected (MOI = 1) wild-type and MAVS KD cells (48 hpi).
Next, we sought to explore the potential role of MDA5 in STING-dependent autophagy. MDA5- deficient cells were therefore constructed by transducing sh-MDA5 to Vero cells and the protein level of MDA5 was examined; Western blot analysis showed that the expression of MDA5 was significantly reduced in sh-MDA5-transduced cells (Fig. 7A). The roles of MDA5 in the STING pathway and in autophagy were determined in sh-MDA5 cells. Intriguingly, infection with PPRV was unable to upregulate STING and failed to activate ATF6 to induce autophagy (Fig. 7B and Fig. S3), indicating that MDA5 is the essential sensor that works upstream of STING to induce autophagy during PPRV infection. Not surprisingly, compared with controls, MDA5-deficient cells were more sensitive to PPRV infection, as demonstrated with dramatically higher N, virus mRNA levels, and viral titers (Fig. 7C to E). This effect was associated with the basic role of MDA5 in initiating innate responses against RNA viruses; in addition, the inactivation of TBK1, IRF3, and NF-κB pathways in MDA5-deficent cells infected with PPRV may confer more permission for viral replication (Fig. 7F). Taken together, these results suggest that it is MDA5 and not cGAS or RIG-I that is essential for STING-dependent autophagy.
FIG 7.
MDA5 is essential for STING-dependent autophagy. (A) MDA5 silencing efficiency was verified by Western blotting. β-Tubulin was used as a loading control. (B) Western blot analysis of LC3, STING, and cleaved ATF6 levels in PPRV-infected (MOI = 1) and uninfected MDA5 KD cells (48 hpi). β-Tubulin was used as a loading control. (C) Western blot analysis of PPRV N protein in PPRV-infected wild-type (MOI = 1) and MDA5 KD cells (48 hpi). (D) PPRV mRNA level in PPRV-infected (MOI = 1) wild-type and MDA5 KD cells (48 hpi) were measured by qPCR. The data show the means ± SD (n = 3). ***, P < 0.001. (E) Wild-type and MDA5 KD cells were infected with PPRV (MOI = 1), and virus titers were measured by TCID50 (48 hpi). The data show the means ± SD (n = 3). **, P < 0.01. (F) Western blot detection of phosphorylated TBK1, IRF3, and NF-κB levels in PPRV-infected (MOI = 1) and uninfected MDA5 KD cells (48 hpi). β-Tubulin was used as a loading control.
Upregulation of STING and activation of ATF6 do not require autophagy.
Autophagy is a conserved key process of eukaryotes to maintain cellular homeostasis with coordination of several ATG proteins. Upon autophagy activation, ATGs are recruited to the subdomains close to ER to play essential roles (47). To determine the role of autophagy in ER homeostasis, inhibition of autophagy was achieved by transducing shRNAs targeting ATG7 or ATG5 to Vero cells, and the protein levels of ATG7 and ATG5 were assessed via Western blotting (Fig. 8A and 9A). ATG7 works at the initiation of autophagy to activate the ubiquitin-like proteins, such as ATG12. Once activated by ATG7, ATG12 forms a complex with ATG5 and ATG16L, which is responsible for the elongation of phagophore. Knockdown of ATG7 or ATG5 can block the autophagy process (48). As expected, in the absence of either ATG7 or ATG5, infection of PPRV did not induce autophagy (Fig. 8B and 9B; see also Fig. S4A and B). However, the upregulation of MDA5 and STING and the activation of ATF6 still occurred in PPRV-infected cells, regardless of the absence of ATG7 or ATG5 (Fig. 8B and 9B). The deficiency of ATG7 or ATG5 inhibited virus-specific N protein expression, as Western blotting revealed (Fig. 8C and 9C). Furthermore, quantitative analysis showed that the mRNA levels of PPRV in ATG7- or ATG5-deficient cells were markedly decreased compared to wild-type controls (Fig. 8D and 9D), and similar trends were also observed in a tissue culture 50% infective dose (TCID50) assay (Fig. 8E and 9E), which were consistent with previous reports that PPRV makes use of autophagy to facilitate its replication (38–40). These results imply that upregulation of MDA5 and STING and activation of ATF6 do not require autophagy.
FIG 8.
The absence of ATG7 had no effect on the upregulation of STING. (A) ATG7 silencing efficiency was verified by Western blotting. β-Tubulin was used as a loading control. (B) Western blot analysis of LC3, STING, cleaved ATF6, and MDA5 levels in PPRV-infected (MOI = 1) and uninfected ATG7 KD cells (48 hpi). β-Tubulin was used as a loading control. (C) Western blot analysis of PPRV N protein in PPRV-infected wild-type and ATG7 KD cells (MOI = 1, 48 hpi). (D) PPRV mRNA levels in PPRV-infected wild-type and ATG7 KD cells (MOI = 1, 48 hpi) were measured by qPCR. The data show the means ± SD (n = 3). *, P < 0.05. (E) Wild-type and ATG7 KD cells were infected with PPRV (MOI = 1), and virus titers were measured by TCID50 (48 hpi). The data show the means ± SD (n = 3). *, P < 0.05.
FIG 9.
Infection with PPRV upregulated STING in ATG5-deficient cells. (A) ATG5 silencing efficiency was verified by Western blotting. β-Tubulin was used as a loading control. (B) Western blot analysis of LC3, STING, cleaved ATF6, and MDA5 levels in PPRV-infected (MOI = 1) and uninfected ATG5 KD cells (48 hpi). β-Tubulin was used as a loading control. (C) Western blot analysis of PPRV N protein in PPRV-infected wild-type and ATG5 KD cells (MOI = 1, 48 hpi). (D) PPRV mRNA levels in PPRV-infected wild-type and ATG5 KD cells (MOI = 1, 48 hpi) were measured by qPCR. The data show the means ± SD (n = 3). *, P < 0.05. (E) Wild-type and ATG5 KD cells were infected with PPRV (MOI = 1), and virus titers were measured by TCID50 (48 hpi). The data show the means ± SD (n = 3). *, P < 0.05.
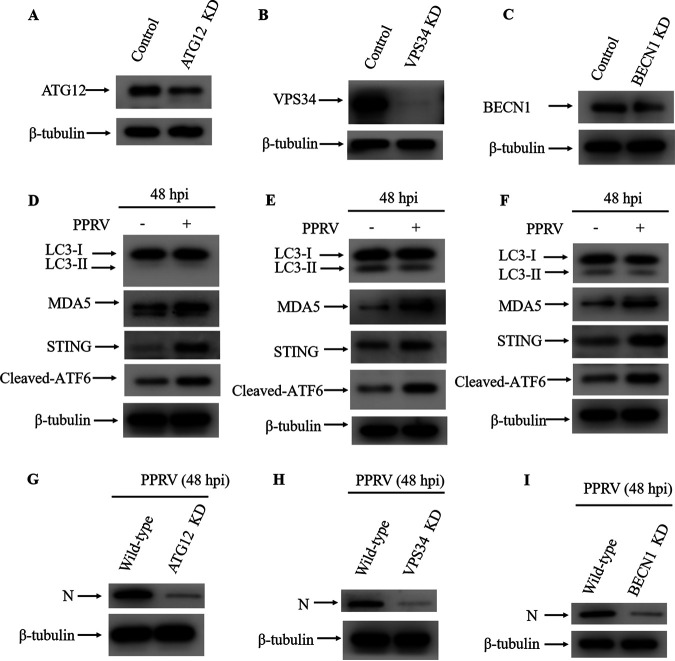
PPRV-mediated autophagy induction is dependent on ATG12, VPS34, and BECN1.
To investigate the molecular mechanism of STING-mediated autophagy, the key molecules of the canonical autophagy pathway, ATG12, VPS34, and BECN1, were inhibited using shRNAs. The process of phagophore formation is controlled by phosphoinositide 3-kinase (P13K) complex, which consists of the catalytic subunit of P13K, vacuolar protein sorting 34 (VPS34), VPS15, ATG14, and Beclin 1 (BECN1) (49–52). ATG12 conjugates with ATG5 and acts as an E3-like enzyme, transferring LC3 to phosphatidylethanolamine (PE) in the autophagosome membrane in the last step (53, 54). As Western blot analysis showed, ATG12, VPS34, and BECN1 were all substantially reduced in cells that had been transduced with the corresponding shRNA (Fig. 10A to C). To determine the role of ATG12, VPS34, and BECN1 in STING-mediated autophagy during PPRV infection, autophagy levels in ATG12-, VPS34-, or BECN1-deficient cells were examined by Western blotting. In the absence of ATG12, VPS34, or BECN1, autophagy was abrogated, while MDA5 and STING were still upregulated and ATF6 was activated in the infected cells compared to uninfected cells (Fig. 10D to F). In addition, reduction of viral protein was observed in ATG12-, VPS34-, or BECN1-deficient cells (Fig. 10G to I). These data demonstrate that ATG12, VPS34, and BECN1 are required for the induction of PPRV-induced autophagy.
FIG 10.
PPRV-induced autophagy is dependent on ATG12, VPS34 and BECN1. (A) ATG12 silencing efficiency was verified by Western bloting. β-Tubulin was used as a loading control. (B) VPS34 silencing efficiency was verified by Western blotting. β-Tubulin was used as a loading control. (C) BECN1 silencing efficiency was verified by Western blotting. β-Tubulin was used as a loading control. (D) Western blot analysis of LC3, STING, cleaved ATF6, and MDA5 levels in PPRV-infected (MOI = 1) and uninfected ATG12 KD cells (48 hpi). β-Tubulin was used as a loading control. (E) Western blot analysis of LC3, STING, cleaved ATF6, and MDA5 levels in PPRV-infected (MOI = 1) and uninfected VPS34 KD cells (48 hpi). β-Tubulin was used as a loading control. (F) Western blot analysis of LC3, STING, cleaved ATF6, and MDA5 levels in PPRV-infected (MOI = 1) and uninfected BECN1 KD cells (48 hpi). β-Tubulin was used as a loading control. (G) Western blot analysis of PPRV N protein in PPRV-infected wild-type and ATG12 KD cells (MOI = 1, 48 hpi). (H) Western blot analysis of PPRV N protein in PPRV-infected wild-type and VPS34 KD cells (MOI = 1, 48 hpi). (I) Western blot analysis of PPRV N protein in PPRV-infected wild-type and BECN1 KD cells (MOI = 1, 48 hpi).
DISCUSSION
Peste des petits ruminants virus, a member of the Paramyxoviridae family within the Morbillivirus genus, causes highly infectious, fatal viral disease in small ruminants and has been rapidly spreading in many parts of Africa, the Middle East, and Asia. Although vaccination is considered an effective way to control PPR, the heat-sensitive nature of the vaccines against PPRV and high costs for its production greatly limit their application in areas with a hot climate. It has been reported by several research groups that PPRV infection leads to autophagy; however, the upstream molecular mechanisms of autophagy induction are unclear. In the present study, we found that PPRV activated autophagy through the RNA sensor MDA5, which acts through STING to activate ATF6 (see Fig. S5 in the supplemental material).
Intracellular antiviral responses can be initiated through the recognition of the double-stranded RNAs (dsRNAs) generated by the replication of invading viruses. The cytoplasmic helicase proteins RIG-I and MDA5 have been identified as dsRNA sensors. Although RIG-I and MDA5 share similar signaling features (55) and structural homology (56), a growing body of evidence suggests that the two detectors discriminate among different ligands to trigger the innate immune response to RNA viruses (57–59). Hiroki et al. found that MDA5 recognizes poly(I·C) and RIG-I detects in vitro-transcribed dsRNAs. RNA viruses were also differentially recognized by RIG-I and MDA5. RIG-I is essential in eliciting the responses against several RNA viruses, including Newcastle disease virus, Sendai virus, and VSV (57). Recently, RIG-I has been implicated in the recognition of RNA moieties that contain 5′-triphosphate ends (60–62) and of RNAs that have complex secondary structures (58, 63). By comparison, MDA5 is essential to signaling uniquely elicited during picornavirus infections or in the presence of poly(I·C) (57, 64). Loo et al. found that in cultured fibroblasts, RIG-I and MDA5 were both dispensable for signaling triggered by reovirus and DENV, whereas RIG-I was essential for signaling triggered by influenza A virus, influenza B virus, and human respiratory syncytial virus (65). Here, we provide the evidence that MDA5 has essential roles in the initiation of PPRV-induced autophagy signaling.
The function and modulation of STING has been well-studied under either self or foreign origin cytosolic DNA stimulation (66). However, its role and mechanism during RNA virus infection remain largely elusive. Several possible mechanisms by which STING might restrict RNA virus replication have been explored (18, 25, 27, 31, 44). It has been reported that STING influences IFN expression during RNA virus infection (18, 31, 32); in contrast to this, several reports indicated that STING does not regulate RNA-induced IFN expression (25, 26). We found that PPRV infection indeed activated IFN signaling, and the loss of STING inhibited the activation of this pathway (data not shown). However, with the genetic incompetence of Vero cells to produce IFN, we were unable to measure and determine the role of STING in PPRV-induced IFN expression. In fact, the activities of STING are not only to induce IFN by which cells restrict viral replication. More recently, Franz et al. uncovered that STING has a translation-inhibitory function during VSV infection or upon transfection of synthetic RIG-I like receptor (RLR) ligands (44), indicating that STING is a multifunctional mediator in host defense against RNA viruses, but the mechanisms by which STING modulates the cellular response to RNA virus infection need to be clarified. In the present study, we found that STING is responsible for PPRV-mediated UPR and autophagy.
Autophagy induction has been identified as an evolutionarily conserved function of the STING signaling pathway. Gui et al. found that during DNA virus infection or cGAMP stimulation, STING is essential for autophagy initiation (67). Liu et al. showed that STING directly activates autophagy by recruiting LC3 (68). Julien et al. found that STING induces autophagy via sensing the viability-associated PAMP c-di-AMP upon infection with the bacterium Listeria innocua (69). These studies indicate that the induction of autophagy is one of the means by which STING counteracts DNA viruses and bacteria. We found that STING is essential for authophagy induced by the RNA virus foot and mouth disease virus (70); however, whether STING plays a role in PPRV-induced autophagy is unknown. Here, we first proved that STING is essential for PPRV-induced ATF6-dependent autophagy signaling, indicating the importance of this pathway for sensing viral pathogens and modulating cellular responses. PPRV replication is facilitated by autophagy (38–40), and the deficiency of ATF6, STING, and MDA5 prevents induction of autophagy. However, in each case the absence of the protein decreased replication of PPRV. One explanation could be that loss of these proteins enhances replication by reducing interferon signaling, but Vero cells have defects in interferon signaling.
DNA virus infection leads to trafficking and subsequent degradation of STING in the perinuclear lysosomal compartment (71, 72). Unlike DNA viruses, RNA virus infection does not cause degradation of STING (44, 72). In contrast, infection of some RNA viruses seems to upregulate the expression of STING (73). In line with this, we found that STING was upregulated during PPRV infection. It is likely that STING mediates antiviral responses to DNA and RNA viruses by distinct and context-dependent mechanisms (74). The coordinated action between RIG-I and STING in RNA virus-induced cellular responses has been reported (18, 73). STING contributes to the sensing of RNA viruses through the RNA sensor RIG-I, but not the related RLR protein MDA5. Moreover, the absence of STING does not affect the host response to MDA5-dependent stimuli, namely, infection with the RNA virus encephalomyocarditis virus (18). A recent study demonstrated that STING triggers a global translation inhibition in a RIG-I/MDA5-dependent but MAVS-independent way (44). Our work showed that during PPRV infection, STING associated with MDA5 rather than RIG-I to induce MAVS-independent UPR, which is a common strategy that cells often use to inhibit translation and maintain homeostasis of the ER. Little is known about the mechanisms that RIG-I and MDA5 use to recognize different RNA virus infections and to transduce signals to STING after sensing viral RNA.
In this study, we explored the molecular pathway that contributes to autophagy in response to PPRV infection, and we identified STING as an adaptor protein between cellular stress responses and innate immune responses. Though autophagy is a potential target for drug development, the components of the autophagy pathway (ATG genes) have been difficult to identify (37). Our work provides a deeper understanding of these pathways and sheds light on the potential molecular targets of both DNA and RNA viruses.
MATERIALS AND METHODS
Cell culture.
African green monkey kidney (Vero) cells were obtained from ATCC (CCL-81). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 μg/mL penicillin, and 100 μg/mL streptomycin as monolayers in cell culture flasks or dishes at 37°C in a humidified atmosphere of 5% CO2 in air (Binder catalog number CB160).
Antibodies and reagents.
The rabbit polyclonal antibodies anti-STING (D1V5L; catalog number 50494S) and anti-LC3B (catalog number 2775s), anti-phospho-NF-κB (93H1; catalog number 3033S), anti-IRF3 (D614C; catalog number 11904S), anti-phospho-IRF3 (S396 and 4D4G; catalog number 4947P), and anti-phospho-TBK1/NAK XP (Ser172 and D52C2; catalog number 5483S) and mouse monoclonal antibody anti-NF-κB (L8F6; catalog number 6956T) were from Cell Signaling Technology. Mouse monoclonal antibody anti-β-tubulin (catalog number 66240-1-Ig) and rabbit polyclonal antibodies anti-XBP1 (catalog number 24864-1-AP), anti-ATF4 (catalog number 10835-1-AP), anti-EIF2S1 (catalog number 11170-1-AP), anti-PERK (catalog number 24390-1-AP), anti-MAVS (catalog number 14341-1-AP), anti-IFIH1 (anti-MDA-5; catalog number 21775-1-AP), and anti-DDX58 (anti-RIG-I; catalog number 20566-1-AP) were obtained from Proteintech. Mouse monoclonal antibody anti-ATF6 (catalog number ab11909) and rabbit polyclonal antibodies anti-ATF6 (catalog number ab37149), anti-VPS34 (catalog number ab233437), anti-BECN1 (catalog number ab62557), anti-ATG12 (catalog number ab155589), anti-phospho-EIF2S1 (E90; Ser51; catalog number ab32157), anti-ATG7 (catalog number ab133528), and rabbit recombinant monoclonal antibody anti-NAK/TBK1 (EP611Y; catalog number ab40676) were from Abcam. Rabbit polyclonal antibody anti-phospho-PERK (Thr981; catalog number sc-32577) was from Santa Cruz Biotechnology. Rabbit polyclonal antibody anti-cGAS (catalog number ABF124) was from EMD Millipore Corp. Rabbit polyclonal antibody anti-ATG5 (catalog number NB110-53818) was obtained from Novus Biologicals. Horseradish peroxidase conjugated goat anti-mouse IgG secondary antibody (catalog number 170-6516) and goat anti-rabbit IgG secondary antibody (catalog number 170-6515) were from Bio-Rad. ER-Tracker Red (catalog number E34250) was from Invitrogen.
Virus propagation.
PPRV Nigeria 75/1 strain was obtained from Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Science (Lanzhou, Gansu, People’s Republic of China). Stock virus was applied to monolayers of Vero cells for adsorption. After 60 min at 37°C, monolayers were washed with phosphate-buffered saline (PBS) to remove unbound virus and after that replenished with fresh DMEM containing 2% FBS. Supernatant fluids were harvested at 6 days postinfection and clarified at 10,000 × g for 30 min before storage at −80°C.
TCID50 assay.
Vero cells cultured in 96-well plates were inoculated with 100-μL volumes of 10-fold serial viral dilutions. Each dilution had 8 duplications. Following adsorption at 37°C for 1 h, virus supernatants in the wells were discarded and cultures were supplemented with fresh medium with 2% FBS. Cells were incubated for another 168 h. Viral titers were determined by the Reed and Muench method (75) and expressed as the TCID50.
Transmission electron microscopy.
Vero cells were mock infected or infected with PPRV Nigeria 75/1 at a multiplicity of infection (MOI) of 1 for 48 h. After infection for 48 h, cells were scraped and harvested by centrifugation at 10,000 rpm for 5 min. Cells were fixed in 2.5% glutaraldehyde overnight at 4°C and then washed three times with PBS, followed by postfixing with 1% osmium tetroxide for 3 h at 4°C with shaking. After three washes with PBS, samples were dehydrated in a series of graded ethanol solutions and embedded in Spurr’s plastic resin. Cells were then polymerized at 70°C in a drying oven overnight. Ultrathin sections of 70 nm were made using an ultramicrotome (Ultracut R, Leica, Germany), mounted onto copper mesh grids, and stained with uranyl acetate and lead citrate. A JEM1230 transmission electron microscope (JEOL, Japan) was used to obtain images of the sections.
Western blotting.
Vero cells were harvested and washed with cold PBS at the indicated time points, then lysed in protease inhibitor and phosphatase inhibitor-containing RIPA lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS; Beyotime catalog number P0013B) supplemented with 1% 100 mM phenylmethylsulfonyl fluoride. Whole-cell extracts were clarified by centrifugation at 16,000 rpm for 10 min at 4°C. Protein concentrations were determined using a bicinchoninic acid protein assay kit (Thermo Scientific catalog number 23225). Protein samples were denatured in equivalent 2× SDS-PAGE sample buffer (Sigma-Aldrich catalog number S3401) by heating for 5 min at 95°C. Proteins were separated on a 10% electrophoresis acrylamide gel and then were transferred to polyvinylidene fluoride membranes (Millipore catalog number ISEQ00010) at 200 mA for 2 h. Membranes were blocked with 5% nonfat milk powder in Tris-buffered saline with Tween 20 (TBST) for 2 h at room temperature (RT) and then incubated with primary antibodies overnight at 4°C. After washing with TBST three times, membranes were incubated with peroxidase-conjugated secondary antibodies for 1 h at RT. Bound antibodies were visualized using the Clarity Western ECL substrate (Bio-Rad). Mouse horseradish peroxidase-coupled monoclonal antibody specific to β-tubulin (Proteintech catalog number 66240-1-Ig) was used as a loading control. Bands were detected using a GE Health Care Amersham Imager 600 in automatic exposure mode to make sure that bands were not saturated.
Quantitative real-time PCR.
Cells were harvested at 48 hpi, and total RNA was extracted using the RNeasy Plus Universal minikit (Qiagen catalog number 73404) according to the given protocol. The first-strand cDNA was synthesized using the Maxima H Minus cDNA synthesis master mix with dsDNase (Thermo Scientific catalog number M1682). PowerUp SYBR green master mix (Applied Biosystems catalog number 1801040) was used to perform the quantitative real-time PCR, and the thermal cycling conditions were set as the manufacturer’s manual indicated. The primers used in qPCR were as follows: PPRV forward, 5′-AGAGTTCAATATGTTRTTAGCCTCCAT-3′; PPRV reverse, 5′-TTCCCCARTCACTCTYCTTTGT-3′; GAPDH forward, 5′-CGAGATCCCTCCAAAATCAA-3′; GAPDH reverse, 5′-TGACGATCTTGAGGCTGTTG-3′.
RNA interference.
To construct lentiviral shRNA vectors, shRNAs were designed using BLOCK-iT RNAi Designer (Invitrogen). The shRNAs used in this study were all synthesized by Sangon Biotech (Shanghai, China) and cloned into a pLKO.1-TRC cloning vector following a standard protocol. Vero cells cultivated in 6-well cell culture plates were transfected with RNA interference oligonucleotides using Lipofectamine 3000 (Invitrogen) based on the manufacturer’s instructions. The targeted sequences were as follows: ATG7, 5′-GCAAATGAGATATGGGAATCC-3′; ATG5, 5′-GCTTCGAGATGTGTGGTTTGG-3′; ATF6, 5′-GCATTTGGAAGCAGCAAATGA-3′; MDA5, 5′-GGGTGGTGATGATGAGTATTG-3′; STING, 5′-GCATTACAACCACCTGCTACG-3′; cGAS, 5′-GCTACTACGAGCACGTGAAGA-3′; RIG-I, 5′-GCCCTAGACCATGCAGGTTAT-3′; MAVS, 5′-GCTGAAGACAAGACCTATAAG-3′; ATG12, 5′-GCTGTGGGAGATACTCCTATT-3′; VPS34, 5′-GGATATCAACGTCCAGCTTAA-3′; BECN1, 5′-GGTCTAAGACGTCCAACAACA-3′.
Lentivirus packaging and infection.
For packaging lentivirus, 1.5 μg psPAX2 packaging plasmid (Addgene catalog number 12260), 1 μg pMD2.G envelope plasmid (Addgene catalog number 12259), and 2 μg pLKO.1 plasmid were cotransfected into 4 × 106 Lenti-X 293T cells with Lipofectamine 3000 transfection reagent (Thermo catalog number L3000015). Supernatant was collected at 36 hpi, filtered, and stored at −80°C. Vero cells were incubated with viral particles in the presence of 8 μg/mL Polybrene (Solarbio catalog number H8761) for 24 h and treated with 5 μg/mL puromycin (Invitrogen catalog number A1113803) for 3 days. Protein expression levels were determined by Western blot analysis.
Immunofluorescence.
For immunofluorescence, Vero cells were seeded into glass-bottom dishes and infected with PPRV Nigeria 75/1 at an MOI of 1 for 48 h. The cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.2% Triton X-100 for 10 min, blocked in 5% bovine serum albumin in PBS for 1 h at room temperature, and incubated with the primary antibody at 4°C overnight followed by incubation with a fluorescent secondary antibody (Alexa Fluor 488; Thermo catalog number A11070) at RT for 1 h. Nuclei were labeled by staining with 4′,6-diamidino-2-phenylindole (DAPI) in the mounting medium (Vector catalog number H-1500). Images were obtained using a confocal fluorescence microscope (SP8, Leica, Germany).
ACKNOWLEDGMENTS
This work was supported by Southwest Minzu University Research Startup Funds (125900/16011211013, 2021115), Gansu Province Science and Technology Planning Project (20YF3WA008), and the National Natural Science Foundation of Sichuan Province (2022NSFSC0073).
We gratefully thank Guangqing Liu (Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Shanghai, China) for providing the mouse monoclonal antibody against the N protein of PPRV.
We declare no conflict of interest, financial or otherwise.
Footnotes
Supplemental material is available online only.
Contributor Information
Zhidong Zhang, Email: zhangzhidong@swun.edu.cn.
Yanmin Li, Email: liyanmin@swun.edu.cn.
Tom Gallagher, Loyola University Chicago.
REFERENCES
- 1.Abubakar M, Mahapatra M, Muniraju M, Arshed MJ, Khan EH, Banyard AC, Ali Q, Parida S. 2017. Serological detection of antibodies to Peste des petits ruminants virus in large ruminants. Transbound Emerg Dis 64:513–519. 10.1111/tbed.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalafalla AI, Saeed IK, Ali YH, Abdurrahman MB, Kwiatek O, Libeau G, Obeida AA, Abbas Z. 2010. An outbreak of peste des petits ruminants (PPR) in camels in the Sudan. Acta Trop 116:161–165. 10.1016/j.actatropica.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Zakian A, Nouri M, Kahroba H, Mohammadian B, Mokhber-Dezfouli MR. 2016. The first report of peste des petits ruminants (PPR) in camels (Camelus dromedarius) in Iran. Trop Anim Health Prod 48:1215–1219. 10.1007/s11250-016-1078-6. [DOI] [PubMed] [Google Scholar]
- 4.Kumar N, Maherchandani S, Kashyap SK, Singh SV, Sharma S, Chaubey KK, Ly H. 2014. Peste des petits ruminants virus infection of small ruminants: a comprehensive review. Viruses 6:2287–2327. 10.3390/v6062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu F, Li J, Li L, Liu Y, Wu X, Wang Z. 2018. Peste des petits ruminants in China since its first outbreak in 2007: a 10-year review. Transbound Emerg Dis 65:638–648. 10.1111/tbed.12808. [DOI] [PubMed] [Google Scholar]
- 6.Singh RK, Balamurugan V, Bhanuprakash V, Sen A, Saravanan P, Pal Yadav M. 2009. Possible control and eradication of peste des petits ruminants from India: technical aspects. Vet Ital 45:449–462. [PubMed] [Google Scholar]
- 7.Jagtap SP, Rajak KK, Garg UK, Sen A, Bhanuprakash V, Sudhakar SB, Balamurugan V, Patel A, Ahuja A, Singh RK, Vanamayya PR. 2012. Effect of immunosuppression on pathogenesis of peste des petits ruminants (PPR) virus infection in goats. Microb Pathog 52:217–226. 10.1016/j.micpath.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Truong T, Boshra H, Embury-Hyatt C, Nfon C, Gerdts V, Tikoo S, Babiuk LA, Kara P, Chetty T, Mather A, Wallace DB, Babiuk S. 2014. Peste des petits ruminants virus tissue tropism and pathogenesis in sheep and goats following experimental infection. PLoS One 9:e87145. 10.1371/journal.pone.0087145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maan S, Kumar A, Gupta AK, Dalal A, Chaudhary D, Gupta TK, Bansal N, Kumar V, Batra K, Sindhu N, Kumar A, Mahajan NK, Maan NS, Mertens PPC. 2018. Concurrent infection of Bluetongue and Peste-des-petits-ruminants virus in small ruminants in Haryana State of India. Transbound Emerg Dis 65:235–239. 10.1111/tbed.12610. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar J, Sreenivasa BP, Singh RP, Dhar P, Bandyopadhyay SK. 2003. Comparative efficacy of various chemical stabilizers on the thermostability of a live-attenuated peste des petits ruminants (PPR) vaccine. Vaccine 21:4728–4735. 10.1016/s0264-410x(03)00512-7. [DOI] [PubMed] [Google Scholar]
- 11.Singh RP, De UK, Pandey KD. 2010. Virological and antigenic characterization of two Peste des Petits Ruminants (PPR) vaccine viruses of Indian origin. Comp Immunol Microbiol Infect Dis 33:343–353. 10.1016/j.cimid.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Singh RP. 2011. Control strategies for peste des petits ruminants in small ruminants of India. Rev Sci Tech 30:879–887. 10.20506/rst.30.3.2079. [DOI] [PubMed] [Google Scholar]
- 13.Saravanan P, Sen A, Balamurugan V, Bandyopadhyay SK, Singh RK. 2008. Rapid quality control of a live attenuated Peste des petits ruminants (PPR) vaccine by monoclonal antibody based sandwich ELISA. Biologicals 36:1–6. 10.1016/j.biologicals.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Goubau D, Deddouche S, Reis e Sousa C. 2013. Cytosolic sensing of viruses. Immunity 38:855–869. 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding SW. 2010. RNA-based antiviral immunity. Nat Rev Immunol 10:632–644. 10.1038/nri2824. [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Wu J, Du F, Chen X, Chen ZJ. 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791. 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, Shu HB. 2008. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29:538–550. 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa H, Barber GN. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455:674–678. 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 6:981–988. 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 20.Seth RB, Sun L, Ea CK, Chen ZJ. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122:669–682. 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 21.McWhirter SM, Tenoever BR, Maniatis T. 2005. Connecting mitochondria and innate immunity. Cell 122:645–647. 10.1016/j.cell.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. 2005. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell 19:727–740. 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Cai X, Wu J, Cong Q, Chen X, Li T, Du F, Ren J, Wu YT, Grishin NV, Chen ZJ. 2015. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347:aaa2630. 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]
- 24.Lin R, Mamane Y, Hiscott J. 1999. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol Cell Biol 19:2465–2474. 10.1128/MCB.19.4.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa H, Ma Z, Barber GN. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461:788–792. 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. 2013. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341:1390–1394. 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing Y, Chen J, Tu J, Zhang B, Chen X, Shi H, Baker SC, Feng L, Chen Z. 2013. The papain-like protease of porcine epidemic diarrhea virus negatively regulates type I interferon pathway by acting as a viral deubiquitinase. J Gen Virol 94:1554–1567. 10.1099/vir.0.051169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Q, Cao X, Lu J, Huang B, Liu YJ, Kato N, Shu HB, Zhong J. 2013. Hepatitis C virus NS4B blocks the interaction of STING and TBK1 to evade host innate immunity. J Hepatol 59:52–58. 10.1016/j.jhep.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Holm CK, Rahbek SH, Gad HH, Bak RO, Jakobsen MR, Jiang Z, Hansen AL, Jensen SK, Sun C, Thomsen MK, Laustsen A, Nielsen CG, Severinsen K, Xiong Y, Burdette DL, Hornung V, Lebbink RJ, Duch M, Fitzgerald KA, Bahrami S, Mikkelsen JG, Hartmann R, Paludan SR. 2016. Influenza A virus targets a cGAS-independent STING pathway that controls enveloped RNA viruses. Nat Commun 7:10680. 10.1038/ncomms10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Yang X, Zheng Y, Yang Y, Xing Y, Chen Z. 2014. SARS coronavirus papain-like protease inhibits the type I interferon signaling pathway through interaction with the STING-TRAF3-TBK1 complex. Protein Cell 5:369–381. 10.1007/s13238-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, Gutman D, Maringer K, Bernal-Rubio D, Shabman RS, Simon V, Rodriguez-Madoz JR, Mulder LC, Barber GN, Fernandez-Sesma A. 2012. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog 8:e1002934. 10.1371/journal.ppat.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu CY, Chang TH, Liang JJ, Chiang RL, Lee YL, Liao CL, Lin YL. 2012. Dengue virus targets the adaptor protein MITA to subvert host innate immunity. PLoS Pathog 8:e1002780. 10.1371/journal.ppat.1002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesseraud S, Avril P. 2020. Autophagy in farm animals: current knowledge and future challenges. Autophagy 17:1809–1827. 10.1080/15548627.2020.1798064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizushima N. 2018. A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol 20:521–527. 10.1038/s41556-018-0092-5. [DOI] [PubMed] [Google Scholar]
- 35.Levine B, Kroemer G. 2019. Biological functions of autophagy genes: a disease perspective. Cell 176:11–42. 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen M, Rubinsztein DC, Walker DW. 2018. Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol 19:579–593. 10.1038/s41580-018-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhardwaj M, Leli NM, Koumenis C, Amaravadi RK. 2020. Regulation of autophagy by canonical and non-canonical ER stress responses. Semin Cancer Biol 66:116–128. 10.1016/j.semcancer.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang B, Xue Q, Guo J, Wang X, Zhang Y, Guo K, Li W, Chen S, Xue T, Qi X, Wang J. 2020. Autophagy induction by the pathogen receptor NECTIN4 and sustained autophagy contribute to peste des petits ruminants virus infectivity. Autophagy 16:842–861. 10.1080/15548627.2019.1643184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang B, Xue Q, Qi X, Wang X, Jia P, Chen S, Wang T, Xue T, Wang J. 2018. Autophagy enhances the replication of Peste des petits ruminants virus and inhibits caspase-dependent apoptosis in vitro. Virulence 9:1176–1194. 10.1080/21505594.2018.1496776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Wu S, Lv J, Feng C, Deng J, Wang C, Yuan X, Zhang T, Lin X. 2013. Peste des petits ruminants virus exploits cellular autophagy machinery for replication. Virology 437:28–38. 10.1016/j.virol.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 41.van Anken E, Braakman I. 2005. Versatility of the endoplasmic reticulum protein folding factory. Crit Rev Biochem Mol Biol 40:191–228. 10.1080/10409230591008161. [DOI] [PubMed] [Google Scholar]
- 42.Kroemer G, Mariño G, Levine B. 2010. Autophagy and the integrated stress response. Mol Cell 40:280–293. 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchberger A, Bukau B, Sommer T. 2010. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell 40:238–252. 10.1016/j.molcel.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Franz KM, Neidermyer WJ, Tan YJ, Whelan SPJ, Kagan JC. 2018. STING-dependent translation inhibition restricts RNA virus replication. Proc Natl Acad Sci USA 115:E2058–E2067. 10.1073/pnas.1716937115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollien J, Weissman JS. 2006. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313:104–107. 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 46.Ma P, Li L, Jin L, Zhang D, Cao X, Guo F, Zhao Y, Bai J, Ma Z, Shang Y, Ma XX. 2020. Antiviral responses of ATG13 to the infection of peste des petits ruminants virus through activation of interferon response. Gene 754:144858. 10.1016/j.gene.2020.144858. [DOI] [PubMed] [Google Scholar]
- 47.Molino D, Nascimbeni AC, Giordano F, Codogno P, Morel E. 2017. ER-driven membrane contact sites: evolutionary conserved machineries for stress response and autophagy regulation? Commun Integr Biol 10:e1401699. 10.1080/19420889.2017.1401699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. 2005. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169:425–434. 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itakura E, Kishi C, Inoue K, Mizushima N. 2008. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 19:5360–5372. 10.1091/mbc.e08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, Maejima I, Shirahama-Noda K, Ichimura T, Isobe T, Akira S, Noda T, Yoshimori T. 2009. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol 11:385–396. 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 51.Sun Q, Fan W, Chen K, Ding X, Chen S, Zhong Q. 2008. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA 105:19211–19216. 10.1073/pnas.0810452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, Heintz N, Yue Z. 2009. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol 11:468–476. 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. 2000. A ubiquitin-like system mediates protein lipidation. Nature 408:488–492. 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 54.Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. 1998. A protein conjugation system essential for autophagy. Nature 395:395–398. 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 55.Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo Y-M, Gale M, Akira S, Yonehara S, Kato A, Fujita T. 2005. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol 175:2851–2858. 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 56.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5:730–737. 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 57.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh C-S, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105. 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 58.Sumpter R, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol 79:2689–2699. 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. 2007. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol 81:1401–1411. 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann K-K, Schlee M, Endres S, Hartmann G. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997. 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 61.Pichlmair A, Schulz O, Tan CP, Näslund TI, Liljeström P, Weber F, Reis e Sousa C. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′ phosphates. Science 314:997–1001. 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 62.Plumet S, Herschke F, Bourhis JM, Valentin H, Longhi S, Gerlier D. 2007. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS One 2:e279. 10.1371/journal.pone.0000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saito T, Hirai R, Loo Y, Owen D, Johnson C, Sinha S, Akira S, Fujita T, Gale M. 2007. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci USA 104:582–587. 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. 2006. Essential role of MDA-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA 103:8459–8464. 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loo Y-M, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, García-Sastre A, Katze MG, Gale M. 2008. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol 82:335–345. 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Q, Sun L, Chen ZJ. 2016. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 17:1142–1149. 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 67.Gui X, Yang H, Li T, Tan X, Shi P, Li M, Du F, Chen ZJ. 2019. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 567:262–266. 10.1038/s41586-019-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu D, Wu H, Wang C, Li Y, Tian H, Siraj S, Sehgal SA, Wang X, Wang J, Shang Y, Jiang Z, Liu L, Chen Q. 2019. STING directly activates autophagy to tune the innate immune response. Cell Death Differ 26:1735–1749. 10.1038/s41418-018-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moretti J, Roy S, Bozec D, Martinez J, Chapman JR, Ueberheide B, Lamming DW, Chen ZJ, Horng T, Yeretssian G, Green DR, Blander JM. 2017. STING senses microbial viability to orchestrate stress-mediated autophagy of the endoplasmic reticulum. Cell 171:809–823.e813. 10.1016/j.cell.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang R, Qin X, Yang Y, Zhu X, Zhao S, Zhang Z, Su Q, Zhao Z, Yin X, Meng X, Zhang Z, Li Y. 2022. STING1 is essential for an RNA-virus triggered autophagy. Autophagy 18:816–828. 10.1080/15548627.2021.1959086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konno H, Konno K, Barber G. 2013. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell 155:688–698. 10.1016/j.cell.2013.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ni G, Konno H, Barber GN. 2017. Ubiquitination of STING at lysine 224 controls IRF3 activation. Sci Immunol 2. 10.1126/sciimmunol.aah7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y, Goulet ML, Sze A, Hadj SB, Belgnaoui SM, Lababidi RR, Zheng C, Fritz JH, Olagnier D, Lin R. 2016. RIG-I-mediated STING upregulation restricts herpes simplex virus 1 infection. J Virol 90:9406–9419. 10.1128/JVI.00748-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ni G, Ma Z, Damania B. 2018. cGAS and STING: at the intersection of DNA and RNA virus-sensing networks. PLoS Pathog 14:e1007148. 10.1371/journal.ppat.1007148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Epidemiol 27:493–497. 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S5. Download jvi.01375-22-s0001.pdf, PDF file, 0.7 MB (767.7KB, pdf)